Abstract
Although historically studied in the context of cancer, recent literature has highlighted the importance of the highly conserved serine/threonine kinase CK2 in inflammatory disorders. Most strikingly, CK2 is a major regulator of the Th17/Treg axis, relevant to many T-cell driven autoimmune disorders including Multiple Sclerosis (MS).
Keywords: Protein Kinase CK2, CD4+ T cells, Th17/Treg Axis, Multiple Sclerosis, Experimental Autoimmune Encephalomyelitis
TEXT
Protein kinase CK2, or Casein Kinase II, is a highly conserved serine/threonine kinase distributed ubiquitously in eukaryotic organisms [1]. Structurally, CK2 can exist in a tetramer composed of 2 catalytic subunits, CK2α and or CK2α′, associated with 2 regulatory subunits, termed CK2β. Importantly, each subunit is encoded by a separate gene, CSKN2A1 (CK2α), CSKN2A2 (CK2α′), and CSNK2B (CK2β), and each can function independently of its association in the tetramer. Therefore, CK2α and CK2α′ maintain their kinase activity outside of their association with CK2β, which functions to modulate activity and confer substrate specificity [1]. CK2 is truly unique in its immense promiscuity. It has been reported to phosphorylate over 500 known target proteins and is estimated to be responsible for up to 10% of the human phosphoproteome [1]. Therefore, CK2 activity can regulate numerous and diverse cellular processes including transcription, translation, cell cycle progression and survival [1, 2]. As such, many cancer types exhibit aberrant CK2 activity linked to tumor-promoting processes and a poorer prognosis [2]. Although much of the current knowledge of CK2 comes from extensive studies in the context of cancer, roles for CK2 have more recently been described in inflammatory disorders and the functions of immune cells, as summarized in Table 1.
Table 1. Major Findings Regarding the Role of CK2 in Inflammatory Diseases.
The major findings of publications describing the role of CK2 during inflammatory diseases, and the model, targeting approach, and major cell types and signaling pathways demonstrated to be involved are listed chronologically. Emodin, apigenin, TBB, and TBCA are CK2 inhibitors used widely before the generation of next-generation small molecule inhibitors such as CX-4945. Abbreviations: GN – glomerulonephritis; GBM – glomerular basement membrane; AS-ODN – antisense oligodeoxynucleotide; EAE – experimental autoimmune encephalomyelitis; IEC – intestinal epithelial cells; DSS – dextran sodium sulfate; TBB – 4,5,6,7-tetrabromobenzotriale; TBCA – tetrabromocinnamic acid; moDC – monocyte-derived dendritic cells.
| Major Findings | Animal Model(s) Utilized | CK2 Targeting Approach(es) | Major Cell Type and/or Signaling Pathway(s) Involved | Publication |
|---|---|---|---|---|
| - CK2α expression is elevated in kidney during GN. - CK2 inhibition is protective in rat models of GN. |
- Rat Anti-GBM GN - Rat Anti-Thy1 GN |
- AS-ODN against CK2α - Emodin - Apigenin |
Not described | [3] |
| - CD5-CK2 interaction in CD4+ T cells promotes EAE, associated with the generation of IFN-γ+IL-17A+ CD4+ T cells. | - Mouse EAE | - CD5-CK2 binding/activation deficient mice. | CD4+ T cells | [9] |
| - CK2α expression and nuclear localization are enhanced during mouse and human colitis. - CK2 protects IECs from inflammation-mediated apoptosis and promotes wound healing. |
- Mouse DSS Colitis - Mouse Salmonella enterica colitis |
- TBB - TBCA - Emodin |
Wnt/β-catenin in IECs | [4] |
| - CK2β expression is required for ILT3+ Tregs to suppress Th2 mediated lung inflammation. | - Spontaneous mouse lung inflammation. | - Csnk2bfl/fl-Foxp3-Cre mice | CD4+ T cells | [13] |
| - CK2β expression in CD4+ T cells promotes EAE through regulation of the Th17/Treg axis. | - Mouse EAE | - Csnk2bfl/fl-CD4-CreERT2 mice | Akt/mTOR and STAT3 in CD4+ T cells | [14] |
| - CK2 is activated in human moDCs and promotes DC activation and T cell polarization in response to ACD-related contact sensitizers. | NA | - CX-4945 | - Human moDCs | [7] |
