Abstract
Tumor-specific CD8+ T cells often fail to elicit effective antitumor immune responses due to an inability to expand into a substantial effector population and persist long-term in vivo. Using an adoptive transfer model of cancer immunotherapy, we demonstrate that constitutive Eomes expression in tumor-specific CD8+ T cells improves tumor rejection and survival. The increase in tumor rejection was associated with an increased number and persistence of CD8+ T cells in lymphoid tissues during acute tumor rejection, tumor regrowth and in mice that remained tumor-free. Constitutive Eomes expression increased expression of CD25, and this was associated with enhanced interleukin-2 (IL-2) responsiveness and tumor-specific CD8+ T cell proliferation. Moreover, constitutive Eomes expression improved cell survival. Taken together, our data suggest that constitutive Eomes expression enhances CD8+ T cell proliferation and survival, in part through the enhancement of IL-2 responsiveness through CD25 induction.
Introduction
The role of CD8+ T cells in mediating antitumor immune responses has been well documented, yet a major limitation in the field remains the generation of a substantial population of tumor-specific CD8+ T cells that persist in vivo.1, 2 Adoptive immunotherapy aims to increase both the number and specificity of tumor-reactive CD8+ T cells and has yielded promising results in patients with metastatic melanoma.3, 4 Current adoptive cell transfer therapies require a significant expansion period to generate billions of tumor-specific CD8+ T cells before transfer.5 Recent studies have highlighted the importance of proliferative potential and persistence of CD8+ T cells in adoptive cell therapy.6–8 The ability to increase the expansion and survival of adoptively transferred cells in vivo would provide more practical means of treatment for cancer patients.
The T-box transcription factors T-bet and Eomesodermin (Eomes) have been implicated in CD8+ T cell effector activity and memory specification in models of acute viral infection.9–13 The role of Eomes in promoting CD8+ T cell-mediated antitumor immune responses is poorly understood. Our lab and others have demonstrated a marked increase in Eomes expression in tumor-specific CD8+ T cells following treatment with an agonistic α4-1BB (CD137/TNFSF9) antibody.14–16 Our study showed that endogenous expression of Eomes was required for 4-1BB-agonist-mediated tumor rejection. Agonistic 4-1BB antibody treatment has been shown to improve the antitumor immune response in various ways such as promoting CD8+ T cell expansion, preventing T cell exhaustion, promoting cytokine production and supporting T cell persistence.16–18 Other studies have demonstrated impaired tumor infiltration and tumor rejection in mice treated with CD8+ T cells lacking Eomes.19, 20 These findings prompted us to examine whether Eomes expression alone was sufficient to mediate effective CD8+ T cell-mediated tumor rejection.
To address whether augmented expression of Eomes was sufficient to promote CD8+ T cell-mediated tumor rejection, we utilized adoptively transferred CD8+ T cells constitutively expressing Eomes in a mouse model of lymphoma. We found that constitutive expression of Eomes in tumor-specific CD8+ T cells improved recipient mouse survival following adoptive transfer, and this survival was associated with an increase in the number of adoptively transferred cells in lymphoid tissues and the tumor. We further observed that constitutive Eomes expression increased cell proliferation and survival and this effect was associated with an Eomes-dependent increase in CD25 expression, and enhanced interleukin-2 (IL-2) responsiveness. Our findings suggest that Eomes expression alone is sufficient to improve tumor rejection efficacy by increasing both CD8+ T cell responsiveness to IL-2 and the number of tumor-specific T cells in an antitumor immune response.
Methods
Mice
Mice were bred, housed and utilized in accordance with University of Maryland School of Medicine Institutional Animal Care and Use Committee Guidelines. C57BL/6 and OT-1 mice were initially purchased from The Jackson Laboratory.
Antibodies
Cells were stained with fluorochrome-labeled antibodies to Eomes(clone Dan11mag), Thy1.1(clone His51), CD8a(clone 53-6.7), CD25(clone Pc61.5), CD122(clone TM-b1), CD44(clone Im7), CD69(clone H1.2f3), CD62L(clone Mel-14), Granzyme b(clone NGZB) and perforin(clone eBioOMAK-D) purchased from eBioscience (Thermo Fisher Scientific, Waltham, MA) Flow data were acquired on an Accuri C6 (BD Biosciences, San Jose, CA) and analyzed using FlowJo software (Tree Star Inc., Ashland, OR).
Cell staining and flow cytometry
Tumors and lymph tissues were harvested and prepared as previously described.16 Cells were stained with fluorochrome-labeled antibodies to cell surface molecules for 30 minutes at 4°C prior to fixation and permeabilization (FoxP3/Transcription Factor Staining Buffer Set, eBioscience) and stained with fluorochrome-labeled antibodies to intracellular antigens. For analysis of cytokine production, cells were re-stimulated with OVA peptide (1μg/mL, AnaSpec Inc., Fremont, CA) for 4 hours. Brefeldin A (10μg/mL, Life technologies, Carlsbad, CA) was added to the media to inhibit protein secretion. Cells were fixed with 4% PFA/PBS and permeabilized in saponin buffer (1% BSA and 0.1% Saponin in PBS) prior to staining with fluorochrome-labeled anti-IFNγ(clone Xmg1.2, eBioscience) and anti-TNFα(clone Mp6-xt22, eBioscience). For analysis of phosphorylated STAT5 expression, cells were cultured in media without IL-2 for 4 hours prior to stimulation with IL-2 of the indicated dose for 15 minutes. Cells were fixed with IC fixation buffer (eBioscience) and then methanol. Fixed cells were washed with PBS and stained with fluorochrome-labeled anti-Stat5(Y694) antibody (clone SRBCZX, eBioscience). Data acquisition was performed on an Accuri C6 (BD Biosciences) flow cytometer. Gating based on CD8 and Thy1.1 surface staining (simultaneous) and subsequent analysis was performed using FlowJo software (Tree Star Inc.).
Mouse lymphoma model
EG7 cells were purchased from ATCC and cultured as previously described.16 1 × 106 EG7 cells in 200 μL phosphate-buffered saline (PBS) were subcutaneously (s.c.) injected into the right side flank of 6–12 week old C57BL/6 recipient mice. The inguinal lymph node adjacent to the tumor was considered the tumor-draining lymph node, and the contralateral inguinal lymph node was used as a control. Tumor size was measured at the indicated time points as previously described.16 Mice were euthanized and tissues were harvested three days after T cell transfer (acute rejection) or when the tumor area reached 3cm2 (tumor regrowth).
CD8+ T cell activation and in vitro culture
Single cell suspensions were prepared from the spleen, flank lymph nodes and axillary lymph nodes of OT-1 donor mice. CD8+ T cells were magnetically isolated by negative selection (EasySep Mouse CD8+ T Cell Isolation Kit, STEMCELL Technologies, Vancouver, Canada). Isolated CD8+ T cells were activated in 24-well culture plates (2 × 106/well) with immobilized anti-CD3 and anti-CD28 antibodies and cultured in T cell media (IMDM+ 10% FBS+ 2mM β-mercaptoethanol) supplemented with IL-2 of the indicated dose (1u – 100u/mL).
Adoptive T cell transfer
CD8+ OT-1 T cells were activated and cultured in T cell media with 100u/mL IL-2. Two days after retroviral transduction, successfully transduced cells were magnetically enriched using either biotinylated anti-Thy1.1 antibody and streptavidin microbeads (Miltenyi Biotech, Bergisch Gladbach, Germany) or EasySep™ Mouse CD90.1 Positive Selection Kit (STEMCELL Technologies). Thy1.1+-enriched cells were cultured in complete media with 100u/mL IL-2 for an additional day. On day 4, CD8+ T cells were resuspended in PBS and 2 × 106 cells/200 μL of the cell suspension was transferred to each recipient mouse by intraperitoneal (i.p.) injection.
