Abstract
With the use of contemporary tools and techniques, it has become possible to more precisely tune the biochemical mechanisms associated with using nonviral vectors for gene delivery. Consequently, nonviral vectors can incorporate numerous vector compositions and types of genetic cargo to develop diverse genetic therapies. Despite these advantages, gene delivery strategies using nonviral vectors have poorly translated into clinical success due to preclinical experimental design considerations that inadequately predict therapeutic efficacy. Furthermore, the manufacturing and distribution processes are critical considerations for clinical application that should be considered when developing therapeutic platforms. This review evaluates potential avenues towards improving the transition of gene delivery technologies from in vitro assessment to human clinical therapy.
Gene therapy technology and limitations
Gene therapy is a promising therapeutic strategy predicated on rectifying pathogenic diseases or other chronic ailments through genetic modification (e.g., cancer). At its foundation, gene therapy involves the intentional modulation of gene expression patterns through the delivery of exogenous genetic material such as DNA, messenger RNA (mRNA), microRNA (miRNA), RNA interference (RNAi) molecules such as small interfering RNAs (siRNA) or small hairpin RNAs (shRNA), and antisense oligonucleotides (AONs). Due to properties such as negative charge or large size, most biomacromolecules require vectors for delivery [1]. Over the last twenty years, the number of clinical trials evaluating gene-delivery technology has steadily increased [2], but these trials have only yielded five products globally, and none in the United States [3]. These products, none of which were approved prior to 2003, include: Gendicine, Oncorine, Rexin G, Neovasculgen, and Glybera. Unfortunately, nearly 95% of clinical trials have failed to proceed beyond Phase II studies [2], illustrating that there are potent challenges to developing clinically-relevant gene therapies.
Of the clinical trials listed in Table 1, ~90% utilized viral vectors such as adenoviruses, adeno-associated viruses (AAVs), lentiviruses, or retroviruses. Although the development of viral vectors has substantially advanced gene delivery technology, they possess several inherent shortcomings: limited DNA packaging capacity, complex production processes, broad tropism, cytotoxicity, immunogenicity, and tumorigenicity [4]. Nonviral vectors possess the potential to address many of these issues, thanks to recent advances in material science, nucleic acid chemistry, and nanobiotechnology [5].
Table 1.
Breakdown of US gene therapy clinical trials.
| Disease | Vector | Gene Target | Trial Number |
|---|---|---|---|
|
| |||
| Phase I | |||
|
| |||
| Leber's Hereditary Optic Neuropathy (LHON) | Adeno-Associated Virus | G11778G ND4 | NCT02161 380 |
| Late-Onset Pompe Disease | Adeno-Associated Virus | α-Glucosidase | NCT02240 407 |
| Late Infantile Neuronal Ceroid Lipofuscinosis | Adeno-Associated Virus | CLN2 | NCT01161 576 |
| Parkinson's Disease | Adeno-Associated Virus | Aromatic L-Amino Acid Decarboxylase |
NCT01973 543 |
| α1-Antitrypsin Deficiency | Adeno-Associated Virus | α1-Antitrypsin | NCT02168 686 |
| Leber Congenital Amaurosis | Adeno-Associated Virus | RPE65 | NCT00821 340 |
| MERTK-Associated Retinal Disease | Recombinant Adeno-Associated Virus |
MERTK | NCT01482 195 |
| Sickle Cell Disease | Lentivirus (ex novo) | Anti-Sickling (βAS3) | NCT02247 843 |
| Fanconi Anemia | Lentivirus | Fanconi Anemia Complementation Group A |
NCT01331 018 |
| Recurrent Malignant Glioma | Retroviral Replicating Vector | Cytosine Deaminase | NCT01156 584 |
| Solid Tumors | Liposome | RB94 | NCT01517 464 |
|
| |||
| Phase II | |||
|
| |||
| Heart Failure | Adeno-Associated Virus | Sarcoplasmic Reticulum Calcium ATPase |
NCT01966 887 |
| Blindness Caused by Choroideremia | Adeno-Associated Virus | Rab-Escort Protein 1 | NCT01461 213 |
| Hemophilia B | Adeno-Associated Virus | Human Factor IX | NCT02484 092 |
| Malignant Pleural Effusion | Recombinant Adenovirus | p53 | NCT02429 726 |
| Malignant Melanoma and Other Solid Tumors | Adenoviral serotype 5 | CD40L | NCT01455 259 |
| β-Thalassemia Major | Lentivirus (ex novo) | β-A(T87Q)-Globin | NCT01745 120 |
| HIV-1 | Lentivirus | shRNA For CCR5, HIV-1 Fusion Inhibitor, C46 |
NCT01734 850 |
| Transfusion Dependent β-Thalassemia | GLOBE Lentivirus | Human β-Globin | NCT02453 477 |
| Wiskott-Aldrich Syndrome | Retrovirus | Wiskott-Aldrich Syndrome Protein (WASP) |
NCT01410 825 |
| Severe Combined Immunodeficiency, X-linked (SCID-X1) |
Self-Inactivating (SIN) Gammaretrovirus |
IL-2RG | NCT01129 544 |
| X-linked Chronic Granulomatous Disease (X-CGD) | SIN Gammaretrovirus | gp91phox Subunit of NADPH Oxidase Complex |
NCT01906 541 |
| Leukaemia | GMP Grade Retrovirus | Wilms Tumor 1 | NCT01621 724 |
| Non-Hodgkin Lymphoma | Autologous T Cells | CD19 CAR | NCT02134 262 |
|
| |||
| Phase III | |||
|
| |||
| Myocardial Ischemia | Adenovirus Serotype-5 | Fibroblast Growth Factor-4 | NCT01550 614 |
| Duchenne Muscular Dystrophy | Lentivirus | AON for Exon 51 of Dystrophin |
NCT02255 552 |
| Painful Diabetic Neuropathy | Non-Viral | Hepatocyte Growth Factor | NCT02427 464 |
To become clinically relevant, nonviral vectors must better tolerate the challenges of a physiological environment. Conventional research and development of nonviral vectors has been focused on altering the vector’s material composition or formulation to improve the efficiency of in vitro gene transfer or to prevent premature degradation of the vector during in vivo circulation [6-8]. Unfortunately, solutions from this paradigm are typically validated in experimental systems that poorly represent the in vivo environment. Most in vitro tests are conducted using adherent immortal cell lines, which are typically transfected for long durations under non-physiological conditions. Consequently, such experiments fail to account for complications associated with organism-level gene delivery, such as renal clearance, serum inactivation, off-target delivery, and nuclear translocation in somatic cells. Positive outcomes in oversimplified in vitro tests do not guarantee success in animal model validations. Furthermore, even success in animal models does not guarantee clinical success. Current animal testing methods cannot fully address translation to human physiology, and there are economic considerations associated with the clinical setting.
In this review, we summarize the challenges that impede the clinical translation of gene delivery technology and discuss potential strategies for overcoming each barrier. We highlight compositional considerations for nonviral gene delivery vectors, in vitro and in vivo experimental design strategies that will circumvent, if not eliminate, limitations that have previously stymied clinical success of gene delivery technology.
Compositional considerations
Genetic cargo
Because developing gene delivery strategies often requires testing many combinations of different vectors and cargo, there is a risk of inefficiently applying resources to test less viable options. This can be avoided by evaluating only vector and cargo combinations ideally suited for the intended application. Careful consideration of the specific advantages and disadvantages associated with a particular genetic cargo and/or delivery vector to reduce the amount of compositional design options to a manageable number.
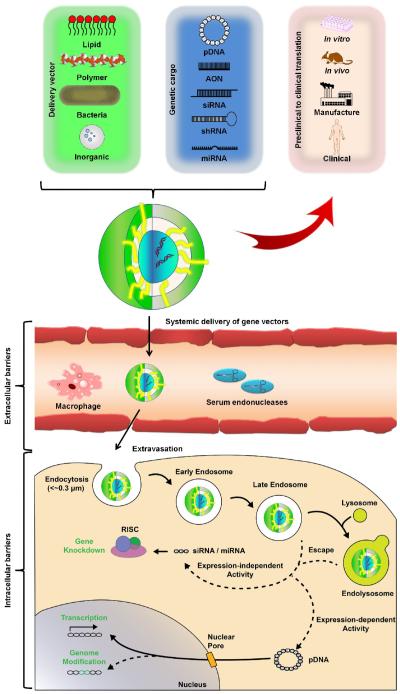
Nucleic acid payloads utilized in gene delivery applications can be divided into two groups based on their mechanism of action: i) transiently-active payloads (e.g., DNAs, AONs, and RNAs) and ii) genome editing systems. Transiently-active molecules can be further segmented into expression-dependent or -independent subdivisions. These subgroups differ in traits such as the type of nucleic acid cargo (DNA or RNA), location of activity (nucleus or cytoplasm), and mechanism of action (transgene expression or RNA interference), respectively. The most common expression-dependent systems consist of circular, double-stranded DNA constructs, termed “plasmids” (pDNA), which facilitate gene delivery by driving expression of the transgene encoding the protein of interest (Figure 1). These plasmids contain several basic components: the transgene expression system (i.e., promoter, gene of interest, and terminator), regulatory signals, antibiotic resistance marker, origin of replication, and the remaining bacterially-derived plasmid backbone (BB). The native plasmid systems are only active in vivo for only 1-2 months, but derivatives of these systems have demonstrated improved expression durations extended to several months/years while simultaneously boosting the expression magnitude of the transgene by 10- to 1,000-fold [9, 10]. For example, plasmids that are devoid of the BB have demonstrated prolonged and sustained efficacy compared to traditional pDNA [11, 12]. The extension of activity is due to the reduction of a silencing phenomenon that occurs when ~1 kb or more of DNA is placed outside of the transcriptional cargo (between 5’ end of promoter and 3’ end of poly A site) [13]. Strategies such as these expand therapeutic options for expression-dependent systems, especially within circumstances where traditional plasmids do not provide an adequate therapeutic timeframe or when permanent genetic changes are undesirable.
