Abstract
When fathers leave the family, mothers are at increased risk of developing depression and anxiety disorders. In biparental, socially monogamous prairie voles (Microtus ochrogaster), sudden bond disruption increases passive stress-coping, indicative of depressive-like behavior, and acts as chronic stressor in both males and females. However, the consequences of separation in lactating prairie vole mothers are unknown. In the present study, following 18 days of cohousing, half of the prairie vole pairs were separated by removing the male. In early lactation, maternal care was unaffected by separation, whereas anxiety-related behavior and passive stress-coping were significantly elevated in separated mothers. Separation significantly increased corticotropin-releasing factor (CRF) mRNA expression in the paraventricular nucleus of the hypothalamus under basal conditions, similar to levels of paired females after acute exposure to forced swim stress. A second cohort of lactating prairie voles was infused intracerebroventricularly with either vehicle or the CRF receptor antagonist D-Phe just prior to behavioral testing. The brief restraining during acute infusion significantly decreased arched back nursing in vehicle-treated paired and separated groups, whereas in the D-Phe-treated separated group the behavior was not impaired. Furthermore, in the latter, anxiety-related behavior and passive stress-coping were normalized to levels similar to vehicle-treated paired mothers. In conclusion, maternal investment is robust enough to withstand loss of the partner, whereas the mother’s emotionality is affected, which may be - at least partly - mediated by a CRF-dependent mechanism. This animal model has potential for mechanistic studies of behavioral and physiological consequences of partner loss in single mothers.
Keywords: Anxiety-related behavior, corticotropin-releasing factor, maternal behavior, passive stress-coping, partner loss, paraventricular nucleus
1. Introduction
The predominant form of family relationship in humans is biparental care [1–3], which occurs in less than 10 % of all mammalian species [4]. In biparental family structures, the father supports the mother in caring for the young. However, when mothers experience the loss of the partner by separation or divorce the odds of developing emotional dysfunction increase, as rates of depression and generalized anxiety disorder increase compared with married - or even never-married - mothers (e.g. [5–7]). In such separated or divorced mothers, the risk for emotional disturbances is thought to be triggered by parenting stressors, like childcare responsibilities, social isolation or financial difficulties, among others [8–11]. In turn, greater parental stress is associated with a higher risk of marital separation [12]. Importantly, parenting stressors are not as prominent for married or even never-married mothers [5, 13]. It is surprising that almost no data are available on the neurobiological basis of emotional changes following partner separation in females.
Among the few biparental mammals are the socially monogamous prairie voles (Microtus ochrogaster), which are a valuable animal model to study complex family dynamics ([14] for review see [15, 16]). The prairie vole mothers express the full range of maternal components including lactation, thereby providing nutrition in the first days of life making the mothers’ presence indispensable for the survival of the young. In addition, the prairie vole fathers also substantially contribute to caring for the offspring [17]. The pattern of paternal behavior includes retrieving and grooming the pups, carrying food to older offspring, providing them with warmth (huddling), and defending the nest to ensure protection and safeness, all of which can occur in the absence of the mother (babysitting) (for review see [4]). Despite their important role in rearing the young, the presence of prairie vole fathers is somewhat dispensable for the mere survival of the offspring. Still, losing the father results in the pups receiving less care, as the single mother does not fully compensate for the loss of paternal care [14, 18–20], which results in slowed maturation and impaired adult social behavior, including delayed partner preference formation, in the offspring [18]. Hence, such a family constellation likely increases the offspring’s demand for maternal behavior, thereby creating a situation similar to what can be observed in human single mothers (see above).
Recently, prairie voles have been studied in terms of physiological and psychological consequences of partner-loss [21–26]. In males, separation from the pair-bonded female partner increases passive stress-coping, indicative of depressive-like behavior ([21, 27, 28] for review see [15, 29]), in conjunction with adrenal hypertrophy, higher basal levels of stress hormones as well as increased corticotropin-releasing factor (CRF) mRNA expression in the bed nucleus of the stria terminalis (BNST) [21] and CRF-immunoreactivity in the PVN [24]. Based on these findings in males, we aimed to study the emotional and behavioral consequences of separation from the male partner in lactating female prairie voles, including caring for the offspring as a single mother. Interestingly, in nature, approximately one-third of prairie vole family units are single mothers [30], as they typically remain with the offspring, when the male has left the family unit [31, 32].
Here, we studied the socially monogamous Illinois prairie vole [33]. Naïve females were co-housed with males and left undisturbed until post-pairing day 18 when half of the pairs were separated [14, 18]. Following parturition, we monitored maternal care, anxiety-related behavior on the elevated plus-maze (EPM), and passive stress-coping in the forced swim test (FST), and brains were analyzed for CRF mRNA expression. Furthermore, based on our findings in separated males [21], a second cohort of prairie vole mothers additionally received daily central infusions of D-Phe, a CRF receptor 1/2 antagonist, to potentially prevent separation-induced changes in behavior and emotionality.
2. Material and Methods
2.1 Animals
All animals were sexually naïve adult male and female prairie voles from the laboratory breeding colony originally derived from field-captured voles in Illinois, USA. After weaning at 21 days of age, subjects were housed in same-sex sibling pairs or trios under standard laboratory conditions (14:10h light-dark cycle, lights on at 0600 h; 20°C, 60% humidity and free access to water and Purina rabbit chow). Each female vole (70–100 days of age) was paired with an unfamiliar male. On post-pairing day 18, the female partner was either separated from the male or they continued to stay together until the end of the experiment following an established protocol [14, 18]; separation earlier than post-pairing day 18 has a higher prevalence for termination of pregnancy [34]. In the present study, separation and/or surgery on post-pairing day 18 did not result in termination of pregnancy nor did it affect reproductive parameters in any of the females. The average gestation length was 23.3 days ± 0.2 (no surgery, non-separated: 23.2 ± 0.4; separated: 23.2 ± 0.5; surgery, non-separated: 22.8 ± 0.2; separated: 23.5 ± 0.3) and the average number of delivered pups was 3.4 ± 0.2 (no surgery, non-separated: 3.8 ± 0.4; separated: 2.8 ± 0.6; surgery, non-separated: 4.2 ± 0.3; separated: 3.3 ± 0.3). All behavioral tests were performed between 0800 h and 1200 h.
The animal studies were conducted in accordance with the guidelines of the National Institute of Health and were approved by Emory University’s Institutional Animal Care and Use Committee.
2.2 Experimental design
In Experiment 1, lactating female prairie voles were tested for maternal care on lactation day (LD) 1, for anxiety-related behavior on the EPM on LD 2, and for passive stress-coping in the FST on LD 3. In Experiment 2, pregnant female prairie voles were implanted with an intracerebroventricular (ICV) guide cannula at the time of separation from the male partner, i.e. on post-pairing day 18. Following parturition, all behavioral tests were performed as described for Experiment 1, except that the subjects were acutely infused ICV with vehicle (VEH) or the CRF receptor antagonist D-Phe before testing. In both experiments, lactating prairie voles from all groups were tested in random order to exclude possible order effects.
