Abstract
Background
Acute Kidney Injury (AKI) is a common complication of cardiopulmonary bypass surgery (CPB) in children. Several promising post-operative AKI biomarkers have been identified, but no pre-operative biomarkers are available. We evaluated the association of urinary uromodulin (uUMOD) with post-operative AKI.
Methods
101 children undergoing CPB were enrolled. Urine was collected prior to CPB. AKI was defined as ≧50% increase in serum creatinine from pre-operative baseline within 48h of surgery.
Results
Forty-seven patients (47%) developed AKI. 92% of participants in the lowest quartile of pre-operative uUMOD concentrations developed AKI, compared to 8% in the highest quartile. Patients with pre-operative uUMOD levels in the lowest quartile had 132.3 times increased risk of post-operative AKI versus the highest quartile. Raw uUMOD levels were significantly lower in patients with AKI vs. no AKI. Significance was unchanged after correcting uUMOD levels for urinary creatinine. Receiver operating characteristic analysis showed pre-operative uUMOD strongly predicted post-operative AKI with area under the curve (AUC) 0.90. Stepwise logistic regression analysis revealed a model combining uUMOD and bypass time predicted AKI with p<0.001. Neither RACHS Score nor age improved the model’s ability to predict AKI. Independent analysis demonstrated that while bypass time was associated with AKI, the predictive ability of bypass time (AUC 0.77) was less than that of pre-operative uUMOD levels (AUC 0.9).
Conclusions
Children with lowest pre-operative levels of uUMOD have greatly increased risk of AKI post CPB. If uUMOD were used to risk stratify patients undergoing CPB, clinical measures could be taken to minimize AKI development.
Keywords: Acute Kidney Injury (AKI), Cardiopulmonary Bypass Surgery (CPB), Biomarker, uromodulin (UMOD), Tamm-Horsfall Protein (THP)
Introduction
Acute kidney injury (AKI) is a common and serious complication of cardiopulmonary bypass surgery (CPB), occurring in up to 50% of pediatric patients undergoing the procedure [1, 2]. Depending on the definition of AKI used, the mortality rate ranges between 20% and 79% [1, 3]. Risk factors for AKI after CPB include age <2 years, history of prior surgeries, and longer bypass time [1, 4, 5].
Much research has been dedicated to the discovery of postoperative biomarkers to predict AKI and its severity. Proteins that mark tubular damage, such as neutrophil gelatinase-associated lipocalin (NGAL), interleukin 18 (IL-18) and kidney injury molecule-1, have shown promise as potential early diagnostics of AKI [6–11]. However, there are currently no pre-operative measures in use to determine a patient’s predisposition to developing AKI after CPB. Recent studies have demonstrated that lower urinary excretion of uromodulin (UMOD), also known as Tamm-Horsfall Protein (THP), measured in adult patients prior to CPB, is associated with higher odds of developing AKI post-surgery [12].
UMOD is the most abundant protein excreted in urine. It is a glycoprotein that is expressed in the thick ascending limb (TAL) of the Loop of Henle and was first purified by Igor Tamm and Frank Horsfall in 1952 [13–15]. Mutations in the UMOD gene are associated with medullary cystic kidney disease and autosomal dominant tubulointerstitial kidney disease [16, 17]. In healthy kidneys, UMOD is thought to have a role in salt transport in the tubules, innate immunity and protection against kidney stones [18].
In this study, we set out to determine if pre-operative levels of urinary UMOD are associated with risk of AKI in a cohort of pediatric patients undergoing CPB.
Methods
Patients
This study was approved by the Cincinnati Children’s Hospital Medical Center Internal Review Board and was carried out in accordance with the Declaration of Helsinki. All patients <18 years of age undergoing cardiac surgery with CPB at our center between June 2005 and July 2007 were approached for study inclusion. Written informed consent was obtained from the legal guardian of each patient, and assent was obtained from patients aged 11 years and older at the time of enrollment. Patients with pre-existing renal insufficiency (serum creatinine (SCr) >2 times age-adjusted normal range) were excluded. Past nephrotoxin use, such as contrast or aminoglycoside, was not an exclusion factor as long as the SCr was normal at the time of surgery. Complexity of surgery was categorized according to the Risk Adjustment for Congenital Heart Surgery 1 (RACHS-1) scoring system [19]. Urine samples for biomarker analysis were obtained immediately before CPB and stored in aliquots at −80°C. SCr was measured at baseline, immediately after surgery and at least daily in the post-operative period.
