Abstract
Behavioral assessments in rats are overwhelmingly conducted during the day, albeit that is when they are least active. This incongruity may preclude optimal performance. Hence, the goal of this study was to determine if differences in neurobehavior exist in traumatic brain injured (TBI) rats when assessed during the day vs. night. The hypothesis was that the night group would perform better than the day group on all behavioral tasks. Anesthetized adult male rats received either a cortical impact or sham injury and then were randomly assigned to either Day (1:00 – 3:00 p.m.) or Night (7:30 – 9:30 p.m.) testing. Motor function (beam-balance/walk) was conducted on postoperative days 1–5 and cognitive performance (spatial learning) was assessed on days 14–18. Corticosterone (CORT) levels were quantified at 24 hr and 21 days after TBI. No significant differences were revealed between the TBI rats tested during the Day vs. Night for motor or cognition (p’s < 0.05). CORT levels were higher in the Night-tested TBI and sham groups at 24 hr (p < 0.05), but returned to baseline and were no longer different by day 21 (p > 0.05), suggesting an initial, but transient, stress response that did not affect neurobehavioral outcome. These data suggest that the time rats are tested has no noticeable impact on their performance, which does not support the hypothesis. The finding validates the interpretations from numerous studies conducted when rats were tested during the day vs. their natural active period.
Keywords: beam-walk, controlled cortical impact, CORT, functional recovery, learning and memory, Morris water maze, traumatic brain injury
1. Introduction
Traumatic brain injury (TBI) affects two million people in the United States each year and several million more worldwide [1]. Survivors of TBI are often left with long-term motor and cognitive disabilities [2] and unfortunately limited treatment options. Therefore, evaluating therapeutic strategies in the laboratory that may translate to the clinic is of utmost importance. To this end, numerous studies have been conducted assessing various forms of therapeutic interventions [3–5]. Nevertheless, while some preclinical studies are positive, the lack of translational success indicates that something is amiss between the bench-and-bedside [6,7]. Several explanations exist, such as heterogeneity of the injury in the clinic vs. homogeneity in the laboratory, differences in therapeutic dosing regimens, and timing of drug administration. However, another important, and often overlooked, methodological issue is timing of behavioral manipulations. Specifically, when during the circadian cycle are the subjects tested?
In preclinical TBI studies, rats are the overwhelming animal model used to assess the efficacy of various therapies. However, rats are nocturnal and therefore may be more engaged in the tasks at hand (i.e., behavioral assessments) when evaluated during their natural activity state. Moreover, most of the experimental manipulations are conducted during the day when the research laboratories, but not the rats, are most active, which suggests that the traditional experimental paradigm may not be ideal. Hence, the primary goal of this study was to investigate the relationship between circadian phase and neurobehavioral performance after TBI. The hypothesis was that the night group would perform better than the day group in all behavioral tasks. The secondary goal was to determine whether stress, as determined by increased corticosterone (CORT) levels might mitigate behavioral differences. The rationale is based on the hypothalamic-pituitary-adrenal (HPA) axis displaying a characteristic circadian pattern of corticosterone release, with lower levels at the beginning of the light phase and higher levels at the onset of the active dark phase. Rodents display a differential HPA axis response after behavioral testing depending of the circadian phase [8]. Changes in CORT levels modify the sensibility to stress [9], affect performance in behavioral tasks [10], and could influence the susceptibility to TBI. It has been proposed that TBI increases HPA axis reactivity during the post-injury period [11] and causes a blunted response on the chronic phase [12]. However, this evidence is still controversial, as corticosteroid insufficiency has also been observed after TBI [13]. Moreover, light exposure during testing could act as a potent zeitgeber altering the circadian HPA axis response.
The findings will yield important insight regarding whether the behavioral evaluations currently being conducted during the light phase are appropriate or if researchers need to be more cognizant regarding circadian rhythms.
