Abstract
Advanced pancreatic ductal adenocarcinoma (PDAC) has typically been resistant to chemotherapy and immunotherapy; therefore, novel strategies are needed to enhance therapeutic response. Cholecystokinin (CCK) has been shown to stimulate growth of pancreatic cancer. CCK receptors (CCKRs) are present on pancreatic cancer cells, fibroblasts, and lymphocytes. We hypothesized that CCKR blockade would improve response to immune checkpoint antibodies by promoting influx of tumor-infiltrating lymphocytes (TILs) and reducing fibrosis. We examined the effects of CCKR antagonists or immune checkpoint blockade antibodies alone or in combination in murine models of PDAC. Monotherapy with CCKR blockade significantly decreased tumor size and metastases in SCID mice with orthotopic PDAC, and in C57BL/6 mice, it reduced fibrosis and induced the influx of TILs. Immune-competent mice bearing syngeneic pancreatic cancer (Panc02 and mT3-2D) that were treated with the combination of CCK receptor antagonists and immune checkpoint blockade antibodies survived significantly longer with smaller tumors. Tumor immunohistochemical staining and flow cytometry demonstrated that the tumors of mice treated with the combination regimen had a significant reduction in Foxp3+ T-regulatory cells and an increase in CD4+ and CD8+ lymphocytes. Masson’s trichrome stain analysis revealed 50% less fibrosis in the tumors of mice treated with CCKR antagonist compared to controls and compared to checkpoint antibody therapy. CCKR antagonists given with immune checkpoint antibody therapy represent a novel approach for improving survival of PDAC. The mechanism by which this combination therapy improves the survival of PDAC may be related to the decreased fibrosis and immune cells of the tumor microenvironment.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-017-2077-9) contains supplementary material, which is available to authorized users.
Keywords: Cholecystokinin, Tumor microenvironment, Fibrosis, Tumor-infiltrating lymphocytes
Introduction
The gastrointestinal peptides, cholecystokinin (CCK) [1, 2], and gastrin [3], have been shown to stimulate growth of pancreatic cancer. Researchers have used a decapeptide analog of CCK, cerulein, to accelerate pancreatic carcinogenesis in animal models, such as the nitrosamine model [4] or in the KRAS transgenic mouse model [5]. Gastrin is found in the fetal pancreas [6, 7], but levels rapidly decrease to zero after birth in the pancreas, and gastrin expression is then only detected in the stomach [8]. However, gastrin is re-expressed during pancreatic carcinogenesis in early pancreatic intraepithelial neoplasia (PanIN) lesions [9] and is overexpressed in pancreatic cancer where it regulates the growth of pancreatic cancer by an autocrine mechanism [10, 11]. Both gastrin and CCK stimulate the growth of pancreatic cancer through G-protein-coupled CCK receptors [12, 13], and these receptors are markedly overexpressed in cancer. When gastrin is silenced by RNA interference [14], pancreatic cancer growth is significantly decreased. In addition, if the cholecystokinin receptor (CCKR) is downregulated [15], or the CCKRs are blocked with antagonists [13, 16], pancreatic cancer growth is inhibited.
Three types of CCKRs have been characterized and sequenced. The CCK-A receptor is the predominant type found in normal rodent pancreas [17], whereas the CCK-B receptor is the form found in the normal human pancreas [18, 19]. Of interest, when the rodent pancreas undergoes malignant transformation as a result of azaserine treatment [20] or under the influence of mutant KRAS [21], the rodent pancreas expresses the CCK-B receptor type. The third variety of CCK receptor, the CCK-C receptor, is a splice variant of the CCK-B receptor that occurs only in human PDAC patients with a germline single nucleotide polymorphism (rs1800843) [22, 23]. Although human pancreatic cancer may have both CCK-A and CCK-B receptor subtypes [19, 24], PDAC growth is mediated through the CCK-B receptor type and its splice variant [16]. A clinical trial using CCKR blockade was conducted many years ago to treat human subjects with advanced PDAC, and unfortunately, this trial failed because a selective CCK-A receptor antagonist was used rather than a CCK-B antagonist [25]. A number of receptor-specific CCKR antagonists have been developed that have high affinity to either the CCK-A or CCK-B receptor and others are under investigation [26]. L364,718 (devazepide) is a highly potent and selective antagonist to the CCK-A receptor [27], the predominant receptor variety in mouse pancreas and in mouse Panc02 pancreas cancer [28]. Proglumide [29] is a less potent nonselective antagonist and inhibits the actions of peptides at both the CCK-A receptor and the CCK-B receptor.
CCKRs have also been identified on rodent tissue fibroblasts [30], rodent pancreatic stellate [31] cells, and also on human pancreatic stellate cells [32]; when these receptors are stimulated, the fibroblasts become activated to produce desmoplastic stroma characteristic of the microenvironment of pancreatic cancer [33, 34]. Evidence that CCKRs play a role in the dense fibrosis of the pancreas cancer microenvironment was demonstrated when CCKR blockade with proglumide inhibited fibrosis in the mutant KRAS murine model [21].
Apart from its normal physiologic role in digestion, proliferative effects on cancer epithelial cells, and collagen promoting effects from pancreatic stellate cells, CCK-8 also serves as an immunomodulatory peptide. Immune cells also have CCKRs [35] that respond to CCKR blockade to change their cytokine expression signature. CCK can influence the action of specific CD4+ T cell subsets by regulating antigen-presenting cell functions, and this effect is blocked with CCKR antagonists [35]. Zhang and colleagues [36] showed that CCK peptide administration suppressed Th1 phenotype while enhancing Th2 development and cytokine production; CCKR antagonists blocked this effect. Because CCKRs are found on three components of the pancreatic cancer microenvironment, (pancreatic epithelial cells, cancer-associated fibroblasts (CAFs), and immune cells), use of pharmaceutical agents that block the signaling at this receptor may be useful in improving therapy to pancreatic cancer.
We hypothesized that CCKR blockade would change the immune signature and decrease fibrosis of the tumor microenvironment to make pancreatic cancer more receptive to immune therapy with immune checkpoint blockade. In this investigation, we studied the outcome of CCKR blockade on primary tumor growth and metastases, fibrosis and tumor-infiltrating lymphocytes (TILs) of the microenvironment, and survival in murine models of PDAC alone and in combination with immune checkpoint antibodies.
Materials and methods
Cell lines
Panc02 cells, a murine pancreatic cancer cell line which is syngeneic to C57BL/6 mice, [37] were a gift from Professor Corbett (Wayne State University, MI, USA). Cells were cultured in DMEM: F12 media with 10% FBS. Histologically, Panc02 cancer cells resemble human pancreatic cancer, in that they are ductal epithelium; in vivo, the tumors are locally invasive and metastatic, and Panc02 tumors are associated with a dense fibrotic microenvironment. Panc02 cells express the CCK-A receptor [28]. This cell line has been used for decades when studying PDAC in an immune-competent model.
The second cell line used in this investigation mT3-2D (mT3) was obtained from the laboratory of Dr. David Tuveson (Cold Spring Harbor) [38]. This murine pancreatic cancer cell line was developed from organoids isolated from mutant KrasLSL-G12D; Pdx1-Cre mouse PDAC lesions [38] and it has mutant KRAS, similar to human PDAC. The cell line is also syngeneic to C57BL/6 mice; and therefore, capable of being studied in immune-competent mice.
