Summary
Multiple myeloma (MM) is a lethal haematological malignancy that arises in the context of a tumour microenvironment that promotes resistance to apoptosis and immune escape. In the present study, we demonstrate that co-culture of MM cells with stromal cells results in increased resistance to cytotoxic and biological agents as manifested by decreased rates of cell death following exposure to alkylating agents and the proteosome inhibitor, bortezomib. To identify the mechanism of increased resistance, we examined the effect of the co-culture of MM cells with stroma cells, on expression of the MUC1 oncogene, known to confer tumour cells with resistance to apoptosis and necrosis. Co-culture of stroma with MM cells resulted in increased MUC1 expression by tumour cells. The effect of stromal cell co-culture on MUC1 expression was not dependent on cell contact and was therefore thought to be due to soluble factors secreted by the stromal cells into the microenvironment. We demonstrated that MUC1 expression was mediated by interleukin-6 and subsequent up-regulation of the JAK-STAT pathway. Interestingly, the effect of stromal cell co-culture on tumour resistance was partially reversed by silencing of MUC1 in MM cells, consistent with the potential role of MUC1 in mediating resistance to cytotoxic-based therapies.
Keywords: multiple myeloma, MUC1, stroma cells, STAT-3, bortezomib
While the advent of biological therapies has resulted in improved disease response and long term outcomes in patients with multiple myeloma (MM), the majority of patients ultimately succumb to progressive disease due to the emergence of resistant clonal populations (Chauhan et al, 1996; Palumbo & Anderson, 2011). Defining the critical mechanisms responsible for disease resistance is vital for understanding disease biology and the development of more effective therapeutic strategies.
A unique aspect of MM is the complex interaction between the tumour cells and the bone marrow (BM) microenvironment that creates a protective niche against cytotoxic and immunological mediated injury (Lemaire et al, 2011; Manier et al, 2012). Exposure to stromal cells promotes MM cell resistance to apoptosis and the generation of an immunosuppressive milieu (Gorgun et al, 2013). These effects are mediated by direct contact with accessory cells, such as plasmacytoid dendritic cells (DCs), regulatory T cells (Dosani et al, 2015) and myeloid derived suppressor cells (Gorgun et al, 2013), as well as soluble factors such as interleukin 6 (IL6) (Romano et al, 2014; Görguün et al, 2015), a multifunctional cytokine that has been shown to play a crucial role in growth and survival of MM cells within the BM milieu. IL6 is predominantly produced and secreted by BM stromal cells (BMSCs) (Rougier et al, 1998), mediating MM cell growth, proliferation, preventing apoptotic cell death and promoting myeloma cell survival, and conferring drug resistance (Hardin et al, 1994; Lichten-stein et al, 1995; Chauhan et al, 1996, 1997; Catlett-Falcone et al, 1999; Gupta et al, 2001; Shain et al, 2009). IL6 activates multiple pathways, including the Janus kinase 2 (JAK2)/signal transducer and activator of transcription (STAT3) cascade (Catlett-Falcone et al, 1999; French et al, 2002; Shain et al, 2009). Immune modulatory drugs, such as lenalidomide, that exhibit significant potency as anti-myeloma agents, are thought to derive their efficacy in part by disrupting the stro-mal–MM cell interactions (Quach et al, 2010).
MUC1 is an oncoprotein that is aberrantly expressed in the majority of solid tumours and haematological malignancies (Li et al, 2003; Kawano et al, 2008; Raina et al, 2009; Yin et al, 2010; Stroopinsky et al, 2013; Jain et al, 2015). Furthermore, MUC1 was shown to be aberrantly expressed by the examined MM cell lines and primary patient samples (Takahashi et al, 1994; Burton et al, 1999; Treon et al, 1999; Paydas et al, 2001; Cloosen et al, 2006; Baldus et al, 2007; Kawano et al, 2008; Yin et al, 2010). MUC1 has been shown to regulate critical aspects of tumourigenesis, including autonomous self-renewal, cell proliferation, resistance to apoptosis, and tissue invasion (Schroeder et al, 2003; Ren et al, 2004; Huang et al, 2005; Kawano et al, 2008; Rajabi et al, 2012; Alam et al, 2014; Kharbanda et al, 2014; Bouillez et al, 2016). MUC1 is comprised of an N-terminus that is shed and a transmembrane C-terminus that, upon activation, undergoes dimerization and translocation to the nucleus to interact with downstream effectors such as nuclear factor (NF)-κB and β-catenin/wnt, which in turn modulate myeloma growth and survival (Huang et al, 2005; Kawano et al, 2008). We have developed a cell penetrating peptide (GO-203), which inhibits MUC1 via intercalation at the CQC motif. Inhibition of the MUC1-C subunit with GO-203 prevents dimerization and downstream signalling (Zhou et al, 2011; Yin et al, 2012). Exposure to the MUC1 inhibitor potently induces apoptosis in MM cells in vitro, results in disease regression in a xenogenic murine model, and is synergistic with an anti-myeloma agent bortezomib (BZT). Interestingly, MUC1 inhibition with GO-203 did not affect the viability of normal B cells (Yin et al, 2010). Targeting MUC1-C with GO-203 was shown to be synergistic with BZT in suppressing TP53 induced glycolysis regulatory phosphatase (TIGAR)-mediated regulation of reactive oxygen species (ROS) levels which provided an experimental rationale for combining GO-203 with BZT (Yin et al, 2014).
These findings strongly suggest that MUC1 plays a significant role in the evolution of therapeutic resistance in MM patients. However, the role of MUC1 in the stromal–MM interactions has not been defined.
In this study, we demonstrate that co-culture of MM with stromal cells results in increased resistance to cytotoxic and biological agents as manifested by decreased rates of apoptosis and cell death after exposure to alkylating agents and the proteasome inhibitor BZT. To identify the mechanism of increased resistance, we examined the effect of stromal cell co-culture on expression of the MUC1 oncogene, known to confer tumour cells resistance to apoptotic cell death. Co-culture of stroma with MM cells resulted in increased MUC1 expression by tumour cells. The effect of stromal cell co-culture on MUC1 expression was not dependent on cell contact and was therefore thought to be due to soluble factors secreted by the stromal cells into the microenvironment. We have shown that MUC1 expression was mediated by IL6 and subsequent up-regulation of the JAK-STAT3 pathway. We further demonstrated that the effect of stromal cell co-culture on tumour resistance was partially reversed by silencing of MUC1 in MM cells, consistent with the potential role of MUC1 in mediating resistance to cytotoxic-based therapies.
Materials and methods
Multiple myeloma patient derived cells and cell lines
MM human cell lines RPMI-8226 (termed RPMI) and U266 were purchased from American Type Cell Collection (ATCC) and cultured in growth media consisting of RPMI 1640 media (Cellgro, Manassas, VA, USA) supplemented with heat-inactivated 10% Fetal Bovine Serum (Sigma, St. Louis, MO, USA), 100 iu/ml penicillin, and 100 μg/ml streptomycin (Cellgro). RPMI-8226 and U266 cells were transduced with a lentiviral vector expressing a MUC1 shRNA (MUC1shRNA; Sigma) or with a scrambled control shRNA vector (CshRNA; Sigma). Cells that were transduced with the vectors were cultured in the presence of puromycin. HS5 human stromal cell line was obtained from ATCC and cultured in Dulbecco's Modified Eagle Medium (DMEM) (ATCC) supplemented with heat-inactivated 10% Fetal Bovine Serum (Sigma), 100 iu/ml penicillin, and 100 μg/ml streptomycin (Cellgro).
