Abstract
Medication nonadherence contributes to morbidity/mortality, but adherence interventions yield small and inconsistent effects. Understanding the mechanisms underlying initiation and maintenance of adherence could improve interventions. The National Institutes of Health (NIH) support adherence research, but it is unclear whether existing NIH-funded research incorporates mechanisms. We conducted a systematic review to determine the proportion of NIH-funded adherence trials that have tested hypothesized mechanisms of intervention effects.
We included randomized and quasi-randomized NIH-funded trials with medication adherence in adults as the primary outcome. Studies were identified by searching electronic databases from inception to 6/2016, references, and clinicaltrials.gov.
Two of 18 (11%) NIH-funded trials tested a hypothesized mechanism of an intervention’s effect on medication adherence. Another 44 studies with medication adherence as a secondary outcome were described in protocol form, and are either ongoing or never published results, but none mentioned mechanism tests. Overall, 3% of NIH-funded trials with adherence as an outcome conducted, or plan to conduct, tests of behavior change mechanisms.
These results mirror previous findings that very few studies of behavior change interventions actually test the mechanism by which the intervention is hypothesized to improve health behaviors. We must understand mechanisms if we are to improve the effectiveness of interventions.
Keywords: medication adherence, mechanisms, interventions
Introduction
The global rise in chronic disease has been referred to as a “slow-motion disaster” (Chan, 2011). Chronic diseases, including cardiovascular disease, diabetes, cancer, and obesity, are the most common and costly causes of morbidity and mortality across the globe. As of 2012, approximately half of all adults in the United States (U.S.)—117 million individuals—reported having at least one chronic health condition, and 1 in 4 adults were afflicted by multiple chronic conditions (Ward, Schiller, & Goodman, 2014). These chronic diseases account for more than 60% of deaths worldwide (Bloom et al., 2011), as well as the vast majority of health care spending in most countries (86% as of 2010 in the US) (Gerteis et al., 2014). Further, the global economic burden of life lost due to chronic diseases is expected to double from 2010 to 2030, with 2030 estimates exceeding $40 trillion in 2010 U.S. dollars (Bloom et al., 2011).
Human behaviors are among the most critical factors that determine chronic disease onset and management, with behavior accounting for approximately 40% of the risk associated with preventable premature deaths in the U.S (Mokdad, Marks, Stroup, & Gerberding, 2004; Yoon, Bastian, Anderson, Collins, & Jaffe, 2014). One of the most crucial health behaviors for chronic disease prevention and management is medication adherence. Indeed, organizations such as the Agency for Healthcare Research and Quality and the World Health Organization have called for the implementation of strategies for improving medication adherence as a means for improving global health. However, no such implementation has yet occurred, and as many as 50% of patients remain nonadherent to treatments prescribed for chronic health conditions (Naderi, Bestwick, & Wald).
The case of nonadherence to antihypertensive medications illustrates the current state of affairs. Nearly one in three (80 million) US adults have a diagnosis of hypertension, and 45% of them have uncontrolled hypertension (BP>140/90 mmHg), which substantially increases their risk for stroke and heart disease, at a cost of $48 billion annually (Cutler et al., 2008; Mozaffarian et al., 2016). A recent meta-analysis of six studies reported that the odds of controlled BP was 3.4 (95% CI, 1.6–7.4) times greater among adherent versus nonadherent patients (DiMatteo, 2004). Nonadherent patients are at 38% increased risk for cardiovascular (CV) events compared to those who are adherent (Bramley, Gerbino, Nightengale, & Frech-Tamas, 2006; Ho, Bryson, & Rumsfeld, 2009; Mazzaglia et al., 2009), but among Medicare beneficiaries, 25% refill their antihypertensives less than 80% of the time (Ritchey et al., 2016). Further, within 1 year of being prescribed an antihypertensive, 50% of all hypertensive patients will have stopped taking it all together (Dragomir et al., 2010; Vrijens, Vincze, Kristanto, Urquhart, & Burnier, 2008).
Simulation research has become a useful tool in providing researchers, clinicians, and policy-makers with informative estimates of implementation cost-effectiveness, quality-adjusted life years, and drug costs for chronic diseases and their respective treatments (Murray, Evans, Acharya, & Baltussen, 2000). Using epidemiological and computer-simulation models, researchers have been able to estimate the cost-effectiveness of hypertension treatments in relation to adherence rates. Simulation models suggest that treating hypertension according to clinical guidelines is cost-effective, but becomes low value costing greater than $50,000 per quality adjusted life year when refill rates dip below 50% (Moise et al., 2016). Hypertension nonadherence costs are associated with higher disease-related medical costs and higher hospitalization rates (Sokol, McGuigan, Verbrugge, & Epstein, 2005). Estimates have placed hypertension nonadherence costs anywhere between $1836 and $4170 per capita, as measured through disease-related cost (drug cost, medical cost, or utilization) associated with the target condition identified by pharmacy and medical claims (Nasseh, Frazee, Visaria, Vlahiotis, & Tian, 2012; Roebuck, Liberman, Gemmill-Toyama, & Brennan, 2011). Despite affordable and effective clinical recommendations, medication nonadherence act as a significant and expensive barrier to treatment.
