Abstract
Background
Growing evidence suggests that exposure to per- and polyfluoroalkyl substances (PFASs) may disrupt lipid homeostasis and liver function, but data in children are limited.
Objective
We examined the association of prenatal and mid-childhood PFAS exposure with lipids and alanine aminotransferase (ALT) levels in children.
Methods
We studied 682 mother-child pairs from a Boston-area pre-birth cohort. We quantified PFASs in maternal plasma collected in pregnancy (median 9.7 weeks gestation, 1999–2002) and in child plasma collected in mid-childhood (median age 7.7 years, 2007–2010). In mid-childhood we also measured fasting total (TC), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), and ALT. We then derived low-density lipoprotein cholesterol (LDL-C) from TC, HDL-C, and TG using the Friedewald formula.
Results
Median (interquartile range, IQR) perfluorooctane sulfonate (PFOS), perfluorooctanoate (PFOA), and perfluorodecanoate (PFDeA) concentrations in child plasma were 6.2 (5.5), 4.3 (3.0), and 0.3 (0.3) ng/mL, respectively. Among girls, higher child PFOS, PFOA, and PFDeA concentrations were associated with detrimental changes in the lipid profile, including higher TC and/or LDL-C [e.g., β per IQR increment in PFOS = 4.0 mg/dL (95% CI: 0.3, 7.8) for TC and 2.6 mg/dL (−0.5, 5.8) for LDL-C]. However, among both boys and girls, higher plasma concentrations of these child PFASs were also associated with higher HDL-C, which predicts better cardiovascular health, and slightly lower ALT, which may indicate better liver function. Prenatal PFAS concentrations were also modestly associated with improved childhood lipid and ALT levels.
Conclusions
Our data suggest that prenatal and mid-childhood PFAS exposure may be associated with modest, but somewhat conflicting changes in the lipid profile and ALT levels in children.
Keywords: per- and polyfluoroalkyl substances, lipids, liver function, pregnancy, childhood
1. Introduction1
Per- and polyfluoroalkyl substances (PFASs) – synthetic compounds used in a wide range of industrial and consumer products, including stain-resistant coatings for upholstery and fabrics, pesticide additives, coatings for food packaging, and fire-retardant foams (Lindstrom et al. 2011) – have structural homology with fatty acids (Fletcher et al. 2013) and may have endocrine-disrupting properties (Braun 2017). Evidence suggests that PFAS exposure may contribute to lipid- and liver enzyme-related metabolic disturbances (Steenland et al. 2010) through activation of the peroxisome proliferator-activated receptors (PPAR) alpha (α) (Wolf et al. 2008) and gamma (γ) (Vanden Heuvel et al. 2006), and/or altered expression of lipid transport- and metabolism-related genes (Fletcher et al. 2013).
Most animal studies have shown that PFAS exposure can induce beneficial changes in circulating lipids, including lower total cholesterol (TC) and triglycerides (TG) (Kennedy et al. 2004; Lau et al. 2007; White et al. 2011). Human studies have reported conflicting associations of PFASs with lipids, with cross-sectional studies in adults and children reporting associations of higher PFASs concentrations with detrimental [i.e., higher circulating TC, low-density lipoprotein cholesterol (LDL-C), and TG] (Costa et al. 2009; Eriksen et al. 2013; Fitz-Simon et al. 2013; Geiger et al. 2014; Nelson et al. 2010; Sakr et al. 2007a; Sakr et al. 2007b; Starling et al. 2014; Steenland et al. 2009; Zeng et al. 2015) and beneficial changes in lipid profile [i.e., higher high-density lipoprotein cholesterol (HDL-C)] (Chateau-Degat et al. 2010; Starling et al. 2014). One of two published studies that explored associations of prenatal PFASs with mid-childhood lipids observed non-linear associations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) (two of the most prevalent and commonly studied PFASs) with serum lipids (TC and LDL-C): at low PFAS concentrations (lower tertile), PFAS-related associations with lipids were beneficial and at high PFAS concentrations (middle and upper tertiles), PFAS-related associations with lipids were detrimental (Maisonet et al. 2015). The other study observed beneficial associations of prenatal perfluorohexane sulfonate (PFHxS) plasma concentrations with TG z-scores measured in early childhood (Manzano-Salgado et al. 2017). These studies did not examine the association between postnatal PFAS exposure and childhood lipids.
Recent cross-sectional and cohort studies in adults have investigated the association between PFASs and liver enzymes – markers of hepatocellular dysfunction – with inconsistent results (Alexander et al. 2007; Costa et al. 2009; Darrow et al. 2016; Emmett et al. 2006; Gallo et al. 2012; Gleason et al. 2015; Lin et al. 2009; Sakr et al. 2007a; Sakr et al. 2007b). For example, PFAS concentrations have been positively associated with alanine aminotransferase (ALT) levels in some population-based (Darrow et al. 2016; Gallo et al. 2012; Gleason et al. 2015) and occupational studies (Alexander and Olsen 2007; Lin et al. 2009; Sakr et al. 2007a), but not in others (Costa et al. 2009; Sakr et al. 2007b). To our knowledge, no study has examined the association of prenatal or postnatal PFAS exposure with liver enzymes in children.
Project Viva is a prospective pre-birth cohort designed to study the extent to which events during early development affect health outcomes over the lifespan. In previous analyses of PFASs in Project Viva, we observed modest associations with increased adiposity and risk of obesity in girls, but not boys, in mid-childhood (Mora et al. 2017). However, we found no adverse effects of early-life PFAS exposure on leptin, adiponectin, or homeostatic assessment of insulin resistance (HOMA-IR) in mid-childhood; in fact, children with higher plasma concentrations of some PFASs had lower insulin resistance (Fleisch et al. 2017). In light of these findings, and given that animal data have shown that early-life exposure to PFASs may disrupt lipid metabolism and induce hepatotoxic effects (Kennedy et al. 2004; Lau et al. 2007; White et al. 2011), we evaluated the extent to which PFAS concentrations in prenatal and mid-childhood plasma were associated with childhood lipids and ALT in Project Viva.
2. Methods
2.1. Study population
Pregnant women were enrolled in Project Viva from 1999 to 2002 during their first prenatal visit to Atrius Harvard Vanguard Medical Associates, a multi-specialty group practice in Eastern Massachusetts (Oken et al. 2015). Of 2,128 live singleton offspring, 1,776 (84%) children had PFAS concentrations measured in maternal non-fasting plasma collected in early pregnancy [median (range) 9.7 (4.8–21.4) weeks gestation, n = 1,645] or in child fasting plasma collected in mid-childhood [median age (range) 7.7 (6.7–11.0) years, n = 653]. Of these 1,776 children, 682 (38%) had lipids or ALT measured in fasting mid-childhood plasma samples (same samples used to measure PFAS; see Figure A.1).
Institutional Review Boards of participating sites approved all study protocols. All mothers provided written informed consent at each study visit and children provided verbal assent at the mid-childhood visit. The involvement of the Centers for Disease Control and Prevention (CDC) laboratory did not constitute engagement in human subjects research.
2.2. Prenatal and child PFAS measurements
Maternal and child plasma samples were shipped to the Division of Laboratory Sciences at the CDC and analyzed for concentrations of eight PFAS analytes: PFOS, PFOA, PFHxS, perfluorononanoate (PFNA), 2-(N-ethyl-perfluorooctane sulfonamido) acetate (Et-PFOSA-AcOH; also known as EtFOSAA), 2-(N-methyl-perfluorooctane sulfonamido) acetate (Me-PFOSA-AcOH; also known as MeFOSAA), perfluorodecanoate (PFDeA), and perfluorooctane sulfonamide (PFOSA; also known as FOSA). Child plasma samples were also analyzed for concentrations of linear and branched isomers of PFOS and PFOA (we did not measure linear and branched isomers in our maternal samples because they were analyzed earlier): n-perfluorooctane sulfonate (n-PFOS), perfluoromethylheptane sulfonates (Sm-PFOS), perfluorodimethylhexane sulfonates (Sm2-PFOS), n-perfluorooctanoate (n-PFOA), and branched perfluorooctanoates (Sb-PFOA). All samples were analyzed using online solid-phase extraction coupled with isotope dilution high-performance liquid chromatography-tandem mass spectrometry, as previously described (Fleisch et al. 2017; Harris et al. 2017; Sagiv et al. 2015). Limits of detection (LOD) were 0.1 ng/mL for all PFASs except for PFOS concentrations in prenatal plasma (0.2 ng/mL). Values below the LOD were replaced with LOD divided by the square root of 2.
2.3. Lipids and ALT levels in mid-childhood
We measured fasting TC, HDL-C, TG, and ALT using enzymatic assays, in the same plasma samples used for quantification of mid-childhood PFASs. We then calculated LDL-C using the Friedewald formula: TC-(HDL-C)-(TG × 0.2) (Friedewald et al. 1972). We also calculated the ratio of TC to HDL-C, an indicator of the detrimental portion of the lipid profile (Millan et al. 2009).
2.4. Potential confounders and predictors of lipids and ALT levels
We collected information on maternal age, marital status, education, parity, smoking habits, and household income using in-person interviews at study enrollment. We also assessed maternal albumin concentrations and glomerular filtration rate (GFR), two markers of pregnancy hemodynamics that have been hypothesized as potential confounders of PFAS-outcomes associations (Savitz 2014; Verner et al. 2015). We measured albumin and creatinine concentrations in the same early pregnancy plasma specimens used for PFASs quantification and then calculated GFR using the Cockroft-Gault formula [GFR-CG = (140-age) × weight (kg) × 1.04/serum creatinine (μmol/L)] (Cockcroft et al. 1976). Albumin is the primary binding protein for PFASs (D’Eon J et al. 2010) and also an indicator for plasma volume expansion during pregnancy.
We abstracted date of delivery from medical records. We collected information on child sex, race/ethnicity, breastfeeding duration, fast food and soda intake, physical activity, and screen time using mailed questionnaires and in-person interviews throughout childhood. Lastly, we measured child height and weight at the mid-childhood visit and calculated body mass index (BMI) as weight (kg)/height (m)2.
2.5. Statistical analyses
We decided a priori to include in our analyses only PFASs with a detection frequency of 70% or more (Lubin et al. 2004), which included six prenatal (PFOS, PFOA, PFNA, PFHxS, EtFOSAA, and MeFOSAA) and five mid-childhood PFASs (PFOS, PFOA, PFNA, PFHxS, and PFDeA).
We examined associations of prenatal and mid-childhood PFAS plasma concentrations with lipids and ALT in mid-childhood using multivariable linear regression models. We modeled PFAS concentrations as continuous variables, with point estimates representing the change in outcome per interquartile range (IQR) increment in exposure in order to facilitate comparisons across PFAS analytes with different distributions within our study population. To evaluate nonlinearity, we fitted generalized additive models (GAM) with penalized spline smooth terms for the exposures (all pGAM > 0.05); we also modeled PFAS concentrations in quartiles to limit the influence of extreme values. We interpreted effect estimates based on their magnitude and precision.
We identified potential confounders and known predictors of the outcomes of interest (i.e. maternal education, smoking during pregnancy, gestational age at blood draw, and child’s sex, race/ethnicity, and age at lipids/ALT measurements) using directed acyclic graphs. These covariates were included a priori in regression models. We assessed other covariates reported in the literature [household income, maternal marital status, maternal plasma albumin concentrations and GFR during pregnancy (only for prenatal PFASs), breastfeeding duration; and child’s physical activity, screen time, and fast food and soda consumption (only for mid-childhood PFASs)] by adding them, one at a time, to the final models, but none of them materially changed the PFAS coefficients. Missing values (< 10%) for covariates (i.e., marital status, maternal education, annual household income, smoking status, and child race/ethnicity) were imputed by randomly selecting a value from the dataset (Lubin et al. 2004). Because we were missing data on child race/ethnicity for almost 10% of the study participants, we also created a separate variable for which we substituted the missing values with maternal race/ethnicity. We found minimal differences between the randomly imputed and substitution variables, so we decided to adjust our models for the former in order to be consistent with our imputation method.
Because previous studies have observed sex-specific associations between PFASs and lipids (Eriksen et al. 2013; Frisbee et al. 2010), we examined whether child sex modified the exposure-outcome associations using a product interaction term between child sex and prenatal or mid-childhood PFAS IQRs (or quartiles) and also stratifying by sex.
