Abstract
Proanthocyanidins (PACs) find wide applications for human use including food, cosmetics, dietary supplements, and pharmaceuticals. The chemical complexity associated with PACs has triggered the development of various chromatographic techniques, with countercurrent separation (CCS) gaining in popularity. This study applied the recently developed DESIGNER (Depletion and Enrichment of Select Ingredients Generating Normalized Extract Resources) approach for the selective enrichment of trimeric and tetrameric PACs using centrifugal partition chromatography (CPC). This CPC method aims at developing PAC based biomaterials, particularly for their application in restoring and repairing dental hard tissue. A general separation scheme beginning with the depletion of polymeric PACs, followed by the removal of monomeric flavan-3-ols and a final enrichment step produced PAC trimer and tetramer enriched fractions. A successful application of this separation scheme is demonstrated for the four polyphenol rich plant sources: grape seeds, pine bark, cinnamon bark, and cocoa seeds. Minor modifications to the generic DESIGNER CCS method were sufficient to accommodate the varying chemical complexities of the individual source materials. The step-wise enrichment of PAC trimers and tetramers was monitored using normal phase TLC and Diol-HPLC-UV analyses. CPC proved to be a reliable tool for the selective enrichment of medium size oligomeric PACs (OPACs). This method plays a key role in the development of dental biomaterials considering its reliability and reproducibility, as well as its scale-up capabilities for possible larger-scale manufacturing.
Keywords: Centrifugal partition chromatography, oligomeric proanthocyanidins, diol-HPLC, dental biomaterials, degree of polymerization, enrichment
1 Introduction
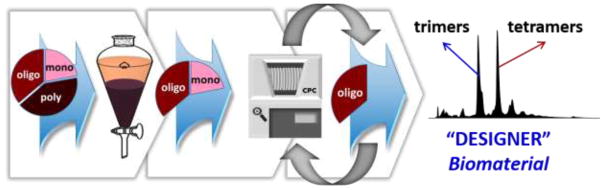
The concept of chemical subtraction entails the selective removal of targeted component(s) from a complex mixture or an extract. It was first developed in 2008 and experimentally demonstrated by the removal of benzoic acid from cranberry juice fractions using countercurrent separation (C S) [1]. Due to the broad applicability of this technique in the field of natural product research, the concept was further developed to encompass both the selective enrichment and/or the depletion of extracts, guided by the intended biological application. The term DESIGNER (Deplete and Enrich Select Ingredients to Generate Normalized Extract Resources) was, thus, coined and the methodology was demonstrated for bioactive prenylated flavonoids from the extract of Humulus lupulus [2]. The current study demonstrates a new application of the DESIGNER methodology for the preparation of proanthocyanidin based biomaterials (Figure 1) that were prepared for dental hard tissue applications.
Figure 1.
Application of the DESIGNER concept for the selective enrichment of PACs. Schematic representation of the methodology used for the preparation of trimer and tetramer enriched DESIGNER material from various proanthocyanidin-enriched plant sources.
Proanthocyanidins (PACs) are oligomers of monomeric flavan-3-ols. PACs are ubiquitously present and structurally complex metabolites that plants synthesize as a predatory defense mechanism. Many food plants such as grape seeds, green tea, mangosteen pericarp, cinnamon bark, cocoa seeds, and cranberries are particularly rich in PACs. The increasing number of reports on the in vivo and in vitro bioactivities of PACs is primarily contingent upon their anti-oxidant and anti-inflammatory activity, as well as their anti-infective potential [3–6]. In fact, most recently, FDA approved the plant based oligomeric PAC (OPAC) mixture obtained from Croton lechleri, FulyzaqR (crofelemer, now sold under the brand name Mytesi™), for the alleviation of HIV-associated diarrhea (FDA FulyzaqR approval) [7,8].
Recent efforts aimed at the development of PAC-based biomaterials have resulted in several chemical separation, optimization, and dentin biomechanical evaluation studies for prolonging the lifespan of composite based dental restorations [9]. Through recently performed in-vitro studies on dentin, it was observed that the OPACs, especially trimers and tetramers, as well as their galloylated analogues, are potent dentin biomodifiers [10–12]. Certain OPACs are capable of structurally modifying dentin via inter-molecular and inter-microfibrillar collagen cross-linking. Optimum molecular size (degree of polymerization) and shape (relative/absolute stereochemistry) of the OPACs are the most important factors for this biomimetic property. Thus, the present study focuses on the application of CPC methodology for the preparation of PAC trimer and tetramer enriched (DESIGNER) biomaterials.
