Abstract
As they differentiate, thymocytes encounter spatially restricted cues critical for differentiation and selection of a functional, self-tolerant T cell repertoire. Sequential migration of developing T cells through distinct thymic microenvironments is enforced by the ordered expression of chemokine receptors. Herein, we provide an updated perspective on T cell differentiation through the lens of recent advances that illuminate the dynamics of chemokine-driven thymocyte migration, localization, and interactions with stromal cells. We consider these findings in the context of earlier groundwork exploring the contribution of chemokines to T cell development, recent advances regarding the specificity of chemokine signaling, and novel techniques for evaluating the T cell repertoire. We suggest future research should amalgamate visualization of localized cellular interactions with downstream molecular signals.
Potential contributions of chemokines to the roadmap of T cell development
T cells develop in the thymus, where they interact with stromal cells that deliver signals essential for thymocyte survival, proliferation, differentiation, and T cell receptor (TCR) repertoire selection. The thymic stromal compartment is comprised of hematopoietic cells, including dendritic cells (DC), and non-hematopoietic cells, including endothelial cells (EC) and thymic epithelial cells (TEC) (see Glossary), which are organized in the two major thymic compartments, the cortex and the medulla. As thymocytes differentiate, they sequentially migrate through thymic regions, where they interact with localized stromal subsets (Figure 1, Box 1). Thymocyte-stromal cell interactions are critical at each stage of thymocyte development, including recruitment of thymic seeding progenitors (TSP), T-lineage commitment, βselection, positive selection, negative selection, regulatory T cell (Treg) induction, agonist selection, and thymocyte egress. Thus, elucidating the mechanisms by which developing thymocytes traffic through and interact with the thymic microenvironment will not only enlighten our understanding of T cell differentiation, but will also inform therapeutic strategies to improve T cell production for inherited or induced immunodeficiencies[1].
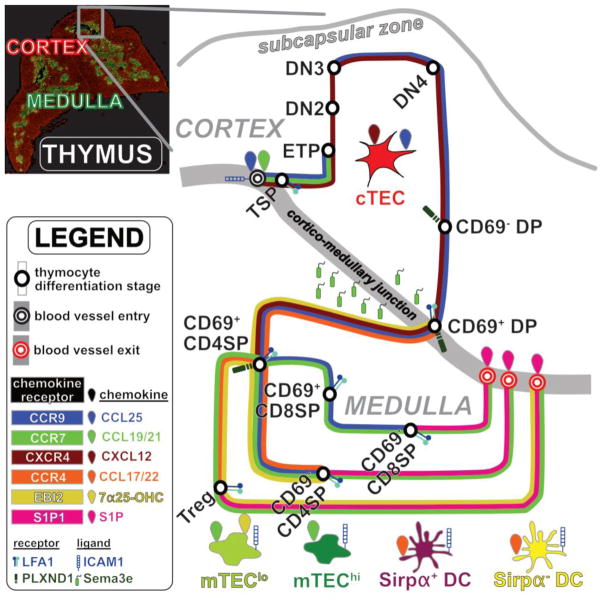
Figure 1.
Chemokines coordinate the transit map of thymocyte differentiation.
Immunofluorescence image of a thymus cross-section (top left) reveals organization into distinct cortical and medullary compartments. An expanded view (right) depicts the migratory pathway of a thymocyte as a transit system, in which the differentiating thymocyte is successively guided through different regions of the thymus by altered expression of distinct chemokine receptors (also see Box 1). Expression of chemokine receptors by thymocyte subsets is denoted by colored lines along the route of thymocyte differentiation. Stromal cell subsets depicted include cTEC, MHCIIlo mTEC (mTEClo), MHCIIhi mTEC (mTEChi), Sirpα+DC, Sirpα−DC, and EC at thymic entry and exit sites. These stromal cells express chemokines and other adhesion molecules to promote recruitment of and interactions with distinct thymocyte subsets.
BOX 1. Overview of T cell Development.
Thymic seeding progenitors (TSP) enter the thymus through vasculature at the CMJ[15]. ECs promote TSP thymic entry, survival, and differentiation[18–20]. TSP give rise to early thymocyte progenitors (ETP; CD3−CD4−CD8−c-Kit+CD44+CD25−) in the thymic parenchyma, which differentiate into double negative 2 cells (DN2; CD3−CD4−CD8−c-Kit+CD44+CD25+) that migrate into the mid-cortex. ETP and DN2 cells interact with cTEC, to activate signaling molecules such as NOTCH1, IL-7R, and CXCR4 that promote their survival, differentiation, and/or T-lineage commitment (reviewed in [31,32]). T-lineage committed DN2 thymocytes rearrange TCRβ gene segments, and differentiate into double negative 3 cells (DN3; CD3−CD4−CD8−c-Kit−CD44−CD25+). DN2 and DN3 cells migrate towards the thymic capsule. DN3 cells accumulate at the SCZ, where they undergo β-selection, ensuring survival, proliferation and differentiation of only those cells that productively rearranged a TCRβ gene (reviewed in [32]). β-selection requires signaling through the pre-TCR, composed of TCRβ paired with pre-Tα. Following β-selection, thymocytes transiently progress through the double negative 4 stage (DN4; CD3−CD4−CD8−c-Kit−CD44−CD25−), and then, in the postnatal mouse thymus, upregulate CD8 and proliferate as immature CD8 single positive cells (CD8 ISP; CD3−CD4−CD8+), before expressing CD4 to become pre-selection double positive cells (CD69− DP; CD3−CD4+CD8+CD69−). CD69−DP cells, which are quiescent and located throughout the cortex, initiate TCRα gene rearrangements before undergoing positive selection. Positive selection enables survival of only those DP cells that express a TCRαβ receptor capable of signaling in response to self-pMHC complexes presented by cTEC. Following positive selection, DP cells rapidly upregulate CD69 (CD69+ DP; CD3−CD4+CD8+CD69+). Strong TCR signaling can lead to negative selection at the CD69+DP and subsequent stages (reviewed in [51]). CD69+ DP cells migrate into the medulla, where they downregulate either CD4 or CD8 to become immature CD4 or CD8 single positive cells (CD69+ CD4SP; CD3+CD4+CD8−CD69+or CD69+ CD8SP; CD3+CD4−CD8+CD69+). SP cells interact with mTEC, DC, and B cells in the medulla that display highly diverse self-pMHC complexes to induce central tolerance. Central tolerance ensures that thymocytes that receive a strong TCR signal in response to self-pMHC complexes on medullary APCs undergo either apoptosis, through negative selection, or differentiation into the regulatory T cell lineage (Treg; CD3+CD4+Foxp3+CD25+), through agonist selection. SP cells mature in the medulla, where they downregulate CD69 (CD69− CD4SP; CD3+CD4+CD8−CD69− or CD69− CD8SP; CD3+CD4− CD8+CD69−), prior to exiting the thymus through vasculature at the CMJ to join the repertoire of T cells in the periphery.
