Abstract
Background
Drug eluting balloon (DEB) is a new therapeutic option for treatment of obstructive coronary lesions in percutaneous coronary intervention (PCI). There is limited data on the safety and efficacy of DEB in Asian patients in contemporary clinical registries. We evaluated the clinical efficacy and safety of SeQuent Please paclitaxel-eluting balloon in our cohort of South-East Asian patients in real world clinical practice.
Methods
Between January 2010 to November 2012, 320 patients (76% male, mean age 61.3 ± 11.2 years) with a total of 337 coronary lesions were treated with SeQuent Please drug-eluting balloon (DEB). The primary endpoint was major adverse cardiac events (MACE) ie a composite of cardiovascular death, target vessel related myocardial infarction (MI) and target lesion revascularization (TLR) at 9 months follow-up.
Results
The majority of patients presented with acute coronary syndrome (76%).The most common indication for the use of DEB was small vessel disease (54%) followed by instent restenosis (21%), bifurcation lesions (6%) and others (19%). An average of 1.23 ± 0.5 DEB were used per patient, with mean DEB diameter of 2.6 ± 0.6 mm and average total length of 24.0 ± 11.1 mm.
At 9 months follow-up, 5.3% of patients developed MACE. MACE was mainly driven by TLR(4%) followed by target vessel related myocardial infarction (2.6%) and cardiovascular death (1%).
Conclusion
SeQuent Please DEB was a safe and effective treatment modality in our cohort of South-East Asian patients with a low incidence of MACE observed at 9 months follow-up.
Keywords: Drug-eluting balloon, Percutaneous coronary intervention, South-East Asia, Registry, Outcomes
1. Introduction
Drug-eluting balloon [1] (DEB) has emerged as a new therapeutic option to treat obstructive coronary artery disease (CAD) in percutaneous coronary intervention (PCI). Non-stent-based local drug delivery using DEB has several advantages as it:
-
1)
inhibits excessive neointimal hyperplasia following balloon angioplasty of a diseased native coronary artery without leaving a permanent metallic frame
-
2)
avoid a “stent-in-stent” approach in previously stented arteries
-
3)
eliminate the risk of stent thrombosis
-
4)
reduce the duration of dual anti-platelet therapy (DAPT).
The clinical efficacy of DEB has been well proven in randomized controlled trials [2], [3] in the treatment of bare metal stent instent restenosis ( BMS ISR) when compared to uncoated balloon/paclitaxel-coated stent with sustainable results [4] observed at long term follow-up. Favourable results are also seen with the use of DEB in drug-eluting stent (DES) ISR [5], [6] although robust data is lacking for the combined use of DEB and BMS [7], [8] in routine PCI and also in specific lesions subsets [9], [10], [11] like small vessel, bifurcation lesion, etc. Similar to DES, DEB has been used in “off-label” indications in the “real world” and there is limited data [12] on its clinical efficacy and safety in Asian patients in contemporary clinical registries. We therefore sought to evaluate the clinical efficacy and safety of SeQuent Please DEB (B. Braun, Melsungen, Germany) in our cohort of South-East Asian patients in “real world” clinical practice.
2. Materials and Methods
2.1. Study Population
From January 2010 to November 2012, a total of 320 symptomatic patients with native coronary lesions, instent restenosis (ISR) and saphenous venous graft lesions (total of 337 lesions) were treated with SeQuent Please DEB at our institution.
2.2. Interventional procedure
All PCIs were performed using standard techniques and according to contemporary practice guidelines. All patients were treated with aspirin 100 mg prior to the procedure and indefinitely thereafter. Patients also received clopidogrel ( an oral loading dose of 600 mg followed by 75 mg daily) before the procedure, followed by a minimum of 1 month in patients who received DEB alone and a minimum of 3 months in patients who received BMS implantation in combination with DEB. Additional duration of clopidogrel treatment was at the discretion of the attending physician.