We conducted several sensitivity analyses to assess the robustness of our results. First, given that a few studies have linked specific PFAS isomers to health outcomes (Bao et al. 2017; Yu et al. 2015), we examined the extent to which different isomers of mid-childhood PFASs were associated with lipids and ALT. We considered all isomer concentrations with a detection frequency of 70% or more [n-PFOS, Sm-PFOS, and n-PFOA (all with 99.5% detectable values) (CDC 2015; Fleisch et al. 2017)]. Second, we included all prenatal PFASs in the prenatal covariate-adjusted models and all mid-childhood PFASs in the mid-childhood models to examine co-pollutant confounding. Finally, because prenatal PFAS concentrations have been positively associated with adiposity (Mora et al. 2017) and adiposity is an important determinant of lipids in childhood (Dai et al. 2009; Plourde 2002), we included mid-childhood BMI (kg/m2) in both combined and sex-stratified models to explore its potential effect as mediator of the PFAS-lipids associations.
3. Results
3.1. Participants’ characteristics
On average, pregnant women included in the present analyses were 32.4 years old at enrollment [standard deviation (SD) = 5.6]. Approximately 90% were married or cohabitating with their partners, 58% were multiparous, 65% had a college degree or greater, 62% had an annual household income higher than $70,000, and 69% had never smoked (Table 1). Fifty-two percent of children were boys and 59% were white. Maternal and child characteristics were similar among boys and girls (Table 1).
Table 1.
Characteristics [n (%) or median (P25-P75)] of Project Viva study participants included in present analyses.
| All children (n = 682) |
Boys (n = 356) |
Girls (n = 326) |
p-valuea | |
|---|---|---|---|---|
| Maternal/family characteristics | ||||
| Age at enrollment (years) | ||||
| <20 | 27 (4.0) | 14 (3.9) | 13 (4.0) | 0.99 |
| 20-<35 | 434 (63.6) | 226 (63.5) | 208 (63.8) | |
| ≥35 | 221 (32.4) | 116 (32.6) | 105 (32.2) | |
| Married/cohabitatingb | ||||
| No | 73 (10.7) | 35 (9.8) | 38 (11.7) | 0.44 |
| Yes | 609 (89.3) | 321 (90.2) | 288 (88.3) | |
| Parity | ||||
| 0 | 287 (42.1) | 152 (42.7) | 135 (41.4) | 0.85 |
| 1 | 254 (37.2) | 129 (36.2) | 125 (38.3) | |
| ≥2 | 141 (20.7) | 75 (21.1) | 66 (20.3) | |
| Educationb | ||||
| <College graduate | 242 (35.5) | 133 (37.4) | 109 (33.4) | 0.56 |
| College graduate | 245 (35.9) | 124 (34.8) | 121 (37.1) | |
| Graduate school | 195 (28.6) | 99 (27.8) | 96 (29.5) | |
| Annual household income (USD $)a | ||||
| <40,000 | 120 (17.6) | 58 (16.3) | 62 (19.0) | 0.64 |
| 40,000–70,000 | 141 (20.7) | 74 (20.8) | 67 (20.6) | |
| >70,000 | 421 (61.7) | 224 (62.9) | 197 (60.4) | |
| Smoking statusb | ||||
| Never smoked | 473 (69.4) | 241 (67.7) | 232 (71.2) | 0.62 |
| Former smoker | 133 (19.5) | 73 (20.5) | 60 (18.4) | |
| Smoked during pregnancy | 76 (11.1) | 42 (11.8) | 34 (10.4) | |
| Gestational age at prenatal PFAS measurements (weeks)c | 9.6 (8.6–10.7) | 9.6 (8.7–10.7) | 9.7 (8.4–10.7) | 0.75 |
| Child characteristics | ||||
| Race/ethnicityb | ||||
| Black | 159 (23.3) | 84 (23.6) | 75 (23.0) | 0.93 |
| White | 403 (59.1) | 208 (58.4) | 195 (59.8) | |
| Other | 120 (17.6) | 64 (18.0) | 56 (17.2) | |
| BMI in mid-childhood (kg/m2) | 16.6 (15.4–18.4) | 16.4 (15.4–17.9) | 16.7 (15.3–18.8) | 0.08 |
| Age at mid-childhood PFAS and lipids/ALT measurements (years) | 7.7 (7.4–8.4) | 7.7 (7.4–8.5) | 7.7 (7.3–8.2) | 0.16 |
Abbreviations: BMI, body mass index; PFAS, per- and polyfluoroalkyl substances; ALT, alanine aminotransferase.
p-values are for chi-square or Kruskal-Wallis tests across the different categories of each characteristic.
Missing data before simple random imputation: 5 (0.7%) for marital status, 4 (0.6%) for maternal education, 67 (9.8%) for annual household income, 1 (0.2%) for smoking status, and 65 (9.5%) for child race/ethnicity.
Only available for 561 mother-child pairs (299 boys and 262 girls) with prenatal PFAS concentrations.
Mid-childhood PFOS, PFOA, PFNA, and PFHxS concentrations were lower than prenatal concentrations quantified in maternal samples (Table 2). Spearman correlation coefficients of PFAS concentrations measured in prenatal plasma ranged between 0.20 and 0.72 and in mid-childhood plasma ranged between 0.14 and 0.79 (strongest correlation for PFOS and PFOA; see Table A.1). Correlations of the same PFASs measured in prenatal versus mid-childhood plasma ranged between 0.08 and 0.40 (see Table A.1). Prenatal and mid-childhood PFAS concentrations did not vary by child sex (Table 2).
Table 2.
Distribution of prenatal PFAS concentrations and mid-childhood PFAS (ng/mL), lipid (mg/dL) and ALT (U/L) concentrations in the study population.
| All childrena
|
Boysa
|
Girlsa
|
||||
|---|---|---|---|---|---|---|
| %>LODb | Range | Median (P25-P75) | Median (P25-P75) | Median (P25-P75) | p-valuec | |
| Exposures | ||||||
| Prenatal PFAS | ||||||
| PFOS | 100.0 | 4.6–168.0 | 24.6 (17.9–34) | 24.3 (17.8–34.4) | 24.7 (18.2–32.9) | 0.92 |
| PFOA | 100.0 | 0.9–22.4 | 5.4 (3.9–7.6) | 5.5 (4.0–7.3) | 5.4 (3.8–7.9) | 0.53 |
| PFNA | 99.0 | 0.1–2.6 | 0.6 (0.5–0.9) | 0.6 (0.5–0.9) | 0.6 (0.5–0.9) | 0.56 |
| PFHxS | 99.7 | 0.1–43.2 | 2.4 (1.6–3.8) | 2.5 (1.6–3.8) | 2.2 (1.4–3.9) | 0.22 |
| EtFOSAA | 99.6 | 0.1–21.2 | 1.2 (0.1–21.2) | 1.1 (0.7–1.8) | 1.2 (0.7–1.9) | 0.72 |
| MeFOSAA | 100.0 | 0.1–29.7 | 2.0 (1.3–3.2) | 2.0 (1.3–3.1) | 2.0 (1.3–3.2) | 0.96 |
| Mid-childhood PFAS | ||||||
| PFOS | 99.5 | 0.1–51.4 | 6.2 (4.2–9.7) | 6.3 (4.2–9.8) | 6.1 (4.1–9.6) | 0.49 |
| n-PFOS | 99.5 | 0.1–34.2 | 4.4 (3.0–7.0) | 4.4 (3.0–7.1) | 4.5 (2.9–6.9) | 0.57 |
| sm-PFOS | 99.5 | 0.1–16.8 | 1.7 (1.1–2.8) | 1.8 (1.2–2.9) | 1.7 (1.1–2.7) | 0.33 |
| sm2-PFOS | 1.4 | 0.1–0.4 | 0.1 (0.1–0.1) | 0.1 (0.1–0.1) | 0.1 (0.1–0.1) | 0.86 |
| PFOA | 99.5 | 0.1–14.3 | 4.3 (3.0–6.0) | 4.4 (3.1–6.0) | 4.2 (3.0–6.1) | 0.69 |
| n-PFOA | 99.5 | 0.1–13.8 | 4.1 (3.0–5.7) | 4.1 (3.0–5.7) | 4.0 (2.8–5.7) | 0.56 |
| sb-PFOA | 57.3 | 0.1–2.4 | 0.2 (0.1–0.4) | 0.2 (0.1–0.4) | 0.2 (0.1–0.4) | 0.71 |
| PFNA | 99.5 | 0.1–25.7 | 1.5 (1.1–2.3) | 1.5 (1.1–2.2) | 1.5 (1.1–2.3) | 0.83 |
| PFHxS | 99.5 | 0.1–56.8 | 1.9 (1.2–3.4) | 1.9 (1.2–3.8) | 1.9 (1.2–3.2) | 0.16 |
| PFDeA | 88.1 | 0.1–1.9 | 0.3 (0.2–0.5) | 0.3 (0.2–0.5) | 0.4 (0.2–0.5) | 0.26 |
| Mid-childhood outcomesd | ||||||
| Lipids | ||||||
| Total cholesterol | 78.0–288.0 | 160.0 (141.0–176.0) | 160.0 (140.0–178.0) | 159.0 (144.0–175.0) | 0.90 | |
| LDL-C | 10.1–183.0 | 90.6 (76.5–103.9) | 89.4 (74.5–103.9) | 92.1 (79.9–104.6) | 0.11 | |
| HDL-C | 24.7–144.4 | 55.8 (47.7–64.5) | 57.5 (49.8–66.3) | 54.3 (46.5–62.3) | <0.01 | |
| Total/HDL-C × 100 | 135.6–753.0 | 281.5 (243.1–332.6) | 277.4 (236.3–314.1) | 289.3 (250.0–346.8) | <0.01 | |
| Triglycerides | 13.0–756.0 | 52.0 (41.0–68.0) | 51.0 (41.0–67.0) | 54.0 (42.0–70.0) | 0.13 | |
| Liver enzyme | ||||||
| ALT | 8.0–76.0 | 19.0 (16.0–23.0) | 19.0 (16.0–24.0) | 19.0 (16.0–23.0) | 0.93 | |
Abbreviations: PFAS, per- and polyfluoroalkyl substances; LOD, limit of detection; GM, geometric mean; GSD, geometric standard deviation; PFOS, perfluorooctane sulfonate; PFOA, perfluorooctanoate; PFNA, perfluorononanoate; PFHxS, perfluorohexane sulfonate; PFDeA, perfluorodecanoate; EtFOSAA, 2-(N-ethyl-perfluorooctane sulfonamido) acetate; MeFOSAA, 2-(N-methyl-perfluorooctane sulfonamido) acetate; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; ALT, alanine aminotransferase.
Children with prenatal PFAS concentrations = 551 (294 boys and 257 girls), with mid-childhood PFAS concentrations = 649 (343 boys and 306 girls), with lipids = 629 (327 boys and 302 girls), and with ALT levels = 635 (333 boys and 302 girls).
LOD for all analytes except prenatal PFOS: 0.1 ng/mL. LOD for prenatal PFOS: 0.2 ng/mL.
p-values are for Kruskal-Wallis tests comparing boys and girls.
All enzymatic assays were approved by the Food and Drug Administration for clinical use. Coefficients of variation ranged between 1.6–1.7% for total cholesterol, 1.7–3.3% for HDL-C, 1.7–1.8% for triglycerides, and 3.3–4.4% for ALT
Overall, median (IQR) TC in mid-childhood was 160.0 (35.0) mg/dL, LDL-C 90.6 (27.4) mg/dL, HDL-C 55.8 (16.8) mg/dL, TC/HDL-C ratio (× 100) 281.5 (89.5), TG 52.0 (27.0) mg/dL, and ALT 19.0 (7.0) U/L (Table 2). About 17%, 13%, and 15% of children had high TC (≥ 200 mg/dL), LDL-C (≥ 130 mg/dL), and TG (≥ 100 mg/dL), whereas 8% had low HDL-C (<40 mg/dL) (NIH 2012). Among boys, we observed higher HDL-C [median (IQR) 57.5 (16.5)] than among girls [54.3 (15.8); p < 0.05; Table 2]. Among girls, we found higher TC/HDL-C ratios [289.2 (96.8)] than among boys [277.4 (77.8); p < 0.05].