Stated simply, medications cannot be effective if patients do not take them, and the entire healthcare system is negatively affected by nonadherence (Chowdhury et al., 2013; Sokol et al., 2005). The scope and consequences of the problem are clear. Thus, nonadherence—in cardiovascular disease and other chronic conditions – is a national priority in order to reduce avoidable costs and improve patient outcomes (Anderson, 1987). One major issue remaining for researchers and policy makers is to determine what should be done to improve adherence.
Many randomized controlled trials (RCTs) have evaluated adherence interventions, yet systematic reviews have repeatedly concluded that, despite decades of research, there is no gold-standard or consistently effective intervention (Fahey, Schroeder, & Ebrahim, 2006; Gwadry-Sridhar et al., 2013; Haynes, Ackloo, Sahota, McDonald, & Yao, 2008; Kripalani, Yao, & Haynes, 2007; McDonald, Garg, & Haynes, 2002). While some interventions result in modest improvements in adherence, very few are sufficient to impact disease onset or course. Further, those few interventions that have statistically significantly (but not substantially) improved medication adherence are complex and multifaceted, thus requiring heavy investments of resources to effectively implement (Fahey et al., 2006). Moreover, the complexity of the interventions, and the field’s historical lack of commitment to early phase testing of target mechanisms of behavior change, have made it challenging to understand which components of the intervention “worked” and should be retained for future intervention development. As a result, these medication adherence interventions (perhaps rightly) are not being widely adopted by health care systems. So how do we develop potent, generalizable, and scalable adherence interventions that are so urgently needed (Bosworth et al., 2011)?
The answer is certainly not to continue to do what we have always done. In the prevailing paradigm, many iterations of the cycle of pilot studies→multicomponent conventional RCT→post-hoc exploratory analysis→revision via new pilot studies→revised RCT is required to optimize a behavioral intervention. Factoring in the time from submission to funding for each step in this cycle, it is easy to see how this laborious approach yields outdated, suboptimal interventions. Indeed, the multiple iterations that are required for optimization never occur. Similarly, this conventional approach does not apply a standardized taxonomy to the labeling of intervention components, or hypothesized mechanisms of action. This has created confusion about how to generalize knowledge about previous interventions -- knowledge that is essential to accurately replicating, faithfully implementing, and further optimizing interventions. So after decades of research, years of investigator and participant time devoted RCTs, and millions of dollars to support these trials, we still know little about how to improve medication adherence in patients at risk for, or managing, chronic diseases.
Behavioral medicine researchers might look to other approaches to developing “treatments” for medication nonadherence. Ironically, one such approach can be borrowed from the field of experimental medicine that is responsible for developing many of the medications that patients are not adhering to. The experimental medicine approach focuses on early phase identification of intermediate targets on the causal pathway to disease, reliable measurement of those targets, and demonstration that a compound engages that target, followed by observation of the influence of target engagement on relevant disease outcomes. One reason that behavior change interventions have been largely ineffective, particularly with respect to medication adherence interventions, is that the field has not systematically used this approach to identify underlying cognitive, affective, neurological, interpersonal, and behavioral mechanisms that drive behavior change.
Observational research provides insight on where to look for potential intermediate targets (also known as mechanisms) on the pathway to medication nonadherence. For example, patient beliefs about medications and their relationship with prescribing physicians have been repeatedly shown to be associated with noninitiation and premature discontinuation of medications. (Zeber et al., 2013) Depression,(Kronish et al., 2013) PTSD,(Kronish, Edmondson, Goldfinger, Fei, & Horowitz, 2012; Kronish, Edmondson, Li, & Cohen, 2012; Kronish, Lin, Cohen, Voils, & Edmondson, 2014) anxiety sensitivity,(Alcántara et al., 2014) and other psychological phenomena have also been associated with nonadherence. These factors may do so in part because of their influence on patients’ beliefs in their ability to adhere to medications or in their actual capability of adhering to complex regimens.(Edmondson, 2014; Edmondson, Horowitz, Goldfinger, Fei, & Kronish, 2013) These findings suggests that promising intermediate targets for medication adherence interventions might relate to self-efficacy or self-regulation strength, for example. Unfortunately, few adherence interventions are designed to influence mechanisms, and even fewer report tests of the intervention’s influence on these putative mechanisms. As a result, cumulative behavior change science based on evidence of known mechanisms of action has remained elusive.
To foster the development of a comprehensive, rigorous, and transparent science of behavior change, the National Institutes of Health (NIH) spearheaded systematic efforts aimed at transforming behavior change research that are interdisciplinary, innovative, and rigorous. Indeed, the initiatives of the NIH Science of Behavior Change (SOBC) Common Fund Program represent a fundamental change in the way that research on the initiation, personalization, and maintenance of behavior change is being conducted and applied. The SOBC Program aims to improve the field of behavior change research by focusing on mechanisms of behavior change across a broad range of health-related behaviors and outcomes. Furthermore, rather than perpetuating a balkanized science where basic scientists study the neurobiological, affective, and motivational mechanisms that underlie behavior change in isolation from applied scientists working to develop behavior change interventions, the SOBC Program supports cross-cutting research that integrates basic and translational science.(Czajkowski et al., 2015; Onken, Carroll, Shoham, Cuthbert, & Riddle, 2014) The goal of the program is to implement a mechanisms-focused, experimental medicine approach to behavior change research. With this approach, researchers identify putative intervention targets that are highly relevant to understanding mechanisms of behavior change and then develop and test measures of these targets. Researchers subsequently engage these targets through experimentation and/or intervention, and assess the degree to which target engagement produces desired behavior changes. The experimental medicine approach thus provides a systematic, rigorous, and reproducible method for identifying the mechanisms that drive successful behavior change. Ultimately, this knowledge will be used to develop more effective and efficient behavioral interventions by providing a set of validated measures of early phase intervention targets, and a systematic method for developing new measures of as yet undiscovered targets.