2.3. Use of DEB during PCI
The SeQuent Please DEB catheter was loaded with paclitaxel 3 ug/mm2. The length of the DEB catheter was chosen to exceed the target lesion for at least 2 mm ( at both proximal and distal ends). The catheter(s) was inflated for 30 to 60 s with a minimum of 7 atm. Predilation of the diseased coronary segment with a uncoated balloon/scoring balloon/cutting balloon before the use of DEB was encouraged. BMS was implanted if the angiographic result after DEB alone therapy was not satisfactory due to significant recoil/residual stenosis or dissection (Type C-F). For those opting for the combined use of DEB and BMS as primary therapy, the length of DEB had to exceed the length of the implanted BMS ( this principle was also applied for bail-out stenting).
2.4. End-Points and Definitions
The primary end-point was major adverse cardiac event (MACE) ie a composite of cardiovascular (CVS) death, target vessel related myocardial infarction (MI) and target lesion revascularization (TLR) at 9 months follow-up. Secondary end-points include individual components of MACE and target lesion thrombosis.
Death from CVS causes was defined as death due to acute MI, cardiac perforation or tamponade, arrhythmia, a complication of the PCI procedure or as any death in which a CVS cause could not be ruled out.
Target-vessel related MI was defined as the presence of new Q waves in at least 2 contiguous leads on electrocardiogram (concordant with the intervened target lesion) with elevation in cardiac troponin or in creatine kinase/creatine kinase-MB above the upper limit of the normal range, or in the absence of pathologic Q waves, MI was diagnosed in the presence of an elevation in cardiac troponin or in creatine kinase > 2 times the upper limit of normal. TLR was defined as any repeat revascularization (percutaneous or surgical) secondary to a stenosis > 50% within the stent or within 5 mm proximal or distal to the stented segment. Target lesion thrombosis was defined according to the Academic Research Consortium [13] criteria for definite and probable stent thrombosis. In our study, we defined native coronary artery as small vessel when reference vessel diameter ≤ 2.8 mm and as de novo lesion when reference vessel diameter > 2.8 mm. Our retrospective study conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the institution's human research committee.
2.5. Statistical analysis
Continuous variables were expressed as mean ± standard error of mean. Dichotomous variables were expressed as counts and percentages. Statistical comparisons were performed using Student’s t test or Fisher’s exact test, as appropriate. Multivariate regression analysis was performed ( using an enter regression model) to evaluate predisposing factors for TLR, in which each entered variable had p value < 0.1 based on univariate analysis. Calculations were performed using SPSS software (version 16.0; SPSS, Inc., Chicago, Illinois). All p-values were 2-sided and p-values < 0.05 were considered statistically significant.
3. Results
Table 1 show the baseline clinical characteristics of the study patients. The mean age of the patients at presentation was 61.3 ± 11.2 years with male preponderance (76%).
Table 1.
Baseline Clinical Characteristics of Patients.
| N = 320 | |
|---|---|
| Mean age (years) | 61.3 ± 11.2 |
| Male:Female,n, % | 242: 78 (76 : 24) |
| Ever smokers,n,% | 173 (54.1) |
| Diabetes,n, % | 155 (48.4) |
| Hyperlipidemia,n,% | 261 (81.6) |
| Hypertension,n,% | 257 (80.3) |
| Previous myocardial infarction,n,% | 102 (32.0) |
| Previous PCI,n,% | 137 (42.8) |
| Previous CABG,n,% | 24 (7.5) |
| LVEF(%) | 45 ± 13 |
| Presentation: | |
| STEMI,n,% | 48 (15) |
| NSTEMI/UAP,n,% | 194 (61) |
| Stable angina,n,% | 78 (24) |
*PCI denotes percutaneous coronary intervention, CABG coronary artery bypass graft, LVEF left ventricular ejection fraction, STEMI ST elevation myocardial infaction, NSTEMI/UAP denotes non STEMI/unstable angina pectoris.
Diabetes mellitus (DM) was present in 155 patients (48.4%) and 137 patients (43%) had history of prior stenting. The mean left ventricular function was 45 ± 13 %. The majority (76%) of patients presented with acute coronary syndrome (ACS).
