Abstract
Background and Objective
Data from clinical and preclinical models of relapse suggest that progesterone attenuates cocaine-seeking behavior. In a recent study, we found that cocaine-dependent women reported greater subjective responses to cues that were preceded by a stressor than cocaine-dependent men. The objective of this study was to examine the impact of endogenous progesterone on the subjective and endocrine responses to a drug-paired cue that was preceded by a stressor in cocaine-dependent women.
Methods
Cocaine-dependent women with low (<4 ng/ml; n=16) and high (≥4 ng/ml; n=9) plasma progesterone levels received either the alpha-2 adrenergic receptor antagonist yohimbine (21.6 mg) or placebo before each of two cocaine-cue exposure sessions. Participants were tested under both conditions in a counterbalanced, double-blind fashion. Data were collected after study drug administration, immediately and at 5, 30, and 60 minutes after the cue.
Results
The anxiety response to the cue was differentially modified by progesterone levels under the two administration conditions (condition x progesterone level interaction, F1,23=9.8, p=0.005). Progesterone levels also modified the craving response to the cue differently under the placebo condition as compared to the yohimbine condition (condition x progesterone level interaction, F1,23=13.9, p=0.001). In both cases, high progesterone levels attenuated craving and anxiety response to the cue following yohimbine administration. There was no effect of progesterone levels on salivary cortisol or dehydroepiandrosterone under the placebo condition or under the yohimbine condition.
Conclusions
These preliminary data suggest that high levels of endogenous progesterone attenuate subjective responses to drug-cues that are preceded by a stressor. Importantly, these data support a growing literature demonstrating the protective effects of progesterone on the vulnerability to cocaine relapse in women.
1.0 Introduction
There are significant sex differences in the development and symptomology of substance use disorders, including cocaine dependence. For example, compared to men, women meet criteria for substance use disorders faster and enter treatment programs earlier than men (Anglin et al., 1987; Hernandez-Avila et al., 2004; Westermeyer et al., 2000). In addition, cocaine-dependent women report higher rates of cocaine use and remain abstinent for shorter periods of time (Griffin et al., 1989). Cocaine-dependent women exhibit significant psychiatric, medical, and psychosocial dysfunction (Brady et al., 1999; Najavits et al., 2008; Wong et al., 2002). Thus, the effects of short and long-term drug use are particularly formidable for women. Research focused on understanding the neurobiologic factors that underscore sex differences in drug craving and relapse could have important treatment implications for cocaine-dependent women.
Data from clinical and preclinical studies suggests that ovarian hormones are important factors that contribute to sex and gender differences related to stimulant use. For example, estrogen enhances the reinforcing effects of stimulants and may increase the vulnerability of women to drug craving and relapse (Anker et al., 2007; Evans et al., 2002; Justice et al., 1999, 2000; Larson et al., 2007; Larson et al., 2005; Lile et al., 2007; White et al., 2002). Progesterone appears to have the opposite effects on stimulant-seeking behavior. For example, exogenous progesterone administered to ovariectomized (OVX) and sham-operated (SH) female rodents attenuated self-administration of cocaine under long-access conditions, suggesting that progesterone attenuates drug binging behavior (Larson et al., 2007). In addition, progesterone treatment reduced cocaine-primed reinstatement in SH female rodents and in OVX female rodents that were treated with estrogen (Anker et al., 2007). Human laboratory studies investigating exogenous progesterone administration have found attenuated cue-induced craving in cocaine-dependent individuals (Fox et al., 2013), and reduced weekly cocaine use in post-partum cocaine-dependent women during a 12-week clinical trial (Yonkers et al., 2014). Studies investigating endogenous progesterone demonstrate similar effects. Elevated levels of progesterone were associated with lower reinstatement responding for cocaine in intact freely-cycling female rodents (Feltenstein et al., 2007). Protective effects of endogenous progesterone have also been found in clinical studies of cocaine-dependent women. Sinha and colleagues (2007) examined the effects of endogenous progesterone levels on subjective responses to stress and drug cues using an autobiographical imagery paradigm. Cocaine-dependent women with high progesterone levels reported significantly lower cue- and stress-induced craving and lower cue-induced anxiety than cocaine-dependent women with low progesterone levels. Taken together, these data suggest that among cocaine-dependent women, elevated progesterone attenuates craving responses to triggers of relapse and may impact use outcomes.
Accumulating research demonstrates an important interaction between stress and drug cues in relapse. For example, stress potentiates cue-induced reinstatement of cocaine-seeking behavior in rodents (Banna et al., 2010; Buffalari et al., 2009; Feltenstein et al., 2011; Liu et al., 2002). Of note, compared to male rodents, female rodents exposed to the pharmacological stressor yohimbine exhibit greater stress potentiation of cue-induced cocaine seeking behavior (Feltenstein et al., 2011). Compared to cocaine-dependent men, cocaine-dependent women exhibited significantly greater craving and anxiety to drug cues that were preceded by yohimbine (Moran-Santa Maria et al., 2014). Thus, stress may enhance the salience of drug cues in cocaine-dependent women. To date, the effects of endogenous progesterone on reactivity to drug cues that are preceded by a stressor have yet to be explored in a clinical population. The aim of this study was to assess the impact of endogenous progesterone on reactivity to yohimbine and drug cues in cocaine-dependent women. Yohimbine was used as it is a reliable pharmacological stressor in both human and animal models. We hypothesized that cocaine-dependent women with high progesterone levels would exhibit lower reactivity to yohimbine and the drug cue than cocaine-dependent women with low progesterone levels.
