Abstract
Background
The stress kinase c-jun N-terminal kinase (JNK) is critical in the pathogenesis of cardiac diseases associated with an increased incidence of atrial fibrillation (AF), the most common arrhythmia in the elderly. We recently discovered that JNK activation is linked to the loss of gap junction connexin43 (Cx43) and enhanced atrial arrhythmogenicity. However, direct evidence for JNK-mediated impairment of intercellular coupling (cell-cell communication) in the intact aged atrium is lacking, as is evidence for whether and how JNK suppresses Cx43 in the aged human atrium.
Methods and Results
JNK activity in human atrial samples is correlated with both reduced Cx43 expression and increasing age. Using a unique technique of optical mapping space constant measurement, we found that impaired intercellular coupling and reduced Cx43 were linked to enhanced activation of JNK in intact aged rabbit atria. These JNK-associated alterations were further confirmed in naturally JNK activated aged mice and in cardiac-specific inducible MKK7D (JNK upstream activator) young mice. Moreover, JNK inhibition, using either JNK specific inhibitors in aged wild-type (WT) mice and JNK activator anisomycin-treated young WT mice or JNK1/2 dominant-negative mice with genetically inhibited cardiac JNK activity, completely eliminated these functional abnormalities. Furthermore, we discovered for the first time that long-term JNK activation downregulates Cx43 expression via c-jun suppressed transcriptional activity of the Cx43 gene promoter.
Conclusion
Our results demonstrate that JNK is a critical regulator of Cx43 expression, and that augmented JNK activation in aged atria downregulates Cx43 to impair cell-cell communication and promote the development of AF. JNK inhibition may represent a promising therapeutic approach to prevent or treat AF in the elderly.
Keywords: atrial fibrillation, c-jun N-terminal kinase, gap junction, cell-cell communication
Introduction
Atrial fibrillation (AF) is the most common arrhythmia in humans and is associated with a high risk of morbidity (stroke and heart failure (HF)). Clinical data suggest a 2-fold higher mortality in patients with AF vs. those without AF [1–3]. Although AF is not the direct cause for a high risk of mortality, AF is associated with a 5-fold increased risk for stroke and a 40–90% increase in mortality due to the stroke based on the Framingham study [4]. AF is promoted by various stresses such as aging, increased fibrosis, and atrial dilation. While all deserve to be studied in more detail, aging is inevitable and the most prevalent risk factor for AF. The underlying mechanisms of AF development in the aged heart remain unclear to date.
The c-jun N-terminal kinase (JNK), a stress-response protein kinase, is critical in the development of cardiovascular diseases including heart failure, hypertrophy, and atherosclerosis [5, 6]. Specific links to the formation of the arrhythmia substrate for AF had not been identified until recent findings reported by our laboratory [7]. Specifically, we discovered that heightened JNK activation in the aged rabbit atria links to suppressed gap junction connexin43 (but unchanged connexin40), which promotes AF development [7]. However, whether JNK directly contributes to impaired intercellular coupling (cell-cell communication) in the intact aged atrium is unclear. Also, whether this JNK regulated Cx43 expression also occurs in aged human atria and the underlying molecular mechanism remain completely unknown.
Atrial gap junctions, composed of Cx43 and Cx40, form specialized membrane channel structures that critically influence electrical and chemical signal propagation between adjacent myocytes [8, 9]. Accumulating evidence suggests that gap junction remodeling leads to slowed action potential propagation that promotes the development of cardiac arrhythmias, most likely due to its important role in stabilizing and maintaining reentry arrhythmias [7, 9–12]. However, gap junctional remodeling in atrial tissue from AF patients has been variably reported as an increase or a decrease in one or more connexins (Cx43 and/or Cx40), as a reduction of only one isoform, and as increased heterogeneity in the distribution of the connexins. Each of these results is dependent on concomitant cardiovascular disease, the type of AF patient population, or the animal model used [13]. Although reduced Cx43 has also been reported in aged right atria and sinus node [14, 15], the role of gap junctional protein in slowing conduction velocity (CV) and increasing AF propensity in aged left atrium (LA) without associated AF has not been characterized. The LA is important in AF development since AF often originates from the pulmonary veins and LA area based on accumulated clinical data [16, 17], yet few studies investigating the underlying mechanisms have actually focused on the LA [7]. In the present study, we found an inverse correlation between JNK activation and Cx43 expression in human LA with increasing age (from donors lacking a history of AF or major cardiovascular diseases).
To date, gap junction mediated cell-cell communication has been measured primarily in isolated paired myocytes. While important information has been derived from such studies, the electrophysiologic and biologic interactions of intact preparations are only partially recapitulated in isolated myocytes. By using a unique method of optical mapping intercellular coupling measurements in the intact atrium, we found impaired intercellular coupling along with reduced Cx43 and activated JNK in aged rabbit intact LA.
The causal connection between activated JNK and Cx43 atrial remodeling in AF is critical to understand. Here, we employed aged mice with natural JNK activation, and MKK7D transgenic (Tg) mice with cardiac-specific inducible JNK activation [18]. To further dissect the specific action of JNK and explore potential therapeutic intervention, the rescue effects of JNK inhibition on Cx43 expression, intercellular coupling, and arrhythmogenesis were determined using either a JNK specific inhibitor (JNKI, SP600125 or JNKI-XI) [19, 20] or a Tg mouse model with cardiac-specific genetically overexpressed inactive dominant negative mutant JNK1/2 (JNK1/2dn) [21].
Although downregulation of Cx43 is a common feature of diseased hearts (e.g. HF, diabetes, aging) and plays an important role in arrhythmic remodeling [9–11, 22], the underlying molecular mechanisms for Cx43 suppression have not been elucidated. Using several unique molecular and biochemistry assays including cross-linked chromatin-immunoprecipitation (XChIP) and luciferase reporter promoter activity assays and c-jun siRNA knockdown, we report a novel molecular mechanism linking JNK to Cx43: JNK-mediated downregulation of atrial Cx43 mRNA via increased binding of the JNK downstream transcription factor c-jun to the Cx43 promoter, which leads to suppressed transcriptional activity.
Our current studies provide direct evidence for the causative action of JNK in atrial arrhythmogenic remodeling and a novel molecular mechanism underlying JNK-regulated Cx43 gene expression. These results shed new light on JNK modulation as a potential therapeutic approach to improve atrial conduction and prevent or treat AF in the elderly.
Materials and Methods
An expanded Materials and Methods section is available as an online supplement.
Human atrial specimens and animal preparation
Human left atrial tissues were obtained as previously described [10, 11, 23] with approvals from the Human Study Committees of Rush University Medical Center (RUMC), Loyola University Chicago (LUC) and Illinois Gift of Hope Organ Donor Network (GOH). Human tissue samples were de-identified. Studies conformed with the declaration of Helsinki. GOH obtained informed research consent from all the donors or donor’s family members for the current studies.
Young and aged male New Zealand White rabbits and wild-type (WT) C57B/6j mice along with two cardiac-specific mouse models (all with a C57B/6j genetic background) with genetically manipulated JNK activity were used for the current studies (see details in the Online Supplements). All animal studies followed the Guide for the Care and Use of Laboratory Animals (NIH Publication, 8th Edition, 2011) and were approved by the Institutional Animal Care and Use Committees of RUMC, LUC and University of Alabama at Birmingham.
Intercellular coupling measurement and atrial arrhythmia induction ex/in vivo
The conduction velocity and space constant were measured using ex vivo optical mapping in Langendorff-perfused intact atria and in a cultured myocyte line (HL-1). In vivo, AF inductions in sedated live mice were performed and measurements made as previously described and detailed in the Online Supplements [7, 24–26].