Table 2 shows the angiographic features and procedural data of our patients. The majority of patients (78 %) had multi-vessel disease on coronary angiography. The most common target vessel for PCI with DEB was left anterior descending artery (37%), right coronary artery (18%), left circumflex artery (17%) and others (28%). “Others” include side branches ( mostly diagonals followed by posterior descending arteries/posterior left ventricular branches, obtuse marginals), saphenous venous grafts ( all seven cases involved distal venous graft anastomosis) and left main lesions (2 patients).
Table 2.
Baseline Angiographic Features and Procedural Data of Patients.
| N = 320 | |
|---|---|
| No.of obstructive coronary artery: | |
| 1,n,% | 70 (22) |
| 2,n,% | 112 (35) |
| 3,n,% | 138 (43) |
| Target vessel: | |
| LAD,n,% | 124 (37) |
| RCA,n,% | 62 (18) |
| LCx,n,% | 58 (17) |
| Others,n,% | 93 (28) |
| Lesion type: | |
| Small vessel,n,% | 182 (54) |
| ISR,n,% | 72 (21) |
| Bifurcation,n,% | 20 (6) |
| De novo,n,% | 17 (5) |
| Others,n,% | 46 (14) |
| PCI Approach: | |
| DEB alone therapy ,n,% | 276 (82) |
| DEB and bare metal stenting,n,% | 61 (18) |
| Mean number of DEB per patient | 1.2 ± 0.5 |
| Mean diameter of DEB, mm | 2.6 ± 0.6 |
| Total length of DEB (average),mm | 24.0 ± 11.1 |
| Glycoprotein IIb/IIIa inhibitors,n,% | 232 (72) |
* LAD denotes left anterior descending, RCA right coronary artery, LCx left circumflex, ISR instent restenosis, DEB drug-eluting balloon.
Glycoprotein IIb/IIIa inhibitors were administered in 232 patients (73%). The most common indication for the use of DEB in our registry was small vessel disease (54%) followed by ISR (21%), bifurcation lesions (6%), de novo lesions (5%) and others (14%). Of the 72 ISR, DEB intervention was performed for 56% BMS ISR and 44% DES ISR. Based on Mehran classification for ISR, DEB was largely used for focal ISR (Mehran type I: 56%) followed by Mehran type II (28%), Mehran Type IV (12%) and Mehran Type III (4%).
DEB alone therapy was the predominant approach (82% of patients ) during PCI whereas DEB followed by bare metal stenting was performed for the remaining 18% of patients. An average of 1.23 ± 0.5 DEB were used per patient, with mean DEB diameter of 2.6 ± 0.6 mm and average total length of 24.0 ± 11.1 mm.
For the initial 320 patients, 5 patients died during index hospitalization and 11 patients were lost to follow-up. Table 3 summarizes the clinical outcomes of 304 patients at 9 months follow-up. A total of 16 patients (5.3%) developed MACE at 9 months follow-up.
Table 3.
Clinical Outcomes at 9 months Follow-up.
| N = 304 | |
|---|---|
| MACE,n,%, | 16(5.3) |
| Cardiovascular death,n,% | 3 (1) |
| TLR,%,n | 12 (4) |
| Target vessel related MI,n,% | 8 (2.6) |
| Target lesion thrombosis,n,% | 0 (0) |
MACE denotes major adverse cardiac events, TLR target lesion revascularization, MI myocardial infarction
MACE was mainly driven by TLR (4%) followed by target vessel related MI (2.6%) and CVS death (1%). There was no reported target lesion thrombosis. Factors associated with TLR by univariate analysis ( TLR group vs non-TLR group) were older age at presentation (67.2 ± 11.2 years vs 60.6 ± 10.9 years, p = 0.04), diabetes mellitus (75% vs 47%, p = 0.07), DES ISR (25% versus 8.6%, p = 0.08), DEB alone therapy during PCI (58.3% vs 83%, p = 0.04), mean number of DEB per patient (1.5 ± 0.67 vs 1.23 ± 0.47, p = 0.06) and total DEB length (31.7 ± 14.6 vs 23.9 ± 11 mm, p = 0.02). However no independent predictors of TLR were identified by multi-variable analysis. Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7 illustrates our clinical experiences with the use of DEB in “real world”clinical practice.
Fig. 1.