2.0 Methods
2.1 Participants
This study is a secondary analysis of a larger study designed to determine if cocaine-dependent subjects would have altered craving following yohimbine administration and drug cues as compared to placebo (Moran-Santa Maria et al., 2014). Only cocaine-dependent women were included in the present analysis. Cocaine-dependent women were recruited via advertisements over a 48-month period. Written informed consent was obtained from each participant before the study assessments were administered. All procedures were conducted in accordance with Good Clinical Practice Guidelines and the Declaration of Helsinki, and received Institutional Review Board (IRB) approval. Inclusion criterion included (1) Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) criteria for cocaine-dependence in the 90-days prior to the study. Exclusion criterion included (1) DSM-IV criteria for substance dependence except caffeine, nicotine, alcohol or marijuana within the past 60 days; (2) pregnancy, nursing, or ineffectual means of birth control; (3) premenstrual dysphoric disorder; (4) history/current hematological, endocrine, cardiovascular, pulmonary, renal, gastrointestinal, or neurological diseases; (5) history/current psychotic, panic, eating, or bipolar affective disorders; (6) current depression or PTSD; (7) history/current medical diseases that could affect HPA axis hormones; (8) synthetic glucocorticoid or exogenous steroid therapy within 30 days of testing; (9) psychotropic medications (with the exception of selective serotonin reuptake inhibitors), opiates or opiate antagonists, benzodiazepines, antipsychotics, b-blockers and other medications that might interfere with HPA axis hormones; (10) acute illness or fever; (11) body mass index > 35; and (12) aversion or inability to remain abstinence from alcohol and other drugs of abuse (except nicotine) for three days prior to the study procedures.
2.2 Assessment
Participants meeting pre-screening criteria were evaluated for study eligibility with the Mini-International Neuropsychiatric Interview (MINI) (Sheehan et al. 1998). The substance use module of the Structured Clinical Interview for DSM-IV (SCID-IV) was used to assess current and lifetime substance use disorders (First et al. 1994). Substance use in the ninety days prior to the study was assessed using the Time-Line Follow-Back (Sobell and Sobell 1992). A medical history and physical examination were completed to assess for medical exclusions. Participants meeting inclusion criteria and no exclusion criteria were scheduled to complete the study procedures.
2.3 Study Procedures
Subjects participated in two cue reactivity sessions on consecutive days. On day 1 of testing, participants arrived at the Medical University of South Carolina’s Clinical and Translational Research Center (CTRC) at 10:00 a.m. Upon arrival, urine pregnancy tests were administered. All participants were practicing some form of non-hormonal birth control (e.g. condoms, barriers, abstinence) throughout the study. Smokers were provided with a nicotine patch. Self-reports, urine drug screens (Roche Diagnostics, Indianapolis, Indiana), and breathalyzer tests (AlcoSensor III, Intoximeters, Inc., St. Louis, Missouri) were used to assess abstinence. If the pregnancy and drug tests were negative (with the exception of THC), study procedures continued.
The study utilized a double-blind placebo-controlled design. At 11:00 a.m. on the morning of the first study visit, a blood sample was collected. This blood sample was used to assay ovarian hormone levels (see assays below). Salivary cortisol and dehydroepiandrosterone (DHEA) samples were also collected at 11:00 a.m. using the passive drool method (Salimetrics, LLC, State College, PA). One hour later (12:00 p.m.), participants received either yohimbine or a matching placebo capsule (see medication administration below). Study participants were provided with a standard lunch and were seated in a CTRC testing room where they were allowed to read until the testing procedures began. Subjective and endocrine response data were collected at 1:40 p.m. and again at 1:55 p.m. The cue reactivity session began at 2:00. The session started with a scripted imagery exercise. Briefly, the participants were instructed to release any tension in the shoulders, back and neck and to breathe deeply. Afterwards, the participants were asked to remember a time when they were using cocaine, and to recall as much detail as possible including their physical response (increased heart rate and breathing) and the “rush” at the first hit of cocaine. Participants were then asked to view and handle cocaine cues. For those who use crack cocaine, this consisted of a small bag of simulated crack cocaine, a crack pipe, a lighter, and a $20 bill. For powder or intravenous users, cues consisted of simulated cocaine, a mirror, a razor, and a $20 bill. Participants examined and handled the cues for two-minutes. Afterwards, the subjects watched a five-minute film depicting cocaine use. This combination of “in-vivo” cues and cocaine use depiction has produced significant craving and physiologic activity in previous studies (Coffey et al. 2002; Saladin et al. 2003). Data were collected immediately after the cues, and again 5, 30-, and 60-minutes post-cue exposure. Participants were then escorted to a private room in the Medical University Hospital where they spent the night. The next day, participants returned to the CTRC and repeated the study procedures described above in the other study condition (i.e. yohimbine or placebo). Participants then spent a second night in the hospital. The following morning, the participants were debriefed, compensated and discharged from the CTRC.
2.4 Medication Dosing and Administration
Participants received yohimbine (Spectrum Laboratories, Inc., New Brunswick, New Jersey) 21.6 mg and a matching placebo capsule in randomized counterbalanced order. Medication administration occurred under the observation of research personnel to assure ingestion. The MUSC Investigational Drug Service compounded the yohimbine and placebo capsules and managed the study randomization procedures. Half of the participants were randomized to yohimbine on day 1 and half to placebo. The dose of yohimbine and timing of administration were selected based on previous studies (O’Carroll et al. 1999; Swann et al. 2005).