The primary outcome measure was AKI, defined as a 50% or greater increase in SCr above pre-procedural baseline within 48 hours post-surgery, comprising modified KDIGO criteria [20]. Secondary outcomes included bypass time and length of hospital stay.
Biomarker measurements
Urinary UMOD was measured using a clinically available ELISA kit, per manufacturer’s instructions (EHUMOD, Thermo-Fisher Scientific, Waltham, MA). Intra- and inter-assay variability were 10% and 12%, respectively. Urine creatinine was measured using an enzymatic creatinine assay on a Dimension RXL clinical analyzer (Siemens, Munich, Germany). Coefficients of variability for the creatinine measurements were 2.4% (intra) and 4.2% (total).
Statistics
Statistical analysis was performed using SigmaPlot 12.5 (Systat Software, Inc., Chicago, IL). Demographics, baseline measurements and clinical outcomes were summarized and compared between AKI and no AKI patients. For continuous variables, median and inter-quartile ranges (IQR) were reported and Wilcoxon rank sum test was used to test for group differences. For categorical variables, frequencies and proportions were reported and Chi-square test or Fisher’s exact test was used to test for group differences. For UMOD measurements, median and IQR were calculated and subjected to Mann-Whitney Rank Sum Analysis. UMOD IQR was used to categorize patients into quartiles to calculate odds ratios for development of AKI. To determine association of clinical variables and UMOD levels with AKI, a stepwise logistic regression analysis was performed. Independent receiver operator characteristic curves (ROC) were also calculated for each variable (UMOD, length of stay, RACHS score and bypass time), and the area under the curve (AUC) was calculated to determine the discriminatory power for each variable to predict AKI. In all tests, p-values ≤0.05 were considered significant.
Results
Patient characteristics
One-hundred and one patients <18 years of age undergoing on-pump cardiac surgery at our center were enrolled in this prospective study. Forty seven (47%) developed AKI. Patient demographic and clinical characteristics can be found in Table 1. There were no differences between AKI and non-AKI patients in terms of age, sex, race, prior CPB or RACHS score. Bypass times and length of hospital stay were significantly increased in AKI patients (p <0.001).
TABLE 1.
PATIENT CHARACTERISTICS
| Characteristics | No AKI n = 54 | AKI n = 47 | p value |
|---|---|---|---|
| Age, years | 3.9 (0.7–6.6) | 1.8 (0.5–5.3) | 0.17 |
| Male | 30 (55.6) | 26 (55.3) | 0.86 |
| White race | 48 (88.9) | 42 (89.4) | 0.81 |
| Prior CPB | 32 (59.2) | 19 (40.4) | 0.09 |
| Bypass time, minutes | 93 (67.0–114.5) | 152 (103.0–182.0) | < 0.001 * |
| Hospital stay, days | 4 (3.0–6.0) | 7 (5.0–13.0) | < 0.001 * |
| Rachs score | 0.65 | ||
| 1 | 7 (13.0) | 7 (14.9) | |
| 2 | 26 (48.1) | 25 (53.2) | |
| 3 | 18 (33.3) | 13 (27.7) | |
| 4 | 2 (3.7) | 0 (0) | |
| 5 | 1 (1.9) | 1 (2.1) | |
| 6 | 0 (0) | 1 (2.1) |
Values are median (interquartile range) for continuous variables (with p values from Wilcoxon rank sum test) or n (%) for categorical variables (with p values from chi-square test or Fisher exact test).
deems significance p < 0.05.
AKI – acute kidney injury; CPB – cardiopulmonary bypass.
uUMOD and AKI
Table 2 shows that 92% of the participants in the lowest quartile of urinary UMOD levels developed AKI, compared to 8% in the highest quartile. Odds ratios demonstrated that having a uUMOD level in the lowest quartile yielded a 132.3 times increased risk of developing AKI vs. the highest quartile. Raw UMOD levels were significantly lower in patients with AKI (4.739μg/ml; 25th 2.454; 75th 6.483) vs. no AKI (13.701μg/ml; 9.022, 20.999) (Figure 1a). Significance did not change with urine creatinine correction (Figure 1b). ROC analysis of pre-operative uUMOD levels demonstrated an AUC of 0.90 for prediction of AKI (p<0.001) (Figure 2). Stepwise logistic regression analysis revealed that a model combining UMOD (F statistic 33) and bypass time (F statistic 32) could predict AKI with a p<0.001. Neither RACHS Score (F statistic 2.1, p = 0.15) or age (F statistic 1.6, p = 0.2) added to the ability of the model to predict AKI. Independent analysis demonstrated that while bypass time was associated with AKI, the predictive ability of bypass time AUC (0.77) was less than that of the pre-operative urinary UMOD levels (AUC 0.90) (Figure 2).