Characterization of CCK receptors on mT3 pancreatic cancer cells
To confirm that mT3 cancer cells express CCKRs, total RNA was extracted from mT3 cells with an RNeasy Plus Mini Kit (Qiagen, Germantown, MD) and 1 μg was subjected to RT-PCR in a SimpliAmp Thermal Cycler (Applied Biosystems, Carlsbad, CA) for the evaluation of CCK-A and CCK-B receptor expression status. Reverse transcription was performed under the following conditions: 95 °C × 30 s (denaturation), 60 °C × 1 min (annealing), and 72 °C for 30 s (elongation) ×35 cycles using murine CCK-A receptor primers: 5′CTTTTCTGCCTGGATCAACCT3′ (forward); 5′ACCGTGATAACCAGCGTGTTC3′ (Reverse). The murine primers for the CCK-B receptor were as follows: forward primer 5′GATGGCTGCTACGTGCAACT3′ and reverse primer 5′CGCACCACCCGCTTCTTAG3′. HPRT was used as a reference gene and primers were: 5′TCCTCCTCAGACCGCTTT3′ (forward), 5′TTTTCCAAATCCTCGGCATAATG3′ (reverse). PCR products were evaluated by gel electrophoresis in a 2% agarose gel. Confirmation of CCK-B receptor protein expression was confirmed with immunofluorescence with CCK-B receptor antibody (1:200; Abcam ab77077, Toronto, Canada) conjugated to Dylight 488 (ab201799). Nuclei were stained with Hoescht: NucBlue™ (Thermofisher).
Animal models
Three different murine models were used in this investigation: (1) severe combined immune-deficient (B6.CB17-Prkdc scid/SzJ; SCID) mice to study the role of CCKR blockade on orthotopic cancer growth and metastasis independent of immune cells, (2) immune-competent Pdx1-Cre/LSL-KrasG12D transgenic mice for a pancreatic carcinogenesis model to study the role of CCKR blockade on the pancreatic stellate cells and immune cells of the pancreas, and (3) immune-competent C57BL/6 mice to study the role of CCKR antagonist in combination with immune checkpoint antibodies on growth and survival of mice bearing syngeneic PDAC tumors. Institutional guidelines for care and use of laboratory animals were followed throughout the study in accordance with protocols approved by the Georgetown University Institutional Animal Use and Care Committee.
In the following series of experiments, Panc02 cells (1 × 106) were injected either orthotopically as previously described [14] or subcutaneously (1 × 106 and 2 × 106) in mice. mT3 cells (1 × 105) were grown subcutaneously in immune-competent syngeneic mice. Subcutaneous tumor volumes were measured with calipers weekly in animals bearing subcutaneous tumors using the formula Length × (width)2 × 0.5.
Treatments
Two different CCKR antagonists were analyzed in this investigation. L364,718 (Tocris Bioscience, Bristol, UK) is a selective CCK-A receptor antagonist [27] which is the primary type of the CCKR in Panc02 murine cancer cells. L364,718 was administered at a dose of 4 mg/kg three times a week by an intraperitoneal injection. The other CCKR antagonist used was proglumide (Tocris) which is an orally bioavailable nonselective antagonist that blocks both the CCK-A and CCK-B receptors but has greater affinity for the CCK-B receptor [39]. Proglumide is water soluble and was administered orally in the drinking water at a concentration of 0.1 mg/mL, or approximately 30 mg/kg/d per mouse, a dose we previously found was effective in blocking the CCKR in vivo [21].
Two immune checkpoint blockade antibodies were used in this investigation. The antibody to the programmed cell death protein 1 (PD-1-Ab) (Bio X cell, West Lebanon, NH) was administered intraperitoneally to mice at a dose of either 125 μg x3 injections or 200 μg every 3 days. The antibody to cytotoxic T–lymphocyte-associated antigen 4 (CTLA-4 Ab; Bio-X cell) was given at a dose of 200 µg every 3 days by intraperitoneal injections.
Study design
To demonstrate the importance of the endogenous immune system in regulating growth and metastases of pancreatic cancer, we compared tumor mass 4 weeks after orthotopic inoculation of 106 Panc02 cells in the pancreas of SCID (N = 20) versus C57BL/6 mice (N = 28). Ten mice in each group were treated with L364,718, (4 mg/kg) three times a week and the other mice were treated with PBS (controls). Four weeks after tumor inoculation, mice were euthanized, tumors removed and weighed, and the number of metastases was counted. TILs were evaluated in tumors of the C57BL/6 mice by immunohistochemistry and fibrosis assessed with Masson’s trichrome stain.
The second series of experiments was performed to analyze the role of CCKR antagonist monotherapy on the TILs of the pancreas microenvironment during pancreatic carcinogenesis using the Pdx1-Cre/LSL-KrasG12D transgenic mouse. After the mice reached 3–4 months of age, when PanINs and fibrosis are established, mice were treated with either proglumide (in the drinking water) or untreated water. After 4 months of therapy, the pancreata were excised and compared to the pancreas of age-matched litter mates of mice on untreated water. Tissues were stained for fibrosis and CD3+ lymphocytes.
Another series of experiments was performed in C57BL/6 mice to evaluate the role of CCKR blockade in combination with immune checkpoint antibody therapy. Each series of experiments was performed using 40 mice with syngeneic murine tumors. The first two experiments tested the effects of L364,718 and the PD-1 Ab in mice injected with Panc02 cells (1 × 106 or 2 × 106). The 3rd experimental design examined the role of the CTLA-4 Ab and proglumide in mice inoculated with 1 × 106 Panc02 cancer cells. In the 4th experiment, the mT3 cells (1 × 105) were injected and mice were treated with PBS, proglumide, the PD-1 antibody, or the combination of both compounds. Animal treatments were initiated 1 week after cancer cell inoculation to assure all animals in the study had a measurable tumor and not to interfere with tumor initiation. The primary end point was tumor volume (1000 mm3) or mouse survival. Tumors were excised and examined histologically, and by immunohistochemistry, or by flow cytometry for immune cells.
Histology and immunohistochemistry
Pancreatic tumors or whole pancreas (KRAS study) were excised after treatment and the tissues were paraffin-embedded, sectioned, and stained with hematoxylin and eosin, and Masson’s trichrome stain for fibrosis evaluation. Some tumors were stained with an antibody to α-SMA (Abcam; Ab124964 at 1:6000). Tumor sections were also stained with either CD3 antibodies (1:85; DAKO, Carpinteria, CA); CD8 antibodies (1:75; eBioscience, San Diego, CA); CD4 antibodies (1:225; eBioscience), or Foxp3 antibody (1:30; eBioscience) and immunoreactive cells counted manually. Fibrosis was determined by Masson’s trichrome staining, and a quantitative fibrosis analysis was done in a blinded fashion according to a protocol by Kennedy [40].
Flow cytometry
Tumors were harvested when they reached approximately 1000 mm3. They were processed and stained as previously described [41].
Statistics
The results of immune cell analysis were expressed as mean ± standard error of the mean (SEM). Comparisons were made by ANOVA and student’s t test. Bonferroni corrections were made for multiple comparisons. Survival was analyzed by Kaplan–Meier analysis and differences by hazard ratios using Prism Software (GraphPad software, Inc.). Where data was skewed, a nonparametric Kruskal–Wallis statistical method was performed.
