Abstract
The serotonergic dorsal raphé nucleus (DRN) expresses glucocorticoid receptors (GR), and systemic glucocorticoids have been shown to regulate expression and activity of tryptophan hydroxylase isoform 2, the rate-limiting enzyme for serotonin synthesis in brain. We have used intra-DRN injection of pseudotyped adeno-associated virus AAV2/9 transducing either green fluorescent protein (GFP control) or Cre recombinase (DRN GR deletion) in floxed GR mice to determine if DRN GR directly regulate DRN mRNA levels of tryptophan hydroxylase 2 (tph2). In a separate set of similarly-treated floxed GR mice, we also measured limbic forebrain region concentrations of serotonin (5-hydroxytryptamine; 5-HT) and its major metabolite, 5-hydroxyindoleacetic acid (5-HIAA). DRN GR deletion increased tph2 mRNA levels in the dorsal, lateral wing, and caudal parts of the DRN without altering tissue concentrations of 5-HT, 5-HIAA, or the 5-HIAA/5-HT ratio in limbic forebrain regions. We conclude that DRN GR inhibit DRN tph2 gene expression in mice without marked effects on serotonin metabolism, at least under basal conditions at the circadian nadir. These data provide the first evidence of localized control of DRN tph2 mRNA expression by DRN GR in mice.
Keywords: glucocorticoid, serotonin, tryptophan hydroxylase, dorsal raphé nucleus, 5-hydroxyindoleacetic acid (5-HIAA)
INTRODUCTION
Serotonin has been implicated in the pathology of anxiety and depression. Dysregulation of serotonin in affective disorders has been inferred from the effects of antidepressants on serotonin signaling, and from the ability of tryptophan depletion to cause relapse in some patients [7, 14, 36]. The dorsal raphé nucleus (DRN) supplies many of the serotonergic projections to brain regions relevant to anxiety and depression [11, 14, 37]. DRN expression of serotonin receptors or tryptophan hydroxylase isoform 2, the rate-limiting enzyme for central nervous system serotonin synthesis, has been found to change in depressed patients and in response to antidepressant treatment [1, 14, 15]. These findings suggest the DRN is important to the etiology and treatment of affective disorders.
DRN serotonergic neurons express glucocorticoid receptors (GR; [16]), and manipulation of systemic glucocorticoid levels alters DRN expression of tryptophan hydroxylase activity, tryptophan hydroxylase 2 protein (Tph 2) and mRNA (tph2) levels in rodents (reviewed in [3, 8, 36]. DRN GR gene expression can be regulated by antidepressants, and antidepressant effects on tph2 expression can be glucocorticoid-dependent [17, 18], suggesting that DRN GR contribute to antidepressant effects on serotonin transmission. Moreover, since glucocorticoids can cause symptoms of depression or anxiety in humans [20] and depression- or anxiety-like behavioral responses in animals [8, 9, 28, 32–34, 36], glucocorticoid effects on DRN serotonergic neurons could be relevant to the causes and treatment of mood disorders. Supporting this possibility, we have shown that GR gene deletion from the DRN reduces anxiety- and depression-like behavior in mice [36].
To date, glucocorticoid effects on DRN serotonergic neurons have only been demonstrated after manipulating systemic glucocorticoids by adrenalectomy or systemic glucocorticoid treatment. It is unclear if GR in the DRN per se are required for the effects of circulating glucocorticoids on DRN serotonin neuron function. To address this question, we have performed virally-transduced DRN GR gene deletion in floxed GR mice and measured tph2 mRNA levels as a marker of serotonin synthesis capacity [26] and measured tissue concentrations of serotonin and its primary metabolite, 5-hydroxyindoleacetic acid (5-HIAA), in corticolimbic targets of the DRN as an index of serotonin transmission [10].
MATERIALS AND METHODS
Animals
Animal use was approved by the Institutional Animal Care and Use Committee of Albany Medical College and complied with the NIH Guide for the Care and Use of Laboratory Animals [19] and European Commission Directive 2010/63/EU. Mice with a floxed exon 2 of the GR gene (Jackson Laboratories stock number 012914; Bar Harbor, ME, USA) were bred on a pure C57BL/6J background for over ten generations. Mice were housed on a 12:12 light cycle (lights-on, 0700 h) and given unrestricted access to standard rodent chow and water.
Viral vectors
Recombinant pseudotyped adeno-associated virus (AAV) vectors were provided and packaged by the Gene Therapy Center and Vector Core of the University of Massachusetts Medical School, Worcester, MA, USA [12]. The pseudotyped AAV contained genetic material from AAV serotype 2 packaged in the capsid of AAV serotype 9 (AAV2/9) and were designed to transduce expression of Cre recombinase (AAV2/9-Cre) or green fluorescent protein (AAV2/9-GFP) under the control of a universal promoter consisting of the cytomegalovirus immediate-early (CMV IE) enhancer and 260 bp (nucleotides −1261 to −1001) of the chicken β-actin 6 promoter (personal communication, Li Zhong, University of Massachusetts Medical School). We have shown that the AAV2/9 pseudotype efficiently transduces Cre-mediated GR deletion from the DRN of floxed GR mice [36].