Biochemical assays
Immunoblotting and cell lysate fractionation were performed as previously described with minor modification [7, 10, 11, 22]. Activity of activator protein-1 (AP-1), composed of JNK downstream transcription factor c-jun and ATF2, was assessed using an AP-1-luciferase reporter vector as previously described [27]. The status of AP1 binding to the Cx43 gene promoter region was assessed using cross-linked chromatin-immunoprecipitation (XChIP) assay. Cx43 promoter activity was measured using a newly constructed vector containing a firefly luciferase reporter gene with inserted Cx43 promoter sequence. Functional impact of JNK downstream transcription factor c-jun on Cx43 promoter activity was assessed in cells with a c-jun specific knockdown using the siRNA approach as previously described with modification [11].
Statistical analysis
All data are presented as Mean ± SEM. Differences between multiple groups or any two groups were evaluated using one-way ANOVA with Post-hoc Tukey test or Student t-test. A p < 0.05 was considered to be significant.
Results
Activated JNK and markedly reduced Cx43 in human atria with increasing age
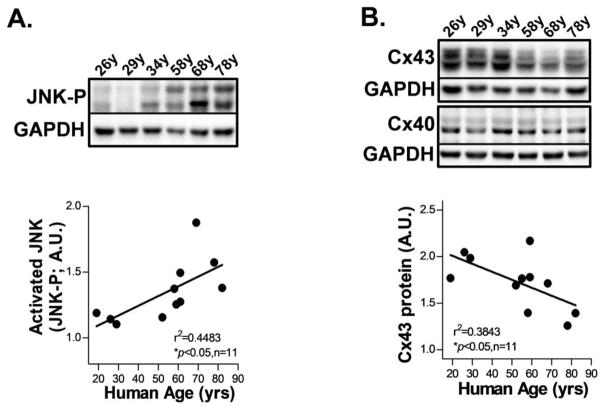
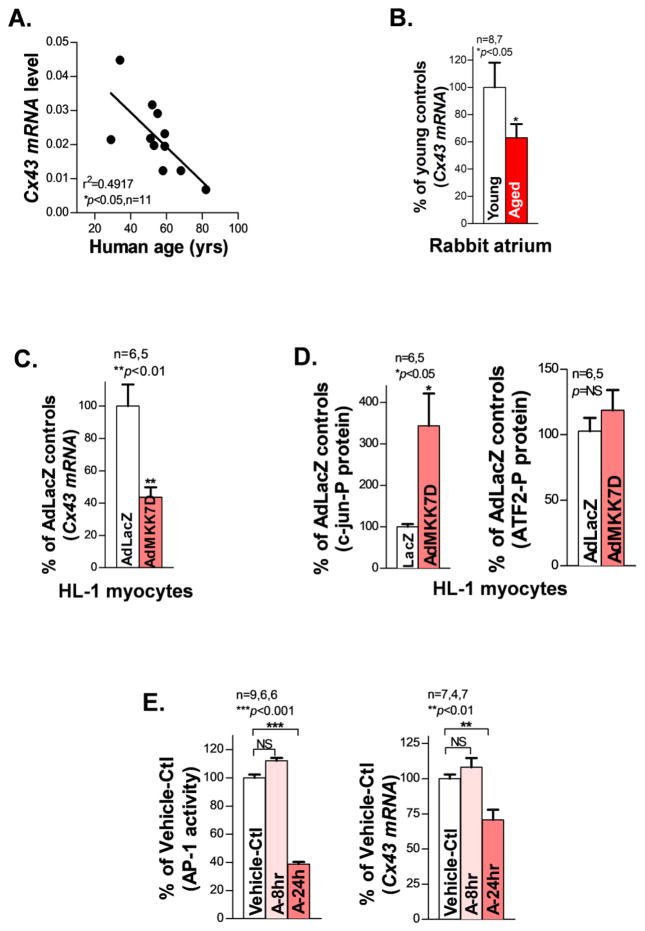
We first assessed JNK proteins in human atria obtained from organ donors lacking any history of AF or major cardiovascular diseases (Supplemental_Table. S1). JNK activation was significantly increased in human atrium with increasing age as assessed by immunoblotting with a phospho-specific antibody that recognized the activated form of phosphorylated total JNK proteins (JNK-P; Fig.1A), while the total JNK proteins remained unchanged (Supplemental_Figs. S1A–S1B). Moreover, gap junction Cx43 proteins were markedly reduced in human atrium with increasing age, while the abundant atrial Cx40 proteins were unchanged (Fig.1B). Meanwhile, the JNK upstream regulators MKK7 and MKK4 showed enhanced activation evidenced by increased phosphorylation in aged atrium (Supplemental_Figs. S1C–S1D, S1F and Supplemental_Table. S2). On the other hand, phosphorylation of stress-activated kinase, p38 (which is not directly involved in the JNK pathway) was unaltered (Supplemental_Figs. S1E, S1F).
Figure 1.
Enhanced JNK activation in human atria with increasing age. A–B. Representative immunoblotting images and quantitative data of phosphorylated JNK (JNK-P; A) and gap junction Cx43 (B) proteins expression with increased human age.
Activated JNK, reduced junctional Cx43, and impaired intercellular coupling in aged rabbit intact atria
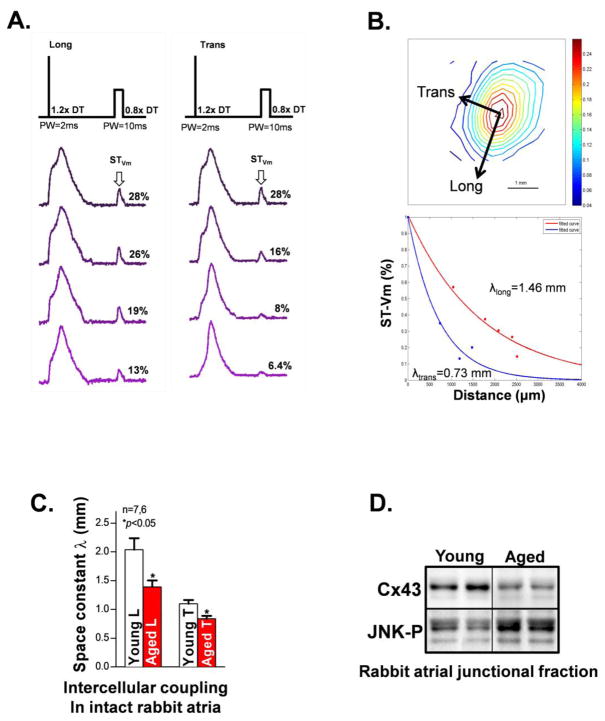
The abundance of gap junction channels impacts coupling between adjacent myocytes [8, 9]. Measurement of intercellular coupling in intact tissue (without structural disruptions) is critical for understanding the functional role of JNK-driven Cx43 suppression in aged atrium. Using a unique approach of optical mapping space constant measurement in intact atrium, we found a significantly reduced space constant λ in both longitudinal and transverse directions, which reflects augmented intercellular resistance (reduced intercellular coupling) in aged rabbit intact atrium LA (vs young controls, Figs.2A–2C). This impaired intercellular coupling is consistent with slowed atrial conduction velocity from the same rabbits prior to the space constant measurement (Supplemental_Fig. S2A). These results are also supported by our previous finding of reduced Cx43 and slowed atrial CV in the aged rabbit LA, along with markedly increased inducibility and duration of burst pacing-induced atrial arrhythmias [7].
Figure 2.