Bone marrow aspirate samples were obtained from patients with active MM as per an institutionally approved protocol. Mononuclear cells were isolated by Ficoll density centrifugation (Histopaque-1077; Sigma) and cultured in growth media as described above. Stromal cell cultures were generated from the adherent fraction that was cultured in RPMI 1640 media (Cellgro) supplemented with heat-inactivated 15% human serum albumin (Sigma), 100 iu/ml penicillin and 100 μg/ml streptomycin (Cellgro). For some experiments, plasma cells were isolated by CD138 magnetic bead separation using the MiniMacs CD138 cell isolation kit (Miltenyi Biotec, San Diego, CA, USA).
Immunoblot analysis
Cell lysates were prepared as described (Yin et al, 2012). Soluble proteins were analyzed by immunoblotting with anti-MUC1-C (Neomarkers, Fremont, CA, USA), Phosphorylated (p)-STAT3 (Cell Signaling Technologies, Danvers, MA, USA), STAT3 (Cell Signaling Technologies) and anti–β-actin (Sigma).
Quantitative real time polymerase chain reaction
Quantitative real time polymerase chain reaction (qPCR) was performed on cDNA synthesized from total cell RNA using the Thermoscript RT-PCR system (Invitrogen, Waltham, MA, USA). The SYBR green qPCR assay (Applied Biosystems, Foster City, CA, USA) was used with diluted cDNA. The samples were amplified using the ABI Prism 7000 Sequence Detector (Applied Biosystems). Forward and reverse primers were MUC1 Fwd (5′-TACCGATCGTAG CCCCTATG-3′), Rev (5′-CTCACCAGCCCAAACAGG-3′) and GAPDH Fwd (5′-CCATGGAGAAGGCTGGGG-3′) Rev (5′-CAAAGTTGTCATGGATGACC-3′). Statistical significance was determined by the Student's t-test.
Detection of MUC1 expression by flow cytometry
Multiple myeloma cells were analyzed for MUC1 expression by multichannel flow cytometric analysis. Cells were incubated with MAb DF3 (anti-MUC1-N) or a control mouse IgG1 for 30 min, followed by secondary labelling of the cells with phycoerythrin (PE)-conjugated goat anti-mouse IgG for an additional 30 min. The cells were then incubated with fluorescein isothiocyanate (FITC)-conjugated anti-CD38 or anti-CD138 MAbs and fixed in 2% paraformaldehyde. Stained cells were analyzed by flow cytometry using Kaluza for Gallios software (http://www.beckman.com/coulter-flow-cytometry/software/kaluza-for-galios-acquisition-software).
Cytotoxicity assays
Multiple myeloma cells were seeded in white flat-bottomed 96-well plates at 20 000 cells per well for cell lines and 200 000 cells per well for primary MM cells. Cells were then treated with increasing concentration of BZT, melphalan (Mel), cyclophosphamide (Cy) and MUC1-C inhibitor GO-203, alone or in combination. The cells were incubated for 72 h at 37°C in a 5% CO2 humidified incubator. Following incubation, the cell viability was assessed using the CellTiter-Glo ® (CTG) CellTiter-Glo (Promega, WI, USA) Luminescent Cell Viability Assay. Raw luminescence values were obtained from each well using a luminometer. Drug synergy was assessed using CompuSyn software program (http://www.combosyn.com) in which a combination index (CI) <1 considered as synergistic and >1 considered as antagonistic. Each experiment was performed in triplicate. Additionally, dead cells were detected by addition of 0.1 mg/ml propidium iodide (PI) and apoptotic cells were detected by Annexin V (FITC) apoptosis detection kit (BD Biosciences, San Jose, CA, USA) using flow cytometry.
Co-culture experiments
1 5 × 106 HS5 stroma cell line or 2 × 105 MM patient BM-derived stroma cells were plated in a 100-mm petri dish or 6-well transwell plate overnight. Following incubation, 1 × 105/ml MM cell lines or 106/ml primary MM cells were plated without a transwell or in the upper chamber of transwell for 48–72 h. Following co-culture, the cells were collected, washed and analyzed. For direct contact experiments, the MM cells were isolated using flow cytometric sorting of CD138+ or CD38+ cells.
Alternatively, to evaluate the effect of the supernatant on MM tumour cells, 1.5 × 106 HS5 stroma cells were plated in a petri dish for 48 h or until confluence. The supernatant was collected following centrifugation. MM cells were re-suspended in the collected supernatant at a concentration of 1 × 105/ml and cultured for 48 h.
Cytokines
Increasing concentrations of IL6, IL8, granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF) (R&D systems, Minneapolis, MN, USA) were added directly to the MM cell lines RPMI-8226 and U266 for 24–72 h. The cells were then subjected to flow cytometric analysis for MUC1 analysis as described above.
STAT3 and JAK2 inhibitors
RPMI-8226 and U266 MM cells were incubated with 300 nmol/l STAT3 Inhibitor VI (EMD Millipore, Taunton, MA, USA) for 2 h and 1 μmol/l JAK Inhibitor I (EMD Milli-pore) for 1 h. Following incubation, the cells were washed in RPMI medium and incubated in the presence of 10 ng/ml IL6 for an additional 3 h. The cells were then harvested, washed and cultured in complete media for 24 h prior to analysis.
Results
MUC1 expression is associated with drug resistance in MM cells
We first examined the role of MUC1, an oncogene that is highly expressed in several MM cell lines (Fig S1), in mediating therapeutic resistance in the absence of interaction with stromal elements. This was done by comparing drug sensitivity of the control RPMI and U266 human myeloma cell lines to the corresponding cell line in which MUC1 was silenced by lentiviral transduction with MUC1-specific shRNA (Fig 1A). Consistent with its role in mediating protection from cellular injury, silencing of MUC1 was associated with significantly increased sensitivity to drug induced killing by Cy, Mel and BZT in RPMI (Fig 1B) and U266 cells (Fig 1C) as detected by a luminescent cell viability assay, which quantifies the presence of ATP, an indicator of metabolically active cells. To further examine the effect of MUC1 in mediating resistance to cytotoxic therapy, we similarly examined the effect of GO-203, a cell penetrating peptide that inhibits MUC1 signalling by preventing homo-dimerization necessary for nuclear translocation and interaction with downstream effectors. Exposure of RPMI and U266 cells to sub-lethal doses of GO-203 markedly increased their sensitivity to Cy, Mel and BZT (Fig 2A and B). Analysis of these findings demonstrated potent synergy between GO-203 and cytotoxic therapy with CI of 0.3 and 0.1 for RPMI and U266, respectively (synergy defined as <1.0).
Fig 1.