Compared to mothers of children included in the present analysis (n = 682; Table 1), mothers from the initial cohort (n = 2,128) were more likely to be nulliparous (48%), have an annual household income of 40,000–70,000 USD$ (23%), and had slightly higher plasma PFAS concentrations [e.g., median (interquartile range, IQR) PFOS and PFOA concentrations in mothers with PFAS measurements during pregnancy (n = 1,645) 25.7 (16.0) and 5.8 (3.8) ng/mL]; their children were also more likely to be white (67%) or have other race/ethnicity (20%; data not shown).
3.2. Associations of prenatal PFAS with lipids and ALT in mid-childhood
We did not find strong associations of prenatal plasma PFAS concentrations with lipids and ALT measured in mid-childhood in analyses of boys and girls combined (Table 3). However, among girls, we found that higher prenatal PFOS and PFOA concentrations were associated with a beneficial lipoprotein profile, including lower TG and TC/HDL-C ratio (× 100) [e.g., β per IQR increment in PFOS = −4.2 mg/dL (95% confidence interval (CI): −9.2, 0.8) for TG, pINT = 0.04; and −7.7 (95% CI: −16.0, 0.6) for TC/HDL-C, pINT = 0.08; Table 3]. Prenatal PFOA plasma concentrations were also associated with higher HDL-C, a predictor of better cardiovascular health, among girls [β per IQR increment = 1.2 mg/dL (95% CI: −0.8, −3.2), pINT = 0.03; Table 3]. In quartile analyses, we found overall, but not strictly monotonic, lower TG and TC/HDL-C ratio (× 100) and higher HDL-C across higher quartiles (Q3 and/or Q4) of PFOS and/or PFOA concentrations among girls (versus Q1; data not shown). Lastly, almost all prenatal PFAS exposures were negatively associated with ALT levels among girls [e.g., β per IQR increment in MeFOSAA = −1.2 U/L (95% CI: −1.9, −0.5); pINT = 0.05; Table 3].
Table 3.
Adjusted linear regression coefficients for associations of prenatal PFAS concentrations with lipid and ALT levels in mid-childhood among all children and stratified by child sex.
| All childrena
|
Boysb
|
Girlsc
|
||
|---|---|---|---|---|
| β (95% CI) | β (95% CI) | β (95% CI) | pint | |
| Lipids | ||||
| Total cholesterol (mg/dL) | ||||
| PFOS | 0.8 (−1.6, 3.3) | 1.4 (−1.9, 4.8) | −0.4 (−3.5, 2.7) | 0.24 |
| PFOA | 0.5 (−2.3, 3.4) | 1.4 (−2.8, 5.7) | 0.0 (−3.8, 3.9) | 0.63 |
| PFNA | 0.2 (−2.4, 2.8) | 2.0 (−2.3, 6.2) | −1.4 (−4.8, 1.9) | 0.23 |
| PFHxS | 0.5 (−1.1, 2.2) | 0.6 (−2.4, 3.6) | 0.4 (−1.3, 2.2) | 0.99 |
| EtFOSAA | 0.1 (−1.2, 1.4) | 0.0 (−1.9, 1.9) | 0.0 (−1.6, 1.6) | 0.68 |
| MeFOSAA | −0.1 (−2.1, 2.0) | 0.3 (−2.1, 2.8) | −0.1 (−3.2, 2.9) | 0.46 |
| LDL cholesterol (mg/dL) | ||||
| PFOS | 0.5 (−1.5, 2.6) | 0.6 (−2.2, 3.5) | −0.1 (−2.8, 2.5) | 0.38 |
| PFOA | 0.7 (−1.8, 3.1) | 2.2 (−1.5, 5.8) | −0.7 (−4.0, 2.6) | 0.18 |
| PFNA | 0.5 (−1.8, 2.8) | 2.3 (−1.3, 6.0) | −1.2 (−4.0, 1.7) | 0.12 |
| PFHxS | 0.5 (−0.9, 1.9) | 0.4 (−2.3, 3.2) | 0.5 (−0.9, 1.8) | 0.98 |
| EtFOSAA | 0.5 (−0.5, 1.6) | 0.2 (−1.2, 1.7) | 0.7 (−0.7, 2.1) | 0.86 |
| MeFOSAA | 0.1 (−1.8, 2.0) | 0.6 (−1.7, 2.9) | −0.3 (−3.0, 2.4) | 0.38 |
| HDL cholesterol (mg/dL) | ||||
| PFOS | 0.6 (−0.5, 1.7) | 0.6 (−0.8, 2.0) | 0.6 (−0.9, 2.1) | 0.93 |
| PFOA | −0.2 (−1.6, 1.2) | −1.2 (−3.1, 0.6) | 1.2 (−0.8, 3.2) | 0.03 |
| PFNA | 0.2 (−1.2, 1.6) | −0.1 (−2.0, 1.8) | 0.3 (−1.7, 2.3) | 0.41 |
| PFHxS | 0.1 (−0.5, 0.7) | 0.0 (−1.0, 1.0) | 0.2 (−0.5, 0.8) | 0.48 |
| EtFOSAA | −0.2 (−0.9, 0.5) | −0.2 (−1.0, 0.7) | −0.4 (−1.6, 0.9) | 0.89 |
| MeFOSAA | −0.2 (−1.0, 0.6) | −0.4 (−1.2, 0.5) | 0.4 (−1.2, 2.0) | 0.65 |
| Total/HDL-C × 100 | ||||
| PFOS | −3.4 (−9.1, 2.4) | −0.8 (−8.8, 7.3) | −7.7 (−16.0, 0.6) | 0.08 |
| PFOA | −1.0 (−8.7, 6.8) | 6.9 (−3.6, 17.5) | −10.4 (−21.1, 0.2) | 0.01 |
| PFNA | −1.8 (−8.9, 5.3) | 5.2 (−4.8, 15.3) | −7.8 (−17.4, 1.7) | 0.02 |
| PFHxS | −0.1 (−3.4, 3.1) | 1.3 (−5.2, 7.8) | −1.0 (−4.6, 2.7) | 0.37 |
| EtFOSAA | 0.1 (−3.1, 3.3) | 0.1 (−4.0, 4.2) | −0.0 (−4.9, 4.9) | 0.59 |
| MeFOSAA | −0.6 (−6.1, 5.0) | 1.6 (−4.2, 7.4) | −4.8 (−14.5, 4.9) | 0.22 |
| Triglycerides (mg/dL) | ||||
| PFOS | −1.4 (−4.6, 1.8) | 1.0 (−2.2, 4.2) | −4.2 (−9.2, 0.8) | 0.04 |
| PFOA | 0.2 (−3.3, 3.8) | 2.5 (−2.0, 6.9) | −2.4 (−8.1, 3.3) | 0.13 |
| PFNA | −2.5 (−5.8, 0.8) | −1.5 (−4.9, 1.9) | −2.9 (−8.1, 2.4) | 0.35 |
| PFHxS | −0.6 (−2.0, 0.8) | 0.6 (−1.9, 3.1) | −1.1 (−3.1, 1.0) | 0.22 |
| EtFOSAA | −1.0 (−2.4, 0.4) | −0.3 (−2.1, 1.5) | −1.6 (−3.3, 0.1) | 0.43 |
| MeFOSAA | −0.1 (−2.1, 2.0) | 0.5 (−1.2, 2.1) | −1.3 (−7.7, 5.0) | 0.55 |
| Liver enzymes | ||||
| ALT (U/L) | ||||
| PFOS | −0.4 (−1.1, 0.2) | −0.2 (−1.2, 0.8) | −0.7 (−1.4, 0.1) | 0.71 |
| PFOA | −0.5 (−1.3, 0.2) | −0.5 (−1.6, 0.6) | −0.7 (−1.7, 0.4) | 0.96 |
| PFNA | −0.1 (−0.9, 0.6) | 0.3 (−1.0, 1.6) | −0.6 (−1.4, 0.2) | 0.32 |
| PFHxS | −0.1 (−0.4, 0.2) | −0.0 (−0.6, 0.6) | −0.1 (−0.4, 0.2) | 0.75 |
| EtFOSAA | −0.1 (−0.5, 0.2) | 0.1 (−0.4, 0.6) | −0.4 (−0.8, 0.0) | 0.35 |
| MeFOSAA | −0.2 (−1.1, 0.7) | 0.2 (−0.7, 1.1) | −1.2 (−1.9, −0.5) | 0.05 |
Abbreviations: PFAS, per- and polyfluoroalkyl substances; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; ALT, alanine aminotransferase; PFOS, perfluorooctane sulfonate; IQR, interquartile range; PFOA, perfluorooctanoate; PFNA, perfluorononanoate; PFHxS, perfluorohexane sulfonate; EtFOSAA, 2-(N-ethyl-perfluorooctane sulfonamido) acetate; MeFOSAA, 2-(N-methyl-perfluorooctane sulfonamido) acetate.
Estimates are presented as change (95% confidence intervals) in outcome for each interquartile range increment in exposure. Models were adjusted for maternal education, prenatal smoking, gestational age at blood draw, and child’s sex, race/ethnicity, and age at lipids/ALT measurements.
Children with lipid and ALT measurements = 512 and 508, respectively.
Boys with lipid and ALT measurements = 272 and 273, respectively.
Girls with lipid and ALT measurements = 240 and 235, respectively.
3.3. Associations of child PFAS with lipids and ALT in mid-childhood
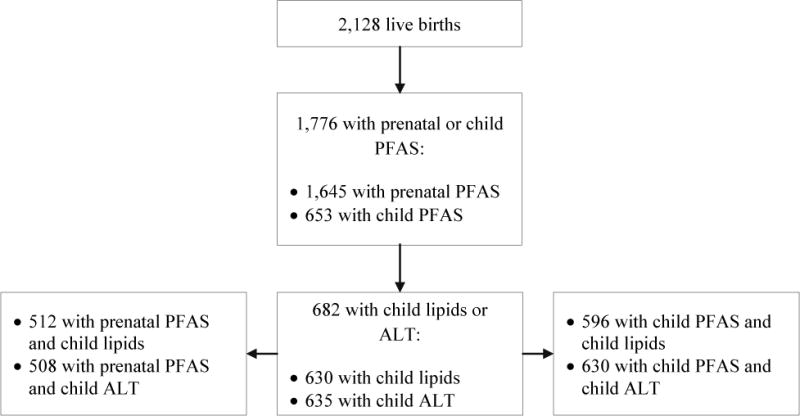
Mid-childhood PFAS plasma concentrations were associated with both detrimental (increased TC and LDL-C) and beneficial (increased HDL-C and decreased TG) changes in circulating lipid profile in combined (all children) and sex-stratified analyses (Table 4). Specifically, among girls, higher mid-childhood PFOS, PFOA, and PFDeA concentrations were associated with a higher TC and LDL-C [e.g., β per IQR increment in PFOS = 4.0 mg/dL (95% CI: 0.3, 7.8) for TC, pINT = 0.19; and 2.6 mg/dL (95% CI: −0.5, 5.8) for LDL-C, pINT = 0.20; Table 4]. While not strictly monotonic, we found overall higher TC and LDL-C across higher quartiles of PFOS, PFOA, and PFDeA among girls (see Figure A.2).
Table 4.