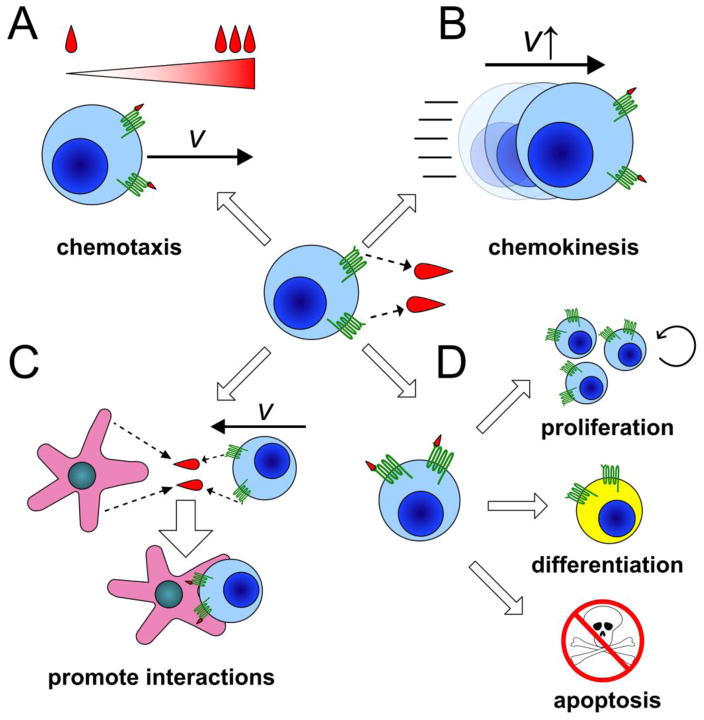
Chemokine receptors, members of the G protein-coupled receptor (GPCR) superfamily, are essential for directing thymocyte motility, localization, and interactions with stromal cells. About 20 chemokine receptors and 50 chemokines have been identified[2]. Chemokine receptors are differentially expressed by thymocyte subsets[3,4], while chemokines are expressed by spatially restricted stromal cells [5] (Figure 1). Chemokines can exert diverse biological effects: they are best known for promoting chemotaxis towards areas with elevated chemokine concentrations, but can also induce chemokinesis, integrin-mediated cell-cell adhesion, survival, proliferation, and differentiation[2] (Figure 2). Individual chemokine receptors can bind multiple ligands, and individual chemokines can bind multiple receptors. Interestingly, distinct ligands can induce different conformational changes in the same receptor, resulting in activation of alternate signaling pathways, or “biased signaling”[6]. Given their spatial confinement and diverse biological activities, chemokines are ideally poised to contribute to thymocyte localization, interactions with stromal cells, and resultant signals that drive T cell differentiation.
Figure 2.
Chemokine receptors can have multiple modes of action in the thymus.
Signaling through chemokine receptors (center) can result in a wide array of cellular outcomes, including A) chemotaxis in response to a concentration gradient formed by chemokines, B) chemokinesis, C) cell-cell interactions, and/or D) alterations in cell cycle progression, differentiation, and/or survival. All of these chemokine receptor activities have been shown to impact different stages of thymocyte differentiation.
Here, we review the contribution of chemokines and chemokine receptors to postnatal thymocyte differentiation and selection (Table 1). We focus on conclusions drawn from mouse studies, unless otherwise specified. Notably, chemokine receptor expression and chemokine responsiveness are largely conserved between mouse and human thymocytes [13]. Because chemokines promote cell motility, we also focus on live imaging approaches. In many of these studies, thymocyte migration was imaged in ex vivo thymic slices [7–9] because technical constraints preclude intravital imaging within the central medullary region of the postnatal thymus. We note, however, that intravital imaging of the thymus has been achieved in zebrafish and medaka embryos [10,11] and in the cortex of transplanted neonatal mouse thymi [12]. We place findings from live imaging studies of adult thymocyte migration in the context of recent updates regarding TCR repertoire selection. Finally, we consider how results from multiple current imaging approaches could be considered together to further our understanding of the contribution of chemokines to thymocyte differentiation.
Table 1.
Thymic chemokines receptors/GPCRs, ligands and their respective functions.
| Chemokine Receptors | Expression on | Ligand chemokines | Presentation from | Function | Reference |
|---|---|---|---|---|---|
| CXCR4 | TSP, ETP, DN2, DN3, DN4, CD8 ISP, CD69− DP, CD69+ DP | CXCL12 | cTEC | Survival and localization of DN cells; Co-stimulation of the pre-TCR to mediate survival and differentiation during β-selection | [33,36] |
| CCR9 | TSP, DN3-4, CD8 ISP, CD69− DP, CD69+ DP, CD69+ CD4SP, CD69+ CD8SP, pDC | CCL25 | cTEC | TSP thymic entry; Potentially DN2-3 cell cortical localization | [21,34,38] |
| CCR7 | TSP, CD4SP, Treg, CD8SP, Sirpα−DC, Sirpα+DC | CCL19, CCL21 | mTEC | TSP thymic entry, Cortical to medullary migration of SP cells needed for negative selection and mTEC maturation; rapid SP motility; TCR signaling; thymic Treg recirculation | [8,21,22,25,35,53,54,56] |
| CCR4 | CD69+ DP, CD69+ CD4SP | CCL17, CCL22 | DC | Cortical to medullary migration of post-positive selection DP and CD4SP cells needed for negative selection against low-avidity self-antigens | [55,56] |
| EBI2 | CD69+ CD4SP, Treg | 7α25-OHC | mTEClo | Medullary accumulation of CD4 SP cells; rapid SP motility needed for negative selection against low-avidity self-antigens | [58] |
| S1P1 | CD69− CD4SP, CD69− CD8SP | S1P | CMJ pericytes | Impacts thymic egress of mature thymocytes and Tregs | [92,95,96] |
| CCR2 | Sirpα+DC | CCL2 | Perivascular regions | Migration and localization of thymic Sirpα+DC required for tolerance against peripheral antigens | [79] |
| XCR1 | Sirpα−DC | XCL1 | mTEChi | Localization of Sirpα− DC in medulla needed for Treg generation | [80] |
Import of hematopoietic progenitors into the thymus
Import of TSP, whose identity has been reviewed elsewhere[14], is tightly regulated. TSP enter the thymus through blood vessels at the cortico-medullary junction (CMJ)[15]. There are a total of ~160 TSP niches in the adult mouse thymus, with ~10 available at any given time[16]. To enter the thymus, TSP adhere to VCAM-1 (vascular cell adhesion molecule-1), ICAM-1 (intercellular adhesion molecule-1), and P-selectin on thymic EC [17]. P-selectin expression is upregulated on EC when TSP cellularity diminishes[17], suggesting a positive feedback loop through which EC recruit TSP as space becomes available. Thymic EC are heterogeneous, and both KitL+ EC[18] and P-selectin+Ly6clo EC[19,20] have been implicated as TSP portals. However, these two candidates differ in expression of genes critical for differentiation of immature thymocytes, such as Cxcl12 and Dll4, leaving open the question of which serves as the true TSP niche.
The chemokine receptors CCR7 and CCR9 are also required for TSP entry [21,22]. The CCR7 ligands CCL19 and CCL21 are displayed by thymic EC [23,24], but they are expressed by TECs rather than by ECs themselves [18,25]. As TSP enter the EC niche, CCR7 and CCR9 likely promote tight binding via inside-out-signaling that increases integrin affinity for VCAM-1 and ICAM-1 on EC (Figure 1). CCR7 is subject to biased signaling[26], that is the differential engagement of signaling pathways by ligands of the same receptor, such that CCL19 and CCL21 may play distinct roles in TSP entry. However, the cellularity of immature thymocytes was not reduced in mice singly or doubly deficient for CCL19 and/or CCL21a[20,25]. These findings likely reflect redundancy between CCR7 and CCR9 in supporting thymic entry[21,22]. Thus, studies on a Ccr9−/− background are needed to determine if CCL19 versus CCL21 play distinct roles in TSP entry.
Live imaging approaches, such as two-photon microscopy (2PM) (Figure 3), could help identify the TSP niche and clarify mechanisms of thymic entry by enabling direct visualization of progenitor recruitment to EC subsets genetically labeled with fluorescent reporters. However, the rarity of seeding events imposes technical challenges. Histo-cytometry[27] could also be used to visualize and quantify interactions between TSP and EC subsets, along with downstream signaling, potentially clarifying the identity of the thymic EC niche (Figure 3). Because T cell reconstitution following bone marrow transplantation is clinically desirable [28], but limited by recruitment of T cell progenitors [21], elucidating mechanisms underlying TSP recruitment throughout the lifespan would be valuable.
Figure 3.