Figure 1.
General gene delivery mechanism. Upon assembly of the chosen nucleic acid cargo with the delivery vector construct, the composite particles must traverse various extracellular barrier (e.g., serum endonucleases) followed by gaining cellular entry through endocytosis (depicted in the figure) or by other means. Following uptake, particles modulate gene expression by either in the cytosol (expression-independent) or in the nucleus (expression-dependent). Regardless of the strategy selected, a gene delivery vector must successfully navigate in vitro and in vivo testing and GMP manufacturing prior to entering clinical testing.
A second set of transient-active molecules consists of synthetic nucleotide sequences (DNA, RNA, and chemical analogues) that serve to reduce and/or eliminate expression of specific protein(s) (Figure 1). Although these molecules are predominantly expression-independent, they can also be packaged into the plasmid-based systems described previously [14, 15]. The most commonly used types of these molecules are RNAs, (i.e., siRNA [16], shRNA [17], and miRNA [18]). Typically, siRNA and miRNA are employed to facilitate translation-inhibition of mRNA transcribed from the targeted gene(s) by directing RNA-induced silencing complex (RISC) activity [19, 20]. Although RNA possesses considerably lower stability (~1 hour in plasma) compared to DNA [21], the advantage of RNA-based gene therapy is the associated reduction in immunogenicity and mutagenesis [22]. Furthermore, a highly successful gene knockdown using siRNA can reduce targeted gene’s expression by 80–95% without reducing cell viability [23]. In addition, RISC’s modulation of target gene expression does not require nuclear translocation, which is the bottle-neck of expression-based systems. One drawback, however, is that the expression level of as many as 100 genes may be altered by a single siRNA transcript [24]. Alternatively, unlike RNA-based molecules, AONs interfere with mRNA translation through steric-hindrance of ribosomal binding in a sequence-specific manner, improving targeting specificity [25], but at the cost of decreasing knockdown efficiency (20–80%) [26]. Despite numerous advantages, off-target interactions with non-specific mRNA sequences, insufficient delivery to the targeted cells, and high manufacturing-costs of practice–grade AONs in sufficient quantities, impairs the wide- scale adoption of particular strategy [27, 28].
Unlike transient-active molecules, genome-editing systems such as zinc-finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and clustered regularly interspaced short palindromic repeats (CRISPRs) introduce permanent genomic changes into host cells through gene correction, disruption, and whole-gene insertion, respectively [29]. Of these systems, CRISPR-Cas, has garnered the most attention recently due to its ease of design and implementation; however, improvements in efficiency and specificity are still necessary [30]. For example, a recent study conducted in human zygotes achieved an editing efficiency of only 25%, far below clinically relevant levels [31]. Even though permanent gene modification in humans is a promising avenue of research, ethical concerns will hinder widespread adoption for the foreseeable future.
Selecting the appropriate material for building the Vector
Once the clinical application and therapeutic genetic cargo have been determined, the composition of the delivery vector is the next consideration. Non-viral vectors can be broadly classified as either biomaterial or biologically-derived agents.
Biomaterial-based vectors
Conventional biomaterials are either lipid- or polymer-based and contain a net-positive charge [32-35]. These constructs can encapsulate or attach to the gene expression systems via electrostatic interactions, or other physical/chemical mechanisms [4]. Alternatively, researchers have recently developed inorganic nanoparticle-based nucleic acid delivery systems that act as single- entity agents capable of gene transfection and regulation without the need of auxiliary carriers or cationic transfection agents. Of these, gold nanoshell (NS)-based delivery vehicles are designed to control the release of genetic cargo by light-inducible mechanisms with pulsed laser irradiation [36]. For these NS-based systems, attachment of the genetic cargo is mediated by the electrostatic attachment between the negatively charged phosphate backbone of the nucleic acids and the cationic region of the gold nanoshells. Another system, spherical nucleic acid (SNA) nanoparticle conjugates, (gold nanoparticles covalently functionalized with small interfering RNA duplexes) mediate activity by interacting with scavenger receptors in the cell membrane, thus increasing cellular uptake [37, 38]. Regardless of the inorganic system, the most common attachment mechanism is by gold nanoparticles functionalized with alkylthiolated oligonucleotides through Au-S bonds [39].
The chemical structure of vectors can be altered through tuning of electrostatic interactions in order to reduce the risk of aggregation and ineffective delivery [54]. Specifically, vector stability can be increased through the formation of a hydrophilic shield via grafting of polyethylene glycol (PEG) [55, 56]. PEGylation inhibits premature clearance by masquerading vectors as water particles [57]. For example, PEGylated liposome with 15 mol % modification exhibited a higher blood circulating concentration (ID/mL) at 6 h after intravenous administration. The extent of improved stabilization was positively correlated with the length of PEG, ranging from 1 kDa to 40 kDa [58]. Furthermore, the size and shape of gene delivery vector can impact its blood clearance rate, tissue distribution, endothelial uptake, intracellular transport, accumulation after endocytosis [59] [60]. Generally, vectors with sharp corners and edges have an increased probability of cytoplasmic translocation and a decreased probability of cellular excretion, which is a preferable trait for achieving high transfection efficiencies [61]. Although vector shape is a critical gene delivery parameter, its effects on biological interactions are not as clear as its physical behavior (e.g. degradation profiles). Finally, ligand-receptor modifications can be introduced to target specific cells and limit unintended off-target effects [62].
Biologically-derived vectors
Unlike biomaterial-based vectors, nonviral biologically-derived agents have unique features for mediate and enhance gene delivery efficacy. Engineered bacteria (e.g., Escherichia coli), for example, can deliver a diverse range of biomolecules in addition to nucleic acids [42-45]. Furthermore, E. coli possesses several traits that are advantageous for gene delivery: i) economically-feasible cell-growth kinetics, ii) established molecular biology techniques, iii) general size- restricted targeting, iv) adjuvant-like physical composition, v) superior innate or engineered endosomal escape mechanisms compared to biomaterials and vi) greater stability than biomaterial vectors [46, 47]. However, biologically-derived vectors often require complex synthesis and purification schemes and extended lead times are required when reengineering a vector for new situations. Hybrid combinations of biomaterial and biological vectors have been explored to harness the innate advantages associated with both delivery vector classes, further expanding the capabilities of gene delivery by combining two powerful experimental areas (i.e., polymer chemistry and molecular biology) [47-49].
Taking into account physiological factors
Gene delivery outcomes are mediated by cellular entry, achieved through either non-specific or receptor-mediated uptake mechanisms. However, some gene delivery strategies can bypass the uptake process entirely to gain immediate access to the cytosol [40]. For example, bactofection of mammalian cells (through either active invasion or passive uptake by phagocytic cells) can deliver pre-made mature mRNA to the cytoplasm of the target cell for immediate translation by the host cell machinery [41]. Upon uptake, genetic cargo is then transported intracellularly in endosomal vesicles prior to release into the cytosol through vector-specific mechanisms. These include lipid mixing or flip-flop mechanisms facilitated by cationic lipids or the proton sponge effect prompted by cationic polymers [1]. Alternatively, controlled release of the genetic cargo from inorganic nanoparticle- based nucleic acid delivery systems is triggered by light through either a thermal or non- thermal mechanism [36]. Other issues to be taken into account include vector stability in physiological media, circulation residence time, cytotoxicity, immunogenicity, off-target cellular uptake, and poor activity in non-dividing cells [50, 51]. Furthermore, if gene delivery vectors are to be administered directly into circulation, their residence time can be reduced through various environmental factors such as serum inactivation, enzymatic degradation, complement-mediated clearance, and reticuloendothelial system recognition [52, 53]. Therefore, vector composition must be considered to enable optimal gene delivery potential since the chemical, physical, and biological structure will primarily dictate the final gene delivery outcomes (Figure 2).
