Abstract
Purpose
Lymphadenectomy is a well established practice for many urological malignancies but its role in renal cell carcinoma is less clear. Our primary objective was to determine whether lymphadenectomy impacted survival in patients with fully resected, high risk renal cell carcinoma.
Materials and Methods
Patients with fully resected, high risk, nonmetastatic renal cell carcinoma were randomized to adjuvant sorafenib, sunitinib or placebo in the ASSURE (Adjuvant Sorafenib and Sunitinib for Unfavorable Renal Carcinoma) trial. Lymphadenectomy was performed for cN+ disease or at surgeon discretion. Patients treated with lymphadenectomy were compared to patients in the trial who did not undergo lymphadenectomy. The primary outcome was overall survival associated with lymphadenectomy. Secondary outcomes were disease free survival, factors associated with performing lymphadenectomy and surgical complications.
Results
Of the 1,943 patients in ASSURE 701 (36.1%) underwent lymphadenectomy, including all resectable patients with cN+ and 30.1% of those with cN0 disease. A median of 3 lymph nodes (IQR 1–8) were removed and the rate of pN+ disease in the lymphadenectomy group was 23.4%. There was no overall survival benefit for lymphadenectomy relative to no lymphadenectomy (HR 1.14, 95% CI 0.93–1.39, p = 0.20). In patients with pN+ disease who underwent lymphadenectomy no improvement in overall or disease-free survival was observed for adjuvant therapy relative to placebo. Lymphadenectomy did not confer an increased risk of surgical complications (14.2% vs 13.4%, p = 0.63).
Conclusions
The benefit of lymphadenectomy in patients undergoing surgery for high risk renal cell carcinoma remains uncertain. Future strategies to answer this question should include a prospective trial in which patients with high risk renal cell carcinoma are randomized to specific lymphadenectomy templates.
Keywords: kidney, carcinoma, renal cell, lymph node excision, chemotherapy, adjuvant, mortality
Lymphadenectomy in the presence of clinically negative lymph nodes (cN0) is a well established practice for most genitourinary malignancies, including prostate, bladder and penile cancers.1–3 Yet current guidelines for RCC recommend LND only in the presence of clinically suspicious nodes.4,5 Retrospective analyses of high risk RCC offer conflicting evidence of an oncologic benefit for LND.6,7 A single randomized trial, EORTC 30881, demonstrated no survival advantage for LND in the setting of localized cN0 RCC. However, since most patients in this trial had a low rate of pathological lymph node involvement (4%) and low grade (77%), low stage (68% stage I–II) disease, this population may not have represented the cohort most likely to benefit.8 Thus, defining the ideal patient in whom LND would be beneficial remains elusive.
The survival impact of adjuvant systemic therapy in patients with high risk RCC is similarly controversial. Historically adjuvant radiotherapy, hormonal therapy and cytotoxic chemotherapy have demonstrated no measureable survival benefit in this population.9 Two recent trials, S-TRAC (Sunitinib as Adjuvant Treatment for Patients at High Risk of Recurrence of Renal Cell Carcinoma Following Nephrectomy) and ASSURE, provided disparate evidence of benefit with the former demonstrating improved DFS in patients who received adjuvant sunitinib and the latter showing no DFS difference between adjuvant sunitinib or sorafenib vs placebo.10,11 Since the goal of adjuvant systemic therapy is to eradicate micrometastatic disease after surgery, a multimodal approach, including the removal of clinically suspicious lymph nodes at nephrectomy followed by systemic therapy, may better select patients for the adjuvant approach.
We performed a secondary analysis of the ASSURE (ECOG-ACRIN E2805) randomized trial,11 focusing on the role of LND in patients with high risk, fully resected RCC. Our primary objective was to assess whether LND impacted OS in these patients. Secondary objectives were to examine differences in DFS conferred by LND, the oncologic benefit of adjuvant therapy in patients with pN+ disease who underwent LND, the extent of LND performed, factors predicting LND and complications associated with LND.
PATIENTS AND METHODS
Patient Characteristics and Setting
The methodology of the ASSURE trial was recently published.11 Briefly, after nephrectomy patients at high risk were randomly assigned 1:1:1 to sunitinib, sorafenib or placebo from April 2006 to December 2010. The primary outcome measure was intent to treat DFS between each experimental group and the placebo group. Patients at high risk were defined as having pT1b G3-4 N0 (or pNX with cN0) M0 to T (any) G (any) N+ M0. In patients with cN+ disease complete resection was required. Of the patients 98.5% enrolled in the study postoperatively.