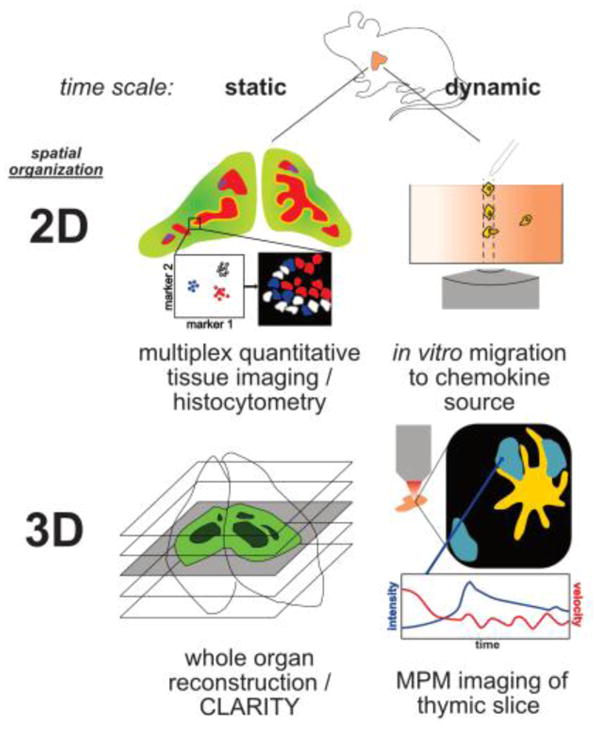
Static and dynamic imaging modalities visualize different aspects of thymocyte differentiation.
(2D static) Histo-cytometry uses multiplex immunofluorescence on tissue sections to identify and quantify distinct cellular populations, cellular interactions, and activation of signaling pathways. This technique combines quantitative information with spatial resolution, but is unable to resolve cellular kinetic parameters. (3D static) Tissue clearing methods such as CLARITY[103], can be used along with multiplex immunofluorescence and imaging modalities such as light sheet microscopy to reconstruct and quantify cells types within varied tissue depths. Although this technique cannot resolve kinetic parameters, it enables quantification of cell types and interactions throughout an entire organ. One disadvantage of this approach is the computational demands of the large datasets. (2D dynamic) In vitro imaging can be used to assess cellular migration to determine whether a given cell subset can undergo chemotaxis/chemokinesis in response to a specific chemokine. A caveat is that it does not provide information about whether that response if physiologically relevant within tissue, as chemokine expression and localization are tightly controlled. (3D dynamic) Live-cell 2PM can be used to assess kinetic parameters of cellular migration in live tissues, including directionality, velocity, confinement, cell-cell interactions, and molecular signaling events. This approach has the advantage of providing kinetic and spatial information within the physiologic tissue. However, imaging the whole thymus intravitally is technically challenging. Thus, these different imaging modalities provide complementary information, and can be integrated to maximize insights into T cell development.
Outward migration of immature thymocytes to the subcapsular zone
After thymic entry, thymocytes differentiate from ETP to DN2 to DN3 stages, while progressively migrating outwards from the CMJ towards the subcapsular zone (SCZ) (Figure 1, Box 1). ETP remain at the CMJ for ~10 days[29], while proliferating and initiating commitment to the T cell lineage. DN2 thymocytes migrate into the central cortex where they complete T-lineage specification and begin rearrangement of TCRβ chain genes. DN3 thymocytes accumulate at the SCZ, where those that pass β-selection proliferate extensively[15]. Cortical migration depends on adhesive interactions between integrins on DN thymocytes and VCAM-1 on cTECs[30]. In addition to providing adhesion molecules, cTECs express ligands that activate NOTCH1, c-Kit and IL-7R, which are required for T-lineage commitment, proliferation, and/or survival at the DN stages (reviewed in[31,32]).
Although the coordinated outward migration of developing DN thymocytes suggests that differentiation cues are restricted to cortical microenvironments, neither the functional significance of DN subset localization nor the mechanisms by which DN migrate towards the capsule have been elucidated. CXCL12 and CCL25, which are expressed by cTECs, have been suggested to promote outward migration of DN thymocytes, which express the cognate receptors, CXCR4 and CCR9, respectively[33,34]. However, CXCL12 and CCL25 are distributed fairly uniformly throughout the cortex[35], which is inconsistent with a chemotactic gradient emanating from the SCZ. Although T-lineage specific Cxcr4 deficiency impairs DN cell differentiation and localization to the central cortex[36], these phenotypes likely reflect a role for CXCR4 in survival and proliferation during β-selection, rather than for outward cortical migration[33]. Likewise, while premature expression and deletion of CCR9 impaired accumulation of DN3 cells at the SCZ[37,38], DP cells continue to express functional CCR9[34,37], but do not accumulate at the SCZ. Thus, CCR9 signaling is not likely to induce the outward cortical migration of DN thymocytes. Resolving the contribution of chemokine receptors to directional migration of DN thymocytes will require alternate techniques, such 2PM to monitor migration of live thymocyte subsets in situ (Figure 3).
Guidance of DP cells back to the CMJ and positive selection
After β-selection, DP thymocytes rearrange TCRα chain genes, while progressively accumulating near the medulla[8] (Figure 1). Mechanisms regulating inward migration of DP thymocytes have yet to be identified. Within the cortex, DP cells undergo positive selection to enforce self-MHC restriction (Box 1). 2PM imaging studies, in which DP motility and intracellular calcium levels were monitored, revealed that positive selection is associated with multiple transient TCR activation events over the course of 24 hours [7, 39,40]. These studies suggest a model in which DP cells scan pMHC displayed by multiple cTEC, integrating progressively less frequent TCR signals to reach a threshold for positive selection. An earlier in vitro study observed sustained signaling during positive selection [41], while a second study inferred that positive selection was confined to single cTEC niches based on flow cytometric analysis of reaggregate cultures [42]. Thus, live-cell imaging in the context of the thymic microenvironment was able to refine the model of positive selection to include more dynamic thymocyte motility and TCR signals than indicated by in vitro approaches.
Pre-selection DP cells migrate slowly in the cortex in an apparent random walk [43], but preferentially accumulate near the CMJ, where they migrate more rapidly[8], possibly promoting cTEC scanning needed for positive selection. Consistent with this model, proteins that modulate DP motility impact positive selection. For example, GIT2 suppresses actin reorganization during migration [44]. Counterintuitively, live-cell 2PM imaging revealed that GIT2-deficiency diminished DP thymocyte velocities. GIT2 was required to attenuate chemokine receptor signals, enabling DP cells to escape areas with high CXCL12 concentrations. Thus, GIT2 deficiency reduced cTEC scanning and impaired positive selection[44]. PlexinD1, whose ligand Sema3e is produced in the medulla[45], has also been implicated in modulating DP motility. A recent 2PM study revealed decreased motility of PlexinD1-deficient DP cells[46], presumably due to defects in releasing integrin catch bonds in response to chemokine or TCR signals that mediate binding to TEC[46,47]. Surprisingly, positive selection was unimpaired in PlexinD1-deficient mice[47], possibly reflecting the subtle decline in DP motility [46]. Thus, it remains to be determined if chemokines promote scanning and positively selecting interactions between DP cells and cTEC.
Notably, pre-positive selection DP cells are confined to the cortex[8], such that they are sequestered from mTEC and instead encounter cTEC, which express the thymoproteasome that generates unique peptides that may be essential for positive selection of some MHC-I restricted TCRs [48,49]. While CXCR4 has been implicated in confining human DP to the cortex in thymic slices [50], murine CXCR4 deficiency does not result in inappropriate medullary localization of DP cells [33,36]. Thus, mechanisms regulating cortical confinement of preselection mouse DP thymocytes have yet to be identified, and live-cell 2PM imaging will likely prove useful identifying such signals and validating their impact on human thymocytes.