Intracerebral virus injection
Male mice (7–9 weeks old) received intra-DRN injections under ketamine-xylazine anesthesia. AAV2/9-Cre or -GFP was diluted to 1.45 × 1013 genomic copies/ml and delivered in two, 600 nl injections (AP −4.4 mm, ML 0 mm, DV −3.4 mm and AP −4.7 mm, ML 0 mm, DV −3.5 mm from bregma). Mice were given 1 mM indomethacin to drink for analgesia [36]. After a 3-week recovery, mice were killed by decapitation in the undisturbed state within 2h of lights-on, during the inactive phase of their circadian cycle. This time point was chosen because systemic glucocorticoids have been shown to inhibit DRN tph2 mRNA expression in mice at this time [18]. Brains were removed and frozen on dry ice.
Tryptophan hydroxylase 2 (tph2) in situ hybridization histochemistry
In situ hybridization histochemistry was performed in 10 μm brain sections as previously described, using 35S-labeled antisense cRNA probes complementary to an Nco I digest of the full-length mouse tph2 cDNA [17, 18]. Slides were exposed for 22 h to phosphorimager screens with identical 14C standards (American Radiolabeled Chemicals, St. Louis, MO, USA). Tph2 hybridization signals were analyzed semi-quantitatively in 50 μm resolution autoradiograms (Typhoon 9210 phosphorimager; GE Health Care, Niskayuna, NY, USA). DRN subregions were outlined using the criteria of Donner et al. [8]. Densitometric readings from each subregion were corrected for background of a similarly-sized, non-expressing area and normalized to the 14C standards. Values for each mouse were averaged from readings from all sections containing the specific DRN subregion.
Serotonin (5-hydroxytryptamine; 5-HT) and 5-hydroxyindoleacetic acid (5-HIAA) content
Frozen punches were collected from coronal forebrain sections, extracted in acetate buffer, and analyzed by high pressure liquid chromatography with electrochemical detection (HPLCED) for 5-HT and 5-HIAA as previously described [9]. Detection limits were 1.69 pg for 5-HT and 1.22 pg for 5-HIAA. Punches (310–410 μm in diameter) were collected from the medial prefrontal cortex (bregma +1.94 mm, including portions of the pre- and infralimbic regions), nucleus accumbens (bregma +1.18 mm, including portions of the core and shell regions), bed nucleus of the stria terminalis (bregma +0.02 mm), paraventricular nucleus of the hypothalamus (bregma −0.94 mm), basolateral amygdala (bregma −1.46 mm), central amygdala (bregma −1.46 mm), and the CA1 subfield of the dorsal and ventral hippocampus (3 punches per subfield at bregma −1.94 and −3.16 mm, respectively; [29]). Serotonin and 5-HIAA content is expressed relative to protein content (BCA Protein Assay Kit, ThermoFisher, Rockford, IL, USA).
GR and Tph2 immunohistochemistry
Slide-mounted 10 μm sections were on alternate slides from those used for tph2 in situ hybridization histochemistry or were obtained from brainstems reserved from mice used for HPLC-ED analysis. Sections were fixed in 4% phosphate-buffered paraformaldehyde (30 min, 4 °C), washed (Tris-buffered saline; TBS), reacted with 1% hydrogen peroxide, blocked in TNB (0.1 M Tris, 0.15 M NaCl, 0.5% blocking reagent from Perkin Elmer, Boston, MA, USA), and incubated (48 h, 4 °C) in 1:1000 rabbit anti- mouse GR (sc-1004, Santa Cruz Biotechnology, Santa Cruz, CA, USA) in TNB with 0.3% Triton X-100. GR immunoreactivity was revealed with 1:1500 horseradish peroxidase-conjugated donkey anti-rabbit IgG in TNB (Perkin Elmer), followed by 1:50 Cy5-labeled TSA in amplification diluent (Perkin Elmer). Slides were then washed and incubated 48 h in 1:500 goat anti-mouse Tph2 (208477, USBio, Salem, MA, USA), followed by 1:800 Alexafluor 555-donkey anti-goat (ThermoFisher, Waltham, MA, USA), with antibodies diluted in TBS with 3% donkey serum and 0.3% Triton X-100. Slides were coverslipped with 50 mg/ml n-propylgallate in 0.2 M Tris and imaged with a Zeiss AxioImager M2 microscope (Carl Zeiss Microscopy, Peabody, MA, USA) using the same exposure conditions for each fluorophore.
