Abstract
People’s ability to perceive rapidly presented targets can be disrupted both by voluntary encoding of a preceding target and by spontaneous attention to salient distractors. Distinctions between these sources of interference can be found when people search for a target in multiple rapid streams instead of a single stream: voluntary encoding of a preceding target often elicits subsequent perceptual lapses across the visual field, whereas spontaneous attention to emotionally salient distractors appears to elicit a spatially localized lapse, giving rise to a theoretical account suggesting that emotional distractors and subsequent targets compete spatiotemporally during rapid serial visual processing. We used gaze-contingent eye-tracking to probe the roles of spatiotemporal competition and memory encoding on the spatial distribution of interference caused by emotional distractors, while also ruling out the role of eye-gaze in driving differences in spatial distribution. Spontaneous target perception impairments caused by emotional distractors were localized to the distractor location regardless of where participants fixated. But when emotional distractors were task-relevant, perceptual lapses occurred across both streams while remaining strongest at the distractor location. These results suggest that spatiotemporal competition and memory encoding reflect a dual-route impact of emotional stimuli on target perception during rapid visual processing.
The mechanisms that drive visual awareness act fast, with people able to detect targets that flash by for a mere 13 milliseconds (Potter, Wyble, Hagmann, & McCourt, 2014). Yet, such mechanisms can be easily disrupted. For example, in the widely studied attentional blink, reporting of one target within a rapid serial visual stream impairs people’s abilities to report a second target that follows soon after (Chun & Potter, 1995; Raymond, Shapiro, & Arnell 1992). Phenomenally similar disruptions are caused by attention grabbing distractors even when people are not meant to report them (Folk, Leber, & Egeth, 2008; Maki & Mebane, 2006; Spalek, Falcon, & Di Lollo, 2006; Visser, Bischof, & Di Lollo, 2004). This spontaneous impact appears to be particularly enhanced when the distractors are emotionally powerful, an effect known as emotion-induced blindness (EIB; Arnell, Killman, & Fijavz, 2007; Most, Chun, Widders, & Zald, 2005; Most & Wang, 2011; Wang, Kennedy, & Most, 2012).
Insight into the mechanisms underlying such disruptions (and thus into the mechanisms underlying rapid perception) might be gained by noting distinctions between such phenomenally similar effects. For example, whereas the attentional blink has sometimes been found to extend across the visual field (Lunau & Olivers, 2010; but see Kristjansson & Nakayama, 2002), spontaneous disruptions caused by featurally salient distractors appear to be greater when the distractors appear away from – rather than at – the location of the target (e.g., Moore & Weissman, 2011). Meanwhile, spontaneous disruptions caused by emotional distractors have been found to exhibit the opposite pattern: they are particularly robust when targets and distractors appear in the same location as each other. In one study, participants monitored two simultaneous rapid serial streams of images for a single target image, and an emotional distractor could appear either in the same stream as the target or in the opposite stream. Target disruption caused by the emotional picture – EIB – occurred primarily when the target and distractor appeared in the same stream as each other, a spatial localization that was not apparent following non-emotional distractors (Most & Wang, 2011).
Based on this spatially localized pattern, EIB has been proposed to reflect relatively early spatiotemporal competition between a target and distractor, with emotional distractors dominating due to tendencies to prioritize emotional information (Wang et al., 2012). This is distinct from theoretical accounts of the attentional blink (AB), which have largely converged on the notion that the AB stems from relatively late or central processing stages, such as visual working memory interference or disruption of a top-down target template (e.g., Chun & Potter, 1995; Di Lollo, Kawahara, Ghorashi, & Enns, 2005; Shapiro, Raymond, & Arnell, 1994). These processing stages may come into play, as well, in attentional blink tasks that incorporate emotional stimuli as targets; for example, the AB has been found to be larger when the first target is an emotional stimulus (Schwabe & Wolf, 2010; Schwabe et al., 2011; Ihssen & Keil, 2009).
Notably, this distinction appears to map onto an independently developed model of attentional dynamics within rapid serial presentations (Wyble & Swan, 2015). According to this model, perceptual failures can stem from several information processing bottlenecks. For example, stimuli that appear close in time and in the same location compete with each other in a mutually suppressive manner, as they compete to drive the neural response of a shared receptive field (Desimone & Duncan, 1995; Keysers & Perrett, 2002). In this case, stimuli with particular salience (such as emotional stimuli) can gain the competitive edge, and this “competitive interference” yields spatially localized perceptual deficits such as those found in EIB. In contrast, when stimuli are selected for encoding into visual working memory, as is necessary when people report the first target in the AB, this process causes a suppression of attention across the visual field (Wyble & Swan, 2015).
It is important to note, however, that although the spatially localized nature of EIB might support a spatiotemporal competition account of spontaneous interference, which may be distinguishable from the impact of a distractor that is task-relevant, a plausible alternative is that such a pattern arises as a function of where participants look. In this scenario, participants may only be registering one stream of images at a time (and neglecting the other stream), which would result in a pattern of performance strikingly similar to the results that were observed for EIB (see Figure 1). This alternative explanation assumes that unless stimuli are fixated and attended, the images will not be processed. While this assumption goes against findings that show that emotional stimuli are processed even when not fixated or goal-relevant (e.g., MacLeod, Mathews, & Tata, 1986), it is possible that the fast presentation of complex stimuli presented in two simultaneous streams makes the task demands too difficult to monitor both streams at the same time.
Figure 1. Alternative account predictions.
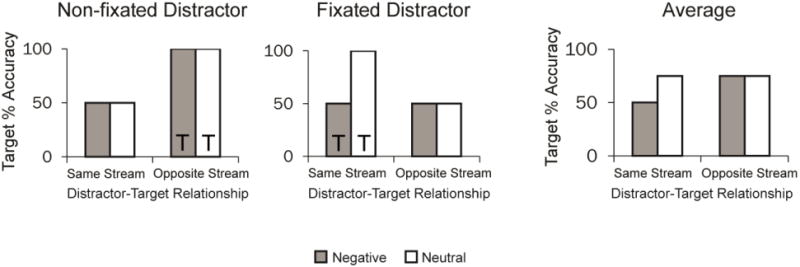
Predicted results according to an account that the spatial specific impairment in emotion-induced blindness results from participants fixating at only one stream at a time. The streams in which targets appear are represented with a T. See text for details.
It is also worth noting that previous AB studies have not found a spatially localized interference, suggesting that participants tend to look at multiple locations during RSVP tasks (e.g., Lunau & Olivers, 2010). These findings suggest that the overall spatial pattern in AB primarily reflects a later stage of working memory – rather than an interference at an earlier stage of representational processing – since impairment occurs no matter the spatial relationship between two targets. However, rather than simple alphanumeric characters traditionally used in AB studies, complex stimuli like images or words are typically used in EIB studies, making it possible that participants may attend to only one stream at a time with more complex stimuli in an AB or EIB task.