Medullary entry of post-positive selection thymocytes
2PM live-cell imaging revealed that within hours of initiating positive selection, DP thymocytes upregulate CD69 and migrate into the medulla [40], where they are subject to central tolerance[51]. Chemokine receptors play an essential role in trafficking post-positive selection thymocytes into the medulla (Figure 1, Box 1). CCR7 and CCR4 are critical for thymocyte medullary entry, and are thus required for central tolerance[52–55]. CCR4 is expressed shortly after positive selection on DP cells and is maintained on immature CD4 single positive (SP) thymocytes [55,56], while CCR7 is upregulated on mature SP cells[56]. The CCR4 ligands CCL17 and CCL22 are expressed by thymic DC, while the CCR7 ligands CCL19 and CCL21 are expressed by mTEC [35,57]. Thus, gradients of both chemokine sets emanate from the medulla[5]. Live-cell 2PM imaging studies revealed that CCR4 is required for medullary entry of early post-positive selection DP cells[55], while CCR7 is required for chemotaxis of SP cells towards the medulla and accumulation therein[8]. A recent study demonstrated that despite expression of both CCL19 and CCL21 in the medulla, Ccl21a is uniquely required for medullary accumulation of SP cells[25], indicating that CCR7 is subject to biased signaling. In addition to CCR4 and CCR7, the GPCR EBI2 is expressed by CD4SP cells, while its ligand is produced in the medulla [58]. 2PM imaging also identified a role for EBI2 in promoting SP medullary accumulation. Consistent with these findings, EBI2-deficiency resulted in inefficient negative selection[58]. Thus, regulated expression of chemokine receptors by post-positive selection thymocytes is critical for their medullary localization which is needed for central tolerance.
Recent studies have revealed that more thymocytes are negatively selected at the CCR7− DP stage than at the SP stage[59,60]. Because of the central role CCR7 plays in medullary accumulation, these studies presupposed that CCR7− DP cells were localized to the cortex, and concluded that clonal deletion occurs mainly in the cortex. However, within 24 hours of initiating positive selection, thymocytes relocate to the medulla, but remain at the DP stage[40]. CCR4 can induce medullary entry of post-positive selection CCR7− DP cells[55]. Thus, it remains to be resolved whether CCR7−DP that undergo deletion encounter negatively selecting ligands in the cortex versus the medulla. Notably, when cTEC expressed negatively selecting antigens, they could induce TCR signaling, but clonal deletion required thymic DC[61], which are localized in the medulla. These studies raise the intriguing possibility that positive and negative selection are not discretely compartmentalized into the cortex and medulla, respectively, but overlap as maturing DP thymocytes engage in serial TCR signaling to enforce positive selection and lineage specification while traveling into the medulla, where negatively selecting ligands are localized. Live imaging studies to monitor TCR activation[62] and apoptosis[63] would be useful for resolving the timeline and localization of positive and negative selection events.
Orchestration of medullary interactions that establish central tolerance
Chemokine receptors also promote interactions between autoreactive thymocytes and antigen presenting cells (APC) during the induction of central tolerance. mTEC and DC are the major APC subsets in the medulla. Remarkably, single cell RNAseq studies revealed that, collectively, mature mTEC express >90% of the proteome, enabling them to display the majority of self-antigens T cells will encounter throughout the body[64,65]. These studies confirmed that expression of many tissue-restricted antigens (TRA) was dependent on the transcriptional regulator Aire. Interestingly, ectopic expression of Aire in cTEC failed to recapitulate TRA expression or to prevent tissue-specific autoimmunity[66], indicating that mTEC have additional properties, potentially differences in epigenetic landscapes, that render them permissive for AIRE-mediated expression of TRAs to enforce self-tolerance. mTEC can induce negative selection by directly presenting TRA to autoreactive thymocytes[67–69]. However, any given TRA is expressed by only 1–3% of mature mTEC[64,65]. Thus, thymocytes must efficiently scan APC for cognate antigens, and GPCR contribute to this process. Both CCR7 and EBI2, which are required for efficient central tolerance, promote rapid SP motility[8,58], enabling scanning of numerous APC. Furthermore, 2PM imaging studies showed that MST1, which is required for high-affinity integrin-mediated adhesion, promotes efficient interactions between SP cells and mTEC during negative selection[70]. CCL21-induced integrin-mediated migration on ICAM-1 substrates was impaired in Mst1−/− CD4SP thymocytes, further suggesting that CCR7 signaling likely promotes interactions between thymocytes and mTEC that express ICAM-1[70]. Consistent with this possibility, CCR7 promotes T cell:APC interactions in secondary lymphoid organs[71], but additional studies on the role of CCR7 in thymocyte:mTEC interactions will be required to confirm this activity in the thymus.
In addition to TEC, DC are also required for thymic central tolerance[72]. There are three major subsets of thymic DC: Sirpα+DC, Sirpα−DC and plasmacytoid DC (pDC). Sirpα+DC, which present antigens acquired from the blood and peripheral tissues[73], as well as from mTEC[74] are important for negative selection and Treg induction[75]. Sirpα−DC have also been shown to mediate selection of some Treg clones[76], although studies in Batf3−/− mice call into question whether Sirpα−DC are generally needed for DC-mediated negative selection[73] or Treg induction[75]. pDC can also induce negative selection by presenting antigens acquired in the periphery[77]. Taken together, thymocytes likely must scan multiple DC subsets to encounter the diverse pMHC that ensure self-tolerance. 2PM imaging showed that CCR4 was required for efficient interactions between CD4SP thymocytes and DC[55]. It remains to be determined whether CCR4 promotes interactions with only a subset of DC, and whether other chemokine receptors play a similar role for CD8SP:DC interactions. A recent imaging study showed that PlexinD1 signaling in response to medullary Sema3e regulates both SP motility and integrin mediated adhesion in response to chemokine signals[46], likely accounting for the defective negative selection of PlxnD1−/− thymocytes[47]. Whether PlexinD1 regulates interactions with mTEC and/or DC remains to be determined.
Chemokine receptors also promote recruitment of DC and B cells to the thymus and influence their intrathymic localization. Sirpα+DC and pDC, migrate into the thymus, while Sirpα−DC differentiate intrathymically[78]. CCR2 and CCR9 are required for recruitment of Sirpα+DC and pDC, respectively, to the thymus[77,79]. Sirpα−DC express XCR1, while XCL1 is expressed by MHCIIhi mTEC cells in an Aire-dependent manner[68,80]. Sirpα−DC can present Aire-dependent mTEC-derived antigens to induce Treg selection[76,81], and proximity between Sirpα−DC and mTEC is likely required for antigen transfer[74]. Consistent with this, medullary accumulation of Sirpα−DC is impaired in Xcl1−/− mice, resulting in defective central tolerance[80]. Another recent study showed that mature Sirpα+DC and Sirpα−DC express functional CCR7. In the absence of CCR7, survival of mature Sirpα−DC was diminished. This resulted in both reduced antigen transfer from mTEC to Sirpα−DC, and an increase in the proportion of Sirpα+DC, a subset that induced enhanced Treg generation[82]. Thus, chemokine receptor signaling can alter the composition of thymic APC subsets in multiple ways to impact the repertoire of T cells and Treg. B cells also induce thymic negative selection[83,84] and are absent in Ccr7−/− thymi[85], potentially contributing to autoimmunity in Ccr7−/− mice. We have yet to fully elucidate the mechanisms by which chemokine receptors impact medullary APC, with downstream consequences for thymocyte selection.