Surgeons completed a form after surgery documenting 3 categories, including 1) surgical performance and staging, including type of surgery—radical vs partial nephrectomy, laterality, approach—open vs laparoscopic, adrenalectomy— yes vs no vs partial, lymphadenectomy—yes vs no, grossly positive yes vs no and extranodal extension yes vs no), surgical margin status, histological type, presence of sarcomatoid features and pathological tumor stage, 2) intraoperative surgical complications and 3) extent of LND if performed. Although the protocol required the removal of all suspicious nodes, the performance of LND was at the discretion of the operative surgeon and templates were not standardized. Surgical complication reporting included wound infection, wound dehiscence and/or fistula, pneumonia, cardiopulmonary complication, hemorrhage, renal failure and specified other.
Paraffin embedded tumor samples were required for central review. Patients had to be treatment naïve for kidney cancer, have good ECOG performance status (0 or 1), and have normal liver and hematological function. The criterion for kidney function was creatinine clearance greater than 30 ml per minute. The treatment regimen was 54 weeks of sunitinib orally 50 mg per day for the first 28 days of each 6-week cycle, sorafenib orally 400 mg twice daily throughout all cycles or placebo. Study participation required written informed consent.
Statistical Analysis
The Fisher and Mehta exact tests were used to test for univariate associations of surgery type with patient characteristics, disease and surgical course. For continuous variables the Wilcoxon rank sum test was applied. A logistic regression model was created to explore multivariable associations with LND. Cox proportional hazards models were used to identify factors associated with an increased risk of OS and DFS. The Collet model building methods were also applied.12 Factors that were significant at p = 0.05 on univariate analysis were retained in the model. Because there were no statistically significant differences in OS or DFS among patients treated with sorafenib, sunitinib or placebo,11 patients from all arms were included in analysis.
RESULTS
Surgical forms were available for 1,942 of the 1,943 patients (99.9%). LND was performed in 701 of enrolled patients (36.1%). A median of 3 lymph nodes (IQR 1–8) were examined. Only 124 LNDs (17.7%) yielded greater than 10 nodes. Disease was pN+ in 164 cases (23.4%), of which 99.2% were deemed grossly positive. Patients with cN+ disease were more likely to undergo LND than those with cN0 disease (99.4% vs 30.1%, p <0.001). Only 10 patients (1.98%) with cN0 disease who underwent LND had pN+ disease, suggesting high negative predictive value of a benign-appearing lymph node on computerized tomography. Only 1 patient with cN+ had unresectable lymphadenopathy and did not undergo LND. There were no differences in reported surgical complications between the groups (14.2% for LND vs 13.4%, p = 0.63).
Lymph Node Dissection Category
Patient Characteristics
Median patient age was 56 (range 19–84) (supplementary table 1, http://jurology.com/). Of the patients 67.4% were male, 92.7% were white and 84.6% had good ECOG performance status (ECOG 0). Those who underwent LND were more often symptomatic at presentation (70.7% vs 62.1%, p <0.001). No differences were noted in age, gender, race, ethnicity, ECOG performance status, history of deep venous thrombosis/pulmonary embolism or history of cardiovascular disease (all p >0.05).
Surgical Characteristics
Surgery location was divided among academic centers (44.9% of cases), CCOP sites (24.9%) and affiliate sites (30.3%) (supplementary table 2, http://jurology.com/). Of the patients 94.5% underwent radical nephrectomy and the preferred surgical approach was open in 57.2%. Significant differences between patients who did vs did not undergo LND were noted in institution, primary surgery type (radical vs partial nephrectomy), surgical approach (open vs laparoscopic) and whether adrenalectomy was performed (all p <0.05). No difference was noted in positive surgical resection margins (LND 9.1% vs 8.0%, p = 0.27).
Tumor Characteristics
Tumors were clear cell in 81.7% of cases and Fuhrman grade 3–4 in 66.1% (table 1). When stratified by stage, 182 tumors (9.4%) were stage 1, 480 (24.7%) were stage II, 1,256 (64.7%) were stage III and 24 (1.2%) were stage IV. Tumor factors associated with the performance of LND were laterality, disease extent, grade, stage and presence of sarcomatoid features (all p <0.05). Histological subtype did not differ between the LND groups (p = 0.09).