Retroviral constructs
The MSCV-IRES-Thy1.1 retroviral vector (MiT) was designed by the Marrack Lab.21 Eomes and T-bet cDNA was subcloned into the MiT backbone to generate Eomes-MiT and T-bet-MiT constructs, respectively.
Retroviral transduction
MiT retroviral genomes were packaged into retrovirus by co-transfecting 293T cells with MiT vector plasmid and helper plasmids and viral supernatants were harvested at 48 hours. One day following T cell activation, OT-1 CD8+ T cells were transduced with MiT virus in complete media (IMDM+ 10% FBS) in 24-well, RetroNectin-coated plates (Clontech, Mountain View, CA). Cell culture plates containing cells and virus were centrifuged at 2500rpm for 2 hours. After the centrifugation, viral supernatants were replaced with fresh media with IL-2 and transduced cells were cultured for another three days. The cultures were split 1:2 daily.
Proliferation and apoptosis/cell death assays
For proliferation assays, activated CD8+ OT-1 cells were stained with CFSE prior to retroviral transduction as previously described.22 CFSE-labeled cells were cultured in T cell media containing IL-2 of the indicated dose for 3 days. For analysis of apoptosis and cell death, activated CD8+ OT-1 cells were cultured in T cell media containing IL-2 of the indicated dose for 3 days and then labeled with AnnexinV (BD biosciences) to assay apoptosis and fixable viability dye eFluor 780 (eBioscience) to label dead cells. CFSE staining and apoptosis/cell death were assayed using an Accuri C6 flow-cytometer (BD Biosciences).
Statistical Analysis
Statistical analyses were performed as indicated using the 2-tailed Student t test or log-rank test. A P value of less than 0.05 was considered significant.
Results
Constitutive expression of Eomes, but not T-bet, in tumor-specific CD8+ T cells improves survival in an adoptive transfer model of cancer immunotherapy
To evaluate the antitumor efficacy of constitutive Eomes expression in tumor-specific CD8+ T cells, we utilized an adoptive transfer model in which CD8+ OT-1 transgenic T cells specifically target OVA-expressing EG7 lymphoma cells.23, 24 CD8+ OT-1 cells transduced with Eomes, T-bet or empty-vector retrovirus (Eomes-OT-1, T-bet-OT-1 and control-OT-1) were transferred into EG7 tumor-bearing mice (Fig. 1A). To measure the relative increase in Eomes expression after viral transduction, transduced OT-1 cells were harvested from tumors 3 days after transfer. In our prior work, agonistic 4-1BB-antibody (α4-1BB) treatment led to a marked increase in Eomes expression in tumor-infiltrating CD8+ T cells.16 This result prompted us to assess whether constitutive expression of Eomes via retroviral transduction achieved comparable levels of Eomes expression as did α4-1BB treatment. Eomes expression in Eomes OT-1 cells was increased to approximately the same degree (Fig. 1B), suggesting that Eomes is expressed at a high physiologic level following transduction.
Fig. 1. Constitutive expression of Eomes, but not T-bet, in tumor-specific CD8+ T cells improves survival in a murine model of lymphoma.
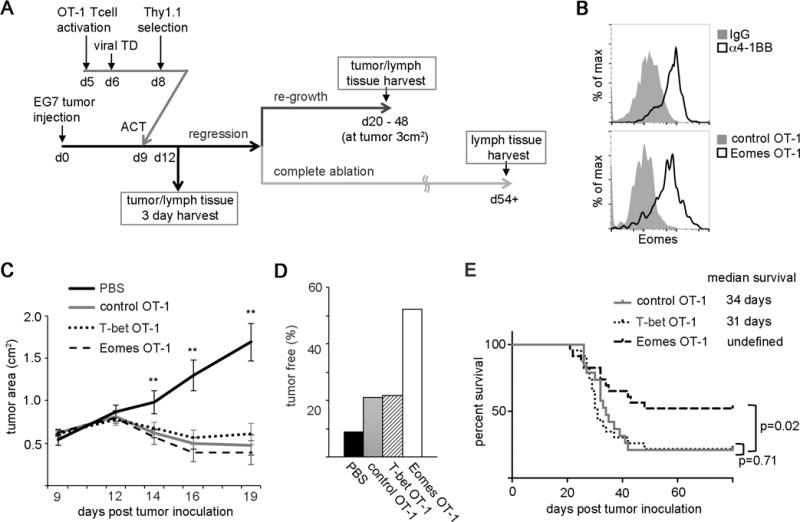
E.G7-OVA (EG7) cells were injected subcutaneously (s.c.) into the rear flank of mice 9 days prior to adoptive T cell transfer. OT-1 CD8+ T cells transduced with retrovirus expressing Eomes, T-bet or empty retrovirus (Eomes-OT-1, T-bet-OT-1 and control OT-1) were transferred intraperitoneally (i.p.) into mice bearing EG7 tumors at day 9.
(A) Experimental diagram of adoptive T cell transfer of retrovirus transduced OT-1 cells to EG7 tumor bearing mice. TD, transduction; ACT, adoptive cell transfer
(B) Histograms comparing Eomes expression in tumor-infiltrating CD8+ T cells 3 days after injection with an agonistic anti-4-1BB antibody (top panel) and Eomes expression in tumor-infiltrating OT-1 cells 3 days after adoptive transfer in Eomes-transduced cells (bottom panel). Histograms are representative of at least 3 samples.
(C) Tumor area average over time following transfer of Eomes-OT-1, T-bet-OT-1, control OT-1 or PBS injection in tumor-bearing mice. Statistical analyses were performed by Student t test; **, p<0.01.
(D) Percentage of tumor-free mice at 50 days post tumor inoculation.
(E) Kaplan-Meier curve depicting survival in the three OT-1 T cell recipient groups shown in (C), with median survival in days listed. The p-values between control OT-1 and Eomes OT-1, control OT-1 and T-bet OT-1 recipients were calculated using the log-rank test.
(C – E) Data are representative of 8 experiments using total of 11, 19, 23, 23 mice for recipients of PBS, control-OT-1, T-bet-OT-1 and Eomes-OT-1 recipient mice respectively.
We then wanted to address whether constitutive expression of Eomes or T-bet in tumor-specific CD8+ T cells improved tumor rejection. Utilizing our EG7/OT-1 adoptive transfer model, we measured tumor growth over time in mice receiving Eomes-OT-1, T-bet-OT-1 or control OT-1 cells. In the absence of adoptive T cell transfer, mice failed to control EG7 tumor growth. Consistent with previous reports,25–27 we observed acute tumor regression after adoptive transfer of OT-1 T cells roughly 12 days post-transfer (Fig. 1C). After initial tumor regression, some of the tumors regrew, leading to eventual euthanasia, while others remained tumor-free (Fig. 1A). We did not observe a significant difference in the rate of initial tumor regression between recipients of control OT-1, T-bet-OT-1 and Eomes-OT-1 cells, however, treatment with Eomes-OT-1 cells reduced the occurrence of tumor regrowth. Among 23 mice treated with Eomes-OT-1 cells, 12 (52%) mice survived without tumor recurrence whereas 5 out of 23 (22%) mice treated with T-bet OT-1 cells and 4 out of 19 (21%) mice treated with control-OT-1 cells remained tumor-free (Fig. 1D). Kaplan-Meier analysis of these data revealed a significant increase in median survival in recipients of Eomes-OT-1 cells compared to control-OT-1 recipients, whereas treatment with T-bet-OT-1 cells did not improve survival relative to the control (Fig. 1E). These data suggest that constitutive Eomes expression in CD8+ T cells is sufficient to reduce the occurrence of tumor regrowth and enhance survival in a mouse lymphoma model.