Table 2.
| uUMOD ug/ml | N (%) of AKI events | Odds Ratios (vs Q4) | 95% CI | p value |
|---|---|---|---|---|
| Total | 47 (46.5) | |||
| Q1: < 4.6 | 23 (92.0) | 132.3 | 17.1–1020.5 | < 0.001 * |
| Q2: 4.6–8.5 | 17 (65.3) | 21.7 | 4.1–113.7 | < 0.001 * |
| Q3: 8.51–14.4 | 5 (20.0%) | 2.9 | 0.5–16.4 | 0.42 |
| Q4: >14.4 | 2 (8.0%) |
Odds Ratios to develop AKI based on quartile (Q) level of urinary UMOD.
deems significance p < 0.05.
AKI acute kidney injury, uUMOD urinary uromodulin.
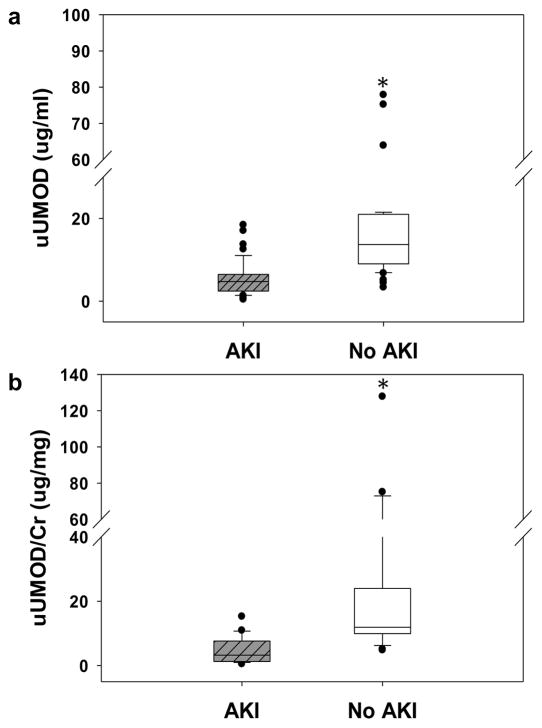
Figure 1.
a) Raw UMOD levels were significantly lower in patients with AKI (4.739 μg/ml; 25th 2.454; 75th 6.483) vs. no AKI (13.701 μg/ml; 9.022, 20.999) b) Significance did not change with urine creatinine correction. uUMOD – urinary uromodulin; AKI – acute kidney injury; Cr – creatinine.
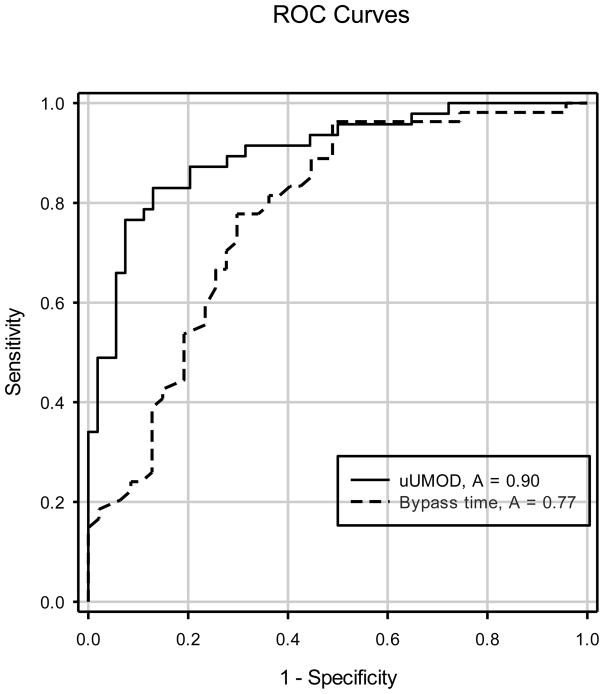
Figure 2.