Tolerance mechanisms shaping the T cell repertoire
Technologies enabling quantification of antigen-specific T cells have clarified the impact of central tolerance on the polyclonal TCR repertoire. A recent study using pMHC tetramers coupled with flow cytometric analysis of mouse T cells subsets revealed that antigens ubiquitously-expressed in the thymus induce negative selection, antigens sparsely expressed induce negative selection coupled with Treg induction, and antigens expressed only in peripheral organs do not induce tolerance[86]. Analysis of human peripheral blood with pMHC tetramers also revealed that negative section does not eliminate all autoreactive T cells, but those that persist are anergic[87], adding anergy to the arsenal of tolerance mechanisms that could be induced either in the thymus or the periphery. We do not yet understand the basis for alternate fate specification of a given autoreactive T cell clone. The niche for intrathymic Treg induction is limited in both mice and humans[88]. At least in mice, IL-2 production by thymic DC may control the niche size[89]. Competition for the thymic Treg niche niche may account for selection of thymocytes expressing TCRs with high affinity for self-pMHC into the Treg lineage [90]. Nonetheless, a fine line exists between negative selection and Treg induction, and modulating thymocyte:APC interactions can shift this balance. Thus, CCR4-deficiency reduces thymocyte:DC interactions, impairs negative selection, and diverts autoreactive cells into the Treg lineage [55], perhaps by keeping TCR signaling below a threshold for deletion.
TCR repertoire sequencing has also refined our understanding of thymic selection. A recent study indicated that mTEC versus DC negatively select and induce Treg generation of distinct TCR clones [76]. This study also indicated that Sirpα−DC were particularly important for acquiring mTEC-derived antigens to induce Treg generation. Interestingly, another recent study in which the TCR repertoires of mosue T cells and Treg were compared revealed that AIRE was particularly critical for generation of tissue-protective Treg[91], but that one such Treg clone required Sirpα+DC for selection[75]. Together, these findings indicate that thymic APC present non-overlapping antigens to induce complete central tolerance, and highlight the importance of understanding mechanisms that enable SP thymocytes to efficiently scan multiple APC during their medullary residence.
Medullary maturation and thymocyte egress
Thymic egress is also highly regulated, ensuring thymocytes have sufficient time to encounter self-antigens before exiting the thymus through blood vessels at the CMJ[92]. A recent study further delineated SP maturation, which is associated with upregulation of CD62L, Qa2, CCR7, KLF2, and MHCI, and downregulation of CD24, CD69, and CCR9[93]. Although CCR7 was initially implicated in egress of neonatal thymocytes[23], recent works shows it is not required for export of recent thymic emigrants (RTE) in adults[94]. In contrast, the GPCR S1P1 is central to thymic egress[95,96]. Mature SP thymocytes undergo S1P1-dependent chemotaxis in response to S1P, which is expressed by pericytes[92], resulting in trans-endothelial migration and exit from the thymus. Transcription of S1p1 is induced by KLF2, which is induced by FOXO1[97,98]. In turn, FOXO1 is activated by diminished TCR signaling (reviewed in[99]). However, it is not clear why TCR signaling would decrease as SP cells mature, given the abundance of self-pMHC in the medulla. One possibility is that miR181, which suppresses expression of proteins that counteract TCR signals, is down-regulated in mature SP thymocytes[100]. Alternatively, because distinct pMHC are displayed in the cortex and medulla[49], migration of SP into the medulla, or restricted access to APC as SP mature within the medulla, could promote termination of the TCR signaling that was induced by positively selecting ligands. Live cell imaging could resolve whether cessation of positively selecting signals occurs in distinct thymic environments.
Expression of IL-4Rα by thymic stroma was recently identified as a novel mechanism impacting thymocyte egress[101]. In IL-4Rα−/− mice, mature SP thymocytes accumulated in thymic perivascular spaces and egress was impaired. Interestingly, thymic-resident invariant natural killer T cells (iNKT) were identified as the relevant source of IL-4 and IL-13. Although mTEC responded to IL-4 and IL-13 stimulation, it remains to be determined if TEC are the stromal subset that requires IL-4Rα signaling to mediate egress. Notably, IL-4Rα and S1P1 regulated egress through independent mechanisms[101]. Live-cell imaging and histo-cytometry could reveal whether SP interactions with EC and pericytes are impacted by IL-4Rα deficiency. Coming full circle from thymic entry, it will also be important to determine if distinct thymic EC subsets support egress of mature thymocytes, as increasing export could improve T cell output with age.
Concluding Remarks
Foundational studies established that chemokine receptors had the potential to direct developing thymocytes to specific thymic microenvironments[3–5]. Numerous studies since have revealed specific roles for chemokine receptors in multiple stages of T cell differentiation, spanning TSP thymic entry to emigration of RTE. Because chemokine expression is spatially segregated within tissues, and because chemokine receptor signaling can contribute to many distinct processes, analyzing the impact of chemokines by in vitro methods may not reflect thei physiologic contributions. However, live-cell imaging along with quantitative static imaging techniques such as histo-cytometry combined with 3D reconstructions (Figure 3), have great potential to reveal physiologic contributions of distinct chemokines and chemokine receptors to thymocyte differentiation (Outstanding Questions Box). Similar approaches could be used to study intrathymic differentiation of innate cells, as well as to identify mechanisms of age-associated changes in T cell differentiation[57,102]. Such insights into the processes underlying thymocyte selection could be used clinically to boost or restore production of a healthy T cell repertoire throughout the lifespan[1].
OUTSTANDING QUESTIONS BOX.
What is the functional consequence of differential localization of immature thymocytes to specific regions within the thymic cortex?
What restricts DP thymocytes to the cortex but promotes their return towards the medulla?
Do chemokines contribute to differential interactions with APC that promote tolerance in the cortex and/or medulla?
Can we use updated imaging modalities to visualize thymocyte-stromal cell interactions and resultant signaling to clarify mechanisms that result in alternative cell fates, such as negative selection versus Treg induction?
Can we develop techniques to image dynamics of rare thymocytes subsets, like ETP or iNKT cells, to gain insight into the dynamic interactions that govern their differentiation and function?
How can we translate the dynamic cellular interactions and molecular signals that govern mouse thymocytes selection to humans?
TRENDS BOX.
Chemokine receptor signaling controls thymocyte migration and enables thymocytes to successively engage with spatially segregated stromal cells that provide signals essential for different steps of differentiation and selection.
Recent advances in repertoire analysis have refined our understanding of how selection impacts the polyclonal TCR repertoire.
Imaging approaches have elucidated the dynamics of cellular interactions during selection, and have the potential to advance our understanding of both cellular and molecular mechanisms that drive selection of thymocyte subpopulations in situ.
The integration of multiple imaging approaches will provide novel insight into mechanisms governing generation of a functional, non-autoreactive T cell repertoire, with important clinical perspectives for reconstituting T cell function in immunodeficient individuals.
GLOSSARY
- Agonist selection
The process by which TCR-mediated recognition of self-pMHC promotes differentiation of thymocytes into alternative T cell lineages, including Treg, iNKT, and intraepithelial lymphocytes.
- β-selection
Developmental checkpoint during which DN3 thymocytes that have successfully rearranged and expressed a TCRβ chain signal through the pre-TCR to promote their survival and proliferation.
- Central Tolerance
The outcome of selection processes in the thymus by which thymocytes that express a TCR with sufficient affinity for self-pMHC undergo either negative selection or diversion to the Treg lineage.
- Chemokine
Secreted cytokine containing conserved cysteine motifs[2]. They can be soluble or spatially restricted by binding to glycosaminoglycans on proteoglycans in cell membranes. Chemokines are ligands for specific chemokine receptors.
- Chemokine receptor
Members of the class A rhodopsin-like family of G protein-coupled receptors that associate with Gαi heterotrimeric G proteins[6]. Chemokine receptors are responsible for a wide array of biological functions, including but not limited to trafficking and positioning of leukocytes throughout the body by inducing movement towards higher concentrations of chemokine ligands.
- Chemokinesis
Chemokine-induced stimulation of cell motility without a prescribed directionality.
- Chemotaxis
Chemokine-induced directional migration of a cell.