Tumor-specific CD8+ T cells constitutively expressing Eomes are present in increased numbers in the tumor-draining lymph node during tumor regression
To begin to delineate the mechanisms through which constitutive Eomes expression could enhance CD8+ T cell mediated-tumor rejection, we harvested tumors and lymphoid tissues from tumor-bearing recipients 3 days after adoptive transfer for evaluation of CD8+ T cells. At this time point, tumors were beginning to regress. While overall OT-1 cell numbers were few and there was variability between recipients, a statistically significant increase in the number of OT-1 cells was observed in tumor-draining lymph nodes (TDLN) in recipients of Eomes-OT-1 cells (Fig. 2). This difference was observed when considering both the total number of OT-1 cells in the TDLN and the percent of CD8+ T cells in the TDLN that were transferred OT-1 cells (Fig. 2A–B). Such differences were not observed in recipients of T-bet-OT-1 cells relative to control. In all three groups, Eomes OT-1, T-bet OT-1, and control OT-1, we consistently observed larger number of OT-1 cells in TDLN compared to control LN to the degree that the y-axis for the TDLN graph in Figure 2 is an order of magnitude greater than the y-axis for the control LN graph.
Fig. 2. Tumor-specific CD8+ T cells constitutively expressing Eomes are present in increased numbers in the tumor-draining lymph node during tumor regression.

OT-1 CD8+ T cells transduced with retrovirus encoding Eomes, T-bet or empty retrovirus (Eomes-OT-1, T-bet-OT-1 and control OT-1) were transferred into recipient mice bearing E.G7-OVA (EG7) tumors. Tumors and lymphoid tissues were harvested 3 days after adoptive T cell transfer. Donor CD8+ T cells were distinguished from host CD8+ T cells based on the expression of Thy1.1. Results are shown in box-whisker plots. Boxes indicate the middle 50% of samples, horizontal lines indicate the median value, and upward and downward whiskers indicate the highest and lowest values respectively. (A) Percentage of Thy1.1+ cells in the total CD8+ T cell population in the specified tissue. (B) Total numbers of Thy1.1+ CD8+ T cells in the specified tissue (n= 5 to 12). Statistical analyses were performed by Student t test; *, p<0.05; and **, p<0.01.
These results suggest that an Eomes-dependent increase in tumor-specific CD8+ T cell numbers may contribute to improved tumor rejection.
Constitutive Eomes expression promotes the long-term persistence of tumor-specific CD8+ T cells in lymphoid tissues throughout tumor-regrowth or ablation
The finding that constitutive Eomes expression increased the number of tumor-specific CD8+ T cells in the acute immune response prompted us to examine whether this result would be observed at longer time points, when tumors recurred or were completely ablated. For mice in which the tumor recurred, we harvested tumor and lymphoid tissues when tumors measured 3cm2. We observed increased numbers of Eomes-OT-1 cells in the tumor, tumor-draining lymph node, spleen and bone marrow. These differences were statistically significant when comparing the percentage of the Thy1.1+ population (OT-1 cells) to the total CD8+ population as well as the total number of OT-1 cells in each tissue (Fig. 3A–B). For the mice that remained tumor-free, lymphoid tissues were harvested at least 54 days after tumor ablation (Fig. 3C–D). At this time point, we observed an increased number of Eomes-OT-1 cells in all lymphoid tissues. Again, these differences were statistically significant when comparing the percentage of the Thy1.1+ population to the total CD8+ population as well as the total number of OT-1 cells in each tissue. To confirm continued constitutive expression of Eomes in recipients of Eomes OT-1 cells with tumor-regrowth post transfer, we evaluated Eomes expression in OT-1 cells from tumors and TDLN of Eomes OT-1 and control OT-1 recipients after tumor regrowth to 3 cm2. Continued constitutive expression of Eomes in Eomes OT-1 CD8+ T cells was observed in every case (Fig. S1). These results suggest that increased numbers of tumor-specific CD8+ T cells associated with constitutive expression of Eomes persist long-term, both when tumors regrow and long after tumor ablation.
Fig. 3. Constitutive Eomes expression promotes the long-term persistence of tumor-specific CD8+ T cells in lymphoid tissues throughout tumor-regrowth or ablation.
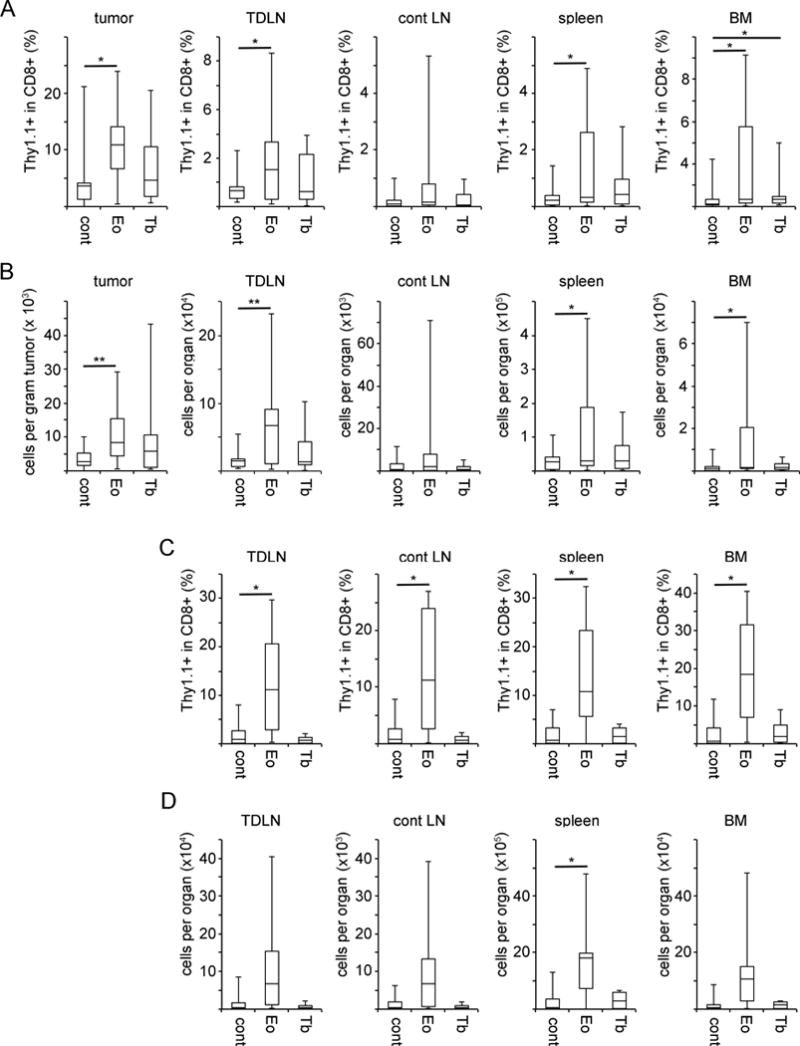
OT-1 CD8+ T cells transduced with retrovirus encoding Eomes, T-bet or empty retrovirus (Eomes-OT-1, T-bet-OT-1 and control OT-1) were transferred into recipient mice bearing E.G7-OVA (EG7) tumors. In mice in which the tumor recurred, tumor and lymphoid tissues were harvested when the tumor size reached 3cm2 (A and B, n = 10–18). In recipient mice in which tumors were completely ablated, lymphoid tissues were harvested at least 54 days after tumor clearance (C and D, n = 3–10). Donor CD8+ T cells were distinguished from host CD8+ T cells based on the expression of Thy1.1. (A and C) Percent of Thy1.1+ CD8+ T cells in the total CD8+ T cell population in the specified tissues. (B and D) Total numbers of Thy1.1+ CD8+ T cells in the specified tissues. Statistical analyses were performed by Student t test;*, p<0.05; and **, p<0.01.