Markedly reduced intercellular electrical coupling and reduced junctional Cx43 in the aged rabbit intact atrium. A. Representative action potential traces obtained from the pacing site to a distance of ~2.5mm subjected to pacing at 1.2x diastolic threshold (DT) with a 2ms pulse width (PW) to generate a train of full action potentials and followed by a sub-threshold (ST, 0.8x DT; PW=10ms) extra stimulus to obtain the STVm response (arrow). In response to a ST extra stimulus, directional differences in the spatial decay of Vm were readily identified with longitudinal (left panel) and transverse (right panel) coupling of atrial myocytes in region measured. B. A representative isochronal map from ST data and analyzed STVm decay from longitudinal and transverse directions over distance. C. Summarized data showing reduced intercellular electrical coupling in the aged rabbit atrium at both longitudinal (L) and transverse (T) myocardial fiber directions. D. Immunoblotting images of reduced junctional (membrane located) Cx43 along with enhanced activation of JNK in the aged rabbit atrium.
Because gap junctions are the primary membrane structures between adjacent cardiac myocytes, we fractionated the membrane (junctional) and cytosolic (non-junctional) fractions of aged rabbit atrial tissue. We discovered markedly reduced junctional Cx43 along with enhanced activation of cell membrane-localized JNK in aged rabbit atrium (Fig.2D & Supplemental_Fig. S2B). Reduced junctional Cx43 is also consistent with our previous findings of reduced immunostained junctional Cx43 in the aged rabbit atrium [7, 23]. Thus, JNK associated junctional Cx43 reduction could be responsible for the impaired cell-cell communication in the aged intact atrium.
JNK activation reduces junctional Cx43 and impairs intercellular coupling in cultured HL-1 myocytes
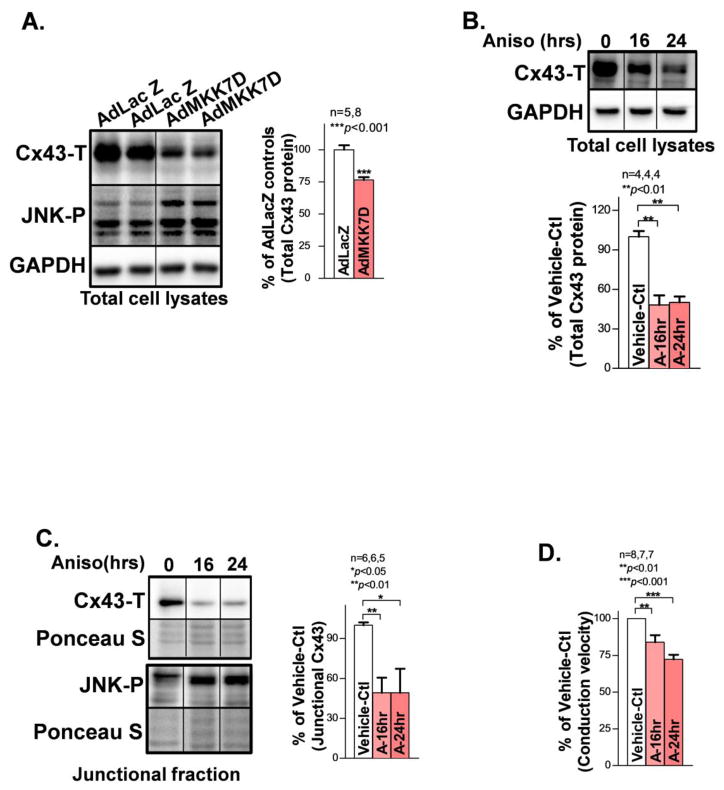
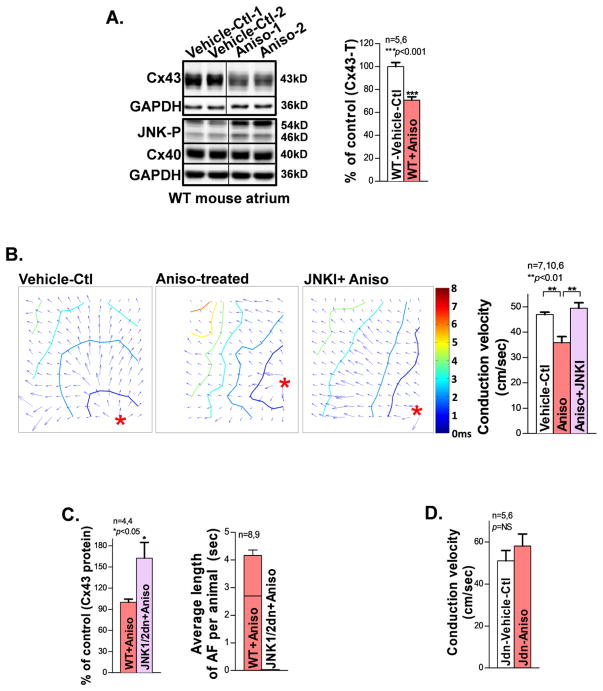
To assess the functional impact of JNK activation in atrial myocytes, we employed a well-characterized cultured HL-1 atrial myocyte line [7, 26]. We overexpressed constitutively activated JNK upstream regulator MKK7 with AdMKK7D (adenoviral-encoded constitutively active MKK7) or treated with the JNK activator anisomycin for 24hrs to activate JNK in cultured HL-1 myocytes. We found that JNK activation resulted in significantly reduced total Cx43 proteins compared to vehicle-controls (Figs.3A–3B). Moreover, reduced total Cx43 protein was associated with concomitantly decreased junctional Cx43 (Triton-X100 insoluble membrane fraction; Fig.3C) and slowed CV (Fig.3D) in JNK activated HL-1 myocytes (anisomycin-treated for 16hrs or 24hrs) vs. vehicle-controls. These results establish that JNK reduces junctional Cx43, which in turn impairs intercellular coupling between atrial myocytes.
Figure 3.
JNK reduces junctional Cx43 and impairs intercellular electrical coupling between myocytes. A. Immunoblotting images showing suppressed Cx43 in cultured HL-1 myocytes with overexpressed constitutively active JNK upstream activator MKK7D. B. Progressively reduced Cx43 proteins also found in JNK activated myocytes treated with anisomycin (Aniso) for 16hrs or 24hrs compared to vehicle-controls (Vehicle-Ctl). C. Representative immunoblotting images showing a reduced amount of junctional Cx43 in anisomycin-treated (16hrs, and 24hrs) HL-1 atrial myocytes compared to Vehicle-Ctl. Ponceau staining images suggest even loading between the immunoblotting samples. D. Summarized data of CV between the three groups analyzed from recorded optical mapping data in cultured HL-1 confluent monolayers.