2. Materials and methods
2.1. Subjects
Fifty-three adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 300–325 g on the day of surgery were paired housed in ventilated polycarbonate rat cages and maintained in a temperature (21 ± 1°C) and light (on 7:00 a.m. to 7:00 p.m.) controlled environment with food and water available ad libitum. After one week of acclimatization all rats underwent a single day of beam-walk training, which consisted of 3–5 successive approximation trials to traverse the beam. Following training the rats were randomly assigned to TBI + Day testing (n=14), TBI + Night testing (n=14), Sham + Day testing (n=5), and Sham + Night testing (n=5) groups that underwent all behavioral assessments and were sacrificed at 3 weeks to quantify corticosterone (CORT) levels. Groups assigned to day testing were assessed from 1:00 – 3:00 p.m., while those assigned to testing after the lights went out were evaluated from 7:30 – 9:30 p.m. An additional 20 rats were assigned to the same group conditions (n=5 each), but were sacrificed for acute CORT assessment at 24-hr (Day testing) or 30.5-hr (Night testing), which was immediately after motor behavior. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh. Every attempt was made to limit the number of rats used and to minimize suffering.
2.2. Surgery
TBI was produced during the light phase (1:00 p.m. to 3:00 p.m.), for all groups, as previously described [14,15]. Briefly, following surgical anesthesia with 4% isoflurane, a controlled cortical impact (CCI) injury of moderate severity (2.8 mm tissue deformation at 4 m/sec) was produced. Immediately after the CCI, anesthesia was discontinued and the incision was promptly sutured. The rats were subsequently extubated and assessed for acute neurological outcome. Sham rats underwent similar surgical procedures, but were not subjected to the impact.
2.3. Acute neurological evaluation
Hind limb reflexive ability was assessed immediately after the cessation of anesthesia by gently squeezing the rats’ hind paw every 5 sec and recording the time to elicit a withdrawal response. Return of the righting reflex was determined by the time required to turn from the supine to prone position three consecutive times.
2.4. Motor performance
Established beam-balance and beam-walk tasks were used to assess motor function. Briefly, the beam-balance task consists of placing the rat on an elevated and narrow beam (1.5 cm wide) and recording the time it remains on for a maximum of 60 sec. The beam-walk task, modified from that originally devised by Feeney and colleagues [16], consists of training/assessing rats using a negative-reinforcement paradigm to escape a bright light and white noise by traversing an elevated narrow beam (2.5 × 100 cm) and entering a darkened goal box. Performance was assessed by recording the elapsed time to traverse the beam. Rats were tested for beam-balance and beam-walk performance prior to surgery to establish a baseline measure and again on post-operative days 1–5 (except the groups sacrificed at 24-hr who only received testing on day 1). Testing consisted of three trials (60 sec allotted time with an inter-trial interval of 30 sec) per day on each task. If a rat was unable to traverse the beam the maximum time of 60 sec was recorded. The average daily scores for each rat were used in the statistical analyses.
2.5. Cognitive function: acquisition of spatial learning
A Morris water maze (MWM) task that is sensitive to cognitive function after TBI was utilized [14,15,17]. The pool (180 cm diameter; 60 cm high) was filled with tap water (26 ± 1°C) to a depth of 28 cm and was situated in a room with salient visual cues. The platform, a clear Plexiglas stand (10 cm diameter, 26 cm high), was positioned 26 cm from the maze wall in the southwest quadrant and held constant for each rat. Spatial learning began on post-operative day 14 and consisted of providing a block of four daily trials (4-min inter-trial interval) for five consecutive days (14–18) to locate the submerged platform (i.e., invisible to the rat). On day 19 the platform was raised 2 cm above the water surface (i.e., visible to the rat) as a control procedure to determine the contributions of non-spatial factors on cognitive performance. For each daily block of trials, the rats were placed in the pool facing the wall at each of the four possible start locations in a randomized manner. Each trial lasted until the rat climbed onto the platform or until 120 sec had elapsed, whichever occurred first. Rats that failed to locate the platform within the allotted time were manually guided to it. Once on the platform the rats remained on it for 30 sec before being placed in a heated incubator between trials. The times of the 4 daily trials for each rat were averaged and used in the statistical analyses.
2.6. Cognitive function: probe trial (memory retention)
One day after the final acquisition training session (i.e., day 19), all rats were given a single probe trial to measure retention. Briefly, the platform was removed and the rats explored the pool for 30 sec. The percent time spent in the target quadrant was used in the statistical analysis. The data were obtained using ANY-maze video tracking software.