2.3 Behavioral tests
2.3.1 Maternal care in the home cage
Maternal care was observed for 10 s every 2nd min according to an established protocol in rats [21, 22]. The behavior was monitored from 0800 h to 0900 h under undisturbed conditions (Experiment 1 and 2) as well as immediately after ICV infusion for 90 min (Experiment 2). In Experiment 1, the data are shown for a total of 60 min with a maximal count of 30 observations. In Experiment 2, the data are shown in 30 min blocks with a maximal count of 15 observations per block. The occurrence of the following maternal parameters was scored: arched back nursing (ABN; nursing in a quiescent kyphosis), blanket nursing posture/in contact with the pups, licking/grooming the pups; together, these parameters account for “mother on nest”.
2.3.2 Fathers’ affiliative behavior in the home cage
In Experiment 2, the behaviors of the fathers were monitored using the method described for the mothers. Here, the behaviors scored were: blanket posture on the pups/being in contact with the nursing mother (blanket/contact), total sum of parental behavior consisting of blanket/contact together with nest building, licking/grooming the pups (on nest), grooming the female partner (partner-grooming) or themselves (self-grooming) (adapted from [14, 18]).
2.3.3 Anxiety-related behavior on the elevated plus maze (EPM)
Lactating prairie voles were tested on the EPM 10 min after ICV infusion as described in male voles [21]. Briefly, a conflict situation was created between the animal’s exploratory drive and its innate fear of open and exposed areas as demonstrated in rats [35] and voles [36–38]. The EPM consisted of an elevated (100 cm) plus-shaped aluminum platform with two closed (40 cm high walls out of dark PVC, < 20 lux) and two open arms (each 60 × 10 cm, 80 lux), connected at the center by a neutral zone (10 × 10 cm). Before each test, the surface of the EPM was cleaned with water containing a low concentration of a detergent and dried. The vole was placed in the neutral zone with its head facing a closed arm. The behavior was recorded with a video/computer system (Plus-maze V2.0, Ernst Fricke, Germany). A trained observer blind to the animals’ treatments analyzed the following parameters during the 5-min exposure: time spent on and entries into the open and closed arms as well as the neutral zone. The data were used to calculate (I) the percentage of time spent on the open arms vs. total time on all arms, and (II) the percentage of entries into open arms vs. entries into all arms.
2.3.4 Forced swim test (FST)
Ten minutes after treatment infusion, lactating female voles were exposed to the FST for 5 min in a 4 L glass beaker (15 cm in diameter) filled to a height of 20 cm with tap water (24 ± 1°C). The behaviors were scored by a trained observer blind to the animals’ treatment. The following behaviors were recorded according to our previous studies in male prairie voles [21, 22] using an automatic timer software package (Eventlog, Robert Hendersen, Grand Valley State University, Allendale, MI, USA): (1) struggling, defined as movements during which the forelimbs brake the water’s surface; (2) swimming, defined as movements of the fore and hind limbs resulting in forward motion without breaking the water surface, including diving, and (3) floating, defined as the behavior during which the animal uses limb movement to maintain its equilibrium without any movement of the trunk, a measure for passive stress-coping.
2.4 In situ hybridization for CRF mRNA expression
In Experiment 1 on LD 3, lactating female voles from both paired and separated groups were either left undisturbed in the home cage (basal condition) or exposed to the FST (stress condition) resulting in four groups termed female paired basal/stress or female separated basal/stress. After termination of the FST, mothers were returned to their offspring; 120 min later (or similar in the basal groups), female voles were rapidly euthanized via CO2 asphyxiation, and the brains were collected, flash-frozen and stored at −80°C until cryo-sectioning into 16 μm slices.
The in situ hybridization for CRF mRNA was performed in the PVN and the BNST using a highly specific single, 35S-labeled oligonucleotide probe (5′ ggc ccg cgg cgc tcc aga gac gga tcc cct gct cag cag ggc cct gca) following an established protocol [39, 40]. Afterwards, the slides were air-dried and exposed to BioMax MR film (Eastman Kodak, Rochester, New York, USA) for 8 weeks. Slides from all groups were measured for each subject to provide individual means. Expression of mRNA was measured as gray density with ImageJ 1.32j (National Institutes of Health, http://rsb.info.nih.gov/ij/) by an experienced experimenter blind to the treatment. Background activity was subtracted from measured areas to yield values for specific binding.
2.5 Acute blocking of CRF receptors ICV
2.5.1 Implantation of an ICV guide cannula on post-pairing day 18
Using isoflurane inhalation anesthesia, pregnant female prairie voles were stereotaxically implanted with a guide cannula (stainless steel; 21 G; length: 8 mm) 2 mm above the left ventricle (A/P − 0.6 mm; ML + 1.0 mm; DV − 2.0 mm; for details see [41]). The guide cannulas were closed using a stylet. After surgery and regaining full consciousness, female voles were either single-housed or placed back to their male partners. Following, the pregnant female voles were handled twice daily to adapt them to the ICV infusion procedure.
2.5.2 ICV infusions of CRF receptor antagonist
On LD 1 to 3, lactating vole mothers received an acute ICV infusion of either VEH (2 μl Ringer’s solution; pH adjusted to 7.4) or of the nonspecific CRF receptor 1/2 antagonist D-Phe [(D-Phe12, Nle21,38, α-Me-Leu37)-CRF (12–41); 2 μg/2 μl; Bachem, Montreal, Canada]. The dose was chosen based on a previous behavioral study in lactating mice [42]. VEH or D-Phe was infused slowly into the conscious and briefly restrained vole mothers (experimenter immobilizing the vole in a towel) using a 27 G ICV cannula inserted into the guide cannula and extending it by 2 mm. After ICV infusion, the cannula was kept in place for 30 s to allow substance diffusion. Afterwards, the lactating vole mothers were placed back in the home cage. For maternal care observation on LD 1, recording of the mothers’ behavior continued immediately after treatment infusion. On LD 2 and 3, i.e. EPM and FST, respectively, the lactating vole mothers received the drug infusion 10 min prior to testing.
2.6 Histology
At the end of Experiment 2, lactating mothers were euthanized via CO2 asphyxiation. Blue ink (0.5 μl) was injected via the guide cannula using the ICV infusion system in order to verify the correct infusion site by presence of ink in the ventricles in a coronal brain cut.
2.7 Statistics
The statistical tests performed were independent t-test (maternal care from Experiment 1, EPM, FST, in situ hybridization), or two-way analysis of variance for repeated measures (ANOVA; maternal care from Experiment 2; factor separation condition: paired/separated; factor treatment; factor time). Where appropriate, Newman-Keuls post hoc tests for pair-wise comparisons were applied. In cases, in which we a priori predicted specific outcomes, planned comparisons of specific contrasts were performed. Statistics were performed using GB-Stat 10.0 (Dynamic Microsystems, Silver Springs, MD, USA). Data are presented as mean + SEM; significance was accepted at p ≤ 0.05, and effect size estimations were indicated by eta squared values or Cohens d.