JNK specific actions in Cx43 and intercellular electrical coupling in intact mouse atria; JNK inhibition reversed these changes
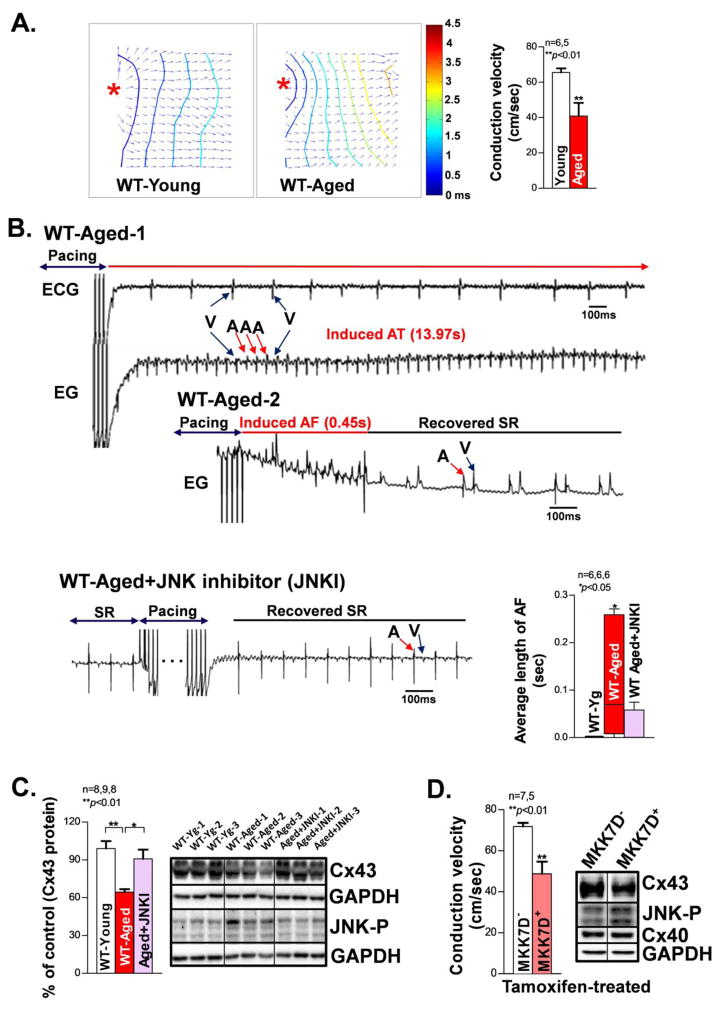
JNK-specific effects on atrial conduction in intact atria were assessed in optically mapped aged mouse hearts. We found that aged intact mouse atria exhibited significantly slowed CV, reflecting dampened propagation of membrane potentials in response to electrical pacing at a cycle length (CL) of 100 ms, as compared with young controls (Fig.4A). Moreover, these aged mice showed markedly increased duration of pacing induced AF using a unique in vivo intra-cardiac arrhythmia induction technique in live animals (Fig.4B). Furthermore, activated JNK was associated with significantly reduced Cx43 in these aged mouse atria (vs. young controls; Fig.4C), consistent with the results from aged human atria (Fig.1A) and aged rabbit atria (as we have shown previously) [7]. Strikingly, we discovered that JNK inhibition with a JNK inhibitor (JNKI) in aged WT mice (10 days in vivo treatment) significantly reduced pacing-induced AF and reversed Cx43 protein expression as compared with untreated aged WT mice (Figs.4B–4C, Supplemental_Fig. S3). Since the remodeling of gap junctions has been linked to the maintenance of arrhythmias [8, 9, 12, 13], JNK suppressed Cx43 in the aged atrium likely contributes to the increased duration of pacing-induced AF.
Figure 4.
Reduced Cx43, slower CV, and increased pacing-induced AF in JNK activated aged WT mice and cardiac-specific inducible MKK7D young mice. A. Representative isochronal maps from young and aged mouse intact atria subjected to pacing at a CL of 100ms (* indicates the pacing sites). Summarized optical mapping CV data show that aged mouse atria exhibited significantly slowed conduction compared to that of young controls. B. Representative electrograms (EG) and ECG traces of an episode of AT/AF (V=ventricular signal, A=atrial signal) induced by burst pacing (50Hz, 2sec) in two aged WT mice and sinus rhythm conversion right after burst pacing in a JNK inhibitor treated aged WT mouse. Pooled data of significantly increased average duration of pacing-induced AF in vivo per attempt per animal in aged WT mice compared to that of young controls. JNK inhibition strikingly attenuated this increased AF in the aged WT mice. C. Summarized data showing increased JNK activation is associated with reduced Cx43 protein in the aged mouse atria, while JNK inhibition markedly increased Cx43. D. Reduced Cx43 is associated with a slower CV in intact atria from cardiac specific inducible MKK7D young Tg mice (MKK7D+) with robustly activated JNK upon transient tamoxifen treatment compared to MKK7D negative littermates (MKK7D−) treated with tamoxifen.
Cardiac specific tamoxifen-inducible MKK7D Tg mice were studied to assess the functional role of activated JNK in the slowing of CV and Cx43 suppression, without interfering with other potential confounding factors in the aged heart [28]. Induction of constitutively activated MKK7, an upstream activator of JNK, led to significant atrial JNK activation and Cx43 downregulation as well as significantly slowed atrial CV in tamoxifen-treated MKK7D mice (Fig.4D). Similarly, treatment with the JNK activator anisomycin in WT young mice in vivo for 8 days resulted in a 30% decrease in Cx43 protein in the atrium versus vehicle-treated WT controls (Fig.5A). Moreover, CV in the anisomycin-treated WT mouse atrium slowed by 36% versus vehicle-treated WT at a pacing CL of 100ms (Fig.5B).
Figure 5.
Slowing of CV and increased pacing-induced AF in pharmacologically JNK activated young WT mice, while JNK inhibition abolished these changes. A. Immunoblotting images and pooled quantitative data of reduced Cx43 protein in JNK activator anisomycin-treated mouse atria compared to vehicle-controls (Vehicle-Ctl). B. Representative isochronal maps of intact atria subjected to pacing at a CL of 100ms (*indicates the pacing site) from vehicle-control and anisomycin-treated young WT mice with and without pretreatment of the JNK. Summarized CV data suggesting JNK inhibition prevented anisomycin-induced slowing of CV. C. Pooled data showing that JNK inhibition in cardiac-specific overexpression of inactive JNK1/2 dominant negative (Jdn) Tg mice prevented anisomycin-induced Cx43 down-regulation and enhanced AF propensity compared to anisomycin-treated WT littermates. D. Pooled optical mapping data of normal CV in anisomycin-treated JNK1/2dn (Jdn) mouse atria compared to that of untreated Vehicle-Ctl littermates.
To further establish causality between JNK-mediated Cx43 suppression and slowing of atrial conduction and AF, several approaches were used. First, we inhibited JNK in anisomycin-challenged young WT mice using the JNKI for in vivo pre-treatment and found that JNK inhibition completely abolished anisomycin-induced slow conduction (Fig.5B; far right) as compared with WT mice treated with anisomycin alone. Next, anisomycin effects were also assessed in cardiac-specific JNK1/2dn Tg mice with overexpression of inactive dominant-negative JNK1&2 proteins [21]. We found that JNK1/2 inhibition not only prevented anisomycin-mediated suppression of Cx43 protein and pacing-induced AF in live mice (Fig.5C), but also attenuated the slowing of CV in intact atria (Fig.5D). These results indicate that Cx43 suppression and conduction impairment are mediated by JNK, which are consistent with the changes found in the aged atrium. Taken together, our results establish that activated JNK suppresses Cx43, which in turn impairs intercellular coupling in the intact mouse atrium, and ultimately enhances AF susceptibility.
JNK suppressed Cx43 mRNA expression leads to impaired intercellular coupling
To understand how JNK suppresses Cx43, Cx43 mRNA was measured using real-time qPCR. With increasing age, human atria exhibited significantly reduced Cx43 mRNA expression (Fig.6A and Supplemental_Table. S3). Similarly, aged rabbit atria showed ~40% reduction in Cx43 mRNA compared to young controls (Fig.6B). These results are consistent with significantly decreased total Cx43 protein in the aged human (Fig.1B) and rabbit atria [7]. Moreover, AdMKK7D overexpression in cultured HL-1 myocytes also led to 45% reduction in Cx43 mRNA compared to AdLacZ-infected controls (Fig.6C), while comparable reduction of Cx43 proteins was also shown in Fig.3A.
Figure 6.
JNK leads to suppressed Cx43 mRNA expression and reduced AP-1 activity. A–C. Pooled quantitative qPCR data showing downregulated Cx43 mRNA expression in both aged human and rabbit atria as well as AdMKK7D overexpressed HL-1 myocytes compared to controls. D. Pooled quantitative immunoblotting data showing increased phosphorylation of JNK downstream transcription factor c-jun (c-jun-P) and unchanged ATF2 (ATF2-P) in AdMKK7D infected HL-1 atrial myocytes compared to AdLacZ-infected controls. E. Summarized data showing that AP-1 luciferase activity in anisomycin-treated HEK293 cells is positively correlated with Cx43 mRNA expression in anisomycin-treated HL-1 myocytes vs vehicle-controls (Vehicle-Ctl).