2.7. Corticosterone (CORT) levels
CORT levels were quantified with a commercially available ELISA kit (R&D Systems, Parameter™, Minneapolis, MN, USA, Cat# KGE009). Serum samples were obtained from trunk blood at 24 hr for the Day tested groups and approximately 30.5 – 32.5 hr for the Night tested rats and again at 21 days after TBI or sham injury for both groups (during the day or night paralleling the behavioral schedule). Briefly, anesthetized rats underwent a thoracotomy and ~5 mL of blood was taken via the heart, then allowed to clot in BD (Franklin Lakes, NJ, USA) red top collection vacutainers, and subsequently spun at 4000 g for 10 min. Serum was collected, aliquoted, and stored at −80°C until use. Assay procedures followed the manufacturer’s instructions. Optical densities were obtained at an absorbance wavelength of 450 nm with a plate correction of 570 nm. A 4-parameter logarithmic curve for standards was established and the results were calculated based on these plate standards. All samples were run in duplicate.
2.8. Data analyses
Statistical analyses were performed using StatView 5.0.1 (Abacus Concepts, Inc., Berkeley, CA) by a researcher blinded to group conditions. Only after the analyses were concluded and the code was broken did the statistician know the correct order of the groups. The motor and cognitive data were analyzed by repeated-measures analysis of variance (ANOVA). The acute neurological assessment, probe trial, visible platform, swim speed, and CORT levels were analyzed by one-factor ANOVAs. When the ANOVA showed a significant effect, the Newman-Keuls post-hoc test was utilized to determine specific group differences. The results are expressed as the mean ± standard error of the mean (S.E.M.) and are considered significant when p values were ≤ 0.05.
3. Results
3.1. Acute neurological evaluation
No significant differences were observed among the TBI groups for return of hind limb reflex ability after a brief paw pinch (right TBI Day = 140.6 ± 5.3 sec and right TBI Night = 146.3 ± 4.2 sec; left TBI Day = 145.3 ± 5.3 and left TBI Night = 151.0 ± 4.2 sec) or righting reflex latency (TBI Day = 352.7 ± 16.5 sec and TBI Night = 368.5 ± 11.5 sec) following the cessation of anesthesia [p’s > 0.05]. As expected, the sham controls had significantly shorter hind limb reflexes (right TBI Day = 13.2 ± 0.6 sec and right TBI Night = 14.6 ± 1.6 sec; left TBI Day = 17.2 ± 1.0 and left TBI Night = 19.0 ± 1.7 sec) and righting reflex latency (TBI Day = 109.8 ± 6.0 sec and TBI Night = 120.8 ± 4.1 sec).
3.2. Motor performance: beam-balance and beam-walk
No baseline differences were observed among the groups as all rats balanced on the beam for the full 60 sec (Fig. 1A). For the post-surgical data, the repeated-measures ANOVA revealed significant Group [F3,34 = 8.559, p = 0.0002] and Day [F5,170 = 10.476, p < 0.0001] differences, as well as a significant Group x Day interaction [F15,170 = 4.14, p < 0.0001]. The post-hoc analysis revealed that the Sham controls, which were able to balance for the full 60 sec were significantly better than both TBI groups [p < 0.05], regardless of when tested, and did not differ from one another [p > 0.05]. Additionally, no difference was revealed between the TBI Day and TBI Night groups [p > 0.05]. Regarding the beam-walk data, no significant differences were observed among any of the groups in the time to traverse the beam prior to surgery (Fig. 1B). However, after surgery, the repeated-measures ANOVA revealed significant Group [F3,34 = 28.236, p < 0.0001] and Day [F5,170 = 32.389, p < 0.0001] differences, as well as a significant Group x Day interaction [F15,170 = 10.652, p < 0.0001]. Like the beam-balance findings, the post-hoc analysis revealed that the Sham controls were significantly better than both TBI groups [p < 0.05], and did not differ from one another [p > 0.05]. No difference was revealed between the TBI Day and TBI Night groups [p > 0.05].
Fig. 1.