To illustrate the alternative account that non-fixated images are not processed, consider the case in which participants fixate only one of two streams of images and the distractor appears in that stream (such that the distractor is “fixated”; see middle panel of Figure 1). In this case, when participants are fixating at the location of the distractor, targets that appear in the same stream as the distractor should elicit the typical pattern of emotion-induced blindness (like the single stream version of this effect), while targets that appear in the opposite stream would likely be missed altogether and accuracy for reporting the target would be at chance (because participants are not fixating the stream where the target appears). Alternatively, consider the case in which participants are fixating the stream of images where the distractor does not appear (left panel in Figure 1). Targets that appear in the same stream as the distractor will likely be missed and accuracy would be at chance (because participants are not attending to that stream), but targets that appear in the opposite stream from the distractor will be well-reported, since participants were fixating on that stream and likely did not process the distractor in the other stream. Altogether, if performance in the two-stream EIB task was based on participants fixating on just one stream at a time, averaging across conditions where the distractor appeared in the stream participants were fixating and not fixating would yield results that make it seem that impairment from emotional stimuli is limited to the “same-stream condition” (right panel in Figure 1). Thus, the predictions in this account match the spatially localized pattern usually observed in two-stream versions of EIB. Lending credence to this alternative account, average baseline performance (when no distractor is presented) in correctly reporting the target rotation in the two-stream EIB task tends to be around 75% (e.g., Most & Wang, 2011). As chance performance is 50% and perfect performance is 100%, baseline performance should average to around 75% if performance is a result of participants fixating to only one stream. As such, the current research cannot differentiate between the accounts of fixating to one stream at a time and the spatiotemporal competition account.
To tease apart these two potential accounts of the spatially localized impairment caused by emotional distractors, we used gaze-contingent stimulus presentation to manipulate where distractors and targets appeared in relation to participants’ eye-gaze. By placing the distractor in a specific position relative to a participant’s fixation location, we were able to control, at the very point at which the distractor appeared, whether it was placed in the fixated stream of images or the non-fixated stream of images. We chose to use a gaze-contingent approach to place distractors based on where participants were fixating (rather than, say, have them attend only to the central region between the two streams) for two reasons. First, the gaze-contingent approach encouraged participants to freely view the stimuli and give little reason to separate their overt and covert attention (which are usually tightly coupled; see Deubel & Schneider, 1996), whereas focusing on the center of the streams would encourage a separation between where participants attended and where they kept their gaze. Second, the gaze-contingent approach limited additional task demands, such that participants did not have to maintain fixation in a certain place and simultaneously attend to the rapid streams. While the spatiotemporal competition account would predict impairments in both the fixated and non-fixated stream conditions, the alternative fixate-to-one-stream account would instead predict impairments only in the fixated stream condition, and not in the non-fixated stream condition (as illustrated in Figure 1).
Previous studies of EIB have demonstrated that the spatially localized impairment is stronger for negative distractors than for neutral distractors (Most & Wang, 2011; Wang & Most, 2017). In these designs, neutral distractors share qualities with negative distractors, such as semantic distinctiveness (typically depicting people or animals) from other items in the RSVP streams, but they differ from the negative images in their absence of obvious emotional content. However, findings suggest that the underlying mechanisms involved in EIB may be activated for neutral stimuli as well. In a recent study, both negative and neutral distractors demonstrated a spatially localized interference when the target appeared at lag-1, whereas only negative distractors elicited a spatially localized interference when it appeared at lag-2 (Wang & Most, 2017). This suggests that EIB elicited by emotional and neutral distractors may be mechanistically similar, but of longer duration in the emotionally negative condition. In contrast, Wang and Most (2017) found that featural distractors, which differed from other stimuli in the stream only from a visual feature but not because of the semantic “meaning” in the image (e.g., colored images among otherwise grayscale images), elicited no spatially specific effect (or the opposite spatial pattern). Thus, featural distractors may attract attention toward their spatial location because of their shared visual properties with goal-relevant targets, whereas negative distractors may compete for representation at their spatial location because of their strong conceptual meaning (Wang & Most, 2017; see also Moore & Weissman, 2011). Given the potential overlap in mechanisms engaged by emotional and semantically distinctive, neutral distractors, as well as the possibility that these mechanisms differ from those engaged by featural distractors, in the current study we compare performance following negative emotional distractors with performance following featural distractors. In both cases, distractors are featurally distinct, but negative distractors have an added conceptual meaning, which is what is implicated to drive a spatially localized impairment (Wang & Most, 2017).
Experiment 1 tested the hypothesis that emotional distractors would cause impaired target perception in trials when distractors and targets appeared in the same stream, regardless of where the participant was fixating – as predicted by the spatiotemporal competition account for EIB. This was in contrast to the prediction of the fixate-to-one-stream account, which would predict such impairment when the distractor was fixated and the target appeared in the same fixated stream, but not in the case of the non-fixated distractor condition. In Experiment 2, we tested the spatial pattern of interference under conditions in which “distractor” items were made task relevant by a test of memory for them. To avoid cross-condition contamination that would likely result from participants treating distractors as relevant in some conditions and irrelevant in others, the impact of task-relevance was examined by comparing between experiments.
Experiment 1
Method
Participants
Sixty-two participants were recruited from the community via the UNSW Sydney Paid Sona system (mean age=23.6 years, SD=5.8; 32 female, 30 male). Participants were compensated $15 for completing the experiment. All participants gave informed consent and the experiment was approved by the UNSW Sydney Human Research Ethics Approval Panel (Psychology).
Materials and Procedure
The experiment was conducted using a Tobii TX-300 eyetracker. The monitor had a refresh rate of 60Hz, and the eyetracker had a 300Hz temporal and 0.15° spatial resolution. Stimuli were presented and responses made through the Psychophysics Toolbox for Matlab (Brainard, 1997; Pelli, 1997). Head position was fixed via a chin rest ~60 cm away from the screen. Importantly, participants were allowed to move their eyes freely between the two streams of images throughout the experiment.
The experiment was composed of 20 blocks of 18 trials (360 trials in total). On every trial, participants saw a fixation point in the center of the screen for 500ms, a blank screen for 200ms, followed by two simultaneous, rapid streams of images (see Figure 2). Images were presented against a black background, and one image per stream was presented for one “frame”. There were 12 frames per trial, presented at a rate of 100 ms/frame. The two streams were vertically separated by 100 pixels (2.5 degrees visual angle (dva) - each 50 pixels from the vertical center of the screen). Stimuli were images sized to 320 pixels wide and 240 pixels high (8.1 × 6.1 dva).
Figure 2. Schematic of a partial trial in Experiment 1.

Participants reported the direction of the one rotated picture that appeared in either of two simultaneously presented RSVP streams (presentation rate 100ms/frame). The distractor item - a colored negative image or colored scene image - appeared either one frame or two frames before the target, which was also colored. All other images in the stream were grayscale. The distractor appeared either in the same stream or opposite stream from the target.
Every trial contained one target image, one distractor image, and 22 filler images to make up the remaining images in the two streams. 252 grayscale images of upright landscape and architectural scenes served as the filler images. The target image was always a colored landscape image that came from a bank of eighty-four “target” images, and these images were rotated either 90° clockwise or counterclockwise on each trial.
An additional 160 images served as the “distractors”. Distractors were also colored images, but were not rotated. There were 80 negative, emotional distractors (colored images depicting medical injuries, threatening animals, or grotesque scenes), and 80 “featural” distractors (colored images depicting upright landscape or architectural scenes). Featural distractors were different images than those used as the filler images, but represented similar content and were also collected from publicly available sources. This was a deviation from previous emotion-induced blindness studies, which usually use “neutral” images (e.g., neutral images of people or animals) to compare performance with emotional distractors (e.g., Most & Wang, 2011). We made this change to the standard procedure to minimize the amount of potentially “meaningful” content displayed in the images. Neutral distractors usually impair target performance in the direction of spatial localization (particularly at very early lags; Wang & Most, 2017), perhaps due to the “meaningful” content they contain (people or animals) compared to the filler items. Featural distractors in this experiment were more similar to filler images in terms of meaningful content, but differed by being presented in color, a feature they shared with the target. This was important as a way to compare two physically salient stimulus types (colored negative and colored featural) to purely isolate the effects of distraction by the “meaningful” content in the negative distractors. Negative, emotional distractors were gathered from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 2008) and from publicly available sources.