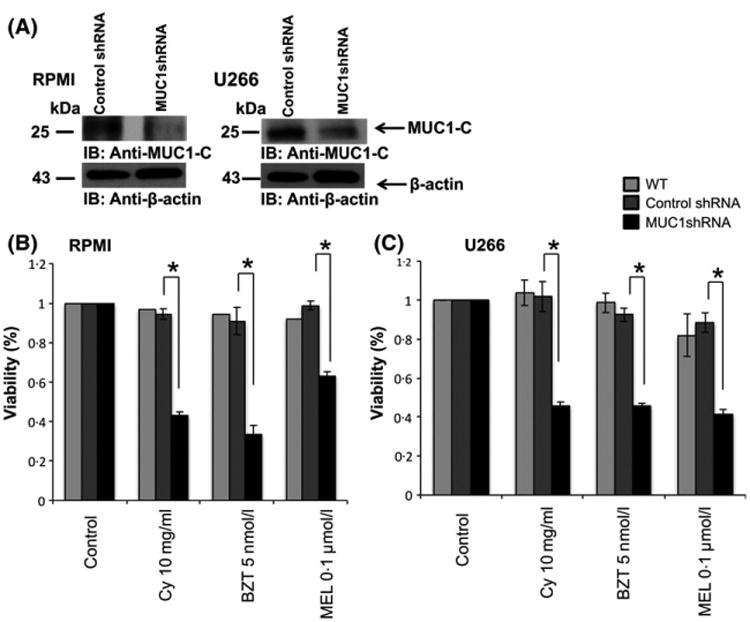
MUC1 expression is associated with drug resistance in multiple myeloma (MM) cells. MUC1-C was silenced in RPMI and U266 MM cells using lentiviral transduction with a shRNA hairpin sequence against MUC1-C. (A) RPMI, U266 cells and their MUC1 silenced counterparts were harvested and lysates were immunoblotted for the expression of MUC1-C using anti-CT2 monoclonal antibody. (B, C) Multiple myeloma cells were treated for 48 h with a variety of anti-myeloma agents in the indicated concentrations, after which viable cell number was quantitated by ATP dependent bioluminescence. BZT, bortezomib; Cy, cyclophosphamide; Mel, melphalan; WT, wild type. “★” indicate statistical significance (p<0.05).
Fig 2.
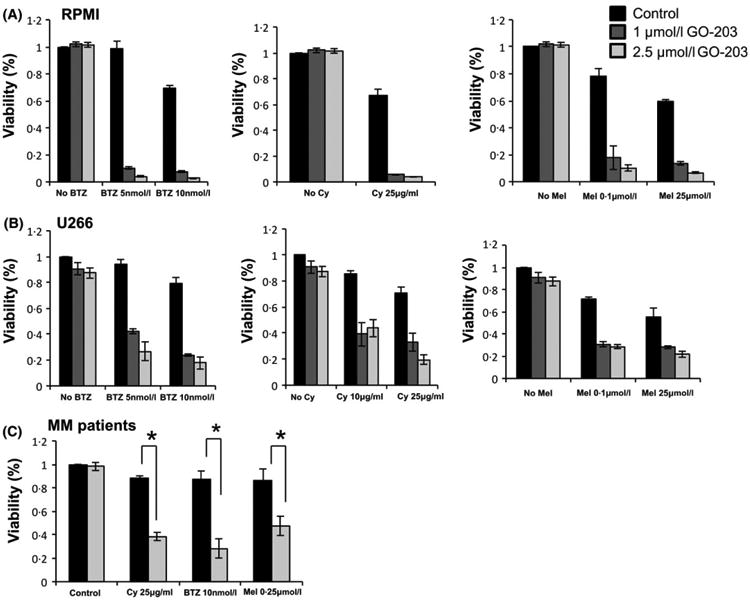
MUC1-C inhibition with peptide inhibitor GO-203 leads to increased susceptibility to anti-myeloma agents. (A) RPMI and (B) U266 cell lines were treated with bortezomib (BZT), cyclophosphamide (Cy) or melphalan (MEL) at the indicated concentrations with or without the addition of GO-203 at the indicated concentrations. Following 48-h of culture, viable cell number was measured using an ATP dependent bioluminescence assay. (C) Bone marrow mononuclear cells were isolated from patients with active MM. CD138+ plasma cells were isolated using magnetic bead separation. The cells were treated with BZT, Cy MEL at the indicated concentrations, with or without the addition of 2 5 μmol/l MUC1 inhibitor GO-203 for 48 h, after which viable cell number was measured using an ATP dependent bioluminescence assay (n 3). “★” indicate statistical significance (p<0.05).
Similarly, GO-203 demonstrated synergy with Cy, Mel and BZT in targeting patient primary MM cells. BM mononuclear cells were obtained from three patients with active MM; on average, 93% of the tumour cells in the samples expressed MUC1. The tumour cell population was then isolated using CD138 magnetic bead selection, and treated with sublethal doses of GO-203 and anti-myeloma agents as described above (Fig 2C).
Of note, exposure of MUC1 silenced MM cells to MUC1 inhibitor alone did not affect the viability of the MM cells as opposed to the control MM cells, confirming the specificity of MUC1 inhibition with GO-203 (Fig S2).
Co-culture of MM cells with BMSC increases MUC1 expression on MM cells
Having demonstrated the protective effect of MUC1 expression on resistance of MM to cytotoxic injury, we sought to examine the effect of stromal cells on MUC1 expression by MM cells. We first examined the effect of co-culture of RPMI and U266 immortalized MM cells with the stromal cell line HS5. Co-culture with HS5 resulted in significant increase in MUC1 expression as determined by the mean fluorescent intensity (MFI) quantified by flow cytometric analysis (Fig 3A). Of note, MUC1 expression intensified with increased duration of exposure to stroma in the co-culture (Fig 3B). These findings were confirmed by Western blot analysis in which co-culture with stroma demonstrated a marked increase in detectable MUC1 in both RPMI and U266 MM cells (Fig 3C). Consistent with these findings a rise in MUC1 mRNA expression was detected by qPCR analysis (Fig 3D). Co-incubation of RPMI MM cells with stromal cultures established from patient-derived BM aspirates resulted in increased MUC1 expression by the tumour cells (Fig 3E, n = 3). Primary patient-derived MM cells were also noted to increase MUC1 expression when co-cultured with stromal cell line HS5 with the mean increase of 100% in MUC1 expression, as demonstrated in a representative fluorescence-activated cell sorting (FACS) plot (Fig 3F; n = 4). These findings demonstrate that MUC1 expression is induced in MM cells by co-culture with stromal cells.
Fig 3.