Data analysis
Eleven mice received intra-DRN AAV2/9-GFP injections (5 for tph2 in situ hybridization histochemistry and 6 for HPLC analysis), and 12 mice received injections of AAV2/9-Cre (6 for tph2 in situ hybridization histochemistry and 6 for HPLC analysis). One AAV2/9-Cre-injected mouse with residual DRN GR immunoreactivity was excluded from tph2 in situ hybridization analysis, and one medial prefrontal cortex punch was lost from the GFP control group for HPLC-ED analysis. Therefore, n = 5 for all groups for tph2 in situ hybridization analysis and the GFP control medial prefrontal cortex HPLC-ED samples, whereas n = 6 for other groups for HPLC-ED.
Data were analyzed using linear mixed model (LMM) analyses (PASW Statistics 24.0.0.0 for Windows, SPSS Inc., Chicago, IL, USA). These analyses separately modeled tph2 mRNA expression, 5-HT concentrations, 5-HIAA concentrations, and 5-HIAA:5-HT ratios for each brain region and group, assuming an unstructured covariance structure. Post hoc analyses were by Fisher’s Least Significant Difference (LSD) tests when main effects were significant. This approach provided comparable results to those of ANOVA and Bonferroni correction but more efficiently accounted for multiple measures from the same mouse, while accommodating missing data and complex covariance structures. Data are expressed as mean ± SEM; significance was set at P < 0.05.
RESULTS
Virally-transduced DRN GR deletion
Figure 1 shows representative GR expression in floxed GR mice with DRN injections of adeno-associated virus AAV2/9 transducing either GFP as a control or Cre recombinase producing DRN GR deletion. GR expression extends from bregma −4.36 to −4.96 in the DRN and is negligible in the median raphé nucleus in mice [17, 36]. GR deletion was defined [36] as the absence of GR immunoreactivity in the entire DRN, with DRN boundaries delineated by Tph2 immunoreactivity. GR expression in neighboring regions such as the locus coeruleus was unaffected in mice with DRN GR deletion (Figure 1, bottom row).
Figure 1.
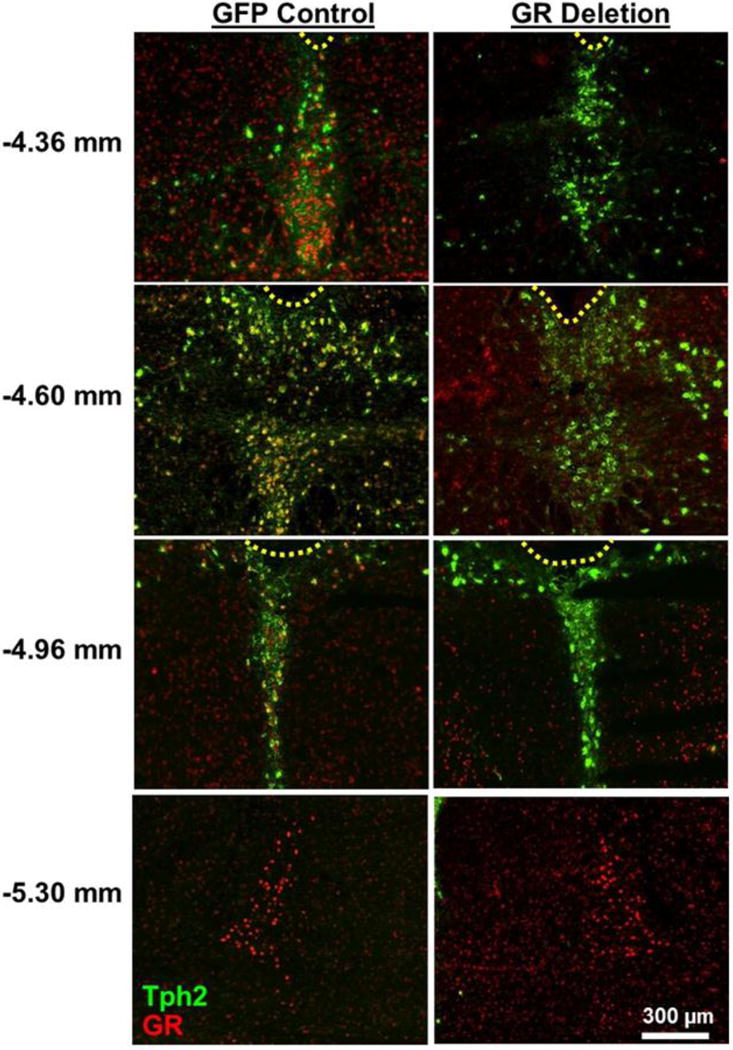
Representative images of glucocorticoid receptor (GR) expression in the dorsal raphé nucleus (DRN, bregma −4.36 mm to −4.96 mm) and locus coeruleus (bregma −5.30 mm) in floxed GR mice after DRN injections of AAV2/9 virus transducing green fluorescent protein (GFP control) or Cre recombinase (GR deletion). Tryptophan hydroxylase isoform 2 immunoreactivity (Tph2; green pseudocolor) indicates the boundaries of the DRN, while GR immunoreactive nuclei are shown in red pseudocolor. Yellow dashed lines indicate the cerebral aqueduct; the fourth ventricle is above and medial to locus coeruleus images. Numbers on left indicate distance from bregma [29].