3. Results
3.1 Maternal care was unchanged after partner-loss
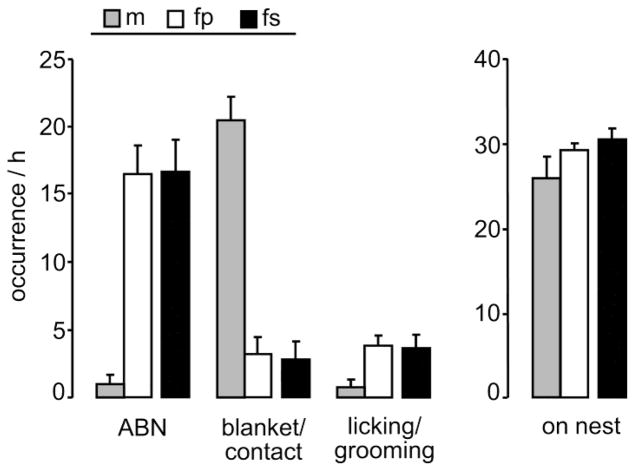
Previous studies revealed that prairie vole mothers do not alter their maternal investment when being a single parent [14, 18]. Hence, we predicted the same outcome in the present study, and performed planned comparisons using t-tests. The amount of maternal care provided by lactating mothers that were either with their male partners or had been separated on post pairing day 18 did not differ (Fig. 1, white and black bars, respectively). In detail, both groups spent similar amount of time on ABN (p = 0.86), blanket nursing/contact (p = 0.70), licking/grooming (p = 0.29), or total time on the nest (p = 0.36). However, the amount of care received by the pups from paired parents also included the care provided by the fathers, which provided additional paternal care, especially blanket/contact (Fig. 1, gray bars).
Figure 1. Unchanged maternal care in single prairie vole mothers.
Female prairie voles were cohabitated in pairs with males until day 18 of pairing, after which half of the females were separated from their male partners (fs) whereas the other half stayed paired (fp). On lactation day 1, maternal care in the home cage was monitored for 60 min starting at 8:00 A.M. under undisturbed conditions. Behaviors monitored were arched back nursing (ABN), blanket nursing position/direct contact with the pups (blanket/contact), licking/grooming the pups, and total occurrence of pup-directed behavior (on nest). Maternal care was similar between fp and fs mothers, whereas the fathers (m) contributed to offspring care of fp mothers. Data are presented as mean + SEM, n = 6 per group.
3.2 Increased anxiety-related behavior and passive stress-coping after partner-loss
In male [21–26] as well as in non-lactating female prairie voles [23], separation from the partner results in increased anxiety-related behavior and passive stress-coping. Therefore, we predicted a similar outcome in the present study, and performed planned comparisons using t-tests. Here, separation from the male partner increased anxiety-related behavior of lactating mothers on the EPM as reflected by reduced percentage of entries into open arms (p = 0.04; d = −1.6) as well as a trend towards decreased percentage of time spent on the open arms (p = 0.06; d = −1.3) compared with non-separated mothers (Fig. 2A). The total number of entries did not differ between the groups (non-separated: 23.6 ± 2.2; separated: 19.5 ± 5.2). Furthermore, in the FST, separated female mothers spent more time displaying passive stress-coping (floating) when compared with non-separated mothers (p = 0.05; d = 1.0), indicative of increased depressive-like behavior (Fig. 2B).
Figure 2. Increased emotionality in single prairie vole mothers.
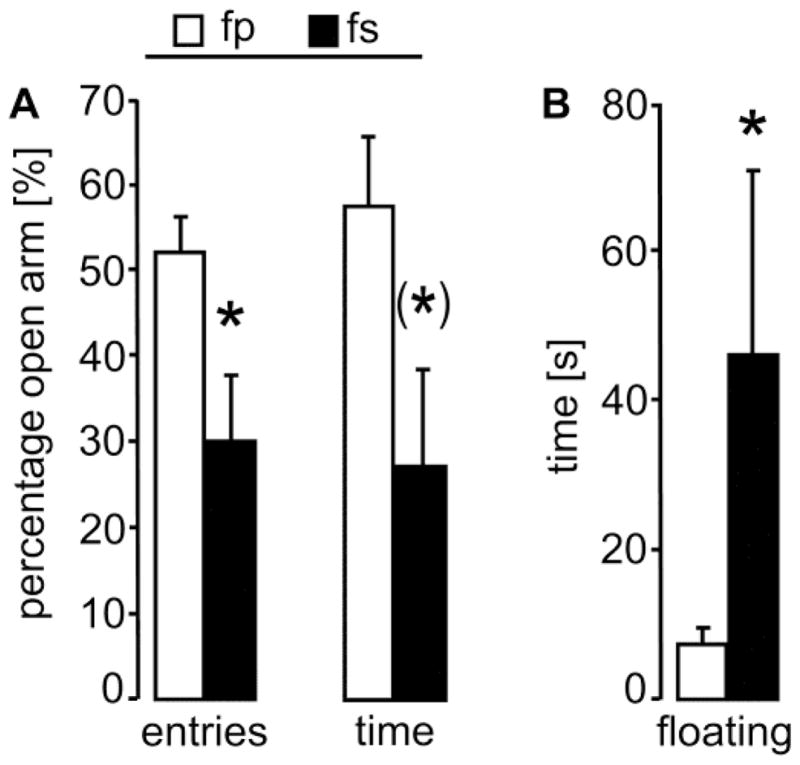
In the morning of lactation days 2 and 3, prairie vole mothers were tested for 5 min on the elevated plus-maze for anxiety-related behavior and in the forced swim test for passive stress-coping, respectively. (A) On the elevated plus-maze, fs mothers displayed higher anxiety-related behavior compared to fp mothers, indicated by a significantly less percentage of entries into and a trend towards less percentage of time spent on the open arms. (B) In the forced swim test, fs mothers spent more time on passive stress-coping (floating), resembling increased depressive-like behavior. For further details, see legend to Fig. 1. Data are presented as mean + SEM, n = 5 – 6 per group. * p ≤ 0.05 versus fp; (*) p = 0.06.
3.3 Increased CRF mRNA expression after partner-loss or acute stress
In male prairie voles, separation from the female partner results in increased CRF mRNA expression in the BNST [21] and CRF-immunoreactivity in the PVN [24]. Therefore, we predicted changes in the CRF mRNA due to separation and/or stress in the present study, and performed planned comparisons using t-tests. Statistics revealed that within the PVN CRF mRNA expression was increased in separated mothers under non-stress conditions (p = 0.03; d = 1.7) as well as in non-separated mothers under stress conditions (p = 0.01; d = 2.3) compared with the basal non-separated group (Fig. 3). Within the BNST, CRF mRNA expression did not differ between the groups (data not shown).
Figure 3. Changes in CRF mRNA expression after separation or stressor-exposure in prairie vole mothers.