Results
CCKR antagonist monotherapy blocks tumor growth and metastases
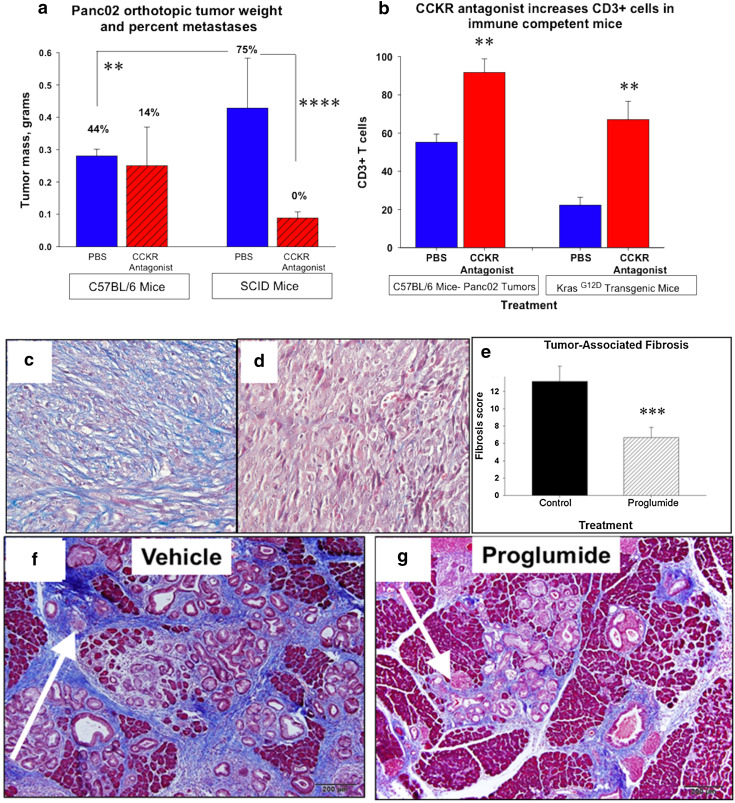
Orthotopic Panc02 tumor weights were two to threefold greater in SCID mice compared to C57BL/6 mice (Fig. 1a) suggesting that the endogenous immune system in the wild-type mouse is restraining tumor growth. The CCKR antagonist, L364,718, significantly reduced the primary tumor weight in SCID mice but not in immune-competent mice. Seventy-five percent of the SCID mice had metastases to liver, mesenteric lymph nodes, and/or lung. There were no metastases in the SCID mice that were treated with the CCKR antagonist. Although CCKR antagonist therapy did not alter the primary tumor size in immune-competent mice at the dose used, the CCKR antagonist decreased metastases. Since the CCKR antagonist monotherapy was effective in decreasing primary tumor growth in the immune-deficient mouse, this suggests that inhibition of tumor growth and metastases with CCKR antagonism is at least in part independent of the host immune system.
Fig. 1.
Monotherapy with CCKR antagonist inhibits primary tumor growth and alters the pancreas microenvironment. a Panc02 pancreatic cancer growth is increased in SCID mice compared to C57BL/6 mice (**p < 0.01). Tumor growth is suppressed in SCID mice when the animals are treated with a CCK receptor antagonist (****p < 0.0001) but not in the immune-competent mice. The percentage of mice with metastases is shown above each respective column. CCKR antagonist therapy reduced metastases in C56BL/6, mice and there were no metastases in SCID mice treated with the CCKR antagonist. b CD3+ tumor-infiltrating lymphocytes significantly increase in the pancreatic cancer microenvironment with CCKR antagonist treatment in both C57BL/6 mice bearing Panc02 tumors and in the mutant KRAS transgenic mice (**p < 0.01). c Trichrome stain of Panc02 tumor from a vehicle-treated mouse demonstrating marked fibrosis. d Trichrome stain of a Panc02 tumor from a mouse treated with the CCKR antagonist proglumide demonstrates marked decreased fibrosis. e Quantitation of fibrosis from Panc02 tumors of untreated mice (control) is significantly greater than tumors of mice treated with proglumide (***p < 0.001). f Trichrome stain of a pancreas from the control mutant KRAS transgenic mouse shows extensive fibrosis. g Trichrome stain from an age-matched mutant KRAS transgenic mouse that was treated with the CCK receptor antagonist proglumide, showed significantly diminished fibrosis. (Figures f and g were reproduced with permission from Pancreas (2014); [21])
CCKR antagonist monotherapy significantly modifies the tumor microenvironment
Orthotopic tumors from the C57BL/6 immune-competent mice were analyzed for tumor-infiltrating lymphocytes. Mice treated with L364,718 had a significant increase in CD3+ lymphocytes by immunohistochemistry compared to the tumors from the PBS control mice (Fig. 1b). In the Pdx1-Cre/LSL-KrasG12D transgenic mouse experiments, the mice that received proglumide monotherapy exhibited a significant increase in the CD3+ cells in the mouse pancreas compared to control animals (Fig. 1b). Orthotopic Panc02 tumors from PBS-treated mice showed extensive fibrosis in the tumor microenvironment (Fig. 1c) and this fibrosis was significantly decreased in tumors of the mice treated with CCKR blockade (Fig. 1d). Differences in mean fibrosis scores with CCKR treatment (Fig. 1e) were confirmed by quantitative analysis [40]. In the pancreatic carcinogenesis experiments using the mutant Pdx1-Cre/LSL-KrasG12D mouse, proglumide also significantly reversed fibrosis of the pancreas microenvironment (Fig. 1f, g). These data show that CCKR antagonist monotherapy alters the pancreas microenvironment by decreasing fibrosis and increasing TILs.
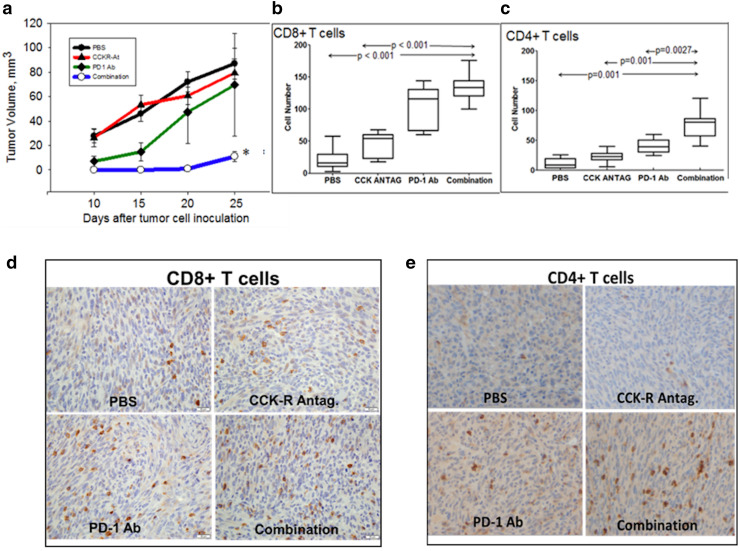
Combination therapy with CCKR antagonist L364,718 and PD-1 immune checkpoint antibody inhibits growth of Panc02 pancreatic cancer
In the next series of experiments, we evaluated whether CCKR antagonist therapy could improve responsiveness to immune checkpoint antibodies in immune-competent mouse models. Panc02 tumors increased in size in the mice treated with PBS (controls). The CCKR antagonist L364,718 monotherapy, and PD-1 antibody monotherapy (at the lower dose—125 μg × 3 injections) did not significantly decrease the tumor size (Fig. 2a). In contrast, mice receiving the combination of the CCKR antagonist and the PD-1 antibody had tumors 22-times smaller compared to the PBS group (Fig. 2a). Immunohistochemistry revealed a lack of CD8+ (Fig. 2b) and CD4+ (Fig. 2c) cells in the tumors from the PBS control animals. Immunoreactivity for both CD4 and CD8 increased in tumors treated with the PD-1 antibody or the CCKR antagonist L364,718. The greatest number of CD8+ and CD4+ cells were noted in tumors of mice treated with the combination therapy. Individual group immunohistochemistry stains are shown for CD8+ tumor-infiltrating lymphocytes (Fig. 2d) and CD4+ tumor-infiltrating lymphocytes (Fig. 2e).