Constitutive Eomes expression promotes IFNγ production in CD8+ T cells
Because we observed improved survival in tumor-bearing mice following adoptive transfer with Eomes-OT-1 cells, we sought to determine if Eomes was influencing the antitumor activity of these cells in addition to increasing cell number. In acute immune responses, Eomes controls CD8+ T cell effector function through induction of interferon gamma (IFNγ), perforin, and granzyme B.11, 12 To address the impact of constitutive Eomes expression on CD8+ T cell effector activity in the antitumor immune response, Eomes-OT-1 and control-OT-1 cells were cultured for 3 days and assayed for effector molecule expression by flow cytometry. Consistent with the previously defined role for Eomes,12, 28 constitutive expression resulted in an approximately 15% increase in the percentage of IFNγ-producing cells (Fig. 4A–B). There was no observed difference in TNFα, granzyme B and perforin expression between Eomes-OT-1 and control-OT-1 cells (Fig. 4B–C). This result confirms that Eomes increases IFNγ production in tumor-specific CD8+ T cells.
Fig. 4. Constitutive Eomes expression promotes IFNγ production in CD8+ T cells.
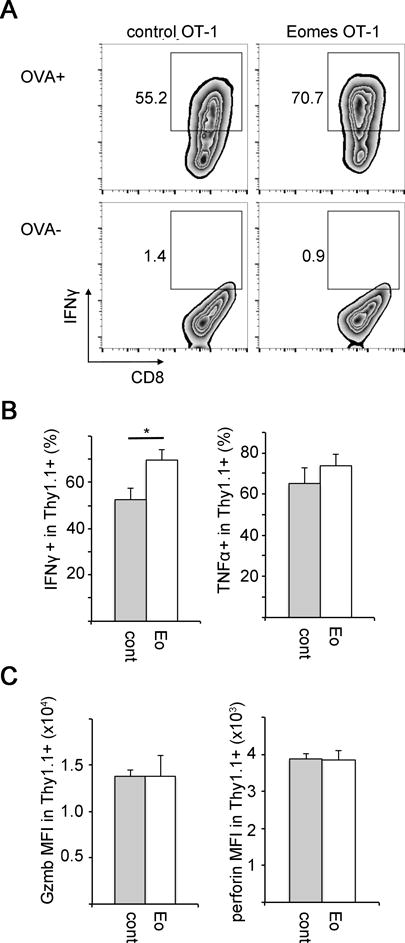
OT-1 CD8+ T cells from transduced with retrovirus encoding Eomes or empty retrovirus (Eomes-OT-1 and control OT-1 cells) were cultured in vitro for 3 days and then re-stimulated with OVA peptide for 4 hours. Expression of interferon gamma (IFNγ), TNFα, granzyme B and perforin were analyzed by flow-cytometry.
(A) Representative FACS plot showing IFNγ expression in CD8+ Thy1.1+ OT-1 cells. Gates represent cells expressing IFNγ in response to OVA stimulation (upper plots) and without OVA stimulation (lower plots). Data are representative of at least three experiments.
(B) Percentage of IFNγ-expressing and TNFα-expressing cells in the CD8+ Thy1.1+ OT-1 cells after OVA stimulation. Gating for IFNγ-expressing cells is shown in A. Bars represent mean values over 2 experiments with standard error (n=5).
(C) MFI of granzyme B and perforin in unstimulated CD8+ Thy1.1+ OT-1 cells. Bars represent mean values over 2 experiments with standard error (n=5). Statistical analyses were performed by Student t test; *, p<0.05.
Constitutive Eomes expression increases CD25 expression prior to adoptive transfer
The observed increase in Eomes-OT-1 cell number in the TDLN 3 days post-transfer prompted us to investigate the expression of early activation markers associated with terminal effector or memory precursor fates prior to adoptive transfer. Surface protein expression was tested 3 days after in vitro T cell activation by flow-cytometry. Prior studies have identified CD25, CD44 and CD69 as T cell activation markers whereas CD122 and CD62L are expressed at low levels in effector cells and associated with central and stem-like memory cells.11, 29, 30 We observed a significant increase in CD25 expression but no difference in CD44 or CD69 expression between Eomes-OT-1 and control-OT-1 cells (Fig. 5). We observed no significant difference in the expression levels of CD122 and CD62L, suggesting that constitutive Eomes expression may not affect memory precursor differentiation prior to adoptive transfer.
Fig. 5. Constitutive Eomes expression increases CD25 expression prior to adoptive transfer.
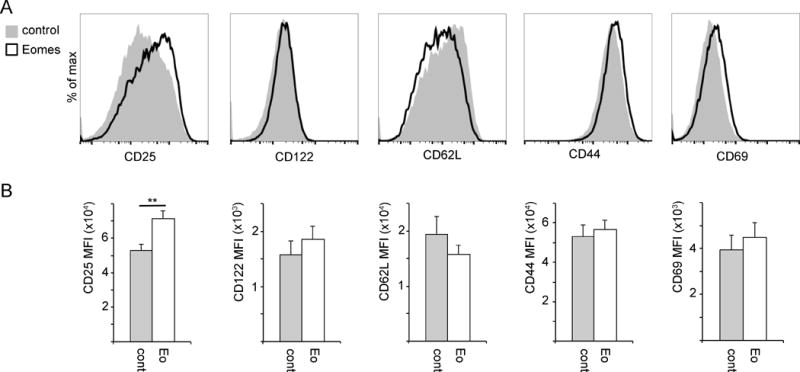
Surface expression of T cell activation and memory/effector differentiation markers were analyzed in vitro by flow cytometry 2 days after transduction with Eomes or control retrovirus. Data shown are gated on CD8+/Thy1.1+ cells. (A) Representative histograms showing expression of the indicated markers in control (gray) and Eomes-expressing (white) CD8+ T cells. (B) MFI of indicated proteins shown as the mean with standard error (n=8 to 15). Statistical analyses were performed by Student t test; **, p<0.01.
Increased CD25 expression associated with Eomes expression enhances IL-2 responsiveness and increases IL-2-dependent proliferation
In CD8+ T cells, IL-2 signaling occurs through the high affinity IL-2 receptor complex consisting of IL2Rα (CD25), IL2Rβ (CD122) and the common gamma chain (γc).31 Signaling through this receptor promotes multiple aspects of T cell immunity such as in-vitro expansion32, 33 and effector molecule expression.34, 35 High expression of CD25 increases CD8+ T cell proliferation in an IL-2-dependent manner.36 We hypothesized that increased CD25 expression as a result of constitutive Eomes expression would augment IL-2 signaling and promote an increase in cell number in lymphoid tissues. IL-2 binds to the IL-2R complex and promotes the recruitment and transphosphorylation of receptor associated Janus kinases (JAK1 and JAK3), which phosphorylate tyrosine residues on the cytoplasmic tails of IL2Rβ and γc and recruit signal transducer and activator of transcription protein 5 (Stat5). JAK1 and JAK3 phosphorylate Stat5 at Y694 and Y699. Stat5 then dissociates from the receptor, dimerizes in the cytosol and translocates to the nucleus and binds STAT5 response elements.37–40 Therefore, we measured phosphorylated Stat5 levels as a marker of IL-2 signal input. We observed a significant increase in pStat5 in Eomes-OT-1 cells relative to control, suggesting an increase in responsiveness to IL-2 with constitutive Eomes expression (Fig. 6A).
Fig. 6. Increased CD25 expression associated with Eomes expression enhances IL-2 responsiveness and increases IL-2 dependent proliferation and survival.