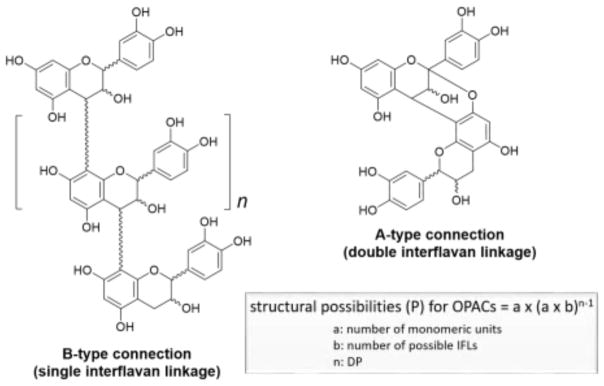
The structural possibilities for OPACs increase exponentially based on the variable monomeric units, the type (A vs. B) and position of the interflavan linkages (IFLs), the degree of polymerization (DP), and the inherent stereochemistry of the monomers (Figure 2). In consequence, separation of OPACs from the already chemically complex plant extracts has been a significant chromatographic challenge, even with the development of advanced analytical techniques [13]. Despite the apparent similarities of the basic OPAC skeletons, their structural differences deceive the untrained human eye in both 2D and 3D molecular representations, but, in fact, the variances are major and give rise to distinct chromatographic fingerprints and biological potential for each plant source. Consequently, specific chromatographic methods need to be adapted according to the starting material, despite the goal of separating the same general class of compounds. The chromatographic elution order of the different classes of PACs and the number of steps required to achieve the desired separation depends on the specific PAC chemistry of each plant source.
Figure 2.
Representative structures of proanthocyanidins and their theoretical structural possibilities based on the monomeric units, linkage types, and degree of polymerization (DP).
While hydrodynamic CCS methods employing HSCCC (high speed countercurrent chromatography) have been used frequently [14–18], their success depends on the specific type of the target molecules. For instance, HSCCC has enabled successful separation of green tea polyphenols that mainly represent mixtures of the flavan-3-ol monomers, catechin, epicatechin, epigallocatechin gallate, and epicatechin gallate, along with galloylated dimers [15]. Separation of grape seed extract by HSCCC yields relatively poor resolution of the dimers and leads to co-elution of all higher oligomers [18]. In fact, as documented in the literature, PAC research hardly extends beyond dimers owing to the increasing complexity of the higher order oligomers with regards to chromatography as well as structural analyses. The current study presents a scheme for preparative separation that yields the desired, most bioactive DP 3+4 cuts from the PAC-rich extracts using source-dependent, minor solvent system modifications and employing CPC methodology. The effect of step-wise enrichment of trimers and tetramers from grape seed and cocoa extract on dentin biomodification is also demonstrated using dentin stiffness as the means of evaluation of biomechanical enhancement.
2 Experimental
2.1 Materials
Grape seed extract (Vitis vinifera, VV) was kindly donated by Polyphenolics MegaNatural Gold Grape Seed Extract, Madera, California, USA (No. 206112508-01/122112505-01). Cocoa extract (Theobroma cacao, TC) was obtained from Barry Callebaut Ltd., NCE- PO804-WW41 [polyphenol extract], Meulan, France, (No. 700900001). Pine bark extract (Pinus massoniana, M) was obtained from Xi’an Chukang Biotechnology Co. Ltd., China (No. PB120212). Cinnamon stem-bark powder (Cinnamomum verum, CV) was purchased from Oregon’s Wild Harvest, Sandy, Oregon, USA (No. CIN-07011p-OMH01) and extracted in house using 70 % acetone, and the extract was dried and lyophilized. All solvents were of analytical grade and obtained from Fisher Scientific (Fair Lawn, NJ, USA) or Sigma-Aldrich (St. Louis, MO, USA).
2.2 Instruments
A centrifugal partition chromatography (CPC) extractor, SCPC-250-B (Armen Instrument Gilson Inc. SAS, France), was used. Diol-HPLC profiles were acquired on a Waters 600 HPLC (Waters, USA) instrument using a Develosil 5 μm Diol 100A 250 × 4.6 mm column (Phenomenex, Torrance, CA, USA) equipped with a Waters 2996 PDA detector.
2.3 Preparation of trimeric and tetrameric PAC enriched fractions
A three-step separation scheme was employed beginning with the depletion for polymeric PACs, followed by a monomer knock-out step and a trimer and tetramer enrichment in the final step (Figure 1). Slight modifications were made for individual methods based on the plant source, level of complexity and type of PAC chemistry/composition, as indicated below. A flow rate of 30 (for TC, PM and CV) or 35 ml/min (for VV) and rotation speed of 2500 rpm was used throughout. All the CPC separations were performed at room temperature without additional temperature control. An analytical Diol HPLC column was used for the monitoring of CPC fractions. The HPLC mobile phase consisted of acetonitrile/acetic acid (98/2) as solvent and methanol/water/acetic acid (95/3/2) as solvent B. solvent gradient beginning at 7 % B for 5 min, increasing up to 37.6 % B from 5–57 min and finally reaching 100 % B at 60 min was used. This was followed by washing and re-equilibration with a total run time of 80 minutes [19].