JNK regulates target gene expression via the downstream transcription factor AP-1 complex; c-jun and ATF2 are the important components within this complex [27, 29, 30]. We found that JNK activation was associated with substantially increased c-jun phosphorylation but unchanged ATF2 in AdMKK7D-infected HL-1 atrial myocytes (Fig.6D). This increased phosphorylation of c-jun was also consistently discovered in the aged rabbit (Supplemental_Figs. S4). Next, we transfected AP-1 luciferase vector in cultured HEK293 cells followed by anisomycin treatment for 24hrs. In response to JNK activation, AP-1 luciferase activity significantly decreased by 63% compared to vehicle-controls (Fig.6E). Studies indicate that short-term (<90min) treatment with anisomycin may suppress gene expression. However, we found that myocytes treated with anisomycin for 8hrs showed a 12% increase in AP-1 activity. Moreover, HL-1 cells treated with anisomycin for 8hrs exhibited slightly elevated Cx43 mRNA (Fig.6E) along with a slightly increased CV vs. vehicle-controls (Supplemental_Fig. S5). In contrast, reduced AP-1 activity along with attendant reduction of Cx43 mRNA (Fig.6E) and junctional proteins (Fig.3C) were associated with a significantly slowed CV in myocytes treated with anisomycin for 24hrs (Supplemental_Fig. S5). These results suggest that JNK-mediated Cx43 downregulation and reduced intercellular electrical coupling is a dynamic action and likely controlled through the JNK-regulated downstream transcription complex AP-1.
JNK downregulates Cx43 mRNA expression via c-jun suppressed Cx43 promoter activity
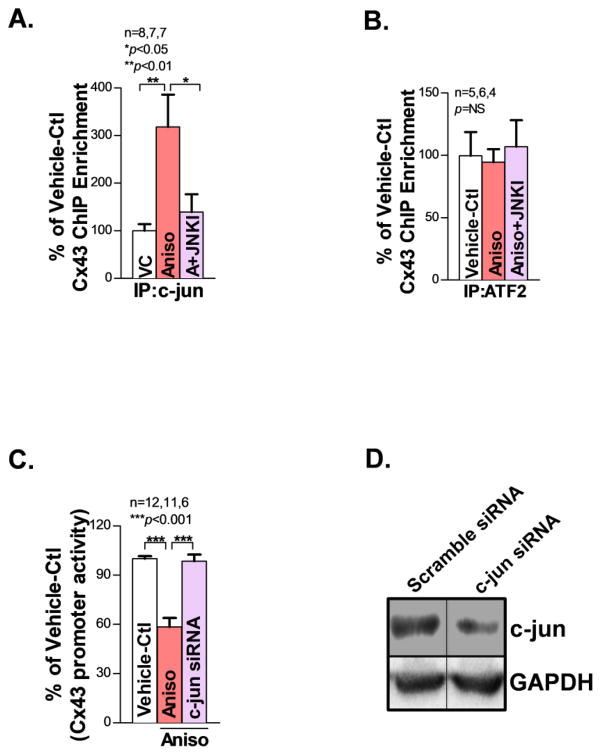
AP-1 regulates target gene expression by binding to or dissociating from the AP-1 consensus site(s) in the promoter region of target genes [27, 29, 30]. Since the Cx43 gene contains AP-1 binding sites in its promoter region [30], we first performed silico analysis of the mouse Cx43 genomic sequence and identified a highly conserved c-jun binding site 1210 bp at the upstream site of Cx43 translational start codon region. Next, we performed XChIP assays and discovered that c-jun not only directly binds the Cx43 promoter but also showed increased c-jun protein binding after anisomycin treatment in cultured HL-1 atrial myocytes as compared with vehicle-controls (Fig.7A). In contrast, JNK activation did not significantly alter the protein binding of ATF2 (another component of the AP-1 complex) to the Cx43 promoter, assessed using an ATF2 antibody pull-down XChIP assay (Fig.7B). Moreover, JNK inhibition using the JNKI significantly reduced binding of c-jun to the Cx43 promoter as assessed by XChIP assay (Fig.7A), while ATF2 binding was not affected (Fig.7B, far right bar). These results establish that JNK enhances c-jun binding to the Cx43 promoter.
Figure 7.
JNK down-regulates Cx43 expression via c-jun suppressed Cx43 gene promoter activity. A–B. Pooled data of ChIP assay showing markedly increased binding of c-jun protein but unchanged ATF2 to the Cx43 promoter in anisomycin-treated HL-1 atrial myocytes compared to vehicle-controls (VC or Vehicle-Ctl). Summarized data of XChIP assay showing that JNK inhibition markedly reduced immunoprecipitated c-jun to the Cx43 promoter (A, far right bar), but not ATF2 (B, far right bar). C. Significantly reduced Cx43 promoter activity in anisomycin(Aniso)-treated (JNK activated) HEK293 cells but c-jun siRNA knockdown (A+c-jun siRNA) reversed Cx43 promoter activity to the normal baseline. Both vehicle-control and anisomycin-treated cells were transfected with the scramble sRNA. D. Immunoblotting images showing the effective c-jun protein knockdown evidenced by significantly reduced c-jun proteins in c-jun siRNA transfected HEK293 cells compared to scramble siRNA-transfected controls.
To assess whether this JNK transcriptionally regulates Cx43 gene promoter activity, we inserted 1181 bp of Cx43 genomic sequence encompassing the putative AP-1 binding sites [30] upstream of a firefly luciferase reporter vector. As shown in Fig.7C, the activity of firefly luciferase downstream of Cx43 promoter was significantly reduced (by 45%) in HEK293 cells treated with anisomycin for 24hrs versus untreated controls. Strikingly, c-jun siRNA knockdown completely reversed anisomycin-mediated suppression of Cx43 promoter activity (Fig.7C and Fig.7D). Taken together, our data demonstrate that long-term JNK activation downregulates Cx43 expression via c-jun-mediated suppression of the Cx43 promoter.
Discussion
Here we report for the first time that JNK activation and Cx43 suppression occur in the human atrium with increasing age. We further provide direct evidence of JNK-mediated Cx43 downregulation and impaired intercellular coupling in the intact atrium. Moreover, we reveal an underlying molecular mechanism showing that JNK downregulates Cx43 gene expression via enhanced binding of c-jun proteins to the Cx43 promoter, which in turn suppresses Cx43 gene promoter activity. Finally, functional rescue with JNK inhibition suggests that JNK modulation could be a novel therapeutic approach to improve atrial conduction and alleviate AF.
Causal connection between JNK, impaired intercellular coupling, and AF in the intact atrium
The role of JNK in cell growth and survival is well established. Emerging evidence suggests a functional impact of activated JNK on slowed CV [31, 32]. Our laboratory reported for the first time that activated JNK is linked to enhanced AF propensity in the aged rabbit heart [7]. However, direct functional evidence remains lacking for the role of JNK in intercellular coupling and AF in aged intact atria. It is known that intercellular coupling critically determines the speed of action potential propagation within atrial myocardium. Traditional intercellular coupling studies generally use isolated myocyte pairs. However, isolated myocytes lack the spatial integrity of electrically coupled neighboring myocytes within an intact three-dimensional myocardial structure. The optically mapped space constant measurement developed by Akar and Rosenbaum [25] provides a unique way to assess intercellular coupling that can be tailored to focus on a small region in the intact myocardium. In the current studies, we applied this technique and discovered that JNK activation is indeed linked to impaired intercellular coupling along with slowed CV in the aged intact atrium (Fig.2A–2C & Supplemental_Fig. S2A). This was further supported by findings of slowed CV in the aged mouse atrium (Fig.4A).