Figure 2.
Compositional design considerations for three representative properties of nonviral gene delivery vectors. Genetic cargo is surrounded by a coating (green outline) that provides protection and/or cell-specific targeting (top left). Furthermore, the vector itself can be designed from a variety of different chemical compositions (top right) and constructed into various shapes (bottom left). This flexibility in compositional design allows researchers to tune gene delivery vectors for specific clinical applications.
Experimental Strategies
In vitro setup and assay design
Efficacy
One limitation associated with in vitro testing of gene delivery pertains to the assays used to determine delivery efficacy (Table 2). For expression-dependent systems, transfection efficiency is typically determined using transgenic cargo driving expression of fluorescent or luminescent protein such as enhanced green fluorescent protein (EGFP) or firefly luciferase respectively, due to the ease of measuring and quantifying the expression level of these signal proteins. Although, these reporter protein techniques are often used interchangeably to validate gene delivery strategies, each separately describes only one metric of efficacy. For example, EGFP (and other proteins assessed via fluorescence-activated cell sorting [FACS]) are excellent at describing population-based expression metrics such as the percentage of cells expressing a desired transgene (i.e., gene delivery efficiency), whereas luciferase is better equipped to quantify gene expression magnitude within a population (i.e., the total protein expressed). Individually, these metrics provide an incomplete look at gene delivery, but when coupled with an assessment of cytotoxicity, characterization of gene delivery efficacy is improved and has been used as a predictor for in vivo success [46, 47, 65].
Table 2.
General gene delivery clinical translation hurdles.
| Conventional Approach | Limitations | Potential Solutions |
|---|---|---|
| Cytotoxicity | ||
|
| ||
| MTT, MTS assay |
|
|
| Transfection efficiency | ||
|
| ||
| Fluorescent reporter (e.g., EGFP) |
|
|
| Use of layered or suspended cells |
|
|
| Use of immortalized cell lines (e.g., Hela cells) |
|
|
| Long duration of transfection (~4 h) |
|
|
| Vector stability | ||
|
| ||
| Tests are run in simple formulation |
|
|
| Protection of cargo and subsequent release at target site. |
|
|
Alternatively, gene delivery can be utilized to modulate gene expression therapeutically by decreasing expression of a target gene (e.g., RNAi). The efficacy RNAi to inhibit expression of target genes is generally assessed by detecting reduced levels of mRNA and proteins. This typically involves assays such as quantitative PCR (qPCR) or RNA sequencing (RNA-Seq) for RNA measurements or western blots for protein measurements. However, while conducting these experiments, it is important to evaluate potential off-target effects [66, 67]. This is especially important considering that such off target effects can reduce cellular viability by nearly 80%, potentially compromising cytotoxicity tests [24]. The benefit of using qPCR and RNA-Seq is that they can also evaluate the expression of housekeeping genes to provide a baseline for expression. One important consideration in delivery of RNAi cargo is that the level of gene expression does not directly indicate that the desired phenotypic change has been elicited. This may occur in situations where only a small amount of protein is necessary to perform a specific cellular function or when the function of the target gene can be achieved through alternative means, thus targeting multiple genes for RNAi-based gene therapy is often desirable [68]. This was demonstrated in a study which increased repression of tumor growth from two-fold using individual siRNAs to seven-fold using three different siRNAs [69]. It is therefore necessary to supplement gene expression assays with additional tests that determine phenotypic impact of the delivered genetic cargo.
Cytotoxicity
One of the important properties evaluated with in vitro gene delivery is cytotoxicity, generally assessed by the MTS and MTT assays, which determine cell viability through a colorimetric measurement of cellular mitochondrial activity (Table 2). Within in vitro confines, stress-induced increases in mitochondrial activity can lead to underestimations of cytotoxicity [70]. Furthermore, conditions such as pH and the presence of metal ions can interfere with the assay, further confounding results [71]. To address these limitations, it is desirable to confirm the results with additional experiments. For instance, techniques such as microscopy or the growth of colony forming units on a plate can enable visual estimations of cell viability to support information gleaned from MTS and MTT assays. Ultimately, the conventional settings for such tests represent an artificial environment that cannot fully imitate the complexity of an in vivo system. However, in vitro cytotoxicity experiments represent a critical aspect of preclinical research and substantial cell damage measured with these assays is an initial indicator of vector-associated drawbacks. Alternatively, low levels of cytotoxicity are a promising start to gene delivery therapies that must be tested further in more physiologically-relevant settings [72, 73].
Integrity of the vector
Once delivery vectors are introduced in vivo, they are subjected to various forms of inactivation or clearance that are not accounted for in in vitro tests (Table 2). In addition to mechanisms for bodily clearance, there are various components of human serum that interfere with gene delivery through interactions with the delivery vector [74]. Typically, when the binding affinity of the vector to albumin is low (<106 M) the kinetics for such processes are often relatively slow compared the rates of clearance and tissue distribution [74]. However, when the binding affinity is higher (107-109 M), binding to serum proteins becomes significant, and should be considered in in vitro models [74]. These limitations can be accounted for with accommodations to current in vitro tests to improve their simulation of blood composition (e.g., adding serum) and circulatory flow.
To protect the cargo from degradation through mechanisms such as macrophage uptake, approaches like PEGylation can be used [75]. Unfortunately, the PEGylation of vectors often interferes with effective gene delivery in vivo by impairing membrane interactions [76]. It is therefore desirable to design modified cationic polymers that improve gene delivery by protecting their cargo, until preferentially degrading near the desired delivery target [77, 78]. The initial in vitro experiments conducted should ensure that both processes are performed in series, rather than in parallel. For instance transfection experiments using vector that has been previously subjected to stability testing helps ensure that the surface modification is capable of both providing protection and degrading to enable cellular uptake. Alternatively, transfection efficiencies could be determined by using a microfluidic device that subjects the vector to physiological conditions prior to reaching the target cells. Upon successful delivery to the targeted cell or tissue, the foreign expression system must successfully navigate additional barriers including escape of endocytosis (<2% success rate) [79, 80]. Upon endosomal escape, the expression system may require successful nuclear translocation, which is dependent on the type of cell being targeted. Nuclear translocation is considered to be a highly inefficient process that depends upon disruption of the nuclear membrane, which typically occurs during cell division [81]. This becomes problematic when the cellular target for gene delivery is a slowly growing or non-dividing cell line. In such cases, translocation must occur through nuclear pores, which have size- and signal-related (i.e., nuclear localization signal) constraints [82]. Therefore, it is necessary for in vitro experiments to employ a cell type that displays similar metabolic and mitotic rates as the intended in vivo target.
Replicating in vivo environments
An additional consideration is to ensure that the physical design of in vitro experiments accurately represents in vivo conditions. For instance, many in vitro studies evaluate gene delivery using cells that are adhered to a surface [83, 84]. A limitation associated with this design is that in the undesirable case of vector aggregation, aggregates can still interact with cells and demonstrate gene delivery [1, 85]. This can lead to the detection of false-positives that will likely be unsuccessful in vivo [1]. Furthermore, experiments often utilize long-duration transfections containing high concentrations of DNA not realizable under physiological conditions. More specifically, a several-hour long transfection, chosen to optimize expression within cells in a static closed environment [86], fails to account for the convective transport associated with circulatory flow. As such, transfection efficiencies determined under such in vitro conditions will likely be dissimilar to in vivo performance since the vector’s ability to deliver its cargo to the desired tissue while also rapidly flowing through the circulatory system is not being challenged (Figure 3A). An attractive alternative would be to utilize microfluidic technology to more accurately represent the kinetics and transport conditions relevant for physiological gene delivery [87, 88]. Furthermore, utilizing 2D or 3D microenvironments can affect gene delivery by modulating cellular physiology [89]. Cells cultured in 3D environment interact and remodel their microenvironment and can develop into complex structures, effectively recapitulating in vivo phenotypes. Gene delivery experiments utilizing 3D environments provide the ability to assess challenges such as mass transport, physical forces, and cell microenvironment interactions that are important in in vivo systems. Yet, 3D cultures have not become the standard approach in the field of gene delivery due to difficulty in the cellular and molecular analyses [90].
Figure 3.
Physical design considerations of in vitro systems vs. in vivo conditions. Gene therapy studies typically incubate delivery transfection agents (red [A] and blue [B]) under conditions that are not representative of systemic in vivo circulation. One such condition is the use of long incubation times (A), which are physiologically unrealizable and can be avoided by using microelectromechanical systems (MEMS). Additionally, in vitro measurements of preferential cell uptake are usually validated using homogenous cell populations in media lacking relevant levels of potentially interfering serum proteins (green particles) (B). Transfection studies can be improved by determining delivery specificity metrics in physiological media with heterogeneous cell populations to accurately predict non-specific interactions with serum proteins and off-target delivery respectively.