There were an equal number of trials with negative and featural distractors. There were also 40 additional trials with “no distractor” (2 per block), in which another “filler” image was placed in the stream where a distractor would have usually been presented.
Depending on the trial, the distractor appeared at serial frame position 3 through 7, and the target appeared either one position (lag-1) or two positions (lag-2) after the distractor. We expected performance to be impaired by negative distractors at both lag 1 and lag 2 based on previous EIB studies (Kennedy & Most, 2015), but used these two lags in order to minimize any explicit expectation for when the target would appear. Every distractor was presented once in the experiment at lag-1, and once at lag-2.
The placement of the distractor was manipulated in relation to the participant’s point of fixation. Depending on the trial, the distractor was presented either in the same stream that participants were fixating or in the opposite stream (non-fixated). The position of the target was manipulated orthogonally to this factor: on half of all trials the target would appear in the same stream as the distractor and on the remaining half it would appear in the opposite stream.
At the end of the trial, participants indicated the direction that the target image was rotated. Participants heard a bell through headphones if they answered correctly but heard nothing if they answered incorrectly.
Before starting the experiment, participants engaged in a 5-point eyetracker calibration procedure. They then started the EIB task, first with 8 practice trials to get used to the task. Practice trials did not have any distractors, started at 200ms/frame, and progressed to the experiment speed of 100ms/frame. Practice trials were not included in the analyses.
Gaze-contingent analysis
Participants’ eye-gaze was tracked throughout the experiment. On every distractor-present trial, the distractor placement was determined according to the location of the participant’s gaze. This was achieved by measuring eye position during the 200-ms immediately prior to the distractor onset. The algorithm then searched backwards through this period of eye-gaze data for a block of 50ms of “valid” eye-gaze (i.e., data without missing samples due to blinks). The average position of eye-gaze over this 50ms was then attributed to either one of the streams of images or the background. A participant was determined to be fixating at one stream of images if gaze was biased towards that image relative to the centre of the screen. We used a 25 pixel (0.6 dva) buffer around the images which therefore incorporates trials in which eye-gaze was substantially biased towards one image (within at least 25 pixels of the boundary) and away from the other image (at least 75 pixels from the boundary), but not falling directly on an image. The experiment was programmed such that throughout the experiment, when participants were determined to be looking at one of the streams during the 50ms time frame, the distractor was then presented in one of the two streams, depending on the trial type (same or opposite stream). If the average position of a participant’s gaze was determined to be in an otherwise blank region of the screen (in the center of the screen, or to the left or right of the images) during the time when the gaze location was assessed, the distractor would appear randomly in one of the two streams. These trials were excluded from the analyses.
Results
The median number of valid gaze-contingent trials across all conditions per participant was 255.5 trials (mean=229.3 trials; SD=75.2 trials), with a range from 62 to 346 trials out of the total 360 trials. Data were collapsed across lags 1 and 21.
Target Performance Accuracy
We used target accuracy (correctly reporting the direction of the rotated target) as our primary dependent variable (see Figure 3). A 2 (distractor fixation: fixated vs. non-fixated) × 2 (distractor-target relationship: same stream vs. opposite stream) × 2 (distractor type: negative vs. featural) ANOVA revealed a significant effect of distractor fixation, F(1,61)=14.825, p<.001, ηp2=.196, with generally better target accuracy when the distractors were in the non-fixated stream. That is, when distractors appeared in the stream that participants were not fixating, participants were better able to report the target rotation. There was also a significant main effect of distractor-target relationship, F(1,61)=21.341, p<.001, ηp2=.259, with worse overall accuracy when the target was positioned in the opposite stream to the distractor compared to when the target was in the same stream as the distractor (discussed in more detail below). The main effect of distractor type was also significant, F(1,61)=53.770, p<.001, ηp2=.469, such that negative distractors elicited worse performance than featural distractors, consistent with traditional EIB findings.
Figure 3. Experiment 1 target accuracy.
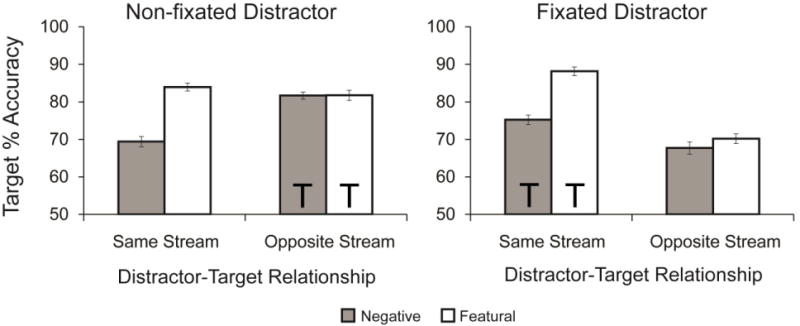
In Experiment 1, impairment from emotional distractors was localized both when the distractor was fixated and non-fixated. EIB was observed when targets appeared in the same stream as distractors – regardless of whether the participants were fixating on the distractor stream or when fixating at the opposite stream. When the target and distractor appeared in opposite streams, no EIB was observed. Error bars represent standard error, and Ts represent the stream in which the targets appeared.
The interaction between distractor-target relationship and distractor type was significant, F(1,61)=74.367, p<.001, ηp2=.549, with greater emotion-induced impairment when the distractor and target appeared in the same stream than when they appeared in opposite streams. There was also a significant interaction between distractor fixation and distractor-target relationship, F(1,61)=55.289, p<.001, ηp2=.475, with better performance when the target appeared in the stream that was fixated at the moment the distractor appeared (also discussed below). There was no significant interaction between distractor fixation and distractor type, F<1, or between all three factors, F(1,61)=1.233, p=.271, ηp2=.020. The non-significant 3-way interaction suggests that EIB was localized to the position of the distractor regardless of the position of eye-gaze, consistent with a spatiotemporal competition account for emotion-induced blindness. That is, negative distractors impaired performance significantly more than featural distractors when the target and distractor appeared in the same stream (compared to when they appeared in different streams), both when the distractor was fixated, t(61) =7.622, p < .001, dz =0.947, and when it was not fixated, t(61) =7.862, p < .001, dz =0.999. However, when the distractor and target appeared in opposite streams, there was no difference in the impairment from negative distractors compared to featural distractors, either when the distractor was fixated, t(61) =1.296, p = .200, dz=0.165, or when it was not fixated, t<1, conditions.2
An influence of distractor fixation was also revealed in several conditions of our experiment. Notably, when the target was in a opposite stream from the distractor, performance was worse when the distractor was fixated (negative: t(61) = 6.242, p < .001, dz =0.793, featural: t(61) = 5.575, p < .001, dz =0.708). This was not surprising, since when the distractor and target appeared in opposite streams, a non-fixated distractor indicates that participants were fixating at the stream the target would soon appear, while the opposite is true for a fixated distractor. As such, we did seem to find an effect of where participants were fixating on ability to report the target, however the spatially localized impairment (EIB) was not accounted for simply by where participants were fixating.