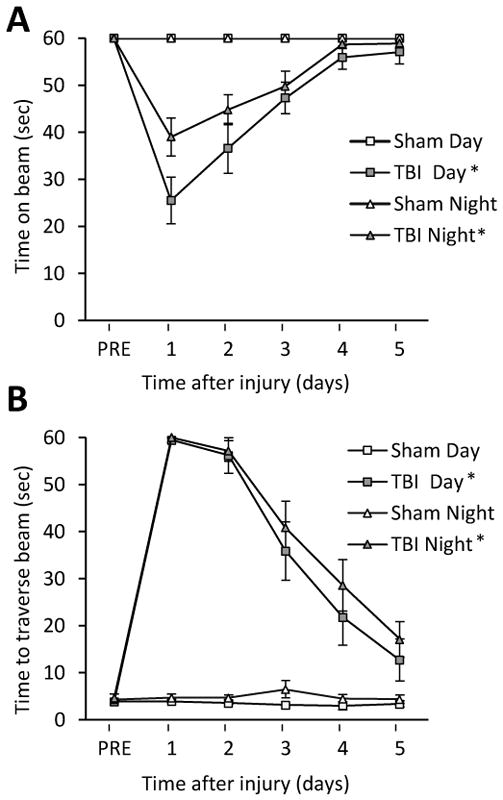
Fig. 1AB. Mean (± S.E.M.) time (sec) balancing on an elevated narrow beam prior to, and after, TBI or sham injury (A). * p < 0.05 vs. Sham Day and Sham Night. No differences were revealed between the TBI groups, regardless of when tested, nor between the Sham controls [p > 0.05]. Mean (± S.E.M.) time (sec) to traverse an elevated narrow beam after TBI or sham injury (B). * p < 0.05 vs. Sham Day and Sham Night. No differences were revealed between the TBI groups, regardless of when tested, nor between the Sham controls [p > 0.05].
3.3. Cognitive function: acquisition of spatial learning and probe trial (memory retention)
Analysis of the spatial learning data revealed significant Group [F3,34 = 21.501, p < 0.0001] and Day [F4,136 = 16.616, p < 0.0001] differences. The post-hoc analysis revealed that the Sham groups did not differ from one another [p > 0.05] and were significantly better at learning the location of the escape platform relative to both the TBI Day and TBI Night groups [p < 0.05]. No difference was revealed between the TBI Day and TBI Night groups [p > 0.05; Fig. 2A]. Analysis of the probe trial (i.e., memory retention) data revealed a significant Group effect [F3,34 = 9.698, p < 0.0001]. The post-hoc revealed that the Sham Day and Sham Night groups spent a greater percentage of the allotted time in the target quadrant (41.3 ± 1.9 % and 38.7 ± 2.1 %, respectively) relative to the TBI Day and TBI Night groups (26.9 ± 2.0 % and 27.8 ± 1.6 %, respectively) [p’s < 0.05]. No difference was revealed between the TBI Day and TBI Night groups [p > 0.05; Fig. 2B]. Lastly, no significant differences in swim speed (range = 28.8 ± 2.1 cm/sec to 36.2 ± 3.3 cm/sec) were observed among the TBI or Sham groups [p > 0.05], but there was a difference in the time to reach the visible platform, with both Sham groups locating the platform quicker than the TBI groups [p < 0.05; Fig. 2A].
Fig. 2.
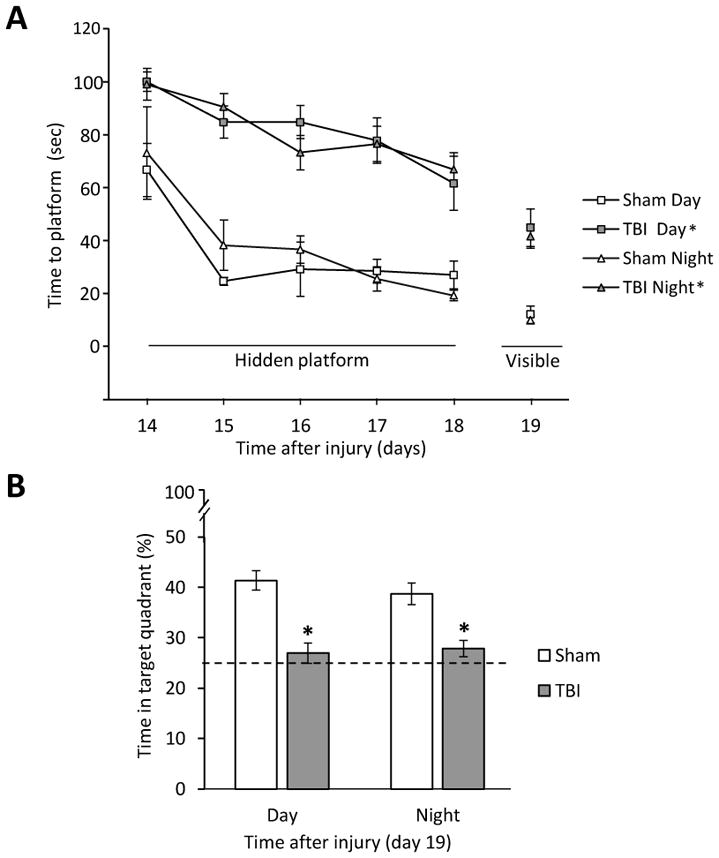
Fig. 2AB. Mean (± S.E.M.) time (sec) to locate a hidden and visible platform in the MWM (A). * p < 0.05 vs. Sham Day and Sham Night. No differences were revealed between the TBI groups, regardless of when tested, nor between the Sham controls [p > 0.05]. Mean (± S.E.M.) percent time spent in the target quadrant (B). The histogram shows the % time that each group spent in the target quadrant and the horizontal dashed line at depicts chance (25%) level of exploration of the target quadrant. * p < 0.05 vs. Sham Day and Sham Night. No differences were revealed between the TBI groups, regardless of when tested, nor between the Sham controls [p > 0.05].