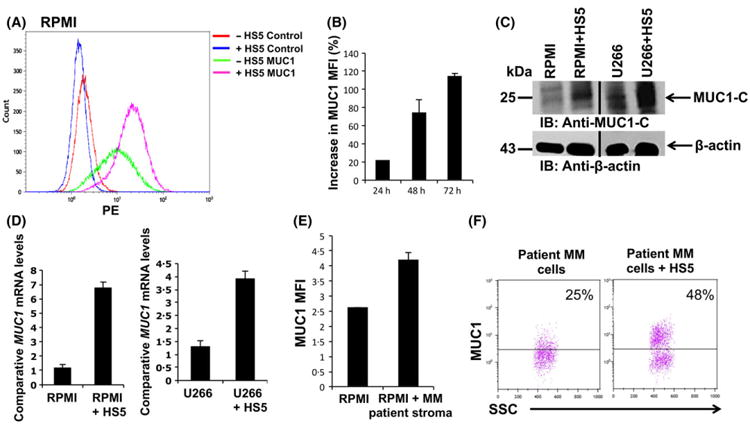
Co-culture of multiple myeloma (MM) cells with bone marrow stromal cells (BMSC) leads to increased MUC1 expression (A) 1 × 105/ml RPMI MM cells were co-cultured with 1.5 × 106 HS5 stroma cell line, after which MUC1 maximum fluorescence intensity (MFI) was measured using flow cytometry after gating on CD138+ tumour cells. The values are obtained relatively to the appropriate isotope control. Representative FACS plot is shown (n = 5) (B) RPMI MM cells were co-cultured with HS5 stromal as described above for 24, 48 and 72 h. MUC1 expression on MM cells was detected using flow cytometry compared to isotype control at each indicated time point. Percent increase in MUC1 MFI in the gated CD138+ MM cells following the addition of HS5 cells as opposed to RPMI cells alone was calculated and presented as a summary of three independent experiments. (C) RPMI and U266 MM cell lines were co-cultured with HS5 cells for 72 h. BMSC were removed from co-culture and immunoblots were performed with the indicated antibodies. (D) 1 × 105/ml RPMI and U266 were co-cultured with 1.5 × 106 HS5 stroma cells for 4 h, after which HS5 cells were removed, RNA was isolated from the MM cells and expression of the indicated genes were measured using qPCR and normalized to the expression of GAPDH (E) RPMI cells were incubated with stroma cells generated from bone marrow mononuclear cells obtained from patients with MM and then analyzed by flow cytometry for MUC1 expression. MUC1 MFI detected on gated CD138+ tumour cells is presented as summary of three independent experiments. (F) MM tumour cells were isolated from bone marrow mononuclear cells obtained from patients with MM. The tumour cells were co-cultured with HS5 stromal cells for 72 h and MUC1 was detected by flow cytometry. Representative FACS plot is shown (n = 4). [Colour figure can be viewed at wileyonlinelibrary.com].
Stromal-cell induced resistance of MM cells to proteasome inhibitors is partially reversed by MUC1 silencing
We subsequently evaluated whether the demonstrated increase in MUC1 levels following the co-culture of MM cells with BMSCs might render MM cells more resistant to anti-myeloma agents. Indeed, increased MUC1 expression on RPMI MM cells following co-culture with HS5 stroma cells was associated with augmented resistance to BZT. Co-culture of MM cell line with HS5 cells resulted in decreased killing at normally lethal doses of BZT in-vitro (Fig 4A). RPMI MM cells were treated with increasing doses of BZT in the presence or absence of HS5 cells. Following 48 h of incubation, cell viability was evaluated using a luminescent cell viability assay (Fig 4A). The results were confirmed using Annexin V and PI staining as demonstrated in representative FACS plots (Fig 4B).
Fig 4.
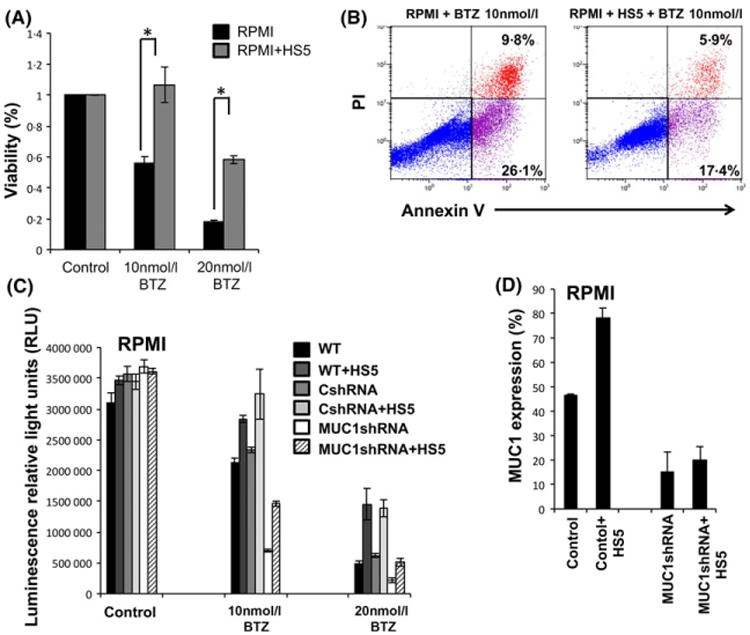
Co-culture of multiple myeloma cells with bone marrow stromal cells confers bortezomib resistance. (A) RPMI cell line was co-cultured with HS5 stromal cell line for 72 h and then was incubated with bortezomib in the indicated concentration for 48 h after which viable cell number was quantitated by ATP dependent bioluminescence (B) or subjected to annexin V/propidium iodide staining and flow cytometry was performed (B, representative experiment). (C) Wild type (WT), Control shRNA (CshRNA) RPMI and MUC1 silenced RPMI cells were co-cultured with HS5 cell line in transwells for 72 h. Subsequently, bortezomib was added to the cultures in the indicated concentration for an additional 48 h after which viable cell number was quantitated by ATP dependent bioluminescence. (D) MUC1 levels were detected in RPMI cells following co-culture with HS5 as detected by flow cytometry and presented as mean of two independent experiments. “★” indicate statistical significance (p<0.05). [Colour figure can be viewed at wileyonlinelibrary.com].
Furthermore, to investigate the role of MUC1 in mediating MM resistance associated with stromal cell culture, we silenced MUC1 in the co-cultured MM cells using a MUC1-specific shRNA. MUC1 silencing did not affect the proliferation of un-treated RPMI cells. However, silencing of MUC1 expression in RPMI cells resulted in increased sensitivity of MM cells to BZT. Interestingly, MUC1 silencing in RPMI cells resulted in a significant loss of the protection conferred by co-culture with HS5 cells (Fig 4C).
Of note, co-culture of the MUC1-silenced RPMI cells with stromal cells resulted in the partial recovery of MUC1 expression, suggesting that MUC1 expression remains somewhat dynamic in the lentiviral silenced cells (Fig 4D). While this did not fully restore the resistant phenotype in MUC1-silenced cells, improved survival was noted as compared to MUC1-silenced cells not co-cultured with stroma (Fig 4C).
IL6 produced by BMSC induces MUC1 expression on myeloma cells through the JAK2-STAT3 pathway
To determine whether the enhanced MUC1 expression on MM cells after co-culture with BMSC is mediated by soluble factors or dependent on cell–cell contact, we compared the induction of MUC1 by direct stromal co-cultures with transwell cultures. In transwell cultures, the MM and stromal cells were segregated, preventing direct cell contact but enabling them to share a common culture media. MUC1 expression was similarly increased in both the co-culture and transwell cultures, suggesting the effect was primarily mediated by soluble factors that did not require cell contact (Fig S3).
We then sought to identify the factors residing in the microenvironment responsible for augmenting MUC1 expression on MM cells. Remarkably, the addition of recombinant IL6 resulted in a significant increase in MUC1 expression on both RPMI and U266 MM cells (Fig 5A) while exposure to GM-CSF, G-CSF or IL8, additional factors known to be produced my stromal cells (Roecklein & Torok-Storb, 1995), did not increase MUC1 expression. In order to extend our investigations, we co-cultured RPMI and U266 MM cells with HS5 stromal cells with the addition of IL6 neutralizing antibodies (NA) or control antibodies. Consistent with our findings, the addition of anti-IL6 to the co-culture of MM and stromal cells was shown to abrogate the increase in the MUC1 levels on the MM cells (Fig 5B).