Effect of DRN GR deletion on DRN levels of tph2 mRNA
In mice, glucocorticoids have been found to inhibit DRN gene expression of tph2, the predominant tryptophan hydroxylase isoform in brain [3, 17, 18, 36]. To determine if GR expressed specifically within the DRN mediate the glucocorticoid effects on DRN tph2 gene expression, we measured tph2 mRNA at the beginning of the light cycle in floxed GR mice with virally-transduced, GFP expression or Cre-mediated DRN GR deletion. We focused our analyses on the dorsal part of the DRN (DRD), the lateral wings (LW), the caudal DRN (DRC), and the interfascicular part of the DRN (DRI) because these regions have the highest GR expression [36] and project to regions associated with emotion-related behavior ([14]; Figure 2A). There was a significant main effect of DRN GR deletion to increase tph2 mRNA expression within the DRN (Figure 2B; F(1,31) = 21.84, P < 0.0001), but no interaction between DRN GR deletion and DRN subregion on tph2 mRNA expression. Post hoc tests revealed that virally-transduced DRN GR deletion significantly increased tph2 mRNA levels in the DRD, LW, and DRC of the DRN. Effects of DRN GR deletion on tph2 mRNA expression in the DRI did not reach statistical significance (P = 0.072; Figure 2B).
Figure 2.
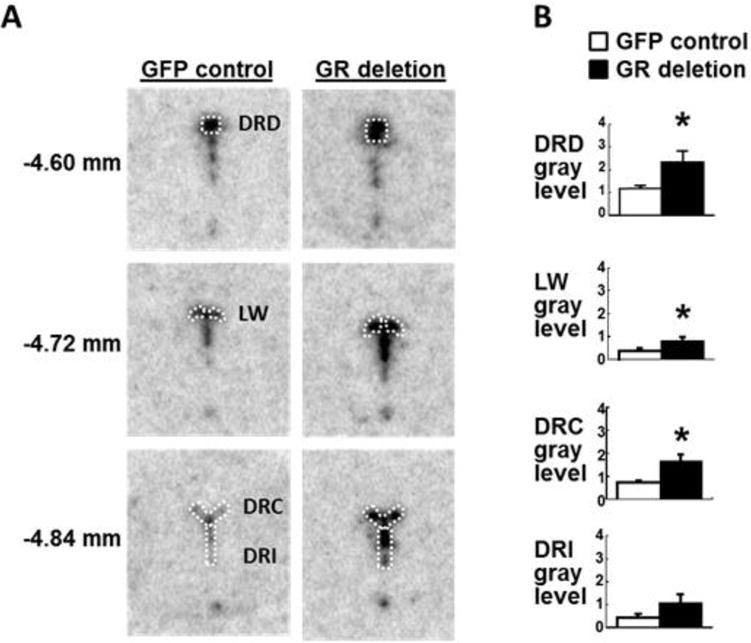
A, Representative examples of tryptophan hydroxylase isoform 2 (tph2) in situ hybridization histochemistry in the DRN of floxed GR mice after DRN injections of AAV2/9 virus transducing green fluorescent protein (GFP control) or Cre recombinase (GR deletion). White outlines show the dorsal DRN (DRD), the lateral wings (LW), the caudal DRN (DRC), and the interfascicular DRN (DRI), as defined by Donner et al. [8]. B, Semi-quantitative analysis of tph2 in situ hybridization histochemistry in GFP control (white bars) and DRN GR deletion groups (black bars). Numbers on the left indicate distance from bregma [29]. Mice were euthanized 3 weeks after DRN injection under basal conditions within 2h of lights-on.
*, P < 0.05 vs. GFP control; n = 5 per group.
Effect of DRN GR deletion on 5-HT and 5-HIAA tissue concentrations
To determine if DRN GR also regulate measures of serotonin transmission, we analyzed tissue concentrations of serotonin (5-HT) and its major metabolite, 5-hydroxyindoleacetic acid (5-HIAA; [10]) in micropunches of forebrain regions collected from an additional set of mice killed early in the light cycle after DRN injections of AAV2/9-Cre or AAV2/9-GFP. Regions were selected for analysis because they receive serotonergic afferents from the DRN [9, 14, 37] or exhibit glucocorticoid-dependent changes in serotonergic tone [3, 4, 6, 9, 21–24, 30]. Despite the increase in tph2 mRNA, we did not detect changes in 5-HT or 5-HIAA concentrations or in 5-HIAA to 5-HT ratios in any of the selected regions in undisturbed mice at the circadian nadir (Table 1).