ROC curve analysis of uUMOD and bypass time for discrimination of AKI. The AUC for uUMOD was 0.90 vs 0.77 for Bypass time. Both values reached statistical significance (p = 0.001). uUMOD – urinary uromodulin; AKI – acute kidney injury; ROC – receiver operating characteristic; AUC – area under the curve.
uUMOD and nonrenal outcomes
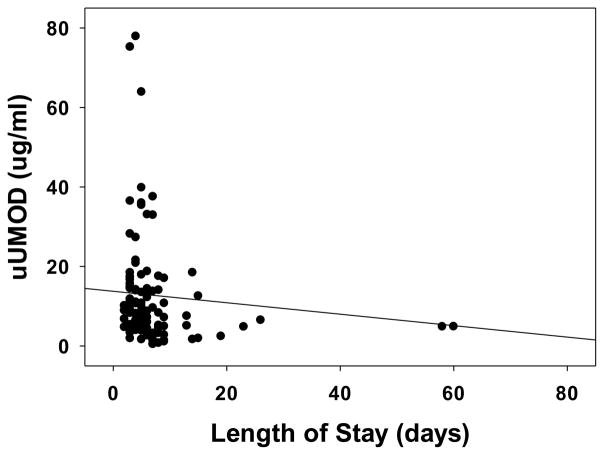
Lower pre-operative concentrations of uUMOD were also associated with a longer length of hospital stay (r = −0.31, p = 0.002) (Figure 3).
Figure 3.
Association of uUMOD with Non-Renal Outcomes. Spearman correlation showed that uUMOD was negatively correlated with length of hospital stay. (r = −0.31, p = 0.002). uUMOD – urinary uromodulin.
Discussion
This is the first study to show that lower pre-operative levels of urinary UMOD in children undergoing CBP is associated with increased risk of AKI. We found that raw UMOD levels were significantly lower at baseline (p <0.001) in patients who developed AKI after CPB than those who did not develop AKI. In fact, we found that patients in the lowest quartile of baseline UMOD concentrations had a 132.5 times increased odds of developing AKI post-surgery than those patients in the highest quartile. While increased bypass time was also associated with AKI (AUC 0.77), baseline urinary UMOD levels were more predictive of AKI (AUC = 0.90), independent of complexity of surgery or age. We also found that baseline urinary UMOD levels were negatively associated with the length of hospital stay, likely due to increased incidence of AKI in patients with low baseline UMOD levels.
AKI is a common and serious complication of CPB in both children and adults. It occurs at a high rate in children (20–60%) and is associated with higher in hospital mortality and increased length of stay [20–22]. Due to the complications associated with AKI, it is also associated with a significant financial burden. In the United States, it is estimated that AKI accounts for increased hospitalization costs of $5.4 – 24 billion annually [23]. In the adult population, AKI after CPB is associated with increased risk of CKD and hypertension [24, 25]. In the pediatric population, higher incidences of CKD and hypertension at 5 years following CPB have been identified [21].
The production of UMOD is restricted to the TAL of the Loop of Henle and the most proximal portion of the distal tubule [26]. UMOD is a glycoprotein with the capability of binding to a large number of ligands [27]. UMOD has been shown to bind to cells, immunoglobulins, cytokines, crystals and ions. One of the physiological roles proposed for UMOD is to bind harmful products to remove them from the tubular fluid [28]. UMOD is typically associated with the apical membrane domain of the TAL, but has been shown in mice to translocate to the basolateral domain of the TAL, the interstitium and the S3 segment of proximal tubules in the recovery period post AKI [29]. This was hypothesized to contribute to potential cross-talk between the TAL and the S3 segment to downregulate inflammatory molecules in proximal tubules. The authors demonstrated that in a mouse model of ischemia reperfusion injury, the S3 tubular cells in UMOD deficient mice expressed a high level of Macrophage inflammatory protein (MIP-2) - a neutrophil-attracting chemokine – and were surrounded by neutrophils. It is thought that the basolateral translocation of UMOD interacts with the S3 segments to reduce expression of MIP-2 and therefore decrease damage due to inflammation in the tubules [30].