Fig. 2.
Combination therapy with PD-1 antibody and CCKR antagonist inhibits pancreatic cancer growth. a Tumor volumes were not significantly smaller in immune-competent mice treated with PD-1 antibody or CCKR antagonist monotherapy (L-364,718) compared to PBS-treated controls. In contrast, tumor volumes of mice receiving both the CCKR antagonist and the immune checkpoint blockade antibody were significantly smaller (*p < 0.05). b The number of CD8+ cells significantly increased in mice treated with the combination therapy. c CD4+ cells in the tumors also increased in number with combination therapy. d Immunohistochemistry of mouse Panc02 tumors shows positive immunoreactivity for CD8+ cells with each treatment and the greatest in the combination group. e Immunohistochemistry of tumors demonstrates CD4+ stained cells for each treatment (mag 20×)
Effects of CCKR blockade with proglumide and PD-1 antibody on survival of mice with greater tumor burden
In another experiment, immune-competent mice received twice the number of Panc02 cells (2 × 106) to increase tumor burden. This experiment utilized the CCKR antagonist, proglumide and the PD-1 antibody. The Kaplan–Meier survival curve for this experiment is shown in supplementary Figure 1. On day 45 when all the PBS control-treated mice had died, 60% of the mice treated with the combination of PD-1 antibody and proglumide were still alive (p = 0.0009). Treatment with either PD-1 antibody or proglumide prolonged life by 5 days and the combination prolonged life by 10 days. This experiment demonstrates that even with a greater tumor burden, combination therapy with a CCKR antagonist and immune checkpoint antibody improves the survival of mice bearing Panc02 pancreatic tumors.
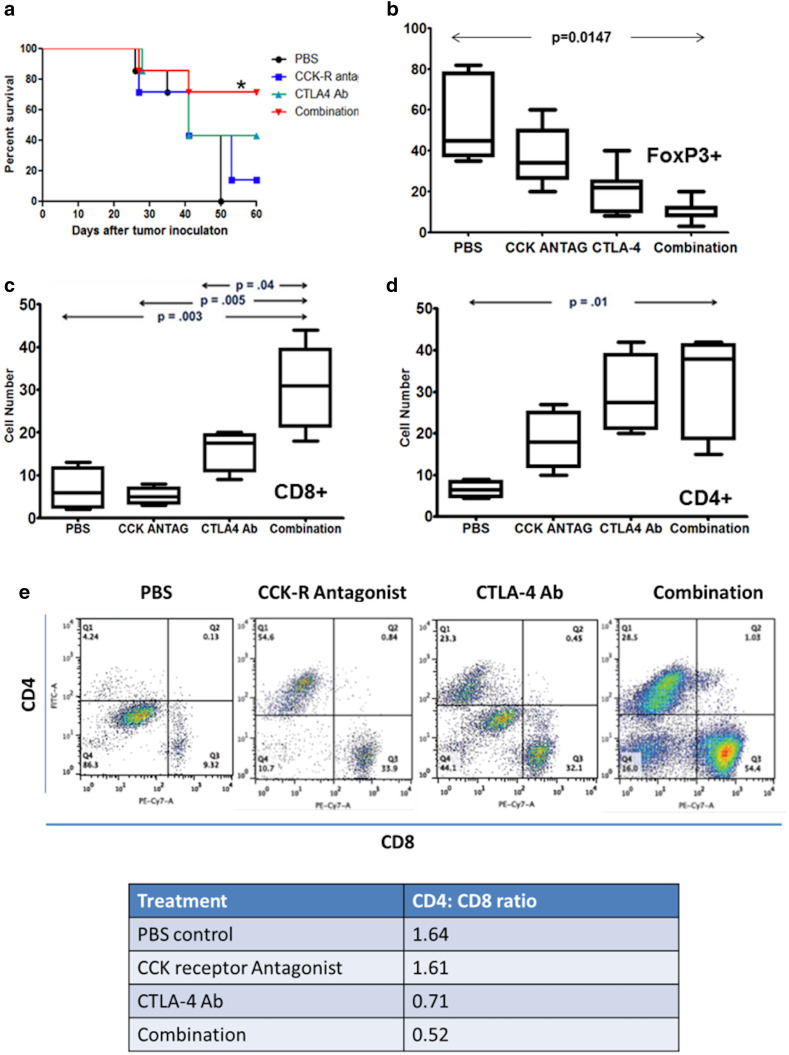
Combination therapy with CCKR antagonist L364,718 and CTLA-4 immune checkpoint antibody prolongs the survival of Panc02 pancreatic cancer-bearing mice
Kaplan–Meier analysis revealed improved survival of mice bearing Panc02 tumors treated with the combination of L364,718 and CTLA-4 Ab compared to PBS, or CCKR antagonist and CTLA-4 monotherapy (Fig. 3a). All PBS-treated control animals had died by day 50, while 42% of the CCKR antagonist treated, 42% of the CTLA4-Ab treated, and 71% of the combination-treated mice were still alive (p < 0.05). By day 90, only one mouse was still alive and it was in the combination-treated group.
Fig. 3.
Effects of CCKR blockade with L364,718 and CTLA-4 antibody on survival and tumor immune cells. a Kaplan–Meier survival curve for immune-competent C57BL/6 mice bearing Panc02 subcutaneous tumors show that all control animals (PBS) had died by day 50 when 42% of the CCKR antagonist (CCK) treated, 42% of the CTLA4-Ab (CTLA4) treated, and 71% of the combination-treated mice were still alive (*p < 0.05). b Foxp3+ (Tregs) immune cells decrease in the tumor microenvironment with combination therapy. c CD8+ cell numbers increased in pancreatic cancer treated with the combination of CTLA-4 immune checkpoint blockade antibody and CCKR antagonism. d CD4+ cell numbers were increased also in tumors of mice treated with the combination of the CCKR antagonist and a CTLA-4 immune checkpoint blockade antibody. e CD4+ and CD8+ tumor-infiltrating lymphocytes analysis by flow cytometry. Although both populations of T cells increased with therapy, the increase in the number of infiltrating CD8+ cells was greater. The CD4:CD8 ratio decreased significantly with the combination therapy using both the CCK receptor antagonist and the CTLA-4 antibody
Immunohistochemical stains of tumors obtained at necropsy showed that Foxp3+ cells were significantly increased in control mice (Fig. 3b). The number of Foxp3+ cells decreased with treatment and was significantly diminished in tumors of mice treated with the combination therapy (**p = 0.0147). Average CD8+ (Fig. 3c) and average CD4+ (Fig. 3d) cell numbers were increased in the Panc02 tumors of mice treated with CCKR antagonism and CTLA-4 antibody, and markedly increased with the combination of both agents. Evaluation by flow cytometry confirmed the immunohistochemical stains (Fig. 3e). Since the number of CD8+ cells exceeded the influx of CD4+ cells, the CD4:CD8 ratio decreased with combination treatment.