All SOBC Network projects are expected to have an adherence focus, in part because medication adherence has such a large impact on health outcomes and costs, but also because mechanisms underlying adherence to one chronic disease regimen are likely to be applicable to other chronic disease and, potentially, health behaviors more broadly. Furthermore, one of the goals of the SOBC Research Network is to develop a registry of validated measures of potential mechanisms that play a key role in health behaviors (i.e., intermediate targets) and that can be incorporated in future trials and studies of adherence interventions. In these efforts, the SOBC Research Network aims to define and operationalize a systematic development pipeline for including new measures in the registry, akin to the drug development pipeline.
Yet, there is a gap in understanding the frequency and types of behavioral mechanisms that have been studied in a manner consistent with the SOBC’s experimental medicine approach. Thus, we undertook a systematic review to identify the proportion NIH funded medication adherence trials that have tested the mechanisms by which their effect on adherence operates through a hypothesized mechanistic target, consistent with the SOBC experimental medicine approach. Further, we sought to determine which types of mechanisms have been tested in this manner.
Methods
Search Strategy and selection criteria
We sought to determine the proportion of NIH-funded behavioral intervention trials to improve medication adherence in adults that measured and tested hypothesized mechanisms of behavior change, as well as to identify the types of mechanisms already being studied. Potentially relevant articles were identified by searching the biomedical electronic databases Ovid MEDLINE, EMBASE, The Cochrane Library, CINAHL, and PsycINFO, and NIH Reporter. Databases were searched from inception to June 2016. All relevant subject headings and free-text terms were used to represent medication adherence and behavior change. We applied a search filter to limit results to studies funded by NIH only (see Appendix for all search strategies). Ongoing studies were also sought through Clinicaltrials.gov. Additional records were identified by scanning the reference lists of relevant studies and reviews, by employing the Similar Articles feature in PubMed, and the Cited Reference Search in ISI Web of Science. Randomized and quasi-randomized studies were included. We excluded observational studies that included cross-sectional, retrospective, prospective or longitudinal designs. Only studies funded by the National Institutes of Health were included. This review protocol was pre-registered in Prospero (2016:CRD42016043626).
Database Construction and Coding
Two review authors independently scanned the title and/or abstract of all search results to determine which studies required further assessment. Two review authors then investigated all potentially-relevant articles as full text. Full articles were retrieved for further assessment if the information given in the title/abstract suggested that the study 1) was NIH-funded 2) was an intervention to change behavior in medication adherence, 3) was randomized or quasi-randomized, and 4) included participants of 18 years of age or older. At each stage of search and data extraction, discrepancies were resolved through consensus or recourse to a third review author.
Data extraction
For studies that met the inclusion criteria, two review authors independently abstracted key participant and intervention characteristics, as well as information about hypothesized and tested mechanisms. We report data on: year of publication, condition, medication type, adherence assessment, and mechanism(s).
Strategy for data synthesis
Our initial plan for synthesizing data was to examine whether interventions were more effective when mechanisms were measured, and to analyze the differential association of classes of mechanisms with intervention effect size. We also planned to perform a sensitivity analysis for RCT vs quasi randomized studies. Due to the paucity of studies that included mechanisms, we did not synthesize the data.
Results
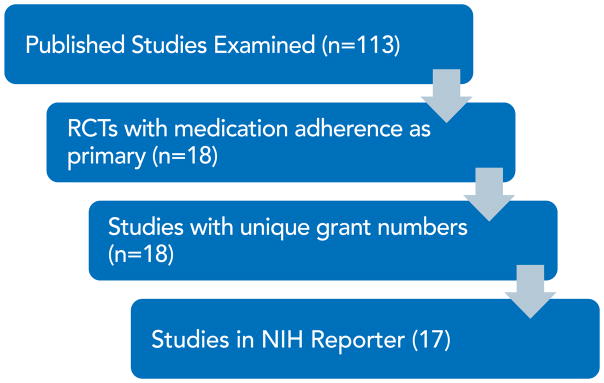
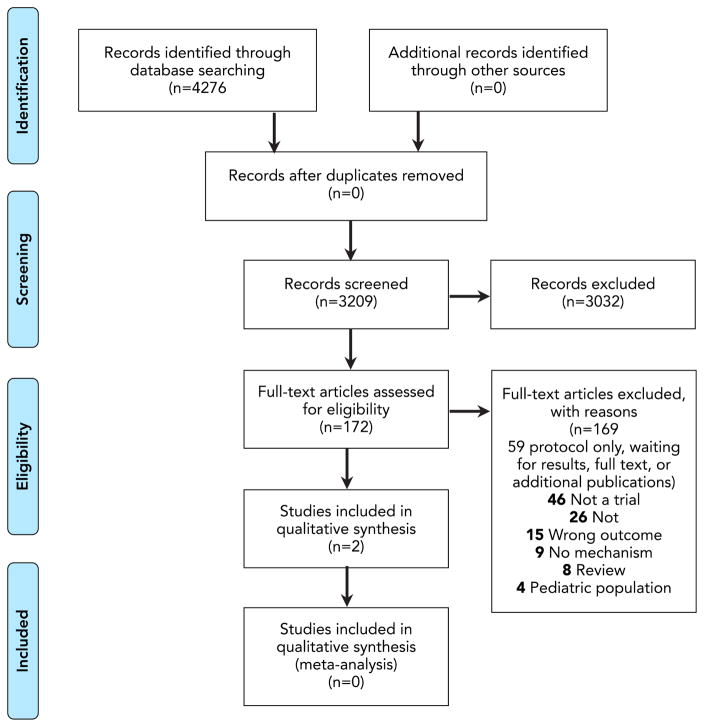
The PRISMA diagram in Figure 1 describes the review process. After removing duplicate records, we searched the titles and abstracts of 3,209 published articles, other published works, and NIH Reporter records. The two independent reviewers identified 172 articles that required full text review, and 18 were determined to be NIH funded studies of interventions with adherence as the primary outcome (Figure 2). Of those, 2 reported testing hypothesized mechanisms of action.(Feaster et al., 2010; Kopelowicz et al., 2015) Another 44 studies were identified as NIH intervention studies for which adherence is a secondary outcome, but none have yet published on that outcome, nor on tests of the intervention’s influence on a hypothesized mechanism.