2.5 Assays
Salivary cortisol and DHEA levels were measured by enzyme-linked immunosorbent assay (ELISA) using commercially available kits (Salimetrics, LLC, State College, PA). The DHEA ELISA kit has a lower sensitivity limit of 5 pg/ml. The cortisol ELISA kit has a lower sensitivity level of <0.003 μg/dl. For progesterone measurements, plasma samples were analyzed using a 125I Progesterone radioimmunoassay Kit (MP Biomedicals, Santa Ana, CA). The average limit of detection was 0.02 ng/ml. Hormone levels below the detectable limit were set to the individual detection limits of each assay. Sample values were calculated from known standards run within each assay, with an average intra-assay variation (%CV) of 11.9%. Inter-assay variation was 12.0%. For measurement of total estrogens [combined concentrations of unconjugated estradiol-17β (E2) and estrone (E1)], plasma samples were analyzed using a 125I Total Estrogens radioimmunoassay Kit (MP Biomedicals, Santa Ana, CA). The average limit of detection was 0.5 pg/ml. Hormone levels below the detectable limit were set to the individual detection limits of each assay. Sample values were calculated from known standards run within each assay, with an average intra-assay variation (%CV) of 5.7%. Inter-assay variation was 10.9%.
2.6 Subjective Responses
A modified version of the Within Session Rating Scale (WSRS; Childress et al. 1986) was used to assess subjective craving and anxiety response at each assessment timepoint during the procedure (pre-cue, 0-, 5-, 30-, and 60-minutes post-cue). This 0 to 10 visual analogue scale is anchored with the adjectival modifiers (0 = “not at all” to 10 = “extremely”) and includes items that assess craving, anxiety, distress, and mood. Craving and anxiety scores were based on single-item participant responses to the questions “Do you crave cocaine?” and “How anxious do you feel?”, respectively.
2.7 Analysis
Participants with plasma progesterone levels of <4 ng/ml were categorized into the “low” progesterone group (n=16; range 0.15–2.0 ng/ml). Participants with plasma progesterone levels of ≥ 4 ng/ml were placed into the “high” progesterone group (n=9; range 4.0–26.0 ng/ml). Plasma progesterone levels of ≤ 2.0 ng/ml and 3–31 ng/ml have been used to predict the follicular and luteal phases respectively (Kletzky et al., 1975; Sinha et al., 2007; Sofuoglu et al., 1999). To verify homogeneity of groups at pre-treatment baseline, a Wilcoxon rank sum test was performed on continuous demographic and clinical measures; for categorical demographic and clinical measures, a Pearson Chi-square test was applied (Fisher’s exact test was performed when appropriate). To assess the impact of progesterone level on neuroendocrine and subjective measures of stress and craving following cue presentation, linear mixed effects models using restricted maximum likelihood estimation were developed using all serially measured time points following the administration of the study drug (yohimbine/placebo). Preliminary models contain progesterone group, study drug, time, and all appropriate interactions. Cortisol and DHEA levels were collected prior to the administration of yohimbine or placebo and used as covariates in the analysis models. To achieve residual normality, the endocrine outcomes were log10 transformed. All comparisons and statistical analysis were adjusted for baseline levels where available. The order of drug administration was included as a covariate in each of the models, however, no effects of the order of study drug administration were determined to significantly affect the parameter or variance estimates. Model based estimates were used to test all planned hypothesis. Pairwise comparisons between progesterone level groups were assessed for differential response to the presentation of the cue as well as possible interaction effects using all post cue time points (adjusted for pre-cue drug response level). Additionally, 8 participants in the low progesterone group were determined to have abnormal menstrual cycles (i.e. post-menopausal, hysterectomy); a secondary analysis was performed to test the durability of results in a normally cycling sub-population of cocaine dependent females. All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). Significance was assessed at α= 0.05 and no adjustment for multiple testing was applied to reported p-values.
3.0 Results
3.1 Participant Characteristics
Baseline demographics and clinical measures are presented in Table 1. The average age of the study group was 42.1 (SD=±9.5) years and 44% were Caucasian. There were no significant differences in age, race, employment status, smoking status, days since last cocaine use, past treatment for cocaine dependence, or total years of cocaine use between the high and low progesterone groups.
Table 1.