Our data is consistent with earlier studies in both adults and neonates. Garimella et al. [12], found that lower pre-surgery levels of uUMOD in adults undergoing on-pump cardiac surgery had increased odds of AKI, but the significance of the data in their population was not as strong as ours, and more evident in cases of severe AKI. They also demonstrated that lower pre-surgery uUMOD was inversely related to peak SCr post-surgery. This is consistent with the idea that UMOD has a protective effect on renal tubules. The slight differences in our results could be due to a number of variables. First, children have fewer comorbid conditions than adult patients, rendering biomarkers in children being subjected to fewer confounding variables. Secondly, the ELISA kit used by Garimella et al. [12] was discontinued by the manufacturer, necessitating a different kit for our analysis. Similarly, Askenazi, et al. [31] demonstrated 62% lower levels of uUMOD in critically-ill neonates with AKI than in those without. In this setting it was not predictive, since the children already had AKI, but had a good discriminatory value (AUC 0.77) to distinguish AKI from non-AKI patients.
Our study has several strengths. We used a well characterized patient cohort that has been useful in evaluating the predictive value of tubular markers to predict AKI post-surgery [9]. While we used a different commercially available ELISA kit than in previous studies [12, 32], the results from the kit used in this study correlated well with the referenced kit (data not shown). To our knowledge, this is the first study to assess pre-operative levels of uUMOD in an attempt to determine AKI risk in pediatric patients undergoing CPB. Our results were consistent with adult studies, but had a much higher degree of significance (p=0.03 in adults vs 0.001 in our study) [12]. We also determined that the association of urinary UMOD with AKI (AUC = 0.90) was independent of age, RACHS score or bypass time.
Our study is not without limitations. This was a single center study with a relatively small sample size. As such, we were not able to stratify patients by severity of AKI. Stratification of AKI by pRIFLE criteria, for instance, could be informative to determine whether there is a direct correlation between uUMOD levels and AKI severity. Lastly, our samples had been stored at −80°C for 10 years at the time of our analysis. Studies have shown that storage of urine for greater than 8 months does yield a small, but significant decrease in uromodulin as measured by ELISA [33], but there is no reason to expect that this decrease would be influenced differentially in samples from patients with AKI vs their non-AKI counterparts.
In conclusion, assessment of risk factors prior to CPB in children, including urinary UMOD levels, could allow physicians to implement strategies to reduce AKI risk and improve overall outcomes for these patients. While this concept needs further exploration in a larger multi-center cohort to allow further validation and stratification for AKI severity, combining pre-operative risk assessment with post-operative monitoring of tubular damage markers such as NGAL could yield significant improvement in the care of CPB patients.
Acknowledgments
This work was funded by NIH P50 DK096418 to PD.
Footnotes
Disclosures
None
References
- 1.Li S, Krawczeski CD, Zappitelli M, Devarajan P, Thiessen-Philbrook H, Coca SG, Kim RW, Parikh CR TRIBE-AKI Consortium. Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery: a prospective multicenter study. Crit Care Med. 2011;39:1493–1499. doi: 10.1097/CCM.0b013e31821201d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jefferies JL, Devarajan P. Early detection of acute kidney injury after pediatric cardiac surgery. Prog Pediatr Cardiol. 2016;41:9–16. doi: 10.1016/j.ppedcard.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pedersen KR, Povlsen JV, Christensen S, Pedersen J, Hjortholm K, Larsen SH, Hjortdal VE. Risk factors for acute renal failure requiring dialysis after surgery for congenital heart disease in children. Acta Anaesthesiol Scand. 2007;51:1344–1349. doi: 10.1111/j.1399-6576.2007.01379.x. [DOI] [PubMed] [Google Scholar]