Figure 1.
PRISMA Diagram
Figure 2.
Number of NIH-funded trials with medication adherence as primary outcome
The study described by Feaster and colleagues targeted interpersonal processes, broadly speaking, as an approach to improving adherence to HIV medications in patients in recovery from substance abuse. More specifically, consistent with Structural Ecosystems Theory (SET), the authors sought to test a family-based intervention that was intended to reduce psychological distress, family-related irritation, which in turn were expected to decrease risk of relapse and improve medication adherence. Importantly, the authors had previously tested the impact of such an intervention on these intermediate targets (Feaster et al., 2010; Szapocznik et al., 2004). Despite this theory-driven, experimental medicine approach, the intervention did not produce the hypothesized improvement in HIV medication adherence. The study described by Kopelowicz and colleagues tested the impact of a multifamily group intervention on three proposed mechanisms relevant to the Theory of Planned Behavior (subjective norms-social influences, attitudes toward medication, perceived behavioral control of resources) that were in turn postulated to influence adherence to antipsychotic medication in patients with schizophrenia. They also used path analysis to test whether changes in measures of these mechanisms mediated the intervention’s effect on medication adherence. They learned that their intervention was mediated by changes in subjective norms, but not by changes in attitudes toward medications nor in perceived behavioral control.
Discussion
In this systematic review, we found that only 3% of NIH funded trials with adherence as an outcome conducted, or explicitly plan to conduct, tests of hypothesized mechanisms of behavior change to determine the pathways by which interventions may improve medication adherence. These results agree with recent work by behavior change researchers in the UK, who found a similar dearth of mechanism tests in behavior change trials more generally. Carey, Michie and colleagues (in press) recently reported that few behavior change intervention studies mention mechanisms, and only 3% of all behavior change trials across health behavior targets actually test the mechanism by which the intervention is hypothesized to improve health behaviors.
Of course, in many fields, journal guidelines for manuscript length and authors’ decisions about how best to communicate their findings necessitate that manuscripts describing the mechanism by which an intervention produces change are published after the “main paper” describing the intervention’s effect on its intended behavioral target. As such, reviews such as ours would necessarily underestimate the proportion of studies that report the mechanism of influence (as mechanism papers may follow the main paper by months or years). However, even if another 25% of the studies included in this review were to subsequently publish a paper describing the mechanism of the intervention’s effect (which is unlikely, given the year of publication of most studies), many would simply test mediation paths across a number of plausible mechanisms and report on those post hoc tests. In that case, the fundamental problem that is the focus of this manuscript would remain. Namely, that behavioral interventions are not currently designed and optimized to influence target mechanisms on the causal pathway to behavior change from the outset. Indeed, the overwhelming majority of trials do not even mention mechanisms. Therefore, when an intervention works, we cannot completely trust that we know why it worked, and when it fails, we do not know why it failed.