Data from the “no distractor” conditions (trials in which no colored distractor was present) were not included in the ANOVA described above but also reflected a benefit to targets appearing in the stream being fixated. Performance in the two baseline conditions differed significantly, t(61) = 4.618, p < .001, dz = 0.586; in “no distractor” trials, participants performed better when the target appeared in the stream participants were fixating before the target was presented (M=92.8%, SD=10.5%), compared to when the target appeared in the opposite stream they were fixating (M=85.6%, SD=13.4%). For completeness of analysis, performance was impaired in both negative and featural distractor conditions compared to the “no distractor” baseline performance in all conditions except the distractor fixated, same stream condition (distractor fixated, same stream condition, t(61) = 1.441, p=.155, dz=0.183; all other conditions Fs>5.5, ps<.001).
The performance in featural distractor conditions revealed the impact of featurally salient distraction. A 2 (distractor fixation: fixated vs. non-fixated) × 2 (distractor-target relationship: same stream vs. opposite stream) ANOVA revealed that featural distractor performance differed based on distractor fixation, F(1,61)=10.011, p=.002, ηp2=.141, such that performance was worse for fixated distractors compared to non-fixated distractors. There was also a significant distractor-target relationship effect, F(1,61)=71.584, p<.001, ηp2=.540, such that performance was better when featural distractors and targets appeared in the same stream, compared to opposite streams. The distractor fixation × distractor-target relationship interaction was also significant, F(1,61)=33.084, p<.001, ηp2=.352. In the distractor fixated condition, performance was best when the distractor and target appeared in the same stream (M=88.1%, SD=10.2%), but worst when the distractor and target appeared in the opposite streams (M=70.2%, SD=13.9%). In non-fixated distractor conditions, there was little difference between same stream (M=83.9%, SD=10.5%) and opposite stream (M=81.8%, SD=9.8%) conditions.
Discussion
Emotion-induced blindness (EIB) was observed when the distractor and target appeared in the same stream but not when they appeared in opposite streams, an effect that replicates a number of previous demonstrations (Most & Wang, 2011; Wang & Most, 2017). Furthermore, Experiment 1 provides the first evidence that this effect does not depend on where participants were fixating when the distractor was presented. This result is consistent with a spatiotemporal competition account for EIB, which suggests that emotional distractors and subsequent targets compete for representation when presented close in time and in a similar location. This pattern of data is inconsistent with the alternative account, which predicted that the spatially localized impairment in EIB would be dependent upon where participants were fixating when the distractor appeared.
We observed some effects of where participants were fixating. Unsurprisingly, participants were more likely to correctly report targets that appeared at the stream they were fixating than targets that appeared in the stream they were not fixating. However, the spatially localized impairment was observed above and beyond the influence of where participants fixated. Moreover, consistent with previous findings (Wang & Most, 2017), featural distractors impaired target perception more when the distractor and target appeared in opposite streams, particularly when the featural distractor was fixated. This was different to the effect observed for negative distractors, which caused greater impairment when the distractor and target appeared in the same stream. Taken together, these results suggest that while both featural and negative distractors were featurally similar to targets and different from other items in the stream, featural distractors may attract attention to and benefit stimuli that appear at their spatial location, whereas negative distractors seem to compete for representation at their spatial location, regardless of where participants fixated.
Experiment 2
While EIB demonstrates a spatially localized impairment, several attentional blink (AB) studies demonstrate that performance impairment from a task-relevant target will spread across spatial locations (Lunau & Olivers, 2010). One quality that differentiates EIB and AB tasks is the task-relevance of the first attention-grabbing stimulus: in the AB participants have to identify the first target and encode it into working memory, whereas in EIB distractors are best ignored. This difference may be important: according to one model of perceptual failures within rapid serial presentations, the encoding of a first target into working memory leads to suppressed processing of subsequent items across the visual field; in contrast, spontaneous competition between temporally neighboring items leads to spatially localized interference (Wyble & Swan, 2015). Perhaps the distractors from Experiment 1 would impair target perception across space, like in the AB, when the task requires participants to encode them into working memory.
In Experiment 2, we included a recognition test for the distractors in order to render distractors relevant to the task and to encourage encoding of them into memory. The aim of Experiment 2 was therefore to determine if the impairment from emotional distractors is still spatially localized even when the distractors are task-relevant.
Method
Participants
Fifty-nine participants completed Experiment 2 and were recruited through the community via the University of New South Wales “Paid Sona” system (mean age=25.4 years, SD=6.8; 35 female, 24 male). Participants were compensated $15 for completing the study. Data from three participants (two male) were excluded from the analyses: two performed at or below chance, while the other fixated at one of the streams in only seven trials throughout the entire experiment (more than three standard deviations below the median number of fixated trials, reported below), and therefore had very few trials on which the main fixation-contingent manipulation could operate. All participants gave informed consent and the experiment was approved by the UNSW Sydney Human Research Ethics Approval Panel.
Materials and Procedure
Experiment 2 was designed in a similar way to Experiment 1 with some exceptions. In Experiment 2, due to an oversight in experimental design, the “no distractor” condition always presented the target in the opposite stream to where participants were looking. This change was not important for the main analyses, but did differ from Experiment 1, such that the performance in the “no distractor” condition was only provided by the trials in which participants fixated at the stream in which the target would appear.
In contrast to Experiment 1, participants completed a memory test for the colored distractors at the end of each block of 18 trials. Participants were told to remember the colored distractor in each trial. This change rendered the distractors task-relevant, as in the typical AB. There were 160 additional images (80 negative and 80 featural) in Experiment 2 that served as foils in the memory tests. These images matched the negative distractors and the featural distractors in content type and emotional quality, but were never presented in the EIB trials. For each memory test, participants saw a screen with 16 negative images arranged in a four by four grid, eight of which that had actually appeared in that block, and eight foils that had not. Participants were given the instructions that “Eight of these pictures appeared in the most recent block. Please click on them.” When a participant chose an image, it was surrounded with a white border, and they could not choose it again. An identical memory test was then employed for the featural distractors (16 images containing eight images that had appeared in that block of trials as featural distractors, and eight foils that had not). The next block of trials began after the second memory test.
Results
The median number of gaze-contingent trials per participant was 266.5 trials (mean=248.6 trials, SD=82.4 trials), with a range from 57 to 357 trials out of the total 360 trials. Like Experiment 1, data were collapsed across lags 1 and 2.3
Target Performance Accuracy
Like Experiment 1, the main variable of interest in Experiment 2 was accuracy in reporting the target’s rotation (see Figure 4). A 2 (distractor fixation: fixated vs. non-fixated) × 2 (distractor-target relationship: same stream vs. opposite stream) × 2 (distractor type: negative vs. featural) ANOVA revealed a significant effect of distractor fixation, F(1,55)=18.050, p<.001, ηp2=.247, with better target performance when the distractor was non-fixated than when it was fixated. There was also a main effect of distractor-target relationship, F(1,55)=18.890, p<.001, ηp2=.256, with worse performance when the distractor and target appeared in opposite streams. The main effect of distractor type was also significant, F(1,55)=82.573, p<.001, ηp2=.600, with worse performance after negative distractors compared to featural distractors.
Figure 4. Experiment 2 target accuracy.