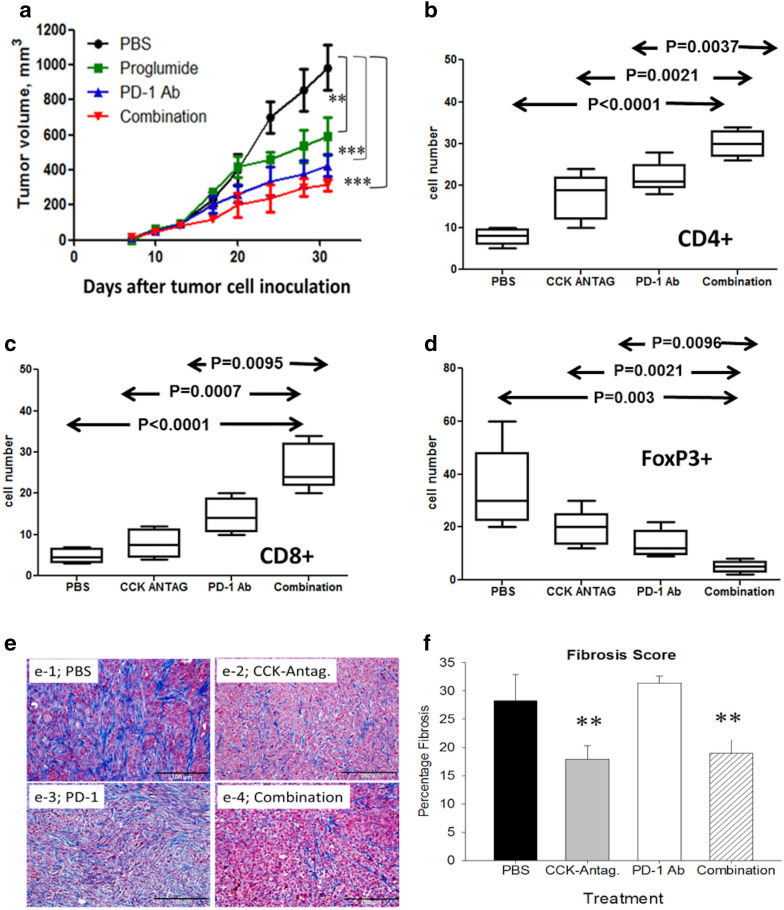
CCKR antagonist proglumide and PD-1 antibody therapy decrease tumor growth and improve survival in mice with mT3 pancreatic cancers
To validate the effects of CCKR antagonism and immune checkpoint blockade antibody therapy, an additional PDAC cell line (mT3) in an immunocompetent mouse model was performed. Analysis of the CCKR type in mT3 murine pancreatic cancer cells by RT-PCR showed that mT3 cells expressed CCK-B but not CCK-A receptor mRNA (Supplementary Figure 2a). CCK-B receptor protein was confirmed by immunofluorescence (Supplementary Figure 2b). Therefore, mT3 cells have characteristics that are representative of human pancreatic cancer, e.g., CCK-B receptor type and mutant KRAS.
Growth of mT3 tumors was significantly slower in mice treated with proglumide or PD-1 antibody monotherapy compared to PBS controls (Fig. 4a). In this experiment, a higher dosing regimen of PD-1 antibody (200 µg every 3 days) may have resulted in the more pronounced inhibitory effect on tumor size compared to the dose used (125 µg × 3 injections) for the Panc02 experiments. Alternatively, the mT3 cancers may be more susceptible to immune checkpoint antibodies. When CCKR blockade was used in combination with the PD-1 antibody, tumor size (Fig. 4a) was significantly decreased. In fact, mice with mT3 pancreatic cancers treated with proglumide or with PD-1 antibody monotherapy survived an average of 12 days longer than PBS control mice. Mice bearing mT3 pancreatic tumors that received the combination of both therapies survived more than 20 days longer than PBS-treated controls.
Fig. 4.
Effects of CCKR blockade with proglumide and PD-1 antibody on growth of mT3 murine pancreatic cancer. a Growth curves over time shows that CCKR antagonist monotherapy (**p < 0.01), PD-1 monotherapy (***p < 0.001), combination therapy (***p < 0.001) significantly slowed tumor growth of mT3 tumors compared to PBS controls. b CD4+ tumor-infiltrating lymphocytes increased with each of the treatments compared to PBS control-treated mice. c CD8+ tumor-infiltrating lymphocytes increase with each of the treatments compared to PBS. d Foxp3+ (Tregs) decrease with each of the treatments compared to PBS control-treated mice. e Control-treated mice mT3 tumors also exhibited dense fibrosis as shown by Masson’s trichrome stain (e-1). Fibrosis was decreased in tumors of mice treated with the CCKR antagonist (e-2) but not in tumors of mice treated with PD-1 antibody monotherapy (e-3). Combination treatment decreased tumor-associated fibrosis similar to that of proglumide-treated mice (e-4). f Quantitative analysis of intratumoral fibrosis demonstrated significant differences in mice treated with proglumide by Kruskal–Wallis analysis (**p < 0.01)
CD4 + TILs (Fig. 4b) and the CD8+ lymphocytes (Fig. 4c) increased significantly in the mice with mT3 tumors and were most pronounced with the combination of the CCKR antagonist and the PD-1 antibody. Likewise, the number of Foxp3+ cells decreased with CCKR antagonism, PD-1 inhibition, and combined therapy (Fig. 4d). Fibrosis as demonstrated by Masson’s trichrome stain was high in the PBS-treated tumors (Fig. 4e-1), and was noticeably less in mice treated with proglumide (Fig. 4e-2) and in mice treated with the combination therapy (Fig. 4e-4). PD-1 antibody monotherapy did not alter tumor-associated fibrosis (Fig. 4e-3), confirming that the anti-fibrotic effect was a result of CCKR antagonism. Quantitative analysis of the intratumoral fibrosis confirmed that proglumide-treated mice had significantly less fibrosis (p < 0.01) than PBS controls and PD-1 monotherapy-treated mice (Fig. 4f). Activated fibroblasts stained positive for α-SMA, and the α-SMA immunoreactivity was somewhat decreased with proglumide, but this change was not statistically significant (supplementary Fig. 3) indicating that other fibrosis-associated proteins may be altered by the proglumide.
Discussion
Our study demonstrates a novel therapeutic approach using CCKR blockade to enhance the response of immunotherapy in pancreatic cancer, a characteristically immune-resistant cancer. Since CCKRs have been reported on pancreatic cancer cells, CAFs, and lymphocytes, our investigation herein looked at all three cell types to study the effects of CCKR antagonism in different models of pancreatic carcinogenesis and established pancreatic cancer. In the immune-deficient SCID mouse, we showed that monotherapy with CCKR blockade decreased the orthotopic PDAC tumor size and metastases suggesting that some growth inhibitory effects mediated through the CCKR are independent of the immune system and due to prevention of peptide signaling at the cancer epithelial cell. The same dosing schedule was less effective in decreasing primary tumor growth in immune-competent mice, but did decrease metastases. These results suggest that CCKR antagonist monotherapy has anti-tumor and anti-metastatic properties. One reported mechanism by which L364,718, decreases Panc02 invasiveness in vitro and metastases in Panc02 orthotopic tumors is related to changes in tumor VEGF-A levels [28].