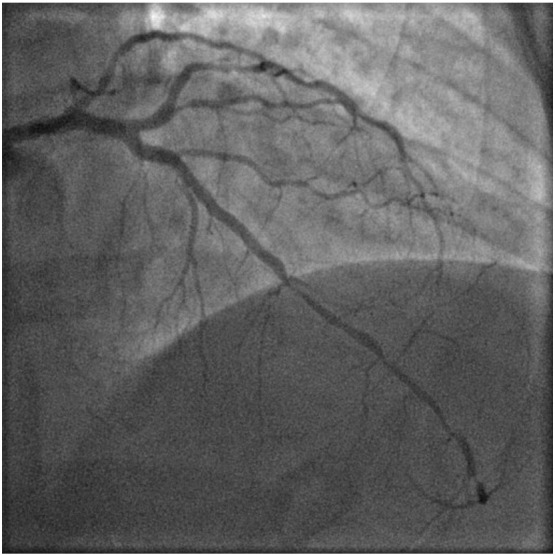
Baseline angiography showing diffuse lesion in mid to distal LAD artery.
Fig. 2.
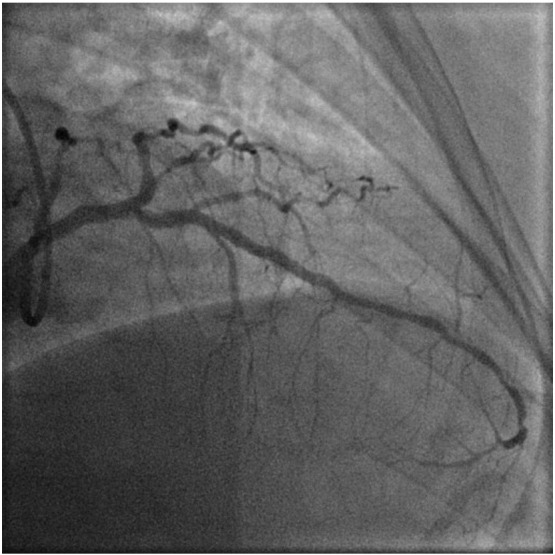
Final angiographic result of mid to distal LAD ( after treatment with DEB alone therapy).
Fig. 3.
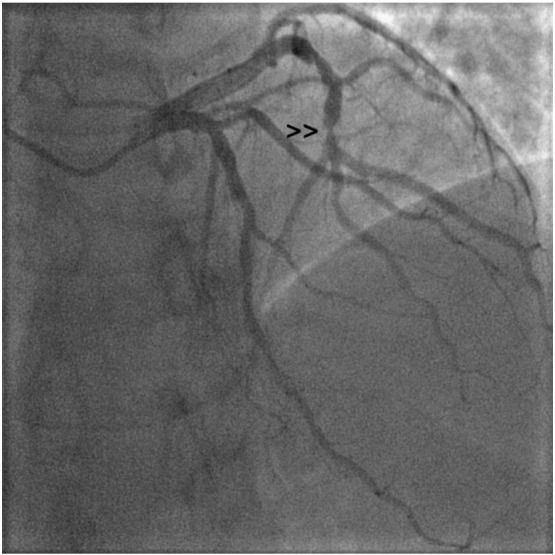
Baseline angiography showing a critical trifurcation stenosis in distal LCx artery ( lesion site highlighted by small arrowhead >>).
Fig. 4.
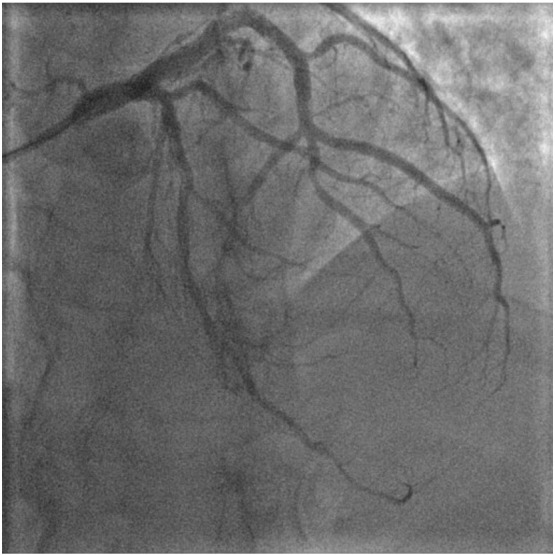
Final angiographic result of distal LCx artery ( after treatment with DEB alone therapy).