Table 1.
Tumor characteristics by lymph node dissection category
| No. Lymph Node Dissection (%)
|
Total No. (%) | p Value | ||
|---|---|---|---|---|
| No | Yes | |||
| Overall | 1,241 | 701 | 1,942 | |
| Laterality: | ||||
| Lt | 587 (47.3) | 395 (56.4) | 982 (50.6) | <0.0001 |
| Rt | 653 (52.6) | 304 (43.4) | 957 (49.3) | |
| Bilat | 1 (0.1) | 2 (0.3) | 3 (0.2) | |
| Gross disease extent: | ||||
| Unifocal | 1,090 (93.6) | 595 (90.4) | 1,685 (92.4) | 0.02 |
| Multifocal | 75 (6.4) | 63 (9.6) | 138 (7.6) | |
| Unknown | 76 | 43 | 119 | |
| Fuhrman grade: | ||||
| 1 | 32 (2.6) | 16 (2.3) | 48 (2.5) | 0.0002 |
| 2 | 418 (34.0) | 187 (27.0) | 605 (31.4) | |
| 3 | 566 (46.0) | 330 (47.6) | 896 (46.6) | |
| 4 | 215 (17.5) | 161 (23.2) | 376 (19.5) | |
| Unknown | 10 | 7 | 17 | |
| Primary histological type: | ||||
| Conventional clear cell Ca | 1,005 (82.8) | 535 (79.6) | 1,540 (81.7) | 0.09* |
| Papillary Ca | 90 (7.4) | 59 (8.8) | 149 (7.9) | |
| Chromophobe | 75 (6.2) | 36 (5.4) | 111 (5.9) | |
| Mixed histology, greater than 25% clear cell | 34 (2.8) | 21 (3.1) | 55 (2.9) | |
| Mixed histology, less than 25% clear cell | 10 (0.8) | 21 (3.1) | 31 (1.6) | |
| Unclassified/unknown | 27 | 29 | 56 | |
| Sarcomatoid features: | ||||
| No | 1,159 (93.5) | 608 (87.0) | 1,767 (91.2) | <0.0001 |
| Yes | 80 (6.5) | 91 (13.0) | 171 (8.8) | |
| Unknown | 2 | 2 | 4 | |
| AJCC stage: | ||||
| I | 159 (12.8) | 23 (3.3) | 182 (9.4) | <0.0001† |
| II | 331 (26.7) | 149 (21.3) | 480 (24.7) | |
| III | 737 (59.4) | 519 (74.0) | 1,256 (64.7) | |
| IV | 14 (1.1) | 10 (1.4) | 24 (1.2) | |
Clear cell vs other.
Between group comparison of low—AJCC 1 and 2 vs high—AJCC 3 and 4.
Independent Factors Associated with Lymph Node Dissection
When adjusting for grade, diagnosis method, disease extent and whether kidney embolization was performed, LND was less commonly done at CCOP sites (OR 0.61, 95% CI 0.48–0.79, p <0.001) and affiliate sites (OR 0.74, 95% CI 0.59–0.95, p = 0.02) than at academic institutions (table 2). Surgical factors associated with LND included radical nephrectomy (vs partial nephrectomy OR 2.48, 95% CI 1.41–4.36, p = 0.002), open surgery (vs laparoscopic surgery OR 2.61, 95% CI 2.11–3.24, p <0.001) and concomitant adrenalectomy (vs no adrenalectomy OR 1.73, 95% CI 1.19–2.50, p 0.004). Patients were less likely to undergo LND if=the tumor was on the right side than on the left side (OR 0.74, 95% CI 0.60–0.91, p = 0.004) and more likely to undergo LND if the tumor had sarcomatoid features (OR 1.99, 95% CI 1.40–2.81, p <0.001). Patients with higher AJCC stage cancer were also more likely to undergo LND (AJCC stage 2 vs 1 OR 2.05, 95% CI 1.24–3.38, p = 0.005 and 3 vs 1 OR 2.91, 95% CI 1.81–4.69, p <0.001).
Table 2.