- 4.Picca S, Principato F, Mazzera E, Corona R, Ferrigno L, Marcelletti C, Rizzoni G. Risks of acute renal failure after cardiopulmonary bypass surgery in children: a retrospective 10-year case-control study. Nephrol Dial Transplant. 1995;10:630–636. [PubMed] [Google Scholar]
- 5.Dittrich S, Dahnert I, Vogel M, Stiller B, Haas NA, Alexi-Meskishvili V, Lange PE. Peritoneal dialysis after infant open heart surgery: observations in 27 patients. Ann Thorac Surg. 1999;68:160–163. doi: 10.1016/s0003-4975(99)00312-4. [DOI] [PubMed] [Google Scholar]
- 6.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 7.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 8.Parikh CR, Mishra J, Thiessen-Philbrook H, Dursun B, Ma Q, Kelly C, Dent C, Devarajan P, Edelstein CL. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2006;70:199–203. doi: 10.1038/sj.ki.5001527. [DOI] [PubMed] [Google Scholar]
- 9.Krawczeski CD, Goldstein SL, Woo JG, Wang Y, Piyaphanee N, Ma Q, Bennett M, Devarajan P. Temporal relationship and predictive value of urinary acute kidney injury biomarkers after pediatric cardiopulmonary bypass. J Am Coll Cardiol. 2011;58:2301–2309. doi: 10.1016/j.jacc.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Endre ZH, Pickering JW, Walker RJ, Devarajan P, Edelstein CL, Bonventre JV, Frampton CM, Bennett MR, Ma Q, Sabbisetti VS, Vaidya VS, Walcher AM, Shaw GM, Henderson SJ, Nejat M, Schollum JB, George PM. Improved performance of urinary biomarkers of acute kidney injury in the critically ill by stratification for injury duration and baseline renal function. Kidney Int. 2011;79:1119–1130. doi: 10.1038/ki.2010.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parikh CR, Devarajan P, Zappitelli M, Sint K, Thiessen-Philbrook H, Li S, Kim RW, Koyner JL, Coca SG, Edelstein CL, Shlipak MG, Garg AX, Krawczeski CD. Postoperative biomarkers predict acute kidney injury and poor outcomes after pediatric cardiac surgery. J Am Soc Nephrol. 2011;22:1737–1747. doi: 10.1681/ASN.2010111163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garimella PS, Jaber BL, Tighiouart H, Liangos O, Bennett MR, Devarajan P, El-Achkar TM, Sarnak MJ. Association of Preoperative Urinary Uromodulin with AKI after Cardiac Surgery. Clin J am Soc Nephrol. 2017;12:10–18. doi: 10.2215/CJN.02520316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamm I, Horsfall FL., Jr A mucoprotein derived from human urine which reacts with influenza, mumps, and Newcastle disease viruses. J Exp Med. 1952;95:71–97. doi: 10.1084/jem.95.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pruijm M, Ponte B, Ackermann D, Paccaud F, Guessous I, Ehret G, Pechère-Bertschi A, Vogt B, Mohaupt MG, Martin P-Y, Youhanna SC, Nägele N, Vollenweider P, Waeber G, Burnier M, Devuyst O, Bochud M. Associations of Urinary Uromodulin with Clinical Characteristics and Markers of Tubular Function in the General Population. Clin J Am Soc Nephrol. 2016;11:70–80. doi: 10.2215/CJN.04230415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Achkar TM, Wu XR, Rauchman M, McCracken R, Kiefer S, Dagher PC. Tamm-Horsfall protein protects the kidney from ischemic injury by decreasing inflammation and altering TLR4 expression. Am J Physiol Renal Physiol. 2008;295:F534–544. doi: 10.1152/ajprenal.00083.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hart TC, Gorry MC, Hart PS, Woodard AS, Shihabi Z, Sandhu J, Shirts B, Xu L, Zhu H, Barmada MM, Bleyer AJ. Mutations of the UMOD gene are responsible for medullary cystic kidney disease 2 and familial juvenile hyperuricaemic nephropathy. J Med Genet. 2002;39:882–892. doi: 10.1136/jmg.39.12.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards N, Olinger E, Adam J, Kelly M, Schiano G, Ramsbottom SA, Sandford R, Devuyst O, Sayer JA. A novel homozygous UMOD mutation reveals gene dosage effects on uromodulin processing and urinary excretion. Nephrol Dial Transplant. 2017 doi: 10.1093/ndt/gfx066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rampoldi L, Scolari F, Amoroso A, Ghiggeri G, Devuyst O. The rediscovery of uromodulin (Tamm-Horsfall protein): from tubulointerstitial nephropathy to chronic kidney disease. Kidney Int. 2011;80:338–347. doi: 10.1038/ki.2011.134. [DOI] [PubMed] [Google Scholar]