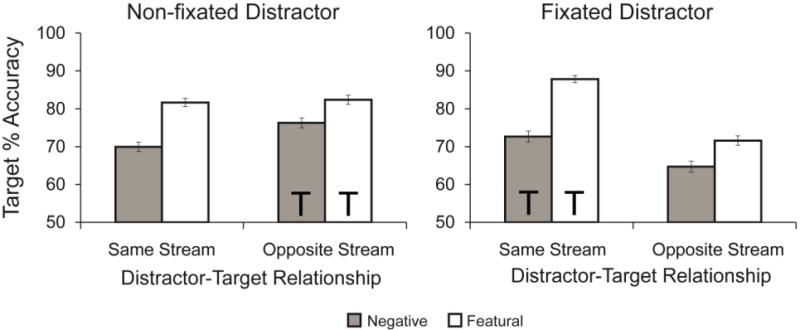
In Experiment 2, EIB was observed when targets appeared in the same stream as distractors – both when participants fixated at the distractor stream and when fixating at the opposite stream. When the target and distractor appeared in opposite streams, EIB was also observed. However, the emotion-induced impairment was greater when distractors and targets appeared in the same stream, compared to when they appeared in opposite streams. Error bars represent standard error, and Ts represent the streams in which the targets appeared.
As predicted, there was also a significant interaction between distractor-target relationship and distractor type, F(1,55)=15.229, p<.001, ηp2=.217, with greater emotion-induced impairment when distractors and targets appeared in the same stream compared to when they appeared in opposite streams. The distractor fixation by distractor-target relationship interaction was also significant, F(1,55)=43.577, p<.001, ηp2=.442, which suggests that for fixated distractor trials, participants were more accurate in detecting the target in the same stream, while for non-fixated distractor trials they performed equally well whether the target appeared in the same or opposite stream as the distractor. There was no significant interaction between distractor fixation and distractor type, F(1,55)=1.763, p=.190, ηp2=.031, or all three factors, F(1,55)=0.703, p=.406, ηp2=.013.4
The impairment from negative distractors compared to featural distractors was greater when the distractor and target appeared in the same stream compared to opposite streams, regardless of whether the distractor was fixated or not. Poorer target reporting was observed following negative compared to featural distractors occurring in the same stream when both the distractor was fixated, t(55) =8.176, p < .001, dz =1.092, and when the distractor was non-fixated, t(55) =6.747, p < .001, dz =0.902. Interestingly, unlike in Experiment 1, it appears that there was some degree of EIB when the distractor and target were in opposite streams, such that negative distractors also significantly impaired performance compared to the featural distractors in both the distractor fixated, t(55) =3.725, p < .001, dz =0.498, and distractor non-fixated conditions, t(55) =3.509, p=.001, dz =0.469. Thus, while the emotion-specific impairment was greater when the distractors appeared in the same stream as the target, the negative distractors also led to greater impairment when they appeared in the opposite stream to the target.
Performance in the baseline “no distractor” condition (M=93.2, SD=7.8) was higher than performance in all distractor-present trial conditions, confirming that both featural and negative distractors impaired performance (Fs>4.45, ps<.001).
A 2 (distractor fixation: fixated vs. non-fixated) × 2 (distractor-target relationship: same stream vs. opposite stream) ANOVA revealed featural distractor performance to be worse for fixated distractors compared non-fixated distractors, F(1,55)=7.126, p=.010, ηp2=.115. The distractor-target relationship effect was also significant, F(1,55)=37.971, p<.001, ηp2=.408. Performance was better when the featural distractor and target appeared in the same stream, compared to opposite streams. The distractor fixation × distractor-target relationship interaction was also significant, F(1,55)=43.752, p<.001, ηp2=.443. Target performance benefited when the distractor was fixated and the target appeared in the same stream (M=87.8%, SD=8.7%), and was impaired when the distractor was fixated and the target appeared in the opposite stream (M=71.6%, SD=13.0%). When the distractor was non-fixated, performance was not as affected whether the distractor and target appeared in the same stream (M=81.7%, SD=11.7%) or opposite stream (M=82.4%, SD=11.8%).
Memory Performance
We next examined the results from the memory tests for distractors, to see if the different trial types affected memory for the distractors in the streams (Figure 5). Memory accuracy was calculated as the percentage of correct responses on the memory test (chance performance was 4/8=50%).
Figure 5. Experiment 2 distractor memory performance.
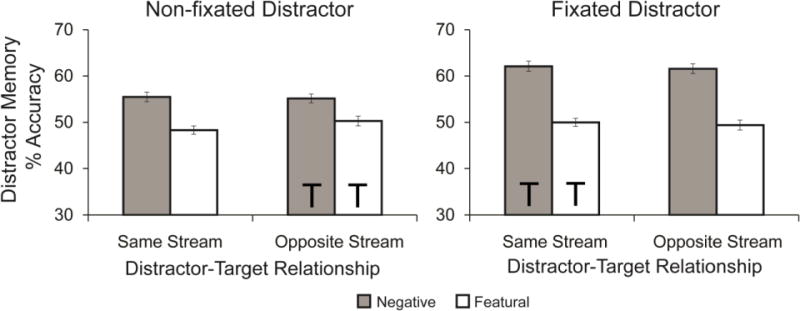
Participants remembered distractors better when they were negative compared to when they were featural distractors. They also remembered distractors better when the distractors were fixated, compared to non-fixated. Error bars represent standard error, and Ts represent the streams in which the targets appeared.
There was a difference in memory performance across the different trial conditions. A 2 (distractor fixation) × 2 (distractor-target relationship) × 2 (distractor type) revealed a significant main effect of distractor fixation, F(1,55)=25.895, p<.001, ηp2=0.320, with better memory for distractors that were fixated. There was also a significant main effect of distractor type, F(1,55)=107.933, p<.001, ηp2=0.662, with better memory for negative distractors than featural distractors. Participants did not remember featural distractors better than chance, (M=49.4%, SD=3.3%, t(55) =1.448, p = .154, dz =0.193), but did remember negative distractors better than chance (M=58.6%, SD=5.4%, t(55) =11.911, p < .001, dz =1.592). There was no main effect of distractor-target relationship, F<1. There was a significant distractor type by target fixation interaction, F(1,55)=11.854, p=.001, ηp2=0.177, suggesting that recognition of fixated distractors compared to when they were non-fixated was larger for negative than for featural distractors. Subsequent t-tests revealed that recognition was greater for negative distractors when they were fixated compared to non-fixated, t(55)=5.472, p<.001, dz=0.731, but that there was no difference in memory performance for featural distractors when they were fixated compared to non-fixated, t<1. No other interaction effects reached statistical significance (ps>.05). Together, these results demonstrate that negative distractors were recognized to a greater extent than featural distractors, and that negative distractors in the fixated stream were remembered better than those in the non-fixated stream.
Comparing target performance accuracy between experiments
In Experiment 2, participants demonstrated impaired performance after emotional distractors both when the distractor and target appeared in the same stream and when they appeared in opposite streams. In contrast, in Experiment 1 the EIB effect was limited to the conditions in which the distractor and the target appeared in the same stream. Taken together, these results suggest that the increased task-relevancy of the distractors in Experiment 2 increased the potential of the emotional distractors to impair target detection across different regions of space.
