Abstract
The single most common cause of chronic pancreatitis (CP, a serious inflammatory disease) is chronic alcohol abuse, which impairs hepatic alcohol dehydrogenase (ADH, a major ethanol oxidizing enzyme). Previously, we found ~5 fold greater fatty acid ethyl esters (FAEEs), and injury in the pancreas of hepatic ADH deficient (ADH−) vs. hepatic normal ADH (ADH+) deer mice fed 3.5g% ethanol via liquid diet daily for two months. Therefore, progression of ethanol-induced pancreatic injury was determined in ADH− deer mice fed ethanol for four months to delineate the mechanism and metabolic basis of alcoholic chronic pancreatitis (ACP). In addition to a substantially increased blood alcohol concentration and plasma FAEEs, significant degenerative changes, including atrophy and loss of acinar cells in some areas, ultrastructural changes evident by such features as swelling and disintegration of endoplasmic reticulum (ER) cisternae and ER stress were observed in the pancreas of ethanol-fed ADH− deer mice vs. ADH+ deer mice. These changes are consistent with noted increases in pancreatic injury markers (plasma lipase, pancreatic trypsinogen activation peptide, FAEE synthase and cathepsin B) in ethanol-fed ADH− deer mice. Most importantly, an increased levels of pancreatic glucose regulated protein (GRP) 78 (a prominent ER stress marker) were found to be closely associated with increased phosphorylated eukaryotic initiation factor (eIF) 2α signaling molecule in PKR-like ER kinase branch of unfolded protein response (UPR) as compared to X box binding protein 1S and activating transcription factor (ATF)6 – 50kDa protein of inositol requiring enzyme 1α and ATF6 branches of UPR, respectively, in ethanol-fed ADH− vs. ADH+ deer mice. These results along with findings on plasma FAEEs, and pancreatic histology and injury markers suggest a metabolic basis of ethanol-induced pancreatic injury, and provide new avenues to understand metabolic basis and molecular mechanism of ACP.
Keywords: Ethanol, Alcohol dehydrogenase, Deer mice, Alcoholic chronic pancreatitis, ER membrane stress, Fatty acid ethyl esters
INTRODUCTION
The medical and social consequences of high alcohol consumption and chronic alcohol abuse are one of the most expensive health problems in society today, costing ~$250 billion to the U.S. economy and ~100,000 deaths annually (NIAAA, 2000; Sacks et al., 2015). Chronic alcohol abuse is the single most common cause of chronic pancreatitis (CP) associated with a considerable morbidity and mortality (Lankisch and Banks, 1998). Alcoholic chronic pancreatitis (ACP) is a relapsing/continuing inflammatory disorder of the exocrine pancreas, characterized by distinct morphological changes including irregular sclerosis that resulting in permanent loss of exocrine parenchyma and its associated functions, and may lead to multiple co-morbidities including diabetes and/or pancreatic cancer (Clemens and Mahan, 2010; Kaphalia, 2011; Herreros-Villanueva et al., 2013). Surprisingly, many alcoholic patients die before reaching the clinical stages of the ACP (Pitchumoni et al., 1984; Singh and Simsek, 1990; Lankisch and Banks, 1998). Although a direct relationship between alcohol abuse and CP has been described in several clinical studies, mechanism(s) and the metabolic basis of ACP are not well understood due to a lack of a suitable animal model.