Besides gap junctional remodeling, sodium channels and interstitial fibrosis also contribute to conduction velocity. We and other groups have previously reported unchanged interstitial fibrosis and unaltered AP dV/dtMax along with sodium channel expression in the aged atria [7, 23, 33]. Note that these aged atria were obtained from healthy aged hearts (i.e. those with normal cardiac function, no AF history or any major cardiac disease. Further, direct Na current measurements by Dr. Boyden’s group indicate there is unchanged sodium channel function in aged canine atrial myocytes [34]. Thus, our findings compliment those findings reported by others and us and suggest Cx43 downregulation plays a critical role in slowing conduction velocity.
Aging is a known risk factor for AF in humans, however there are no clear age-related molecular pathways known to promote AF. The MAPKs are composed of a family of signaling cascades, which act as critical regulators of cell survival and growth in response to both intrinsic and extrinsic stress challenges [5]. In response to stress, both JNK and p38 pathways, the two major members of the MAPK family, could be activated [6, 25]. However, JNK and p38 have opposite functions (activation or suppression) in cellular senescence, which are dependent on cellular context and cell type [35–37]. Accumulating evidence suggests that aged hearts exhibit increased intrinsic stress and higher susceptibility to extrinsic stress stimuli [38, 39]. While altered JNK and p38 have been reported in aged ventricular muscle preparations, our current and previous findings indicate enhanced JNK activation but unchanged p38 in the aged human (Supplemental_Fig. S1E–S1F) and rabbit atrium [7, 40]. This JNK activation was linked to enhanced phosphorylation of its upstream regulators MKK4 and MKK7 (Supplemental_Fig. S1C, D) as suggested by previous studies [7, 41, 42]. However, other age-related confounding factors could also be involved in the atrial remodeling. Therefore, the casual relationship between JNK and AF was further established in the current studies using several unique Tg mouse models with cardiac-specific inhibition or activation of JNK. Our data establish that JNK critically impairs intercellular coupling that in turn results in slowing of atrial conduction and enhanced AF susceptibility. Moreover, the rescue effect of JNK inhibition, using either a genetic or in vivo pharmacological approach, demonstrate a causal connection between JNK activity and atrial arrhythmogenic remodeling.
JNK-induced Cx43 suppression in slowed CV and AF maintenance
Atrial gap junction channels predominantly contain Cx40 and Cx43 [43]. While conflicting results of atrial CV were found in a mouse model with Cx40 deletion [44–47], results from human atria [8] and synthesized cultured strands of atrial myocytes from Cx40 knockout and Cx43 knockout mice [48] all suggest that the decreased ratio of Cx43 to total connexins correlated with slowed CV, while a decreased Cx40 ratio was linked to increased CV. In addition, reduced Cx43 caused by Cx43 gene mutation was reported to have slowed atrial conduction and enhanced AF inducibility [7, 8, 44, 48]. Moreover, we demonstrate that activated JNK reduces junctional Cx43 that leads to slowed CV and increased atrial arrhythmogenicity in the aged heart, while JNK inhibition with a JNK specific inhibitor completely reversed age-associated Cx43 reduction and AF propensity (Fig.4B).
Results of MKK7-mediated JNK activation (either MKK7D Tg mice or AdMKK7D infected cultured myocytes) in young mice further support a fundamental link between JNK-mediated Cx43 downregulation and slowed atrial CV. In addition, these JNK specific actions, independent of other potential confounding factors in the aged heart, were further confirmed in JNK activator anisomycin-treated WT young mice and JNK inhibited JNK1/2dn mice. While our current data demonstrated similar JNK actions in anisomycin-challenged preparations as seen in the aged heart, we are aware that anisomycin can also activate the p38 MAPK pathway as Ogawa et al [49] reported in a rat-liver-derived epithelial cell line. However, activation of JNK and p38 are known to be cellular context and type dependent [37]. With a comparable level of JNK activation in the model system (anisomycin and MKK7D) and aged hearts, the striking rescue effects observed with JNK specific inhibition using genetic or pharmacological JNK inhibition in either aged mice or JNK activator anisomycin-treated JNK1/2dn young mice provided strong evidence for the JNK-specific regulation of Cx43 expression. This is consistent with the results from anisomycin-treated neonatal rat ventricular myocytes [18]. Moreover, our previous findings of improved CV with either JNK inhibition using a JNK specific inhibitor or wild-type Cx43 overexpression with a reversal of slowed conduction between JNK activated HL-1 myocytes also support a causative role for JNK-mediated Cx43 downregulation in the pathogenesis of impaired atrial CV [7]. Taken together, our results establish that age-related impairment of atrial conduction is dependent upon JNK-specific suppression of Cx43 expression and resultant functional abnormalities that lead to increased AF propensity.
Novel molecular mechanism underlying JNK-mediated suppression of Cx43
In general, JNK regulates downstream gene expression through the AP-1 complex that induces target gene expression by binding the AP-1 consensus site(s) in the promoter region of target genes [29]. The AP-1 transcription complex has been shown to regulate the basal level of Cx43 gene expression in neonatal rat myocytes [30]. AP-1 luciferase activity in vitro assay in 24hrs Aniso-treated cells revealed a reduced activity of AP-1. However, 8hrs Aniso-treated cells showed a 12% increase in AP-1 activity, which was associated with slightly elevated Cx43 mRNA. These results not only support the important role of JNK in Cx43 expression but also demonstrate a negative regulation of long-term JNK activation in Cx43 expression. While another report tested the ramifications of JNK inhibition on Cx43 expression [50], how JNK regulates Cx43 promoter activity in myocytes has not been reported to date. Here, we show for the first time that long-term JNK activation increases the binding of c-jun, but not ATF2, to the Cx43 promoter. And, this JNK-enhanced binding of c-jun to the Cx43 promoter is critical in the Cx43 promoter activity, as the JNK specific ablation (either JNK inhibition or JNK downstream target c-jun knockdown) completely prevented this aniso-mediated suppression of Cx43 promoter activity. Collectively, we have established a novel molecular mechanism underlying JNK-mediated downregulation of Cx43 through c-jun-dependent inhibition of the Cx43 gene promoter.
Conclusions and future implications
Enhanced susceptibility of AF is a serious health problem in the aging population and to date, pharmacological AF treatment and prevention strategies for the elderly remain ineffective. The current study demonstrates a causal link between JNK and impaired atrial cell-cell communication and ultimately increased propensity for atrial arrhythmias. We also discovered a novel underlying molecular mechanism of JNK suppressed Cx43 gene expression that contributes to the atrial arrhythmogenic remodeling. Our current results point to a critical role for c-jun in Cx43 expression and, expose a need to explore the responsible c-jun binding sites as well as to understand the detailed relationship between enhanced c-jun binding and other co-transcription factors in aging and possibly other pathological conditions.
Taken together, our results suggest that manipulation of JNK signaling may represent a novel therapeutic target that might be exploited to prevent and treat AF in aged or diseased hearts. Success of any pharmacological approach would require JNK and/or tissue specificity. Further, AF may involve atrial remodeling (molecular or structural) as well as various comorbid cardiac conditions. These factors individually or in concert could conceivably influence the dynamics and/or extent of JNK activation. Although many unknowns clearly remain, our findings present a new and interesting potential therapeutic target for addressing AF.
Supplementary Material
Highlights.
Augmented JNK in aged human and animal atria impairs cell coupling and promotes AF.
JNK suppresses Cx43 expression via increased binding of c-jun to the Cx43 promoter.