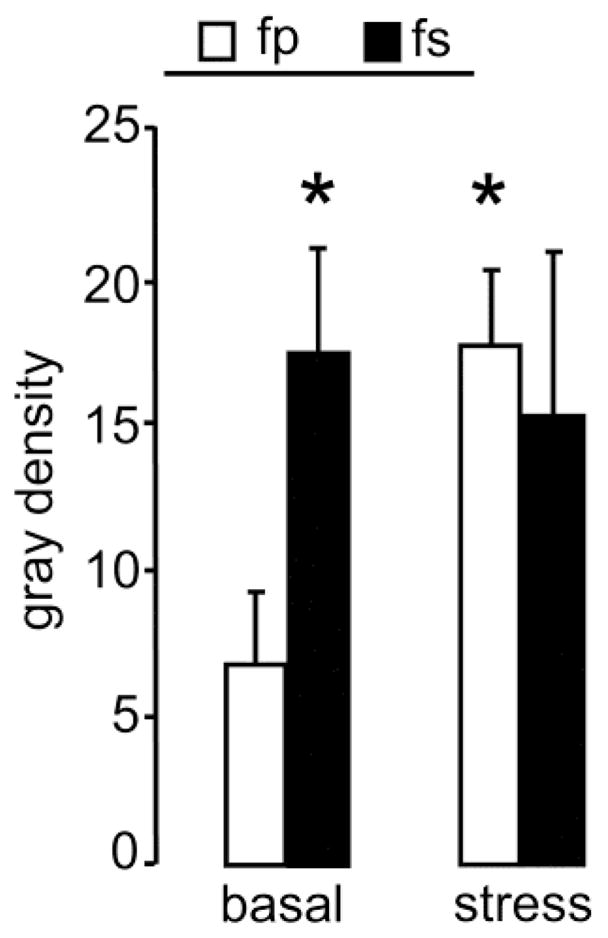
On lactation day 3, prairie vole mothers of each group were either exposed to the forced swim test (see Fig. 3; stress) or left undisturbed (basal). Voles were sacrificed 120 min later, brains were taken, and processed for CRF mRNA in situ hybridization. Expression CRF mRNA in the PVN was increased in stress fp and basal fs mothers compared with basal fp mothers. For further details, see legend to Fig. 1. Data are presented as mean + SEM, n = 5 – 6 per group. * p < 0.05 versus basal fp.
3.4 Central D-Phe infusion rescued maternal care after brief restraining
When performing a repeated measures ANOVA over all groups, the amount of ABN differed between the groups depending on treatment (F2,68 = 8.136, p = 0.003; η2 = 0.12) and time (F4,68 = 6.562, p = 0.002; η2 = 0.18), and there was an interaction (F8,68 = 2.265, p = 0.033; η2 = 0.12; Fig. 4A). The post hoc test revealed that after ICV infusion under brief restraining at +30 min, the separated mothers treated with D-Phe showed significantly more ABN compared with both VEH-treated groups (p < 0.01). To dissect the treatment effect over time, a repeated measures ANOVA within each group was performed, which revealed significant differences (VEH-treated non-separated: F4,28 = 4.312, p = 0.009; η2 = 0.38; VEH-treated separated: F4,28 = 4.268, p = 0.010; η2 = 0.36; D-Phe-treated separated: F4,28 = 3.511, p = 0.022; η2 = 0.28). In detail, the amount of ABN significantly dropped immediately after ICV infusion/brief restraining at +30 min in both VEH-treated groups (p < 0.05, in each group), but not in the D-Phe-treated group. Subsequently, both VEH-treated groups had recovered back to pre-infusion levels at +90 min, now significantly differing from the drop at +30 min (p < 0.05, in each group). Further ANOVA analyses of the other behaviors revealed no effects for blanket/contact (Fig. 4B), licking/grooming (Fig. 4C), total amount of mothers on nest (data not shown), self-grooming (Fig. 4D), and partner-grooming (data not shown). However, as ABN was affected by the treatment, we performed planned comparisons on the other behaviors using t-tests on pre- (−30 min) versus post-infusion (+30 min) within each group. Statistics revealed in both VEH-treated groups decreased occurrence of blanket/contact (fp VEH: p = 0.009; d = 1.9; fs VEH: p = 0.014; d = 1.3; Fig. 4B). In addition, VEH-treated non-separated mothers showed increased licking/grooming of the pups (p = 0.011; d = 1.8; Fig. 4C) as well as elevated self-grooming (p = 0.022; d = 1.7; Fig. 4D). No other behaviors and/or treatment groups were affected by the treatment (data not shown).
Figure 4. Maternal care before and after central CRF receptor blockade in prairie vole mothers.
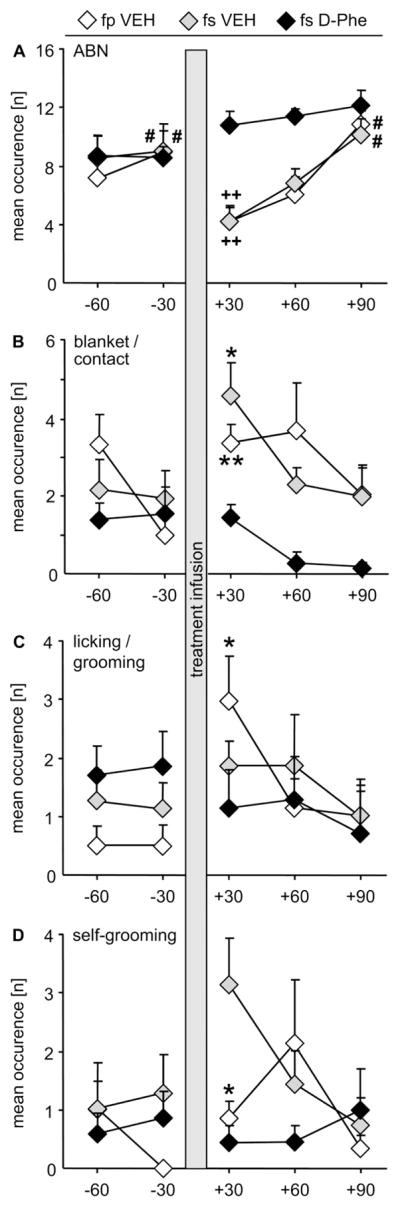
Female prairie voles were fitted with an ICV guide cannula on pregnancy day 18, on which pairs were either separated or stayed paired. On lactation day 1, maternal care in the home cage was monitored at 8:00 A.M. for 60 min, followed by acute infusion of vehicle (VEH) or the CRF receptor 1/2 antagonist D-Phe under brief restraining. Afterwards, behavioral observations continued for 90 min. (A) Arched back nursing (ABN) did not change throughout the test in fs D-Phe mothers, but dropped in both VEH-treated groups immediately after treatment infusion, i.e. +30 min, and returned to pre-infusion levels at +90 min. Both VEH-treated groups showed immediately after treatment more (B) blanket nursing position/direct contact with the pups (blanket/contact; fp VEH, fs VEH), (C) licking/grooming the pups (fp VEH), and (D) self-grooming (fp VEH, fs VEH). For further details, see legends to Fig. 1, 2. Data are presented as mean + SEM, n = 6 – 7. ++ p < 0.01 versus fs D-Phe at same time point; # p < 0.05 versus “+30” within same group; ** p < 0.01, * p < 0.05 versus pre-infusion time point.