3.4. Corticosterone (CORT) levels
Analysis of the CORT data showed a significant Group [F7,29 = 4.812, p = 0.0011] difference. The post-hoc analysis revealed that the TBI Night and Sham Night groups exhibited higher CORT levels 24-hr after surgery relative to the Sham Day group at 24-hr [p < 0.05]. Additionally, the TBI Night group differed from the TBI Day group at 24-hr [p < 0.05]. No differences were observed among any of the groups at 21 days [p > 0.05; Fig. 3].
Fig. 3.
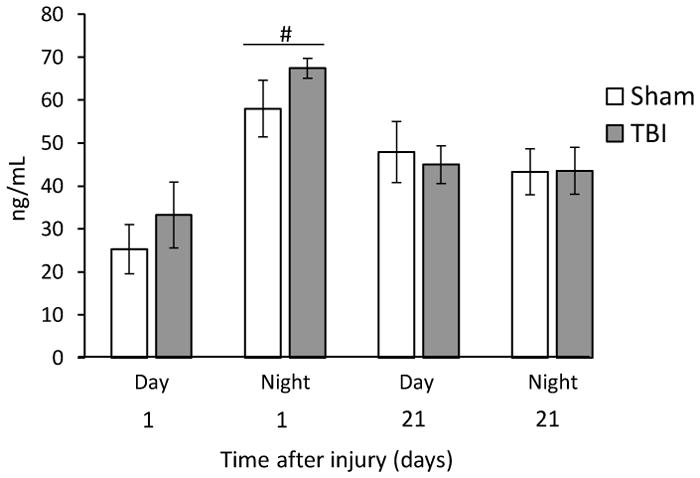
Mean (± S.E.M.) ng/mL serum CORT concentrations at 24-hr (Day 1) and 21 days after TBI or sham injury. The Day and Night groups were sacrificed according to their behavioral testing schedule. CORT levels after behavioral testing were not affected by TBI. No differences were observed between Sham and TBI animals. The only differences were between the Night and Day groups at 24-hr (#p < 0.05).
4. Discussion
The present study was designed to test the hypothesis that rats sustaining a TBI would recover motor and cognitive function better when tested during the night versus the day. The basis for the rationale is that rats are nocturnal, and therefore should be more active and alert during the dark phase, which would lead to optimal performance via focused symptom relevant experience. In contrast, albeit awake, rats that are tested during the day would presumably be less alert and would not perform to their full ability.
No significant differences were revealed between the TBI groups regardless of whether tested during the day or during the night in any of the behavioral assessments. Beam-balance performance was fully recovered in both groups by the last day of testing, whereas beam-walking ability was still significantly, and equally, impaired in both TBI groups relative to the sham controls at the end of the five days of testing. There was also no difference in the acquisition of spatial learning, assessed in a MWM task, between the two TBI groups as they performed almost identically to one another and both were significantly impaired relative to the sham controls, which were also not impacted by the time of testing as both performed similarly.