| - CK2α expression and CK2 kinase activity are induced upon T cell activation. - CK2 activity promotes EAE, associated with regulation of the Th17/Treg axis and pathogenic Th17 cell maturation. |
- Mouse EAE | - CX-4945 | Akt/mTOR, STAT3, STAT5 and SMAD2/3 in CD4+ T cells | [10] |
CK2 has well characterized interactions with signaling pathways involved in regulating inflammatory responses. For example, CK2 activity promotes the activation of the NF-κB, PI3K/Akt/mTOR and JAK/STAT pathways utilized by immune cells to respond to changes in inflammatory environments [2]. In addition, multiple groups have demonstrated changes in CK2 at sites of inflammation. Yamada et al., demonstrated that CK2 expression was enhanced in inflammatory lesions in a rat model of glomerulonephritis. The group further demonstrated that pharmacologic inhibition of CK2 was sufficient to prevent renal pathology, although the major cell type in which CK2 was acting was not described [3]. Similarly, Koch et al., described enhanced CK2 expression, activity and nuclear localization in lesions during murine and human intestinal inflammation, and noted this pattern was especially true in intestinal epithelial cells. The group further described a protective role for CK2 in promoting intestinal epithelial cell homeostasis through protection against cytokine-induced apoptosis [4]. These studies provide important evidence for the importance and potentially complex roles of CK2 in the pathogenesis of inflammatory diseases.
CK2 has been shown to have cell-specific functions in both innate and adaptive immune cells. The Nikolajczyk group has characterized a critical role for CK2 in IRF4- and NF-κB-regulated transcription of the cytokine IL-1β in monocytes [5, 6]. In addition, de Bourayne et al., recently described a critical role for CK2 in the ability of monocyte-derived dendritic cells to mature and produce cytokines necessary to polarize effector T cells in response to chemicals related to allergic contact dermatitis [7]. The most well described function of CK2 within the immune system, however, is as an intrinsic regulator of effector CD4+ T cell responses. The first evidence of the importance of CK2 to CD4+ T cell biology came from studies of the costimulatory molecule and upstream activator of CK2, CD5. The Raman group demonstrated critical roles for the CD5-CK2 interaction in regulating the threshold for T cell anergy as well as cytokine production during experimental autoimmune encephalomyelitis (EAE), a T cell-driven murine model of MS [8, 9]. In addition, our group described a significant increase in the expression of CK2 subunits, most strikingly the catalytic subunit CK2α, in response to activation via the T cell receptor (TCR) and costimulatory molecule CD28 [10]. Importantly, the increase in expression is indeed consistent with an increase in overall CK2 kinase activity [10], providing evidence that activated CD4+ T cells utilize may CK2 to support effector functions.
The most striking function of CK2 in CD4+ T cells is as a dominant regulator of the Th17/Treg axis. Utilizing the small molecule inhibitor CX-4945, which inhibits both CK2α and CK2α′ activity, as well as siRNA knockdown of CK2α, our group demonstrated CK2 activity promotes Th17 cell differentiation and effector functions at the expense of anti-inflammatory Tregs [10]. Two major factors controlling the fate of Th17 and Treg differentiation are the strength of activating signals through the TCR and CD28, mediated by the Akt/mTOR pathway, and the balance of cytokine-induced activation of STAT3 and STAT5. Consistent with the knowledge of CK2 signaling from other cell types, inhibition of CK2 was associated with decreased activation the Akt/mTOR downstream of CD4+ T cell activation and decreased IL-6- induced phosphorylation of STAT3 [10]. Therefore, the current proposed mechanism by which CK2 regulates the Th17/Treg axis involves promotion of signaling through these Th17-favoring pathways, while inhibition of CK2 allows Treg-promoting signals to predominate (Figure 1).
Figure 1. Proposed Regulation of CD4+ T Cell Signaling and Differentiation by CK2.