Adjusted linear regression coefficients for associations of mid-childhood PFAS concentrations with lipid and ALT levels in mid-childhood among all children and stratified by child sex.
| All childrena
|
Boysb
|
Girlsc
|
||
|---|---|---|---|---|
| β (95% CI) | β (95% CI) | β (95% CI) | pint | |
| Lipids | ||||
| Total cholesterol (mg/dL) | ||||
| PFOS | 1.8 (−0.2, 3.7) | 0.5 (−1.8, 2.9) | 4.0 (0.3, 7.8) | 0.19 |
| PFOA | 2.6 (−0.5, 5.7) | 1.2 (−3.0, 5.4) | 5.2 (0.4, 9.9) | 0.26 |
| PFNA | 0.6 (−0.7, 1.9) | 0.4 (−1.2, 1.9) | 1.0 (−1.4, 3.4) | 0.72 |
| PFHxS | −0.3 (−1.0, 0.5) | −0.5 (−1.5, 0.4) | 0.2 (−1.0, 1.3) | 0.38 |
| PFDeA | 6.8 (3.6, 10.1) | 5.3 (0.8, 9.8) | 9.2 (4.3, 14.1) | 0.25 |
| LDL cholesterol (mg/dL) | ||||
| PFOS | 0.8 (−0.9, 2.5) | −0.2 (−2.2, 1.9) | 2.6 (−0.5, 5.8) | 0.20 |
| PFOA | 0.9 (−1.8, 3.6) | −0.3 (−3.9, 3.4) | 3.0 (−1.0, 7.0) | 0.27 |
| PFNA | 0.2 (−0.8, 1.3) | 0.1 (−1.0, 1.2) | 0.4 (−1.7, 2.5) | 0.86 |
| PFHxS | −0.2 (−0.9, 0.4) | −0.5 (−1.4, 0.3) | 0.3 (−0.6, 1.3) | 0.17 |
| PFDeA | 3.2 (0.6, 5.8) | 1.9 (−1.7, 5.4) | 5.3 (1.2, 9.4) | 0.21 |
| HDL cholesterol (mg/dL) | ||||
| PFOS | 1.5 (0.4, 2.5) | 1.2 (0.0, 2.3) | 2.0 (0.0, 4.0) | 0.37 |
| PFOA | 1.5 (0.1, 2.9) | 1.5 (−0.4, 3.3) | 1.8 (−0.4, 4.0) | 0.54 |
| PFNA | 0.2 (−0.3, 0.7) | 0.1 (−0.6, 0.7) | 0.5 (−0.4, 1.3) | 0.52 |
| PFHxS | 0.0 (−0.3, 0.4) | 0.1 (−0.3, 0.5) | −0.1 (−0.5, 0.4) | 0.83 |
| PFDeA | 4.3 (2.6, 6.0) | 3.7 (1.2, 6.2) | 5.2 (3.0, 7.3) | 0.34 |
| Total/HDL-C × 100 | ||||
| PFOS | −4.4 (−9.4, 0.6) | −5.0 (−10.3, 0.3) | −3.9 (−14.7, 6.8) | 0.87 |
| PFOA | −2.7 (−10.1, 4.7) | −5.4 (−14.1, 3.3) | 0.6 (−12.4, 13.5) | 0.80 |
| PFNA | 0.1 (−2.0, 2.3) | 0.7 (−2.1, 3.5) | −0.9 (−4.5, 2.7) | 0.51 |
| PFHxS | −0.6 (−2.2, 1.0) | −1.1 (−3.1, 0.9) | 0.2 (−2.5, 3.0) | 0.56 |
| PFDeA | −10.1 (−18.2, −2.1) | −8.0 (−18.0, 1.9) | −12.3 (−25.3, 0.7) | 0.53 |
| Triglycerides (mg/dL) | ||||
| PFOS | −2.5 (−4.3, −0.6) | −2.3 (−4.3, −0.3) | −3.1 (−7.0, 0.9) | 0.34 |
| PFOA | 1.0 (−2.4, 4.5) | −0.2 (−3.4, 3.0) | 2.0 (−4.5, 8.4) | 0.74 |
| PFNA | 0.9 (−0.2, 2.0) | 1.1 (−0.1, 2.3) | 0.8 (−1.7, 3.3) | 0.87 |
| PFHxS | −0.4 (−1.0, 0.3) | −0.3 (−1.2, 0.6) | −0.5 (−1.3, 0.3) | 0.42 |
| PFDeA | −3.6 (−8.2, 1.0) | −1.3 (−4.6, 2.0) | −6.3 (−15.5, 2.9) | 0.31 |
| Liver enzymes | ||||
| ALT (U/L) | ||||
| PFOS | −0.3 (−0.9, 0.2) | −0.3 (−1.2, 0.5) | −0.4 (−1.0, 0.3) | 0.74 |
| PFOA | −0.7 (−1.4, 0.0) | −0.6 (−1.6, 0.4) | −0.9 (−1.8, 0.0) | 0.42 |
| PFNA | −0.3 (−0.5, −0.1) | −0.4 (−0.6, −0.2) | −0.2 (−0.5, 0.1) | 0.39 |
| PFHxS | 0.0 (−0.2, 0.2) | −0.1 (−0.4, 0.2) | 0.1 (−0.1, 0.4) | 0.30 |
| PFDeA | −0.3 (−1.2, 0.5) | 0.1 (−1.3, 1.4) | −0.9 (−1.8, −0.1) | 0.16 |
Abbreviations: PFAS, per- and polyfluoroalkyl substances; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; ALT, alanine aminotransferase; PFOS, perfluorooctane sulfonate; IQR, interquartile range; PFOA, perfluorooctanoate; PFNA, perfluorononanoate; PFHxS, perfluorohexane sulfonate; PFDeA, perfluorodecanoate.
Estimates are presented as change (95% confidence intervals) in outcome for each interquartile range increment in exposure. Models were adjusted for maternal education, prenatal smoking, and child’s sex, race/ethnicity, and age at lipids/ALT measurements.
Children with lipid and ALT measurements = 596 and 630, respectively.
Boys with lipid and ALT measurements = 314 and 332, respectively.
Girls with lipid and ALT measurements = 282 and 298, respectively.
Paradoxically, among boys and girls combined, higher mid-childhood PFOS, PFOA, and PFDeA plasma concentrations were associated with higher HDL-C and/or lower TG [e.g., β per IQR increment in PFOS = 1.5 mg/dL (95% CI: 0.4, 2.5) for HDL-C and −2.5 mg/dL (95% CI: −4.3, −0.6) for TG; Table 4]. Child PFDeA plasma concentrations were also associated with a lower TC/HDL-C ratio (× 100) [β per IQR increment = −10.1 (95% CI: −18.2, −2.1); Table 4], with consistently larger effect estimates in children in higher quartiles (Q3 and/or Q4) of PFDeA plasma concentrations (versus Q1; see Figure A.2).
We found that higher child PFOA and PFNA concentrations were associated with slightly lower ALT [adjusted β per IQR increment = −0.7 U/L (95% CI: −1.4, 0) for PFOA, and −0.3 U/L (95% CI: −0.5, −0.1) for PFNA] among boys and girls combined (pINT > 0.20; Table 4). In addition, higher mid-childhood PFDeA concentrations were associated with modestly lower ALT among girls [adjusted β per IQR increment = −0.9 U/L (95% CI: −1.8, −0.1), pINT = 0.16; Table 4]. PFHxS concentrations were not associated with consistent changes in lipids or ALT (Table 4).
3.4. Sensitivity analyses
Linear and branched isomers of PFOS and PFOA measured in mid-childhood plasma had similar patterns of association with lipids and ALT as total concentrations of PFOS and PFOA in analyses of boys and girls combined [e.g., β per IQR increment in n-PFOS = 1.4 (95% CI: 0.4, 2.5) for HDL-C, and −2.6 mg/dL (95% CI: −4.4, −0.8) for TG], with stronger associations among girls than among boys (see Table A.2). When we included all prenatal PFASs in the same prenatal models, we continued to observe mostly null PFAS-outcome associations (data not shown). However, when we included all mid-childhood PFASs in the same mid-childhood models, associations of PFDeA concentrations with lipids marginally strengthened while associations with PFOS and PFOA tended to weaken (see Table A.3; all variance inflation factors <10). We also observed an association between PFOA concentrations and TG [β per IQR increment = 11.1 mg/dL (95% CI: 2.1, 20.1)], after adjusting for all mid-childhood PFASs in the same model. The inclusion of mid-childhood BMI in the models did not change the main effect estimates (data not shown).
4. Discussion
In this prospective Boston-area pre-birth cohort, we observed that higher mid-childhood PFOA, PFOS, and PFDeA plasma concentrations were associated with detrimental changes in the lipid profile, including higher TC and LDL-C, and particularly among girls. We also found that higher prenatal and mid-childhood PFOS, PFOA, and/or PFDeA concentrations were associated with some beneficial changes in the lipid profile, including slightly higher HDL-C, lower TG, and/or lower TC/HDL-C ratio, again mainly among girls. Higher prenatal and mid-childhood PFAS concentrations were also associated with potentially better liver function in children, indicated by slight decreases in ALT.
Conflicting findings from these analyses as well as previous analyses of PFASs in Project Viva (Fleisch et al. 2017; Mora et al. 2017) make it challenging to draw any firm conclusions about the impact of prenatal and early life PFAS exposure on childhood metabolic function. However, there are a some notable parallels, including stronger associations of PFASs with higher adiposity (Mora et al. 2017) and poorer lipid profiles among girls. In addition, we found notable beneficial effects of PFASs with insulin resistance (Fleisch et al. 2017) and some lipids also among girls. Diverging directions of PFAS-related associations with metabolic function may be biologically plausible given that these chemicals have multiple mechanisms of action. For example, PFOS and PFOA have been shown to be associated with altered expression of lipid transport- and metabolism-related genes in humans (Fletcher et al. 2013), which may result in increased cholesterol. On the other hand, growing evidence suggests that PFASs activate the peroxisome proliferator-activated receptor (PPAR) alpha (α) (Wolf et al. 2008), and like fibric acid derivatives which also work through this mechanism, may raise HDL-C and lower TG and LDL-C (Derosa et al. 2017; Pawlak et al. 2015). Additionally, PPAR gamma (γ) activation may explain the inverse association of PFASs with liver enzymes (Pawlak et al. 2015; Ratziu et al. 2016) and insulin sensitivity (Janani et al. 2015). Further research on the biological mechanisms underlying the associations of PFAS exposure with lipids, liver enzymes, and other cardiometabolic outcomes would help to disentangle these complex relationships. In addition, it is important to note that it remains unclear whether animal-based evidence can be extrapolated to humans, as differences in toxicokinetics (Hundley et al. 2006) and PPAR-α ligand specificities (Oswal et al. 2013) between rodents and humans have been documented.
Regardless of the mechanistic underpinnings, most studies that have examined the associations of PFAS exposure and lipids (measured in fasting or non-fasting samples) in children and adolescents have reported adverse relationships (Frisbee et al. 2010; Geiger et al. 2014; Lin et al. 2009; Maisonet et al. 2015; Manzano-Salgado et al. 2017; Timmermann et al. 2014; Zeng et al. 2015). Higher child PFOS and PFOA serum concentrations were associated with higher TC and LDL-C at ages 12–15 years in Taiwan (Zeng et al. 2015) and ages 12–18 years across the United States (Geiger et al. 2014). Higher child PFNA and perfluorobutane sulfonate serum concentrations were also associated with higher TC and/or LDL-C in Taiwanese adolescents ages 12–15 years (Zeng et al. 2015). Less consistent associations have been reported for PFASs and TG (Frisbee et al. 2010; Geiger et al. 2014; Maisonet et al. 2015; Manzano-Salgado et al. 2017; Timmermann et al. 2014; Zeng et al. 2015), and null associations were observed for HDL in most studies (Frisbee et al. 2010; Geiger et al. 2014; Lin et al. 2009; Maisonet et al. 2015; Zeng et al. 2015). One of two studies that have examined the association of prenatal PFAS exposure with lipids to date found that higher prenatal PFHxS plasma concentrations were associated with higher TG measured at 4 years in Spanish boys and girls combined (Manzano-Salgado et al. 2017). This study also observed that higher prenatal PFOA plasma concentrations were associated with higher HDL-C in girls, but lower HDL-C in boys. In a British birth cohort study of girls, higher prenatal PFOS and PFOA serum concentrations were associated with higher TC and LDL-C (measured at 7–15 years) at lower PFAS concentrations, but modestly lower TC and LDL-C at higher PFAS concentrations (Maisonet et al. 2015). Similar nonlinear exposure-response relationships with TC and LDL-C were reported in a cross-sectional study of children and adolescents ages 1–18 years from a highly PFAS-exposed community in the Mid-Ohio Valley, United States (Frisbee et al. 2010).
Given that there is suggestive epidemiological evidence for nonlinear associations of PFASs with lipids (Frisbee et al. 2010; Maisonet et al. 2015; Steenland et al. 2010), we hypothesize that inconsistencies between these studies and ours could be due to (1) differences in prenatal and mid-childhood PFAS concentrations between study populations and across time periods and (2) how the directionality of the PFAS-outcome associations may vary depending on these PFAS concentrations. For example, prenatal PFOS and PFOA plasma concentrations in our study population, measured when concentrations peaked in the U.S. population (1999–2002) and before they declined due to industry’s voluntary phase out of these compounds (Sagiv et al. 2015), were higher than serum concentrations reported in the prospective study of British girls, which was conducted ~10 years earlier (1990–1992) (Maisonet et al. 2015). PFAS concentrations in the current study were also higher than prenatal plasma concentrations measured in the INMA birth cohort study (2003–2008) (Manzano-Salgado et al. 2017). In contrast, mid-childhood PFOS and PFOA plasma concentrations in Project Viva participants (2007–2010) were similar to serum concentrations reported in children (12–19 years old) from the 2007–2008 NHANES cycle (CDC 2015) and other U.S. children and adolescents (1999–2008) (Frisbee et al. 2010; Geiger et al. 2014), but lower than plasma concentrations observed in Danish children (1997) (Timmermann et al. 2014). Mid-childhood PFDeA plasma concentrations in our study population were lower than serum concentrations measured in Taiwanese adolescents (2009–2010) (Zeng et al. 2015).