These behavioral findings do not support the hypothesis, and indeed contrast with studies in non-TBI rats reporting better MWM performance during night testing [18,19]. However, the effects of circadian rhythms on MWM outcomes are not clear as some studies suggest that day and night differences in cognitive outcome may be dependent on the complexity of the task with less complex tests failing to show differences [20] while more complex tasks, such as the evaluation of working memory, do show differences [20,21]. Additionally, others report strain dependent effects [22]. One possible explanation regarding the lack of behavioral differences between groups tested during the day or night is that TBI disrupted circadian rhythms. Indeed, studies in both rodents and humans have shown such disruptions [23,24]. Disrupting circadian rhythms may manifest in the lack of day-night continuity, which in turn does not impact behavioral outcome. Nonetheless, that the uninjured sham controls did not differ from one another in any behavioral task whether tested during the day or night suggests that a TBI-induced disruption of circadian rhythms does not fully account for the findings.
Behavioral testing was not affected by changes in CORT levels after TBI. Twenty-four hours after surgery, we observed an increase in CORT levels in response to behavioral testing only during the night in both TBI and sham rats. Light, noise, and exposure to the elevated beam are mildly aversive stimuli that could elicit the activation of the HPA axis during the first days of testing; however, both day and night tested rats showed similar CORT levels 21 days after surgery, suggesting that they habituate to handling regardless of the injury. Studies of HPA axis reactivity after TBI are conflicting. While some groups report that the stress response is heightened during the sub-acute post-injury period [11] and blunted in the chronic phase [12], other groups observe a critical illness-related corticosteroid insufficiency during the first week after TBI [13]. However, these effects are dependent on injury severity [25]. Here, CORT levels after testing did not differ between TBI and sham groups, thus indicating that increased CORT observed during the night could be related to behavioral manipulations rather than TBI-induced stress hyperactivity. In agreement with this, it has been observed that one day post-injury, plasma CORT levels were comparable in sham and injured animals after a mild stressor [26]. Moreover, forced, but not voluntary, exercise continuously elevated nocturnal CORT levels in both fluid percussion injured and sham control rats during the first week after injury [27]. Additionally, recent studies suggest that TBI has no effect on basal or foot shock induced CORT levels 34 days after injury [28], thus suggesting a differential CORT stress response conditional on the nature and time of day of the stimulus. Lastly, the decrease in CORT at 21 days may be due to handling over the two weeks of behavioral manipulations [29].
5. Conclusion
In summary, the results suggest that the neurobehavioral and cognitive assays evaluated in this study after TBI do not appear to be circadian-phase dependent as there were no differences in outcomes between rats when tested either during the day or the night, despite similar injury severities. Additionally, while CORT levels were higher in the TBI and sham groups tested at night 24 hr after surgery, they returned to baseline and were no longer different by day 21, which suggests an initial, but transient, stress response that did not affect neurobehavioral outcome. Hence, these data suggest that the time rats are tested has no measurable impact on their performance, which does not support the hypothesis. The finding validates the interpretations from numerous studies conducted when rats were tested during the day vs. their natural active period.
Highlihts.
Neither motor nor cognitive recovery differed between rats tested during the day vs. the night, for their respective TBI and sham groups
CORT levels were higher in the night-tested rats on day 1, but returned to baseline by day 21
The findings validate interpretations from studies conducted during the day vs. the rats’ natural active period.