Fig. 5.
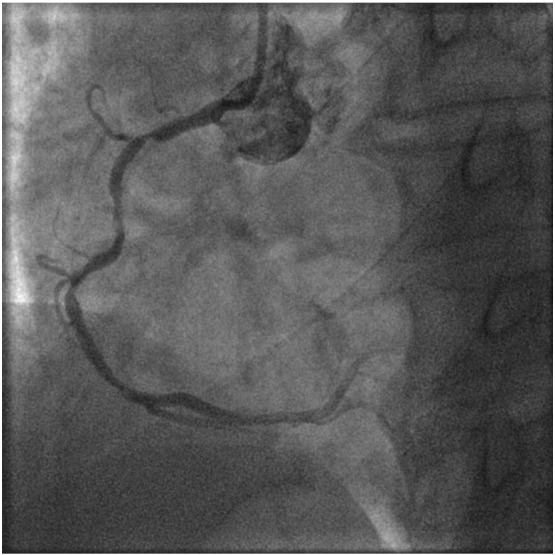
Baseline angiography showing a diffuse obstructive lesion in proximal to mid RCA which was also moderately calcified.
Fig. 6.
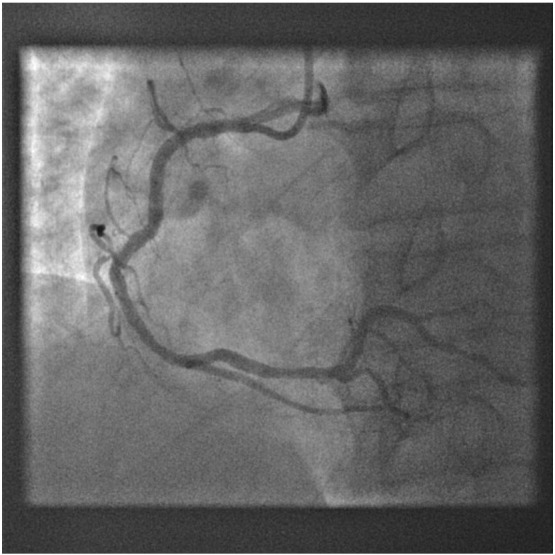
Final angiographic result of RCA after treatment with DEB and bare metal stenting.
Fig. 7.
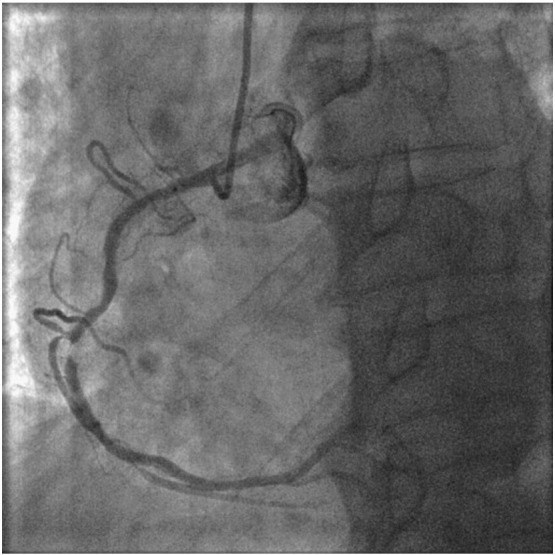
Relook angiogram at 5 months showing focal ISR in mid RCA.
4. Discussion
To our knowledge, this is the largest registry in the South-East Asian region evaluating the use of DEB in an all-comer group of patients in the “real world”. We found that the use of SeQuent Please DEB was a safe and effective treatment modality for South-East Asian patients in the “real world” setting. The intermediate term clinical outcomes in our cohort of patients were good with a low incidence of MACE.
In recent years, DEB has emerged as a viable therapeutic option for treating CAD as the current DES technology [14] has limitations like late stent thrombosis and prolonged DAPT.