2.3.1 Grape seed extract DESIGNER fraction
As a first step, crude grape seed extract (VV) was partitioned between methyl acetate (Ma)/water to achieve depletion of polymeric PACs, yielding a polymer knockout (PKO) fraction as described previously [12]. The VV-PKO was then subjected to separation on a CPC extractor (CPC-E) to produce a monomer depleted fraction (MKO), using the solvent system (SS) HEMaWat 0.8/4/1/4 in descending mode. Stationary phase retention (Sf) was 80 % before injection. In step 3, the VV-MKO was subjected to CPC-E, resulting in the depletion of dimeric PACs and enrichment of trimers and tetramers. For this step, HEMaWat 0.4/4/1/4 was used in ascending mode with an Sf of 76 % before injection.
2.3.2 Cocoa extract DESIGNER fraction
Consistent with the step 1 used for VV (2.1.1), solvent partitioning was used for the removal of polymeric PACs from TC extract. Separation via CPC-E in ascending mode using HEMWat 1/10/1/10 was performed to achieve monomer depletion (TC-MKO) from the TC-PKO with an Sf of 80 %. In the final step, the TC-MKO was further separated by CPC-E using HEMaWat 0.4/4/1/4 in ascending mode (Sf = 83 %) to yield the trimer and tetramer enriched product.
2.3.3 Pine extract DESIGNER fraction
Pine extract (PM) was suspended in water and partitioned with ethyl acetate instead of methyl acetate to afford pine polymer depleted extract. The polymer knockout fraction (PM-PKO) was further fractionated by CPC-E using a HEMaWat 2/4/1/4 SS, resulting in a monomer-dimer depleted fraction PM-MDPKO (PM-monomer/dimer/polymer knockout fraction). Fractionation was performed in descending mode (Sf of 45%). Then PM-MDPKO was subjected to the second CPC-E step in ascending mode (Sf = 64%) with a HEMaWat 0.5/4/1/4 SS to afford the trimer and tetramer enriched fraction.
2.3.4 Cinnamon extract DESIGNER fraction
To produce the trimer and tetramer enriched fraction, the 70 % acetone extract of cinnamon bark (CV) was subjected directly to CPC-E using EtOAc/EtOH/water 6/1/5 as the SS in ascending mode (Sf = 60%).
2.3.5 Evaluation of dentin biomechanical property
Enhancement of dentin strength was evaluated in terms of increase in the modulus of elasticity (ME) of demineralized dentin post-sample treatment as described previously [20,21]. Briefly, the dentin portion of human molars were cut into thin beams of 1.7 × 0.5 × 6.0 mm dimension. These dentin beams were demineralized with phosphoric acid, followed by treatment with the extracts and fractions. The modulus of elasticity (a measure of the mechanical strength) was assessed before and after treatment and compared against the control (no treatment) to evaluate the dentin strength enhancement.
3 Results and Discussion
3.1 Trimers and tetramers as PACs with optimum DP
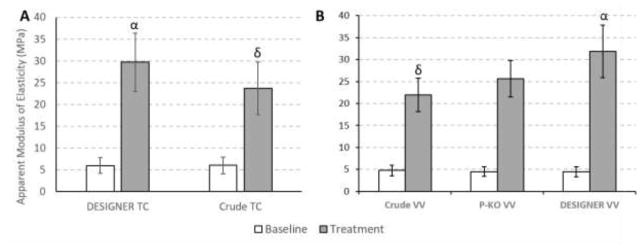
It is commonly reported that the degree of polymerization (DP) of the PACs is one of the primary factors for their biological effects [22–25]. Previous studies have shown that the dentin biomechanical activity increases with increasing DP among the same class of PACs. In particular, OPAC trimers and tetramers are more active than the dimers, which in turn have a better activity than flavanol monomers; however the dentin activity starts plateauing at DP > 4 [12,26]. This suggests that OPCAs with DP 3 to 4 strike the optimum balance between effective cross-linking of dentin-collagen, structural complexity, and associated analytical challenges. In the present study, the effect of the DP on dentin biomechanical activity was demonstrated using enriched fractions from VV and TC. Dentin biomechanical activity, observed as dentin stiffness was measured in terms of the increase in modulus of elasticity (ME) of the demineralized dentin relative to the untreated control (baseline) [9]. It was observed that the dentin bioactivity increased moderately, but significantly, from crude extract to the final trimer and tetramer enriched product with each chromatographic refinement step (Figure 5). The DESIGNER fraction from TC exhibited a 5-fold increase in the ME as compared to the 4-fold increase observed with crude TC. The DESIGNER fraction of VV exhibited a 7.1-fold increase in the ME, as compared to a 5.7-fold increase observed for the polymer KO fraction (step 1) of crude VV and a 4.6-fold increase for the crude VV extract. Additionally, galloylated PACs have higher activity than their non-galloylated counterparts [11]. Furthermore, representing the most potent dentin-active principles, a trimer with one -type and one B-type interflavan linkage (IFL) was identified recently from pine bark extract [10]. This not only supports the general observations regarding the optimum DP of 3 to 4, but also suggests that subtle structural differences caused by the stereochemistry or the positions of the IFLs in PACs can lead to significant differences in their dentin biomechanical effects. Interestingly, these structural differences also affect the chromatographic behavior of the PACs in a source dependent manner. In line with the observations for VV and TC, the trimer and tetramer enriched fractions from PM and CV also exhibited superior dentin enhancing properties compared to the respective crude extracts (data not shown).