Although no published study has examined the effects of prenatal or childhood PFAS exposure on liver enzymes in children, numerous studies in adults have investigated these associations with inconsistent results (Alexander and Olsen 2007; Costa et al. 2009; Darrow et al. 2016; Emmett et al. 2006; Gallo et al. 2012; Gleason et al. 2015; Lin et al. 2009; Sakr et al. 2007a; Sakr et al. 2007b). Higher PFAS concentrations have been associated with higher ALT, a marker of hepatocellular dysfunction commonly used to screen for pediatric nonalcoholic fatty liver disease (NAFLD) (Vos et al. 2017), in most cross-sectional studies (Alexander and Olsen 2007; Darrow et al. 2016; Gallo et al. 2012; Gleason et al. 2015; Lin et al. 2009; Sakr et al. 2007a), whereas prospective studies have reported mostly null associations (Costa et al. 2009; Sakr et al. 2007b). In our analyses, we found that higher prenatal and mid-childhood PFASs were associated with small, and likely clinically insignificant, decreases in ALT in children. Further studies in children would benefit from using more comprehensive assessments of liver function, including measurement of other liver enzymes (e.g., aspartate aminotransferase, albumin, alkaline phosphatase, and total bilirubin), and/or liver imaging technologies.
To our knowledge, no studies have observed sex differences in the associations between PFASs and liver enzymes (Darrow et al. 2016), but a few studies have found differences in PFAS-lipids associations between males and females from different age groups (Eriksen et al. 2013; Frisbee et al. 2010; Maisonet et al. 2015; Manzano-Salgado et al. 2017). In previous analyses of Project Viva, associations of early-life PFAS exposure with greater adiposity (Mora et al. 2017) and lower insulin resistance (Fleisch et al. 2017) were stronger among girls. It is possible that there is a sex-specific susceptibility to PFASs (Eriksen et al. 2013) or that sex differences occur in the expression of lipid transport- or metabolism-related genes (Fletcher et al. 2013). In addition, PFASs could interfere with androgen and/or estrogen activity by disturbing the expression of genes associated with steroid hormone metabolism (Du et al. 2013) and, consequently, affect plasma lipid homeostasis in different ways for males and females (Wang et al. 2011).
Our study has several limitations: (1) we cannot rule out the possibility that selection bias could have arisen due to loss to follow-up because we did not collect data on metabolic outcomes from children excluded from our analyses. (2) We conducted multiple comparisons and this could have led to statistically significant associations by chance. We were careful not to highlight isolated findings, but rather look for patterns in our results. (3) Residual confounding from factors associated with both PFAS exposure and lipids or ALT levels, such as diet and socioeconomic status, could have biased our findings either towards or away from the null. (4) PFASs were modestly to strongly correlated in our study population and including them all in the same model may have compromised the precision of our effect estimates and limited our ability to assess co-pollutant confounding. (5) We cannot dismiss the possibility that the cross-sectional associations of mid-childhood PFAS concentrations with lipids and ALT levels that we observed could be due to reverse causation (Dhingra et al. 2017). (6) While several studies have shown that PFAS concentrations in maternal plasma and serum during pregnancy correlate well with cord blood PFAS concentrations (Manzano-Salgado et al. 2015), suggesting that maternal blood can be used as proxy for fetal exposure to PFASs, the extent to which each PFAS analyte in maternal plasma is transferred to the fetus remains unclear (Pan et al. 2017). (7) Given the large number of prenatal PFDeA plasma concentrations below the LOD and relatively narrow range of mid-childhood PFDeA concentrations, our PFDeA findings should be interpreted cautiously. Additional research is needed to improve our understanding of the potential role of PFDeA on lipids, liver function tests, and other health outcomes.
Our analyses contribute to the existing literature by examining PFAS exposure both during pregnancy and in childhood, two developmental stages during which individuals are potentially more vulnerable to endocrine disruptors (Schug et al. 2011), such as PFASs. Additional strengths of this study include its large sample size and the availability of detailed data on potential confounding variables, mediators and effect modifiers, such as markers of pregnancy hemodynamics, child fast food intake and physical activity, and child BMI.
5. Conclusion
In this Boston-area pre-birth cohort, we found that plasma concentrations of select PFASs during pregnancy and mid-childhood were associated with modest detrimental and/or beneficial changes in circulating lipid profile and ALT levels in children, particularly among girls. Our results are biologically plausible, given the multiple mechanisms of action identified for these chemicals, and are, to some degree, consistent with the existing literature in adults. These findings provide additional evidence supporting a physiologic effect of PFASs and are important due to these toxicants’ persistence in the environment and in humans.
Highlights.
Measured PFASs in maternal plasma in pregnancy and child plasma in mid-childhood
Measured fasting lipids and ALT in mid-childhood
Child PFAS associated with higher TC and LDL-C among girls
Maternal and child PFAS associated with higher HDL-C and lower TG among girls
Maternal and child PFAS associated with decreased ALT levels
Acknowledgments
We thank the participants and staff of Project Viva; K. Kato, A. Patel, and T. Jia for their contribution with PFAS measurements; and S. de Ferranti for her insights on the potential mechanisms of PFAS effects on lipids. This work was supported by the National Institutes of Health (R01ES021447, K24HD069408, P30DK092924, R01HD034568, UG3OD023286, P01ES009605, R25DK096944, and K23ES024803), U.S. Environmental Protection Agency (R82670901 and RD83451301), and Academic Pediatric Association. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the NIH, EPA, or CDC. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the U.S. Department of Health and Human Services.
Appendices
Figure A.1.
Project Viva cohort sample size and participation. A total of 682 participants had data available for at least one exposure and one outcome examined in the present analyses.
Figure A.2.
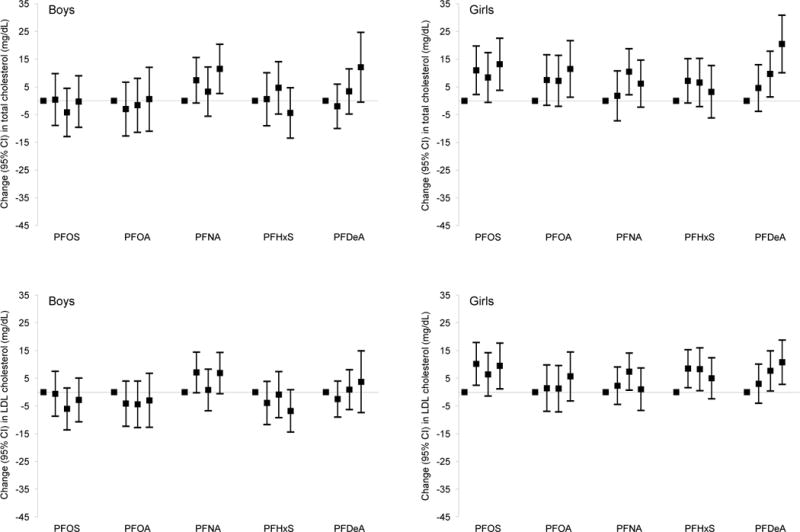
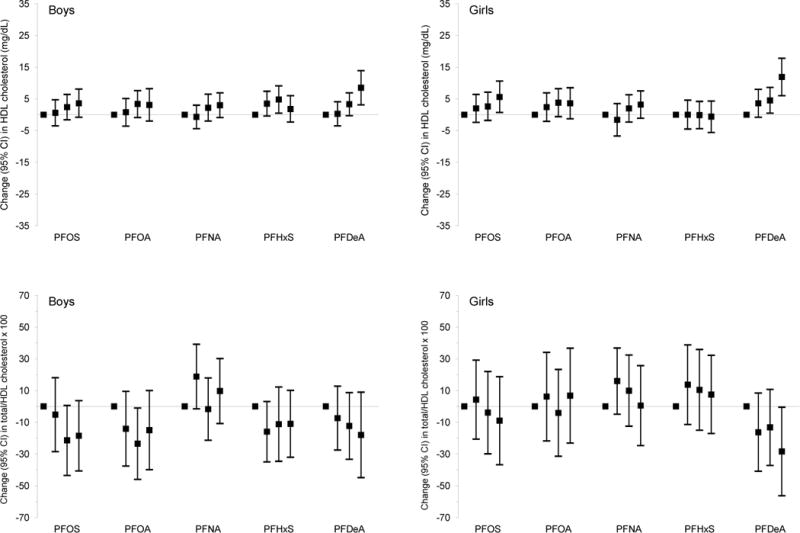
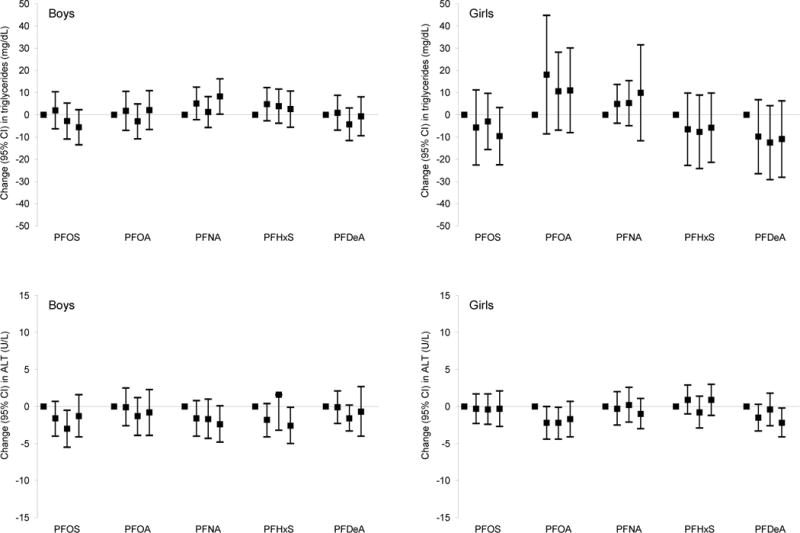
Adjusted linear regression coefficients for associations of mid-childhood PFAS quartiles with lipid and ALT levels in mid-childhood among boysa and girls.b
Abbreviations: PFAS, per- and polyfluoroalkyl substances; LDL, low-density lipoprotein; HDL, high-density lipoprotein; ALT, alanine aminotransferase; PFOS, perfluorooctane sulfonate; PFOA, perfluorooctanoate; PFNA, perfluorononanoate; PFHxS, perfluorohexane sulfonate; PFDeA, perfluorodecanoate.