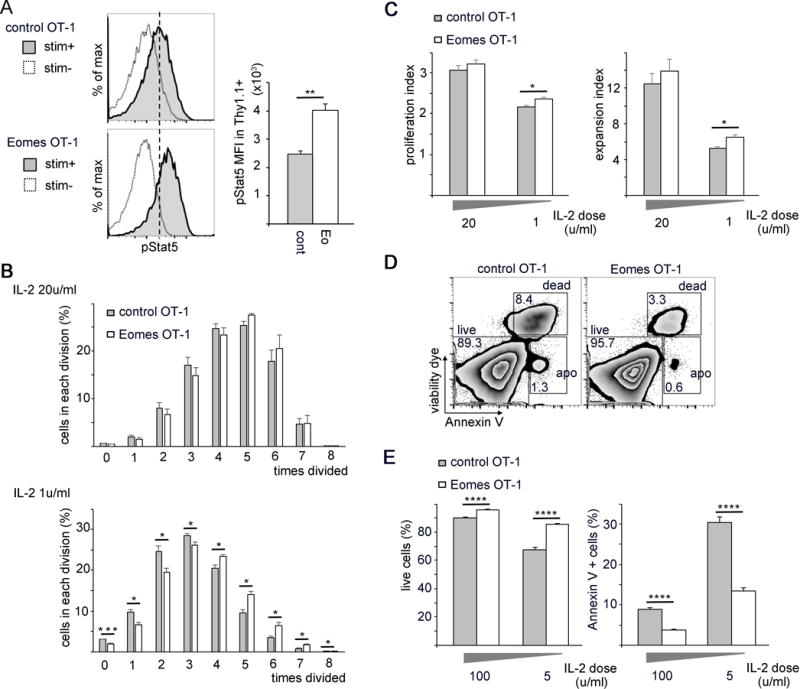
(A) Levels of phosphorylated Stat5 (pStat5) were analyzed by flow-cytometry in Eomes-OT-1 and control-OT-1 CD8+ T cells. Cells were starved with interleukin-2 (IL-2) and then stimulated with 5u/mL IL-2. Histograms are representative of pStat5 expression in stimulated versus unstimulated cells. The bar graph represents the MFI of pStat5 shown as the mean values with standard error (n=3).
(B–C) Cellular proliferation was analyzed by CFSE staining. CD8+ OT-1 cells were stained with CFSE prior to viral transduction. After 3 days of culture with IL-2 of 20u/mL or 1u/mL, CFSE levels were measured by flow-cytometry and plotted as mean values with standard error. (B) Percentage of cells in each division (n=4). (C) Proliferation index and expansion index calculated from cell numbers in each division shown in B (n=4).
(D–E) Control-OT-1 and Eomes-OT-1 cells were cultured in vitro with 100u/mL or 5u/mL IL-2 for 3 days and stained with viability dye and AnnexinV and analyzed by flow cytometry. (D) Representative FACS plots comparing cell viability in control-OT-1 and Eomes-OT-1 cells cultured in 100u/mL IL-2. (E) Bar graphs represent the percentage of live cells and dead or apoptotic cells in the CD8+ OT-1 cells. Bars are plotted as mean values with standard error (n=4). Each experiment was repeated at least 3 times, data shown were from a representative experiment consists of numbers of samples shown above. Statistical analyses were performed by Student t test; *, p<0.05; **, p<0.01; ***, p<0.002; and ****, p<0.0005.
IL-2 was discovered for its role in promoting T cell proliferation following activation in vitro41, 42 and studies have shown that this effect is dose dependent.43 A study using IL2Rα transgenic mice showed that augmented IL-2 signaling increased CD8+ T cell proliferation after adoptive transfer.33 To investigate whether Eomes expression affected T cell proliferation, we CFSE-labeled Eomes-OT-1 and control-OT-1 cells and cultured for 3 days in vitro. We observed that a significantly higher percentage of Eomes-OT-1 cells underwent more cell divisions than control-OT-1 cells when cultured in a lower concentration of IL-2 (1u/mL) but not when cultured in a higher concentration (20u/mL) (Fig. 6B). We then calculated the proliferation index (PI), which represents the average number of divisions that dividing cells have undergone in culture and the expansion index (EI), which demonstrates the fold-expansion of the entire culture. Both the PI and EI were significantly increased in Eomes-OT-1 cells cultured in a lower concentration of IL-2, suggesting that constitutive Eomes expression in CD8+ T cells enhanced both the average number of cell divisions and overall fold-expansion of these cells in culture (Fig. 6C). These results indicate that constitutive Eomes expression increases CD8+ T cell proliferation in low-level IL-2 concentrations, possibly through increasing IL-2 responsiveness via augmented CD25 expression.
Constitutive Eomes expression promotes CD8+ T cell survival
The finding that Eomes increased CD8+ T cell proliferation in a CD25-dependent manner prompted us to investigate whether increased IL-2 responsiveness similarly enhanced CD8+ T cell survival. Prior reports have demonstrated the role for IL-2 in promoting T cell survival through activation of downstream signaling pathways and induction of pro-survival genes.44–46 To analyze cell survival and apoptosis, control-OT-1 and Eomes-OT-1 T cells were cultured for 3 days in high and low IL-2 concentrations (100u/mL and 5u/mL, respectively) and stained with viability dye and AnnexinV. We observed an increase in cell survival and a decrease in apoptosis in Eomes-OT-1 cells compared to control-OT-1 cells (Fig. 6D–E). Although the differences in cell survival and apoptosis appear greater at the lower IL-2 concentration, this finding was significant regardless of IL-2 concentration (Fig. 6E). This result suggests that constitutive Eomes expression promotes CD8+ T cell survival and may contribute to the increase in Eomes-OT-1 cells observed in lymph tissues and the tumor after transfer.
Discussion
Here we report that constitutive Eomes expression improves survival in an adoptive transfer model of cancer immunotherapy and increases the number of adoptively transferred CD8+ T cells in lymphoid tissues and the tumor during acute tumor rejection, tumor re-growth and after tumor ablation. We further demonstrate that Eomes is sufficient to increase the number of tumor-specific CD8+ T cells in the antitumor immune response, as constitutive expression increased cell proliferation and cell survival. This study extends our previous finding in which α4-1BB antibody administration led to high levels of Eomes expression in tumor-infiltrating CD8+ T cells and slowed tumor growth in an Eomes-dependent manner.16 In our current study, we show that constitutive expression of Eomes in tumor-specific CD8+ T cells is sufficient to improve survival in an adoptive transfer model of cancer immunotherapy.
Our study corroborates previous studies examining the role of Eomes in promoting CD8+ T cell-mediated antitumor immunity in a B16 model of melanoma.19, 20 Eomes-knockout (EKO) mice that were vaccinated against tumor antigen and then challenged with live B16 cells were susceptible to tumor growth. Similarly, adoptive transfer of EKO bone marrow cells into irradiated WT mice failed to reject tumors. In both experiments, WT mice and transfer of WT bone marrow cells was sufficient to prevent tumor growth.20 A study by the same group demonstrated reduced numbers of EKO tumor infiltrating lymphocytes (TIL) following adoptive transfer into irradiated WT mice.19 In the present study, we observed that in contrast to the impaired antitumor immunity exhibited in CD8+ T cells lacking Eomes, constitutive expression of Eomes increased the number of transferred cells in lymphoid tissues and the tumor, and this was sufficient to improve tumor rejection.