Hepatic alcohol dehydrogenase (ADH), known to metabolize the majority of ingested alcohol (~90%), is commonly impaired during chronic alcohol abuse and in experimental animals, following chronic ethanol feeding (Nuutinen et al., 1983; Sharkawi, 1984; Panés et al., 1989; 1993; Kaphalia et al., 1996). Although hepatic cytochrome P450 (CYP) 2E1, which oxidizes ethanol to acetaldehyde is induced by chronic alcohol abuse, has been reported to cause oxidative stress (Cederbaum, 2010). However, the km of CYP2E1 is much higher than that of hepatic ADH for ethanol (Lieber, 1997). In addition, acetaldehyde is rapidly metabolized to acetate at the rate it is formed (Rognstead and Grunnet, 1979). An inhibition of hepatic ADH induced either by chronic alcohol feeding, or by chemical inhibition has been shown to facilitate formation of ethanol to fatty acid ethyl esters (FAEEs) via nonoxidative metabolism by several-fold in the pancreas, an organ frequently damaged during chronic alcohol abuse (Laposata and Lange, 1986; Manautou and Carlson, 1991; Werner et al., 2002). Similar results were also reported by our laboratory using hepatic ADH deficient (ADH−) deer mouse model (Kaphalia et al., 2010). High FAEE synthase activity in the pancreatic tissue and its induction during chronic ethanol feeding has been reported by us and others (Kaphalia and Ansari, 2003; Pfützer at al., 2002). Therefore, mammalian pancreas is an important target organ for the synthesis of FAEEs and injury during chronic alcohol abuse (Werner et al., 1997; Wu et al., 2008).
In our previous study, a dose-dependent formation of FAEEs, and injury and endoplasmic reticulum membrane (ER) stress in the pancreas were observed in ADH− deer mice (a natural variant lacking the major ethanol oxidizing ADH 1 isoform in the liver) vs. hepatic ADH normal (ADH+) deer mice fed ethanol daily for two months (Kaphalia et al., 2010). Therefore, the present study was conducted to understand metabolic basis of ethanol-induced pancreatic injury and associated ER stress signaling in ADH− deer mouse model.
MATERIALS AND METHODS
Chemicals, reagents and antibodies
High purity grade FAEEs from Sigma Chemical Co. (St. Louis, MO) and [1-14C] oleic acid from DuPont-New England Nuclear (Boston, MA; specific activity 56 mCi/mmol) were used. QuantiChrom™ Lipase assay kit (BioAssay Systems, Hayward, CA; Cat# DLPS-100), and cathepsin B fluorogenic activity assay kit (Calbiochem Company, San Diego, CA; Cat# CBA 001) were utilized. Polyclonal antibodies raised against a synthetic peptide corresponding to the sequence of human glucose regulated protein (GRP)78 (Cell Signaling, Danvers, MA; Cat# 3183; 1:1000 dilution; MW, 78 kDa), inositol-requiring enzyme (IRE)1α (Novus Biologicals, Littleton, CO; Cat# NB100-2323; 1:1000 dilution; MW, 110 kDa), activating transcription factor (ATF) 6 (Novus Biologicals; Cat# nbp1-40256; 5μg/ml dilution; MW, 90 kDa: full length & 50kDa: active/cleaved), phosphorylated eukaryotic initiation factor (p-eIF)2α (Cell Signaling, Cat# 9721; 1:1000 dilution; MW, 38 kDa), X-box binding protein (XBP)1s (Abcam, Cambridge, MA; Cat# ab37152; 2μg/ml dilution; MW, 29 kDa [isoform 1] & 42 kDa [isoform 2]), proapoptotic protein CCAAT/-enhancer-binding protein homologous protein (CHOP) (Cell Signaling, Cat# 5554; 1:1000 dilution; MW, 27 kDa), phosphorylated protein kinase R-like endoplasmic reticulum kinase (PERK) (Santa Cruz, Dallas, TX; Cat# sc-32577; 1:1000 dilution; MW, 125 kDa), 4-hydroxynonenal (4-HNE) (Abcam, Cat# ab46545; 1:200 dilution), and anti-rabbit IgG and HRP-linked secondary antibody (Cell Signaling, Cat# 7074; 1:1000 dilution) were procured. Unless specified, all other chemicals, reagents and solvents used in the present study were from Sigma Aldrich (St. Louis, MO), Bio-Rad (Hercules, CA) or Fischer Scientific (Hampton, NH).