3.5 Paternal and affiliative behavior of the male partner before removal and after return of the female partner
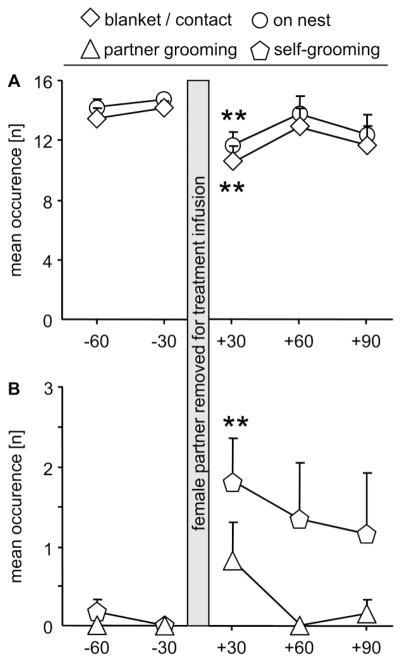
The prairie vole fathers of the unseparated, VEH-treated mothers were affected in their behaviors due to the brief removal of the lactating female partner for infusion (Fig. 5A, B). Performing a repeated measures AVONA for each behavior revealed that the fathers differed in self-grooming over time (F4,28 = 3.11, p = 0.039; η2 = 0.26; Fig. 5B), but the post hoc test revealed no further differences. There were no differences in the occurrence of blanket posture/in contact with the nursing mother (F4,28 = 2.10, p = 0.119; η2 = 0.26; Fig. 5A), total time spent on nest (F4,28 = 2.08, p = 0.122; η2 = 0.25; Fig. 5A), or partner grooming (F4,28 = 2.58, p = 0.069; η2 = 0.29; Fig. 5B). We continued performing planned comparisons using t-tests on pre- (−30 min) versus post-infusion (+30 min), only. Statistics revealed a significant drop in blanket posture/in contact with the nursing mother (p = 0.005; d = −2.1) and total time spent on nest (p = 0.007; d = −1.9), as well as a significant increase in self-grooming (p = 0.007; d = 2.0) whereas partner grooming was not affected.
Figure 5. Affiliative behavior of prairie vole fathers before and after treatment of lactating mothers.
Prairie vole fathers from the non-separated, VEH-treated mothers (see Fig. 4) were monitored for their affiliative behavior before and after removal of their lactating female partners to determine possible effects of the procedure on the fathers. Indeed, immediately after returning the female partners (+30 min), prairie vole fathers dropped in the occurrence of blanket posture on the pups/being in contact with the nursing mother (blanket/contact) and total sum of parental behavior (on nest), whereas self-, but not partner-, grooming was increased. For further details, see legends to Fig. 4. Data are presented as mean + SEM, n = 6. ** p < 0.01 versus pre-infusion time point.
3.6 D-Phe ICV normalized anxiety-related behavior and passive stress-coping in separated mothers
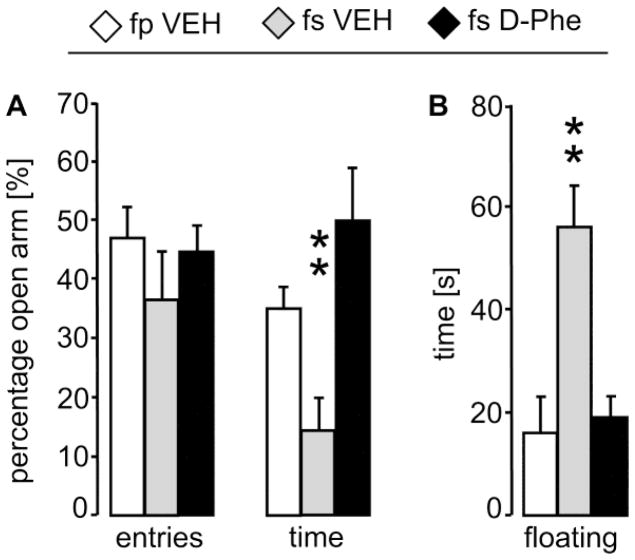
Based on our findings in 3.2 as well as on previous studies [21–26], we predicted that D-Phe treatment decreases emotionality. Therefore, we performed planned comparisons using t-tests. On the EPM, VEH-treated separated mothers spent less percentage of time on the open arms compared with VEH-treated non-separated mothers (p = 0.008; d = −1.8; Fig. 6A), thereby confirming our findings from Experiment 1 (3.2). After D-Phe-treatment, separated mothers spent more percentage of time on the open arms compared with VEH-treated separated mothers (p = 0.005; d = 1.8) making them indistinguishable from the VEH-treated non-separated mothers (p = 0.48; d = −0.4). The percentage of entries into open arms (Fig. 6A) as well as the total number of entries (VEH-treated non-separated: 25.8 ± 1.7; VEH-treated separated: 21.3 ± 2.7; D-Phe-treated separated: 22.7 ± 3.7) did not differ between the groups.
Figure 6. Behavioral and emotional effects of CRF receptor blockade in prairie vole mothers.
(A) On lactation day 2, mothers were tested 10 min after central treatment infusion on the elevated plus-maze for anxiety-related behavior, which was significantly reduced in fs VEH mothers compared with both other groups as indicated by less percentage of time spent on the open arms. (B) On lactation day 3, mothers were tested 10 min after treatment infusion in the forced swim test for passive stress-coping, which was significantly increased in fs VEH mothers compared with both other groups as indicated by a higher time spent floating. For further details, see legends to Fig. 1, 2, 4. Data are presented as mean + SEM, n = 6 – 7. ** p ≤ 0.01 versus both other groups.
In the FST, VEH-treated separated mothers spent more time on floating compared with VEH-treated non-separated mothers (p = 0.004; d = 2.0; Fig. 6B), thereby confirming our findings from Experiment 1 (3.2). Furthermore, D-Phe-treated separated mothers spent less time on floating compared with VEH-treated separated mothers (p = 0.007; d = −1.8), making them indistinguishable from the VEH-treated non-separated mothers (p = 0.74; d = 0.2; Fig. 6B).
4. Discussion
The challenges that a single mother has to face in everyday life are numerous, and the effects on her physical and psychological well-being can be dramatic, including the development of anxiety and depressive disorders [5]. Using lactating prairie vole mothers that have experienced the loss of the male partner in late pregnancy, we now model those findings observed in humans and propose a potential target neuroendocrine system, i.e. the brain CRF system. When separated, prairie vole mothers displayed heightened levels of anxiety-related behavior and passive stress-coping, indicative of depressive-like behavior [21, 22, 27, 28], while their maternal investment was not different from mothers that cared for the young together with their male partner. At the same time, separation caused increased CRF mRNA expression in the PVN; and central acute blockade of CRF receptors 1/2 restored the single mothers’ anxious and passive phenotype back to levels similar to non-separated mothers. Furthermore, when interrupting maternal care by brief restraining, CRF receptors 1/2 antagonism prevented the typical stress-induced drop in arched back nursing.
The finding of unchanged maternal care in single compared with biparental prairie vole mothers is in line with previous studies (Fig. 1); single prairie vole mothers did not compensate for the loss of paternal care [14, 18–20]. That sustained level of maternal care even in the absence of the father is most likely due to the activated OT and/or AVP system during mother-pup interaction as part of the peripartum adaptations triggering the maternal drive [43, 44]. Consequently, from the offspring’s perspective, the young receive less care - as both parents typically occupy the nest an equal amount of time (Fig. 1) - resulting in socio-emotional impairments in adulthood [14, 18]. Such impairments in the offspring might also be caused by the increased emotionality of the single mothers (see Results 3.2, 3.6) as this can be - even non-genomically - transferred to the next generation (animals [45]; humans [46, 47]). In fact, early life experiences can affect species-typical adult nurturing and bonding behaviors in monogamous animals [18, 48, 49].
Interestingly, the presence of the fathers is somewhat dispensable for the mere survival of the offspring (for review see [50]). However, the prairie vole fathers normally also interact with the mothers through, e.g., huddling and licking/grooming (Fig. 1) [14]. These behaviors underlie the positive effects of social buffering, which are mediated via an activated hypothalamic OT system [51]. The latter has the potential to dampen the stress system in lactation in rodents [52]. Hence, the lack of social interaction in single prairie vole mothers might contribute to their anxious and passive phenotype.