The ability to generate large numbers of autologous, tumor-specific T cells in vitro for transfer into patients has yielded promising results in a wide variety of malignancies.47 Current immunotherapies expand T cells in vitro or ex vivo prior to adoptive transfer under culture conditions that differ with respect to the antigens, antigen-presenting cells and cytokines used.48–52 The T cell-intrinsic mechanisms that promote the expansion of tumor-specific T cells in vivo are poorly defined. Here we show that Eomes promotes the in vivo expansion and survival of tumor-specific CD8+ T cells during acute rejection, tumor regrowth and in tumor-free mice. Prior work has established TDLN as major sites of cross-presentation and CD8+ T cell activation during antitumor immune responses, suggesting that activation of tumor-reactive T cells in the draining lymph node is necessary for successful tumor clearance.53–56 In our study, Eomes-OT-1 cells were found in increased numbers in the TDLN during acute tumor rejection. Interestingly, Eomes-OT-1 recipient mice exhibited improved tumor-free survival, suggesting that Eomes expression in tumor-specific CD8+ T cells during the acute antitumor response allows the generation of a larger TIL population, which may confer superior antitumor immunity. Similar to what we observed during acute rejection, the increase in the number and percentage of Eomes-OT-1 cells was maintained in the tumor and lymph tissues of mice that experienced tumor re-growth. It is unclear why some recipients of Eomes-OT-1 cells experienced tumor recurrence despite the increase in tumor-specific CD8+ T cell number, but it is possible that the number of viable Eomes-OT-1 cells in TDLN differed among recipient mice in the acute response, and that a threshold must be met to efficiently reject the tumor. Alternative reasons may also underlie tumor recurrence in some recipients in the face of increased tumor-specific CD8+ T cell numbers after transfer of Eomes OT-1 cells. These reasons may include both tumor-specific factors, such as loss of expression of antigen or MHC, as well as T-cell alterations, such as increased expression of checkpoint inhibitors or decreased expression of co-stimulatory receptors.
In addition to increased numbers of tumor-specific CD8+ T cells, we observed a modest but significant increase in IFNγ production in cells constitutively expressing Eomes, suggesting that Eomes may improve the antitumor response through enhanced cytokine production. Studies have demonstrated the critical role of IFNγ in promoting antitumor immune responses.57–60 Eomes is known to regulate IFNγ production in CD8+ T cells and this finding corroborates previous studies demonstrating a defect in IFNγ production in CD8+ T cells that were Eomes-deficient or that were treated with a dominant-negative form of Eomes.12, 19 The independent contribution of increased cell numbers and enhanced IFNγ production to the antitumor immune response is unclear. It is likely that enhanced cytokine secretion by a larger effector population contributes to tumor rejection.
Expansion of tumor-specific CD8+ T cells is necessary for efficient tumor rejection and prior work supports a role for IL-2 in this expansion.61–63 IL-2 has also been shown to promote T cell expansion during anti-viral immune responses.64 Studies using a transgenic IL-2 receptor have shown that augmented IL-2 signaling can increase T cell proliferation and cell number following adoptive transfer.33, 65 In this study, we show that constitutive Eomes expression in CD8+ T cells augments CD25 (IL2Rα) expression, and this is associated with increased STAT5 phosphorylation in response to IL-2. Strong IL-2 signaling in vitro has been shown to be critical for effective T cell expansion in vivo.66 We observed an IL-2 dependent increase in T cell proliferation in vitro, suggesting that Eomes-OT-1 cells may exhibit enhanced proliferation in vivo in response to low IL-2 concentrations. In addition to proliferation, Eomes expression promoted CD8+ T cell survival. Eomes-OT-1 cells exhibited increased survival and decreased cell death in both IL-2 concentrations tested. Although our data do not demonstrate that Eomes promotes cell survival in an IL-2-depdendent manner, it is well documented that IL-2 signaling promotes T cell survival.43, 44 Taken together, Eomes expression contributes to an increase in cell number by promoting both cell survival and proliferation, at least in part by enhancing IL-2 responsiveness.
In this study, we observed that an increased number of Eomes-OT-1 cells were maintained long-term even after tumor clearance, suggesting that constitutive Eomes expression increased T cell persistence. It is likely that both the increase in early expansion as well as the long-term persistence of transferred T cells can be attributed to increased IL-2 signaling during the activation phase. Strong IL-2 signaling in the early phase of T cell activation is necessary for successful long-term memory maintenance and recall responses.66, 67 Studies show that IL-2 signaling in the early activation phase promotes secondary expansion of T cells.67–69 On the contrary, IL2Rα-deficient mice were shown to demonstrate insufficient recall responses.70 Our research suggests that constitutive Eomes expression enhances IL-2 responsiveness through upregulation of CD25, which maintains long-term tumor control by increasing T cell persistence.
In corroboration with our previous study in which an α4-1BB-mediated increase in Eomes improved tumor rejection, here we demonstrate that increased Eomes expression alone enhanced tumor rejection efficacy. 4-1BB has been shown to be a promising target in improving the antitumor efficacy of CD8+ T cells by promoting CD8+ T cell expansion, preventing exhaustion, promoting cytokine production and supporting T cell persistence.17, 71, 72 Recently, replacement of the CD28 co-stimulation endodomain with 4-1BB led to increased persistence and tumor rejection in CD19-expressing chimeric antigen T cells (CAR T).73, 74 Another study showed that 4-1BB agonism upregulates CD25 expression in CD8+ T cells following in vitro activation, lending to enhanced proliferation.75 Along with previous published reports, our study suggests that the improved tumor rejection observed after α4-1BB treatment may be associated with an Eomes-dependent upregulation of CD25.
This study elucidates a novel role of Eomes in promoting an increase in tumor-specific CD8+ T cell number via CD25 upregulation in an adoptive transfer model of cancer immunotherapy. Our findings provide valuable insights for novel approaches to T cell-directed therapy with the goal of eliciting more efficient tumor control in cancer immunotherapy.
Supplementary Material
Acknowledgments
Source of Funding
This work was supported by National Institute of Health grants (P30CA134274, K08HL93207), the Gabrielle’s Angel Foundation for Cancer Research, The Alliance for Cancer Gene Therapy, and the Marlene and Stewart Greenebaum Comprehensive Cancer Center.