Table 1.
Concentrations (pg/μg protein) of 5-hydroxyindoleacetic acid (5-HIAA) and serotonin (5-hydroxytryptamine, or 5-HT), and ratios of 5-HIAA to 5-HT in punches of the medial prefrontal cortex (mPFC), nucleus accumbens (NAc), bed nucleus of the stria terminalis (BNST), CA1 subfield of the dorsal and ventral hippocampus (dCA1 and vCA1, respectively), paraventricular nucleus of the hypothalamus (PVH), and basolateral and central amygdala (BLA and CeA, respectively) of floxed GR mice with dorsal raphé nucleus (DRN) injections of AAV2/9-GFP (GFP control), or of AAV2/9-Cre with GR deletion in the entire DRN (DRN GR deletion). Mice were euthanized 3 weeks after DRN injection under basal conditions within 2h of lights-on. Data are presented Mean + SEM (n).
| Region | Group | 5-HIAA | 5-HT | 5-HIAA/5-HT |
|---|---|---|---|---|
| mPFC | GFP Control | 2.25 + 0.19 (5) | 2.63 + 0.29 (5) | 0.88 + 0.084 (5) |
| DRN GR Deletion | 2.26 + 0.42 (6) | 2.98 + 0.70 (6) | 0.83 + 0.11 (6) | |
| NAc | GFP Control | 2.03 + 0.47 (6) | 3.11 + 0.83 (6) | 0.68 + 0.034 (6) |
| DRN GR Deletion | 1.39 + 0.31 (6) | 2.18 + 0.49 (6) | 0.64 + 0.043 (6) | |
| BNST | GFP Control | 4.50 + 0.26 (6) | 7.80 + 0.76 (6) | 0.60 + 0.069 (6) |
| DRN GR Deletion | 4.10 + 0.24 (6) | 8.66 + 0.71 (6) | 0.48 + 0.027 (6) | |
| dCA1 | GFP Control | 5.15 + 0.48 (6) | 6.72 + 0.58 (6) | 0.765 + 0.0214 (6) |
| DRN GR Deletion | 5.35 + 0.26 (5) | 7.75 + 1.02 (6) | 0.653 + 0.0462 (6) | |
| vCA1 | GFP Control | 6.67 + 0.54 (6) | 10.20 + 1.28 (6) | 0.679 + 0.0530 (6) |
| DRN GR Deletion | 6.35 + 0.43 (6) | 10.29 + 1.10 (6) | 0.639 + 0.0555 (6) | |
| PVH | GFP Control | 6.73 + 0.51 (6) | 14.15 + 1.04 (6) | 0.48 + 0.032 (6) |
| DRN GR Deletion | 5.19 + 0.94 (6) | 11.69 + 1.79 (6) | 0.57 + 0.18 (6) | |
| BLA | GFP Control | 4.35 + 0.23 (6) | 12.94 + 2.05 (6) | 0.36 + 0.036 (6) |
| DRN GR Deletion | 3.53 + 0.47 (6) | 11.43 + 1.18 (6) | 0.31 + 0.038 (6) | |
| CeA | GFP Control | 4.17 + 0.76 (6) | 8.32 + 0.98 (6) | 0.56 + 0.10 (6) |
| DRN GR Deletion | 4.37 + 1.13 (6) | 10.89 + 1.89 (6) | 0.39 + 0.093 (6) |
DISCUSSION
We have shown for the first time that GR in the DRN specifically influence DRN tph2 mRNA expression. Virally transduced DRN GR deletion increased circadian trough mRNA levels of tph2, which encodes the rate-limiting enzyme for brain serotonin synthesis. These data are consistent with the hypothesis that the DRN GR mediate, at least in part, the inhibitory effects of circulating glucocorticoids on DRN tph2 gene expression in mice at the circadian nadir [3–5, 17, 18, 36]. The potential for control of serotonergic neuron activity suggests that DRN GR could be a therapeutic target in treating affective disorders.
Virally-transduced DRN GR deletion increased tph2 mRNA expression in all DRN subregions examined except the DRI. Prior characterization of mice with DRN GR deletion indicated that circadian glucocorticoid levels were unaffected [36], making it unlikely that changes in tph2 mRNA expression were due indirectly to changes in glucocorticoid levels acting at GR outside the DRN. Our findings therefore suggest that systemic glucocorticoids inhibit DRN tph2 mRNA expression in mice largely via DRN GR. This conclusion agrees with findings that adrenalectomy enhances, while glucocorticoid treatment decreases, tph2 mRNA and Tph2 protein levels in mice [3–5, 17, 18]. However, findings that systemic glucocorticoids augment circadian and stress-induced increases in tph2 mRNA and Tph protein levels in rats [8, 9, 25] could be interpreted as evidence that glucocorticoids stimulate tph2 gene expression. These discrepancies have been attributed to species differences [3]. In addition, since circadian and stress stimuli engage brain regions besides the DRN, manipulation of systemic glucocorticoids employed by these studies could be affecting afferent input to the DRN rather than the DRN itself. Consistent with this possibility, antagonism of CRH input to the DRN blocks glucocorticoid enhancement of stress-induced increases in Tph activity in rats [9]. Our current data support the interpretation that, at least in mice in the early light phase, GR in the DRN mediate inhibitory effects of systemic glucocorticoids on DRN tph2 gene expression.