Paclitaxel is the drug of choice for all the commercially available DEB manufacturers because of its highly lipophilic properties which allows rapid diffusion into the vessel wall and sustained anti-proliferative effect despite its short contact with the vessel wall. The largest clinical evidence [2], [3], [4], [5], [6], [7], [8], [9], [11], [12] has been reported for DEB coated with paclitaxel-iopromide with > 3000 patients evaluated in randomized clinical trials and registries. Currently, the European Society of Cardiology [15] (ESC) guidelines have given a Class IIa approval to DEB for the treatment of BMS ISR as there is robust clinical data [2], [3], [4] supporting its use in this particular lesion subset. Favourable results are also seen with the use of DEB in DES ISR [5], [6] although robust data is lacking for the combined use of DEB and BMS [7], [8] in routine PCI and also in specific lesions subsets [9], [10], [11] like small vessel, diffuse disease, bifurcation lesion, calcified lesion, thrombus-containing lesion, chronic total occlusion, etc. Similar to DES, DEB has been used in “off-label” indications and our study is one of the few [12] to evaluate its clinical efficacy and safety in the “real world”. The clinical profile of patients in the “real world” setting are usually different from those recruited into clinical trials as they often have more complex lesions and certain clinical issues which necessitate a shorter DAPT regimen.
In our registry, the majority of our patients (76%) presented with acute coronary syndrome and the percentage of patients with DM (48.4%) was quite high. Small vessel disease (often with concomitant diffuse disease) was a common feature of our patients on coronary angiography and accounted for the predominant use of DEB (54%) in our registry. Our patients’ clinical characteristics were very different from patients enrolled in the SeQuent Please World Wide Registry [12] which had a small number of Asian patients. For the latter registry, the majority of patients (73%) received treatment with SeQuent Please DEB for BMS/DES ISR and the percentage of patients with DM (36%) was much lower. By comparing the mean number of DEB per patient, mean DEB diameter and average total length of DEB (1.23 ± 0.5, 2.6 ± 0.6 mm and 24.0 ± 11.1 mm) in our registry and the SeQuent Please World Wide Registry registry (1.1 DEBs per lesion, 2.9 ± 0.4 mm and 20.3 ± 5.5 mm), we can infer that our South-East Asian patients had a relatively higher proportion of small vessel and diffuse CAD than their non-Asian counterparts. This could be due to the higher prevalence of DM in our cohort of patients.
DEB maybe a good alternative option for diffuse lesions as outcomes remain relatively unfavorable for stent-based coronary intervention especially those with de novo long CAD. Even in the DES era, studies [16], [17], [18] have shown that the TLR rates can range from 6% to 28% for such lesion subset with higher rates of ISR for paclitaxel DES. A recent study [19] evaluating the full metal jacket use of overlapping everolimus-eluting stents in extra long lesion (> 60 mm) reported an angiographic restenosis of 12.5% at 9 months and a high cardiac death rate at long term follow-up ( possibly related to stent thrombosis).
In our study, DEB alone therapy (82%) was the predominant approach for PCI. This is the preferred PCI strategy as recommended by the German DEB Consensus Group [20] as this approach is associated with a low late loss (which translates into lower rate of TLR). Adequate lesion preparation before application of DEB to culprit lesion is important and we used scoring/cutting balloons in addition to uncoated balloon in approximately 30% of all PCI cases. DEB followed by bare metal stenting was performed for the remaining 18% of cases. This strategy was used as primary therapy (75%) or as bail-out stenting (25%) in cases when we observe significant recoil/residual stenosis or dissection (Type C-F) after deflation of DEB.