Demographic Characteristics and Pre-Cue Outcome Levels
| Characteristic | Cocaine Subjects
|
||
|---|---|---|---|
| All Subjects (n=25) | Low Progesterone (n=16) | High Progesterone (n=9) | |
| Age | 42.1 ± 9.5 | 43.8 ± 10.4 | 39.0 ± 7.1 |
| Caucasian %(n) | 43.5 (10) | 46.7 (7) | 37.5 (3) |
| Never Married %(n) | 47.8 (11) | 46.7 (7) | 50.0 (4) |
| Unemployed (non-student) %(n) | 78.3 (18) | 66.7 (10) | 100 (8) |
| Smoker %(n) | 91.3 (21) | 93.3 (14) | 87.5 (7) |
| Treatment in the Past1 %(n) | 36.0 (9) | 31.3 (5) | 44.4 (4) |
| Total Years of Use3 | 12.9 ± 7.7 | 13.4 ± 8.9 | 11.4 ± 4.0 |
| Days Since Last Use3 | 15.4 ± 7.6 | 17.1 ± 11.4 | 11.9 ± 10.0 |
| Estradiol | 194 ± 102 | 175 ± 105 | 229 ± 93 |
| Progesterone | 6.3 ± 8.7 | 0.7 ± 1.0 | 16.2 ± 7.1* |
| Pre Administration Levels2 | |||
| Cortisol (ng/ml) | 5.7 ± 2.6 | 5.6 ± 2.6 | 5.9 ± 2.7 |
| DHEA (ng/ml) | 0.6 ± 0.4 | 0.6 ± 0.4 | 0.7 ± 0.4 |
| Post Placebo Levels4 | |||
| Craving | 1.8 ± 2.1 | 2.1 ± 2.2 | 1.3 ± 2.0 |
| Anxiety | 1.4 ± 1.7 | 1.6 ± 1.5 | 1.1 ± 2.0 |
| Cortisol (ng/ml) | 4.7 ± 2.4 | 5.1 ± 2.9 | 4.0 ± 1.4 |
| DHEA (ng/ml) | 0.6 ± 0.4 | 0.6 ± 0.3 | 0.7 ± 0.5 |
| Post Yohimbine Levels4 | |||
| Craving | 2.6 ± 1.9 | 3.3 ± 1.9 | 1.6 ± 1.4* |
| Anxiety | 2.1 ± 1.9 | 2.7 ± 1.8 | 1.2 ± 1.8 |
| Cortisol (ng/ml) | 9.0 ± 6.1 | 9.1 ± 6.7 | 8.8 ± 5.3 |
| DHEA (ng/ml) | 0.7 ± 0.4 | 0.8 ± 0.3 | 0.7 ± 0.4 |
Data are presented as means and associated standard deviations or % (n).
p <0.05 as compared to Low Progesterone subjects; Wilcoxon Rank Sums Test
treatment for cocaine abuse or dependence
pre-administration levels taken prior to administration of study drug on the 1st day of cue exposure
Total years of use and days since last use available on 21 of 25 participants
Measurements taken following administration of study drug or placebo prior to presentation of the cue.
3.2 Medication Responses Prior to the Cue
Following medication administration yet prior to cue presentation, both subjective and endocrine responses were assessed and compared between progesterone groups (Table 1). Following administration of the placebo drug, there were no significant differences between high and low progesterone groups in the self-reported levels of anxiety (F1,23=0.5; p=0.507) or craving (F1,23=0.9; p=0.342) or measured levels of cortisol (F1,20=1.0; p=0.336) or DHEA (F1,20=0.3; p=0.604). Following administration of yohimbine, women in the high progesterone group reported significantly lower subjective craving and nominally, although non-significantly, lower anxiety than women in the low progesterone group (F1,23=5.2; p=0.032 and F1,23=3.7; p=0.066; respectively). Neither cortisol or DHEA were significantly different following administration of yohimbine between progesterone groups (F1,19=0.0; p=0.901 and F1,20=0.1; p=0.781; respectively)
3.3 Subjective Responses to the Cue
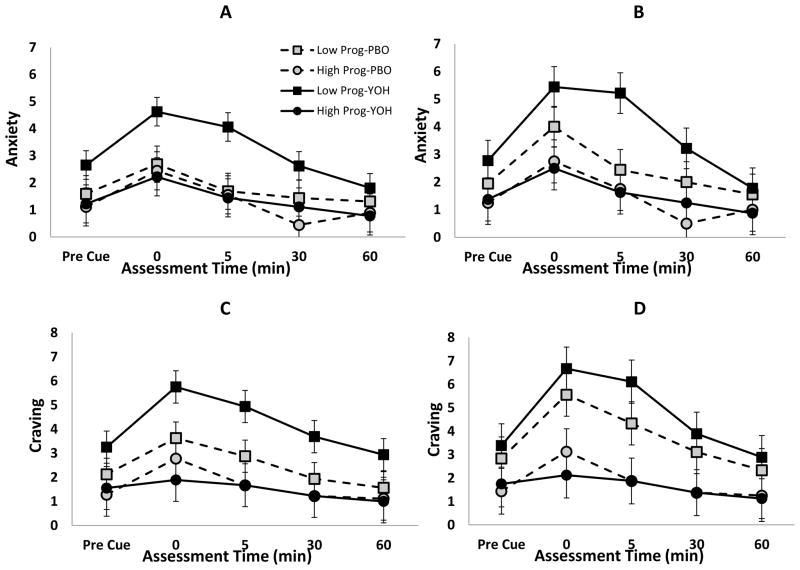
The presentation of the cocaine cue produced a significant anxiety response in both high and low progesterone groups (Cue Response: High Progesterone Δ=1.2±0.5, t92=2.2, p=0.034 and Low Progesterone Δ=1.5±0.4, t92=3.8, p<0.001) as well as under both yohimbine and placebo conditions (Cue Response: Yohimbine Δ=1.5±0.5, t92=3.1, p=0.003 and Placebo Δ=1.2±0.5, t92=2.5, p=0.013; Figure 1A). Anxiety response between those with high and low progesterone levels was significantly different under the yohimbine condition as compared to placebo (Progesterone x Treatment interaction; F1,23=9.8, p=0.005); indicating that progesterone level modified the anxiety response to the cue differentially in the two treatment conditions. Under the yohimbine condition, those with increased progesterone levels had an attenuated anxiety post-cue response profile as compared to those with low progesterone levels (Low Progesterone – High Progesterone Δ=1.8±0.6, t23=2.8, p=0.009). However, under the placebo condition, there was no difference in anxiety response between those with high or low progesterone (Low Progesterone – High Progesterone Δ=0.5±0.6, t23=0.7, p=0.482). Following removal of participants with abnormal menstrual cycles, the anxiety response between those with high and low progesterone levels remained significantly different under the yohimbine condition as compared to placebo (Progesterone x Treatment interaction; F1,15=5.3, p=0.036; Figure 1B).