- G protein-coupled receptors
A large family of seven-transmembrane receptors that interact with and activate intracellular signal transduction molecules, most notably heterotrimeric G proteins and β-arrestin. Binding of an external ligand leads to activation of a wide array of signaling pathways.
- Histo-cytometry
A technique involving the combination of multi-parameter high resolution fluorescence imaging with image processing and computational data visualization to analyze and quantify complex cellular populations and phenotypes, while retaining spatial information.
- Negative selection
The process by which TCR-mediated recognition of pMHC induces apoptosis, or clonal deletion, of autoreactive thymocytes.
- Plasmacytoid dendritic cell (pDC)
Bone marrow-derived hematopoietic cells that produce high levels of type I interferons upon viral infections. pDC can traffic self-antigens to the thymus to mediate central tolerance.
- Positive selection
The process by which low affinity TCR-mediated recognition of pMHC presented by cTEC promotes DP cell survival and differentiation. Positive selection enforces self-MHC restriction on the T cell pool.
- Recent thymic emigrant (RTE)
Phenotypically and functionally immature naïve T cells in the periphery that have recently exited the thymus after T cell development.
- Regulatory T cell (Treg)
A CD4+ T cell subset generated either intrathymically or induced in the periphery, which expresses an autoreactive TCR and can suppress autoreactive T cell effector responses.
- Sirpα+ dendritic cell (Sirpα+DC)
Bone marrow-derived conventional dendritic cell subset (XCR1−CD8α−CD11b+Sirpα+) that differentiates in the periphery, and homes to the thymus. Sirpα+DC can traffic self-antigens into the thymus or capture self-antigens from circulation for display to thymocytes to mediate central tolerance induction.
- Sirpα− dendritic cell (Sirpα−DC)
Bone marrow-derived conventional dendritic cell subset (XCR1+CD8α+CD11b−Sirpα−) that is generated intrathymically and participates in tolerance induction. Sirpα−DC can acquire mTEC-derived antigens, as well as blood-borne antigens to display to thymocytes to mediate central tolerance induction.
- T lineage commitment
The process by which a multipotent T cell precursor undergoes fate restriction via epigenetic changes to commit to a T cell-specific differentiation program, thus excluding its potential to differentiate into alternative lineages.
- Thymic epithelial cell (TEC)
Epithelial cells within the thymus that can be stratified into cortical (cTEC) and medullary (mTEC) subsets. TEC provide signals that are essential for supporting thymocyte differentiation, selection, and survival.
- Thymic seeding progenitor (TSP)
A hematopoietic progenitor subset that is imported into the thymus with the potential to develop into T cells.
- Tissue restricted antigen (TRA)
A protein whose expression is limited to a few peripheral organs. Many TRA are expressed by mature mTEC in an Aire-dependent manner.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chaudhry MS, et al. Thymus: The next (re)generation. Immunol Rev. 2016;271:56–71. doi: 10.1111/imr.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36:705–712. doi: 10.1016/j.immuni.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell JJ, et al. Cutting edge: Developmental switches in chemokine responses during T cell maturation. J Immunol. 1999;163:2353–7. [PubMed] [Google Scholar]
- 4.Kim CH, et al. Differential chemotactic behavior of developing T cells in response to thymic chemokines. Blood. 1998;91:4434–4443. [PubMed] [Google Scholar]
- 5.Bleul CC, Boehm T. Chemokines define distinct microenvironments in the developing thymus. Eur J Immunol. 2000;30:3371–3379. doi: 10.1002/1521-4141(2000012)30:12<3371::AID-IMMU3371>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 6.Zweemer AJM, et al. Bias in chemokine receptor signalling. Trends Immunol. 2014;35:243–252. doi: 10.1016/j.it.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Bhakta NR, et al. Calcium oscillations regulate thymocyte motility during positive selection in the three-dimensional thymic environment. Nat Immunol. 2005;6:143–151. doi: 10.1038/ni1161. [DOI] [PubMed] [Google Scholar]
- 8.Ehrlich LIR, et al. Differential Contribution of Chemotaxis and Substrate Restriction to Segregation of Immature and Mature Thymocytes. Immunity. 2009;31:986–998. doi: 10.1016/j.immuni.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Borgne M, et al. The impact of negative selection on thymocyte migration in the medulla. Nat Immunol. 2009;10:823–830. doi: 10.1038/ni.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, et al. Noninvasive intravital imaging of thymocyte dynamics in medaka. J Immunol. 2007;179:1605–15. doi: 10.4049/jimmunol.179.3.1605. [DOI] [PubMed] [Google Scholar]
- 11.Hess I, Boehm T. Intravital Imaging of Thymopoiesis Reveals Dynamic Lympho-Epithelial Interactions. Immunity. 2012;36:298–309. doi: 10.1016/j.immuni.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa Y, et al. Thymic nurse cells provide microenvironment for secondary T cell receptor rearrangement in cortical thymocytes. Proc Natl Acad Sci. 2012;109:20572–20577. doi: 10.1073/pnas.1213069109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halkias J, et al. Conserved and divergent aspects of human T-cell development and migration in humanized mice. Immunol Cell Biol. 2015;93:716–26. doi: 10.1038/icb.2015.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlenner SM, Rodewald HR. Early T cell development and the pitfalls of potential. Trends Immunol. 2010;31:303–310. doi: 10.1016/j.it.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Lind EF, et al. Mapping precursor movement through the postnatal thymus reveals specific microenvironments supporting defined stages of early lymphoid development. J Exp Med. 2001;194:127–134. doi: 10.1084/jem.194.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ziętara N, et al. Multicongenic fate mapping quantification of dynamics of thymus colonization. J Exp Med. 2015;212:1589–1601. doi: 10.1084/jem.20142143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossi FMV, et al. Recruitment of adult thymic progenitors is regulated by P-selectin and its ligand PSGL-1. Nat Immunol. 2005;6:626–634. doi: 10.1038/ni1203. [DOI] [PubMed] [Google Scholar]
- 18.Buono M, et al. A dynamic niche provides Kit ligand in a stage-specific manner to the earliest thymocyte progenitors. Nat Cell Biol. 2016;18:157–167. doi: 10.1038/ncb3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi Y, et al. LTβR controls thymic portal endothelial cells for haematopoietic progenitor cell homing and T-cell regeneration. Nat Commun. 2016;7:12369. doi: 10.1038/ncomms12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucas B, et al. Lymphotoxin β Receptor Controls T Cell Progenitor Entry to the Thymus. J Immunol. 2016;197:2665–2672. doi: 10.4049/jimmunol.1601189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zlotoff DA, et al. CCR7 and CCR9 together recruit hematopoietic progenitors to the adult thymus. Blood. 2010;115:1897–1905. doi: 10.1182/blood-2009-08-237784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krueger A, et al. CC chemokine receptor 7 and 9 double-deficient hematopoietic progenitors are severely impaired in seeding the adult thymus. Thymus. 2010;115:1906–1912. doi: 10.1182/blood-2009-07-235721. [DOI] [PubMed] [Google Scholar]
- 23.Ueno T, et al. Role for CCR7 ligands in the emigration of newly generated T lymphocytes from the neonatal thymus. Immunity. 2002;16:205–218. doi: 10.1016/s1074-7613(02)00267-4. [DOI] [PubMed] [Google Scholar]
- 24.Ladi E, et al. Thymocyte-dendritic cell interactions near sources of CCR7 ligands in the thymic cortex. Journal of Immunology. 2008;181:7014–23. doi: 10.4049/jimmunol.181.10.7014. [DOI] [PubMed] [Google Scholar]
- 25.Kozai M, et al. Essential role of CCL21 in establishment of central self- tolerance in T cells. J Exp Med. 2017;214:1925–1935. doi: 10.1084/jem.20161864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hauser MA, Legler DF. Common and biased signaling pathways of the chemokine receptor CCR7 elicited by its ligands CCL19 and CCL21 in leukocytes. J Leukoc Biol. 2016;99:869–882. doi: 10.1189/jlb.2MR0815-380R. [DOI] [PubMed] [Google Scholar]