- 19.Jenkins KJ. Risk adjustment for congenital heart surgery: the RACHS-1 method. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2004;7:180–184. doi: 10.1053/j.pcsu.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Devarajan P. Pediatric Acute Kidney Injury: Different From Acute Renal Failure But How And Why. Curr Pediatr Rep. 2013;1:34–40. doi: 10.1007/s40124-012-0003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenberg JH, Zappitelli M, Devarajan P, Thiessen-Philbrook HR, Krawczeski C, Li S, Garg AX, Coca S, Parikh CR. Kidney Outcomes 5 Years After Pediatric Cardiac Surgery: The TRIBE-AKI Study. JAMA Pediatr. 2016;170:1071–1078. doi: 10.1001/jamapediatrics.2016.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li S, Krawczeski CD, Zappitelli M, Devarajan P, Thiessen-Philbrook H, Coca SG, Kim RW, Parikh CR. Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery: a prospective multicenter study. Crit Care Med. 2011;39:1493–1499. doi: 10.1097/CCM.0b013e31821201d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silver SA, Chertow GM. The Economic Consequences of Acute Kidney Injury. Nephron. 2017 doi: 10.1159/000475607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim CC, Tan CS, Chia CM, Tan AK, Choo JC, Kaushik M, Tan HK. Long-Term Risk of Progressive Chronic Kidney Disease in Patients with Severe Acute Kidney Injury Requiring Dialysis after Coronary Artery Bypass Surgery. Cardiorenal Med. 2015;5:157–163. doi: 10.1159/000381068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu CY, Hsu RK, Yang J, Ordonez JD, Zheng S, Go AS. Elevated BP after AKI. J Am Soc Nephrol. 2016;27:914–923. doi: 10.1681/ASN.2014111114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serafini-Cessi F, Malagolini N, Cavallone D. Tamm-Horsfall glycoprotein: biology and clinical relevance. Am J Kidney Dis. 2003;42:658–676. doi: 10.1016/s0272-6386(03)00829-1. [DOI] [PubMed] [Google Scholar]
- 27.Hard K, Van Zadelhoff G, Moonen P, Kamerling JP, Vliegenthart FG. The Asn-linked carbohydrate chains of human Tamm-Horsfall glycoprotein of one male. Novel sulfated and novel N-acetylgalactosamine-containing N-linked carbohydrate chains. Eur J Biochem. 1992;209:895–915. doi: 10.1111/j.1432-1033.1992.tb17362.x. [DOI] [PubMed] [Google Scholar]
- 28.Kumar S. Mechanism of injury in uromodulin-associated kidney disease. J Am Soc Nephrol. 2007;18:10–12. doi: 10.1681/ASN.2006111234. [DOI] [PubMed] [Google Scholar]
- 29.El-Achkar TM, McCracken R, Liu Y, Heitmeier MR, Bourgeois S, Ryerse J, Wu XR. Tamm-Horsfall protein translocates to the basolateral domain of thick ascending limbs, interstitium, and circulation during recovery from acute kidney injury. Am J Physiol Renal Physiol. 2013;304:F1066–1075. doi: 10.1152/ajprenal.00543.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El-Achkar TM, McCracken R, Rauchman M, Heitmeier MR, Al-Aly Z, Dagher PC, Wu XR. Tamm-Horsfall protein-deficient thick ascending limbs promote injury to neighboring S3 segments in an MIP-2-dependent mechanism. Am J Physiol Renal Physiol. 2011;300:F999–1007. doi: 10.1152/ajprenal.00621.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Askenazi DJ, Koralkar R, Hundley HE, Montesanti A, Parwar P, Sonjara S, Ambalavanan N. Urine biomarkers predict acute kidney injury in newborns. J Pediatr. 2012;161:270–275. e271. doi: 10.1016/j.jpeds.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlatzer D, Maahs DM, Chance MR, Dazard JE, Li X, Hazlett F, Rewers M, Snell-Bergeon JK. Novel urinary protein biomarkers predicting the development of microalbuminuria and renal function decline in type 1 diabetes. Diabetes care. 2012;35:549–555. doi: 10.2337/dc11-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Youhanna S, Weber J, Beaujean V, Glaudemans B, Sobek J, Devuyst O. Determination of uromodulin in human urine: influence of storage and processing. Nephrol Dial Transplant. 2014;29:136–145. doi: 10.1093/ndt/gft345. [DOI] [PubMed] [Google Scholar]