We further investigated if increases in DRN tph2 mRNA were associated with measures of increased serotonin concentrations or serotonin metabolism. We did not detect significant effects of DRN GR deletion on 5-HT, 5-HIAA, or their ratio, possibly due to the lag between peak tph2 mRNA and Tph2 protein levels [27]. It was not feasible to determine if DRN 5-HT or 5-HIAA concentrations were altered because of the need to verify GR deletion in the same animals. It is possible that microdialysis would provide a better measure of serotonin release. Alternatively, effects of DRN GR deletion may have been obscured by projections from serotonergic cell groups that were not manipulated in the current study. The median raphé nucleus, which, along with the DRN, provides serotonergic projections to the forebrain [14, 35], was not affected by our injections. The interpeduncular nucleus was also not targeted but may contribute up to a third of serotonergic input to the hippocampus [13].
It is also possible that significant effects of DRN GR deletion on serotonin turnover might have been detected if samples were collected at additional circadian times or after stress. Even conventional behavioral tests, which we used to demonstrate reductions in depression- and anxiety-like behavior after DRN GR deletion [36], involve some degree of stress. Consequently, the elevations in DRN tph2 gene expression that we have identified could be more important to serotonin levels in DRN projection targets under stimulated conditions, including those relevant to emotional behavior.
It remains to be determined if increases in DRN tph2 mRNA are caused by GR loss specifically from serotonin neurons. The universal promoter in the viral constructs may have mediated GR deletion from GABAergic, glutamatergic, or neuropeptidergic DRN neurons [2, 11, 14, 31]. Nevertheless, as an initial demonstration of how DRN GR regulate behavior and DRN function, our studies establish that DRN GR inhibit DRN tph2 mRNA expression.
CONCLUSION
We have novel evidence that GR in the DRN inhibit DRN tph2 gene expression in mice. This study highlights effective methods for deletion of DRN GR in mice, leading to elevation of DRN tph2 mRNA expression and providing new opportunities for future studies of GR influences on serotonin-regulated circuits and behaviors. These effects represent potential targets for the development of novel treatments for mood disorders and for the identification of current medications whose effects on DRN GR [17, 18] may have unique therapeutic benefits.
Highlights.
Dorsal raphé glucocorticoid receptors inhibit DRN tryptophan hydroxylase 2 mRNA
DRN GR deletion did not change basal serotonin or 5-HIAA content of DRN targets
Acknowledgments
We are grateful to Linda Barenboim McCormack for expert assistance. Supported in part by National Institute of Mental Health grant R01MH080394 to LJ.
Abbreviations
- AAV2/9-Cre
AAV2/9-GFP: pseudotyped adeno-associated virus 2/9 transducing either Cre recombinase or green fluorescent protein
- DRN
dorsal raphé nucleus
- DRD, DRC
DRI: dorsal, caudal, and interfascicular parts of the dorsal raphé nucleus
- GR
glucocorticoid receptor
- 5-HT
5-hydroxytryptamine, or serotonin
- HPLC-ED
high performance liquid chromatography with electrochemical detection
- 5-HIAA
5-hydroxyindoleacetic acid
- tph2
tryptophan hydroxylase 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: none.