Fig 5.
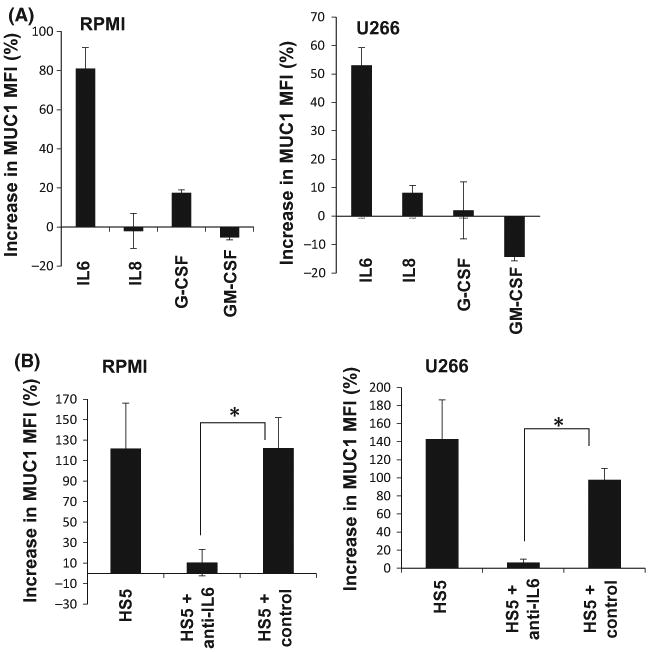
IL6 produced by bone marrow stromal cells induces MUC1 expression on myeloma cells. (A) RPMI and U266 cell lines were incubated with 10 ng/ml IL6, 20 ng/ml IL8, 100 pg/ml G-CSF and 10 ng/ml GM-CSF for 48 h and MUC1 expression was measured by flow cytometry. Per cent increase in MUC1 MFI in multiple myeloma (MM) cells was calculated and presented as a summary of three independent experiments. (B) RPMI and U266 cells were co-cultured for 72 h in transwells with HS5 stroma cells in the presence or absence of anti-IL6 neutralizing antibodies or control antibodies. MUC1 expression was measured by flow cytometry. Per cent increase in MUC1 MFI in the gated MM cells following co-culture with HS5 cells as opposed to RPMI cells alone was calculated and presented as a summary of three independent experiments. “★” indicate statistical significance (p<0.05).
IL6 has been shown to effect downstream signalling via the JAK2-STAT3 pathway. We next explored whether the observed increase in MUC1 levels following the incubation of MM cells with stromal cells is mediated by IL6 through the JAK2-STAT3 pathway. RPMI and U266 cells were incubated with HS5 stromal cells and the levels of phosphorylated and de-phosphorylated STAT3 were detected. Indeed, co-culture of both RPMI and U266 cells with HS5 led to a rapid increase in the expression of activated p-STAT3 that maintained throughout 48 h, as opposed to a transient increase in p-STAT3 following the addition of exogenous IL6 (Figs 6A and S4). Moreover, RPMI cells cultured with recombinant IL6 that were treated with STAT3 inhibitor or JAK2 inhibitor, demonstrated a significant decrease in MUC1 levels as opposed to control (Fig 6B, C). These results suggest that secretion of IL6 by BMSCs might play an important role in upregulation of MUC1 expression on MM cells.
Fig 6.
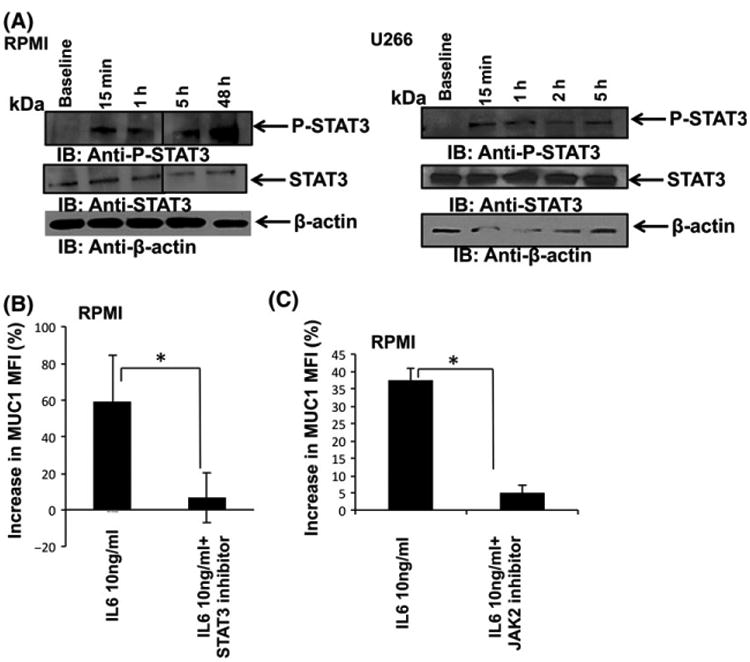
IL6 leads to MUC1 upregulation on multiple myeloma (MM) cells through the Jak-STAT3 pathway. (A) RPMI and U266 MM cells were co-cultured with HS5 stromal cell line for the indicated time points, after which cell lysates were immunoblotted for the expression of active STAT3 using anti-P-STAT3 and anti-STAT3 antibodies. B-actin was used as a loading control. (B) RPMI cells were incubated with 300 nmol/l STAT3 inhibitor or 1 μmol/l JAK2 inhibitor, following which 10 ng/ml IL6 was added to the culture. MUC1 expression was measured by flow cytometry. Per cent increase in MUC1 MFI in MM cells following the addition of STAT3 and Jak2 inhibitors was calculated and presented as a summary of three independent experiments (P = 0-04 and P = 0-002 for STAT3 inhibitor and Jak2 inhibitor respectively). “★” indicate statistical significance (p<0.05).
Discussion
The tumour microenvironment in MM is characterized by complex interactions between the malignant plasma cells and the surrounding stroma that fosters resistance to cytotoxic injury, immunological tolerance and disease growth (Chauhan et al, 1996; Gupta et al, 2001). The precise mechanisms by which stromal cells interact with known oncogenes that induce disease resistance and immune escape have not been fully elucidated.
In the present report we demonstrate that co-culture of MM cells with stromal cells results in the marked upregulation of MUC1 in the tumour population. This effect was observed using stroma derived from patients with myeloma as well as the HS5 cell line derived from normal stromal cells. Heightened levels of MUC1 expression by the MM cells is associated with increased resistant to alkylating agents and the proteasome inhibitor, BZT. Consistent with these findings, silencing of MUC1 in MM cells partially reverses stromal cell-induced MM cell resistance to therapeutic agents.