- 27.Gerner MY, et al. Histo-Cytometry: in situ multiplex cell phenotyping, quantification, and spatial analysis applied to dendritic cell subset micro-anatomy in lymph nodes. Immunity. 2012;37:364–376. doi: 10.1016/j.immuni.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van den Brink MRM, et al. Strategies to enhance T-cell reconstitution in immunocompromised patients. Nat Rev Immunol. 2004;4:856–67. doi: 10.1038/nri1484. [DOI] [PubMed] [Google Scholar]
- 29.Porritt HE, et al. Kinetics of steady-state differentiation and mapping of intrathymic-signaling environments by stem cell transplantation in nonirradiated mice. J Exp Med. 2003;198:957–962. doi: 10.1084/jem.20030837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prockop SE, et al. Stromal cells provide the matrix for migration of early lymphoid progenitors through the thymic cortex. J Immunol. 2002;169:4354–4361. doi: 10.4049/jimmunol.169.8.4354. [DOI] [PubMed] [Google Scholar]
- 31.Rothenberg EV. T cell lineage commitment: Identity and renunciation. J Immunol. 2011;186:6649–6655. doi: 10.4049/jimmunol.1003703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah DK, Zúñiga-Pflücker JC. An overview of the intrathymic intricacies of T cell development. J Immunol. 2014;192:4017–4023. doi: 10.4049/jimmunol.1302259. [DOI] [PubMed] [Google Scholar]
- 33.Trampont PC, et al. CXCR4 acts as a costimulator during thymic β-selection. Nat Immunol. 2010;11:162–170. doi: 10.1038/ni.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wurbel MA, et al. Complex regulation of CCR9 at multiple discrete stages of T cell development. Eur J Immunol. 2006;36:73–81. doi: 10.1002/eji.200535203. [DOI] [PubMed] [Google Scholar]
- 35.Misslitz A, et al. Thymic T cell development and progenitor localization depend on CCR7. J Exp Med. 2004;200:481–491. doi: 10.1084/jem.20040383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plotkin J, et al. Critical role for CXCR4 signaling in progenitor localization and T cell differentiation in the postnatal thymus. J Immunol. 2003;171:4521–4527. doi: 10.4049/jimmunol.171.9.4521. [DOI] [PubMed] [Google Scholar]
- 37.Benz C, et al. Homing of immature thymocytes to the subcapsular microenvironment within the thymus is not an absolute requirement for T cell development. Eur J Immunol. 2004;34:3652–3663. doi: 10.1002/eji.200425248. [DOI] [PubMed] [Google Scholar]
- 38.Uehara S, et al. Premature expression of chemokine receptor CCR9 impairs T cell development. J Immunol. 2006;176:75–84. doi: 10.4049/jimmunol.176.1.75. [DOI] [PubMed] [Google Scholar]
- 39.Melichar HJ, et al. Distinct temporal patterns of T cell receptor signaling during positive versus negative selection in situ. Sci Signal. 2013;6:ra92. doi: 10.1126/scisignal.2004400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ross JO, et al. Distinct phases in the positive selection of CD8+ T cells distinguished by intrathymic migration and T-cell receptor signaling patterns. Proc Natl Acad Sci U S A. 2014;111:E2550–8. doi: 10.1073/pnas.1408482111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daniels MA, et al. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444:724–729. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- 42.Merkenschlager M, et al. Evidence for a single-niche model of positive selection. Proc Natl Acad Sci U S A. 1994;91:11694–8. doi: 10.1073/pnas.91.24.11694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Witt CM, et al. Directed migration of positively selected thymocytes visualized in real time. PLoS Biol. 2005;3:1062–1069. doi: 10.1371/journal.pbio.0030160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phee H, et al. Regulation of thymocyte positive selection and motility by GIT2. Nat Immunol. 2010;11:503–511. doi: 10.1038/ni.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi YI, et al. PlexinD1 Glycoprotein Controls Migration of Positively Selected Thymocytes into the Medulla. Immunity. 2008;29:888–898. doi: 10.1016/j.immuni.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ueda Y, et al. Sema3e/Plexin D1 Modulates Immunological Synapse and Migration of Thymocytes by Rap1 Inhibition. J Immunol. 2016;196:3019–31. doi: 10.4049/jimmunol.1502121. [DOI] [PubMed] [Google Scholar]
- 47.Choi YI, et al. Dynamic control of β1 integrin adhesion by the plexinD1-sema3E axis. Proc Natl Acad Sci. 2014;111:379–384. doi: 10.1073/pnas.1314209111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takada K, et al. TCR affinity for thymoproteasome-dependent positively selecting peptides conditions antigen responsiveness in CD8+ T cells. Nat Immunol. 2015;16:1069–1077. doi: 10.1038/ni.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sasaki K, et al. Thymoproteasomes produce unique peptide motifs for positive selection of CD8(+) T cells. Nat Commun. 2015;6:7484. doi: 10.1038/ncomms8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Halkias J, et al. Opposing chemokine gradients control human thymocyte migration in situ. J Clin Invest. 2013;123:2131–2142. doi: 10.1172/JCI67175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klein L, et al. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see) Nat Rev Immunol. 2014;14:377–91. doi: 10.1038/nri3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nitta T, et al. CCR7-mediated migration of developing thymocytes to the medulla is essential for negative selection to tissue-restricted antigens. Proc Natl Acad Sci U S A. 2009;106:17129–17133. doi: 10.1073/pnas.0906956106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ueno T, et al. CCR7 signals are essential for cortex-medulla migration of developing thymocytes. J Exp Med. 2004;200:493–505. doi: 10.1084/jem.20040643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kwan J, Killeen N. CCR7 directs the migration of thymocytes into the thymic medulla. J Immunol. 2004;172:3999–4007. doi: 10.4049/jimmunol.172.7.3999. [DOI] [PubMed] [Google Scholar]
- 55.Hu Z, et al. CCR4 promotes medullary entry and thymocyte-dendritic cell interactions required for central tolerance. J Exp Med. 2015;212:1947–1965. doi: 10.1084/jem.20150178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cowan JE, et al. Differential Requirement for CCR4 and CCR7 during the Development of Innate and Adaptive αβT Cells in the Adult Thymus. J Immunol. 2014;193:1204–12. doi: 10.4049/jimmunol.1400993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ki S, et al. Global transcriptional profiling reveals distinct functions of thymic stromal subsets and age-related changes during thymic involution. Cell Rep. 2014;9:402–415. doi: 10.1016/j.celrep.2014.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ki S, et al. EBI2 contributes to the induction of thymic central tolerance in mice by promoting rapid motility of medullary thymocytes. Eur J Immunol. 2017 doi: 10.1002/eji.201747020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stritesky GL, et al. Murine thymic selection quantified using a unique method to capture deleted T cells. Proc Natl Acad Sci U S A. 2013;110:4679–84. doi: 10.1073/pnas.1217532110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Daley SR, et al. Helios marks strongly autoreactive CD4+ T cells in two major waves of thymic deletion distinguished by induction of PD-1 or NF-kB. 2013;210:269–285. doi: 10.1084/jem.20121458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McCaughtry TM, et al. Clonal deletion of thymocytes can occur in the cortex with no involvement of the medulla. J Exp Med. 2008;205:2575–84. doi: 10.1084/jem.20080866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hu Z, et al. Detecting T cell activation using a varying dimension Bayesian model. J Appl Stat. 2017 doi: 10.1080/02664763.2017.1290789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dzhagalov IL, et al. Elimination of self-reactive T cells in the thymus: A timeline for negative selection. PLoS Biol. 2013;11:e1001566. doi: 10.1371/journal.pbio.1001566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sansom SN, et al. Population and single-cell genomics reveal the Aire dependency, relief from Polycomb silencing, and distribution of self-antigen expression in thymic epithelia. Genome Res. 2014;24:1918–1931. doi: 10.1101/gr.171645.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meredith M, et al. Aire controls gene expression in the thymic epithelium with ordered stochasticity. Nat Immunol. 2015;16:942–9. doi: 10.1038/ni.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nishijima H, et al. Ectopic aire expression in the thymic cortex reveals inherent properties of Aire as a tolerogenic factor within the medulla. J Immunol. 2015;195:4641–4649. doi: 10.4049/jimmunol.1501026. [DOI] [PubMed] [Google Scholar]