Furthermore, if treatment is intended for specific targets (e.g., tumor masses), in vitro models using direct delivery to layered or suspended cells cannot accurately predict the risk of off-target effects (Figure 3) [91]. It is also important to balance the need for selective targeting of the desired tissue with the clearance rate of the vector itself. These conditions therefore suggest a mechanism for delivery that must be both target-specific and possess a clearance rate that is low enough to ensure that the cargo can be delivered effectively. However, if the clearance time for the gene therapeutic is too long, significant off-target effects can be observed [92]. These concerns can be addressed by avoiding the use of monoclonal cell lines for in vitro tests and determining the specificity of gene delivery in a diverse cellular population in which a specific cell type is the desired target. In addition, if delivery to a mass of cells such as a tumor is desired, it is also important to recognize the transport-limitations of the genetic material into the cell mass [93]. Typically, such processes benefit from the enhanced permeability and retention (EPR) effect in vivo, which results in better transport of macromolecules across the tumor endothelium due to its structural properties [94]. This can enable accumulation of therapeutics at tumor sites that is 10-50 times higher than background [95]. However, not all tumors are equally permissive; it is therefore necessary to possess an understanding of the tumor architecture to inform the design the delivery vector’s particle size [94] and to conduct in vitro tests using tumors with similar physical properties.
Cell type
Another key difference between typical in vitro experiments used to assess gene delivery efficacy and the vector’s actual physiological application is the cell type used in such in vitro tests (Figure 3). Immortal cell lines are often used in in vitro experiments due to their indefinite capacity for cellular division from monoclonal populations. This has the advantage of providing genetically identical cellular populations that can be evaluated over extended durations since they are not prone to senescence. These advantages come at a cost, however, since the cells in human and animal models possess different regulation and unrestrained cellular division is abnormal behavior (and usually only present in cancerous instances). In addition to this inaccurate representation of in vivo cellular behavior, immortalized cell lines further confound in vitro testing due to their continuous and potentially rapid ability to divide and therefore disrupt the nuclear membrane. This can serve to enhance nuclear translocation of transgene elements in immortal cell lines compared to somatic cells present in in vivo models, leading to overestimations of the efficiency of gene delivery [96]. Consequently, the use of immortal cell lines in in vitro gene delivery experiments can yield false- positive results that subsequently fail in in vivo delivery. One potential solution is to complement tests using immortal cell lines with primary cell lines (serving as a better mimic of in vivo cell types) in side-by-side in vitro analyses.
Clinically predictive animal models
Animals are commonly used in biomedical research as models for human disease in the context of gene delivery, as well as drug discovery and development. Furthermore, use of animal models allows for extensive testing to estimate and refine dosage regimens, and predict human safety profiles [97-102]. Arguably, their most prominent role is in the therapeutic assessment (toxicology and activity) of potential gene and drug candidates, with positive results representing the gold-standard of preclinical research [97]. Despite their heavy usage, animal models imperfectly predict human responses, and results from animal models cannot always be replicated in clinical trials. Paradoxically, successful validation in animal models is an entry-requirement for all human-based clinical trials [103]. Therefore, it is important to conduct experiments using animal models in a manner that consistently translates to clinical success.
An ideal model associated with developing gene delivery strategies is one that replicates the manifestation and end-phenotype of a specific human disease while responding similarly to established standard of cares. Specifically, an ideal model should possess all of the following features compared to humans: i) similar biological makeup (anatomy and physiology; genetic context), ii) similar disease causation and pathological response(s) (especially phenotypic end-points of clinical studies), and iii) predictive validity (i.e., humanized mice). However, in most cases, the progression of a disease is not fully understood, which results in non-orthogonal observations and conclusions. Even when disease progression is similar, dissimilarities in the model and patient reactions to chemical entities challenge the context and validity of animal usage. For example, genetically engineered mice (Mus musculus) are the most sophisticated animal models of human cancer, mimicking the pathophysiological and molecular features human malignancy. Though mouse cancer models have contributed to our understanding of cancer biology, several limitations remain, such as restricted subset of tumour types, limited recapitulation of de novo human tumour development, and drug response, thus inhibiting our capacities to develop corresponding gene therapies [104]. Various animal models have been developed to study particular aspects of the disease; however, they must be used in collection to have any predictive validity in potential gene therapy [105- 107]. These shortcomings demonstrate the challenge with animal models beyond providing basic scientific studies and preliminary screening procedures.
Another major pitfall in establishing clinical validity of animal models is the use of genetically identical inbred animal strains in some disease models. Using transgenic animal models enables unprecedented insight into phenotypically-linked disease progression, but fails to properly represent the notoriously diverse genetics, nutrition, and lifestyles present in the human population. Going forward, inbred animal strains may still be utilized to establish or initially reduce the number of potential therapeutic candidates, but use of outbred animal strains or multiple animal strains and/or species would improve the fidelity of translation to human clinical applications. Similarly, evaluation of both animal genders will likely increase predictive validity. Given the substantial shortcomings of any one animal model, researchers are advised to use a battery of individually-diverse animal models. When evaluated collectively, they can be utilized at various stages in the preclinical process to answer specific experimental questions. For example, testing of late-stage pre-development cancer therapeutics in a select array of complementary animal models will better represent the genetic and phenotypic heterogeneity of human cancers that will provide more robust data sets and stronger clinical recommendations [108].
Manufacturing considerations
Several criteria must be met when designing gene delivery vectors that will eventually be produced at industrial scale and used in a clinical setting. Namely, the manufacturing pathway should possess the following features to become feasible:i) ease of fabrication/ uniformity control, ii) inexpensive synthesis, iii) facile purification, iv) noninvasive administration, and v) cheap storage for maintaining stability [109]. Aqueous complexation of lipid/polymer vector and nucleic acid cargo is the most common preparation procedure to form nanoparticles used for administering gene therapy [110]. However, despite the simplicity associated with aqueous formulation, this process demonstrates batch-to-batch variability that is unacceptable in a clinical setting. One potential solution is the application of mechanical preparatory methods, such as PRINT (particle replication in non-wetting templates), that confer particle uniformity [111].
The requirement for commercialization of any gene therapy strategy that is most overlooked in preliminary stages is the need for inexpensive synthesis and formulation. Most nonviral vectors are generally incapable of becoming clinically relevant without extensive chemical modifications that usually require multiple rounds of engineering to perfect. Successful clinical development requires that sufficient gene delivery performance is achieved without incurring expensive synthetic schemes. Interestingly, even when a particular nonviral vector requires an expensive synthesis scheme, the cost is marginal when compared to viral alternatives [3]. More specifically, the development of robust and scalable processes for mass production of viral vectors (which include labor- intensive cell culture and purification steps) remains an economic challenge that impedes potential therapeutic use [112].
With respect to practical application, traditional (needle-based) administration of gene therapeutics is hampered by the following disadvantages: refrigeration-costs for liquid formulations, personnel training requirements for administration, needle-based injuries, and patient compliance. To overcome these, Irvine and coworkers developed a polymer film tattooing technology, which offers a dry skin-patch administration platform that is pain-free and self-administrable [113-116]. The solid phase in situ polyplex formation is based on a layer-by-layer (LBL) formation approach, successfully avoiding the disadvantages of traditional administration methods.
Lastly, the robustness and stability of gene therapy formulations is strongly associated with storage methods. For instance, freeze-drying and vacuum-drying-based storage [117], as well as sugar-based formulations [118], can provide efficient preservation for most genetic materials and vector complexes via water replacement. Unfortunately, however, these processes have not been thoroughly optimized to maintain conformational stability [119], retain biological activity [120], and elongate dose shelf life [121, 122]. The ideal production and distribution process would provide a cost- effective gene therapy agent capable of non-cold storage maintenance to enable global access to therapeutics.
Concluding remarks and future perspectives
In recent years, substantial technological advancements in nonviral delivery vectors and nucleic acid chemistry have reinvigorated exploration of gene therapy as a potent clinical application. Coupled with the maturation of genomics and recombinant DNA technology, these technological advancements may enable gene therapy to provide an avenue for remedying diseases considered “untreatable/undrugable” by conventional approaches. However, despite these strides, the broad clinical application of nonviral vectors has yet to come to fruition. The lack of clinical success is partially caused by the experimental models implemented in preclinical research. When these models inaccurately represent relevant physiological conditions, translation into clinical success is unlikely. Consequently, with revisions to contemporary in vitro and preclinical models, novel gene therapy strategies can be developed that have significant clinical potential.
Outstanding Questions.