Acknowledgments
This work was supported, in part, by NIH grants HD069620, HD069620-S1, NS060005, NS084967 (AEK), NS094950, NS099683 (COB), the University of Pittsburgh Physicians/UPMC Academic Foundation, and the UMPC Rehabilitation Institute (COB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: A global perspective. Neurorehabilitation. 2007;22:341–355. [PubMed] [Google Scholar]
- 2.Millis SR, Rosenthal M, Novack TA, MSherer M, Nick TG, Kreutzer JS, High WM, Jr, Ricker JH. Long-term neuropsychological outcome after traumatic brain injury. J Head Trauma Rehabil. 2011;16:343–355. doi: 10.1097/00001199-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Kline AE, Leary JB, Radabaugh HL, Cheng JP, Bondi CO. Combination therapies for neurobehavioral and cognitive recovery after experimental traumatic brain injury: Is more better? Prog Neurobiol. 2016;142:45–67. doi: 10.1016/j.pneurobio.2016.05.002. https://doi.org/10.1016/j.pneurobio.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wheaton P, Mathias JL, Vink R. Impact of early pharmacotherapy treatment on cognitive and behavioral outcome after traumatic brain injury in adults: a meta-analysis. J Clin Psychopharmacol. 2009;29:468–477. doi: 10.1097/JCP.0b013e3181b66f04. https://doi.org/10.1097/JCP.0b013e3181b66f04. [DOI] [PubMed] [Google Scholar]
- 5.Kokiko ON, Hamm RJ. A review of pharmacological treatments used in experimental models of traumatic brain injury. Brain Inj. 2007;21:259–274. doi: 10.1080/02699050701209964. http://dx.doi.org/10.1080/02699050701209964. [DOI] [PubMed] [Google Scholar]
- 6.Doppenberg EM, Choi SC, Bullock R. Clinical trials in traumatic brain injury: lessons for the future. J Neurosurg Anesthesiol. 2004;16:87–94. doi: 10.1097/00008506-200401000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Menon DK, Zahed C. Prediction of outcome in severe traumatic brain injury. Curr Opin Crit Care. 2009;15:437–441. doi: 10.1097/MCC.0b013e3283307a26. http://dx.doi.org/10.1097/MCC.0b013e3283307a26. [DOI] [PubMed] [Google Scholar]
- 8.Verma P, Hellemans K, Choi FY, Yu W, Weinberg J. Circadian phase and sex effects on depressive/anxiety-like behaviors and HPA axis responses to acute stress. Physiol Behav. 2010;99:276–285. doi: 10.1016/j.physbeh.2009.11.002. https://doi.org/10.1016/j.physbeh.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen S, Vainer E, Matar MA, Kozlovsky N, Kaplan Z, Zohar J, Mathé AA, Cohen H. Diurnal fluctuations in HPA and neuropeptide Y-ergic systems underlie differences in vulnerability to traumatic stress responses at different zeitgeber times. Neuropsychopharmacology. 2015;40:774–790. doi: 10.1038/npp.2014.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luine VN, Spencer RL, McEwen BS. Effects of chronic corticosterone ingestion on spatial memory performance and hippocampal serotonergic function. Brain Res. 1993;616:65–70. doi: 10.1016/0006-8993(93)90193-q. [DOI] [PubMed] [Google Scholar]
- 11.Griesbach GS, Hovda DA, Tio DL, Taylor AN. Heightening of the stress response during the first weeks after a mild traumatic brain injury. Neuroscience. 2011;178:147–158. doi: 10.1016/j.neuroscience.2011.01.028. https://doi.org/10.1016/j.neuroscience.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor AN, Rahman SU, Tio DL, Sanders MJ, Bando JK, Truong AH, Prolo P. Lasting neuroendocrine-immune effects of traumatic brain injury in rats. J Neurotrauma. 2006;23:1802–1813. doi: 10.1089/neu.2006.23.1802. https://doi.org/10.1089/neu.2006.23.1802. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Zhang B, Chai Y, Dong B, Lei P, Jiang R, Zhang J. Methylprednisolone exacerbates acute critical illness-related corticosteroid insufficiency associated with traumatic brain injury in rats. Brain Res. 2011;1382:298–307. doi: 10.1016/j.brainres.2011.01.045. https://doi.org/10.1016/j.brainres.2011.01.045. [DOI] [PubMed] [Google Scholar]