The MUC1 oncogene has been noted in both solid tumours and haematological malignancies and is associated with critical elements of the malignant phenotype, including resistance to apoptotic agents, proliferative capacity, and autonomous self-renewal (Ren et al, 2004; Udhayakumar et al, 2007; Raina et al, 2009, 2014; Alam et al, 2014; Yin et al, 2014; Jain et al, 2015). Downstream effectors such as NFκB and the Wnt/β-catenin pathway have been shown to play a key role in the persistence and survival of MM cells (Schroeder et al, 2003; Udhayakumar et al, 2007; Ahmad et al, 2009). Previous studies have shown that targeting MUC1 via inhibition of downstream signalling results in MM cell death in both in -vitro and in -vivo models (Yin et al, 2010, 2014; Stroopinsky et al, 2013; Tagde et al, 2016). Furthermore, MUC1 inhibition demonstrates synergy with another anti-MM agent, BZT. This is the first report to elucidate the role of MUC1 as a mediator of stromal cell induced tumour resistance.
We investigated the potential mechanism by which stromal cells induced MUC1 expression in the malignant plasma cells and found the effect similarly demonstrated in co-culture and transwell assays, suggesting that soluble factors were responsible. A variety of cytokines were investigated and IL6 was identified as the sole agent that was associated with upregulation of MUC1 expression on MM cells. IL6 has been shown to be a critical factor in the MM cell microenvironment and is associated with the activation of osteoclasts, development of lytic bone lesions, and disease progression (Urashima et al, 1996; French et al, 2002). IL6 is secreted by BMSCs (Rougier et al, 1998), and is known to mediate myeloma cell proliferation and resistance to apoptosis conferring drug resistance (Hardin et al, 1994; Lichtenstein et al, 1995; Chauhan et al, 1996, 1997; Catlett-Falcone et al, 1999; Gupta et al, 2001; Shain et al, 2009). IL6 activates multiple pathways, including the JAK2/signal transducer and activator of transcription (STAT-3) cascade (Catlett-Falcone et al, 1999; French et al, 2002; Shain et al, 2009). Interestingly, in a breast cancer model, activated STAT3 was shown to induce the expression of the MUC1 gene in an autoinductive regulatory loop (Ahmad et al, 2011).
Consistent with its primary role in regulating MUC1 expression, the introduction of a NA directed against IL6 into the stromal/MM cell co-culture resulted in the loss of MUC1 upregulation. The IL6 effects appear to be, in turn, mediated by activation of the JAK-STAT pathway. As described in prior studies, IL6-mediated effects on MUC1 expression appeared to be dependent on JAK-STAT signalling and was abrogated by exposure to JAK-STAT inhibitors.
These data suggest that the MUC1 oncogene provides a pathway that plays a critical role in mediating resistance to injury by cytotoxic and biological therapies. Of note, we have previously demonstrated that MUC1 is a primary regulator of ROS levels in MM cells which is a key regulator of apoptotic injury, differentiation and antigen presentation (Ren et al, 2004; Yin et al, 2010, 2012, 2014).
The identification of MUC1 as an oncogenic target responsible for MM resistance arising from the tumour microenvironment, carries significant implications for the development of novel therapeutic strategies for MM. Several strategies are being developed to effectively target MUC1 via immunotherapeutic and biological agents, including a cell penetrating peptide that disrupts dimerization of the MUC1-C subunit critical for downstream signalling which we recently developed (Yin et al, 2010, 2012, 2014). Preclinical studies demonstrate synergy with biological agents such as BZT, including reversal of resistance in BZT-resistant cell lines (Yin et al, 2014).
We have also developed a tumour vaccine for MM in which patient-derived MM cells are fused with autologous DCs such that a broad array of MM antigens are presented in the context of DC-mediated costimulation. The vaccine potently induces MM-specific immunity in phase I/II studies, is associated with effective targeting of minimal residual disease, and is now being studied in a multicentre randomized trial (Vasir et al, 2005; Rosenblatt et al, 2011, 2013). Combining immunotherapeutic strategies such as this vaccine with disruption of upregulation of MUC1 expression and signalling may be critical to prevent the development of immune resistance and escape.
Supplementary Material
Figure S1. MM cell lines express high levels of MUC1.
Figure S2. MUC1-C inhibition with peptide inhibitor GO-203 in MUC1 silenced MM cells does not affect cell viability.
Figure S3. Increased expression of MUC1 on MM cells is mediated via soluble factors.
Figure S4. IL-6 leads to MUC1 upregulation on MM cells through the Jak-STAT3 pathway.
Footnotes
Author contributions: Michal Bar-Natan, Dina Stroopinsky: designed research, performed research, interpreted data, assisted with manuscript preparation. Katarina Luptakova: designed research, analyzed and interpreted data, performed research. Maxwell Douglas Coll, Arie Apel, Hasan Rajabi, Athalia Rachel Pyzer, Kristen Palmer. Michaela R. Reagan: performed research. Myrna R. Nahas, Rebecca Karp Leaf, Salvia Jain, Jon Arnason: assisted with patient sample acquisition. Irene M. Ghobrial, Kenneth C. Anderson, Donald Kufe: designed research, assisted with manuscript preparation. Jacalyn Rosenblatt, David Avigan: assisted with research design, patient sample acquisition, analyzed and interpreted data, and assisted with manuscript preparation.
Supporting Information: Additional Supporting Information may be found in the online version of this article:
References
- Ahmad R, Raina D, Joshi MD, Kawano T, Ren J, Kharbanda S, Kufe D. MUC1-C oncoprotein functions as a direct activator of the nuclear factor-kappaB p65 transcription factor. Cancer Research. 2009;69:7013–7021. doi: 10.1158/0008-5472.CAN-09-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad R, Rajabi H, Kosugi M, Joshi MD, Alam M, Vasir B, Kawano T, Kharbanda S, Kufe D. MUC1-C oncoprotein promotes STAT3 activation in an autoinductive regulatory loop. Science Signalling. 2011;4:ra9. doi: 10.1126/scisignal.2001426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M, Rajabi H, Ahmad R, Jin C, Kufe D. Targeting the MUC1-C oncoprotein inhibits self-renewal capacity of breast cancer cells. Oncotarget. 2014;5:2622–2634. doi: 10.18632/oncotarget.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldus SE, Palmen C, Thiele J. MUC1 (EMA) expressing plasma cells in bone marrow infiltrated by plasma cell myeloma. Histology and Histopathology. 2007;22:889–893. doi: 10.14670/HH-22.889. [DOI] [PubMed] [Google Scholar]