Can current in vitro gene delivery methodologies be further optimized to result in clinically-predictive results that can expedite the screening process of potential therapeutics?
What will be the best gene expression strategy (i.e., transiently-active vs genome-editing) to target diseases derived from epigenetic switches, which control gene action and have an obscure effect upon disorders like cancer and Alzhemier’s disease?
Can the concept of cellular-targeting or controlled release via rationally designed delivery vectors limit potentially off-target effects and conversely, can these strategies enable the targeted delivery to rare cell types that may be dispersed in various anatomical sites and may also be defined by more than one surface marker?
Even if the all technical barriers are overcome, companies will still face substantial financial concerns: What is a suitable price and payment structure for gene therapy products? Will single administration therapies (i.e., genome-editing) require a different structure? Will radical solutions such as the ‘pay-for-performance model’ disincentivize large pharmaceutical companies to develop new, potentially life-saving products?
Trends.
The rise of CRISPR-mediated genome editing provides a powerful tool for application in genetic engineering applications. Although recent studies using human embryos demonstrated off-target effects, CRISPR may eventually demonstrate utility in gene therapy applications.
Within complicated human systems, there is a greater potential for unintended side effects that may not be observed in preclinical studies. An example of this is the occurrence of leukemia in patients receiving treatment for X-linked Severe Combined Immune Deficiency using adeno-associated viral vectors.
Clinical translation is often impaired by limitations associated with the in vitro experiments conducted to validate the vector formulations. These assays often assess only one aspect of gene delivery in a non-therapeutic context. For instance, many clinical trials have demonstrated limited efficacy due to the loss of transgene expression over time.
Another impediment is the tendency for in vitro experiments to poorly represent physiological conditions. Vector circulation and clearance, as well as nuclear translocation of the genetic cargo are critical aspects of gene delivery that are not adequately addressed using conventional experimental design. For instance, immune responses to treated cells impaired the efficacy of gene therapy approaches for treating haemophilia B and lipoprotein lipase deficiency.
Acknowledgements
Funding Sources
The authors recognize support from a SUNY-Buffalo Schomburg fellowship (CHJ), the UT Austin Joe C. Walter, Jr. Endowed Presidential Scholarship (ABH), UT Austin Bruton Fellowship (ABH) and Mark Diamond Research Grant (MC and CHJ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
CHJ, ABH, and BAP are also employees of Abcombi Bioscience Inc.
REFERENCES
- [1].Jones CH, Chen CK, Ravikrishnan A, Rane S, Pfeifer BA. Overcoming nonviral gene delivery barriers: perspective and future. Mol Pharm. 2013;10:4082–4098. doi: 10.1021/mp400467x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ginn SL, Alexander IE, Edelstein ML, Abedi MR, Wixon J. Gene therapy clinical trials worldwide to 2012 - an update. The Journal of Gene Medicine. 2013;15:65–77. doi: 10.1002/jgm.2698. [DOI] [PubMed] [Google Scholar]
- [3].Jones CH, Hakansson AP, Pfeifer BA. Biomaterials at the interface of nano- and micro-scale vector-cellular interactions in genetic vaccine design. Journal of Materials Chemistry B. 2014;2:8053–68. doi: 10.1039/C4TB01058B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-viral vectors for gene-based therapy. Nature Reviews Genetics. 2014;15:541–55. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- [5].Schlenk F, Grund S, Fischer D. Recent developments and perspectives on gene therapy using synthetic vectors. Therapeutic delivery. 2013;4:95–113. doi: 10.4155/tde.12.128. [DOI] [PubMed] [Google Scholar]
- [6].Alex SM, Sharma CP. Enhanced intracellular uptake and endocytic pathway selection mediated by hemocompatible ornithine grafted chitosan polycation for gene delivery. Colloids and Surfaces B, Biointerfaces. 2014;122:792–800. doi: 10.1016/j.colsurfb.2014.08.023. [DOI] [PubMed] [Google Scholar]
- [7].Vighi E, Ruozi B, Montanari M, Battini R, Leo E. pDNA condensation capacity and in vitro gene delivery properties of cationic solid lipid nanoparticles. Int J Pharm. 2010;389:254–61. doi: 10.1016/j.ijpharm.2010.01.030. [DOI] [PubMed] [Google Scholar]
- [8].Xiao B, Wang X, Qiu Z, Ma J, Zhou L, Wan Y, et al. A dual-functionally modified chitosan derivative for efficient liver-targeted gene delivery. J Biomed Mater Res A. 2013;101:1888–97. doi: 10.1002/jbm.a.34493. [DOI] [PubMed] [Google Scholar]
- [9].Jia F, Wilson KD, Sun N, Gupta DM, Huang M, Li Z, et al. A nonviral minicircle vector for deriving human iPS cells. Nature Methods. 2010;7:197–9. doi: 10.1038/nmeth.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gracey Maniar LE, Maniar JM, Chen ZY, Lu J, Fire AZ, Kay MA. Minicircle DNA vectors achieve sustained expression reflected by active chromatin and transcriptional level. Mol Ther. 2013;21:131–8. doi: 10.1038/mt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lu JM, Williams J, Luke J, Zhang FJ, Kay MA. Mini-Intronic Plasmid (MIP) vector sequences enhance aav-mediated transgene expression in vitro and in vivo. Mol Ther. 2014;22:S112–S3. [Google Scholar]
- [12].Lu J, Zhang F, Kay MA. A mini-intronic plasmid (MIP): a novel robust transgene expression vector in vivo and in vitro. Mol Ther. 2013;21:954–63. doi: 10.1038/mt.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lu J, Zhang F, Kay MA. A Mini-intronic Plasmid (MIP): a novel robust transgene expression vector in vivo and in vitro. Mol Ther. 2013;21:954–63. doi: 10.1038/mt.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Aurisicchio L, Fridman A, Bagchi A, Scarselli E, La Monica N, Ciliberto G. A novel minigene scaffold for therapeutic cancer vaccines. Oncoimmunology. 2014;3:e27529. doi: 10.4161/onci.27529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang HY, Yi WJ, Qin SY, Li C, Zhuo RX, Zhang XZ. Tyroserleutide-based gene vector for suppressing VEGF expression in cancer therapy. Biomaterials. 2012;33:8685–94. doi: 10.1016/j.biomaterials.2012.08.022. [DOI] [PubMed] [Google Scholar]
- [16].Sahay G, Querbes W, Alabi C, Eltoukhy A, Sarkar S, Zurenko C, et al. Efficiency of siRNA delivery by lipid nanoparticles is limited by endocytic recycling. Nat Biotechnol. 2013;31:653–U119. doi: 10.1038/nbt.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gu S, Jin L, Zhang Y, Huang Y, Zhang FJ, Valdmanis PN, et al. The loop position of shrnas and pre-mirnas is critical for the accuracy of dicer processing in vivo. Cell. 2012;151:900–11. doi: 10.1016/j.cell.2012.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Xue W, Dahlman JE, Tammela T, Khan OF, Sood S, Dave A, et al. Small RNA combination therapy for lung cancer. Proc Natl Acad Sci U S A. 2014;111:E3553–E61. doi: 10.1073/pnas.1412686111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang Z, Liu D, Yan X, Wang F, Niu G, Yang M, et al. Biomimetic RNA-silencing nanocomplexes: overcoming multidrug resistance in cancer cells. Angewandte Chemie. 2014;53:1997–2001. doi: 10.1002/anie.201309985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shin S, Kwon HM, Yoon KS, Kim DE, Hah SS. FRET-based probing to gain direct information on siRNA sustainability in live cells: asymmetric degradation of siRNA strands. Molecular BioSystems. 2011;7:2110–3. doi: 10.1039/c1mb05054k. [DOI] [PubMed] [Google Scholar]
- [21].Layzer JM, McCaffrey AP, Tanner AK, Huang Z, Kay MA, Sullenger BA. In vivo activity of nuclease-resistant siRNAs. Rna. 2004;10:766–71. doi: 10.1261/rna.5239604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yamamoto A, Kormann M, Rosenecker J, Rudolph C. Current prospects for mRNA gene delivery. Eur J Pharm Biopharm. 2009;71:484–9. doi: 10.1016/j.ejpb.2008.09.016. [DOI] [PubMed] [Google Scholar]