- 67.Aichinger M, et al. Macroautophagy substrates are loaded onto MHC class II of medullary thymic epithelial cells for central tolerance. J Exp Med. 2013;210:287–300. doi: 10.1084/jem.20122149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hubert FX, et al. Aire regulates the transfer of antigen from mTECs to dendritic cells for induction of thymic tolerance. Blood. 2011;118:2462–2472. doi: 10.1182/blood-2010-06-286393. [DOI] [PubMed] [Google Scholar]
- 69.Hinterberger M, et al. Autonomous role of medullary thymic epithelial cells in central CD4+ T cell tolerance. Nat Immunol. 2010;11:512–519. doi: 10.1038/ni.1874. [DOI] [PubMed] [Google Scholar]
- 70.Ueda Y, et al. Mst1 regulates integrin-dependent thymocyte trafficking and antigen recognition in the thymus. Nat Commun. 2012;3:1098–1113. doi: 10.1038/ncomms2105. [DOI] [PubMed] [Google Scholar]
- 71.Friedman RS, et al. Surface-bound chemokines capture and prime T cells for synapse formation. Nat Immunol. 2006;7:1101–1108. doi: 10.1038/ni1384. [DOI] [PubMed] [Google Scholar]
- 72.Ohnmacht C, et al. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J Exp Med. 2009;206:549–559. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Atibalentja DF, et al. Functional redundancy between thymic CD8α+ and Sirpα+ conventional dendritic cells in presentation of blood-derived lysozyme by MHC class II proteins. J Immunol. 2011;186:1421–31. doi: 10.4049/jimmunol.1002587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kroger CJ, et al. Thymic dendritic cell subsets display distinct efficiencies and mechanisms of intercellular MHC transfer. J Immunol. 2017;198:239–256. doi: 10.4049/jimmunol.1601516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leventhal DS, et al. Dendritic cells coordinate the development and homeostasis of organ-specific regulatory T cells. Immunity. 2016;44:847–859. doi: 10.1016/j.immuni.2016.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perry JSA, et al. Distinct contributions of Aire and antigen-presenting-cell subsets to the generation of self-tolerance in the thymus. Immunity. 2014;41:414–426. doi: 10.1016/j.immuni.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hadeiba H, et al. Plasmacytoid dendritic cells transport peripheral antigens to the thymus to promote central tolerance. Immunity. 2012;36:438–450. doi: 10.1016/j.immuni.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li J, et al. Thymus-homing peripheral dendritic cells constitute two of the three major subsets of dendritic cells in the steady-state thymus. J Exp Med. 2009;206:607–22. doi: 10.1084/jem.20082232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baba T, et al. Crucial contribution of thymic Sirpα+ conventional dendritic cells to central tolerance against blood-borne antigens in a CCR2-dependent manner. J Immunol. 2009;183:3053–3063. doi: 10.4049/jimmunol.0900438. [DOI] [PubMed] [Google Scholar]
- 80.Lei Y, et al. Aire-dependent production of XCL1 mediates medullary accumulation of thymic dendritic cells and contributes to regulatory T cell development. J Exp Med. 2011;208:383–394. doi: 10.1084/jem.20102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ardouin L, et al. Broad and largely concordant molecular changes characterize tolerogenic and immunogenic dendritic cell maturation in thymus and periphery. Immunity. 2016;45:305–318. doi: 10.1016/j.immuni.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 82.Hu Z, et al. CCR7 Modulates the Generation of Thymic Regulatory T Cells by Altering the Composition of the Thymic Dendritic Cell Compartment. Cell Rep. 2017;21:168–80. doi: 10.1016/j.celrep.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Perera J, et al. Self-antigen-driven thymic B cell class switching promotes T cell central tolerance. Cell Rep. 2016;17:387–398. doi: 10.1016/j.celrep.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yamano T, et al. Thymic B cells are licensed to present self-antigens for central T cell tolerance induction. Immunity. 2015;42:1048–1061. doi: 10.1016/j.immuni.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 85.Akirav EM, et al. Resident B cells regulate thymic expression of myelin oligodendrocyte glycoprotein. J Neuroimmunol. 2011;235:33–39. doi: 10.1016/j.jneuroim.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Malhotra D, et al. Tolerance is established in polyclonal CD4+ T cells by distinct mechanisms, according to self-peptide expression patterns. Nat Immunol. 2016;17:187–95. doi: 10.1038/ni.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu W, et al. Clonal Deletion Prunes but Does Not Eliminate Self-Specific αβ CD8+ T Lymphocytes. Immunity. 2015;42:929–941. doi: 10.1016/j.immuni.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thiault N, et al. Peripheral regulatory T lymphocytes recirculating to the thymus suppress the development of their precursors. Nat Immunol. 2015;16:628–634. doi: 10.1038/ni.3150. [DOI] [PubMed] [Google Scholar]
- 89.Weist BM, et al. Thymic regulatory T cell niche size is dictated by limiting IL-2 from antigen-bearing dendritic cells and feedback competition. Nat Immunol. 2015;16:635–640. doi: 10.1038/ni.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moran AE, et al. T cell receptor signal strength in T reg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Malchow S, et al. Aire enforces immune tolerance by directing autoreactive T cells into the regulatory T cell lineage. Immunity. 2016;44:1102–1113. doi: 10.1016/j.immuni.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zachariah MA, Cyster JG. Neural crest-derived pericytes promote egress of mature thymocytes at the corticomedullary junction. Science (80-) 2010;328:1129–1135. doi: 10.1126/science.1188222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xing Y, et al. Late stages of T cell maturation in the thymus involve NF-κB and tonic type I interferon signaling. Nat Immunol. 2016;17:565–574. doi: 10.1038/ni.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cowan JE, et al. CCR7 controls thymus recirculation, but not production and emigration, of Foxp3+ T cells. Cell Rep. 2016;14:1041–1048. doi: 10.1016/j.celrep.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Allende ML, et al. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J Biol Chem. 2004;279:15396–15401. doi: 10.1074/jbc.M314291200. [DOI] [PubMed] [Google Scholar]
- 96.Matloubian M, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–60. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 97.Carlson CM, et al. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature. 2006;442:299–302. doi: 10.1038/nature04882. [DOI] [PubMed] [Google Scholar]
- 98.Kerdiles YM, et al. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat Immunol. 2009;10:176–84. doi: 10.1038/ni.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Love PE, Bhandoola A. Signal integration and crosstalk during thymocyte migration and emigration. Nat Rev Immunol. 2011;11:469–477. doi: 10.1038/nri2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li QJ, et al. miR-181a Is an intrinsic modulator of T cell sensitivity and selection. Cell. 2007;129:147–161. doi: 10.1016/j.cell.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 101.White AJ, et al. A type 2 cytokine axis for thymus emigration. J Exp Med. 2017;214:2205–2216. doi: 10.1084/jem.20170271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Griffith AV, et al. Persistent degenerative changes in thymic organ function revealed by an inducible model of organ regrowth. Aging Cell. 2012;11:169–177. doi: 10.1111/j.1474-9726.2011.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chung K, et al. Structural and molecular interrogation of intact biological systems. Nature. 2013;497:332–337. doi: 10.1038/nature12107. [DOI] [PMC free article] [PubMed] [Google Scholar]