Both increased anxiety-related behavior (Fig. 2A) and passive stress-coping (Fig. 2B) in the single mothers were most likely the consequence of perceiving the separation as chronic stress similar to what is known in separated male prairie voles [21, 23, 24]. In fact, single mothers’ basal CRF mRNA expression within the PVN, the main drive for the stress response, was more than doubled in comparison to paired mothers (Fig. 3). This is in line with findings in males, in which CRF-immunoreactivity is increased after separation [24]. With respect to lactating females, it is a characteristic in rodents that the CRF mRNA expression in the PVN is decreased under basal conditions as part of the peripartum adaptations [40, 52–55]. And these peripartum adaptations are essential to wire the brain for the demands during motherhood like, in the present case, enabling an adequate stress response of the mother [52]. Importantly, our data suggest that the increased CRF signaling may underlie the single mothers’ emotional dysregulation. Indeed, acute suppression of CRF receptor signaling by central infusion of the receptor-nonspecific antagonist D-Phe normalized the single mothers’ heightened anxiety-related behavior (Fig. 6A) as well as their increased passive stress-coping (Fig. 6B). This is consistent with our previous findings in separated male prairie voles [21], as chronic central infusions of D-Phe over 4 – 5 days normalized their increased emotionality as did antagonists specific for one of the two CRF receptor subtypes [21]. Further studies are required to differentiate the receptor subtype-specific roles in the increased emotionality of single prairie vole mothers.
Another interesting finding was the difference in maternal care between the three groups from Experiment 2, i.e. VEH-treated paired and single mothers as well as D-Phe-treated single mothers (Fig. 4). During acute treatment infusion, the mothers were removed from the home cage and briefly restrained, which likely acted as a mild stressor to the females regardless of prior handling. That procedure caused a 50 % reduction in the occurrence of arched back nursing in the VEH-treated paired and single mothers, whereas acute central CRF receptor blockade completely prevented that drop in single mothers. These results are consistent with our findings in lactating rats [40]. However, it was rather surprising that in VEH-treated paired mothers the drop in arched back nursing was as prominent as in VEH-treated single mothers. According to previous studies in pair-bonded female prairie voles, increased stress-related parameters are normalized rapidly when returned to the male partners [51], which in turn display increased consolation behavior, a distinct behavior that can be found in both sexes in prairie voles [56]. The consolation behavior helps to counterbalance the negative effects of stressor-exposure via OT release in the PVN of the experimental prairie vole [51]. Furthermore, intranasal OT also enhances the stress-buffering effects of social support in humans [57]. However, in our experiments, returning the briefly restrained mothers to the male partner and the offspring could not prevent the drop in arched back nursing. One possible explanation is that the brief restraining procedure was either not stressful enough for the lactating mothers to induce the transmission of stress signals to the partner. Alternatively, the separation might have been too short (1 min here versus 60 min in [51, 56]) to evoke increased partner-grooming in the males (Fig. 5), which has been shown to help to swiftly return to physiological levels prior to the stressor exposure [51, 56]. Furthermore, the peripartum adaptation-related increased signaling of the OT system [43, 58], which is particularly prominent within the nucleus accumbens in prairie voles [59–61], might have reached a ceiling level and, thus, the presence of the bonded male was without any stress-buffering effect. However, all those findings emphasize the impact of the CRF system in the behavioral and emotional dysregulations of single prairie vole mothers though the small sample size is a limiting factor of the study.
In humans, ending a long-term relationship often means losing the partner who helped to maintain psychological and physiological homeostasis, and the resulting state is considered one of bio-behavioral dysregulation [62]. In view of the attachment theory, the abandoned, but not the abandoning, partner is more likely to experience feelings of neglect and loneliness [63]. In addition, the chances to develop emotional dysfunctions like depression and anxiety disorders are higher in separated/divorced mothers compared with married mothers [5, 13, 64]. Even homebound women experience such negative effects like feelings of anxiety and depression when separated from the romantic partner only for a few days, e.g. due to work-related travel [63]. This link might also account for mothers separated/divorced from the partner. Single mothers are more likely to stay with the children probably at the former home, i.e. these mothers represent the homebound partner. In addition, the resulting parenting stressors single mothers might have to face, like higher childcare responsibilities, social isolation, and financial difficulties, are potentially increasing the mothers’ risk for emotional disturbances [8–11].
In summary, single prairie vole mothers were not different in their behavioral expression of maternal care from biparental mothers. The persistence in this behavior even under such challenging circumstances as being a single parent ensures the survival of the offspring though they lack the additional paternal care for their proper emotional and social development. However, the emotional outcome of being a single mother is more dramatic; the consequence of being deserted by the male partner is a heightened anxious and passive phenotype. One of the potential underlying mechanisms is increased signaling of the brain CRF system. Future studies might reveal the involvement of other neurotransmitter systems like OT, which has stress-buffering effects but has been found to be impaired in male prairie voles after separation from the female partner. In conclusion, the animal model of separated female prairie vole mothers has the potential for further in-depth investigations of the neurobiological background of postpartum mood disorders.
Highlights.
Abandoned mothers often suffer from emotional dysfunctions
Prairie voles serve as animal model to study biparental care and partner loss
Separated vole mothers showed normal maternal care but increased emotionality
Separation increased CRF mRNA expression in the PVN
Acute central blockade of CRF receptors reversed the negative effects of separation
Acknowledgments
Funding
This work was supported by the Deutsche Forschungsgemeinschaft DFG [grant numbers BO 1958/8-1 to OJB, NE 465/16-1 and NE 465/27-1 to IDN], the DFG Graduate School GRK 2174, the German Ministry of Education and Research BMBF [01EE1401A/OptiMD to IDN], and National Institutes of Health [grant numbers MH-077776, MH096983 to LJY; OD P51OD011132 to Yerkes National Primate Research Center].
The authors would like to thank Catherine Barrett and Lorra Julian, as well as Kathrin Bohrer, Martina Fuchs and Gabriele Schindler for their excellent technical support.