Figure 5.
The dentin mechanical bioassay data for VV and TC DESIGNER fractions. Panel A compares the dentin biomechanical activity between the crude extract and the DESIGNER fraction of TC; Panel B compares the dentin biomechanical activity between crude extract, polymer KO fraction, and the DESIGNER fraction of VV. Untreated demineralized dentin beams are used as the negative control in the bioassay. Symbols (α) and (δ) depict statistically significant differences in modulus of elasticity between groups treated with the DESIGNER fraction and Crude extracts (respectively VV with p<0.001 and TC with p = 0.015).
3.2 Preparative separation on centrifugal partition chromatography
PACs are known to adsorb irreversibly on solid supports, especially on silica based materials, causing smearing on the stationary phase, and reducing the column life. In awareness of the chemical complexity of PAC rich extracts, and owing to the exponentially increasing structural possibilities for oligomers, use of solid stationary phases such as Sephadex LH-20 or C18 silica gel has become nearly indispensable for isolation/purification. Hence, CCS is a superior choice for the preparative-scale separation of PACs due to its high reproducibility, near theoretical recovery, and large loading capacity [13,14,16,17,27–29]. Preliminary fractionation using CCS methodology reduces the complexity of the crude material, thus lowering the chromatographic load on the solid phases in subsequent steps. In case of PACs, the partition of crude mixtures into different DP-based classes is preferred over the multistep isolation of single chemical (O)PAC entities. This enrichment approach is more practical for biomedical applications, and makes a strong case for the application of CCS as a separation method.
The first step in any CCS method development is the choice of an appropriate two-phase SS. Separation of VV using HEMWat 1/10/1/10 has been reported previously [12]. Methyl acetate was employed as one of the solvents as a means of enhancing the resolution between the galloylated and non-galloylated dimeric PACs. However, the method did not result in sufficient resolution between the non-galloylated dimers, trimer, tetramers, and higher order oligomers. Hence, modified SSs in which the methanol contained in the traditional HEMWat systems was replaced with methyl acetate (Ma) were employed. SSs such as EMaWat 3/2/4, HEMaWat 0.5/4/1/4, and HEMaWat 0.8/4/1/4 were short-listed based on the distribution of compounds between the two phases and were evaluated by partition experiments and TLC. SSs were then optimized based on actual CPC fractionation results.
Once a desired CCS SS is selected, one other crucial factor affecting the resolution is the stationary phase retention (Sf). Oligomeric PACs (OPACs) are highly hydrophilic compounds and, hence, require SSs of high polarity (e.g., HEMWat + 7 or even more polar). Due to the relatively low density difference between the upper and lower phases, and the resulting longer settling times, these SSs are retained poorly on hydrodynamic CCS instruments such as the HSCCC, as per our observations. Thus, CPC representing a hydrostatic CCS system was chosen. CPC also has advantages in terms of increased sample loads and flow rates, without decline in stationary phase retention. This allows for more rapid preparative separations, which was one requirement resulting from the general goal of this dentistry/pharmacy inter-disciplinary study aimed at developing intervention materials for clinical applications. To this end, the ability of the Armen CPC-E instrument to finish one cycle of elution-extrusion in less than an hour, frequently as short as 30 mins, depending on the K-values, was a major advantage.