In our investigation, we found that combined therapy with a CCKR antagonist and an immune checkpoint antibody changed the number and type of immune cells in the cancer microenvironment. Some differences in treatment efficacy between the two CCKR antagonists used in our study were observed. A possible explanation for why L364,718 monotherapy was not as effective in inhibiting tumor growth as proglumide may be due to the intermittent administration of L364,718 (three times weekly); proglumide provided a more sustained CCKR blockade since it was administered continuously in the drinking water. Although a higher dose of L364,718 could have been utilized, our preliminary studies showed that higher doses impaired gall bladder contraction and could cause cholecystitis [42]. Another possible explanation for the greater efficacy of proglumide compared to L364,718 is that proglumide blocks both CCK-A and CCK-B receptors, whereas L364,718 blocks just CCK-A receptors. Although Panc02 cells have the CCK-A receptor type, CAFs, pancreatic stellate cells, and lymphocytes have both types of receptors. Therefore, proglumide may have a greater impact on altering the tumor microenvironment to render it more susceptible to immune therapy. For human studies where the CCK-B receptor is the predominant receptor type on the cancer epithelial cells, proglumide would also be the preferred antagonist since it has greater activity at the CCK-B receptor [26, 29]. Proglumide is an older drug that was originally developed for peptic ulcer disease [43] and approved dosages of proglumide (up to 1200 mg), have been given without toxicity. Another potential benefit of using CCKR antagonists in cancer patients is that CCKR blockade may improve pain [44]. Proglumide is currently formulated and in use as an oral preparation in Europe as Protaxon forte (proglumetacin) for control of pain. Therefore, translating this therapy into clinical practice is possible and may offer new insights to treatment of pancreatic cancer.
A variety of treatment strategies have been tried to enhance immune responsiveness of cancers. One approach has been with the use of low-dose cyclophosphamide [45, 46] to decrease the immunosuppressive Tregs. Another recent approach has been the use of toll-like receptor agonists to increase the response to tumor vaccines and immune checkpoint antibodies [47]. In our investigation, we found that CCKR antagonism and immune checkpoint antibodies also significantly decreased the Tregs, perhaps making the tumors more immune sensitive.
A new finding from this investigation is that the murine cancer cell line, mT3, derived from organoids of the mutant KRAS mouse expresses CCK-B receptors and not CCK-A receptors. Animal models to study cancer progression and therapeutics are essential but these animal models are most useful when they resemble human cancers, particularly with respect to genetics, inflammation, and the immune system in carcinogenesis and therapy. Hence, athymic nude mice bearing human cancer explants are being replaced by genetically modified animal models with intact immune systems. Panc02 cancer cells have traditionally been used to create a syngeneic immune-competent murine model of pancreatic cancer. However, unlike human pancreatic cancer, Panc02 cells express wild-type KRAS and CCK-A receptors. Since CCK-B receptors become expressed in the Pdx1-Cre/LSL-KrasG12D mouse under the influence of mutated KRAS [21], it is not surprising that the mT3 cells derived from this model also express the CCK-B receptors and more closely resemble human pancreatic cancer. Findings from our work may influence cell lines used in future studies in immune-competent murine models of PDAC.
Another new discovery from our investigation is that CCKR blockade decreases tumor-associated fibrosis that has been proposed to impair therapy [34]. Proglumide reverses fibrosis by the interference of gastrin or CCK signaling with the CCK receptors on pancreatic stellate cells to prevent activation [31, 32]. We showed that CCKR antagonist therapy decreased fibrosis in the pancreas of orthotopic tumors and fibrosis in the mutant KRAS model of carcinogenesis. Pancreatic stellate cells and fibroblasts possess both CCK-A and CCK-B receptor types and complete inhibition of collagen is reported with blockade of both receptors [31]. In the current study, we showed that CCKR antagonist therapy prevented activation and fibrosis from both pancreatic stellate cells (orthotopic model) and CAFs (subcutaneous tumors). CCKR have also been described on fibroblasts in the periphery [30] (away from the pancreas) and the CCKR antagonist therapy was also effective for tumors remote from the pancreas suggesting that this therapy would be effective for even metastatic lesions or perhaps other cancers associated with extensive fibrosis. Several methods have been tried to decrease the fibrosis associated with the pancreatic cancer tumor microenvironment [48–51] in an attempt to improve therapy. Some have tried to decrease tumor-associated macrophages using compounds, such as PF-04136309 that blocks CCL2-CCR2 chemokine axis [52]. Investigators are also studying the use of hyaluronidase to decrease tumor-associated fibrosis and improve chemotherapy [53]. All these strategies demonstrate that by decreasing fibrosis of the tumor microenvironment, therapy to pancreatic cancer can be improved. Unfortunately, many of the methods have systemic toxicity and unlike our approach, these other approaches do not directly attack the tumor epithelial cells, metastatic cells, or recruit effector T-lymphocytes. As a result, these other methods typically require secondary chemotherapeutic agents, such as gemcitabine to decrease growth of the cancer. One potential advantage of using the CCKR as a target for pancreatic cancer treatment is that there are CCKRs located on all three important components of the tumor including the cancer cells, the CAFs, and the immune cells. Given the poor prognosis of advanced pancreatic cancer, novel treatments such as the use of CCKR antagonists should be explored in the clinic.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Grant support: Supported by the National Institutes of Health, National Cancer Institute grants CA50633, and CA194745. These studies were conducted in part at the Lombardi Comprehensive Cancer Center Histopathology & Tissue Shared resource and in the Preclinical Imaging Research Laboratory which is supported in part by NIH/NCI grant P30-CA051008.
Abbreviations
- CAFs
cancer-associated fibroblasts
- CCK
cholecystokinin
- CCKR
cholecystokinin receptor
- CTLA-4
cytotoxic T–lymphocyte-associated antigen 4
- DMEM
Dulbecco’s Modified Eagle Medium
- PanIN
pancreatic intraepithelial neoplasia
- PDAC
pancreatic ductal adenocarcinoma
- PD-1
programmed cell death protein-1
- SCID
severe combined immune deficiency
- α-SMA
alpha smooth muscle actin
- TILs
tumor-infiltrating lymphocytes
- Tregs
T-regulatory lymphocytes
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Statement on the welfare of animals
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Human subjects
This article does not contain any studies with human participants performed by any of the authors.