References
- 1.Bach-Mizrachi H, Underwood MD, Kassir SA, Bakalian MJ, Sibille E, Tamir H, Mann JJ, Arango V. Neuronal tryptophan hydroxylase mRNA expression in the human dorsal and median raphe nuclei: major depression and suicide. Neuropsychopharmacology. 2006;31:814–824. doi: 10.1038/sj.npp.1300897. [DOI] [PubMed] [Google Scholar]
- 2.Challis C, Boulden J, Veerakumar A, Espallergues J, Vassoler FM, Pierce RC, Beck SG, Berton O. Raphe GABAergic neurons mediate the acquisition of avoidance after social defeat. J Neurosci. 2013;33:13978–13988. doi: 10.1523/JNEUROSCI.2383-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen GL, Miller GM. Advances in tryptophan hydroxylase-2 gene expression regulation: new insights into serotonin-stress interaction and clinical implications. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:152–171. doi: 10.1002/ajmg.b.32023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark JA, Flick RB, Pai LY, Szalayova I, Key S, Conley RK, Deutch AY, Hutson PH, Mezey E. Glucocorticoid modulation of tryptophan hydroxylase-2 protein in raphe nuclei and 5-hydroxytryptophan concentrations in frontal cortex of C57/Bl6 mice. Mol Psychiatry. 2008;13:498–506. doi: 10.1038/sj.mp.4002041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark JA, Pai LY, Flick RB, Rohrer SP. Differential hormonal regulation of tryptophan hydroxylase-2 mRNA in the murine dorsal raphe nucleus. Biol Psychiatry. 2005;57:943–946. doi: 10.1016/j.biopsych.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 6.De Kloet ER, Kovács GL, Szabó G, Telegdy G, Bohus B, Versteeg DH. Decreased serotonin turnover in the dorsal hippocampus of rat brain shortly after adrenalectomy: selective normalization after corticosterone substitution. Brain Res. 1982;239:659–663. doi: 10.1016/0006-8993(82)90546-7. [DOI] [PubMed] [Google Scholar]
- 7.Delgado PL. Monoamine depletion studies: implications for antidepressant discontinuation syndrome. J Clin Psychiatry. 2006;67(suppl 4):22–26. [PubMed] [Google Scholar]
- 8.Donner NC, Montoya CD, Lukkes JL, Lowry CA. Chronic non-invasive corticosterone administration abolishes the diurnal pattern of tph2 expression. Psychoneuroendocrinology. 2012;37:645–661. doi: 10.1016/j.psyneuen.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donner NC, Siebler PH, Johnson DT, Villarreal MD, Mani S, Matti AJ, Lowry CA. Serotonergic systems in the balance: CRHR1 and CRHR2 differentially control stress-induced serotonin synthesis. Psychoneuroendocrinology. 2016;63:178–190. doi: 10.1016/j.psyneuen.2015.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans AK, Reinders N, Ashford KA, Christie IN, Wakerley JB, Lowry CA. Evidence for serotonin synthesis-dependent regulation of in vitro neuronal firing rates in the midbrain raphe complex. Eur J Pharmacol. 2008;590:136–149. doi: 10.1016/j.ejphar.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Fu W, Le Maitre E, Fabre V, Bernard JF, Xu ZQD, Hökfelt T. Chemical neuroanatomy of the dorsal raphe nucleus and adjacent structures of the mouse brain. J Comp Neurol. 2010;518:3464–3494. doi: 10.1002/cne.22407. [DOI] [PubMed] [Google Scholar]
- 12.Gao GP, Sena-Esteves M. Introducing genes into mammalian cells: viral vectors. In: Green MR, Sambrook J, editors. Molecular Cloning: A Laboratory Manual. Vol. 2. Cold Spring Harbor Laboratory Press; New York: 2012. pp. 1209–1313. [Google Scholar]
- 13.Hale MW, Lowry CA. Functional topography of midbrain and pontine serotonergic systems: implications for synaptic regulation of serotonergic circuits. Psychopharmacology (Berl) 2011;213:243–264. doi: 10.1007/s00213-010-2089-z. [DOI] [PubMed] [Google Scholar]
- 14.Hale MW, Shekhar A, Lowry CA. Stress-related serotonergic systems: implications for symptomatology of anxiety and affective disorders. Cell Mol Neurobiol. 2012;32:695–708. doi: 10.1007/s10571-012-9827-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamon M, Blier P. Monoamine neurocircuitry in depression and strategies for new treatments, Prog. Neuropsychopharmacol. Biol Psychiatry. 2013;45:54–63. doi: 10.1016/j.pnpbp.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Harfstrand A, Fuxe K, Cintra A, Agnati LF, Zini I, Wikstrom AC, Okret S, Yu ZY, Goldstein M, Steinbusch H, Verhofstad A, Gustafsson JA. Glucocorticoid receptor immunoreactivity in monoaminergic neurons of rat brain. Proc Natl Acad Sci U S A. 1986;83:9779–9783. doi: 10.1073/pnas.83.24.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heydendael W, Jacobson L. Glucocorticoid status affects antidepressant regulation of locus coeruleus tyrosine hydroxylase and dorsal raphé tryptophan hydroxylase gene expression. Brain Res. 2009;1288:69–78. doi: 10.1016/j.brainres.2009.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heydendael W, Jacobson L. Widespread hypothalamic-pituitary-adrenocortical axis-relevant and mood-relevant effects of chronic fluoxetine treatment on glucocorticoid receptor gene expression in mice. Eur J Neurosci. 2010;31:892–902. doi: 10.1111/j.1460-9568.2010.07131.x. [DOI] [PubMed] [Google Scholar]