3.3 Chemistry dictates chromatographic behavior
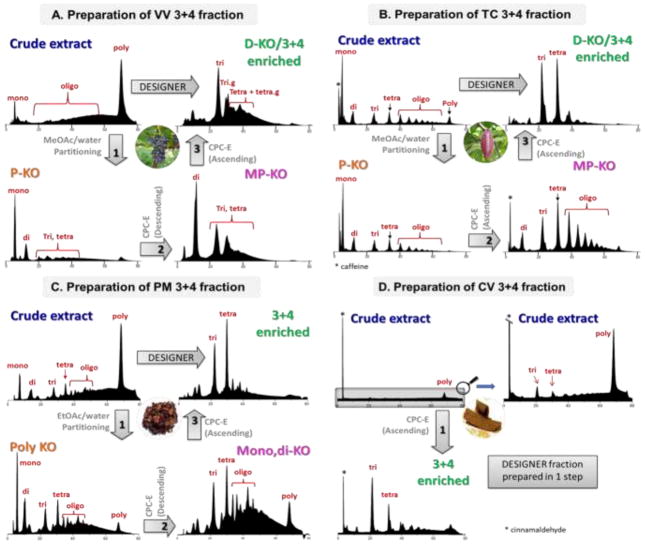
As mentioned in section 3.1, each plant source has its individual (O)PAC profile. Hence, the general separation scheme had to be modified slightly to suit each sample. For instance, both VV and TC primarily contain B-type OPACs (Figure 2), while VV has a much higher polymeric PAC content (Figure 3) compared to TC. This can be seen from the diol-HPLC profiles of crude VV and TC, where the VV chromatogram is dominated by the large polymer peak at 80 mins (Figure 3). Additionally, VV also consists of galloylated OPACs, which further increase its chemical complexity. The elution order for VV PACs is (DPgalloylation) 1, 1g, 2, 2g, 3, 3g, 4…n/ng as opposed to 1, 2, 3, 4, 5…n for TC. The presence of galloylated PACs further reduces the chromatographic resolution. Replacing methanol in the classical HEMWat SSs with methyl acetate (Ma) gave a much improved separation.
Figure 3.
HPLC-diol profiles demonstrating the gradual enrichment of trimers and tetramers. (A) VV, (B) TC, (C) PM, and (D) CV. Diol stationary phase gives separation according to the degree of polymerization (DP). In general, DP of the PACs increases with increasing retention time.
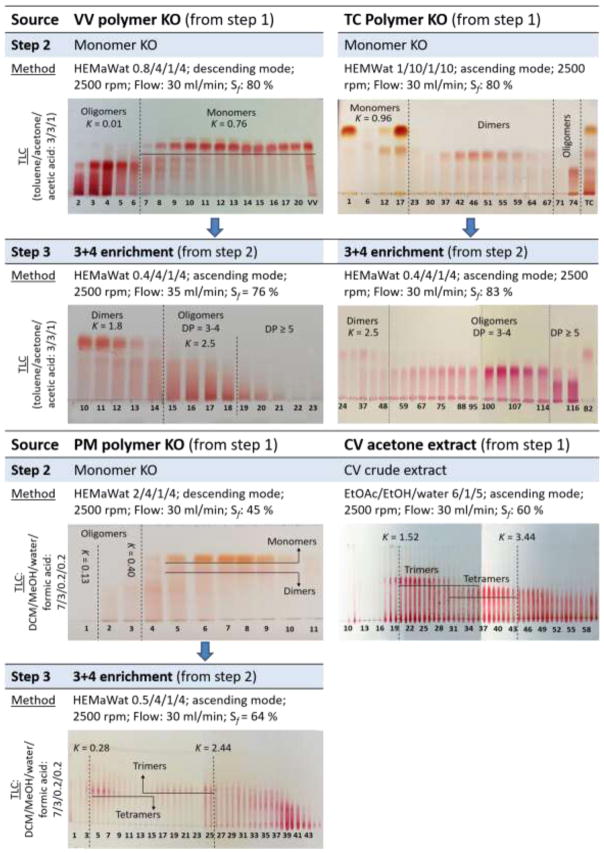
Accordingly, the HEMaWat systems 0.8/4/1/4 and 0.4/4/1/4 were used sequentially in descending and ascending modes, respectively, to achieve trimer and tetramer enrichment of VV PACs. During the monomer KO step (step 2, Figure 4) using HEMaWat0.8/4/1/4 in descending mode, the oligomers eluted right after the void volume, equivalent to a K value of (close to) zero. While the oligomers were unretained, the monomers eluted at K = 0.76. Thus, once the method was developed, during future separations of VV PACs, the entire stationary phase consisted of the monomers and was extruded after elution of oligomer within the first 100 ml. In the final separation step (Step 3, Figure 4), the dimers eluted with K = 1.8, and fractions with trimers and tetramers eluted with K = 2.5. For more accurate fraction control and prior to recombining, the CPC fractions were monitored using diol-HPLC-UV (Figure 3) along with NP-TLC (Figure 4). Once the K-value range was known, the retention volumes of the desired fractions in the subsequent chromatographic runs were calculated, and fractions were collected in a targeted fashion in flasks, as opposed to constant-time collection in test tubes. This avoided the tedious fraction control that usually accompanies CCS experiments and can be necessary even when online UV detection is available. Notably, this K-targeted approach is possible only in CCS systems as elution strictly depends on K-values. As LC employing solid stationary phases does not offer the same K-based approach, CCS is more reproducible from one preparative run to the next.
Figure 4.