Figure 1.
Self-reported Anxiety and Craving following pre-treatment with yohimbine (YOH) or placebo (PBO) in the entire cohort of cocaine dependent women (A, C) as well as the sub-cohort of normally cycling cocaine dependent women (B, D); Pre-cue = immediately prior; post = (0, 5, 30, 60) minutes following completion of cue exposure.
Under the yohimbine condition, the response to the cue elicited a significant increase in craving for those with low progesterone (Cue Response: Δ=2.5±0.7, t92=3.8, p<0.001; Figure 1C), but the response was attenuated and non-significant in those with high progesterone (Cue Response: Δ=0.3±0.9, t92=0.4, p=0.705). The increased craving response to the cue was consistent for both high and low progesterone participants under the placebo condition (Cue Response: High Progesterone Δ=1.5±0.9, t92=1.7, p=0.091 and Low Progesterone Δ=1.5±0.7, t92=2.3, p=0.025). Craving response between those with high and low progesterone levels was significantly different under the yohimbine condition as compared to placebo (Progesterone x Treatment interaction; F1,23=13.9, p=0.001); indicating that progesterone level modified the craving response to the cue differentially in the two treatment conditions. Similar to the reported anxiety levels, those with increased progesterone levels had an attenuated craving profile as compared to those with low progesterone levels (Low Progesterone – High Progesterone Δ=2.6±0.9, t23=3.0, p=0.006) under the yohimbine condition. Following removal of participants with abnormal menstrual cycles, the craving response between those with high and low progesterone levels became statistically non-significant as compared to the full sample under the yohimbine condition as compared to placebo (Progesterone x Treatment interaction; F1,15=3.5, p=0.081; Figure 1D).
3.4 Endocrine Responses to the Cue
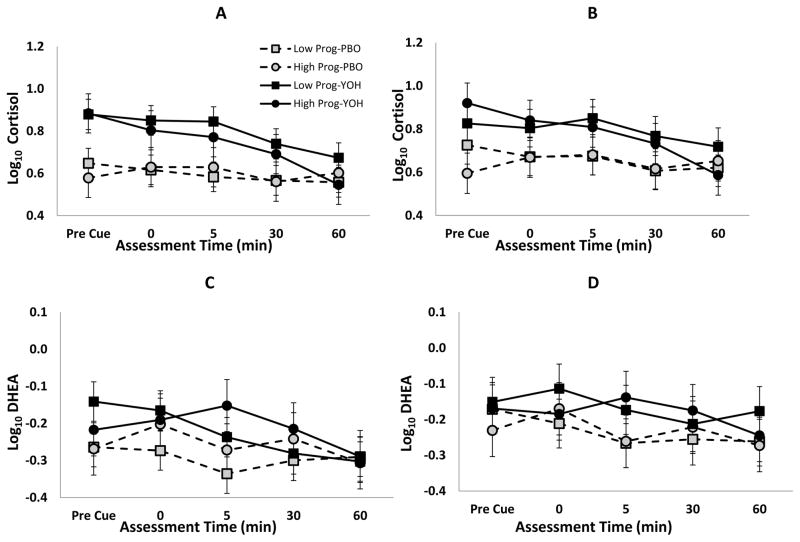
Administration of yohimbine produced significant increases in salivary cortisol levels as compared to placebo (Yohimbine – Placebo Δ=0.17±0.02, F1,20=47.0, p<0.001; Figure 2A). However, there was no evidence of increased cortisol upon presentation of the cocaine cue (Cue Response: Δ=0.02±0.04, t80=0.6, p=0.574) nor were there any differential responses between high and low progesterone across the two treatment conditions (Progesterone x Treatment interaction; F1,20=1.5, p=0.236). In the sub-sample of participants with normal menstrual cycles, the cortisol increase in response to the administration of yohimbine as compared to placebo remained significant (F1,13=19.5, p=0.001; Figure 2B). Similarly, under the yohimbine condition, DHEA levels were slightly increased as compared to placebo (Yohimbine – Placebo Δ=0.06±0.02, t20=2.6, p=0.016; Figure 2C) and no significant response to the cue was seen (Cue Response Δ=−0.02±0.03, t79=−0.5, p=0.651). DHEA levels also did not show any indication of any differential responses between high and low progesterone across the two treatment conditions (Progesterone x Treatment interaction; F1,20=0.3, p=0.609); indicating that progesterone level did not modify the DHEA response to the cue differentially in the two treatment conditions. Although attenuated, the normally cycling sub-group had similar DHEA differences between yohimbine and placebo (F1,13=4.5, p=0.054; Figure 2D).
Figure 2.
Cortisol and DHEA following pre-treatment with yohimbine (YOH) or placebo (PBO) in the entire cohort of cocaine dependent women (A, C) as well as the sub-cohort of normally cycling cocaine dependent women (B, D); Pre-cue = immediately prior; post = (0, 5, 30, 60) minutes following completion of cue exposure.
4.0 Discussion
In this study, we found an association between endogenous progesterone and subjective responses to a drug cue that was preceded by the pharmacological stressor yohimbine. There was no effect of endogenous progesterone on cortisol or DHEA responses to yohimbine and the drug cue. These data are in support of a growing literature demonstrating protective effects of progesterone on subjective responses to triggers of relapse in cocaine-dependent women.