Logistic regression model of factors associated with lymphadenectomy
| OR Point Estimate (95% Wald CI) | p Value (chi-square) | |
|---|---|---|
| Age | 0.98 (0.97–0.99) | 0.001 |
| Institution type (vs academic): | ||
| CCOP | 0.61 (0.48–0.79) | <0.0001 |
| Affiliate | 0.74 (0.59–0.95) | 0.02 |
| Primary nephrectomy (radical vs partial) | 2.48 (1.41–4.36) | 0.002 |
| AJCC stage: | ||
| 2 vs 1 | 2.05 (1.24–3.38) | 0.005 |
| 3 vs 1 | 2.91 (1.81–4.69) | <0.0001 |
| 4 vs 1 | 1.83 (0.68–4.92) | 0.23 |
| Surgical method (open vs laparoscopic) | 2.61 (2.11–3.24) | <0.0001 |
| Side involved (vs lt): | ||
| Rt | 0.74 (0.60–0.91) | 0.004 |
| Bilat | 2.26 (0.18–27.75) | 0.52 |
| Adrenalectomy (vs no adrenalectomy): | ||
| Yes (partial) | 2.82 (2.26–3.52) | <0.0001 |
| Yes (full) | 1.73 (1.19–2.50) | 0.004 |
| Missing | 1.92 (0.93–3.99) | 0.08 |
| Sarcomatoid features (yes vs no) | 1.99 (1.40–2.81) | <0.0001 |
Survival Analyses
After adjustment for risk category, treatment arm, grade, stage, gender, age, performance status, symptoms at diagnosis, lactate dehydrogenase level, anemia, histology and surgery type there was no association between LND and OS (HR 1.14, 95% CI 0.93–1.39, p = 0.20, fig. 1, A) at a median followup of 67.9 months (IQR 56.7–82). LND was associated with worse DFS (HR 1.27, 95% CI 1.10–1.46, p = 0.001, fig. 1, B).
Figure 1.
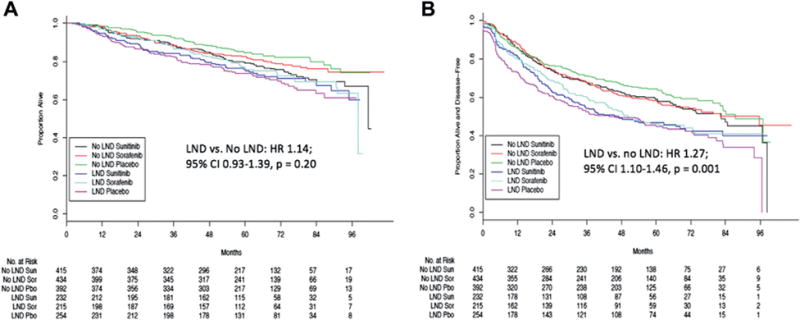
Overall (A) and disease-free (B) survival by adjuvant arm and LND category
Survival analyses were performed to consider the impact of adjuvant systemic therapy in the subset of patients with pN+ disease who underwent LND. There was no difference in 5-year OS in the sunitinib arm (45.7%, 95% CI 32.9–63.6, p = 0.113) or the sorafenib arm (63.7%, 95% CI 51.3–79.3, p = 0.981) vs the placebo arm (64.8%, 95% CI 54.2–77.6, fig. 2, A). Moreover, median DFS in this subset did not differ in the sorafenib arm (21.6 months, 95% CI 13.7–48.4, p = 0.633) vs the placebo arm (24.7 months, 95% CI 14.5–55.4). However, patients with pN+ disease who underwent LND and received sunitinib had worse DFS relative to placebo (median 8.3 months, 95% CI 4.7–17.2, p = 0.002, fig. 2, B).
Figure 2.