Methods employed for the stepwise fractionation by CPC with TLC guided fraction control. For VV, TC and PM, step 1 was solvent partitioning. CPC was used for separation in steps 2 and 3. DESIGNER preparation of CV was done in one step. TLC was used for initial fraction monitoring to group fractions according to the DP of PACs. The outcomes demonstrate the effectiveness of the method from the monomer/dimer knock-out (MKO/DKO) fractions profiles. Diol HPLC profiles of relevant fractions were acquired before combining the fractions consisting of trimers and tetramers for enhanced fraction monitoring. (HEMWat: H - hexane; E - ethyl acetate; Ma- methyl acetate; Wat – water; DCM – dichloromethane; MeOH – methanol; EtOH – ethanol; K: partition coefficient calculated using VR = VM + KVS; Sf: Stationary phase retention; KO: knockout (depletion); DP: degree of polymerization)
In the case of TC, separation was performed using HEMWat 1/10/1/10 and HEMaWat 0.4/4/1/4 in two subsequent steps, both in ascending mode., Based on the literature and in the experience of the authors, separation of PACs from TC is much less complicated compared to the challenges posed by other PAC rich plat sources such a grape seeds or pine bark. This can be attributed to two factors. First, gallate ester moieties are absent in cocoa OPACs, reducing the polarity bandwidth of each DP step. Secondly, the predominance of B-type 4→8 interflavan linkages further limits the chemical diversity of TC OPACs. Collectively, this reduces the chemical complexity of the polyphenolic metabolome of TC and, thus, lowers the separation challenge. Accordingly, step 2 of the TC separation already achieved remarkable resolution between the monomers, dimers, and oligomers using the classic HEMWat system. The subsequent step 3 using HEMaWat 0.4/4/1/4 was employed to enhance the resolution between the trimers, tetramers, and the higher order oligomers, as well as to remove residual dimers.
In contrast to VV and TC, PM and CV extracts mainly consist of -type and/or mixed A/B-type PACs, leading to distinct chromatographic behavior. In the case of PM, using the typical HEMWat systems in ascending mode, it was observed that the dimers co-eluted along with tetramers and did not follow the typical serial elution according to DP. To solve this problem, separation was started with a descending mode (HEMaWat 2/4/1/4) to remove flavanol monomers and dimers simultaneously. Subsequently HEMaWat 0.5/4/1/4 in ascending mode was applied to produce the DP 3+4 enriched fraction. This modified method allowed preparation of the DESIGNER material without loss of the active tetrameric PACs.
On the other hand, as CV is enriched naturally in tri and tetrameric PACs, the medium-DP enriched product could be prepared using a single-step CPC-E without a prior solvent partitioning step. As shown in Figure 3, the tallest peak (*) in the HPLC-diol profile of the extract is cinnamaldehyde, the major component of cinnamon extract. Although this compound remained present in a 3+4 fraction after CPC fractionation, its proportion is very small compared to the PAC content. Accordingly, the single-step CPC fractionation method was effective not only for the removal of monomeric and polymeric PACs, but also substantially subtracted the majority of an inactive major constituent from CV. The number of chromatographic steps required to achieve the desired separation are source dependent, as can be seen from Figure 3. The same figure also shows that VV, PM, and TC exhibit a more complex PAC composition compared to CV.
Conclusions
Preparation of large amounts of material for biological and clinical applications is one of the rate limiting steps in the development of natural product-derived therapeutics. Methodologies such as CCS, specifically CPC, with its enhanced loading capacity, represent a reproducible and robust technique for large-scale separation. Considering the chemical composition of the target material, the purification of single chemical entities (SCEs) from natural PAC mixtures is challenging due to the intrinsic chemical properties of (O)PACs, such as their near identical monomeric units and the complications introduced by the vast stereochemical variability of this compound class. This limits the feasibility of the large-scale production of such SCEs. The availability of fast and reproducible CCS pre-fractionation proves to be a key step in solving the purification challenge. The current study demonstrated that CPC can be highly effective in producing trimeric and tetrameric PAC-enriched materials from complex OPAC mixtures. The successful application of this concept was demonstrated using examples from four different PAC rich plant sources: grape seeds, pine bark, cinnamon bark, and cocoa seeds. Furthermore, dentin biomechanical evaluation of the newly developed DESIGNER biomaterials compared favorably to the crude extracts and demonstrated that each chromatographic step led to the enrichment of PACs with appropriate molecular size for an optimum collagen cross-linking of dentin.
Highlights.