To examine if the differences between the experiments were significant, we compared target performance accuracy in Experiment 1 and Experiment 2. Importantly, a 2 (distractor fixation) × 2 (distractor-target relationship) × 2 (distractor type) × 2 (experiment) ANOVA revealed a significant distractor-target relationship × distractor type × experiment interaction, F(1,116)=5.831, p=.017, ηp2=0.048, which indicates that the localization of the EIB effect differed between the two experiments: the impact of negative stimuli on target detection was localized to when distractors and targets appeared in the same stream in Experiment 1, but had a more diffuse effect in Experiment 2 when these distractors became task-relevant. Separate analyses were conducted on the data across the two experiments, grouped according to whether the target and distractor appeared in the same stream or opposite streams. For the conditions in which they appeared in the same stream, a distractor type × distractor fixation × experiment ANOVA revealed there was no significant distractor type × experiment interaction, F<1, suggesting that the “same-stream EIB effect” was equivalent across the two experiments. However, for the opposite stream conditions, there was a significant distractor type × experiment interaction, F(1,116)=7.542, p=.007, ηp2=0.061, indicating that the EIB effect was greater in Experiment 2 compared to Experiment 1. This lends support to the notion that making distractors task-relevant led to a spatially diffuse effect in Experiment 2.
The main difference between experiments was the task-relevancy of the distractors. We take these data to therefore suggest that whether task-relevant or not, emotion-induced impairment is observed when distractors and targets appear in the same stream suggesting competition at an early representational level. However, for there to be an emotion-induced impairment when the distractor appears in the opposite stream to fixation, the distractors need to be made task-relevant. This suggests that task-relevance may additively impose competition between distractor and targets at later memory stages.
Discussion
In Experiment 2, participants searched for targets that appeared in either of the two streams, and a task-relevant distractor appeared either in the same or opposite stream shortly before it. In line with the results of Experiment 1, negative distractors elicited greater impairments when the distractor and target appeared in the same stream than when they appeared in opposite streams, regardless of where participants were fixating. However, EIB was also observed when the distractor and target appeared in opposite streams. The impact of featural salience was also observed in Experiment 2, such that featural distractors impaired performance more when the distractors and targets appeared in opposite streams, compared to the same stream, especially when the distractor was fixated. These results suggest that – above and beyond their featural salience - the use of task-relevant emotional distractors may result in parallel processes of spatially localized representational competition and task-relevance.
It is interesting to note that relative to when the distractors were irrelevant, performance decrease related to target relevancy primarily followed opposite stream, negative distractors. It may be that this effect of relevance did not generalize to the same stream condition because the perceptual competition between the distractor and target had already compromised the target representation. Moreover, negative distractors were remembered better than featural distractors, so they were likely stronger competitors for memory resources when made task-relevant. Participants did not remember featural distractors as well, and so their competition for memory resources may not have been as strong.
General Discussion
Previous studies have found that, during rapid serial visual presentations, emotional distractors impair perception of targets that appear in that same location, but not of targets that appear in a different location (Most & Wang, 2011). This pattern of data has been suggested to support a spatiotemporal competition account of emotion-induced blindness, whereby emotional distractors and subsequent targets compete for neural representation and for access to further processing, with the emotional distractor biased to win at the cost of the target representation (Wang et al., 2012). In order to rule out an alternative account of these findings, that the spatially localized pattern is a result of participants only fixating on one stream at a time, we tested whether the spatially localized pattern observed in EIB persists regardless of fixation. A spatiotemporal competition account would predict EIB to occur when the distractor and target appear in the same location no matter where the participant is fixating, while the alternative account would predict EIB only when the distractor and target appear in the stream a participant is fixating.
We found that emotional distractors caused spatially localized impairments of target perception independent of where participants were fixating, such that they specifically impaired accuracy for targets that appeared in that same location. These results help rule out the possibility that the localized pattern is an artifact of participants only fixating to one stream at a time, supporting instead an account of EIB that reflects spatiotemporal competition between the distractor and target (Wang et al., 2012).
It is worth noting that we compared the impact of emotional and featural distractors in the present experiments and did not probe the impact of conceptually distinctive, emotionally neutral distractors. This leaves open the possibility that the conceptual distinctiveness of the distractors – rather than their emotional salience per se – drove the localized effect. However, previous research has found that conceptually distinctive, emotionally neutral distractors do not elicit such a pronounced, localized effect, whereas emotional distractors do (Most & Wang, 2011; Wang & Most, 2017). Note also that whether it is conceptual distinctiveness or emotionality that drives the localized effect, the present findings appear to demonstrate that emotion and meaning can help shape perception beyond the role of “peripheral” attentional selection (cf., Firestone & Scholl, 2016). Meanwhile, consistent with our predictions, the distractors that captured attention due to their featural salience did not lead to a spatially localized impairment. In fact, featural distractors sometimes benefitted targets that appeared in their same stream, particularly when the distractor was fixated. This is also consistent with previous findings that distraction is not limited to the same spatial location when it is from stimuli that capture attention because of their features (e.g., Wang & Most, 2017; Moore & Weissman, 2011).
In Experiment 2, when participants were asked to encode the distractors into memory, we further observed disruption when targets and distractors appeared in different locations. This is consistent with results in the AB literature, where a task-relevant first target has been found to disrupt detection of a second target regardless of spatial location (e.g., Lunau & Olivers, 2010): by making distractors task-relevant in Experiment 2, they placed similar attentional demands as the first targets in the AB. This suggests potentially parallel impacts of spatiotemporal competition and suppression from memory encoding in EIB, which may indeed reflect two distinct mechanisms.
Wyble and Swan’s (2015) model describes multiple different aspects of attentional interference during RSVP, including competitive interference and suppression by working memory consolidation. In many ways, this approach to identifying mechanisms that are relevant to both EIB and the attentional blink may represent a more fruitful approach than those pursuing questions as to whether EIB and the attentional blink are two distinct phenomena. It may, for example, be that EIB and the AB have some overlap in the mechanisms involved (e.g., Kennedy et al., 2014; MacLeod et al., 2017). A more important distinction may be between conditions in which the first target is intentionally encoded into working memory (suppression by working memory consolidation) and those in which the first target is task-irrelevant but outcompetes target representations by virtue of its emotional salience (competitive interference). The different spatial patterns across Experiments 1 and 2 suggest that these two sources of attentional interference can work in parallel and operate simultaneously.
Although, as predicted, spatially localized EIB was observed in both fixated and non-fixated streams, we did find some effects that were modulated by where participants were fixating. Overall, targets were better identified when they appeared in the stream participants were fixating, and distractors were better remembered when they were fixated. Eye-gaze is widely used as a marker of attention. It is a measure specifically of overt attention (as opposed to covert attention, see Posner, 1980) and while research suggests that eye-gaze is guided by covert attention, covert and overt attention can operate separately (Hoffman, 1998). It is therefore possible that covert attention could underlie the patterns of impairment beyond that which we were able to capture with an eye-tracker. Nevertheless, the spatially localized pattern, the additive nature of task-relevance across both streams, and the absence of task-demands that would have encouraged decoupling of overt and covert attention, support the scenario that participants were engaged with both streams throughout the trials.
Together, the results of this study hold implications for understanding EIB and, more generally, the attentional dynamics during rapid visual processing. In terms of EIB, emotional distractors appear to primarily impair the detection of targets that appear in the same location regardless of where participants are fixating, which is consistent with a spatiotemporal competition account (Wang et al., 2012). More broadly, the present results suggest that – consistent with conceptual and computational models (e.g., Wyble & Swan, 2015) – the spatial distribution of attentional interference depends in part on whether or not distractors are treated as task-relevant, and thus on the potential engagement of working memory.
Acknowledgments
The experiments reported here were conducted as part of the first author’s PhD thesis at UNSW Sydney. We wish to the thank Ottmar Lipp and Charles Folk for feedback on an early draft of this manuscript. This research was supported by Australian Research Council grant FT120100707 to SBM. Preparation of this manuscript was also supported by the National Institute On Aging of the National Institutes of Health F32AG057162 to BLK. Data for this project are available online at https://osf.io/jkp8q.