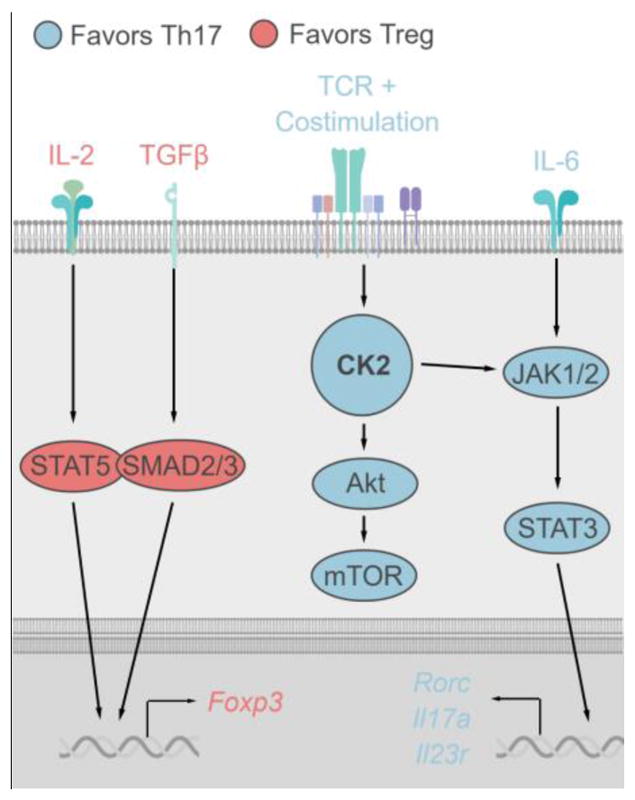
Upon CD4+ T cell activation, CK2 kinase activity is induced. In turn, CK2 promotes the activation of Akt and the downstream kinase mTOR, which is critical for Th17 cell differentiation and plays an essential role in directing the metabolic fate of the cell. In addition to signals downstream TCR activation, CK2 enhances STAT3 activation downstream of IL-6 to promote Th17 cell differentiation, potentially though direct interaction with upstream JAKs. STAT3 then translocates to the nucleus to induce expression of the Th17 transcriptional program including genes such as Rorc, Il7a and Il23r. When CK2 activity is suppressed via pharmacologic inhibition or genetic targeting, Th17-promoting signals are inhibited, allowing for IL-2 mediated STAT5 and TGFβ1-mediated SMAD2/3 activation to induce Foxp3 expression and direct the cell towards the Treg phenotype.
Genetic deletion of CK2α or CK2β is embryonic lethal [11, 12]. As such, the Bopp group utilized a Cre-Lox system to target the regulatory subunit CK2β in subsets of CD4+ T cells. Utilizing Foxp3-Cre, they first demonstrated that CK2β expression was required for a specific population of Tregs to prevent Th2-mediated inflammation in the lung [13]. In addition, the group demonstrated that T cell-specific deletion of CK2β utilizing a tamoxifen-inducible CD4-Cre resulted in the dysregulation of the Th17/Treg axis towards Th17 cell differentiation, as was observed after pharmacologic inhibition of the catalytic subunits utilizing CX-4945 [14]. Together, these results suggest that both the catalytic activity conferred by CK2α and CK2α′ and CK2β-mediated regulatory mechanisms such as substrate specificity are important in the promotion of Th17-promoting signaling pathways during CD4+ T cell activation and lineage commitment.
The Th17/Treg axis is relevant to many autoimmune inflammatory disorders, including MS. As such, targeting of CK2 systemically with pharmacologic inhibition or specifically in CD4+ T cells with CD4-Cre mediated deletion of CK2β resulted in significant protection in the EAE model [10, 14]. Protection in both cases was indeed associated with decreased frequencies of Th17 cells and increased frequencies of Tregs in peripheral immune organs and in the central nervous system at various stages of disease. Interestingly, both active immunization and adoptive transfer models of EAE support that CK2 is not only important in the differentiation of Th17 cells, but also the generation of IFN-γ- and GM-CSF-producing Th17 cells, a subtype particularly pathogenic in the context of neuroinflammation [10]. These findings suggest that CK2 may play multiple roles during T cell activation, differentiation and maturation throughout an inflammatory disease. Considering the importance of these pathogenic Th17 cells in autoimmune diseases, the specific contribution of CK2 to their development warrants more investigation.