Animal experiments
Hepatic alcohol dehydrogenase (ADH) negative phenotype (ADH−) deer mice, a natural genetic variant of Peromyscus maniculatus, and ADH normal (ADH+) deer mice [male, ~1 year-old (equivalent to adult human age), ~20g weight] obtained from Peromyscus Stock Center, University of South Carolina were housed in the UTMB Animal Resource Center in accordance to protocols instituted by UTMB’s Institutional Animal Care and Use Committee. Animals were randomly divided as control and experimental groups for each strain and fed Lieber-DeCarli liquid diet (Dyets Inc., Bethlehem, PA) for a week, followed by increasing concentrations of ethanol from 0 to 3.5g% in the liquid diet for a two week period and then, maintained at a 3.5g% ethanol in the liquid diet daily for an additional four months (Kaphalia et al., 2010). Control animals for each strain were pair-fed with liquid diet containing equivalent ethanol calories, substituted by maltose-dextrin. The diets were prepared and changed daily and the weights of the animals were recorded periodically. Daily intake of liquid diet for each animal, their general health, and signs of distress and morbidity were monitored.
All the animals were sacrificed on day 120 of ethanol feeding, blood was withdrawn directly from the hearts of animals anesthetized with Nembutal™ and transferred into heparinized tubes. A fifty μL aliquot of whole blood was transferred to a gas chromatograph (GC) autosampler vial and analyzed for blood alcohol and acetaldehyde levels (Kaphalia et al., 2010). Remaining blood was centrifuged at 1000 ×g for 5 min, and plasma was separated and stored at −80°C until the analysis. The pancreas was harvested from each animal for gross examination, and immediately cut into small pieces and fixed for histology, immunohistochemistry, electron microscopy or stored at −80°C for biochemical analyses (Bhopale et al., 2006).
Morphological examination
A portion of pancreas was fixed in 10% buffered formalin, dehydrated in 70% ethanol and then embedded in paraffin for hematoxylin and eosin (H&E) or Trichrome staining for histopathologic evaluation. For examining ultrastructural changes, 1–3 mm sections of pancreas were fixed in buffered mixture of formaldehyde and glutaraldehyde and embedded in epoxy plastic. Ultrathin sections were examined with a Philips 201 or CM-100 electron microscope (Bhopale et al., 2006).
Oxidative stress
Oxidative stress was assessed by immunohistochemistry in paraffin embedded thin sections of pancreatic tissue using antibodies against fatty acid oxidation products, 4-HNE (Kaphalia et al., 2010).
Tunnel assay
Paraffin embedded thin sections of pancreas were evaluated for apoptosis by in situ end labeling using TdT-FragEL™ from CalBiochem® according to the manufacturer’s instructions.
Blood alcohol and acetaldehyde levels
The levels of blood alcohol and acetaldehyde were determined by headspace GC as described previously (Kaphalia et al., 2010).
Plasma FAEE analysis
Since plasma FAEE levels are good indicators of pancreatic FAEEs in ADH− deer mice fed ethanol, plasma of ethanol-fed and pair-fed control deer mice was extracted and analyzed for FAEEs as described previously (Kaphalia et al., 2010).
Lipase assay
Plasma lipase was assayed using QuantiChrom™ Lipase assay kit (BioAssay Systems, Hayward, CA) as per manufacturer’s instructions.
FAEE synthase assay
Chronic alcohol consumption is known to induce pancreatic FAEE synthase activity (Pfützer et al., 2002). Therefore, pancreatic FAEE synthase was assayed using [1-14C] oleic acid as substrate as described (Kaphalia et al., 2004).
Cathepsin B assay
Cathepsin B, one of the pancreatic lysosomal hydrolases, is capable of activating trypsinogen to trypsin within the pancreatic acinar cells. FAEEs are also known to increase the fragility of lysosomal membranes and release cathepsin B into the cytoplasmic compartment (Haber et al., 1993). Therefore, cathepsin B was assayed in the supernatant of 16,500 ×g fraction of pancreatic homogenate (5%, wt/vol) prepared in 0.01 M sodium phosphate buffer (pH 6.2) containing 0.1 M NaCl, 0.02% sodium azide, 0.001 M EDTA, leupeptin (1 mg/l), and glycerol (1.5%, vol/vol) by a fluorogenic activity assay kit as per manufacturer’s instructions (Kaphalia et al., 2010).