- [23].Li N, Sun J, Benet ZL, Wang Z, Al-Khodor S, John SP, et al. Development of a cell system for siRNA screening of pathogen responses in human and mouse macrophages. Scientific Reports. 2015;5:9559. doi: 10.1038/srep09559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fedorov Y, Anderson EM, Birmingham A, Reynolds A, Karpilow J, Robinson K, et al. Off-target effects by siRNA can induce toxic phenotype. Rna. 2006;12:1188–96. doi: 10.1261/rna.28106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chan JH, Lim S, Wong WS. Antisense oligonucleotides: from design to therapeutic application. Clinical and Experimental Pharmacology & Physiology. 2006;33:533–40. doi: 10.1111/j.1440-1681.2006.04403.x. [DOI] [PubMed] [Google Scholar]
- [26].Lok CN, Viazovkina E, Min KL, Nagy E, Wilds CJ, Damha MJ, et al. Potent gene-specific inhibitory properties of mixed-backbone antisense oligonucleotides comprised of 2'-deoxy-2'-fluoro-D-arabinose and 2'-deoxyribose nucleotides. Biochemistry-Us. 2002;41:3457–67. doi: 10.1021/bi0115075. [DOI] [PubMed] [Google Scholar]
- [27].Sharma VK, Sharma RK, Singh SK. Antisense oligonucleotides: modifications and clinical trials. MedChemComm. 2014;5:1454–71. [Google Scholar]
- [28].Yee JK, Lin RJ. Antisense oligonucleotides shed new light on the pathogenesis and treatment of spinal muscular atrophy. Mol Ther. 2012;20:8–10. doi: 10.1038/mt.2011.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Moore R, Spinhirne A, Lai MJ, Preisser S, Li Y, Kang T, et al. CRISPR-based self-cleaving mechanism for controllable gene delivery in human cells. Nucleic Acids Res. 2015;43:1297–303. doi: 10.1093/nar/gku1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sander JD, Joung JK. CRISPR-Cas systems for editing, regulating and targeting genomes. Nature Biotechnology. 2014;32:347–55. doi: 10.1038/nbt.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Liang P, Xu Y, Zhang X, Ding C, Huang R, Zhang Z, et al. CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes. Protein & Cell. 2015;6:363–72. doi: 10.1007/s13238-015-0153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ahmad MU, Ali SM, Ahmad A, Sheikh S, Chen P, Ahmad I. Carbohydrate mediated drug delivery: synthesis and characterization of new lipid-conjugates. Chemistry and Physics of Lipids. 2015;186:30–8. doi: 10.1016/j.chemphyslip.2014.10.003. [DOI] [PubMed] [Google Scholar]
- [33].Seguin J, Dhotel H, Kai-Luen R, Bessodes M, Mignet N. Fine tuning of mixed ionic and hydrogen bond interactions for plasmid delivery using lipoplexes. Eur J Pharm Biopharm. 2015;90:63–9. doi: 10.1016/j.ejpb.2014.11.001. [DOI] [PubMed] [Google Scholar]
- [34].Lee D, Lee YM, Jeong C, Lee J, Kim WJ. Bioreducible guanidinylated polyethylenimine for efficient gene delivery. ChemMedChem. 2014;9:2718–24. doi: 10.1002/cmdc.201402293. [DOI] [PubMed] [Google Scholar]
- [35].Islam MA, Park TE, Singh B, Maharjan S, Firdous J, Cho MH, et al. Major degradable polycations as carriers for DNA and siRNA. J Control Release. 2014;193:74–89. doi: 10.1016/j.jconrel.2014.05.055. [DOI] [PubMed] [Google Scholar]
- [36].Huschka R, Barhoumi A, Liu Q, Roth JA, Ji L, Halas NJ. Gene silencing by gold nanoshell-mediated delivery and laser-triggered release of antisense oligonucleotide and siRNA. Acs Nano. 2012;6:7681–91. doi: 10.1021/nn301135w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Jensen SA, Day ES, Ko CH, Hurley LA, Luciano JP, Kouri FM, et al. Spherical nucleic acid nanoparticle conjugates as an RNAi-based therapy for glioblastoma. Science translational medicine. 2013;5:209ra152. doi: 10.1126/scitranslmed.3006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cutler JI, Auyeung E, Mirkin CA. Spherical nucleic acids. J Am Chem Soc. 2012;134:1376–91. doi: 10.1021/ja209351u. [DOI] [PubMed] [Google Scholar]
- [39].Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ. A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature. 1996;382:607–9. doi: 10.1038/382607a0. [DOI] [PubMed] [Google Scholar]
- [40].Loeffler DI, Schoen CU, Goebel W, Pilgrim S. Comparison of different live vaccine strategies in vivo for delivery of protein antigen or antigen-encoding DNA and mRNA by virulence-attenuated Listeria monocytogenes. Infection and immunity. 2006;74:3946–57. doi: 10.1128/IAI.00112-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Baban CK, Cronin M, O'Hanlon D, O'Sullivan GC, Tangney M. Bacteria as vectors for gene therapy of cancer. Bioengineered Bugs. 2010;1:385–94. doi: 10.4161/bbug.1.6.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Parsa S, Wang Y, Rines K, Pfeifer BA. A high-throughput comparison of recombinant gene expression parameters for E. coli-mediated gene transfer to P388D1 macrophage cells. J Biotechnol. 2008;137:59–64. doi: 10.1016/j.jbiotec.2008.07.1815. [DOI] [PubMed] [Google Scholar]
- [43].Parsa S, Wang Y, Fuller J, Langer R, Pfeifer BA. A comparison between polymeric microsphere and bacterial vectors for macrophage P388D1 Gene Delivery. Pharm Res. 2008;25:1202–8. doi: 10.1007/s11095-008-9563-x. [DOI] [PubMed] [Google Scholar]
- [44].Chung TC, Jones CH, Gollakota A, Ahmadi MK, Rane S, Zhang G, et al. Improved Escherichia coli bactofection and cytotoxicity by heterologous expression of bacteriophage PhiX174 lysis gene E. Mol Pharm. 2015;12:1691–1700. doi: 10.1021/acs.molpharmaceut.5b00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Jones CH, Rane S, Patt E, Ravikrishnan A, Chen CK, Cheng C, et al. Polymyxin B treatment improves bactofection efficacy and reduces cytotoxicity. Mol Pharm. 2013;20:4301–4308. doi: 10.1021/mp4003927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Jones CH, Ravikrishnan A, Chen MF, Reddinger R, Ahmadi MK, Rane S, et al. Hybrid biosynthetic gene therapy vector development and dual engineering capacity. Proc Natl Acad Sci U S A. 2014;111:12360–5. doi: 10.1073/pnas.1411355111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Jones CH, Ravikrishnan A, Chen M, Reddinger R, Kamal Ahmadi M, Rane S, et al. Hybrid biosynthetic gene therapy vector development and dual engineering capacity. Proc Natl Acad Sci U S A. 2014;111:12360–5. doi: 10.1073/pnas.1411355111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Jones CH, Chen CK, Chen M, Ravikrishnan A, Zhang H, Gollakota A, et al. PEGylated Cationic Polylactides for Hybrid Biosynthetic Gene Delivery. Mol Pharm. 2015;12:846–856. doi: 10.1021/mp500683c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Jones CH, Gollakota A, Chen M, Chung TC, Ravikrishnan A, Zhang G, et al. Influence of molecular weight upon mannosylated bio-synthetic hybrids for targeted antigen presenting cell gene delivery. Biomaterials. 2015;58:103–11. doi: 10.1016/j.biomaterials.2015.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Singh N, Karambelkar A, Gu L, Lin K, Miller JS, Chen CS, et al. Bioresponsive mesoporous silica nanoparticles for triggered drug release. J Am Chem Soc. 2011;133:19582–5. doi: 10.1021/ja206998x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Guerrero-Cazares H, Tzeng SY, Young NP, Abutaleb AO, Quinones-Hinojosa A, Green JJ. Biodegradable polymeric nanoparticles show high efficacy and specificity at DNA delivery to human glioblastoma in vitro and in vivo. Acs Nano. 2014;8:5141–53. doi: 10.1021/nn501197v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zhang Y, Satterlee A, Huang L. In vivo gene delivery by nonviral vectors: overcoming hurdles? Mol Ther. 2012;20:1298–304. doi: 10.1038/mt.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zhang Y, Satterlee A, Huang L. In vivo gene delivery by nonviral vectors: overcoming hurdles? Mol Ther. 2012;20:1298–304. doi: 10.1038/mt.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Minami K, Okamoto K, Doi K, Harano K, Noiri E, Nakamura E. siRNA delivery targeting to the lung via agglutination-induced accumulation and clearance of cationic tetraamino fullerene. Sci Rep. 2014;4:7. doi: 10.1038/srep04916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Mishra S, Webster P, Davis ME. PEGylation significantly affects cellular uptake and intracellular trafficking of non-viral gene delivery particles. European Journal of Cell Biology. 2004;83:97–111. doi: 10.1078/0171-9335-00363. [DOI] [PubMed] [Google Scholar]