Estimates are presented as change (95% confidence intervals) in outcome for each quartile increment in exposure (exposure quartiles 2–4 vs. quartile 1). Models were adjusted for maternal education, prenatal smoking, and child’s race/ethnicity, and age at lipids/ALT measurements.
aBoys with lipid measurements and PFOS concentrations: quartile 1 (Q1) = 81, quartile 2 (Q2) = 76, quartile 3 (Q3) = 81, and quartile 4 (Q4) = 76; with lipid measurements and PFOA concentrations: Q1 = 76, Q2 = 74, Q3 = 87, and Q4 = 77; with lipid measurements and PFNA concentrations: Q1 = 98, Q2 = 65, Q3 = 74, and Q4 = 77; with lipid measurements and PFHxS concentrations: Q1 = 91, Q2 = 70, Q3 = 67, and Q4 = 86; with lipid measurements and PFDeA concentrations: Q1 = 90, Q2 = 84, Q3 = 100, and Q4 = 40. Boys with ALT measurements and PFOS concentrations: Q1 = 87, Q2 = 76, Q3 = 85, and Q4 = 84; with ALT measurements and PFOA concentrations: Q1 = 80, Q2 = 81, Q3 = 89, and Q4 = 82; with ALT measurements and PFNA concentrations: Q1 = 102, Q2 = 73, Q3 = 81, and Q4 = 76; with ALT measurements and PFHxS concentrations: Q1 = 89, Q2 = 75, Q3 = 73, and Q4 = 95; with ALT measurements and PFDeA concentrations: Q1 = 93, Q2 = 89, Q3 = 105, and Q4 = 45.
bGirls with lipid measurements and PFOS concentrations: Q1 = 75, Q2 = 73, Q3 = 70, and Q4 = 64; with lipid measurements and PFOA concentrations: Q1 = 72, Q2 = 75, Q3 = 63, and Q4 = 72; with lipid measurements and PFNA concentrations: Q1 = 83, Q2 = 66, Q3 = 62, and Q4 = 71; with lipid measurements and PFHxS concentrations: Q1 = 84, Q2 = 67, Q3 = 75, and Q4 = 56; with lipid measurements and PFDeA concentrations: Q1 = 73, Q2 = 68, Q3 = 93, and Q4 = 48. Girls with ALT measurements and PFOS concentrations: Q1 = 77, Q2 = 74, Q3 = 74, and Q4 = 73; with ALT measurements and PFOA concentrations: Q1 = 74, Q2 = 78, Q3 = 70, and Q4 = 76; with ALT measurements and PFNA concentrations: Q1 = 86, Q2 = 74, Q3 = 65, and Q4 = 73; with ALT measurements and PFHxS concentrations: Q1 = 87, Q2 = 69, Q3 = 78, and Q4 = 64; with ALT measurements and PFDeA concentrations: Q1 = 74, Q2 = 72, Q3 = 101, and Q4 = 51.
Wald test p-values for most interaction terms between child sex and mid-childhood PFAS quartiles were not significant [PFOS: for total cholesterol (p = 0.20), for HDL cholesterol (p = 0.88), for total/HDL cholesterol × 100 (p = 0.87), triglycerides (p = 0.75), and ALT (p = 0.62)]; PFOA: for total cholesterol (p = 0.53), for LDL cholesterol (p = 0.67), for HDL cholesterol (p = 0.87), for total/HDL cholesterol × 100 (p = 0.92), triglycerides (p = 0.57), and ALT (p = 0.59); PFNA: for total cholesterol (p = 0.25), for HDL cholesterol (p = 0.99), for total/HDL cholesterol × 100 (p = 0.79), triglycerides (p = 0.94), and ALT (p = 0.72); PFHxS: for total cholesterol (p = 0.67), for HDL cholesterol (p = 0.56), for total/HDL cholesterol × 100 (p = 0.47), triglycerides (p = 0.63); PFDeA: for total cholesterol (p = 0.66), for LDL cholesterol (p = 0.61), for HDL cholesterol (p = 0.65), for total/HDL cholesterol × 100 (p = 0.89), triglycerides (p = 0.71), and ALT (p = 0.30)]. The only exceptions were some interaction terms between child sex and mid-childhood PFOS [for LDL cholesterol (p = 0.10)], PFNA [for LDL cholesterol (p = 0.16)], and PFHxS quartiles [for LDL cholesterol (p = 0.12) and ALT (p = 0.10)].
Table A.1.
Spearman correlation coefficients for prenatal and mid-childhood PFAS concentrations (ng/mL) in the study population (n = 518).
| Prenatal PFAS
|
Mid-childhood PFAS
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposures | PFOS | PFOA | PFNA | PFHxS | EtFOSAA | MeFOSAA | PFOS | n-PFOS | sm-PFOS | PFOA | n-PFOA | PFNA | PFHxS | PFDeA |
| Prenatal PFAS | ||||||||||||||
| PFOS | 1.00 | |||||||||||||
| PFOA | 0.72 | 1.00 | ||||||||||||
| PFNA | 0.67 | 0.56 | 1.00 | |||||||||||
| PFHxS | 0.54 | 0.55 | 0.44 | 1.00 | ||||||||||
| EtFOSAA | 0.52 | 0.38 | 0.20 | 0.21 | 1.00 | |||||||||
| MeFOSAA | 0.46 | 0.41 | 0.24 | 0.28 | 0.49 | 1.00 | ||||||||
| Mid-childhood PFAS | ||||||||||||||
| PFOS | 0.12 | 0.09 | 0.11 | 0.15 | 0.05 | 0.18 | 1.00 | |||||||
| n-PFOS | 0.13 | 0.10 | 0.12 | 0.14 | 0.05 | 0.18 | 1.00 | 1.00 | ||||||
| sm-PFOS | 0.11 | 0.09 | 0.10 | 0.16 | 0.06 | 0.16 | 0.97 | 0.94 | 1.00 | |||||
| PFOA | 0.10 | 0.15 | 0.08 | 0.18 | 0.06 | 0.18 | 0.79 | 0.77 | 0.81 | 1.00 | ||||
| n-PFOA | 0.11 | 0.15 | 0.08 | 0.18 | 0.05 | 0.18 | 0.79 | 0.77 | 0.81 | 1.00 | 1.00 | |||
| PFNA | 0.11 | 0.11 | 0.08 | 0.07 | −0.01 | 0.08 | 0.32 | 0.33 | 0.29 | 0.40 | 0.42 | 1.00 | ||
| PFHxS | 0.12 | 0.12 | 0.08 | 0.40 | 0.05 | 0.17 | 0.68 | 0.66 | 0.70 | 0.61 | 0.61 | 0.14 | 1.00 | |
| PFDeA | 0.13 | 0.10 | 0.11 | 0.08 | 0.06 | 0.11 | 0.60 | 0.61 | 0.55 | 0.70 | 0.72 | 0.54 | 0.37 | 1.00 |
Abbreviations: PFAS, per- and polyfluoroalkyl substances; PFOS, perfluorooctane sulfonate; PFOA, perfluorooctanoate; PFNA, perfluorononanoate; PFHxS, perfluorohexane sulfonate; PFDeA, perfluorodecanoate; EtFOSAA, 2-(N-ethyl-perfluorooctane sulfonamido) acetate; MeFOSAA, 2-(N-methyl-perfluorooctane sulfonamido) acetate.
Table A.2.
Adjusted linear regression coefficients for associations of mid-childhood linear and branched isomers of PFOS and PFOA concentrations with lipid and ALT levels in mid-childhood among all children and stratified by child sex.
| All childrena
|
Boysb
|
Girlsc
|
||
|---|---|---|---|---|
| β (95% CI) | β (95% CI) | β (95% CI) | pint | |
| Lipids | ||||
| Total cholesterol (mg/dL) | ||||
| n-PFOS | 1.8 (−0.2, 3.7) | 0.5 (−1.9, 2.9) | 4.2 (0.5, 7.9) | 0.15 |
| sm-PFOS | 1.8 (−0.4, 3.9) | 0.7 (−1.9, 3.4) | 3.6 (−0.3, 7.5) | 0.36 |
| n-PFOA | 2.9 (−0.2, 6.1) | 1.4 (−2.8, 5.6) | 5.6 (0.8, 10.3) | 0.24 |
| LDL cholesterol (mg/dL) | ||||
| n-PFOS | 0.8 (−0.8, 2.5) | −0.2 (−2.2, 1.8) | 2.8 (−0.3, 6.0) | 0.15 |
| sm-PFOS | 0.7 (−1.2, 2.6) | −0.1 (−2.5, 2.3) | 2.1 (−1.2, 5.4) | 0.43 |
| n-PFOA | 1.0 (−1.7, 3.7) | −0.2 (−3.9, 3.4) | 3.2 (−0.7, 7.1) | 0.25 |
| HDL cholesterol (mg/dL) | ||||
| n-PFOS | 1.4 (0.4, 2.5) | 1.2 (0.1, 2.3) | 2.0 (0.0, 4.0) | 0.38 |
| sm-PFOS | 1.6 (0.4, 2.7) | 1.3 (0.0, 2.6) | 2.1 (−0.1, 4.2) | 0.38 |
| n-PFOA | 1.8 (0.4, 3.3) | 1.7 (−0.2, 3.6) | 2.2 (−0.0, 4.5) | 0.47 |
| Total/HDL-C × 100 | ||||
| n-PFOS | −4.3 (−9.2, 0.7) | −5.0 (−10.2, 0.2) | −3.5 (−14.4, 7.5) | 0.96 |
| sm-PFOS | −4.9 (−10.5, 0.7) | −5.0 (−11.1, 1.0) | −5.4 (−16.6, 5.9) | 0.63 |
| n-PFOA | −3.6 (−10.9, 3.7) | −5.8 (−14.5, 2.9) | −1.1 (−13.7, 11.6) | 0.90 |
| Triglycerides (mg/dL) | ||||
| n-PFOS | −2.6 (−4.4, −0.8) | −2.3 (−4.2, −0.4) | −3.3 (−7.1, 0.5) | 0.33 |
| sm-PFOS | −2.2 (−4.6, 0.2) | −2.3 (−4.7, 0.1) | −2.7 (−7.5, 2.1) | 0.35 |
| n-PFOA | 0.4 (−2.6, 3.5) | −0.3 (−3.5, 2.9) | 0.8 (−5.0, 6.5) | 0.59 |
| Liver enzymes | ||||
| ALT (U/L) | ||||
| n-PFOS | −0.3 (−0.9, 0.3) | −0.3 (−1.2, 0.6) | −0.4 (−1.1, 0.3) | 0.68 |
| sm-PFOS | −0.4 (−0.9, 0.1) | −0.5 (−1.2, 0.3) | −0.4 (−1.0, 0.3) | 0.94 |
| n-PFOA | −0.7 (−1.4, 0.0) | −0.6 (−1.6, 0.4) | −0.9 (−1.8, −0.1) | 0.41 |
Abbreviations: PFOS, perfluorooctane sulfonate; PFOA, perfluorooctanoate; LDL-C, low-density lipoprotein; HDL-C, high-density lipoprotein; ALT, alanine aminotransferase; n-PFOS, n-perfluorooctane sulfonate; IQR, interquartile range; sm-PFOS, perfluoromethylheptane sulfonates; n-PFOA, n-perfluorooctanoate.
Estimates are presented as change (95% confidence intervals) in outcome for each interquartile range increment in exposure. Models were adjusted for maternal education, prenatal smoking, and child’s sex, race/ethnicity, and age at lipids/ALT measurements.
Children with lipid and ALT measurements = 596 and 630, respectively.
Boys with lipid and ALT measurements = 314 and 332, respectively.
Girls with lipid and ALT measurements = 282 and 298, respectively.
Table A.3.
Adjusted linear regression coefficients for associations of mid-childhood PFAS concentrations with lipid and ALT levels in mid-childhood among all children and stratified by child sex (models adjusted for all mid-childhood PFAS simultaneously).