The SOBC program in the US, and related efforts in the UK and around the world are now focusing on applying rigorous scientific techniques to improve behavior change research, and developing validated tools for assessing mechanisms of behavior change for use by the broader behavior change community. For example, as part of the Human Behaviour Change Project, researchers from the University College London Centre for Behaviour Change are focused on the question: What behavior change interventions work, how well, for whom, in what setting, for what behaviors, and why?—by first applying an ontology of behavior change research to the scientific literature, and then partnering with computer scientists to extract and organize relevant information from scientific reports using reasoning and machine learning.(University College London, 2016) These international efforts reflect the growing awareness that a fundamental shift toward an early phase experimental medicine approach to identifying mechanisms of behavior change is necessary and that partnerships with a broad spectrum of scientific disciplines such as decision neuroscience, behavioral economics, affective science, prevention research, developmental psychology among many others will be needed if we wish to achieve our goals of improving medication adherence
If the field of behavioral medicine research is to improve on the small-moderate effect sizes that characterize our interventions, we must begin optimizing interventions to influence the mechanisms that actually drive the initiation and maintenance of behavior change. In doing so, we will not only improve the effectiveness of interventions, but also their generalizability to multiple health behaviors and multiple health conditions and settings. This shift to an experimental medicine approach requires that we also improve the transparency, integrity, and reproducibility of behavior change science. All too often, in fields relevant to behavioral sciences (as well as many other scientific endeavors), there have been missed opportunities to share research findings along with issues related to lack of reproducibility of results and failure to publish negative findings. SOBC Research Network members are embracing openness in their research by sharing their hypotheses and methods with the broader scientific community and the public via http://www.scienceofbehaviorchange.org and the Open Science Framework, a free, open source service of the Center for Open Science.(Nosek et al., 2015)
To improve our ability understand behavior change and effectively support it, our field will need to accomplish the following five tasks: 1) Develop a common experimental medicine approach for understanding behavior change; 2) Develop and test valid measures for assessing mechanisms of behavior change; 3) Optimize interventions through early phase targeting of mechanisms to promote effectiveness; 4) Conduct collaborative research to determine the robustness of measures of mechanisms for the benefit of the entire behavioral science community to reduce inefficiency in scientific efforts; and 5) Increase transparency at all stages of mechanism identification and intervention development. Fortunately, the SOBC Program has been designed to support each of these tasks. Given the paucity of existing published adherence research following this experimental medicine approach, our review suggests that substantial efforts are needed to promote this research agenda to the scientific community.
Since the experimental medicine approach revolutionized the field of drug development, hundreds of medications have been discovered that have contributed to advances in life expectancy and quality of life. Unfortunately, advances in the understanding of how to optimize adherence to these medications have not kept up with the progress in drug discovery. To realize the full benefits of these medications, whether it be statins for reducing cardiovascular disease or antiviral medications for reducing adverse outcomes from HIV, now is the time to apply the experimental medicine approach to the science of human behavior.
Highlights.
Nonadherence to medications is common and associated with poor patient outcomes
Interventions to improve adherence have been moderately successful, at best
The experimental medicine approach could improve interventions
NIH funded adherence interventions have not used an experimental medicine approach
A very small proportion explicitly tested a hypothesized mechanism of action
Acknowledgments
Funding: This research was supported by the National Institute On Aging of the National Institutes of Health under Award Number U24AG052175. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendix
A systematic review of the inclusion of mechanisms of action in NIH-funded intervention trials to improve medication adherence: Search Strategies
MEDLINE (Ovid) 1946 to June Week 2 2016 and Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations June 20, 2016
exp drug therapy/
dt.fs.
(medication$ or drug$).tw.
or/1–3
exp Patient Compliance/
(adhere$ or nonadhere$ or comply or complies or complian$ or noncomplian$ or discontinu$).tw.
5 or 6
4 and 7
Medication Adherence/
8 or 9
exp “National Institutes of Health (U.S.)”/
((national adj2 institute$) or nih).tw.
national library of medicine.tw.
(nci or nei or nhlbi or nhgri or nia or niaid or niams or nibib or nichd or nidcd or nidcr or niddk or nida or niehs or nigms or nimh or ninds or ninr or nlm).tw.
((center adj information technology) or cit).tw.
((center adj scientific review) or csr).tw.
(fogarty international center or fic).tw.
(National Center for Advancing Translational Sciences or NCATS).tw.
((National Center adj2 Integrative Health) or nccih).tw.
or/11–19
10 and 20
exp animals/not humans.sh.
21 not 22
limit 23 to english language
CENTRAL Searched 06/21/2016
-
#1
MeSH descriptor: [Drug Therapy] explode all trees
-
#2
Any MeSH descriptor with qualifier(s): [Drug therapy - DT]
-
#3
(medication* or drug*):ti,ab
-
#4
#1 or #2 or #3
-
#5
MeSH descriptor: [Patient Compliance] explode all trees
-
#6
(adhere* or nonadhere* or comply or complies or complian* or noncomplian* or discontinu*):ti,ab
-
#7
#5 or #6
-
#8
#4 and #7
-
#9
MeSH descriptor: [Medication Adherence] explode all trees