- Bouillez A, Rajabi H, Pitroda S, Jin C, Alam M, Kharbanda A, Tagde A, Wong KK, Kufe D. Inhibition of MUC1-C suppresses MYC expression and attenuates malignant Growth in KRAS mutant lung adenocarcinomas. Cancer Research. 2016;76:1538–1548. doi: 10.1158/0008-5472.CAN-15-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton J, Mishina D, Cardillo T, Lew K, Rubin A, Goldenberg DM, Gold DV. Epithelial mucin-1 (MUC1) expression and MA5 anti-MUC1 monoclonal antibody targeting in multiple myeloma. Clinical Cancer Research. 1999;5:3065s–3072s. [PubMed] [Google Scholar]
- Catlett-Falcone R, Landowski TH, Oshiro MM, Turkson J, Levitzki A, Savino R, Ciliberto G, Moscinski L, Fernandez-Luna JL, Nunez G, Dalton WS, Jove R. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10:105–115. doi: 10.1016/s1074-7613(00)80011-4. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Uchiyama H, Akbarali Y, Urashima M, Yamamoto K, Libermann TA, Anderson KC. Multiple myeloma cell adhesion-induced interleukin-6 expression in bone marrow stromal cells involves activation of NF-kappa B. Blood. 1996;87:1104–1112. [PubMed] [Google Scholar]
- Chauhan D, Kharbanda S, Ogata A, Urashima M, Teoh G, Robertson M, Kufe DW, Anderson KC. Interleukin-6 inhibits Fas-induced apoptosis and stress-activated protein kinase activation in multiple myeloma cells. Blood. 1997;89:227–234. [PubMed] [Google Scholar]
- Cloosen S, Gratama J, van Leeuwen EB, Senden-Gijsbers BL, Oving EB, von Mensdorff-Pouilly S, Tarp MA, Mandel U, Clausen H, Germeraad WT, Bos GM. Cancer specific Mucin-1 glycoforms are expressed on multiple myeloma. British Journal of Haematology. 2006;135:513–516. doi: 10.1111/j.1365-2141.2006.06331.x. [DOI] [PubMed] [Google Scholar]
- Dosani T, Carlsten M, Maric I, Landgren O. The cellular immune system in myeloma-genesis: NK cells and T cells in the development of MM and their uses in immunotherapies. Blood Cancer Journal. 2015;5:e321. doi: 10.1038/bcj.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French JD, Tschumper RC, Jelinek DF. Analysis of IL-6-mediated growth control of myeloma cells using a gp130 chimeric receptor approach. Leukemia. 2002;16:1189–1196. doi: 10.1038/sj.leu.2402516. [DOI] [PubMed] [Google Scholar]
- Gorgun GT, Whitehill G, Anderson JL, Hide-shima T, Maguire C, Laubach J, Raje N, Munshi NC, Richardson PG, Anderson KC. Tumor-promoting immune-suppressive myeloid-derived suppressor cells in the multiple myeloma microenvironment in humans. Blood. 2013;121:2975–2987. doi: 10.1182/blood-2012-08-448548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görgüun G, Samur MK, Cowens KB, Paula S, Bianchi G, Anderson JE, White RE, Singh A, Ohguchi H, Suzuki R, Kikuchi S, Harada T, Hideshima T, Tai YT, Laubach JP, Raje N, Magrangeas F, Minvielle S, Avet-Loiseau H, Munshi NC, Dorfman DM, Richardson PG, Anderson KC. Lenalidomide enhances immune checkpoint blockade-induced immune response in multiple myeloma. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 2015;21:4607–4618. doi: 10.1158/1078-0432.CCR-15-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta D, Treon SP, Shima Y, Hideshima T, Podar K, Tai YT, Lin B, Lentzsch S, Davies FE, Chauhan D, Schlossman RL, Richardson P, Ralph P, Wu L, Payvandi F, Muller G, Stirling DI, Anderson KC. Adherence of multiple myeloma cells to bone marrow stromal cells upregulates vascular endothelial growth factor secretion: therapeutic applications. Leukemia. 2001;15:1950–1961. doi: 10.1038/sj.leu.2402295. [DOI] [PubMed] [Google Scholar]
- Hardin J, MacLeod S, Grigorieva I, Chang R, Barlogie B, Xiao H, Epstein J. Interleukin-6 prevents dexamethasone-induced myeloma cell death. Blood. 1994;84:3063–3070. [PubMed] [Google Scholar]
- Huang L, Chen D, Liu D, Yin L, Kharbanda S, Kufe D. MUC1 oncoprotein blocks glycogen synthase kinase 3beta-mediated phosphorylation and degradation of beta-catenin. Cancer Research. 2005;65:10413–10422. doi: 10.1158/0008-5472.CAN-05-2474. [DOI] [PubMed] [Google Scholar]
- Jain S, Stroopinsky D, Yin L, Rosenblatt J, Alam M, Bhargava P, Clark RA, Kupper TS, Palmer K, Coll MD, Rajabi H, Pyzer A, Bar-Natan M, Luptakova K, Arnason J, Joyce R, Kufe D, Avigan D. Mucin 1 is a potential therapeutic target in cutaneous T-cell lymphoma. Blood. 2015;126:354–362. doi: 10.1182/blood-2015-02-628149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T, Ahmad R, Nogi H, Agata N, Anderson K, Kufe D. MUC1 onco-protein promotes growth and survival of human multiple myeloma cells. International Journal of Oncology. 2008;33:153–159. [PMC free article] [PubMed] [Google Scholar]
- Kharbanda A, Rajabi H, Jin C, Tchaicha J, Kikuchi E, Wong KK, Kufe D. Targeting the oncogenic MUC1-C protein inhibits mutant EGFR-mediated signaling and survival in non-small cell lung cancer cells. Clinical Cancer Research. 2014;20:5423–5434. doi: 10.1158/1078-0432.CCR-13-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire M, Deleu S, De Bruyne E, Van Valckenborgh E, Menu E, Vanderkerken K. The microenvironment and molecular biology of the multiple myeloma tumor. Advances in Cancer Research. 2011;110:19–42. doi: 10.1016/B978-0-12-386469-7.00002-5. [DOI] [PubMed] [Google Scholar]
- Li Y, Liu D, Chen D, Kharbanda S, Kufe D. Human DF3/MUC1 carcinoma-associated protein functions as an oncogene. Oncogene. 2003;22:6107–6110. doi: 10.1038/sj.onc.1206732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein A, Tu Y, Fady C, Vescio R, Berenson J. Interleukin-6 inhibits apoptosis of malignant plasma cells. Cellular Immunology. 1995;162:248–255. doi: 10.1006/cimm.1995.1076. [DOI] [PubMed] [Google Scholar]
- Manier S, Sacco A, Leleu X, Ghobrial IM, Roccaro AM. Bone marrow microenvironment in multiple myeloma progression. Journal of Biomedicine & Biotechnology. 2012;2012:157496. doi: 10.1155/2012/157496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo A, Anderson K. Multiple myeloma. The New England Journal of Medicine. 2011;364:1046–1060. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- Paydas S, Sahin B, Gonlusen G, Hazar B, Zorludemir S. MUC1 expression in plasmacytoma. Leukemia Research. 2001;25:221–225. doi: 10.1016/s0145-2126(00)00111-9. [DOI] [PubMed] [Google Scholar]