- [56].Park YC, Smith JB, Pham T, Whitaker RD, Sucato CA, Hamilton JA, et al. Effect of PEG molecular weight on stability, T(2) contrast, cytotoxicity, and cellular uptake of superparamagnetic iron oxide nanoparticles (SPIONs) Colloids and surfaces B, Biointerfaces. 2014;119:106–14. doi: 10.1016/j.colsurfb.2014.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Pannier AK, Wieland JA, Shea LD. Surface polyethylene glycol enhances substrate-mediated gene delivery by nonspecifically immobilized complexes. Acta Biomater. 2008;4:26–39. doi: 10.1016/j.actbio.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ikeda Y, Nagasaki Y. Impacts of PEGylation on the gene and oligonucleotide delivery system. J Appl Polym Sci. 2014;131 n/a-n/a. [Google Scholar]
- [59].Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9:615–27. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- [60].Kolhar P, Mitragotri S. Polymer Microparticles Exhibit Size and Shape Dependent Accumulation around the Nucleus after Endocytosis. Adv Funct Mater. 2012;22:3759–64. [Google Scholar]
- [61].Chu ZQ, Zhang SL, Zhang BK, Zhang CY, Fang CY, Rehor I, et al. Unambiguous observation of shape effects on cellular fate of nanoparticles. Sci Rep. 2014:4. doi: 10.1038/srep04495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Singh S, Narang AS, Mahato RI. Subcellular Fate and Off-Target Effects of siRNA, shRNA, and miRNA. Pharm Res. 2011;28:2996–3015. doi: 10.1007/s11095-011-0608-1. [DOI] [PubMed] [Google Scholar]
- [63].Wolinsky JB, Colson YL, Grinstaff MW. Local drug delivery strategies for cancer treatment: gels, nanoparticles, polymeric films, rods, and wafers. J Control Release. 2012;159:14–26. doi: 10.1016/j.jconrel.2011.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Underhill GH, Galie P, Chen CS, Bhatia SN. Bioengineering methods for analysis of cells in vitro. Annual review of cell and developmental biology. 2012;28:385–410. doi: 10.1146/annurev-cellbio-101011-155709. [DOI] [PubMed] [Google Scholar]
- [65].Jones CH, Chen M, Ravikrishnan A, Reddinger R, Zhang G, Hakansson AP, et al. Mannosylated poly(beta-amino esters) for targeted antigen presenting cell immune modulation. Biomaterials. 2015;37:333–44. doi: 10.1016/j.biomaterials.2014.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Chen S-H, Zhaori G. Potential clinical applications of siRNA technique: benefits and limitations. European Journal of Clinical Investigation. 2011;41:221–32. doi: 10.1111/j.1365-2362.2010.02400.x. [DOI] [PubMed] [Google Scholar]
- [67].Svoboda P. Off-targeting and other non-specific effects of RNAi experiments in mammalian cells. Current Opinion in Molecular Therapeutics. 2007;9:248–57. [PubMed] [Google Scholar]
- [68].Li S-D, Chono S, Huang L. Efficient Oncogene Silencing and Metastasis Inhibition via Systemic Delivery of siRNA. Mol Ther. 2008;16:942–6. doi: 10.1038/mt.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Song E, Zhu P, Lee SK, Chowdhury D, Kussman S, Dykxhoorn DM, et al. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nature Biotechnology. 2005;23:709–17. doi: 10.1038/nbt1101. [DOI] [PubMed] [Google Scholar]
- [70].Wang P, Henning SM, Heber D. Limitations of MTT and MTS-Based Assays for Measurement of Antiproliferative Activity of Green Tea Polyphenols. PLoS ONE. 2010;5:e10202. doi: 10.1371/journal.pone.0010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kroll A, Pillukat MH, Hahn D, Schnekenburger J. Current in vitro methods in nanoparticle risk assessment: Limitations and challenges. European Journal of Pharmaceutics and Biopharmaceutics. 2009;72:370–7. doi: 10.1016/j.ejpb.2008.08.009. [DOI] [PubMed] [Google Scholar]
- [72].Torubarova NA, Baryshnikov A, Mambetova Ch D, Ni AN. [Antigenic determinants on membranes and monocyte function] Gematologiia i transfuziologiia. 1988;33:34–8. [PubMed] [Google Scholar]
- [73].Dane EL, Chin SL, Grinstaff MW. Synthetic enantiopure carbohydrate polymers that are highly soluble in water and noncytotoxic. ACS Macro Lett. 2013;2:887–90. doi: 10.1021/mz400394r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kratz F, Elsadek B. Clinical impact of serum proteins on drug delivery. Journal of Controlled Release. 2012;161:429–45. doi: 10.1016/j.jconrel.2011.11.028. [DOI] [PubMed] [Google Scholar]
- [75].Tseng Y-C, Mozumdar S, Huang L. Lipid-based systemic delivery of siRNA. Advanced Drug Delivery Reviews. 2009;61:721–31. doi: 10.1016/j.addr.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Holland JW, Hui C, Cullis PR, Madden TD. Poly(ethylene glycol)−lipid conjugates regulate the calcium-induced fusion of liposomes composed of phosphatidylethanolamine and phosphatidylserine. biochemistry. 1996;35:2618–24. doi: 10.1021/bi952000v. [DOI] [PubMed] [Google Scholar]
- [77].Kim YH, Park JH, Lee M, Kim Y-H, Park TG, Kim SW. Polyethylenimine with acid-labile linkages as a biodegradable gene carrier. Journal of Controlled Release. 2005;103:209–19. doi: 10.1016/j.jconrel.2004.11.008. [DOI] [PubMed] [Google Scholar]
- [78].Yin L, Song Z, Kim KH, Zheng N, Tang H, Lu H, et al. Reconfiguring the architectures of cationic helical polypeptides to control non-viral gene delivery. Biomaterials. 2013;34:2340–9. doi: 10.1016/j.biomaterials.2012.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Gilleron J, Querbes W, Zeigerer A, Borodovsky A, Marsico G, Schubert U, et al. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat Biotech. 2013;31:638–46. doi: 10.1038/nbt.2612. [DOI] [PubMed] [Google Scholar]
- [80].Rehman ZU, Hoekstra D, Zuhorn IS. Mechanism of polyplex- and lipoplex-mediated delivery of nucleic acids: real-time visualization of transient membrane destabilization without endosomal lysis. Acs Nano. 2013 doi: 10.1021/nn3049494. [DOI] [PubMed] [Google Scholar]
- [81].Brunner S, Sauer T, Carotta S, Cotten M, Saltik M, Wagner E. Cell cycle dependence of gene transfer by lipoplex, polyplex and recombinant adenovirus. Gene Therapy. 2000;7:401–7. doi: 10.1038/sj.gt.3301102. [DOI] [PubMed] [Google Scholar]
- [82].Fassati A. HIV infection of non-dividing cells: a divisive problem. Retrovirology. 2006;3:74. doi: 10.1186/1742-4690-3-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Govindarajan S, Kitaura K, Takafuji M, Ihara H, Varadarajan KS, Patel AB, et al. Gene delivery into human cancer cells by cationic lipid-mediated magnetofection. International Journal of Pharmaceutics. 2013;446:87–99. doi: 10.1016/j.ijpharm.2013.01.055. [DOI] [PubMed] [Google Scholar]
- [84].Schaffer DV, Lauffenburger DA. Optimization of cell surface binding enhances efficiency and specificity of molecular conjugate gene delivery. Journal of Biological Chemistry. 1998;273:28004–9. doi: 10.1074/jbc.273.43.28004. [DOI] [PubMed] [Google Scholar]
- [85].Ogris M, Steinlein P, Kursa M, Mechtler K, Kircheis R, Wagner E. The size of DNA/transferrin-PEI complexes is an important factor for gene expression in cultured cells. Gene Therapy. 1998;5:1425–33. doi: 10.1038/sj.gt.3300745. [DOI] [PubMed] [Google Scholar]
- [86].van Gaal EVB, van Eijk R, Oosting RS, Kok RJ, Hennink WE, Crommelin DJA, et al. How to screen non-viral gene delivery systems in vitro? Journal of Controlled Release. 2011;154:218–32. doi: 10.1016/j.jconrel.2011.05.001. [DOI] [PubMed] [Google Scholar]
- [87].Kastner E, Verma V, Lowry D, Perrie Y. Microfluidic-controlled manufacture of liposomes for the solubilisation of a poorly water soluble drug. Int J Pharm. 2015;485:122–30. doi: 10.1016/j.ijpharm.2015.02.063. [DOI] [PubMed] [Google Scholar]
- [88].Lai WF. Microfluidic methods for non-viral gene delivery. Current Gene Therapy. 2015;15:55–63. doi: 10.2174/1566523214666141114213915. [DOI] [PubMed] [Google Scholar]