The immense influence of CK2 renders this kinase a promising target in the context of human disease. Growing evidence that CK2 promotes inflammatory functions in multiple cell types has implications for the treatment of inflammatory diseases, but several questions remain. Further studies are necessary to understand the molecular mechanisms by which CK2 regulates immune cell function. In addition, the factors that regulate CK2 expression and activity are not well understood, and appear to be cell type-specific. Moving forward, it is important to understand how CK2 itself is regulated in immune cells during different inflammatory contexts. Evidence of the anti-inflammatory effects of CK2 inhibition in vivo has implications for cancer therapy. CK2 inhibitors are currently being tested in the clinic due to anti-tumor efficacy. Despite the large influence of CK2 on the human phosphoproteome, inhibitors such as CX-4945 have been well tolerated in phase I clinical trials [2]. The growing evidence highlighting a role for CK2 in promoting inflammation suggests that inhibition could have unwanted effects on anti-tumor immunity. These recent studies uncovering complex roles for CK2 in immune cells highlight the importance of thoroughly investigating the consequences of CK2 inhibition as it pertains to different inflammatory environments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nunez de Villavicencio-Diaz T, et al. Protein kinase CK2: intricate relationships within regulatory cellular networks. Pharmaceuticals (Basel) 2017;10(1):27. doi: 10.3390/ph10010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chua MM, et al. CK2 in Cancer: cellular and biochemical mechanisms and potential therapeutic target. Pharmaceuticals (Basel) 2017;10(1):18. doi: 10.3390/ph10010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamada M, et al. Inhibition of protein kinase CK2 prevents the progression of glomerulonephritis. Proc Natl Acad Sci USA. 2005;102(21):7736–7741. doi: 10.1073/pnas.0409818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koch S, et al. Protein kinase CK2 is a critical regulator of epithelial homeostasis in chronic intestinal inflammation. Mucosal Immunol. 2013;6(1):136–45. doi: 10.1038/mi.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang MD, et al. The interleukin-1β gene is transcribed from a poised promoter architecture in monocytes. J Biol Chem. 2006;281(14):9227–9237. doi: 10.1074/jbc.M510700200. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, et al. Dynamic protein associations define two phases of IL-1β transcriptional activation. J Immunol. 2008;181(1):503–512. doi: 10.4049/jimmunol.181.1.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Bourayne M, et al. Protein kinase CK2 controls T-cell polarization through dendritic cell activation in response to contact sensitizers. J Leukoc Biol. 2017;101(3):703–715. doi: 10.1189/jlb.3A0715-320RR. [DOI] [PubMed] [Google Scholar]
- 8.Sestero CM, et al. CD5-dependent CK2 activation pathway regulates threshold for T cell anergy. J Immunol. 2012;189(6):2918–30. doi: 10.4049/jimmunol.1200065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Axtell RC, et al. CD5-CK2 binding/activation-deficient mice are resistant to experimental autoimmune encephalomyelitis: protection is associated with diminished populations of IL-17-expressing T cells in the central nervous system. J Immunol. 2006;177(12):8542–8549. doi: 10.4049/jimmunol.177.12.8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibson SA, et al. Protein kinase CK2 controls the fate between Th17 cell and regulatory T cell differentiation. J Immunol. 2017;198(11):4244–4254. doi: 10.4049/jimmunol.1601912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lou DY, et al. The alpha catalytic subunit of protein kinase CK2 is required for mouse embryonic development. Mol Cell Biol. 2008;28(1):131–139. doi: 10.1128/MCB.01119-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buchou T, et al. Disruption of the regulatory β subunit of protein kinase CK2 in mice leads to a cell-autonomous defect and early embryonic lethality. Mol Cell Biol. 2003;23(3):908–915. doi: 10.1128/MCB.23.3.908-915.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ulges A, et al. Protein kinase CK2 enables regulatory T cells to suppress excessive TH2 responses in vivo. Nat Immunol. 2015;16(3):267–275. doi: 10.1038/ni.3083. [DOI] [PubMed] [Google Scholar]
- 14.Ulges A, et al. Protein kinase CK2 governs the molecular decision between encephalitogenic TH17 cell and Treg cell development. Proc Natl Acad Sci USA. 2016;113(36):10145–10150. doi: 10.1073/pnas.1523869113. [DOI] [PMC free article] [PubMed] [Google Scholar]