- Quach H, Ritchie D, Stewart AK, Neeson P, Harrison S, Smyth MJ, Prince HM. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia. 2010;24:22–32. doi: 10.1038/leu.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina D, Ahmad R, Joshi MD, Yin L, Wu Z, Kawano T, Vasir B, Avigan D, Kharbanda S, Kufe D. Direct targeting of the mucin 1 oncoprotein blocks survival and tumorigenicity of human breast carcinoma cells. Cancer Research. 2009;69:5133–5141. doi: 10.1158/0008-5472.CAN-09-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina D, Uchida Y, Kharbanda A, Rajabi H, Panchamoorthy G, Jin C, Kharbanda S, Scaltriti M, Baselga J, Kufe D. Targeting the MUC1-C oncoprotein downregulates HER2 activation and abrogates trastuzumab resistance in breast cancer cells. Oncogene. 2014;33:3422–3431. doi: 10.1038/onc.2013.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajabi H, Ahmad R, Jin C, Kosugi M, Alam M, Joshi MD, Kufe D. MUC1-C oncoprotein induces TCF7L2 transcription factor activation and promotes cyclin D1 expression in human breast cancer cells. Journal of Biological Chemistry. 2012;287:10703–10713. doi: 10.1074/jbc.M111.323311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Agata N, Chen D, Li Y, Yu WH, Huang L, Raina D, Chen W, Kharbanda S, Kufe D. Human MUC1 carcinoma-associated protein confers resistance to geno-toxic anticancer agents. Cancer Cell. 2004;5:163–175. doi: 10.1016/s1535-6108(04)00020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roecklein BA, TorokStorb B. Functionally distinct human marrow stromal cell lines immortalized by transduction with the human papilloma virus E6/E7 genes. Blood. 1995;85:997–1005. [PubMed] [Google Scholar]
- Romano A, Conticello C, Cavalli M, Vetro C, La Fauci A, Parrinello NL, DiRaimondo F. Immunological dysregulation in multiple myeloma microenvironment. BioMed Research International. 2014;2014:198539. doi: 10.1155/2014/198539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt J, Vasir B, Uhl L, Blotta S, Macna-mara C, Somaiya P, Wu Z, Joyce R, Levine JD, Dombagoda D, Yuan YE, Francoeur K, Fitzgerald D, Richardson P, Weller E, Anderson K, Kufe D, Munshi N, Avigan D. Vaccination with dendritic cell/tumor fusion cells results in cellular and humoral anti-tumor immune responses in patients with multiple myeloma. Blood. 2011;117:393–402. doi: 10.1182/blood-2010-04-277137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt J, Avivi I, Vasir B, Uhl L, Munshi NC, Katz T, Dey BR, Somaiya P, Mills H, Campigotto F, Weller E, Joyce R, Levine JD, Tzachanis D, Richardson P, Laubach J, Raje N, Boussiotis V, Yuan YE, Bisharat L, Held V, Rowe J, Anderson K, Kufe D, Avigan D. Vaccination with dendritic cell/tumor fusions following autologous stem cell transplant induces immunologic and clinical responses in multiple myeloma patients. Clinical Cancer Research. 2013;19:3640–3648. doi: 10.1158/1078-0432.CCR-13-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougier F, Cornu E, Praloran V, Denizot Y. IL-6 and IL-8 production by human bone marrow stromal cells. Cytokine. 1998;10:93–97. doi: 10.1006/cyto.1997.0262. [DOI] [PubMed] [Google Scholar]
- Schroeder JA, Adriance MC, Thompson MC, Camenisch TD, Gendler SJ. MUC1 alters beta-catenin-dependent tumor formation and promotes cellular invasion. Oncogene. 2003;22:1324–1332. doi: 10.1038/sj.onc.1206291. [DOI] [PubMed] [Google Scholar]
- Shain KH, Yarde DN, Meads MB, Huang M, Jove R, Hazlehurst LA, Dalton WS. Beta1 integrin adhesion enhances IL-6-mediated STAT3 signaling in myeloma cells: implications for microenvironment influence on tumor survival and proliferation. Cancer Research. 2009;69:1009–1015. doi: 10.1158/0008-5472.CAN-08-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroopinsky D, Rosenblatt J, Ito K, Mills H, Yin L, Rajabi H, Vasir B, Kufe T, Luptakova K, Arnason J, Nardella C, Levine JD, Joyce RM, Galinsky I, Reiter Y, Stone RM, Pandolfi PP, Kufe D, Avigan D. MUC1 is a potential target for the treatment of acute myeloid leukemia stem cells. Cancer Research. 2013;73:5569–5579. doi: 10.1158/0008-5472.CAN-13-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagde A, Rajabi H, Bouillez A, Alam M, Gali R, Bailey S, Tai YT, Hideshima T, Anderson K, Avigan D, Kufe D. MUC1-C drives MYC in multiple myeloma. Blood. 2016;127:2587–2597. doi: 10.1182/blood-2015-07-659151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Makiguchi Y, Hinoda Y, Kaki-uchi H, Nakagawa N, Imai K, Yachi A. Expression of MUC1 on myeloma cells and induction of HLA-unrestricted CTL against MUC1 from a multiple myeloma patient. Journal of Immunology. 1994;153:2102–2109. [PubMed] [Google Scholar]
- Treon SP, Mollick JA, Urashima M, Teoh G, Chauhan D, Ogata A, Raje N, Hilgers JH, Nadler L, Belch AR, Pilarski LM, Anderson KC. Muc-1 core protein is expressed on multiple myeloma cells and is induced by dex-amethasone. Blood. 1999;93:1287–1298. [PubMed] [Google Scholar]
- Udhayakumar G, Jayanthi V, Devaraj N, Devaraj H. Interaction of MUC1 with beta-catenin modulates the Wnt target gene cyclinD1 in H. pylori-induced gastric cancer. Molecular Carcinogenesis. 2007;46:807–817. doi: 10.1002/mc.20311. [DOI] [PubMed] [Google Scholar]
- Urashima M, Ogata A, Chauhan D, Vidriales MB, Teoh G, Hoshi Y, Schlossman RL, DeCaprio JA, Anderson KC. Inter-leukin-6 promotes multiple myeloma cell growth via phosphorylation of retinoblastoma protein. Blood. 1996;88:2219–2227. [PubMed] [Google Scholar]
- Vasir B, Borges V, Wu Z, Grosman D, Rosenblatt J, Irie M, Anderson K, Kufe D, Avigan D. Fusion of dendritic cells with multiple myeloma cells results in maturation and enhanced antigen presentation. British Journal of Haematology. 2005;129:687–700. doi: 10.1111/j.1365-2141.2005.05507.x. [DOI] [PubMed] [Google Scholar]
- Yin L, Ahmad R, Kosugi M, Kufe T, Vasir B, Avigan D, Kharbanda S, Kufe D. Survival of human multiple myeloma cells is dependent on MUC1 C-terminal transmembrane subunit oncoprotein function. Molecular Pharmacology. 2010;78:166–174. doi: 10.1124/mol.110.065011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Kosugi M, Kufe D. Inhibition of the MUC1-C oncoprotein induces multiple myeloma cell death by down-regulating TIGAR expression and depleting NADPH. Blood. 2012;119:810–816. doi: 10.1182/blood-2011-07-369686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Kufe T, Avigan D, Kufe D. Targeting MUC1-C is synergistic with bortezomib in downregulating TIGAR and inducing ROS-mediated myeloma cell death. Blood. 2014;123:2997–3006. doi: 10.1182/blood-2013-11-539395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Rajabi H, Kufe D. Mucin 1 C-terminal subunit oncoprotein is a target for small-molecule inhibitors. Molecular Pharmacology. 2011;79:886–893. doi: 10.1124/mol.110.070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. MM cell lines express high levels of MUC1.
Figure S2. MUC1-C inhibition with peptide inhibitor GO-203 in MUC1 silenced MM cells does not affect cell viability.
Figure S3. Increased expression of MUC1 on MM cells is mediated via soluble factors.
Figure S4. IL-6 leads to MUC1 upregulation on MM cells through the Jak-STAT3 pathway.