Trypsinogen activation peptide assay
Trypsinogen activation peptide (TAP) is released in the proximal small intestine as a result of the activation of trypsinogen to trypsin. However, premature activation of trypsinogen within the acinar cells in the pancreas, may release TAP into the pancreatic parenchyma (Rinderknecht, 1986). Therefore, TAP was quantified in the pancreatic homogenate using an enzyme-linked immunosorbent TAPKIT (Biotrin International, Dublin, Ireland) as per manufacturer’s instructions.
Protein determination
Pancreatic and plasma protein concentrations were determined according BCA protein assay (Bio-Rad, Hercules, CA).
Western blot analysis for the assessment of ER stress signaling
ER stress and its downstream signaling were assessed by measuring GRP78, a marker of ER stress, and related misfolded protein response (UPR) signaling molecules in the pancreatic post nuclear fraction by Western blot analysis. Briefly, an equal amount of 15,000 ×g supernatant pancreatic homogenate protein was mixed with sample buffer and boiled for 10 minutes. Forty μg of protein from each sample was subjected to SDS-PAGE followed by transfer of proteins on nitrocellulose membrane for Western blot analysis using primary antibodies (Kaphalia et al., 2010). The blots were incubated with horseradish peroxidase conjugated secondary antibodies, and the target proteins were visualized using an ECL detection system (ThermoScientific, Rockford, IL). The protein band intensity was assessed by NIH ImageJ Software (version 1.50i, Bethesda, NIH) and proteins normalized by β-actin values.
Statistical analysis
The data sets were analyzed using the Student’s t-test. The Sigma plot 12.0 program (Systat Software, Inc.) was used for statistical analysis and the results were expressed as mean ± SEM (standard error of mean). A p-value 0.05 was considered statistically significant (n= 5, unless otherwise specified).
RESULTS
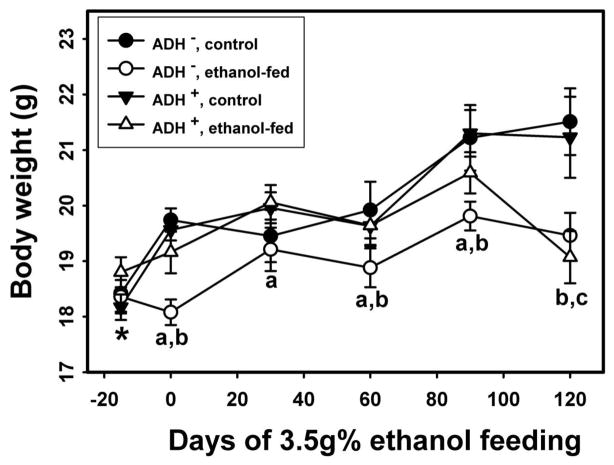
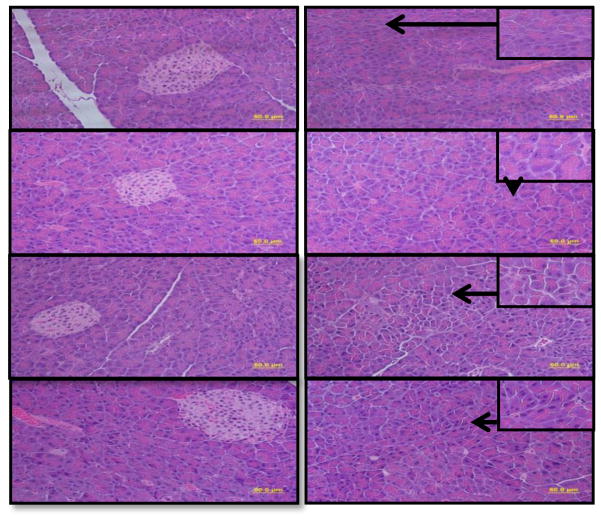
Average body weight of ethanol-fed ADH− and ADH+ deer mice as compared with their respective pair-fed controls are shown in Fig. 1. The initial average body weights before ethanol feeding were similar for both strains. However, the average body weights were significantly low for ethanol-fed ADH− vs. pair-fed control ADH− deer mice on day 0, 60, 90 and 120 days of ethanol feeding. Remarkably, body weights were also significantly low on day 0, 30, 60 and 90 for ethanol-fed ADH− vs. ADH+ deer mice. Except on day 120, average body weights were comparable between ethanol-fed vs. pair-fed control ADH+ deer mice. Morphologic changes observed in the pancreas of ethanol-fed ADH− deer mice were characterized as acinar cell atrophy, which may be appreciated as a paucity of cytoplasm and resultant crowding of slightly smaller and more homogenous nuclei in addition to the loss of acinar cells in some areas (Fig. 2C2). Although no fibrosis was evident using Trichrome staining in the pancreas of both ethanol-fed groups, a few acinar cells showed degenerative changes in ethanol-fed ADH+ deer mice group. The islets of Langerhans were relatively well-preserved and intact in both the ethanol-fed and control groups (Fig. 2A1–D1). A normal histologic appearance was observed in the pancreatic sections of both pair-fed controls (2A2 and 2B2).