References
- 1.Smith JP, Solomon TE, Bagheri S, Kramer S. Cholecystokinin stimulates growth of human pancreatic adenocarcinoma SW-1990. Dig Dis Sci. 1990;35:1377–1384. doi: 10.1007/BF01536744. [DOI] [PubMed] [Google Scholar]
- 2.Smith JP, Kramer ST, Solomon TE. CCK stimulates growth of six human pancreatic cancer cell lines in serum-free medium. Regul Pept. 1991;32:341–349. doi: 10.1016/0167-0115(91)90027-E. [DOI] [PubMed] [Google Scholar]
- 3.Smith JP, Fantaskey AP, Liu G, Zagon IS. Identification of gastrin as a growth peptide in human pancreatic cancer. Am J Physiol. 1995;268:R135–R141. doi: 10.1152/ajpregu.1995.268.1.R135. [DOI] [PubMed] [Google Scholar]
- 4.Howatson AG, Carter DC. Pancreatic carcinogenesis-enhancement by cholecystokinin in the hamster-nitrosamine model. Br J Cancer. 1985;51:107–114. doi: 10.1038/bjc.1985.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carriere C, Young AL, Gunn JR, Longnecker DS, Korc M. Acute pancreatitis markedly accelerates pancreatic cancer progression in mice expressing oncogenic Kras. Biochem Biophys Res Commun. 2009;382:561–565. doi: 10.1016/j.bbrc.2009.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bardram L, Hilsted L, Rehfeld JF. Progastrin expression in mammalian pancreas. Proc Natl Acad Sci USA. 1990;87:298–302. doi: 10.1073/pnas.87.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brand SJ, Fuller PJ. Differential gastrin gene expression in rat gastrointestinal tract and pancreas during neonatal development. J Biol Chem. 1988;263:5341–5347. [PubMed] [Google Scholar]
- 8.Tamiolakis D, Venizelos I, Simopoulos C, Kotini A, Jivannakis T, Papadopoulos N. Does neoplastic gastrin expression remodel the embryonal pattern of the protein? A study in human pancreas. Hepatogastroenterology. 2004;51:249–252. [PubMed] [Google Scholar]
- 9.Prasad NB, Biankin AV, Fukushima N, Maitra A, Dhara S, Elkahloun AG, Hruban RH, Goggins M, Leach SD. Gene expression profiles in pancreatic intraepithelial neoplasia reflect the effects of Hedgehog signaling on pancreatic ductal epithelial cells. Cancer Res. 2005;65:1619–1626. doi: 10.1158/0008-5472.CAN-04-1413. [DOI] [PubMed] [Google Scholar]
- 10.Smith JP, Hamory MW, Verderame MF, Zagon IS. Quantitative analysis of gastrin mRNA and peptide in normal and cancerous human pancreas. Int J Mol Med. 1998;2:309–315. doi: 10.3892/ijmm.2.3.309. [DOI] [PubMed] [Google Scholar]
- 11.Smith JP, Shih A, Wu Y, McLaughlin PJ, Zagon IS. Gastrin regulates growth of human pancreatic cancer in a tonic and autocrine fashion. Am J Physiol. 1996;270:R1078–R1084. doi: 10.1152/ajpcell.1996.270.3.C939. [DOI] [PubMed] [Google Scholar]
- 12.Smith JP, Rickabaugh CA, McLaughlin PJ, Zagon IS. Cholecystokinin receptors and PANC-1 human pancreatic cancer cells. Am J Physiol. 1993;265:G149–G155. doi: 10.1152/ajpgi.1993.265.1.G149. [DOI] [PubMed] [Google Scholar]
- 13.Smith JP, Liu G, Soundararajan V, McLaughlin PJ, Zagon IS. Identification and characterization of CCK-B/gastrin receptors in human pancreatic cancer cell lines. Am J Physiol. 1994;266:R277–R283. doi: 10.1152/ajpregu.1994.266.1.R277. [DOI] [PubMed] [Google Scholar]
- 14.Matters GL, Harms JF, McGovern CO, Jayakumar C, Crepin K, Smith ZP, Nelson MC, Stock H, Fenn CW, Kaiser J, Kester M, Smith JP. Growth of human pancreatic cancer is inhibited by down-regulation of gastrin gene expression. Pancreas. 2009;38:e151–e161. doi: 10.1097/MPA.0b013e3181a66fdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fino KK, Matters GL, McGovern CO, Gilius EL, Smith JP. Downregulation of the CCK-B receptor in pancreatic cancer cells blocks proliferation and promotes apoptosis. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1244–G1252. doi: 10.1152/ajpgi.00460.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith JP, Solomon TE. Cholecystokinin and pancreatic cancer: the chicken or the egg? Am J Physiol Gastrointest Liver Physiol. 2014;306:G91–G101. doi: 10.1152/ajpgi.00301.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wank SA, Harkins R, Jensen RT, Shapira H, de Weerth A, Slattery T. Purification, molecular cloning, and functional expression of the cholecystokinin receptor from rat pancreas. Proc Natl Acad Sci USA. 1992;89:3125–3129. doi: 10.1073/pnas.89.7.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wank SA, Pisegna JR, de Weerth A. Cholecystokinin receptor family. Molecular cloning, structure, and functional expression in rat, guinea pig, and human. Ann N Y Acad Sci. 1994;713:49–66. doi: 10.1111/j.1749-6632.1994.tb44052.x. [DOI] [PubMed] [Google Scholar]
- 19.Weinberg DS, Ruggeri B, Barber MT, Biswas S, Miknyocki S, Waldman SA. Cholecystokinin A and B receptors are differentially expressed in normal pancreas and pancreatic adenocarcinoma. J Clin Invest. 1997;100:597–603. doi: 10.1172/JCI119570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Longnecker DS, Curphey TJ, Lilja HS, French JI, Daniel DS. Carcinogenicity in rats of the nitrosourea amino acid N delta-(N-methyl-N-nitrosocarbamoyl)-L-ornithine. J Environ Pathol Toxicol. 1980;4:117–129. [PubMed] [Google Scholar]
- 21.Smith JP, Cooper TK, McGovern CO, Gilius EL, Zhong Q, Liao J, Molinolo AA, Gutkind JS, Matters GL. Cholecystokinin receptor antagonist halts progression of pancreatic cancer precursor lesions and fibrosis in mice. Pancreas. 2014;43:1050–1059. doi: 10.1097/MPA.0000000000000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith JP, Verderame MF, McLaughlin P, Martenis M, Ballard E, Zagon IS. Characterization of the CCK-C (cancer) receptor in human pancreatic cancer. Int J Mol Med. 2002;10:689–694. [PubMed] [Google Scholar]
- 23.Smith JP, Harms JF, Matters GL, McGovern CO, Ruggiero FM, Liao J, Fino KK, Ortega EE, Gilius EL, Phillips JA., III A single nucleotide polymorphism of the cholecystokinin-B receptor predicts risk for pancreatic cancer. Cancer Biol Ther. 2012;13:164–174. doi: 10.4161/cbt.13.3.18698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rai R, Kim JJ, Tewari M, Shukla HS. Heterogeneous expression of cholecystokinin and gastrin receptor in stomach and pancreatic cancer: an immunohistochemical study. J Cancer Res Ther. 2016;12:411–416. doi: 10.4103/0973-1482.168970. [DOI] [PubMed] [Google Scholar]
- 25.Abbruzzese JL, Gholson CF, Daugherty K, Larson E, DuBrow R, Berlin R, Levin B. A pilot clinical trial of the cholecystokinin receptor antagonist MK-329 in patients with advanced pancreatic cancer. Pancreas. 1992;7:165–171. doi: 10.1097/00006676-199203000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Berna MJ, Jensen RT. Role of CCK/gastrin receptors in gastrointestinal/metabolic diseases and results of human studies using gastrin/CCK receptor agonists/antagonists in these diseases. Curr Top Med Chem. 2007;7:1211–1231. doi: 10.2174/156802607780960519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang RS, Lotti VJ, Chen TB, Kunkel KA. Characterization of the binding of [3H]-(±)-L-364,718: a new potent, nonpeptide cholecystokinin antagonist radioligand selective for peripheral receptors. Mol Pharmacol. 1986;30:212–217. [PubMed] [Google Scholar]