Previously we found that cocaine-dependent women reported greater subjective anxiety in response to yohimbine and a drug cue than cocaine-dependent men (Moran-Santa Maria et al., 2014). In this secondary analysis, cocaine-dependent women with high levels of progesterone reported lower anxiety in response to yohimbine and the drug cue than cocaine-dependent women with low levels of progesterone. Progesterone and its metabolites are potent modulators of the GABAA receptor (Brot et al., 1997; Poisbeau et al., 1997) and produce anxiolytic effects in animal models of anxiety (Bitran et al., 1991; Frye et al., 2006; Picazo et al., 1995). The progesterone metabolite, allopregnenalone, in particular, has been shown to attenuate yohimbine-induced cocaine reinstatement in female rats, but not males (Anker & Carroll, 2010). Data from previous studies of cocaine-dependent women demonstrate that progesterone reduces negative affective responses to drug cues (Fox et al., 2013; Sinha et al., 2007). The present findings suggest that progesterone may also be effective at reducing the anxiogenic effects of drug cues during periods of stress.
Cocaine-dependent women with high levels of progesterone reported lower cue-related craving following the yohimbine challenge than cocaine-dependent women with low progesterone levels. These data are in agreement with the literature demonstrating protective effects of progesterone on cocaine craving in women (Fox et al., 2013; Sinha et al., 2007). Of note, a preclinical study that used yohimbine as a stressor, found that stress potentiation of cue-induced cocaine-seeking behavior was greater during proestrus as compared to all other phases of the estrous cycle (Feltenstein et al., 2011). Proestrus is characterized by high progesterone and estrogen levels (Freeman, 1994). Without direct measures of ovarian hormones, it is difficult to discern the role that progesterone played in the aforementioned findings. It was surprising that we found no effect of progesterone on cue induced craving in the placebo condition. Other clinical studies have found that progesterone attenuates craving in cocaine-dependent women during autobiographical recall of cocaine cues (Fox et al., 2013; Sinha et al., 2007). These disparate findings may be related to differences in experimental design (autobiographical recall versus in vivo cues). It is also possible that differences in estrogen levels may explain these inconsistent study results. Estrogen levels in our study appear to be significantly greater that the estrogen levels observed in Sinha’s study (229 pg/ml versus 69.1 pg/ml). Thus, in the present study higher levels of estrogen may have blocked the protective effects of progesterone on craving that we had anticipated to find under the placebo condition. Given the unique and interactive effects of sex hormones on addictive behaviors and stress responsivity, careful methodological consideration of menstrual phase and hormone levels is critical (see Allen et al., 2016 for review).
There was no effect of progesterone on salivary cortisol or DHEA responses to yohimbine and the drug cue. In addition, both groups exhibited similar endocrine profiles under the placebo condition. Studies of healthy controls demonstrate enhanced glucocorticoid responses to stress during the luteal phase compared to the follicular phase of the menstrual cycle (Kajantie et al., 2006; Kirschbaum et al., 1999; Tersman et al., 1991). Given these data, we had expected that cocaine-dependent women with high levels of progesterone would exhibit lower salivary cortisol responses to the tasks than cocaine-dependent women with low levels of progesterone. Preclinical data suggests that drug use increases corticosteroid binding globulin levels (Nock et al., 1997). Thus, the lack of a difference in glucocorticoid responses in the present study suggests that drug use may alter the regulation of free salivary cortisol levels under conditions of duress.
There are limitations to the present study. This was a secondary analysis of a larger study and the sample size was relatively small, which may have precluded us from finding statistically significant group differences and interactions. In addition, we cannot entirely rule out age as a contributing factor as eight participants in the low progesterone group were post-menopausal or had hysterectomies. That said, durability analysis largely maintained the initial results, with the interaction effect for craving showing a trend, rather than full statistical significance. However, irrespective of etiology (whether age-related or due to individual differences), low endogenous progesterone may increase stress reactivity and drug craving. Lastly, drug abstinence affects subjective responses to drug cues and stress (Fox et al., 2005). However, there were no differences between the groups in number of days abstinent prior to the study visit.
Despite these limitations this is the first study to our knowledge to examine the effects of endogenous progesterone on responses to stress coupled with a drug cue in a clinical population of cocaine-dependent women. Importantly, stress is likely to precede cue-exposure in real-life. Thus, understanding how progesterone affects responses to multiple triggers of relapse may have important treatment implications for cocaine-dependent women. For example, women may be at greater risk for stress-induced relapse during the follicular phase when endogenous progesterone is low, or may demonstrate increased abstinence initiation during the luteal phase when progesterone is high. Translational research on exogenous progesterone administration in cocaine-dependent women has also shown promise and warrants further investigation.
Highlights.