Footnotes
Author note
The experiments reported here were conducted as part of the first author’s PhD thesis at UNSW Sydney.
The results were the same when we included lag into the analyses. A 2 (distractor fixation: fixated vs non-fixated) × 2 (distractor-target relationship: same stream vs opposite stream) × 2 (lag: 1 vs 2) × 2 (distractor type: negative vs featural) revealed significant effects of distractor fixation, F(1,61)=17.268, p<.001, ηp2=.221, distractor-target relationship, F(1,61)=21.870, p<.001, ηp2=.264, and distractor type, F(1,61)=48.330, p<.001, ηp2=.442, but as predicted, no significant effect of lag, F(1,61)=0.006, p=.939, ηp2<.001. Like the analyses collapsed across lag, there was a significant interaction between distractor-target relationship × distractor type, F(1,61)=66.231, p<.001, ηp2=.521, and distractor fixation × stream, F(1,61)=53.715, p<.001, ηp2=.468, and no significant interaction between distractor fixation × distractor type, F(1,61)=0.019, p=.892, ηp2<.001, or distractor fixation × distractor-target relationship × distractor type interaction, F(1,61)=1.411, p=.240, ηp2=.023.
Participants tended to fixate more on the top stream (60.3% trials) than the bottom stream (39.7% trials) on valid gaze-contingent trials. We therefore examined if the interactions we observed were different when participants fixated on the top stream versus the bottom stream, to separate possibly different influences driven from fixating at a particular stream. Note that participants’ head position was not particularly fixed in the vertical center of two streams, making it unsuitable to make interpretations about stimuli that appeared in participants’ top versus bottom visual hemisphere. Nevertheless, given the general preference for the top stream, we examined if this preference affected the analyses.
A 2 (stream fixation: top vs. bottom) × 2 (distractor fixation: fixated vs. non-fixated) × 2 (distractor-target relationship: same stream vs. opposite stream) × 2 (distractor type: negative vs. featural) ANOVA revealed that stream fixation did not significantly interact with any of the interactions we observed (Fs<1.836, ps>.183). Whether participants fixated at the top or bottom stream, results were essentially the same. We ran two separate 2 (distractor fixation: fixated vs. non-fixated) × 2 (distractor-target relationship: same stream vs. opposite stream) × 2 (distractor type: negative vs. featural) ANOVAs for trials when participants fixated at the top stream or the bottom stream, and in both cases, the interaction between distractor-target relationship and distractor type (top: F(1,56)=33.609, p<.001, ηp2=.375; bottom: F(1,45)=16.927, p<.001, ηp2=.273), and the fixation and distractor-target relationship (top: F(1,56)=27.170, p<.001, ηp2=.327; bottom: F(1,45)=11.008, p=.002, ηp2=.197) were significant, and in the same direction as when combined. Neither the fixated × distractor type interaction (top: F(1,56)=.059, p=.808, ηp2=.001; bottom: F(1,45)=1.342, p=.253, ηp2=.029), nor three-way interaction (top: F(1,56)=.944, p=.336, ηp2=.017; bottom: F(1,45)=.050, p=.825, ηp2=.001) were significant in either case. Note that because of the participant driven, gaze-contingent design, not all participants had data for fixating at the top or the bottom stream.
Consistent with Experiment 1, lag did not affect the main findings in Experiment 2. A 2 (distractor fixation: fixated vs non-fixated) × 2 (distractor-target relationship: same stream vs opposite stream) × 2 (lag: 1 vs 2) × 2 (distractor type: negative vs featural) revealed significant main effects of distractor fixation, F(1,55)=17.895, p<.001, ηp2=.245, distractor-target relationship, F(1,55)=21.038, p<.001, ηp2=.277, and distractor type, F(1,55)=82.467, p<.001, ηp2=.600, but no significant effect of lag, F(1,55)=0.232, p=.632, ηp2=.004.
In Experiment 2, participants tended to fixate more on the top stream (73.0% trials) than the bottom stream (27.0% trials) on valid gaze-contingent trials. There were some differences based on which stream a participant was fixating in Experiment 2, though it is worth noting that few participants had enough data to include in our analysis to examine the differences between top and bottom stream performance, and that sometimes participants had only a few trials to represent a particular condition. A 2 (stream fixation: top vs. bottom) × 2 (distractor fixation: fixated vs. non-fixated) × 2 (distractor-target relationship: same stream vs. opposite stream) × 2 (distractor type: negative vs. featural) ANOVA revealed that stream fixation did not significantly interact with any of the two-way interactions we observed (Fs<2.553, ps>.129), but did significantly interact with the three other variables in the stream fixation × distractor fixation × distractor-target relation × distractor type interaction, F(1,17)=5.542, p=.031, ηp2=.246.
Separate 2 (distractor fixation: fixated vs. non-fixated) × 2 (distractor-target relationship: same stream vs. opposite stream) ANOVAs revealed that no matter which stream participants fixated on a given trial, the interaction between distractor-target relationship and distractor type was not significant (top: F(1,45)=2.970, p=.092, ηp2=.062; bottom: F(1,27)=0.248, p=.622, ηp2=.009). This was surprising, given the significant interaction between distractor-target relationship and distractor type across all trials. Again, the smaller sample size due to participants having no or little data in the top or bottom stream may have contributed to this difference. In a similar vein, the fixation and distractor-target relationship was significant when participants fixated at the top stream, but not when participants fixated at the bottom stream (top: F(1,45)=24.705, p<.001, ηp2=.354; bottom: F(1,27)=2.050, p=.164, ηp2=.071). The fixated × distractor type interaction (top: F(1,45)=.439, p=.511, ηp2=.010; bottom: F(1,27)=1.451, p=.239, ηp2=.051), and three-way interaction (top: F(1,45)=2.987, p=.091, ηp2=.062; bottom: F(1,27)=1.006, p=.325, ηp2=.036) were not significant in either case.