Footnotes
Conflicts of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Low BS. Ecological and socio-cultural impacts on mating and marriage systems. In: Dunbar RIM, Barrett L, editors. Oxford Handbook of Evolutionary Psychology. Oxford University Press; New York: 2007. pp. 449–462. [Google Scholar]
- 2.Murdoch GP. Atlas of world cultures. Pittsburgh Press; Pittsburgh: 1981. [Google Scholar]
- 3.Schor EL. American Academy of Pediatrics Task Force on the F. Family pediatrics: report of the Task Force on the Family. Pediatrics. 2003;111:1541–1571. [PubMed] [Google Scholar]
- 4.Woodroffe R, Vincent A. Mother’s little helpers: Patterns of male care in mammals. Trends Ecol Evol. 1994;9:294–297. doi: 10.1016/0169-5347(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 5.Afifi TO, Cox BJ, Enns MW. Mental health profiles among married, never-married, and separated/divorced mothers in a nationally representative sample. Soc Psychiatry Psychiatr Epidemiol. 2006;41:122–129. doi: 10.1007/s00127-005-0005-3. [DOI] [PubMed] [Google Scholar]
- 6.Brown GW, Moran PM. Single mothers, poverty and depression. Psychol Med. 1997;27:21–33. doi: 10.1017/s0033291796004060. [DOI] [PubMed] [Google Scholar]
- 7.Cairney J, Pevalin DJ, Wade TJ, Veldhuizen S, Arboleda-Florez J. Twelve-month psychiatric disorder among single and married mothers: the role of marital history. Can J Psychiatry. 2006;51:671–676. doi: 10.1177/070674370605101007. [DOI] [PubMed] [Google Scholar]
- 8.Pearlin LI, Johnson JS. Marital status, life-strains and depression. Am Sociol Rev. 1977;42:704–715. [PubMed] [Google Scholar]
- 9.Crosier T, Butterworth P, Rodgers B. Mental health problems among single and partnered mothers. The role of financial hardship and social support. Soc Psychiatry Psychiatr Epidemiol. 2007;42:6–13. doi: 10.1007/s00127-006-0125-4. [DOI] [PubMed] [Google Scholar]
- 10.Wade TJ, Veldhuizen S, Cairney J. Prevalence of psychiatric disorder in lone fathers and mothers: examining the intersection of gender and family structure on mental health. Can J Psychiatry. 2011;56:567–573. doi: 10.1177/070674371105600908. [DOI] [PubMed] [Google Scholar]
- 11.Sperlich S, Arnhold-Kerri S, Geyer S. What accounts for depressive symptoms among mothers? The impact of socioeconomic status, family structure and psychosocial stress. Int J Public Health. 2011;56:385–396. doi: 10.1007/s00038-011-0272-6. [DOI] [PubMed] [Google Scholar]
- 12.Kerstis B, Berglund A, Engstrom G, Edlund B, Sylven S, Aarts C. Depressive symptoms postpartum among parents are associated with marital separation: a Swedish cohort study. Scan J Public Health. 2014;42:660–668. doi: 10.1177/1403494814542262. [DOI] [PubMed] [Google Scholar]
- 13.Berkman PL. Spouseless motherhood, psychological stress, and physical morbidity. J Health Soc Behav. 1969;10:323–334. [PubMed] [Google Scholar]
- 14.Ahern TH, Hammock EA, Young LJ. Parental division of labor, coordination, and the effects of family structure on parenting in monogamous prairie voles (Microtus ochrogaster) Dev Psychobiol. 2011;53:118–131. doi: 10.1002/dev.20498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bosch OJ, Young LJ. Oxytocin and Social Relationships: From Attachment to Bond Disruption. Curr Top Behav Neurosci. 2017 doi: 10.1007/7854_2017_10. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pohl TT, Young LJ, Bosch OJ. Lost Connections: Oxytocin and the neural, physiological and behavioural consequences of disrupted relationships. Int J Psychophysiol. doi: 10.1016/j.ijpsycho.2017.12.011. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lonstein JS, De Vries GJ. Sex differences in the parental behavior of rodents. Neurosci, Biobehav, Rev. 2000;24:669–686. doi: 10.1016/s0149-7634(00)00036-1. [DOI] [PubMed] [Google Scholar]
- 18.Ahern TH, Young LJ. The impact of early life family structure on adult social attachment, alloparental behavior, and the neuropeptide systems regulating affiliative behaviors in the monogamous prairie vole (microtus ochrogaster) Front Behav Neurosci. 2009;3:17. doi: 10.3389/neuro.08.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGuire B, Parker E, Bemis WE. Sex differences, effects of male presence and coordination of nest visits in prairie voles (Microtus ochrogaster) during the immediate postnatal period. Am Midl Nat. 2007;157:187–201. [Google Scholar]
- 20.Wang ZX, Novak MA. Influence of social-environment on parental behavior and pup development of meadow voles (Microtus pensylvanicus) and prairie voles (Microtus ochrogaster) J Comp Psychol. 1992;106:163–171. [Google Scholar]
- 21.Bosch OJ, Nair HP, Ahern TH, Neumann ID, Young LJ. The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacology. 2009;34:1406–1415. doi: 10.1038/npp.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bosch OJ, Dabrowska J, Modi ME, Johnson ZV, Keebaugh AC, Barrett CE, et al. Oxytocin in the nucleus accumbens shell reverses CRFR2-evoked passive stress-coping after partner loss in monogamous male prairie voles. Psychoneuroendocrinology. 2016;64:66–78. doi: 10.1016/j.psyneuen.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNeal N, Scotti MA, Wardwell J, Chandler DL, Bates SL, Larocca M, et al. Disruption of social bonds induces behavioral and physiological dysregulation in male and female prairie voles. Auton Neurosci. 2014;180:9–16. doi: 10.1016/j.autneu.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun P, Smith AS, Lei K, Liu Y, Wang Z. Breaking bonds in male prairie vole: Long-term effects on emotional and social behavior, physiology, and neurochemistry. Behav Brain Res. 2014;265:22–31. doi: 10.1016/j.bbr.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNeal N, Appleton KM, Johnson AK, Scotti ML, Wardwell J, Murphy R, et al. The protective effects of social bonding on behavioral and pituitary-adrenal axis reactivity to chronic mild stress in prairie voles. Stress. 2017;20:175–182. doi: 10.1080/10253890.2017.1295444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osako Y, Nobuhara R, Arai YP, Tanaka K, Young LJ, Nishihara M, et al. Partner loss in monogamous rodents: Modulation of pain and emotional behavior in male prairie voles. Psychosom Med. 2017 doi: 10.1097/PSY.0000000000000524. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cryan JF, Mombereau C. In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry. 2004;9:326–357. doi: 10.1038/sj.mp.4001457. [DOI] [PubMed] [Google Scholar]
- 28.Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Gobrogge K, Wang Z. Neuropeptidergic regulation of pair-bonding and stress buffering: Lessons from voles. Horm Behav. 2015;76:91–105. doi: 10.1016/j.yhbeh.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Getz LL, Carter CS. Prairie-vole partnerships. Am Scientist. 1996;84:56–62. [Google Scholar]
- 31.Getz LL, McGuire B. A comparison of living singly and in male-female pairs in the prairie vole, Microtus ochrogaster. Ethology. 1993;94:265–278. [Google Scholar]
- 32.Carter CS, Getz LL. Monogamy and the prairie vole. Sci Am. 1993;268:100–106. doi: 10.1038/scientificamerican0693-100. [DOI] [PubMed] [Google Scholar]
- 33.Roberts RL, Cushing BS, Carter CS. Intraspecific variation in the induction of female sexual receptivity in prairie voles. Physiol Behav. 1998;64:209–212. doi: 10.1016/s0031-9384(98)00042-0. [DOI] [PubMed] [Google Scholar]