- 28.Matters GL, Cooper TK, McGovern CO, Gilius EL, Liao J, Barth BM, Kester M, Smith JP. Cholecystokinin mediates progression and metastasis of pancreatic cancer associated with dietary fat. Dig Dis Sci. 2014;59:1180–1191. doi: 10.1007/s10620-014-3201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hahne WF, Jensen RT, Lemp GF, Gardner JD. Proglumide and benzotript: members of a different class of cholecystokinin receptor antagonists. Proc Natl Acad Sci USA. 1981;78:6304–6308. doi: 10.1073/pnas.78.10.6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh P, Owlia A, Espeijo R, Dai B. Novel gastrin receptors mediate mitogenic effects of gastrin and processing intermediates of gastrin on Swiss 3T3 fibroblasts. Absence of detectable cholecystokinin (CCK)-A and CCK-B receptors. J Biol Chem. 1995;270:8429–8438. doi: 10.1074/jbc.270.15.8429. [DOI] [PubMed] [Google Scholar]
- 31.Berna MJ, Seiz O, Nast JF, Benten D, Blaker M, Koch J, Lohse AW, Pace A. CCK1 and CCK2 receptors are expressed on pancreatic stellate cells and induce collagen production. J Biol Chem. 2010;285:38905–38914. doi: 10.1074/jbc.M110.125534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phillips PA, Yang L, Shulkes A, Vonlaufen A, Poljak A, Bustamante S, Warren A, Xu Z, Guilhaus M, Pirola R, Apte MV, Wilson JS. Pancreatic stellate cells produce acetylcholine and may play a role in pancreatic exocrine secretion. Proc Natl Acad Sci USA. 2010;107:17397–17402. doi: 10.1073/pnas.1000359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Apte MV, Park S, Phillips PA, Santucci N, Goldstein D, Kumar RK, Ramm GA, Buchler M, Friess H, McCarroll JA, Keogh G, Merrett N, Pirola R, Wilson JS. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas. 2004;29:179–187. doi: 10.1097/00006676-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Apte MV, Wilson JS, Lugea A, Pandol SJ. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology. 2013;144:1210–1219. doi: 10.1053/j.gastro.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang JG, Liu JX, Jia XX, Geng J, Yu F, Cong B. Cholecystokinin octapeptide regulates the differentiation and effector cytokine production of CD4 T cells in vitro. Int Immunopharmacol. 2014;20:307–315. doi: 10.1016/j.intimp.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 36.Zhang JG, Cong B, Li QX, Chen HY, Qin J, Fu LH. Cholecystokinin octapeptide regulates lipopolysaccharide-activated B cells co-stimulatory molecule expression and cytokines production in vitro. Immunopharmacol Immunotoxicol. 2011;33:157–163. doi: 10.3109/08923973.2010.491079. [DOI] [PubMed] [Google Scholar]
- 37.Corbett TH, Roberts BJ, Leopold WR, Peckham JC, Wilkoff LJ, Griswold DP, Jr, Schabel FM., Jr Induction and chemotherapeutic response of two transplantable ductal adenocarcinomas of the pancreas in C57BL/6 mice. Cancer Res. 1984;44:717–726. [PubMed] [Google Scholar]
- 38.Boj SF, Hwang CI, Baker LA, Chio II, Engle DD, Corbo V, Jager M, Ponz-Sarvise M, Tiriac H, Spector MS, Gracanin A, Oni T, Yu KH, van BR, Huch M, Rivera KD, Wilson JP, Feigin ME, Ohlund D, Handly-Santana A, Ardito-Abraham CM, Ludwig M, Elyada E, Alagesan B, Biffi G, Yordanov GN, Delcuze B, Creighton B, Wright K, Park Y, Morsink FH, Molenaar IQ, Borel Rinkes IH, Cuppen E, Hao Y, Jin Y, Nijman IJ, Iacobuzio-Donahue C, Leach SD, Pappin DJ, Hammell M, Klimstra DS, Basturk O, Hruban RH, Offerhaus GJ, Vries RG, Clevers H, Tuveson DA. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015;160:324–338. doi: 10.1016/j.cell.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herranz R. Cholecystokinin antagonists: pharmacological and therapeutic potential. Med Res Rev. 2003;23:559–605. doi: 10.1002/med.10042. [DOI] [PubMed] [Google Scholar]
- 40.Kennedy DJ, Vetteth S, Periyasamy SM, Kanj M, Fedorova L, Khouri S, Kahaleh MB, Xie Z, Malhotra D, Kolodkin NI, Lakatta EG, Fedorova OV, Bagrov AY, Shapiro JI. Central role for the cardiotonic steroid marinobufagenin in the pathogenesis of experimental uremic cardiomyopathy. Hypertension. 2006;47:488–495. doi: 10.1161/01.HYP.0000202594.82271.92. [DOI] [PubMed] [Google Scholar]
- 41.Surana R, Wang S, Xu W, Jablonski SA, Weiner LM. IL4 limits the efficacy of tumor-targeted antibody therapy in a murine model. Cancer Immunol Res. 2014;2:1103–1112. doi: 10.1158/2326-6066.CIR-14-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanyu N, Dodds WJ, Layman RD, Hogan WJ, Colton DG. Effect of two new cholecystokinin antagonists on gallbladder emptying in opossums. Am J Physiol. 1991;260:G258–G264. doi: 10.1152/ajpgi.1991.260.2.G258. [DOI] [PubMed] [Google Scholar]
- 43.Miederer SE, Lindstaedt H, Kutz K, Mayershofer R. Efficient treatment of gastric ulcer with proglumide (Milid) in outpatients (double blind trial) Acta Hepatogastroenterol. 1979;26:314–318. [PubMed] [Google Scholar]
- 44.Benedetti F, Amanzio M, Vighetti S, Asteggiano G. The biochemical and neuroendocrine bases of the hyperalgesic nocebo effect. J Neurosci. 2006;26:12014–12022. doi: 10.1523/JNEUROSCI.2947-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abu Eid R, Razavi G, Mkrtichyan M, Janik J, Khleif SN. Old-school chemotherapy in immunotherapeutic combination in cancer, a low-cost drug repurposed. Cancer Immunol Res. 2016;4:377–382. doi: 10.1158/2326-6066.CIR-16-0048. [DOI] [PubMed] [Google Scholar]
- 46.Mkrtichyan M, Najjar YG, Raulfs EC, Abdalla MY, Samara R, Rotem-Yehudar R, Cook L, Khleif SN. Anti-PD-1 synergizes with cyclophosphamide to induce potent anti-tumor vaccine effects through novel mechanisms. Eur J Immunol. 2011;41:2977–2986. doi: 10.1002/eji.201141639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaczanowska S, Joseph AM, Davila E. TLR agonists: our best frenemy in cancer immunotherapy. J Leukoc Biol. 2013;93:847–863. doi: 10.1189/jlb.1012501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amedei A, Niccolai E, Prisco D. Pancreatic cancer: role of the immune system in cancer progression and vaccine-based immunotherapy. Hum Vaccin Immunother. 2014 doi: 10.4161/hv.34392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fang H, Declerck YA. Targeting the tumor microenvironment: from understanding pathways to effective clinical trials. Cancer Res. 2013;73:4965–4977. doi: 10.1158/0008-5472.CAN-13-0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sideras K, Braat H, Kwekkeboom J, van Eijck CH, Peppelenbosch MP, Sleijfer S, Bruno M. Role of the immune system in pancreatic cancer progression and immune modulating treatment strategies. Cancer Treat Rev. 2014;40:513–522. doi: 10.1016/j.ctrv.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 51.Strauss J, Alewine C, Figg WD, Duffy A. Targeting the microenvironment of pancreatic cancer: overcoming treatment barriers and improving local immune responses. Clin Transl Oncol. 2016;18:653–659. doi: 10.1007/s12094-015-1459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nywening TM, Wang-Gillam A, Sanford DE, Belt BA, Panni RZ, Cusworth BM, Toriola AT, Nieman RK, Worley LA, Yano M, Fowler KJ, Lockhart AC, Suresh R, Tan BR, Lim KH, Fields RC, Strasberg SM, Hawkins WG, Denardo DG, Goedegebuure SP, Linehan DC. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol. 2016;17:651–662. doi: 10.1016/S1470-2045(16)00078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hingorani SR, Harris WP, Beck JT, Berdov BA, Wagner SA, Pshevlotsky EM, Tjulandin SA, Gladkov OA, Holcombe RF, Korn R, Raghunand N, Dychter S, Jiang P, Shepard HM, Devoe CE. Phase Ib study of PEGylated recombinant human hyaluronidase and gemcitabine in patients with advanced pancreatic cancer. Clin Cancer Res. 2016;22:2848–2854. doi: 10.1158/1078-0432.CCR-15-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.