- 14.de Witt BW, Ehrenberg KM, McAloon RL, Panos AH, Shaw KE, Raghavan PV, Skidmore ER, Kline AE. Abbreviated environmental enrichment enhances neurobehavioral recovery comparably to continuous exposure after traumatic brain injury. Neurorehabil Neural Repair. 2011;25:343–350. doi: 10.1177/1545968310390520. https://doi.org/10.1177/1545968310390520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radabaugh HL, LaPorte MJ, Greene AM, Bondi CO, Lajud N, Kline AE. Refining environmental enrichment to advance rehabilitation based research after experimental traumatic brain injury. Exp Neurol. 2017;294:12–18. doi: 10.1016/j.expneurol.2017.04.013. https://doi.org/10.1016/j.expneurol.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feeney DM, Gonzalez A, Law WA. Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science. 1982;217:855–857. doi: 10.1126/science.7100929. [DOI] [PubMed] [Google Scholar]
- 17.Hamm RJ. Neurobehavioral assessment of outcome following traumatic brain injury in rats: an evaluation of selected measures. J Neurotrauma. 2001;18:1207–1216. doi: 10.1089/089771501317095241. https://doi.org/10.1089/089771501317095241. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann HJ, Balschun D. Circadian differences in maze performance of C57bl/6 Ola mice. Behav Process. 1992;27:77–83. doi: 10.1016/0376-6357(92)90017-8. https://doi.org/10.1016/0376-6357(92)90017-8. [DOI] [PubMed] [Google Scholar]
- 19.Hauber W, Bareiss A. Facilitative effects of an adenosine A1/A2 receptor blockade on spatial memory performance of rats: selective enhancement of reference memory retention during the light period. Behav Brain Res. 2001;118:43–52. doi: 10.1016/s0166-4328(00)00307-7. [DOI] [PubMed] [Google Scholar]
- 20.Valentinuzzi VSS, Menna-Barreto L, Xavier GF. Effect of circadian phase on performance of rats in the Morris water maze task. J Biol Rhythms. 2004;19:312–324. doi: 10.1177/0748730404265688. [DOI] [PubMed] [Google Scholar]
- 21.Gritton HJ, Kantorowski A, Sarter M, Lee TM. Bidirectional interactions between circadian entrainment and cognitive performance. Learn Mem. 2012;19:126–141. doi: 10.1101/lm.023499.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cain SW, Ko CH, Chalmers JA, Ralph MR. Time of day modulation of conditioned place preference in rats depends on the strain of rat used. Neurobiol Learn Mem. 2004;81:217–220. doi: 10.1016/j.nlm.2004.02.003. https://doi.org/10.1016/j.nlm.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Baumann CR, Werth E, Stocker R, Ludwig S, Bassetti CL. Sleep–wake disturbances 6 months after traumatic brain injury: a prospective study. Brain. 2007;130:1873–1883. doi: 10.1093/brain/awm109. https://doi.org/10.1093/brain/awm109. [DOI] [PubMed] [Google Scholar]
- 24.Willie J, Lim M, Bennett R, Azarion A, Schwetye K, Brody D. Controlled cortical impact traumatic brain injury acutely disrupts wakefulness and extracellular orexin dynamics as determined by intracerebral microdialysis in mice. J Neurotrauma. 2012;29:1908–1921. doi: 10.1089/neu.2012.2404. https://doi.org/10.1089/neu.2012.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor AN, Rahman SU, Sanders NC, Tio DL, Prolo P, Sutton RL. Injury severity differentially affects short- and long-term neuroendocrine outcomes of traumatic brain injury. J Neurotrauma. 2008;25:311–323. doi: 10.1089/neu.2007.0486. https://doi.org/10.1089/neu.2007.0486. [DOI] [PubMed] [Google Scholar]
- 26.McCullers DL, Sullivan PG, Scheff SW, Herman JP. Traumatic brain injury regulates adrenocorticosteroid receptor mRNA levels in rat hippocampus. Brain Res. 2002;947:41–49. doi: 10.1016/s0006-8993(02)02904-9. [DOI] [PubMed] [Google Scholar]
- 27.Griesbach GS, Tio DL, Vincelli J, McArthur DL, Taylor AN. Differential effects of voluntary and forced exercise on stress responses after traumatic brain injury. J Neurotrauma. 2012;29:1426–1433. doi: 10.1089/neu.2011.2229. https://doi.org/10.1089/neu.2011.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Servatius RJ, Marx CE, Sinha S, Avcu P, Kilts JD, Naylor JC, Pang KCH. Brain and serum androsterone is elevated in response to stress in rats with mild traumatic brain injury. Front Neurosci. 2016;10:379. doi: 10.3389/fnins.2016.00379. https://doi.org/10.3389/fnins.2016.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deutsch-Feldman M, Picetti R, Seip-Cammack K, Zhou Y, Kreek MJ. Effects of handling and vehicle injections on adrenocorticotropic and corticosterone concentrations in Sprague-Dawley compared with Lewis rats. J Am Assoc Lab Anim Sci. 2015;54:35–39. [PMC free article] [PubMed] [Google Scholar]