Development of proanthocyanidins (PACs) as restorative dental biomaterials
Separation of PAC trimers/tetramers by centrifugal partition chromatography (CPC)
Advantage of methyl acetate replacing methanol in HEMWat solvent systems for PACs
Evaluation of selectively enriched PAC oligomers in dentin biomechanical assay
Demonstration of DESIGNER extract concept for PAC-rich plant extracts
Acknowledgments
The authors are thankful to Gregoire Audo, of Armen Instruments and Gilson Inc., for providing the CPC extractor instrument and for expert CPC support. This research was supported grant R01 DE021040 from NIDCR/NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen SN, Turner A, Jaki BU, Nikolic D, van Breemen RB, Friesen JB, Pauli GF. An experimental implementation of chemical subtraction. J Pharm Biomed Anal. 2008;46:692–698. doi: 10.1016/j.jpba.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramos Alvarenga RF, Friesen JB, Nikolic D, Simmler C, Napolitano JG, van Breemen R, Lankin DC, McAlpine JB, Pauli GF, Chen SN. K-targeted metabolomic analysis extends chemical subtraction to DESIGNER extracts: selective depletion of extracts of hops (Humulus lupulus) J Nat Prodcuts. 2014;77:2595–2604. doi: 10.1021/np500376g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Andrea G. Pycnogenol: A blend of procyanidins with multifaceted therapeutic applications? Fitoterapia. 2010;81:724–736. doi: 10.1016/j.fitote.2010.06.011. http://dx.doi.org/10.1016/j.fitote.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Latif R. Health benefits of cocoa. Curr Opin Clin Nutr Metab Care. 2013;16:669–674. doi: 10.1097/MCO.0b013e328365a235. [DOI] [PubMed] [Google Scholar]
- 5.Maisuria VB, Los Santos YL, Tufenkji N, Déziel E. Cranberry-derived proanthocyanidins impair virulence and inhibit quorum sensing of Pseudomonas aeruginosa. Sci Rep. 2016;6:30169. doi: 10.1038/srep30169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fumagalli M, Sangiovanni E, Vrhovsek U, Piazza S, Colombo E, Gasperotti M, Mattivi F, De Fabiani E, Dell’Agli M. Strawberry tannins inhibit IL-8 secretion in a cell model of gastric inflammation. Pharmacol Res. 2016;111:703–712. doi: 10.1016/j.phrs.2016.07.028. [DOI] [PubMed] [Google Scholar]
- 7.Tempesta MS. The Discovery of SP-303 (Crofelemer/Fulyzaq), a Novel Polyphenol Isolated from Croton lecheri. Abstr. 44th West. Reg. Meet. Am. Chem. Soc; St. Clara, CA, United States. Oct. 3–6; American Chemical Society; 2013. p. WRM-7. [Google Scholar]
- 8.Patel TS, Crutchley RD, Tucker AM, Cottreau J, Garey KW. Crofelemer for the treatment of chronic diarrhea in patients living with HIV/AIDS. HIV AIDS (Auckl) 2013;5:153–62. doi: 10.2147/HIV.S30948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bedran-Russo AK, Pauli GF, Chen S-N, McAlpine J, Castellan CS, Phansalkar RS, Aguiar TR, Vidal CMP, Napotilano J, Nam J-W, Leme AA. Dentin Biomodification: Strategies, Renewable Resources and Clinical Applications. Dent Mater. 2014;30:62–76. doi: 10.1016/j.dental.2013.10.012. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3972923/pdf/nihms546161.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nam JW, Phansalkar RS, Lankin DC, Bisson J, McAlpine JB, Leme Aa, Vidal CMP, Ramirez B, Niemitz M, Bedran-Russo A, Chen S-N, Pauli GF. Subtle Chemical Shifts Explain the NMR Fingerprints of Oligomeric Proanthocyanidins with High Dentin Biomodification Potency. J Org Chem. 2015;80:7495–7507. doi: 10.1021/acs.joc.5b01082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vidal CMP, Aguiar TR, Phansalkar R, McAlpine JB, Napolitano JG, Chen SN, Araújo LSN, Pauli GF, Bedran-Russo A. Galloyl moieties enhance the dentin biomodification potential of plant-derived catechins. Acta Biomater. 2014;10:3288–3294. doi: 10.1016/j.actbio.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phansalkar RS, Nam JW, Chen SN, McAlpine JB, Napolitano JG, Leme A, Vidal CMP, Aguiar T, Bedran-Russo AK, Pauli GF. galloylated dimeric proanthocyanidin from grape seed exhibits dentin biomodification potential. Fitoterapia. 2015;101:169–178. doi: 10.1016/j.fitote.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yanagida A, Shoji T, Shibusawa Y. Separation of proanthocyanidins by degree of polymerization by means of size-exclusion chromatography and related techniques. J Biochem Biophys Methods. 2003;56:311–322. doi: 10.1016/S0165-022X(03)00068-X. [DOI] [PubMed] [Google Scholar]
- 14.Esatbeyoglu T, Wray V, Winterhalter P. Isolation of dimeric, trimeric, tetrameric and pentameric procyanidins from unroasted cocoa beans (Theobroma cacao L.) using countercurrent chromatography. Food Chem. 2015;179:278–289. doi: 10.1016/j.foodchem.2015.01.130. [DOI] [PubMed] [Google Scholar]