References
- 1.Baitsch L, Fuertes-Marraco SA, Legat A, et al. The three main stumbling blocks for anticancer T cells. Trends in immunology. 2012;33(7):364–72. doi: 10.1016/j.it.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Fesnak AD, June CH, Levine BL. Engineered T cells: the promise and challenges of cancer immunotherapy. Nature reviews Cancer. 2016;16(9):566–81. doi: 10.1038/nrc.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen R, Donia M, Ellebaek E, et al. Long-Lasting Complete Responses in Patients with Metastatic Melanoma after Adoptive Cell Therapy with Tumor-Infiltrating Lymphocytes and an Attenuated IL2 Regimen. Clin Cancer Res. 2016;22(15):3734–45. doi: 10.1158/1078-0432.CCR-15-1879. [DOI] [PubMed] [Google Scholar]
- 4.Hinrichs CS, Rosenberg SA. Exploiting the curative potential of adoptive T-cell therapy for cancer. Immunological reviews. 2014;257(1):56–71. doi: 10.1111/imr.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu R, Forget MA, Chacon J, et al. Adoptive T-cell therapy using autologous tumor-infiltrating lymphocytes for metastatic melanoma: current status and future outlook. Cancer J. 2012;18(2):160–75. doi: 10.1097/PPO.0b013e31824d4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hinrichs CS, Borman ZA, Cassard L, et al. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(41):17469–74. doi: 10.1073/pnas.0907448106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klebanoff CA, Gattinoni L, Palmer DC, et al. Determinants of successful CD8+ T-cell adoptive immunotherapy for large established tumors in mice. Clin Cancer Res. 2011;17(16):5343–52. doi: 10.1158/1078-0432.CCR-11-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klebanoff CA, Gattinoni L, Restifo NP. Sorting through subsets: which T-cell populations mediate highly effective adoptive immunotherapy? J Immunother. 2012;35(9):651–60. doi: 10.1097/CJI.0b013e31827806e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banerjee A, Gordon SM, Intlekofer AM, et al. Cutting edge: The transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. Journal of immunology. 2010;185(9):4988–92. doi: 10.4049/jimmunol.1002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Intlekofer AM, Takemoto N, Wherry EJ, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nature immunology. 2005;6(12):1236–44. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 11.Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nature reviews Immunology. 2012;12(11):749–61. doi: 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pearce EL, Mullen AC, Martins GA, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302(5647):1041–3. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan BM, Juedes A, Szabo SJ, et al. Antigen-driven effector CD8 T cell function regulated by T-bet. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(26):15818–23. doi: 10.1073/pnas.2636938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen S, Lee LF, Fisher TS, et al. Combination of 4-1BB agonist and PD-1 antagonist promotes antitumor effector/memory CD8 T cells in a poorly immunogenic tumor model. Cancer Immunol Res. 2015;3(2):149–60. doi: 10.1158/2326-6066.CIR-14-0118. [DOI] [PubMed] [Google Scholar]
- 15.Curran MA, Geiger TL, Montalvo W, et al. Systemic 4-1BB activation induces a novel T cell phenotype driven by high expression of Eomesodermin. The Journal of experimental medicine. 2013;210(4):743–55. doi: 10.1084/jem.20121190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song C, Sadashivaiah K, Furusawa A, et al. Eomesodermin is required for antitumor immunity mediated by 4-1BB-agonist immunotherapy. Oncoimmunology. 2014;3(1):e27680. doi: 10.4161/onci.27680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munks MW, Mourich DV, Mittler RS, et al. 4-1BB and OX40 stimulation enhance CD8 and CD4 T-cell responses to a DNA prime, poxvirus boost vaccine. Immunology. 2004;112(4):559–66. doi: 10.1111/j.1365-2567.2004.01917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ju SA, Lee SC, Kwon TH, et al. Immunity to melanoma mediated by 4-1BB is associated with enhanced activity of tumour-infiltrating lymphocytes. Immunol Cell Biol. 2005;83(4):344–51. doi: 10.1111/j.1440-1711.2005.01330.x. [DOI] [PubMed] [Google Scholar]
- 19.Li G, Yang Q, Zhu Y, et al. T-Bet and Eomes Regulate the Balance between the Effector/Central Memory T Cells versus Memory Stem Like T Cells. PLoS One. 2013;8(6):e67401. doi: 10.1371/journal.pone.0067401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu Y, Ju S, Chen E, et al. T-bet and eomesodermin are required for T cell-mediated antitumor immune responses. Journal of immunology. 2010;185(6):3174–83. doi: 10.4049/jimmunol.1000749. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell TC, Hildeman D, Kedl RM, et al. Immunological adjuvants promote activated T cell survival via induction of Bcl-3. Nat Immunol. 2001;2(5):397–402. doi: 10.1038/87692. [DOI] [PubMed] [Google Scholar]
- 22.Banerjee A, Schambach F, DeJong CS, et al. Micro-RNA-155 inhibits IFN-gamma signaling in CD4+ T cells. Eur J Immunol. 2010;40(1):225–31. doi: 10.1002/eji.200939381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clarke SR, Barnden M, Kurts C, et al. Characterization of the ovalbumin-specific TCR transgenic line OT-I: MHC elements for positive and negative selection. Immunol Cell Biol. 2000;78(2):110–7. doi: 10.1046/j.1440-1711.2000.00889.x. [DOI] [PubMed] [Google Scholar]
- 24.Moore MW, Carbone FR, Bevan MJ. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988;54(6):777–85. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- 25.Kaluza KM, Thompson JM, Kottke TJ, et al. Adoptive T cell therapy promotes the emergence of genomically altered tumor escape variants. International journal of cancer. Journal international du cancer. 2012;131(4):844–54. doi: 10.1002/ijc.26447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Straetemans T, Berrevoets C, Coccoris M, et al. Recurrence of melanoma following T cell treatment: continued antigen expression in a tumor that evades T cell recruitment. Molecular therapy : the journal of the American Society of Gene Therapy. 2015;23(2):396–406. doi: 10.1038/mt.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hollenbaugh JA, Reome J, Dobrzanski M, et al. The rate of the CD8-dependent initial reduction in tumor volume is not limited by contact-dependent perforin, Fas ligand, or TNF-mediated cytolysis. J Immunol. 2004;173(3):1738–43. doi: 10.4049/jimmunol.173.3.1738. [DOI] [PubMed] [Google Scholar]
- 28.Cruz-Guilloty F, Pipkin ME, Djuretic IM, et al. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. The Journal of experimental medicine. 2009;206(1):51–9. doi: 10.1084/jem.20081242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rolle CE, Carrio R, Malek TR. Modeling the CD8+ T effector to memory transition in adoptive T-cell antitumor immunotherapy. Cancer research. 2008;68(8):2984–92. doi: 10.1158/0008-5472.CAN-07-3040. [DOI] [PubMed] [Google Scholar]
- 30.Ahlers JD, Belyakov IM. Memories that last forever: strategies for optimizing vaccine T-cell memory. Blood. 2010;115(9):1678–89. doi: 10.1182/blood-2009-06-227546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nature reviews Immunology. 2012;12(3):180–90. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- 32.Kündig TM, Schorle H, Bachmann MF, et al. Immune responses in interleukin-2-deficient mice. Science. 1993;262(5136):1059–61. doi: 10.1126/science.8235625. [DOI] [PubMed] [Google Scholar]
- 33.Cheng LE, Ohlen C, Nelson BH, et al. Enhanced signaling through the IL-2 receptor in CD8+ T cells regulated by antigen recognition results in preferential proliferation and expansion of responding CD8+ T cells rather than promotion of cell death. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(5):3001–6. doi: 10.1073/pnas.052676899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cousens LP, Orange JS, Biron CA. Endogenous IL-2 contributes to T cell expansion and IFN-gamma production during lymphocytic choriomeningitis virus infection. J Immunol. 1995;155(12):5690–9. [PubMed] [Google Scholar]
- 35.Pipkin ME, Sacks JA, Cruz-Guilloty F, et al. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32(1):79–90. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valenzuela J, Schmidt C, Mescher M. The roles of IL-12 in providing a third signal for clonal expansion of naive CD8 T cells. Journal of immunology. 2002;169(12):6842–9. doi: 10.4049/jimmunol.169.12.6842. [DOI] [PubMed] [Google Scholar]
- 37.Lin JX, Leonard WJ. The role of Stat5a and Stat5b in signaling by IL-2 family cytokines. Oncogene. 2000;19(21):2566–76. doi: 10.1038/sj.onc.1203523. [DOI] [PubMed] [Google Scholar]