We examined the effect of endogenous progesterone on reactivity following a stressor
High-progesterone cocaine-dependent women showed attenuated subjective reactivity
Progesterone may protect against stress-induced relapse in cocaine-dependent women
Acknowledgments
Funding Sources: This work was supported by the National Institutes of Health [NIDA Grants P50DA016511, K24DA038240]
Footnotes
Disclosure Statement: All authors declare they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen AM, McRae-Clark AL, Carlson S, Saladin ME, Gray KM, Wetherington CL, McKee SA, Allen SS. Determining menstrual phase in human biobehavioral research: A review with recommendations. Exp Clin Psychopharmacol. 2016;24(1):1–11. doi: 10.1037/pha0000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglin MD, Hser YI, McGlothlin WH. Sex differences in addict careers. 2. Becoming addicted. Am J Drug Alcohol Abuse. 1987;13(1–2):59–71. doi: 10.3109/00952998709001500. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Sex differences in the effects of allopregnanolone on yohimbine-induced reinstatement of cocaine seeking in rats. Drug Alcohol Depend. 2010;107(2–3):264–7. doi: 10.1016/j.drugalcdep.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Larson EB, Gliddon LA, Carroll ME. Effects of progesterone on the reinstatement of cocaine-seeking behavior in female rats. Exp Clin Psychopharmacol. 2007;15(5):472–480. doi: 10.1037/1064-1297.15.5.472. [DOI] [PubMed] [Google Scholar]
- Banna KM, Back SE, Do P, See RE. Yohimbine stress potentiates conditioned cue-induced reinstatement of heroin-seeking in rats. Behav Brain Res. 2010;208(1):144–148. doi: 10.1016/j.bbr.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitran D, Hilvers RJ, Kellogg CK. Anxiolytic effects of 3 alpha-hydroxy-5 alpha[beta]-pregnan-20-one: endogenous metabolites of progesterone that are active at the GABAA receptor. Brain Res. 1991;561(1):157–161. doi: 10.1016/0006-8993(91)90761-j. [DOI] [PubMed] [Google Scholar]
- Brady KT, Randall CL. Gender differences in substance use disorders. Psychiatr Clin North Am. 1999;22(2):241–252. doi: 10.1016/s0193-953x(05)70074-5. [DOI] [PubMed] [Google Scholar]
- Brot MD, Akwa Y, Purdy RH, Koob GF, Britton KT. The anxiolytic-like effects of the neurosteroid allopregnanolone: interactions with GABA(A) receptors. Eur J Pharmacol. 1997;325(1):1–7. doi: 10.1016/s0014-2999(97)00096-4. [DOI] [PubMed] [Google Scholar]
- Buffalari DM, See RE. Footshock stress potentiates cue-induced cocaine-seeking in an animal model of relapse. Physiol Behav. 2009;98(5):614–617. doi: 10.1016/j.physbeh.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, McLellan AT, O’Brien CP. Conditioned responses in a methadone population. A comparison of laboratory, clinic, and natural settings. Journal of Substance Abuse Treatment. 1986;3(3):173–179. doi: 10.1016/0740-5472(86)90018-8. [DOI] [PubMed] [Google Scholar]
- Coffey SF, Saladin ME, Drobes DJ, Brady KT, Dansky BS, Kilpatrick DG. Trauma and substance cue reactivity in individuals with comorbid posttraumatic stress disorder and cocaine or alcohol dependence. Drug Alcohol Depend. 2002;65(2):115–127. doi: 10.1016/s0376-8716(01)00157-0. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 2002;159(4):397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Henderson AR, See RE. Enhancement of cue-induced reinstatement of cocaine-seeking in rats by yohimbine: sex differences and the role of the estrous cycle. Psychopharmacology (Berl) 2011;216(1):53–62. doi: 10.1007/s00213-011-2187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug Alcohol Depend. 2007;89(2–3):183–189. doi: 10.1016/j.drugalcdep.2006.12.017. S0376-8716(06)00468-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Sofuoglu M, Morgan PT, Tuit KL, Sinha R. The effects of exogenous progesterone on drug craving and stress arousal in cocaine dependence: impact of gender and cue type. Psychoneuroendocrinology. 2013;38(9):1532–1544. doi: 10.1016/j.psyneuen.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Talih M, Malison R, Anderson GM, Kreek MJ, Sinha R. Frequency of recent cocaine and alcohol use affects drug craving and associated responses to stress and drug-related cues. Psychoneuroendocrinology. 2005;30(9):880–891. doi: 10.1016/j.psyneuen.2005.05.002. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I disorders, patient edition. SCIP-I/P. New York: Biometrics Research, New York State Psychiatric Institute; 1994. [Google Scholar]
- Freeman ME. The neuroendocrine control of the ovarian cycle of the rat. In: Knobil E, Neill JD, editors. The physiology of reproduction. 2. New York, NY: Raven Press; 1994. pp. 613–658. [Google Scholar]
- Frye CA, Rhodes ME. Infusions of 5alpha-pregnan-3alpha-ol-20-one (3alpha,5alpha-THP) to the ventral tegmental area, but not the substantia nigra, enhance exploratory, anti-anxiety, social and sexual behaviours and concomitantly increase 3alpha,5alpha-THP concentrations in the hippocampus, diencephalon and cortex of ovariectomised oestrogen-primed rats. J Neuroendocrinol. 2006;18(12):960–975. doi: 10.1111/j.1365-2826.2006.01494.x. [DOI] [PubMed] [Google Scholar]
- Griffin ML, Weiss RD, Mirin SM, Lange U. A comparison of male and female cocaine abusers. Arch Gen Psychiatry. 1989;46(2):122–126. doi: 10.1001/archpsyc.1989.01810020024005. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Rounsaville BJ, Kranzler HR. Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend. 2004;74(3):265–272. doi: 10.1016/j.drugalcdep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Justice AJ, de Wit H. Acute effects of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 1999;145(1):67–75. doi: 10.1007/s002130051033. [DOI] [PubMed] [Google Scholar]