JNK inhibition may represent a novel therapeutic approach to prevent or treat AF.
Acknowledgments
Funding
This research was supported by American Heart Association (10GRNT3770030 to XA) and National Institutes of Health grants HL113640 & AA024769 (to XA) and HL99014 (to SDP).
We graciously thank Dr. Yibin Wang for his generous gift of MKK7D Tg mice and Mr. Dennis Rollins for his excellent technical assistance.
List of abbreviation
- AF
atrial fibrillation
- Aniso (A)
anisomycin
- Ad
adenoviral
- AP-1
activator protein-1
- CL
cycle length
- CV
conduction velocity
- Cx43
connexin43
- HF
heart failure
- JNK
c-jun N-terminal kinase
- JNKI
JNK inhibitor
- JNK1/2dn
dominant negative mutation of JNK1 and JNK2 proteins
- MAPK
mitogen-activated protein kinase
- MKK7D
constitutively activated mitogen-activated protein kinase kinase 7 with a replacement of S271 and T275 with D (aspartic acid)
- PW
pulse width
- Tg
transgenic
- WT
wild-type
- XChIP
cross-linked chromatin-immunoprecipitation
Footnotes
Disclosures
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114(2):119–25. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 3.Rich MW. Epidemiology of atrial fibrillation. J Interv Card Electrophysiol. 2009;25(1 ):3–8. doi: 10.1007/s10840-008-9337-8. [DOI] [PubMed] [Google Scholar]
- 4.Wolf PA, Benjamin EJ, Belanger AJ, Kannel WB, Levy D, D’Agostino RB. Secular trends in the prevalence of atrial fibrillation: The Framingham Study. Am Heart J. 1996;131(4):790–5. doi: 10.1016/s0002-8703(96)90288-4. [DOI] [PubMed] [Google Scholar]
- 5.Rose BA, Force T, Wang Y. Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale. Physiol Rev. 2010;90(4):1507–46. doi: 10.1152/physrev.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karin M, Gallagher E. From JNK to pay dirt: jun kinases, their biochemistry, physiology and clinical importance. IUBMB Life. 2005;57(4–5):283–95. doi: 10.1080/15216540500097111. [DOI] [PubMed] [Google Scholar]
- 7.Yan J, Kong W, Zhang Q, Beyer EC, Walcott G, Fast VG, et al. c-Jun N-terminal kinase activation contributes to reduced connexin43 and development of atrial arrhythmias. Cardiovascular research. 2013;97(3):589–97. doi: 10.1093/cvr/cvs366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanagaratnam P, Rothery S, Patel P, Severs NJ, Peters NS. Relative expression of immunolocalized connexins 40 and 43 correlates with human atrial conduction properties. J Am Coll Cardiol. 2002;39(1):116–23. doi: 10.1016/s0735-1097(01)01710-7. [DOI] [PubMed] [Google Scholar]
- 9.Saffitz JE, Davis LM, Darrow BJ, Kanter HL, Laing JG, Beyer EC. The molecular basis of anisotropy: role of gap junctions. J Cardiovasc Electrophysiol. 1995;6(6):498–510. doi: 10.1111/j.1540-8167.1995.tb00423.x. [DOI] [PubMed] [Google Scholar]
- 10.Ai X, Pogwizd SM. Connexin 43 downregulation and dephosphorylation in nonischemic heart failure is associated with enhanced colocalized protein phosphatase type 2A. Circ Res. 2005;96(1):54–63. doi: 10.1161/01.RES.0000152325.07495.5a. [DOI] [PubMed] [Google Scholar]
- 11.Ai X, Zhao W, Pogwizd SM. Connexin43 knockdown or overexpression modulates cell coupling in control and failing rabbit left ventricular myocytes. Cardiovascular research. 2010;85(4):751–62. doi: 10.1093/cvr/cvp353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Velden HM, Ausma J, Rook MB, Hellemons AJ, van Veen TA, Allessie MA, et al. Gap junctional remodeling in relation to stabilization of atrial fibrillation in the goat. Cardiovascular research. 2000;46(3):476–86. doi: 10.1016/s0008-6363(00)00026-2. [DOI] [PubMed] [Google Scholar]
- 13.Duffy HS, Wit AL. Is there a role for remodeled connexins in AF? No simple answers. J Mol Cell Cardiol. 2008;44(1):4–13. doi: 10.1016/j.yjmcc.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones SA, Lancaster MK, Boyett MR. Ageing-related changes of connexins and conduction within the sinoatrial node. J Physiol. 2004;560(Pt 2):429–37. doi: 10.1113/jphysiol.2004.072108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhein S, Hammerath SB. Aspects of the intercellular communication in aged hearts: effects of the gap junction uncoupler palmitoleic acid. Naunyn Schmiedebergs Arch Pharmacol. 2001;364(5):397–408. doi: 10.1007/s002100100462. [DOI] [PubMed] [Google Scholar]
- 16.Oral H, Scharf C, Chugh A, Hall B, Cheung P, Good E, et al. Catheter ablation for paroxysmal atrial fibrillation: segmental pulmonary vein ostial ablation versus left atrial ablation. Circulation. 2003;108(19):2355–60. doi: 10.1161/01.CIR.0000095796.45180.88. [DOI] [PubMed] [Google Scholar]
- 17.Pappone C, Oreto G, Rosanio S, Vicedomini G, Tocchi M, Gugliotta F, et al. Atrial electroanatomic remodeling after circumferential radiofrequency pulmonary vein ablation: efficacy of an anatomic approach in a large cohort of patients with atrial fibrillation. Circulation. 2001;104(21):2539–44. doi: 10.1161/hc4601.098517. [DOI] [PubMed] [Google Scholar]
- 18.Petrich BG, Gong X, Lerner DL, Wang X, Brown JH, Saffitz JE, et al. c-Jun N-terminal kinase activation mediates downregulation of connexin43 in cardiomyocytes. Circ Res. 2002;91(7):640–7. doi: 10.1161/01.res.0000035854.11082.01. [DOI] [PubMed] [Google Scholar]
- 19.Angell RM, Atkinson FL, Brown MJ, Chuang TT, Christopher JA, Cichy-Knight M, et al. N-(3-Cyano-4,5,6,7-tetrahydro-1-benzothien-2-yl)amides as potent, selective, inhibitors of JNK2 and JNK3. Bioorganic & medicinal chemistry letters. 2007;17(5):1296–301. doi: 10.1016/j.bmcl.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Bennett BL, Sasaki DT, Murray BW, O’Leary EC, Sakata ST, Xu W, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci U S A. 2001;98(24):13681–6. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang Q, Bueno OF, Wilkins BJ, Kuan CY, Xia Y, Molkentin JD. c-Jun N-terminal kinases (JNK) antagonize cardiac growth through cross-talk with calcineurin-NFAT signaling. EMBO J. 2003;22(19):5079–89. doi: 10.1093/emboj/cdg474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruce AF, Rothery S, Dupont E, Severs NJ. Gap junction remodelling in human heart failure is associated with increased interaction of connexin43 with ZO-1. Cardiovascular research. 2008;77(4):757–65. doi: 10.1093/cvr/cvm083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan J, Thomson JK, Wu X, Zhao W, Pollard AE, Ai X. Novel methods of automated quantification of gap junction distribution and interstitial collagen quantity from animal and human atrial tissue sections. PLoS One. 2014;9(8):e104357. doi: 10.1371/journal.pone.0104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, et al. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009;119(7):1940–51. doi: 10.1172/JCI37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akar FG, Roth BJ, Rosenbaum DS. Optical measurement of cell-to-cell coupling in intact heart using subthreshold electrical stimulation. Am J Physiol Heart Circ Physiol. 2001;281(2):H533–42. doi: 10.1152/ajpheart.2001.281.2.H533. [DOI] [PubMed] [Google Scholar]