-
#10
#8 or #9
-
#11
MeSH descriptor: [National Institutes of Health (U.S.)] explode all trees
-
#12
((national NEAR.2 institute*) or nih):ti,ab
-
#13
“national library of medicine”:ti,ab
-
#14
(nci or nei or nhlbi or nhgri or nia or niaid or niams or nibib or nichd or nidcd or nidcr or niddk or nida or niehs or nigms or nimh or ninds or ninr or nlm):ti,ab
-
#15
(“center for information technology” or cit):ti,ab
-
#16
(“center for scientific review” or csr):ti,ab
-
#17
(“fogarty international center” or fic):ti,ab
-
#18
(“National Center for Advancing Translational Sciences” or “National Center for Complementary and Integrative Health”):ti,ab or (NCATS or nccih):ti,ab
-
#19
#11 or #12 or #13 or #14 or #15 or #16 or #17 or #18
-
#20
#10 and #19
EMBASE (EMBASE.com) 1980 to 21 Jun 2016
-
#20
#10 AND #19 AND [humans]/lim AND [english]/lim AND [embase]/lim
-
#19
#11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18
-
#18
‘national center for advancing translational sciences’:ab,ti OR ‘national center for complementary and integrative health’:ab,ti OR ncats:ab,ti OR nccih:ab,ti
-
#17
‘fogarty international center’:ab,ti OR fic:ab,ti
-
#16
‘center for scientific review’:ab,ti OR csr:ab,ti
-
#15
‘center for information technology’:ab,ti OR cit:ab,ti
-
#14
nci:ab,ti OR nei:ab,ti OR nhlbi:ab,ti OR nhgri:ab,ti OR nia:ab,ti OR niaid:ab,ti OR niams:ab,ti OR nibib:ab,ti OR nichd:ab,ti OR nidcd:ab,ti OR nidcr:ab,ti OR niddk:ab,ti OR nida:ab,ti OR niehs:ab,ti OR nigms:ab,ti OR nimh:ab,ti OR ninds:ab,ti OR ninr:ab,ti OR nlm:ab,ti
-
#13
‘national library of medicine’:ab,ti
-
#12
(national NEXT/2 institute*):ab,ti OR nih:ab,ti
-
#11
‘national health organization’/exp
-
#10
#8 OR #9
-
#9
‘medication compliance’/de
-
#8
#4 AND #7
-
#7
#5 OR #6
-
#6
adhere*:ab,ti OR nonadhere*:ab,ti OR comply:ab,ti OR complies:ab,ti OR complian*:ab,ti OR noncomplian*:ab,ti OR discontinu*:ab,ti
-
#5
‘patient compliance’/exp
-
#4
#1 OR #2 OR #3
-
#3
medication*:ab,ti OR drug*:ab,ti
-
#2
‘drug therapy’/lnk
-
#1
‘drug therapy’/exp
PsycINFO (Ovid) 1806 to June Week 3 2016
exp drug therapy/
(medication$ or drug$).tw.
1 or 2
treatment compliance/
(adhere$ or nonadhere$ or comply or complies or complian$ or noncomplian$ or discontinu$).tw.
4 or 5
3 and 6
((national adj2 institute$) or nih).tw.
national library of medicine.tw.
(nci or nei or nhlbi or nhgri or nia or niaid or niams or nibib or nichd or nidcd or nidcr or niddk or nida or niehs or nigms or nimh or ninds or ninr or nlm).tw.
((center adj information technology) or cit).tw.
((center adj scientific review) or csr).tw.
(fogarty international center or fic).tw.
(National Center for Advancing Translational Sciences or NCATS).tw.
((National Center adj2 Integrative Health) or nccih).tw.
or/8–15
7 and 16
limit 17 to (human and english language)
CINAHL (EBSCOHost) 1981 to June 21, 2016
-
S1
(MH “Drug Therapy+”)
-
S2
TI (medication* OR drug*) OR AB (medication* OR drug*)
-
S3
S1 OR S2
-
S4
(MH “Patient Compliance+”)
-
S5
TI (adhere* OR nonadhere* OR comply OR complies OR complian* OR noncomplian* OR discontinu*) OR AB (adhere* OR nonadhere* OR comply OR complies OR complian* OR noncomplian* OR discontinu*)
-
S6
S6 S4 OR S5
-
S7
S3 AND S6
-
S8
(MH “Medication Compliance”)
-
S9
S7 OR S8
-
S10
(MH “National Institutes of Health (U.S.)+”)
-
S11
TI national N2 institute* OR AB national N2 institute* OR TI nih OR AB nih
-
S12
TI “national library of medicine” OR AB “national library of medicine”
-
S13
TI (nci OR nei OR nhlbi OR nhgri OR nia OR niaid OR niams OR nibib OR nichd OR nidcd OR nidcr OR niddk OR nida OR niehs OR nigms OR nimh OR ninds OR ninr OR nlm) OR AB (nci OR nei OR nhlbi OR nhgri OR nia OR niaid OR niams OR nibib OR nichd OR nidcd OR nidcr OR niddk OR nida OR niehs OR nigms OR nimh OR ninds OR ninr OR nlm)
-
S14
TI “center for information technology” OR AB cit OR TI “center for information technology” OR AB cit
-
S15
TI “center for scientific review” OR AB csr OR TI “center forscientific review” OR AB csr
-
S16
TI “fogarty international center” OR AB fic OR TI “fogarty international center” OR AB fic
-
S17
TI “National Center for Advancing Translational Sciences” OR AB “National Center for Advancing Translational Sciences” OR TI NCATS OR AB NCATS OR TI (“National Center for Complementary and Integrative Health”) OR AB (“National Center for Complementary and Integrative Health”) OR TI NCCIH OR AB NCCIH
-
S18
S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17
-
S19
S9 AND S18 Limiters - English Language; Human
ClinicalTrials.gov Searched June 22, 2016
medication adherence | Closed Studies| Studies with Results | Interventional Studies
NIH Reporter Searched July 22, 2016
(adhere or nonadhere or comply or complies or complian or noncomplian or discontinu) AND (drug or medication) in Advanced Text search. Limited to USA and Territories in Country
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alcántara C, Edmondson D, Moise N, Oyola D, Hiti D, Kronish IM. Anxiety sensitivity and medication nonadherence in patients with uncontrolled hypertension. Journal of Psychosomatic Research. 2014;77(4):283–286. doi: 10.1016/j.jpsychores.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JC. An Approach for Confirmatory Measurement and Structural Equation Modeling of Organizational Properties. Management Science. 1987;33(4):525–541. doi: 10.1287/mnsc.33.4.525. [DOI] [Google Scholar]
- Bloom D, Cafiero E, Jané-Llopis E, Abrahams-Gessel S, Bloom L, Fathima S, … Weinsten C. The global economic burden of noncommunicable diseases. Geneva: World Economic Forum; 2011. [Google Scholar]
- Bosworth HB, Granger BB, Mendys P, Brindis R, Burkholder R, Czajkowski SM, … Granger CB. Medication adherence: a call for action. American Heart Journal. 2011;162(3):412–424. doi: 10.1016/j.ahj.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramley TJ, Gerbino PP, Nightengale BS, Frech-Tamas F. Relationship of blood pressure control to adherence with antihypertensive monotherapy in 13 managed care organizations. Journal of Managed Care Pharmacy. 2006;12(3):239–245. doi: 10.18553/jmcp.2006.12.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan M. Noncommunicable diseases damage health, including economic health. 2011 Retrieved from www.who.int/dg/speeches/2011/un_ncds_09_19/en.