- 19.Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council. Guide for the care and use of laboratory animals. National Academies Press; Washington, DC: 2010. [Google Scholar]
- 20.Jacobson L. The hypothalamic-pituitary-adrenocortical axis: Neuropsychiatric aspects. Compr Physiol. 2014;4:715–738. doi: 10.1002/cphy.c130036. [DOI] [PubMed] [Google Scholar]
- 21.Jhanwar-Uniyal M, Renner K, Bailo M, Luine VN, Leibowitz SF. Serotonin and 5-hydroxyindoleacetic acid levels in discrete hypothalamic areas of the rat brain: relation to circulating corticosterone. Neurosci Lett. 1987;79:145–150. doi: 10.1016/0304-3940(87)90687-2. [DOI] [PubMed] [Google Scholar]
- 22.Johnson DA, Grant EJ, Ingram CD, Gartside SE. Glucocorticoid receptor antagonists hasten and augment neurochemical responses to a selective serotonin reuptake inhibitor antidepressant. Biol Psychiatry. 2007;62:1228–1235. doi: 10.1016/j.biopsych.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Korte-Bouws GA, Korte SM, De Kloet ER, Bohus B. Blockade of corticosterone synthesis reduces serotonin turnover in the dorsal hippocampus of the rat as measured by microdialysis. J Neuroendocrinol. 1996;8:877–881. doi: 10.1046/j.1365-2826.1996.05389.x. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Scholl JL, Tu W, Hassell JE, Watt MJ, Forster GL, Renner KJ. Serotonergic responses to stress are enhanced in the central amygdala and inhibited in the ventral hippocampus during amphetamine withdrawal. Eur J Neurosci. 2014;40:3684–3692. doi: 10.1111/ejn.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malek ZS, Sage D, Pévet P, Raison S. Daily rhythm of tryptophan hydroxylase-2 messenger ribonucleic acid within raphe neurons is induced by corticoid daily surge and modulated by enhanced locomotor activity. Endocrinology. 2007;148:5165–5172. doi: 10.1210/en.2007-0526. [DOI] [PubMed] [Google Scholar]
- 26.Malek ZS, Dardente H, Pevet P, Raison S. Tissue-specific expression of tryptophan hydroxylase mRNAs in the rat midbrain: anatomical evidence and daily profiles. Eur J Neurosci. 2005;22:895–901. doi: 10.1111/j.1460-9568.2005.04264.x. [DOI] [PubMed] [Google Scholar]
- 27.Malek ZS, Pévet P, Raison S. Circadian change in tryptophan hydroxylase protein levels within the rat intergeniculate leaflets and raphe nuclei. Neuroscience. 2004;125:749–758. doi: 10.1016/j.neuroscience.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell JB, Meaney MJ. Effects of corticosterone on response consolidation and retrieval in the forced swim test. Behav Neuroscience. 1991;105:798–803. doi: 10.1037//0735-7044.105.6.798. [DOI] [PubMed] [Google Scholar]
- 29.Paxinos G, Franklin C. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San Diego: p. 2001. [Google Scholar]
- 30.Sillaber I, Montkowski A, Landgraf R, Barden N, Holsboer F, Spanagel R. Enhanced morphine-induced behavioural effects and dopamine release in the nucleus accumbens in a transgenic mouse model of impaired glucocorticoid (type II) receptor function: influence of long-term treatment with the antidepressant moclobemide. Neuroscience. 1998;85:415–425. doi: 10.1016/s0306-4522(97)00607-6. [DOI] [PubMed] [Google Scholar]
- 31.Soiza-Reilly M, Commons KG. Glutamatergic drive of the dorsal raphe nucleus. J Chem Neuroanat. 2011;41:247–255. doi: 10.1016/j.jchemneu.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sterner EY, Kalynchuk LE. Behavioral and neurobiological consequences of prolonged glucocorticoid exposure in rats: relevance to depression. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:777–790. doi: 10.1016/j.pnpbp.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schütz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- 34.Veldhuis HD, De Korte CCMM, De Kloet ER. Glucocorticoids facilitate the retention of acquired immobility during forced swimming. Eur J Pharmacol. 1985;115:211–217. doi: 10.1016/0014-2999(85)90693-4. [DOI] [PubMed] [Google Scholar]
- 35.Vertes RP, Fortin WJ, Crane AM. Projections of the median raphe nucleus in the rat. J Comp Neurol. 1999;407:555–582. [PubMed] [Google Scholar]
- 36.Vincent MY, Jacobson L. Glucocorticoid receptor deletion from the dorsal raphé nucleus of mice reduces dysphoria-like behavior and impairs hypothalamic-pituitary-adrenocortical axis feedback inhibition. Eur J Neurosci. 2014;39:1671–1681. doi: 10.1111/ejn.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waselus M, Valentino R, Bockstaele EV. Collateralized dorsal raphe nucleus projections: a mechanism for the integration of diverse functions during stress. J Chem Neuroanat. 2011;41:266–280. doi: 10.1016/j.jchemneu.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