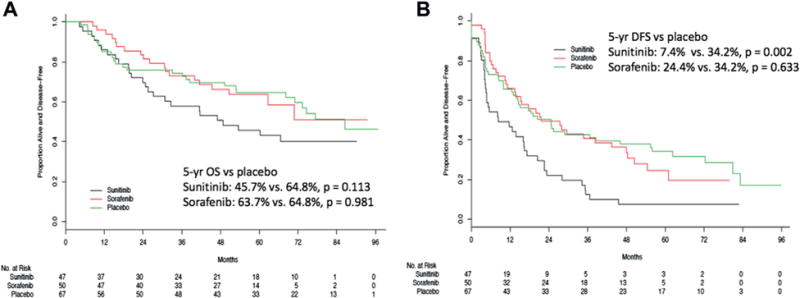
Overall (A) and disease-free (B) survival by adjuvant arm in patients with pN+ who underwent LND
DISCUSSION
These data quantify factors associated with the performance of LND during a randomized clinical trial of adjuvant therapy for RCC. Given that most patients were enrolled postoperatively, it seems likely that surgical decision making reflected current practice and was consistent with published guidelines.4,5 While LND was performed at the discretion of the operating surgeon, LND was almost universal in patients with cN+ disease and it was not routine in those with cN0 disease. The trial also corroborated the notion that regionally positive nodes are often plucked during surgery for high risk RCC rather than removed using standardized dissection templates.13
Independent predictors and nomograms of pN+ disease in advanced RCC have been published to+ guide the decision to perform LND.14–16 Preoperative factors associated with lymph node metastasis include ECOG PS 1 or greater, cN1 stage, increased lactate dehydrogenase, symptomatic presentation and greater tumor size. Pathological predictors of nodal involvement are Fuhrman grade 3–4, sarcomatoid component, tumor size 10 cm or greater, pT3 or pT4 tumor stage and the presence of histological tumor necrosis. While it is not clear that surgeons in the current study prospectively used these established criteria, sarcomatoid features and higher AJCC stage were associated with the performance of LND.
One concern regarding LND is the potential for increased postoperative complications. However, randomized trial data have not demonstrated an increased risk of LND associated complications.8 Similarly our findings showed no difference in complication rates between patients who did and did not undergo LND. Therefore, it appears that LND carries an acceptable risk in patients during renal surgery performed by experienced retroperitoneal surgeons. Importantly these data were contextualized by limiting study eligibility to those who were well enough to be randomized 12 weeks after surgery. Therefore, any enrollment limiting major complications of LND may have been selected out.
The added oncologic benefit of LND in patients with high risk renal cell carcinoma remains unclear. EORTC 30881, the only randomized trial to date, demonstrated no improvement in time to progression or progression free-survival in patients who underwent LND.8 EORTC 30881 has been criticized as being underpowered for its low lymph node positive rate in the LND arm (4%) and the preponderance of low grade (77%), low stage (68%) disease. Conversely retrospective analyses suggested that some patients at high risk may benefit from LND.17,18 However, no retrospective analysis has been able to definitively conclude that improvements in recurrence-free survival were due to LND rather than to the Will Rogers effect.19 Moreover, a recently reported and well controlled, retrospective analysis using propensity scoring demonstrated no survival benefit attributable to LND in patients at high risk.7
The eligibility limitations of our study posed similar problems with selection bias. Performance of LND was an unexpected predictor of earlier recurrence. The most logical explanation of this finding is that surgeons performed LND in patients with higher risk tumors and tumor risk was not adequately controlled in the multivariable model.
The underpinnings of an OS benefit related to aggressive surgery in high risk RCC are derived from the tumor debulking and metastatectomy literature.20,21 In our study LND was independently associated with other markers of aggressive surgical resection such as open surgery, radical nephrectomy and adrenalectomy. Despite this we noted no improvement in OS in patients who underwent more aggressive surgical procedures, including LND. Similar findings have been described for other malignancies for which aggressive surgery is a mainstay in the multimodal management of advanced disease.22 Given the recent results of the STRAC adjuvant RCC trial suggesting improved DFS in patients with high risk, clear cell RCC who received 1 year of adjuvant sunitinib,10 one might expect the same in the highest risk patient group (ie pN+) in ASSURE. The fact that we did not find improvement in DFS or OS in patients with fully resected pN+ disease who received adjuvant sorafenib or sunitinib may suggest incomplete understanding of the optimal patient population in which to deploy adjuvant systemic strategies.23
The principle limitation of our study is selection bias since LND was not an eligibility or randomization requirement in this prospective clinical trial. While the decision to perform LND was surgeon dependent and patients with worse disease appear to have been preferentially selected, patients with any suspicion of cN+ required LND to be considered trial eligible. Therefore, unlike most retrospective studies in which selection bias may decrease the intensity of surgical interventions in patients at higher risk, the nature of this trial enriched the selected population, perhaps providing more powerful insights.
Also, although the trial protocol necessitated the removal of all clinically suspicious lymph nodes, LND templates were not standardized across surgeons. Coupled with the relatively low median lymph node yield, this lack of standardization may have influenced the results.
Further, the potential for recall bias existed since surgeons completed surgical surveys at the time of patient enrollment, which was up to 12 weeks after surgery in more than 95% of the patients enrolled. However, biases associated with the Hawthorne effect were essentially nonexistent for similar design related reasons, making this is an accurate representation of LND in current surgical practice for high risk RCC.