- Justice AJ, de Wit H. Acute effects of estradiol pretreatment on the response to d-amphetamine in women. Neuroendocrinology. 2000;71(1):51–59. doi: 10.1159/000054520. 54520. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31(2):151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61(2):154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kletzky OA, Nakamura RM, Thorneycroft IH, Mishell DR., Jr Log normal distribution of gonadotropins and ovarian steroid values in the normal menstrual cycle. Am J Obstet Gynecol. 1975;121(5):688–694. doi: 10.1016/0002-9378(75)90474-3. [DOI] [PubMed] [Google Scholar]
- Larson EB, Anker JJ, Gliddon LA, Fons KS, Carroll ME. Effects of estrogen and progesterone on the escalation of cocaine self-administration in female rats during extended access. Exp Clin Psychopharmacol. 2007;15(5):461–471. doi: 10.1037/1064-1297.15.5.461. [DOI] [PubMed] [Google Scholar]
- Larson EB, Roth ME, Anker JJ, Carroll ME. Effect of short- vs. long-term estrogen on reinstatement of cocaine-seeking behavior in female rats. Pharmacol Biochem Behav. 2005;82(1):98–108. doi: 10.1016/j.pbb.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Lile JA, Kendall SL, Babalonis S, Martin CA, Kelly TH. Evaluation of estradiol administration on the discriminative-stimulus and subject-rated effects of d-amphetamine in healthy pre-menopausal women. Pharmacol Biochem Behav. 2007;87(2):258–266. doi: 10.1016/j.pbb.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22(18):7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran-Santa Maria MM, McRae-Clark A, Baker NL, Ramakrishnan V, Brady KT. Yohimbine administration and cue-reactivity in cocaine-dependent individuals. Psychopharmacology (Berl) 2014 doi: 10.1007/s00213-014-3555-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najavits LM, Lester KM. Gender differences in cocaine dependence. Drug Alcohol Depend. 2008;97(1–2):190–194. doi: 10.1016/j.drugalcdep.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock B, Wich M, Cicero TJ. Chronic exposure to morphine increases corticosteroid-binding globulin. J Pharmacol Exp Ther. 1997;282(3):1262–1268. [PubMed] [Google Scholar]
- O’Carroll RE, Drysdale E, Cahill L, Shajahan P, Ebmeier KP. Stimulation of the noradrenergic system enhances and blockade reduces memory for emotional material in man. Psychol Med. 1999 Sep;29(5):1083–8. doi: 10.1017/s0033291799008703. [DOI] [PubMed] [Google Scholar]
- Picazo O, Fernandez-Guasti A. Anti-anxiety effects of progesterone and some of its reduced metabolites: an evaluation using the burying behavior test. Brain Res. 1995;680(1–2):135–141. doi: 10.1016/0006-8993(95)00254-n. [DOI] [PubMed] [Google Scholar]
- Poisbeau P, Feltz P, Schlichter R. Modulation of GABAA receptor-mediated IPSCs by neuroactive steroids in a rat hypothalamo-hypophyseal coculture model. J Physiol. 1997;500(Pt 2):475–485. doi: 10.1113/jphysiol.1997.sp022034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saladin ME, Drobes DJ, Coffey SF, Dansky BS, Brady KT, Kilpatrick DG. PTSD symptom severity as a predictor of cue-elicited drug craving in victims of violent crime. Addict Behav. 2003;28(9):1611–1629. doi: 10.1016/j.addbeh.2003.08.037. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, … Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- Sinha R, Fox H, Hong KI, Sofuoglu M, Morgan PT, Bergquist KT. Sex steroid hormones, stress response, and drug craving in cocaine-dependent women: implications for relapse susceptibility. Exp Clin Psychopharmacol. 2007;15(5):445–452. doi: 10.1037/1064-1297.15.5.445. [DOI] [PubMed] [Google Scholar]
- Sobell L, Sobell M. Timeline Follow-Back. In: Litten R, Allen J, editors. Measuring Alcohol Consumption. Humana Press; 1992. pp. 41–72. [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol. 1999;7(3):274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Swann AC, Birnbaum D, Jagar AA, Dougherty DM, Moeller FG. Acute yohimbine increases laboratory-measured impulsivity in normal subjects. Biol Psychiatry. 2005;57(10):1209–1211. doi: 10.1016/j.biopsych.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Tersman Z, Collins A, Eneroth P. Cardiovascular responses to psychological and physiological stressors during the menstrual cycle. Psychosom Med. 1991;53(2):185–197. doi: 10.1097/00006842-199103000-00008. [DOI] [PubMed] [Google Scholar]
- Westermeyer J, Boedicker AE. Course, severity, and treatment of substance abuse among women versus men. Am J Drug Alcohol Abuse. 2000;26(4):523–535. doi: 10.1081/ada-100101893. [DOI] [PubMed] [Google Scholar]
- White TL, Justice AJ, de Wit H. Differential subjective effects of D-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav. 2002;73(4):729–741. doi: 10.1016/s0091-3057(02)00818-3. [DOI] [PubMed] [Google Scholar]
- Wong CJ, Badger GJ, Sigmon SC, Higgins ST. Examining possible gender differences among cocaine-dependent outpatients. Exp Clin Psychopharmacol. 2002;10(3):316–323. doi: 10.1037//1064-1297.10.3.316. [DOI] [PubMed] [Google Scholar]
- Yonkers KA, Forray A, Nich C, Carroll KM, Hine C, Merry BC, … Sofuoglu M. Progesterone Reduces Cocaine Use in Postpartum Women with a Cocaine Use Disorder: A Randomized, Double-Blind Study. Lancet Psychiatry. 2014;1(5):360–367. doi: 10.1016/S2215-0366(14)70333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]