- [89].Lei P, Padmashali RM, Andreadis ST. Cell-controlled and spatially arrayed gene delivery from fibrin hydrogels. Biomaterials. 2009;30:3790–9. doi: 10.1016/j.biomaterials.2009.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Bellis AD, Bernabe BP, Weiss MS, Shin S, Weng S, Broadbelt LJ, et al. Dynamic transcription factor activity profiling in 2D and 3D cell cultures. Biotechnol Bioeng. 2013;110:563–72. doi: 10.1002/bit.24718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Bae YH, Park K. Targeted drug delivery to tumors: Myths, reality and possibility. Journal of Controlled Release. 2011;153:198–205. doi: 10.1016/j.jconrel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Orcutt KD, Rhoden JJ, Ruiz-Yi B, Frangioni JV, Wittrup KD. Effect of small-molecule–binding affinity on tumor uptake in vivo: a systematic study using a pretargeted bispecific antibody. Molecular Cancer Therapeutics. 2012;11:1365–72. doi: 10.1158/1535-7163.MCT-11-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Au JLS, Jang SH, Zheng J, Chen CT, Song S, Hu L, et al. Determinants of drug delivery and transport to solid tumors. Journal of Controlled Release. 2001;74:31–46. doi: 10.1016/s0168-3659(01)00308-x. [DOI] [PubMed] [Google Scholar]
- [94].Zhang Y, Satterlee A, Huang L. In vivo gene delivery by nonviral vectors: overcoming hurdles. Mol Ther. 2012;20:1298–304. doi: 10.1038/mt.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11:812–8. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]
- [96].Wilke M, Fortunati E, van den Broek M, Hoogeveen AT, Scholte BJ. Efficacy of a peptide-based gene delivery system depends on mitotic activity. Gene Therapy. 1996;3:1133–42. [PubMed] [Google Scholar]
- [97].McGonigle P, Ruggeri B. Animal models of human disease: challenges in enabling translation. Biochem Pharmacol. 2014;87:162–71. doi: 10.1016/j.bcp.2013.08.006. [DOI] [PubMed] [Google Scholar]
- [98].Caldwell GW, Masucci JA, Yan Z, Hageman W. Allometric scaling of pharmacokinetic parameters in drug discovery: can human CL, Vss and t1/2 be predicted from in vivo rat data? European Journal of Drug Metabolism and Pharmacokinetics. 2004;29:133–43. doi: 10.1007/BF03190588. [DOI] [PubMed] [Google Scholar]
- [99].Fan J, de Lannoy IA. Pharmacokinetics. Biochem Pharmacol. 2014;87:93–120. doi: 10.1016/j.bcp.2013.09.007. [DOI] [PubMed] [Google Scholar]
- [100].Bass A, Kinter L, Williams P. Origins, practices and future of safety pharmacology. Journal of Pharmacological and Toxicological Methods. 2004;49:145–51. doi: 10.1016/j.vascn.2004.02.007. [DOI] [PubMed] [Google Scholar]
- [101].Ahuja V, Sharma S. Drug safety testing paradigm, current progress and future challenges: an overview. J Appl Toxicol. 2014;34:576–94. doi: 10.1002/jat.2935. [DOI] [PubMed] [Google Scholar]
- [102].Stevens JL, Baker TK. The future of drug safety testing: expanding the view and narrowing the focus. Drug Discov Today. 2009;14:162–7. doi: 10.1016/j.drudis.2008.11.009. [DOI] [PubMed] [Google Scholar]
- [103].Wolfe JH. Gene therapy in large animal models of human genetic diseases. ilar journal / national research council, institute of laboratory animal resources. 2009;50:107–11. doi: 10.1093/ilar.50.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Frese KK, Tuveson DA. Maximizing mouse cancer models. Nature Reviews Cancer. 2007;7:645–58. doi: 10.1038/nrc2192. [DOI] [PubMed] [Google Scholar]
- [105].Achermann Y, Tran B, Kang M, Harro JM, Shirtliff ME. Immunoproteomic Identification of in vivo-produced propionibacterium acnes proteins in a rabbit biofilm infection model. Clinical and Vaccine Immunology : CVI. 2015;22:467–76. doi: 10.1128/CVI.00760-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Trevejo-Nunez G, Chen K, Dufour JP, Bagby GJ, Horne WT, Nelson S, et al. Ethanol impairs mucosal immunity against Streptococcus pneumoniae infection by disrupting interleukin 17 gene expression. Infect Immun. 2015;83:2082–8. doi: 10.1128/IAI.02869-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Grossman TH, Murphy TM, Slee AM, Lofland D, Sutcliffe JA. Eravacycline (TP-434) Is Efficacious in Animal Models of Infection. Antimicrobial agents and chemotherapy. 2015;59:2567–71. doi: 10.1128/AAC.04354-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Mangraviti A, Tzeng SY, Kozielski KL, Wang Y, Jin Y, Gullotti D, et al. Polymeric nanoparticles for nonviral gene therapy extend brain tumor survival in vivo. Acs Nano. 2015;9:1236–49. doi: 10.1021/nn504905q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Bae H, Chu H, Edalat F, Cha JM, Sant S, Kashyap A, et al. Development of functional biomaterials with micro- and nanoscale technologies for tissue engineering and drug delivery applications. J Tissue Eng Regen Med. 2014;8:1–14. doi: 10.1002/term.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Green JJ, Zugates GT, Langer R, Anderson DG. Poly(beta-amino esters): Procedures for Synthesis and Gene Delivery. In: Belting M, editor. Methods in Molecular Biology. Humana Press Inc; 999 Riverview Dr, Ste 208, Totowa, Nj 07512-1165 USA: 2009. pp. 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Chen K, Xu J, Luft JC, Tian S, Raval JS, DeSimone JM. Design of asymmetric particles containing a charged interior and a neutral surface charge: comparative study on in vivo circulation of polyelectrolyte microgels. J Am Chem Soc. 2014;136:9947–52. doi: 10.1021/ja503939n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Ansorge S, Henry O, Kamen A. Recent progress in lentiviral vector mass production. Biochem Eng J. 2010;48:362–77. [Google Scholar]
- [113].DeMuth PC, Min Y, Huang B, Kramer JA, Miller AD, Barouch DH, et al. Polymer multilayer tattooing for enhanced DNA vaccination. Nat Mater. 2013;12:367–76. doi: 10.1038/nmat3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Shah NJ, Hyder MN, Quadir MA, Dorval Courchesne NM, Seeherman HJ, Nevins M, et al. Adaptive growth factor delivery from a polyelectrolyte coating promotes synergistic bone tissue repair and reconstruction. Proc Natl Acad Sci U S A. 2014;111:12847–52. doi: 10.1073/pnas.1408035111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Castleberry SA, Li W, Deng D, Mayner S, Hammond PT. Capillary flow layer-by-layer: a microfluidic platform for the high-throughput assembly and screening of nanolayered film libraries. Acs Nano. 2014;8:6580–9. doi: 10.1021/nn501963q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Min J, Braatz RD, Hammond PT. Tunable staged release of therapeutics from layer-by-layer coatings with clay interlayer barrier. Biomaterials. 2014;35:2507–17. doi: 10.1016/j.biomaterials.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Liang W, Chow MY, Lau PN, Zhou QT, Kwok PC, Leung GP, et al. Inhalable dry powder formulations of siRNA and pH-responsive peptides with antiviral activity against H1N1 influenza virus. Mol Pharm. 2015;12:910–21. doi: 10.1021/mp500745v. [DOI] [PubMed] [Google Scholar]
- [118].Ohtake S, Martin RA, Saxena A, Lechuga-Ballesteros D, Santiago AE, Barry EM, et al. Formulation and stabilization of Francisella tularensis live vaccine strain. Journal of pharmaceutical sciences. 2011;100:3076–87. doi: 10.1002/jps.22563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Kandamchira A, Kanungo I, Fathima NN. Dielectric behaviour and conformational stability of collagen on interaction with DNA. International journal of biological macromolecules. 2012;51:635–9. doi: 10.1016/j.ijbiomac.2012.06.039. [DOI] [PubMed] [Google Scholar]
- [120].Lorincz O, Toke ER, Somogyi E, Horkay F, Chandran PL, Douglas JF, et al. Structure and biological activity of pathogen-like synthetic nanomedicines. Nanomedicine-Uk. 2012;8:497–506. doi: 10.1016/j.nano.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].van der Heijden I, Beijnen JH, Nuijen B. Long term stability of lyophilized plasmid DNA pDERMATT. Int J Pharm. 2013;453:648–50. doi: 10.1016/j.ijpharm.2013.06.010. [DOI] [PubMed] [Google Scholar]
- [122].Ayen WY, Kumar N. A systematic study on lyophilization process of polymersomes for long-term storage using doxorubicin-loaded (PEG)(3)-PLA nanopolymersomes. Eur J Pharm Sci. 2012;46:405–14. doi: 10.1016/j.ejps.2012.03.005. [DOI] [PubMed] [Google Scholar]