References
- Arnell KM, Killman KV, Fijavz D. Blinded by emotion: target misses follow attention capture by arousing distractors in RSVP. Emotion. 2007;7(3):465–477. doi: 10.1037/1528-3542.7.3.465. http://doi.org/10.1167/4.8.359. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10(4):433–436. http://doi.org/10.1163/156856897X00357. [PubMed] [Google Scholar]
- Chun MM, Potter MC. A two-stage model for multiple target detection in rapid serial visual presentation. Journal of Experimental Psychology: Human Perception and Performance. 1995;21(1):109–27. doi: 10.1037//0096-1523.21.1.109. http://doi.org/10.1037/0096-1523.21.1.109. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18(1):193–222. doi: 10.1146/annurev.ne.18.030195.001205. http://doi.org/10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Deubel H, Schneider WX. Saccade target selection and object recognition: Evidence for a common attentional mechanism. Vision Research. 1996;36:1827–1837. doi: 10.1016/0042-6989(95)00294-4. https://doi.org/10.1016/0042-6989(95)00294-4. [DOI] [PubMed] [Google Scholar]
- Di Lollo V, Kawahara J, Ghorashi SMS, Enns JT. The attentional blink: resource depletion or temporary loss of control? Psychological Research. 2005;69(3):191–200. doi: 10.1007/s00426-004-0173-x. http://doi.org/10.1007/s00426-004-0173-x. [DOI] [PubMed] [Google Scholar]
- Firestone C, Scholl BJ. Cognition does not affect perception: Evaluating the evidence for “top-down” effects. Behavioral and Brain Sciences. 2016;39 doi: 10.1017/S0140525X15000965. http://doi.org/10.1017/S0140525X15000965. [DOI] [PubMed] [Google Scholar]
- Folk CL, Leber AB, Egeth HE. Top-down control settings and the attentional blink: Evidence for nonspatial contingent capture. Visual Cognition. 2008;16:616–642. http://dx.doi.org/10.1080/13506280601134018. [Google Scholar]
- Hoffman JE. Visual attention and eye movements. In: Pashler H, editor. Attention. Hove, United Kingdom: Psychology Press; 1998. pp. 119–153. [Google Scholar]
- Ihssen N, Keil A. The costs and benefits of processing emotional stimuli during rapid serial visual presentation. Cognition and Emotion. 2009;23(2):296–326. http://dx.doi.org/10.1080/02699930801987504. [Google Scholar]
- Kennedy BL, Most SB. The rapid perceptual impact of emotional distractors. Plos One. 2015;10(6):e0129320. doi: 10.1371/journal.pone.0129320. http://doi.org/10.1371/journal.pone.0129320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BL, Rawding J, Most SB, Hoffman JE. Emotion-induced blindness reflects competition at early and late processing stages: An ERP study. Cognitive, Affective & Behavioral Neuroscience. 2014;14(4):1485–1498. doi: 10.3758/s13415-014-0303-x. http://doi.org/10.3758/s13415-014-0303-x. [DOI] [PubMed] [Google Scholar]
- Keysers C, Perrett DI. Visual masking and RSVP reveal neural competition. Trends in Cognitive Sciences. 2002;6(3):120–125. doi: 10.1016/s1364-6613(00)01852-0. http://doi.org/10.1016/S1364-6613(00)01852-0. [DOI] [PubMed] [Google Scholar]
- Kristjánsson A, Nakayama K. The attentional blink in space and time. Vision Research. 2002;42:2039–2050. doi: 10.1016/s0042-6989(02)00129-3. http://doi.org/10.1016/S0042-6989(02)00129-3. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-8. University of Florida; Gainesville, FL: 2008. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. [Google Scholar]
- Lunau R, Olivers CNL. The attentional blink and lag 1 sparing are nonspatial. Attention Perception Psychophysics. 2010;72(2):317–325. doi: 10.3758/APP.72.2.317. http://doi.org/10.3758/APP.72.2.317. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. Journal of Abnormal Psychology. 1986;95:15–20. doi: 10.1037//0021-843x.95.1.15. http://doi.org/10.1037/0021-843X.95.1.15. [DOI] [PubMed] [Google Scholar]
- Maki WS, Mebane MW. Attentional capture triggers an attentional blink. Psychonomic Bulletin & Review. 2006;13:125–131. doi: 10.3758/bf03193823. http://doi.org/10.3758/BF03193823. [DOI] [PubMed] [Google Scholar]
- MacLeod J, Stewart BM, Newman AJ, Arnell KM. Do emotion-induced blindness and the attentional blink share underlying mechanisms? An event-related potential study of emotionally-arousing words. Cognitive, Affective, and Behavioral Neuroscience. 2017 doi: 10.3758/s13415-017-0499-7. http://doi.org/10.3758/s13415-017-0499-7. [DOI] [PubMed]
- Moore KS, Weissman DH. Set-specific capture can be reduced by pre-emptively occupying a limited-capacity focus of attention. Visual Cognition. 2011;19(4):417–444. doi: 10.1080/13506285.2011.558862. http://doi.org/10.1080/13506285.2011.558862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Most SB, Chun MM, Widders DM, Zald DH. Attentional rubbernecking: cognitive control and personality in emotion-induced blindness. Psychonomic Bulletin & Review. 2005;12(4):654–61. doi: 10.3758/bf03196754. http://doi.org/10.3758/BF03196754. [DOI] [PubMed] [Google Scholar]
- Most SB, Wang L. Dissociating spatial attention and awareness in emotion-induced blindness. Psychological Science. 2011;22(3):300–5. doi: 10.1177/0956797610397665. http://doi.org/10.1177/0956797610397665. [DOI] [PubMed] [Google Scholar]
- Wang L, Most SB. The cost of seeing the meaning: Conceptual capture by distractors triggers localized target suppression. Visual Cognition. 2017;24(9–10):473–486. http://doi.org/10.1080/13506285.2017.1321076. [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spatial Vision. 1997;10(4):437–442. http://doi.org/10.1163/156856897X00366. [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. http://doi.org/10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Potter MC, Wyble B, Hagmann CE, McCourt ES. Detecting meaning in RSVP at 13 ms per picture. Attention, Percpetion, & Psychophysics. 2014;76:270–279. doi: 10.3758/s13414-013-0605-z. http://doi.org/10.3758/s13414-013-0605-z. [DOI] [PubMed] [Google Scholar]
- Raymond JE, Shapiro KL, Arnell KM. Temporary suppression of visual processing in an RSVP task: an attentional blink? Journal of Experimental Psychology: Human Perception and Performance. 1992;18(3):849–60. doi: 10.1037//0096-1523.18.3.849. http://doi.org/10.1037/0096-1523.18.3.849. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf O. Emotional modulation of the attentional blink: Is there an effect of stress? Emotion. 2010;10(2):283–288. doi: 10.1037/a0017751. http://doi.org/10.1037/a0017751 PMID: 20364906. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Merz CJ, Walter B, Vaitl D, Wolf OT, Stark R. Emotional modulation of the atten- tional blink: The neural structures involved in capturing and holding attention. Neuropsychologia. 2011;49:416–425. doi: 10.1016/j.neuropsychologia.2010.12.037. http://doi.org/10.1016/j.neuropsychologia.2010.12.037 PMID: 21195103. [DOI] [PubMed] [Google Scholar]
- Shapiro KL, Raymond JE, Arnell KM. Attention to visual pattern information produces the attentional blink in rapid serial visual presentation. Journal of Experimental Psychology: Human Perception and Performance. 1994;20(2):357–371. doi: 10.1037//0096-1523.20.2.357. http://doi.org/10.1037/0096-1523.20.2.357. [DOI] [PubMed] [Google Scholar]
- Spalek TM, Falcon LJ, Di Lollo V. Attentional blink and attentional capture: endogenous versus exogenous control over paying attention to two important events in close succession. Perception & Psychophysics. 2006;68:674–684. doi: 10.3758/bf03208767. http://doi.org/10.3758/BF03208767. [DOI] [PubMed] [Google Scholar]
- Visser TA, Bischof WF, Di Lollo V. Rapid serial visual distraction: Task-irrelevant items can produce an attentional blink. Perception & Psychophysics. 2004;66:1418–1432. doi: 10.3758/bf03195008. http://doi.org/10.3758/BF03195008. [DOI] [PubMed] [Google Scholar]
- Wang L, Kennedy BL, Most SB. When emotion blinds: A spatiotemporal competition account of emotion-induced blindness. Frontiers in Psychology. 2012;3 doi: 10.3389/fpsyg.2012.00438. http://doi.org/10.3389/fpsyg.2012.00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyble B, Swan G. Mapping the spatiotemporal dynamics of interference between two visual targets. Attention, Perception, & Psychophysics. 2015;77(7):2331–2343. doi: 10.3758/s13414-015-0938-x. http://doi.org/10.3758/s13414-015-0938-x. [DOI] [PubMed] [Google Scholar]