- 26.Yan J, Thomson JK, Zhao W, Fast VG, Ye T, Ai X. Voltage and calcium dual channel optical mapping of cultured HL-1 atrial myocyte monolayer. J Vis Exp. 2015;(97) doi: 10.3791/52542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Wu S, Ma J, Xia Y, Ai X, Sun J. Bacterial protein AvrA stabilizes intestinal epithelial tight junctions via blockage of the C-Jun N-terminal kinase pathway. Tissue Barriers. 2015;3(1–2):e972849. doi: 10.4161/21688362.2014.972849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petrich BG, Molkentin JD, Wang Y. Temporal activation of c-Jun N-terminal kinase in adult transgenic heart via cre-loxP-mediated DNA recombination. FASEB J. 2003;17(6):749–51. doi: 10.1096/fj.02-0438fje. [DOI] [PubMed] [Google Scholar]
- 29.Gius D, Botero A, Shah S, Curry HA. Intracellular oxidation/reduction status in the regulation of transcription factors NF-kappaB and AP-1. Toxicol Lett. 1999;106(2–3):93–106. doi: 10.1016/s0378-4274(99)00024-7. [DOI] [PubMed] [Google Scholar]
- 30.Teunissen BE, Jansen AT, van Amersfoorth SC, O’Brien TX, Jongsma HJ, Bierhuizen MF. Analysis of the rat connexin 43 proximal promoter in neonatal cardiomyocytes. Gene. 2003;322:123–36. doi: 10.1016/j.gene.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Petrich BG, Eloff BC, Lerner DL, Kovacs A, Saffitz JE, Rosenbaum DS, et al. Targeted activation of c-Jun N-terminal kinase in vivo induces restrictive cardiomyopathy and conduction defects. J Biol Chem. 2004;279(15):15330–8. doi: 10.1074/jbc.M314142200. [DOI] [PubMed] [Google Scholar]
- 32.Tacheau C, Laboureau J, Mauviel A, Verrecchia F. TNF-alpha represses connexin43 expression in HaCat keratinocytes via activation of JNK signaling. J Cell Physiol. 2008;216(2):438–44. doi: 10.1002/jcp.21412. [DOI] [PubMed] [Google Scholar]
- 33.Platonov PG, Mitrofanova LB, Orshanskaya V, Ho SY. Structural abnormalities in atrial walls are associated with presence and persistency of atrial fibrillation but not with age. J Am Coll Cardiol. 2011;58(21):2225–32. doi: 10.1016/j.jacc.2011.05.061. [DOI] [PubMed] [Google Scholar]
- 34.Baba S, Dun W, Hirose M, Boyden PA. Sodium current function in adult and aged canine atrial cells. Am J Physiol Heart Circ Physiol. 2006;291(2):H756–61. doi: 10.1152/ajpheart.00063.2006. [DOI] [PubMed] [Google Scholar]
- 35.Wada T, Stepniak E, Hui L, Leibbrandt A, Katada T, Nishina H, et al. Antagonistic control of cell fates by JNK and p38-MAPK signaling. Cell Death Differ. 2008;15(1):89–93. doi: 10.1038/sj.cdd.4402222. [DOI] [PubMed] [Google Scholar]
- 36.Das M, Jiang F, Sluss HK, Zhang C, Shokat KM, Flavell RA, et al. Suppression of p53-dependent senescence by the JNK signal transduction pathway. Proc Natl Acad Sci U S A. 2007;104(40):15759–64. doi: 10.1073/pnas.0707782104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maruyama J, Naguro I, Takeda K, Ichijo H. Stress-activated MAP kinase cascades in cellular senescence. Curr Med Chem. 2009;16(10):1229–35. doi: 10.2174/092986709787846613. [DOI] [PubMed] [Google Scholar]
- 38.Yang Z, Shen W, Rottman JN, Wikswo JP, Murray KT. Rapid stimulation causes electrical remodeling in cultured atrial myocytes. J Mol Cell Cardiol. 2005;38(2):299–308. doi: 10.1016/j.yjmcc.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 39.Juhaszova M, Rabuel C, Zorov DB, Lakatta EG, Sollott SJ. Protection in the aged heart: preventing the heart-break of old age? Cardiovascular research. 2005;66(2):233–44. doi: 10.1016/j.cardiores.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 40.Peart JN, Gross ER, Headrick JP, Gross GJ. Impaired p38 MAPK/HSP27 signaling underlies aging-related failure in opioid-mediated cardioprotection. J Mol Cell Cardiol. 2007;42(5):972–80. doi: 10.1016/j.yjmcc.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bogoyevitch MA. The isoform-specific functions of the c-Jun N-terminal Kinases (JNKs): differences revealed by gene targeting. Bioessays. 2006;28(9):923–34. doi: 10.1002/bies.20458. [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Destrument A, Tournier C. Physiological roles of MKK4 and MKK7: insights from animal models. Biochim Biophys Acta. 2007;1773(8):1349–57. doi: 10.1016/j.bbamcr.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 43.Verheule S, van Kempen MJ, te Welscher PH, Kwak BR, Jongsma HJ. Characterization of gap junction channels in adult rabbit atrial and ventricular myocardium. Circ Res. 1997;80(5):673–81. doi: 10.1161/01.res.80.5.673. [DOI] [PubMed] [Google Scholar]
- 44.Tuomi JM, Tyml K, Jones DL. Atrial Tachycardia/Fibrillation in the Connexin 43 G60S Mutant (Oculodentodigital Dysplasia) Mouse. Am J Physiol Heart Circ Physiol. 2011 doi: 10.1152/ajpheart.01094.2010. [DOI] [PubMed] [Google Scholar]
- 45.Hagendorff A, Schumacher B, Kirchhoff S, Luderitz B, Willecke K. Conduction disturbances and increased atrial vulnerability in Connexin40-deficient mice analyzed by transesophageal stimulation. Circulation. 1999;99(11):1508–15. doi: 10.1161/01.cir.99.11.1508. [DOI] [PubMed] [Google Scholar]
- 46.Bagwe S, Berenfeld O, Vaidya D, Morley GE, Jalife J. Altered right atrial excitation and propagation in connexin40 knockout mice. Circulation. 2005;112(15):2245–53. doi: 10.1161/CIRCULATIONAHA.104.527325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verheule S, van Batenburg CA, Coenjaerts FE, Kirchhoff S, Willecke K, Jongsma HJ. Cardiac conduction abnormalities in mice lacking the gap junction protein connexin40. J Cardiovasc Electrophysiol. 1999;10(10):1380–9. doi: 10.1111/j.1540-8167.1999.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 48.Beauchamp P, Yamada KA, Baertschi AJ, Green K, Kanter EM, Saffitz JE, et al. Relative contributions of connexins 40 and 43 to atrial impulse propagation in synthetic strands of neonatal and fetal murine cardiomyocytes. Circ Res. 2006;99(11):1216–24. doi: 10.1161/01.RES.0000250607.34498.b4. [DOI] [PubMed] [Google Scholar]
- 49.Ogawa T, Hayashi T, Kyoizumi S, Kusunoki Y, Nakachi K, MacPhee DG, et al. Anisomycin downregulates gap-junctional intercellular communication via the p38 MAP-kinase pathway. J Cell Sci. 2004;117(Pt 10):2087–96. doi: 10.1242/jcs.01056. [DOI] [PubMed] [Google Scholar]
- 50.Wu X, Huang W, Luo G, Alain LA. Hypoxia induces connexin 43 dysregulation by modulating matrix metalloproteinases via MAPK signaling. Mol Cell Biochem. 2013;384(1–2):155–62. doi: 10.1007/s11010-013-1793-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.