| All childrena
|
Boysb
|
Girlsc
|
||
|---|---|---|---|---|
| β (95% CI) | β (95% CI) | β (95% CI) | pint | |
| Lipids | ||||
| Total cholesterol (mg/dL) | ||||
| PFOS | 0.9 (−1.9, 3.8) | 1.0 (−2.7, 4.8) | 0.0 (−5.1, 5.1) | 0.63 |
| PFOA | −3.5 (−8.3, 1.3) | −4.4 (−11.4, 2.5) | −1.2 (−8.4, 6.0) | 0.64 |
| PFNA | 0.1 (−1.3, 1.4) | 0.0 (−1.6, 1.6) | 0.0 (−2.5, 2.5) | 0.99 |
| PFHxS | −0.3 (−1.3, 0.6) | −0.6 (−1.7, 0.5) | 0.2 (−1.3, 1.7) | 0.38 |
| PFDeA | 8.7 (4.6, 12.9) | 8.3 (2.4, 14.3) | 10.0 (3.7, 16.2) | 0.60 |
| LDL cholesterol (mg/dL) | ||||
| PFOS | 0.9 (−1.4, 3.2) | 0.9 (−2.1, 3.8) | 0.3 (−4.0, 4.6) | 0.74 |
| PFOA | −2.5 (−6.8, 1.8) | −2.7 (−9.0, 3.6) | −1.2 (−7.5, 5.1) | 0.86 |
| PFNA | 0.0 (−1.1, 1.0) | −0.1 (−1.3, 1.1) | −0.2 (−2.5, 2.1) | 0.92 |
| PFHxS | −0.3 (−1.0, 0.5) | −0.6 (−1.5, 0.4) | 0.3 (−0.8, 1.5) | 0.19 |
| PFDeA | 4.5 (1.0, 8.0) | 3.7 (−1.1, 8.6) | 6.0 (0.4, 11.7) | 0.46 |
| HDL cholesterol (mg/dL) | ||||
| PFOS | 1.2 (−0.2, 2.7) | 1.2 (−0.5, 2.9) | 1.1 (−1.9, 4.1) | 0.86 |
| PFOA | −3.2 (−5.4, −1.1) | −2.8 (−5.6, 0.0) | −3.2 (−6.7, 0.3) | 0.90 |
| PFNA | −0.2 (−0.6, 0.3) | −0.2 (−0.9, 0.4) | −0.1 (−0.8, 0.7) | 0.80 |
| PFHxS | 0.0 (−0.4, 0.4) | −0.1 (−0.6, 0.5) | 0.0 (−0.6, 0.6) | 0.86 |
| PFDeA | 5.9 (3.7, 8.1) | 5.3 (2.1, 8.5) | 6.5 (3.4, 9.7) | 0.63 |
| Total/HDL-C × 100 | ||||
| PFOS | −5.3 (−11.9, 1.4) | −4.9 (−12.6, 2.7) | −6.9 (−22.1, 8.3) | 0.82 |
| PFOA | 11.8 (−0.2, 23.8) | 4.3 (−9.9, 18.6) | 19.0 (−1.1, 39.2) | 0.41 |
| PFNA | 1.1 (−1.4, 3.5) | 1.6 (−1.7, 4.9) | 0.1 (−4.1, 4.3) | 0.66 |
| PFHxS | −0.4 (−2.2, 1.3) | −0.3 (−2.7, 2.1) | −0.1 (−3.0, 2.8) | 0.91 |
| PFDeA | −15.7 (−26.6, −4.8) | −9.1 (−22.5, 4.2) | −20.0 (−37.7, −2.3) | 0.42 |
| Triglycerides (mg/dL) | ||||
| PFOS | −6.1 (−9.0, −3.2) | −5.3 (−8.7, −1.9) | −7.1 (−13.0, −1.1) | 0.62 |
| PFOA | 11.1 (2.1, 20.1) | 5.4 (0.2, 10.7) | 15.9 (1.6, 30.2) | 0.22 |
| PFNA | 1.4 (0.1, 2.6) | 1.5 (0.2, 2.8) | 1.3 (−1.9, 4.5) | 0.99 |
| PFHxS | −0.1 (−0.8, 0.6) | 0.3 (−0.7, 1.3) | −0.7 (−1.9, 0.6) | 0.21 |
| PFDeA | −8.4 (−17.9, 1.0) | −3.2 (−7.7, 1.2) | −12.9 (−31.7, 5.8) | 0.35 |
| Liver enzymes | ||||
| ALT (U/L) | ||||
| PFOS | 0.0 (−0.8, 0.9) | 0.3 (−1.0, 1.5) | 0.1 (−0.8, 1.0) | 0.93 |
| PFOA | −0.9 (−2.2, 0.5) | −1.1 (−3.0, 0.8) | −1.0 (−2.5, 0.6) | 0.87 |
| PFNA | −0.3 (−0.5, −0.1) | −0.4 (−0.6, −0.2) | −0.1 (−0.4, 0.3) | 0.12 |
| PFHxS | 0.1 (−0.2, 0.3) | −0.1 (−0.4, 0.2) | 0.2 (−0.1, 0.5) | 0.22 |
| PFDeA | 0.4 (−0.8, 1.6) | 1.0 (−0.9, 2.9) | −0.3 (−1.5, 0.8) | 0.26 |
Abbreviations: PFAS, per- and polyfluoroalkyl substances; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; ALT, alanine aminotransferase; PFOS, perfluorooctane sulfonate; IQR, interquartile range; PFOA, perfluorooctanoate; PFNA, perfluorononanoate; PFHxS, perfluorohexane sulfonate; PFDeA, perfluorodecanoate.
Estimates are presented as change (95% confidence intervals) in outcome for each interquartile range increment in exposure. Models were adjusted for maternal education, prenatal smoking, and child’s sex, race/ethnicity, age at lipids/ALT measurements, and all PFAS analytes.
Children with lipid and ALT measurements = 596 and 630, respectively.
Boys with lipid and ALT measurements = 314 and 332, respectively.
Girls with lipid and ALT measurements = 282 and 298, respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: ALT, alanine aminotransferase; BMI, body mass index; CDC, Centers for Disease Control and Prevention; CI, confidence interval; EtFOSAA, 2-(N-ethyl-perfluorooctane sulfonamido) acetate; GAM, generalized additive models; GFR, glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; LOD, limit of detection; MeFOSAA, 2-(N-methyl-perfluorooctane sulfonamido) acetate; n-PFOA, n-perfluorooctanoate; n-PFOS, n-perfluorooctane sulfonate; NAFLD, nonalcoholic fatty liver disease; PFAS, per- and polyfluoroalkyl substances; PFDeA, perfluorodecanoate; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoate; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate; PFOSA, perfluorooctane sulfonamide; PPAR, peroxisome proliferator-activated receptor; TC, total cholesterol; TG, triglycerides; Sb-PFOA, branched perfluorooctanoates; Sm-PFOS, perfluoromethylheptane sulfonates; Sm2-PFOS, perfluorodimethylhexane sulfonates.
Competing financial interests
None of the other authors declares any actual or potential competing financial interests.
References
- Alexander BH, Olsen GW. Bladder cancer in perfluorooctanesulfonyl fluoride manufacturing workers. Ann Epidemiol. 2007;17:471–478. doi: 10.1016/j.annepidem.2007.01.036. https://doi.org/10.1016/j.annepidem.2007.01.036. [DOI] [PubMed] [Google Scholar]
- Bao WW, Qian ZM, Geiger SD, Liu E, Liu Y, Wang SQ, et al. Gender-specific associations between serum isomers of perfluoroalkyl substances and blood pressure among Chinese: Isomers of C8 Health Project in China. Sci Total Environ. 2017:607–608. 1304–1312. doi: 10.1016/j.scitotenv.2017.07.124. https://doi.org/10.1016/j.scitotenv.2017.07.124. [DOI] [PMC free article] [PubMed]
- Braun JM. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat Rev Endocrinol. 2017;13:161–173. doi: 10.1038/nrendo.2016.186. https://doi.org/10.1038/nrendo.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Fourth National Report on Human Exposure to Environmental Chemicals. Atlanta, GA: Centers for Disease Control and Prevention; 2015. Available https://www.cdc.gov/biomonitoring/pdf/fourthreport_updatedtables_feb2015.pdf [accessed 27 February 2017] [Google Scholar]
- Chateau-Degat ML, Pereg D, Dallaire R, Ayotte P, Dery S, Dewailly E. Effects of perfluorooctanesulfonate exposure on plasma lipid levels in the Inuit population of Nunavik (Northern Quebec) Environ Res. 2010;110:710–717. doi: 10.1016/j.envres.2010.07.003. https://doi.org/10.1016/j.envres.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- Costa G, Sartori S, Consonni D. Thirty years of medical surveillance in perfluooctanoic acid production workers. J Occup Environ Med. 2009;51:364–372. doi: 10.1097/JOM.0b013e3181965d80. https://doi.org/10.1097/JOM.0b013e3181965d80. [DOI] [PubMed] [Google Scholar]
- D’Eon JC, Simpson AJ, Kumar R, Baer AJ, Mabury SA. Determining the molecular interactions of perfluorinated carboxylic acids with human sera and isolated human serum albumin using nuclear magnetic resonance spectroscopy. Environ Toxicol Chem. 2010;29:1678–1688. doi: 10.1002/etc.204. https://doi.org/10.1002/etc.204. [DOI] [PubMed] [Google Scholar]
- Dai S, Fulton JE, Harrist RB, Grunbaum JA, Steffen LM, Labarthe DR. Blood lipids in children: age-related patterns and association with body-fat indices: Project HeartBeat! Am J Prev Med. 2009;37:S56–64. doi: 10.1016/j.amepre.2009.04.012. https://doi.org/10.1016/j.amepre.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Darrow LA, Groth AC, Winquist A, Shin HM, Bartell SM, Steenland K. Modeled perfluorooctanoic acid (PFOA) exposure and liver function in a Mid-Ohio Valley community. Environ Health Perspect. 2016;124:1227–1233. doi: 10.1289/ehp.1510391. https://doi.org/10.1289/ehp.1510391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derosa G, Sahebkar A, Maffioli P. The role of various peroxisome proliferator-activated receptors and their ligands in clinical practice. J Cell Physiol. 2017 doi: 10.1002/jcp.25804. [Online 27 March 2017] https://doi.org/10.1002/jcp.25804. [DOI] [PubMed]
- Dhingra R, Winquist A, Darrow LA, Klein M, Steenland K. A study of reverse causation: examining the associations of perfluorooctanoic acid serum levels with two outcomes. Environ Health Perspect. 2017;125:416–421. doi: 10.1289/EHP273. https://doi.org/10.1289/EHP273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du G, Hu J, Huang H, Qin Y, Han X, Wu D, et al. Perfluorooctane sulfonate (PFOS) affects hormone receptor activity, steroidogenesis, and expression of endocrine-related genes in vitro and in vivo. Environ Toxicol Chem. 2013;32:353–360. doi: 10.1002/etc.2034. https://doi.org/10.1002/etc.2034. [DOI] [PubMed] [Google Scholar]
- Emmett EA, Shofer FS, Zhang H, Freeman D, Desai C, Shaw LM. Community exposure to perfluorooctanoate: relationships between serum concentrations and exposure sources. J Occup Environ Med. 2006;48:759–770. doi: 10.1097/01.jom.0000232486.07658.74. https://doi.org/10.1097/01.jom.0000232486.07658.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen KT, Raaschou-Nielsen O, McLaughlin JK, Lipworth L, Tjonneland A, Overvad K, et al. Association between plasma PFOA and PFOS levels and total cholesterol in a middle-aged Danish population. PLoS One. 2013;8:e56969. doi: 10.1371/journal.pone.0056969. https://doi.org/10.1371/journal.pone.0056969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz-Simon N, Fletcher T, Luster MI, Steenland K, Calafat AM, Kato K, et al. Reductions in serum lipids with a 4-year decline in serum perfluorooctanoic acid and perfluorooctanesulfonic acid. Epidemiology. 2013;24:569–576. doi: 10.1097/EDE.0b013e31829443ee. https://doi.org/10.1097/EDE.0b013e31829443ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisch AF, Rifas-Shiman SL, Mora AM, Calafat AM, Ye X, Luttmann-Gibson H, et al. Early-life exposure to perfluoroalkyl substances and childhood metabolic function. Environ Health Perspect. 2017;125:481–487. doi: 10.1289/EHP303. https://doi.org/10.1289/EHP303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher T, Galloway TS, Melzer D, Holcroft P, Cipelli R, Pilling LC, et al. Associations between PFOA, PFOS and changes in the expression of genes involved in cholesterol metabolism in humans. Environ Int. 2013:57–58. 2–10. doi: 10.1016/j.envint.2013.03.008. https://doi.org/10.1016/j.envint.2013.03.008. [DOI] [PubMed]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Frisbee SJ, Shankar A, Knox SS, Steenland K, Savitz DA, Fletcher T, et al. Perfluorooctanoic acid, perfluorooctanesulfonate, and serum lipids in children and adolescents: results from the C8 Health Project. Arch Pediatr Adolesc Med. 2010;164:860–869. doi: 10.1001/archpediatrics.2010.163. https://doi.org/10.1001/archpediatrics.2010.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, Leonardi G, Genser B, Lopez-Espinosa MJ, Frisbee SJ, Karlsson L, et al. Serum perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS) concentrations and liver function biomarkers in a population with elevated PFOA exposure. Environ Health Perspect. 2012;120:655–660. doi: 10.1289/ehp.1104436. https://doi.org/10.1289/ehp.1104436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger SD, Xiao J, Ducatman A, Frisbee S, Innes K, Shankar A. The association between PFOA, PFOS and serum lipid levels in adolescents. Chemosphere. 2014;98:78–83. doi: 10.1016/j.chemosphere.2013.10.005. https://doi.org/10.1016/j.chemosphere.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Gleason JA, Post GB, Fagliano JA. Associations of perfluorinated chemical serum concentrations and biomarkers of liver function and uric acid in the US population (NHANES), 2007–2010. Environ Res. 2015;136:8–14. doi: 10.1016/j.envres.2014.10.004. https://doi.org/10.1016/j.envres.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Harris MH, Rifas-Shiman SL, Calafat AM, Ye X, Mora AM, Webster TF, et al. Predictors of per- and polyfluoroalkyl substance (PFAS) plasma concentrations in 6–10 year old American children. Environ Sci Technol. 2017 doi: 10.1021/acs.est.6b05811. https://doi.org/10.1021/acs.est.6b05811. [DOI] [PMC free article] [PubMed]