- Chowdhury R, Khan H, Heydon E, Shroufi A, Fahimi S, Moore C, … Franco OH. Adherence to cardiovascular therapy: a meta-analysis of prevalence and clinical consequences. European Heart Journal. 2013;34(38):2940–2948. doi: 10.1093/eurheartj/eht295. [DOI] [PubMed] [Google Scholar]
- Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension. 2008;52(5):818–827. doi: 10.1161/HYPERTENSIONAHA.108.113357. [DOI] [PubMed] [Google Scholar]
- Czajkowski SM, Powell LH, Adler N, Naar-King S, Reynolds KD, Hunter CM, … Charlson ME. From ideas to efficacy: The ORBIT model for developing behavioral treatments for chronic diseases. Health Psychology. 2015;34(10):971–982. doi: 10.1037/hea0000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Medical Care. 2004;42(3):200–209. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- Dragomir A, Cote R, Roy L, Blais L, Lalonde L, Berard A, Perreault S. Impact of adherence to antihypertensive agents on clinical outcomes and hospitalization costs. Medical Care. 2010;48(5):418–425. doi: 10.1097/MLR.0b013e3181d567bd. [DOI] [PubMed] [Google Scholar]
- Edmondson D. An enduring somatic threat model of posttraumatic stress disorder due to acute life-threatening medical events. Social and Personality Psychology Compass. 2014;8(3):118–134. doi: 10.1111/spc3.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D, Horowitz CR, Goldfinger JZ, Fei K, Kronish IM. Concerns about medications mediate the association of posttraumatic stress disorder with adherence to medication in stroke survivors. British Journal of Health Psychology. 2013;18(4):799–813. doi: 10.1111/bjhp.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey T, Schroeder K, Ebrahim S. Interventions used to improve control of blood pressure in patients with hypertension. Cochrane Database of Systematic Reviews. 2006;(4):CD005182. doi: 10.1002/14651858.CD005182.pub3. [DOI] [PubMed] [Google Scholar]
- Feaster DJ, Burns MJ, Brincks AM, Prado G, Mitrani VB, Mauer MH, Szapocznik J. Structural Ecosystems Therapy for HIV+ African-American women and drug abuse relapse. Family Process. 2010;49(2):204–219. doi: 10.1111/j.1545-5300.2010.01318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feaster DJ, Mitrani VB, Burns MJ, McCabe BE, Brincks AM, Rodriguez AE, … Robbins MS. A randomized controlled trial of Structural Ecosystems Therapy for HIV medication adherence and substance abuse relapse prevention. Drug and Alcohol Dependence. 2010;111(3):227–234. doi: 10.1016/j.drugalcdep.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerteis J, Israel D, Deitz D, LeRoy L, Ricciardi R, Miller T, Basu J. Multiple chronic conditions chartbook. Rockville, MD: AHRQ Publications; 2014. [Google Scholar]
- Gwadry-Sridhar FH, Manias E, Lal L, Salas M, Hughes DA, Ratzki-Leewing A, Grubisic M. Impact of interventions on medication adherence and blood pressure control in patients with essential hypertension: a systematic review by the ISPOR medication adherence and persistence special interest group. Value in Health. 2013;16(5):863–871. doi: 10.1016/j.jval.2013.03.1631. [DOI] [PubMed] [Google Scholar]
- Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database of Systematic Reviews. 2008;(2):CD000011. doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119(23):3028–3035. doi: 10.1161/CIRCULATIONAHA.108.768986. [DOI] [PubMed] [Google Scholar]
- Kopelowicz A, Zarate R, Wallace CJ, Liberman RP, Lopez SR, Mintz J. Using the theory of planned behavior to improve treatment adherence in Mexican Americans with schizophrenia. Journal of Consulting and Clinical Psychology. 2015;83(5):985–993. doi: 10.1037/a0039346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions: a systematic review. Archives of Internal Medicine. 2007;167(6):540–550. doi: 10.1001/archinte.167.6.540. [DOI] [PubMed] [Google Scholar]
- Kronish IM, Diefenbach MA, Edmondson DE, Phillips LA, Fei K, Horowitz CR. Key barriers to medication adherence in survivors of strokes and transient ischemic attacks. Journal of General Internal Medicine. 2013;28(5):675–682. doi: 10.1007/s11606-012-2308-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronish IM, Edmondson D, Goldfinger JZ, Fei K, Horowitz CR. Posttraumatic stress disorder and adherence to medications in survivors of strokes and transient ischemic attacks. Stroke. 2012;43(8):2192–2197. doi: 10.1161/STROKEAHA.112.655209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronish IM, Edmondson D, Li Y, Cohen BE. Post-traumatic stress disorder and medication adherence: results from the Mind Your Heart study. Journal of Psychiatric Research. 2012;46(12):1595–1599. doi: 10.1016/j.jpsychires.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronish IM, Lin JJ, Cohen BE, Voils CI, Edmondson D. Posttraumatic stress disorder and medication nonadherence in patients with uncontrolled hypertension. JAMA Internal Medicine. 2014;174(3):468–470. doi: 10.1001/jamainternmed.2013.12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- University College London. Human Behavior-Change Project. 2016 Retrieved from www.ucl.ac.uk/human-behaviour-change.