In our study, a total of 16 patients (5.3%) developed MACE at 9 months follow-up and it was mainly driven by TLR (12 patients). Although the number was small, we identified certain clinical features/factors that were predictive of TLR by univariate analysis. These included older age at presention, longer length of DEB and lower use of DEB alone therapy (p < 0.05). There was a trend towards higher rate of TLR when patients have history of DM and underlying DES ISR. This is consistent with the findings of the SeQuent Please World Wide Registry registry [12] which had shown unfavourable DEB results in patients with DM versus non-diabetics and similarly, in DES ISR versus BMS ISR. In our study, 5 patients who were treated with combined DEB and bare metal stenting required re-intervention. All five have long stents implanted ( average total stent length of 37.2 ± 15.3 mm) which likely contributed to its restenosis besides underlying diabetes mellitus which was present in 4 patients. 3 of the patients were also found to have moderate to heavily calcified vessels (Fig. 5, Fig. 6, Fig. 7). For calcified lesions, DEB may not be suitable as the distribution of paclitaxel during balloon inflation may not be homogenous and the drug diffusion into the vessel wall may be suboptimal. The coating of DEB may also be “damaged” during balloon delivery if the coronary segment proximal to target lesion is calcified as a result of friction and resistance.
5. Limitation
There were several limitations to our study. All patients in our study received treatment with SeQuent Please DEB and our results could only be extrapolated to those who had received similar therapy. Whether similar results would be seen with patients receiving other types of DEB is unknown as not all DEBs are equal in terms of clinical efficacy. Furthermore, our study was a single center registry, subject to selection and operator bias.
There is also lack of routine angiographic follow-up in our study which may lead to overestimation of its purported clinical benefit in the “real world”. As many of our treated patients had small vessel disease, they may have developed clinically silent restenosis/occlusion and therefore assumed to be fine on follow-up.
6. Conclusion
We found that the use of SeQuent Please DEB was a safe and effective treatment modality for South-East Asian patients in the “real world” setting. The intermediate term clinical outcomes in our cohort of patients were good with low incidence of MACE. Longer clinical follow-up is necessary to establish its true clinical efficacy and safety.
Footnotes
Declaration of Interest: The authors report no conflicts of interest.
This author takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Available online 27 November 2013
References
- 1.Scheller B., Speck U., Abramjuk C., Bernhardt U., Böhm M., Nickenig G. Paclitaxel balloon coating, a novel method for prevention and therapy of restenosis. Circulation. 2004;110:810–814. doi: 10.1161/01.CIR.0000138929.71660.E0. [DOI] [PubMed] [Google Scholar]
- 2.Scheller B., Hehrlein C., Bocksch W. Treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. N Engl J Med. Nov 16 2006;355(20):2113–2124. doi: 10.1056/NEJMoa061254. [Epub 2006 Nov 13] [DOI] [PubMed] [Google Scholar]
- 3.Unverdorben M., Vallbracht C., Cremers B. Paclitaxel-coated balloon catheter versus paclitaxel-coated stent for the treatment of coronary in-stent restenosis. Circulation. Jun 16 2009;119(23):2986–2994. doi: 10.1161/CIRCULATIONAHA.108.839282. [Epub 2009 Jun 1] [DOI] [PubMed] [Google Scholar]
- 4.Scheller B., Clever Y.P., Kelsch B. Long-term follow-up after treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. JACC Cardiovasc Interv. Mar 2012;5(3):323–330. doi: 10.1016/j.jcin.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Habara S., Mitsudo K., Kadota K. Effectiveness of paclitaxel-eluting balloon catheter in patients with Sirolimus-eluting stent restenosis. J Am Coll Cardiol Intv. 2011;4:149–154. doi: 10.1016/j.jcin.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Rittger H., Brachmann J., Sinha A.M. PEPCAD-DES: a randomized, multicenter, single-blinded trial comparing paclitaxel-coated balloon angioplasty with plain balloon angioplasty in drug-eluting stent restenosis. J Am Coll Cardiol. Apr 10 2012;59(15):1377–1382. doi: 10.1016/j.jacc.2012.01.015. [Epub 2012 Feb 29] [DOI] [PubMed] [Google Scholar]