Finally, the fidelity of followup data related to complications and tumor recurrence were likely better in this prospective clinical trial than what would be expected in an institutional retrospective study.
The uncertain benefit of LND in patients with high risk RCC coupled with no apparent difference in complications at experienced centers offers appropriate equipoise for a clinical trial. Patients with high risk kidney tumors (cT2-3N0M0) could be randomized to no LND vs standard template LND24 with DFS as a primary outcome and OS as 1 secondary outcome. A planned subanalysis could be performed in patients with high risk pathological features. Ideally such a trial could garner support from cooperative groups to answer this important clinical question.
CONCLUSIONS
Lymphadenectomy in patients with high risk, nonmetastatic RCC is safe and carries an acceptably low complication rate. However, the oncologic benefit of LND in this setting remains unproven and LND was not associated with an OS advantage in ASSURE. Therefore, it is important to consider that the risk of LND may outweigh the clinical benefit despite the relatively small incidence of associated complications. Moreover, even in patients with highest risk who have fully resected pN+ disease adjuvant therapy does not appear to confer improved survival. Future strategies to answer this question should include a prospective clinical trial designed to capture patients with high risk RCC.
Supplementary Material
Acknowledgments
The ECOG-ACRIN Cancer Research Group, Drs. Robert L. Comis and Mitchell D. Schnall, Group Co-Chairs, coordinated this study.
Supported by the National Cancer Institute of the National Institutes of Health Awards CA180820, CA180794, CA180821, CA180858, CA180863 and CA180888, and Canadian Cancer Research Institute 021039 and 704970.
Abbreviations and Acronyms
- ACRIN
American College of Radiology Imaging Network
- AJCC
American Joint Committee on Cancer
- ASSURE
Adjuvant Sorafenib and Sunitinib for Unfavorable Renal Carcinoma
- CCOP
Community Cancer Oncology Program
- DFS
disease-free survival
- ECOG
Eastern Cooperative Oncology Group
- EORTC
European Organisation for Research and Treatment of Cancer
- LND
lymph node dissection
- OS
overall survival
- RCC
renal cell carcinoma
Footnotes
No direct or indirect commercial incentive associated with publishing this article.
The corresponding author certifies that, when applicable, a statement(s) has been included in the manuscript documenting institutional review board, ethics committee or ethical review board study approval; principles of Helsinki Declaration were followed in lieu of formal ethics committee approval; institutional animal care and use committee approval; all human subjects provided written informed consent with guarantees of confidentiality; IRB approved protocol number; animal approved project number.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health nor does mention of trade names, commercial products or organizations imply endorsement by the United States government.
References
- 1.Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate cancer, version 1.2016. J Natl Compr Canc Netw. 2016;14:19. doi: 10.6004/jnccn.2016.0004. [DOI] [PubMed] [Google Scholar]
- 2.Witjes JA, Comperat E, Cowan NC, et al. EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol. 2014;65:778. doi: 10.1016/j.eururo.2013.11.046. [DOI] [PubMed] [Google Scholar]
- 3.Clark PE, Spiess PE, Agarwal N, et al. Penile cancer: Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2013;11:594. doi: 10.6004/jnccn.2013.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motzer RJ, Jonasch E, Agarwal N, et al. Kidney cancer, version 3.2015. J Natl Compr Canc Netw. 2015;13:151. doi: 10.6004/jnccn.2015.0022. [DOI] [PubMed] [Google Scholar]
- 5.Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67:913. doi: 10.1016/j.eururo.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Pantuck AJ, Zisman A, Dorey F, et al. Renal cell carcinoma with retroperitoneal lymph nodes: role of lymph node dissection. J Urol. 2003;169:2076. doi: 10.1097/01.ju.0000066130.27119.1c. [DOI] [PubMed] [Google Scholar]