- Mazzaglia G, Ambrosioni E, Alacqua M, Filippi A, Sessa E, Immordino V, … Mantovani LG. Adherence to antihypertensive medications and cardiovascular morbidity among newly diagnosed hypertensive patients. Circulation. 2009;120(16):1598–1605. doi: 10.1161/circulationaha.108.830299. [DOI] [PubMed] [Google Scholar]
- McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: scientific review. JAMA. 2002;288(22):2868–2879. doi: 10.1001/jama.288.22.2868. [DOI] [PubMed] [Google Scholar]
- Moise N, Huang C, Rodgers A, Kohli-Lynch CN, Tzong KY, Coxson PG, … Moran AE. Comparative Cost-Effectiveness of Conservative or Intensive Blood Pressure Treatment Guidelines in Adults Aged 35–74 Years: The Cardiovascular Disease Policy Model. Hypertension. 2016;68(1):88–96. doi: 10.1161/HYPERTENSIONAHA.115.06814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291(10):1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, … Turner MB. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133(4):e38–360. doi: 10.1161/cir.0000000000000350. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Evans DB, Acharya A, Baltussen RM. Development of WHO guidelines on generalized cost-effectiveness analysis. Health Economics. 2000;9(3):235–251. doi: 10.1002/(SICI)1099-1050(200004)9:3<235::AID-HEC502>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Naderi SH, Bestwick JP, Wald DS. Adherence to Drugs That Prevent Cardiovascular Disease: Meta-analysis on 376,162 Patients. American Journal of Medicine. 125(9):882–887. e881. doi: 10.1016/j.amjmed.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Nasseh K, Frazee SG, Visaria J, Vlahiotis A, Tian Y. Cost of medication nonadherence associated with diabetes, hypertension, and dyslipidemia. American Journal of Pharmacy Benefits. 2012;4(2):e41–e47. [Google Scholar]
- Nosek BA, Alter G, Banks G, Borsboom D, Bowman S, Breckler S, … Christensen G. Promoting an open research culture. Science. 2015;348(6242):1422–1425. doi: 10.1126/science.aab2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onken LS, Carroll KM, Shoham V, Cuthbert BN, Riddle M. Reenvisioning Clinical Science: Unifying the Discipline to Improve the Public Health. Clinical Psychological Science. 2014;2(1):22–34. doi: 10.1177/2167702613497932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M, Chang A, Powers C, Loustalot F, Schieb L, Ketcham M, … Hong Y. Vital Signs: Disparities in Antihypertensive Medication Nonadherence Among Medicare Part D Beneficiaries - United States, 2014. MMWR Morbidity and Mortality Weekly Report. 2016;65(36):967–976. doi: 10.15585/mmwr.mm6536e1. [DOI] [PubMed] [Google Scholar]
- Roebuck MC, Liberman JN, Gemmill-Toyama M, Brennan TA. Medication adherence leads to lower health care use and costs despite increased drug spending. Health Affairs. 2011;30(1):91–99. doi: 10.1377/hlthaff.2009.1087. [DOI] [PubMed] [Google Scholar]
- Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Medical Care. 2005;43(6):521–530. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- Szapocznik J, Feaster DJ, Mitrani VB, Prado G, Smith L, Robinson-Batista C, … Robbins MS. Structural ecosystems therapy for HIV-seropositive African American women: effects on psychological distress, family hassles, and family support. Journal of Consulting and Clinical Psychology. 2004;72(2):288–303. doi: 10.1037/0022-006x.72.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijens B, Vincze G, Kristanto P, Urquhart J, Burnier M. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ. 2008;336(7653):1114–1117. doi: 10.1136/bmj.39553.670231.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BW, Schiller J, Goodman R. Multiple chronic conditions among US adults: a 2012 update. Preventing Chronic Disease. 2014;11:130389. doi: 10.5888/pcd11.130389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon PW, Bastian B, Anderson RN, Collins JL, Jaffe HW. Potentially preventable deaths from the five leading causes of death—United States, 2008–2010. Morbidity and Mortality Weekly Report. 2014;63(17):369–374. [PMC free article] [PubMed] [Google Scholar]
- Zeber JE, Manias E, Williams AF, Hutchins D, Udezi WA, Roberts CS, Peterson AM. A systematic literature review of psychosocial and behavioral factors associated with initial medication adherence: a report of the ISPOR medication adherence & persistence special interest group. Value in Health. 2013;16(5):891–900. doi: 10.1016/j.jval.2013.04.014. [DOI] [PubMed] [Google Scholar]