- 15.Savitri Kumar N, Maduwantha WMa, Wijekoon B, Kumar V, Nimal Punyasiri Pa, Sarath I, Abeysinghe B. Separation of proanthocyanidins isolated from tea leaves using high-speed counter-current chromatography. J Chromatogr A. 2009;1216:4295–4302. doi: 10.1016/j.chroma.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 16.Shibusawa Y, Yanagida A, Ito A, Ichihashi K, Shindo H, Ito Y. High-speed counter-current chromatography of apple procyanidins. J Chromatogr A. 2000;886:65–73. doi: 10.1016/S0021-9673(00)00448-9. [DOI] [PubMed] [Google Scholar]
- 17.Shibusawa Y, Yanagida A, Shindo H, Ito Y. Separation of apple catechin oligomers by CCC. J Liq Chromatogr Relat Technol. 2003;26:1609–1621. doi: 10.1081/JLC-120021270. [DOI] [Google Scholar]
- 18.Köhler N, Wray V, Winterhalter P. Preparative isolation of procyanidins from grape seed extracts by high-speed counter-current chromatography. J Chromatogr A. 2008;1177:114–125. doi: 10.1016/j.chroma.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 19.Robbins RJ, Leonczak J, Johnson JC, Li J, Kwik-Uribe C, Prior RL, Gu L. Method performance and multi-laboratory assessment of a normal phase high pressure liquid chromatography-fluorescence detection method for the quantitation of flavanols and procyanidins in cocoa and chocolate containing samples. J Chromatogr. 2009;1216:4831–4840. doi: 10.1016/j.chroma.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Castellan CS, Bedran-Russo AK, Karol S, Pereira PNR. Long-term stability of dentin matrix following treatment with various natural collagen cross-linkers. J Mech Behav Biomed Mater. 2011;4:1343–1350. doi: 10.1016/j.jmbbm.2011.05.003. http://dx.doi.org/10.1016/j.jmbbm.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aguiar TR, Vidal CM, Phansalkar RS, Todorova I, Napolitano JG, McAlpine JB, Chen SN, Pauli GF, Bedran-Russo AK. Dentin biomodification potential depends on polyphenol source. J Dent Res. 2014;93:417–422. doi: 10.1177/0022034514523783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng G, Klein MI, Gregoire S, Singh AP, Vorsa N, Koo H. The specific degree-of-polymerization of A-type proanthocyanidin oligomers impacts Streptococcus mutans glucan-mediated adhesion and transcriptome responses within biofilms. Biofouling. 2013;29:629–640. doi: 10.1080/08927014.2013.794456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bitzer ZT, Glisan SL, Dorenkott MR, Goodrich KM, Ye L, O’Keefe SF, Lambert JD, Neilson AP. Cocoa procyanidins with different degrees of polymerization possess distinct activities in models of colonic inflammation. J Nutr Biochem. 2015;26:827–831. doi: 10.1016/j.jnutbio.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lizarraga D, Lozano D, Briede JJ, van Delft JH, Tourino S, Centelles JJ, Torres JL, Cascante M. The importance of polymerization and galloylation for the antiproliferative properties of procyanidin-rich natural extracts. FEBS J. 2007;274:4802–4811. doi: 10.1111/j.1742-4658.2007.06010.x. [DOI] [PubMed] [Google Scholar]
- 25.Lv Q, Luo F, Zhao X, Liu Y, Hu G, Sun C, Li X, Chen K. Identification of proanthocyanidins from litchi (Litchi chinensis Sonn.) pulp by LC-ESI-Q-TOF-MS and their antioxidant activity. PLoS One. 2015;10:e0120480. doi: 10.1371/journal.pone.0120480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vidal CMP, Leme AA, Aguiar TR, Phansalkar R, Nam JW, Bisson J, McAlpine JB, Chen SN, Pauli GF, Bedran-Russo A. Mimicking the hierarchical functions of dentin collagen cross-links with plant derived phenols and phenolic acids. Langmuir. 2014;30:14887–14893. doi: 10.1021/la5034383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esatbeyoglu T, Jaschok-Kentner B, Wray V, Winterhalter P. Structure Elucidation of Procyanidin Oligomers by Low-Temperature 1 H NMR Spectroscopy. J Agric Food Chem. 2010;59:62–69. doi: 10.1021/jf1032334. [DOI] [PubMed] [Google Scholar]
- 28.Shibusawa Y, Yanagida A, Isozaki M, Shindo H, Ito Y. Separation of apple procyanidins into different degrees of polymerization by high-speed counter-current chromatography. J Chromatogr A. 2001;915:253–257. doi: 10.1016/S0021-9673(01)00575-1. [DOI] [PubMed] [Google Scholar]
- 29.Delaunay JC, Castagnino C, Chèze C, Vercauteren J. Preparative isolation of polyphenolic compounds from Vitis vinifera by centrifugal partition chromatography. J Chromatogr A. 2002;964:123–128. doi: 10.1016/S0021-9673(02)00355-2. [DOI] [PubMed] [Google Scholar]