- 38.Malek TR, Castro I. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity. 2010;33(2):153–65. doi: 10.1016/j.immuni.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rane SG, Reddy EP. Janus kinases: components of multiple signaling pathways. Oncogene. 2000;19(49):5662–79. doi: 10.1038/sj.onc.1203925. [DOI] [PubMed] [Google Scholar]
- 40.Grimley PM, Dong F, Rui H. Stat5a and Stat5b: fraternal twins of signal transduction and transcriptional activation. Cytokine Growth Factor Rev. 1999;10(2):131–57. doi: 10.1016/s1359-6101(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 41.Morgan DA, Ruscetti FW, Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976;193(4257):1007–8. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- 42.Smith KA. Interleukin-2: inception, impact, and implications. Science. 1988;240(4856):1169–76. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- 43.Zambricki E, Shigeoka A, Kishimoto H, et al. Signaling T-cell survival and death by IL-2 and IL-15. Am J Transplant. 2005;5(11):2623–31. doi: 10.1111/j.1600-6143.2005.01075.x. [DOI] [PubMed] [Google Scholar]
- 44.Kelly E, Won A, Refaeli Y, et al. IL-2 and related cytokines can promote T cell survival by activating AKT. Journal of immunology. 2002;168(2):597–603. doi: 10.4049/jimmunol.168.2.597. [DOI] [PubMed] [Google Scholar]
- 45.Lan RY, Selmi C, Gershwin ME. The regulatory, inflammatory, and T cell programming roles of interleukin-2 (IL-2) J Autoimmun. 2008;31(1):7–12. doi: 10.1016/j.jaut.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Lord JD, McIntosh BC, Greenberg PD, et al. The IL-2 receptor promotes lymphocyte proliferation and induction of the c-myc, bcl-2, and bcl-x genes through the trans-activation domain of Stat5. Journal of immunology. 2000;164(5):2533–41. doi: 10.4049/jimmunol.164.5.2533. [DOI] [PubMed] [Google Scholar]
- 47.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348(6230):62–8. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chacon JA, Sarnaik AA, Chen JQ, et al. Manipulating the tumor microenvironment ex vivo for enhanced expansion of tumor-infiltrating lymphocytes for adoptive cell therapy. Clin Cancer Res. 2015;21(3):611–21. doi: 10.1158/1078-0432.CCR-14-1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huarte E, Fisher J, Turk MJ, et al. Ex vivo expansion of tumor specific lymphocytes with IL-15 and IL-21 for adoptive immunotherapy in melanoma. Cancer letters. 2009;285(1):80–8. doi: 10.1016/j.canlet.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Y, Liu S, Hernandez J, et al. MART-1-specific melanoma tumor-infiltrating lymphocytes maintaining CD28 expression have improved survival and expansion capability following antigenic restimulation in vitro. Journal of immunology. 2010;184(1):452–65. doi: 10.4049/jimmunol.0901101. [DOI] [PubMed] [Google Scholar]
- 51.Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. Journal of immunology. 2014;192(12):5451–8. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He J, Tang XF, Chen QY, et al. Ex vivo expansion of tumor-infiltrating lymphocytes from nasopharyngeal carcinoma patients for adoptive immunotherapy. Chin J Cancer. 2012;31(6):287–94. doi: 10.5732/cjc.011.10376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marzo AL, Lake RA, Lo D, et al. Tumor antigens are constitutively presented in the draining lymph nodes. Journal of immunology. 1999;162(10):5838–45. [PubMed] [Google Scholar]
- 54.McDonnell AM, Robinson BW, Currie AJ. Tumor antigen cross-presentation and the dendritic cell: where it all begins? Clin Dev Immunol. 2010;2010:539519. doi: 10.1155/2010/539519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kagamu H, Touhalisky JE, Plautz GE, et al. Isolation based on L-selectin expression of immune effector T cells derived from tumor-draining lymph nodes. Cancer research. 1996;56(19):4338–42. [PubMed] [Google Scholar]
- 56.Maass G, Schmidt W, Berger M, et al. Priming of tumor-specific T cells in the draining lymph nodes after immunization with interleukin 2-secreting tumor cells: three consecutive stages may be required for successful tumor vaccination. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(12):5540–4. doi: 10.1073/pnas.92.12.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dighe AS, Richards E, Old LJ, et al. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Immunity. 1994;1(6):447–56. doi: 10.1016/1074-7613(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 58.Kammertoens T, Schuler T, Blankenstein T. Immunotherapy: target the stroma to hit the tumor. Trends Mol Med. 2005;11(5):225–31. doi: 10.1016/j.molmed.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 59.Platzer C, Richter G, Uberla K, et al. Interleukin-4-mediated tumor suppression in nude mice involves interferon-gamma. European journal of immunology. 1992;22(7):1729–33. doi: 10.1002/eji.1830220710. [DOI] [PubMed] [Google Scholar]
- 60.Prevost-Blondel A, Neuenhahn M, Rawiel M, et al. Differential requirement of perforin and IFN-gamma in CD8 T cell-mediated immune responses against B16.F10 melanoma cells expressing a viral antigen. European journal of immunology. 2000;30(9):2507–15. doi: 10.1002/1521-4141(200009)30:9<2507::AID-IMMU2507>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 61.Crompton JG, Sukumar M, Restifo NP. Uncoupling T-cell expansion from effector differentiation in cell-based immunotherapy. Immunological reviews. 2014;257(1):264–76. doi: 10.1111/imr.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hwang LN, Yu Z, Palmer DC, et al. The in vivo expansion rate of properly stimulated transferred CD8+ T cells exceeds that of an aggressively growing mouse tumor. Cancer research. 2006;66(2):1132–8. doi: 10.1158/0008-5472.CAN-05-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nguyen HH, Kim T, Song SY, et al. Naive CD8(+) T cell derived tumor-specific cytotoxic effectors as a potential remedy for overcoming TGF-beta immunosuppression in the tumor microenvironment. Sci Rep. 2016;6:28208. doi: 10.1038/srep28208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bachmann MF, Oxenius A. Interleukin 2: from immunostimulation to immunoregulation and back again. EMBO Rep. 2007;8(12):1142–8. doi: 10.1038/sj.embor.7401099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheng LE, Greenberg PD. Selective delivery of augmented IL-2 receptor signals to responding CD8+ T cells increases the size of the acute antiviral response and of the resulting memory T cell pool. J Immunol. 2002;169(9):4990–7. doi: 10.4049/jimmunol.169.9.4990. [DOI] [PubMed] [Google Scholar]
- 66.Kim MT, Kurup SP, Starbeck-Miller GR, et al. Manipulating Memory CD8 T Cell Numbers by Timed Enhancement of IL-2 Signals. J Immunol. 2016;197(5):1754–61. doi: 10.4049/jimmunol.1600641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441(7095):890–3. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu A, Zhou J, Marten N, et al. Efficient induction of primary and secondary T cell-dependent immune responses in vivo in the absence of functional IL-2 and IL-15 receptors. J Immunol. 2003;170(1):236–42. doi: 10.4049/jimmunol.170.1.236. [DOI] [PubMed] [Google Scholar]
- 69.D’Souza WN, Lefrançois L. IL-2 is not required for the initiation of CD8 T cell cycling but sustains expansion. J Immunol. 2003;171(11):5727–35. doi: 10.4049/jimmunol.171.11.5727. [DOI] [PubMed] [Google Scholar]
- 70.Bachmann MF, Wolint P, Walton S, et al. Differential role of IL-2R signaling for CD8+ T cell responses in acute and chronic viral infections. Eur J Immunol. 2007;37(6):1502–12. doi: 10.1002/eji.200637023. [DOI] [PubMed] [Google Scholar]
- 71.Bartkowiak T, Curran MA. 4-1BB Agonists: Multi-Potent Potentiators of Tumor Immunity. Front Oncol. 2015;5:117. doi: 10.3389/fonc.2015.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hurtado JC, Kim YJ, Kwon BS. Signals through 4-1BB are costimulatory to previously activated splenic T cells and inhibit activation-induced cell death. Journal of immunology. 1997;158(6):2600–9. [PubMed] [Google Scholar]
- 73.Kawalekar OU, O’Connor RS, Fraietta JA, et al. Distinct Signaling of Coreceptors Regulates Specific Metabolism Pathways and Impacts Memory Development in CAR T Cells. Immunity. 2016;44(2):380–90. doi: 10.1016/j.immuni.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 74.Long AH, Haso WM, Shern JF, et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med. 2015;21(6):581–90. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oh HS, Choi BK, Kim YH, et al. 4-1BB Signaling Enhances Primary and Secondary Population Expansion of CD8+ T Cells by Maximizing Autocrine IL-2/IL-2 Receptor Signaling. PLoS One. 2015;10(5):e0126765. doi: 10.1371/journal.pone.0126765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