- 7.Wöhrle J., Birkemeyer R., Markovic S. Prospective randomised trial evaluating a paclitaxel-coated balloon in patients treated with endothelial progenitor cell capturing stents for de novo coronary artery disease. Heart. 2011;97:1338–1342. doi: 10.1136/hrt.2011.226563. [DOI] [PubMed] [Google Scholar]
- 8.Ali R.M., Degenhardt R., Zambahari R. Paclitaxel-eluting balloon angioplasty and cobalt-chromium stents versus conventional angioplasty and paclitaxel-eluting stents in the treatment of native coronary artery stenoses in patients with diabetes mellitus. EuroIntervention. 2011;(7 Suppl. K):K83–K92. doi: 10.4244/EIJV7SKA15. [DOI] [PubMed] [Google Scholar]
- 9.Unverdorben M., Kleber F.X., Heuer H. Treatment of small coronary arteries with a paclitaxel-coated balloon catheter. Clin Res Cardiol. 2010;99:165–174. doi: 10.1007/s00392-009-0101-6. [DOI] [PubMed] [Google Scholar]
- 10.Latib A., Colombo A., Castriota F. A randomized multicenter study comparing a paclitaxel drug-eluting balloon with a paclitaxel-eluting stent in small coronary vessels: the BELLO (Balloon Elution and Late Loss Optimization) study. J Am Coll Cardiol. Dec 18 2012;60(24):2473–2480. doi: 10.1016/j.jacc.2012.09.020. [Epub 2012 Nov 14. Erratum in: J Am Coll Cardiol. 2013 Apr 16;61(15):1660] [DOI] [PubMed] [Google Scholar]
- 11.Mathey D.G., Wendig I., BoxbergerM Bonaventura K., Kleber F.X. Treatment of bifurcation lesions with a drug-eluting balloon: the PEPCAD V (Paclitaxel Eluting PTCA Balloon in Coronary Artery Disease) trial. EuroIntervention. 2011;(7 Suppl. K):K61–K65. doi: 10.4244/EIJV7SKA11. [DOI] [PubMed] [Google Scholar]
- 12.Wöhrle J., Zadura M., Möbius-Winkler S. SeQuentPlease World Wide Registry: clinical results of SeQuent please paclitaxel-coated balloon angioplasty in a large-scale, prospective registry study. J Am Coll Cardiol. Oct 30 2012;60(18):1733–1738. doi: 10.1016/j.jacc.2012.07.040. [Epub 2012 Oct 3] [DOI] [PubMed] [Google Scholar]
- 13.Cutlip D.E., Windecker S., Mehran R. Academic Research Consortium. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. May 1 2007;115(17):2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 14.Joner M., Finn A.V., Farb A. Pathology of drug eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 15.Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS), European Association for Percutaneous Cardiovascular Interventions (EAPCI), Wijns W., Kolh P., Danchin N. Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2010;31:2501–2555. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 16.Degertekin M., Arampatzis C.A., Lemos P.A. Very long sirolimus-eluting stent implantation for de novo coronary lesions. Am J Cardiol. 2004;93:826–829. doi: 10.1016/j.amjcard.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 17.Tsagalou E., Chieffo A., Iakovou I. Multiple overlapping drug-eluting stents to treat diffuse disease of the left anterior descending coronary artery. J Am Coll Cardiol. 2005;45:1570–1573. doi: 10.1016/j.jacc.2005.01.049. [DOI] [PubMed] [Google Scholar]
- 18.Sharp A.S., Latib A., Ielasi A. Long-term follow-up on a large cohort of “full-metal jacket” percutaneous coronary intervention procedures. Circ Cardiovasc Interv. 2009;2:416–422. doi: 10.1161/CIRCINTERVENTIONS.109.886945. [DOI] [PubMed] [Google Scholar]
- 19.Jim M.H., Yiu K.H., Ho H.H. Angiographic and Clinical Outcomes of Everolimus-Eluting Stent in the Treatment of Extra Long Stenoses (AEETES) J Interv Cardiol. Feb 2013;26(1):22–28. doi: 10.1111/joic.12006. [DOI] [PubMed] [Google Scholar]
- 20.Kleber F.X., Mathey D.G., Rittger H., Scheller B. German Drug-eluting Balloon Consensus Group. How to use the drug-eluting balloon: recommendations by the German consensus group. EuroIntervention. May 2011;(7 Suppl. K):K125–K128. doi: 10.4244/EIJV7SKA21. [DOI] [PubMed] [Google Scholar]