- 34.McGuire B, Russell KD, Mahoney T, Novak M. The effects of mate removal on pregnancy success in prairie voles (Microtus ochrogaster) and meadow voles (Microtus pennsylvanicus) Biol Reprod. 1992;47:37–42. doi: 10.1095/biolreprod47.1.37. [DOI] [PubMed] [Google Scholar]
- 35.Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 36.Hendrie CA, Eilam D, Weiss SM. Effects of diazepam and buspirone on the behaviour of wild voles (Microtus socialis) in two models of anxiety. Pharmacol Biochem Behav. 1997;58:573–576. doi: 10.1016/s0091-3057(97)00278-5. [DOI] [PubMed] [Google Scholar]
- 37.Insel TR, Preston S, Winslow JT. Mating in the monogamous male: behavioral consequences. Physiol Behav. 1995;57:615–627. doi: 10.1016/0031-9384(94)00362-9. [DOI] [PubMed] [Google Scholar]
- 38.Pitkow LJ, Sharer CA, Ren X, Insel TR, Terwilliger EF, Young LJ. Facilitation of affiliation and pair-bond formation by vasopressin receptor gene transfer into the ventral forebrain of a monogamous vole. J Neurosci. 2001;21:7392–7396. doi: 10.1523/JNEUROSCI.21-18-07392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bosch OJ, Kromer SA, Neumann ID. Prenatal stress: opposite effects on anxiety and hypothalamic expression of vasopressin and corticotropin-releasing hormone in rats selectively bred for high and low anxiety. Eur J Neurosci. 2006;23:541–551. doi: 10.1111/j.1460-9568.2005.04576.x. [DOI] [PubMed] [Google Scholar]
- 40.Klampfl SM, Neumann ID, Bosch OJ. Reduced brain corticotropin-releasing factor receptor activation is required for adequate maternal care and maternal aggression in lactating rats. Eur J Neurosci. 2013;38:2742–2750. doi: 10.1111/ejn.12274. [DOI] [PubMed] [Google Scholar]
- 41.Kessler MS, Bosch OJ, Bunck M, Landgraf R, Neumann ID. Maternal care differs in mice bred for high vs. low trait anxiety: impact of brain vasopressin and cross-fostering. Soc Neurosci. 2011;6:156–168. doi: 10.1080/17470919.2010.495567. [DOI] [PubMed] [Google Scholar]
- 42.Gammie SC, Negron A, Newman SM, Rhodes JS. Corticotropin-releasing factor inhibits maternal aggression in mice. Behav Neurosci. 2004;118:805–814. doi: 10.1037/0735-7044.118.4.805. [DOI] [PubMed] [Google Scholar]
- 43.Bosch OJ, Neumann ID. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Horm Behav. 2012;61:293–303. doi: 10.1016/j.yhbeh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 44.Rilling JK, Young LJ. The biology of mammalian parenting and its effect on offspring social development. Science. 2014;345:771–776. doi: 10.1126/science.1252723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bosch OJ. Maternal nurturing is dependent on her innate anxiety: The behavioral roles of brain oxytocin and vasopressin. Horm Behav. 2011;59:201–212. doi: 10.1016/j.yhbeh.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 46.Coplan RJ, Arbeau KA, Armer M. Don’t fret, be supportive! Maternal characteristics linking child shyness to psychosocial and school adjustment in kindergarten. J Abnorm Child Psychol. 2008;36:359–371. doi: 10.1007/s10802-007-9183-7. [DOI] [PubMed] [Google Scholar]
- 47.Rubin K, Burgess K. Parents of aggressive and withdrawn children. In: Bornstein M, editor. Handbook of Parenting. Vol. 1. Lawrence Erlbaum Associates; Hillsdale, NJ: 2002. pp. 383–418. [Google Scholar]
- 48.Bales KL, Lewis-Reese AD, Pfeifer LA, Kramer KM, Carter CS. Early experience affects the traits of monogamy in a sexually dimorphic manner. Dev Psychobiol. 2007;49:335–342. doi: 10.1002/dev.20216. [DOI] [PubMed] [Google Scholar]
- 49.Barrett CE, Arambula SE, Young LJ. The oxytocin system promotes resilience to the effects of neonatal isolation on adult social attachment in female prairie voles. Transl Psychiatry. 2015;5:e606. doi: 10.1038/tp.2015.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bales KL, Saltzman W. Fathering in rodents: Neurobiological substrates and consequences for offspring. Horm Behav. 2016;77:249–259. doi: 10.1016/j.yhbeh.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith AS, Wang Z. Hypothalamic oxytocin mediates social buffering of the stress response. Biol Psychiatry. 2014;76:281–288. doi: 10.1016/j.biopsych.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Slattery DA, Neumann ID. No stress please! Mechanisms of stress hyporesponsiveness of the maternal brain. J Physiol. 2008;586:377–385. doi: 10.1113/jphysiol.2007.145896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnstone HA, Wigger A, Douglas AJ, Neumann ID, Landgraf R, Seckl JR, et al. Attenuation of hypothalamic-pituitary-adrenal axis stress responses in late pregnancy: changes in feedforward and feedback mechanisms. J Neuroendocrinol. 2000;12:811–822. doi: 10.1046/j.1365-2826.2000.00525.x. [DOI] [PubMed] [Google Scholar]
- 54.Lightman SL, Windle RJ, Wood SA, Kershaw YM, Shanks N, Ingram CD. Peripartum plasticity within the hypothalamo-pituitary-adrenal axis. Prog Brain Res. 2001;133:111–129. doi: 10.1016/s0079-6123(01)33009-1. [DOI] [PubMed] [Google Scholar]
- 55.Walker CD, Toufexis DJ, Burlet A. Hypothalamic and limbic expression of CRF and vasopressin during lactation: implications for the control of ACTH secretion and stress hyporesponsiveness. Prog Brain Res. 2001;133:99–110. doi: 10.1016/s0079-6123(01)33008-x. [DOI] [PubMed] [Google Scholar]
- 56.Burkett JP, Andari E, Johnson ZV, Curry DC, de Waal FB, Young LJ. Oxytocin-dependent consolation behavior in rodents. Science. 2016;351:375–378. doi: 10.1126/science.aac4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hurlemann R, Scheele D. Dissecting the role of oxytocin in the formation and loss of social relationships. Biol Psychiatry. 2016;79:185–193. doi: 10.1016/j.biopsych.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 58.Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012;35:649–659. doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 59.Keebaugh AC, Barrett CE, LaPrairie JL, Jenkins JJ, Young LJ. RNAi knockdown of oxytocin receptor in the nucleus accumbens inhibits social attachment and parental care in monogamous female prairie voles. Soc Neurosci. 2015;10:561–570. doi: 10.1080/17470919.2015.1040893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keebaugh AC, Young LJ. Increasing oxytocin receptor expression in the nucleus accumbens of pre-pubertal female prairie voles enhances alloparental responsiveness and partner preference formation as adults. Horm Behav. 2011;60:498–504. doi: 10.1016/j.yhbeh.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Olazábal DE, Young LJ. Oxytocin receptors in the nucleus accumbens facilitate “spontaneous” maternal behavior in adult female prairie voles. Neuroscience. 2006;141:559–568. doi: 10.1016/j.neuroscience.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 62.Sbarra DA, Hazan C. Coregulation, dysregulation, self-regulation: an integrative analysis and empirical agenda for understanding adult attachment, separation, loss, and recovery. Pers Soc Psychol Rev. 2008;12:141–167. doi: 10.1177/1088868308315702. [DOI] [PubMed] [Google Scholar]
- 63.Diamond LM, Hicks AM, Otter-Henderson KD. Every time you go away: changes in affect, behavior, and physiology associated with travel-related separations from romantic partners. J Pers Soc Psychol. 2008;95:385–403. doi: 10.1037/0022-3514.95.2.385. [DOI] [PubMed] [Google Scholar]
- 64.Benzeval M. The self-reported health status of lone parents. Soc Sci Med. 1998;46:1337–1353. doi: 10.1016/s0277-9536(97)10083-1. [DOI] [PubMed] [Google Scholar]