- 7.Gershman B, Thompson RH, Moreira DM, et al. Radical nephrectomy with or without lymph node dissection for nonmetastatic renal cell carcinoma: a propensity score-based analysis. Eur Urol. 2017;71:560. doi: 10.1016/j.eururo.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 8.Blom JH, van Poppel H, Marechal JM, et al. Radical nephrectomy with and without lymphnode dissection: final results of European Organization for Research and Treatment of Cancer (EORTC) randomized phase 3 trial 30881. Eur Urol. 2009;55:28. doi: 10.1016/j.eururo.2008.09.052. [DOI] [PubMed] [Google Scholar]
- 9.Smaldone MC, Fung C, Uzzo RG, et al. Adjuvant and neoadjuvant therapies in high-risk renal cell carcinoma. Hematol Oncol Clin North Am. 2011;25:765. doi: 10.1016/j.hoc.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Ravaud A, Motzer RJ, Pandha HS, et al. Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N Engl J Med. 2016;375:2246. doi: 10.1056/NEJMoa1611406. [DOI] [PubMed] [Google Scholar]
- 11.Haas NB, Manola J, Uzzo RG, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet. 2016;387:2008. doi: 10.1016/S0140-6736(16)00559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collett D. Modelling Survival Data in Medical Research. New York: Chapman and Hall; 1994. p. xvii.p. 347. [Google Scholar]
- 13.Sun M, Trinh QD, Bianchi M, et al. Extent of lymphadenectomy does not improve the survival of patients with renal cell carcinoma and nodal metastases: biases associated with the handling of missing data. BJU Int. 2014;113:36. doi: 10.1111/j.1464-410X.2012.11693.x. [DOI] [PubMed] [Google Scholar]
- 14.Hutterer GC, Patard JJ, Perrotte P, et al. Patients with renal cell carcinoma nodal metastases can be accurately identified: external validation of a new nomogram. Int J Cancer. 2007;121:2556. doi: 10.1002/ijc.23010. [DOI] [PubMed] [Google Scholar]
- 15.Capitanio U, Abdollah F, Matloob R, et al. When to perform lymph node dissection in patients with renal cell carcinoma: a novel approach to the preoperative assessment of risk of lymph node invasion at surgery and of lymph node progression during follow-up. BJU Int. 2013;112:E59. doi: 10.1111/bju.12125. [DOI] [PubMed] [Google Scholar]
- 16.Blute ML, Leibovich BC, Cheville JC, et al. A protocol for performing extended lymph node dissection using primary tumor pathological features for patients treated with radical nephrectomy for clear cell renal cell carcinoma. J Urol. 2004;172:465. doi: 10.1097/01.ju.0000129815.91927.85. [DOI] [PubMed] [Google Scholar]
- 17.Capitanio U, Suardi N, Matloob R, et al. Extent of lymph node dissection at nephrectomy affects cancer-specific survival and metastatic progression in specific sub-categories of patients with renal cell carcinoma (RCC) BJU Int. 2014;114:210. doi: 10.1111/bju.12508. [DOI] [PubMed] [Google Scholar]
- 18.Crispen PL, Breau RH, Allmer C, et al. Lymph node dissection at the time of radical nephrectomy for high-risk clear cell renal cell carcinoma: indications and recommendations for surgical templates. Eur Urol. 2011;59:18. doi: 10.1016/j.eururo.2010.08.042. [DOI] [PubMed] [Google Scholar]
- 19.Albertsen PC, Hanley JA, Barrows GH, et al. Prostate cancer and the Will Rogers phenomenon. J Natl Cancer Inst. 2005;97:1248. doi: 10.1093/jnci/dji248. [DOI] [PubMed] [Google Scholar]
- 20.Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345:1655. doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 21.Zaid HB, Parker WP, Safdar NS, et al. Outcomes following complete surgical metastasectomy for patients with metastatic renal cell carcinoma: a systematic review and meta-analysis. J Urol. 2017;197:44. doi: 10.1016/j.juro.2016.07.079. [DOI] [PubMed] [Google Scholar]
- 22.Horowitz NS, Miller A, Rungruang B, et al. Does aggressive surgery improve outcomes? Interaction between preoperative disease burden and complex surgery in patients with advanced-stage ovarian cancer: an analysis of GOG 182. J Clin Oncol. 2015;33:937. doi: 10.1200/JCO.2014.56.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porta C, Chiellino S. ASSURE vs. S-TRAC: conflicting results of adjuvant treatments for kidney cancer in the era of targeted agents and genomics. Ann Transl Med Suppl. 2016;4:S14. doi: 10.21037/atm.2016.10.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Capitanio U, Becker F, Blute ML, et al. Lymph node dissection in renal cell carcinoma. Eur Urol. 2011;60:1212. doi: 10.1016/j.eururo.2011.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