- Hundley SG, Sarrif AM, Kennedy GL. Absorption, distribution, and excretion of ammonium perfluorooctanoate (APFO) after oral administration to various species. Drug Chem Toxicol. 2006;29:137–145. doi: 10.1080/01480540600561361. https://doi.org/10.1080/01480540600561361. [DOI] [PubMed] [Google Scholar]
- Janani C, Ranjitha Kumari BD. PPAR gamma gene–a review. Diabetes Metab Syndr. 2015;9:46–50. doi: 10.1016/j.dsx.2014.09.015. https://doi.org/10.1016/j.dsx.2014.09.015. [DOI] [PubMed] [Google Scholar]
- Kennedy GL, Jr, Butenhoff JL, Olsen GW, O’Connor JC, Seacat AM, Perkins RG, et al. The toxicology of perfluorooctanoate. Crit Rev Toxicol. 2004;34:351–384. doi: 10.1080/10408440490464705. [DOI] [PubMed] [Google Scholar]
- Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci. 2007;99:366–394. doi: 10.1093/toxsci/kfm128. https://doi.org/10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- Lin CY, Chen PC, Lin YC, Lin LY. Association among serum perfluoroalkyl chemicals, glucose homeostasis, and metabolic syndrome in adolescents and adults. Diabetes Care. 2009;32:702–707. doi: 10.2337/dc08-1816. https://doi.org/10.2337/dc08-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom AB, Strynar MJ, Libelo EL. Polyfluorinated compounds: past, present, and future. Environ Sci Technol. 2011;45:7954–7961. doi: 10.1021/es2011622. https://doi.org/10.1021/es2011622. [DOI] [PubMed] [Google Scholar]
- Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, et al. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect. 2004;112:1691–1696. doi: 10.1289/ehp.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonet M, Nayha S, Lawlor DA, Marcus M. Prenatal exposures to perfluoroalkyl acids and serum lipids at ages 7 and 15 in females. Environ Int. 2015;82:49–60. doi: 10.1016/j.envint.2015.05.001. https://doi.org/10.1016/j.envint.2015.05.001. [DOI] [PubMed] [Google Scholar]
- Manzano-Salgado CB, Casas M, Lopez-Espinosa MJ, Ballester F, Basterrechea M, Grimalt JO, et al. Transfer of perfluoroalkyl substances from mother to fetus in a Spanish birth cohort. Environ Res. 2015;142:471–478. doi: 10.1016/j.envres.2015.07.020. https://doi.org/10.1016/j.envres.2015.07.020. [DOI] [PubMed] [Google Scholar]
- Manzano-Salgado CB, Casas M, Lopez-Espinosa MJ, Ballester F, Iniguez C, Martinez D, et al. Prenatal exposure to perfluoroalkyl substances and cardiometabolic risk in children from the Spanish INMA Birth Cohort Study. Environ Health Perspect. 2017;125:097018. doi: 10.1289/EHP1330. https://doi.org/10.1289/EHP1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan J, Pinto X, Munoz A, Zuniga M, Rubies-Prat J, Pallardo LF, et al. Lipoprotein ratios: Physiological significance and clinical usefulness in cardiovascular prevention. Vasc Health Risk Manag. 2009;5:757–765. [PMC free article] [PubMed] [Google Scholar]
- Mora AM, Oken E, Rifas-Shiman SL, Webster TF, Gillman MW, Calafat AM, et al. Prenatal exposure to perfluoroalkyl substances and adiposity in early and mid-childhood. Environ Health Perspect. 2017;125:467–473. doi: 10.1289/EHP246. https://doi.org/10.1289/EHP246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JW, Hatch EE, Webster TF. Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general U.S. population. Environ Health Perspect. 2010;118:197–202. doi: 10.1289/ehp.0901165. https://doi.org/10.1289/ehp.0901165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2012;128:S213–S256. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken E, Baccarelli AA, Gold DR, Kleinman KP, Litonjua AA, De Meo D, et al. Cohort profile: Project Viva. Int J Epidemiol. 2015;44:37–48. doi: 10.1093/ije/dyu008. https://doi.org/10.1093/ije/dyu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswal DP, Balanarasimha M, Loyer JK, Bedi S, Soman FL, Rider SD, Jr, et al. Divergence between human and murine peroxisome proliferator-activated receptor alpha ligand specificities. J Lipid Res. 2013;54:2354–2365. doi: 10.1194/jlr.M035436. https://doi.org/10.1194/jlr.M035436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Zhu Y, Zheng T, Cui Q, Buka SL, Zhang B, et al. Novel chlorinated polyfluorinated ether sulfonates and legacy per-/polyfluoroalkyl substances: placental transfer and relationship with serum albumin and glomerular filtration rate. Environ Sci Technol. 2017;51:634–644. doi: 10.1021/acs.est.6b04590. https://doi.org/10.1021/acs.est.6b04590. [DOI] [PubMed] [Google Scholar]
- Pawlak M, Lefebvre P, Staels B. Molecular mechanism of PPARalpha action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol. 2015;62:720–733. doi: 10.1016/j.jhep.2014.10.039. https://doi.org/10.1016/j.jhep.2014.10.039. [DOI] [PubMed] [Google Scholar]
- Plourde G. Impact of obesity on glucose and lipid profiles in adolescents at different age groups in relation to adulthood. BMC Fam Pract. 2002;3:18. doi: 10.1186/1471-2296-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L, et al. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-α and -δ, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology. 2016;150:1147–1159.e1145. doi: 10.1053/j.gastro.2016.01.038. [DOI] [PubMed] [Google Scholar]
- Sagiv SK, Rifas-Shiman SL, Webster TF, Mora AM, Harris MH, Calafat AM, et al. Sociodemographic and perinatal predictors of early pregnancy per- and polyfluoroalkyl substance (PFAS) concentrations. Environ Sci Technol. 2015;49:11849–11858. doi: 10.1021/acs.est.5b02489. https://doi.org/10.1021/acs.est.5b02489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakr CJ, Kreckmann KH, Green JW, Gillies PJ, Reynolds JL, Leonard RC. Cross-sectional study of lipids and liver enzymes related to a serum biomarker of exposure (ammonium perfluorooctanoate or APFO) as part of a general health survey in a cohort of occupationally exposed workers. J Occup Environ Med. 2007a;49:1086–1096. doi: 10.1097/JOM.0b013e318156eca3. https://doi.org/10.1097/JOM.0b013e318156eca3. [DOI] [PubMed] [Google Scholar]
- Sakr CJ, Leonard RC, Kreckmann KH, Slade MD, Cullen MR. Longitudinal study of serum lipids and liver enzymes in workers with occupational exposure to ammonium perfluorooctanoate. J Occup Environ Med. 2007b;49:872–879. doi: 10.1097/JOM.0b013e318124a93f. https://doi.org/10.1097/JOM.0b013e318124a93f. [DOI] [PubMed] [Google Scholar]
- Savitz DA. Invited commentary: interpreting associations between exposure biomarkers and pregnancy outcome. Am J Epidemiol. 2014;179:545–547. doi: 10.1093/aje/kwt314. https://doi.org/10.1093/aje/kwt314. [DOI] [PubMed] [Google Scholar]
- Schug TT, Janesick A, Blumberg B, Heindel JJ. Endocrine disrupting chemicals and disease susceptibility. J Steroid Biochem Mol Biol. 2011;127:204–215. doi: 10.1016/j.jsbmb.2011.08.007. https://doi.org/10.1016/j.jsbmb.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starling AP, Engel SM, Whitworth KW, Richardson DB, Stuebe AM, Daniels JL, et al. Perfluoroalkyl substances and lipid concentrations in plasma during pregnancy among women in the Norwegian Mother and Child Cohort Study. Environ Int. 2014;62:104–112. doi: 10.1016/j.envint.2013.10.004. https://doi.org/10.1016/j.envint.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K, Tinker S, Frisbee S, Ducatman A, Vaccarino V. Association of perfluorooctanoic acid and perfluorooctane sulfonate with serum lipids among adults living near a chemical plant. Am J Epidemiol. 2009;170:1268–1278. doi: 10.1093/aje/kwp279. https://doi.org/10.1093/aje/kwp279. [DOI] [PubMed] [Google Scholar]
- Steenland K, Fletcher T, Savitz DA. Epidemiologic evidence on the health effects of perfluorooctanoic acid (PFOA) Environ Health Perspect. 2010;118:1100–1108. doi: 10.1289/ehp.0901827. https://doi.org/10.1289/ehp.0901827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermann CA, Rossing LI, Grontved A, Ried-Larsen M, Dalgard C, Andersen LB, et al. Adiposity and glycemic control in children exposed to perfluorinated compounds. J Clin Endocrinol Metab. 2014;99:E608–614. doi: 10.1210/jc.2013-3460. https://doi.org/10.1210/jc.2013-3460. [DOI] [PubMed] [Google Scholar]
- Vanden Heuvel JP, Thompson JT, Frame SR, Gillies PJ. Differential activation of nuclear receptors by perfluorinated fatty acid analogs and natural fatty acids: a comparison of human, mouse, and rat peroxisome proliferator-activated receptor-alpha, -beta, and -gamma, liver X receptor-beta, and retinoid X receptor-alpha. Toxicol Sci. 2006;92:476–489. doi: 10.1093/toxsci/kfl014. https://doi.org/10.1093/toxsci/kfl014. [DOI] [PubMed] [Google Scholar]
- Verner MA, Loccisano AE, Morken NH, Yoon M, Wu H, McDougall R, et al. Associations of perfluoroalkyl substances (PFAS) with lower birth weight: an evaluation of potential confounding by glomerular filtration rate using a physiologically based pharmacokinetic model (PBPK) Environ Health Perspect. 2015;123:1317. doi: 10.1289/ehp.1408837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos MB, Abrams SH, Barlow SE, Caprio S, Daniels SR, Kohli R, et al. NASPGHAN Clinical practice guideline for the diagnosis and treatment of nonalcoholic fatty liver disease in children: recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) J Pediatr Gastroenterol Nutr. 2017;64:319–334. doi: 10.1097/MPG.0000000000001482. https://doi.org/10.1097/MPG.0000000000001482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Magkos F, Mittendorfer B. Sex differences in lipid and lipoprotein metabolism: it’s not just about sex hormones. J Clin Endocrinol Metab. 2011;96:885–893. doi: 10.1210/jc.2010-2061. https://doi.org/10.1210/jc.2010-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SS, Fenton SE, Hines EP. Endocrine disrupting properties of perfluorooctanoic acid. J Steroid Biochem Mol Biol. 2011;127:16–26. doi: 10.1016/j.jsbmb.2011.03.011. https://doi.org/10.1016/j.jsbmb.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf CJ, Takacs ML, Schmid JE, Lau C, Abbott BD. Activation of mouse and human peroxisome proliferator-activated receptor alpha by perfluoroalkyl acids of different functional groups and chain lengths. Toxicol Sci. 2008;106:162–171. doi: 10.1093/toxsci/kfn166. https://doi.org/10.1093/toxsci/kfn166. [DOI] [PubMed] [Google Scholar]
- Yu N, Wang X, Zhang B, Yang J, Li M, Li J, et al. Distribution of perfluorooctane sulfonate isomers and predicted risk of thyroid hormonal perturbation in drinking water. Water Res. 2015;76:171–180. doi: 10.1016/j.watres.2015.02.047. https://doi.org/10.1016/j.watres.2015.02.047. [DOI] [PubMed] [Google Scholar]
- Zeng XW, Qian Z, Emo B, Vaughn M, Bao J, Qin XD, et al. Association of polyfluoroalkyl chemical exposure with serum lipids in children. Sci Total Environ. 2015:512–513. 364–370. doi: 10.1016/j.scitotenv.2015.01.042. https://doi.org/10.1016/j.scitotenv.2015.01.042. [DOI] [PubMed]