Fig. 1.
Average body weights of ADH− and ADH+ deer mice during four months of ethanol feeding. P ≤ 0.05 for ethanol-fed ADH− vs. ethanol-fed ADH+ deer micea, ethanol-fed ADH− vs. pair-fed control ADH− deer miceb and ethanol-fed ADH+ vs. pair-fed control ADH+ deer micec. * - initial body weight of the mice before feeding 1g% ethanol in the liquid diet. The ethanol concentration was gradually increased to 3.5g% in the liquid diet in two weeks period and maintained for remaining 120 days (n = 10 animals/group).
Fig. 2.
Histopathological evaluation of H&E stained pancreatic tissue sections from ethanol-fed ADH− and ADH+ deer mice (bar = 50 μm): A2) & B2) Pancreas of pair-fed control ADH− & ADH+ deer mice, respectively, showing normal histological appearances. C2) Pancreas of ethanol-fed ADH− deer mice showing degenerative changes in the form of individual acinar cell atrophy. D2) Pancreas of ethanol-fed ADH+ deer mice showing only a few acinar cells with degenerative changes. A1, B1, C1 & D1) Pancreas of ethanol-fed and control groups showing relatively well-preserved and intact islets of Langerhans.
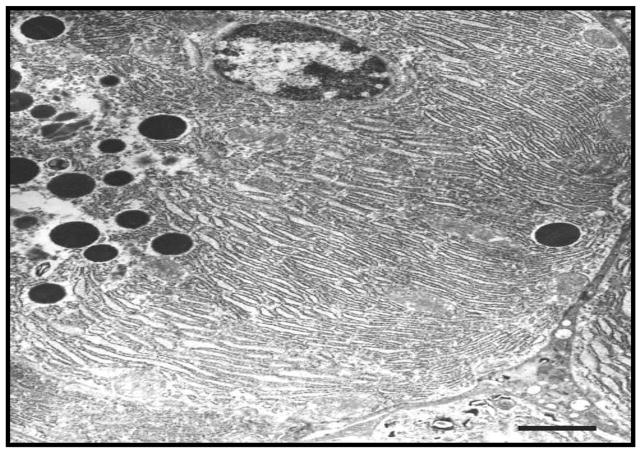
Electron micrographs of the pancreatic sections, showed minimal changes of the endoplasmic reticulum (ER) and ER cisternae, in the form of swelling, in pair-fed controls of both strains (Fig. 3A & B). However, ethanol-fed ADH− deer mice exhibited ER stress as evidenced by such changes as swelling/dilation of ER and some disintegration of cisternae (Fig. 3C). On the other hand, ethanol-fed ADH+ deer mice showed formation of multiple small vacuoles in some cells and a significantly less marked disintegration of granular ER (Fig. 3D). Oxidative stress as well as apoptotic cell death were not significantly altered between the ethanol-fed ADH− and ADH+ deer mice (data not shown).
Fig. 3.
Ultrastructural changes in the pancreas of ethanol-fed ADH− vs. ADH+ deer mice (bar = 1μm): A) & B) Pair-fed control groups of ADH− & ADH+ deer mice, respectively, showing minimal changes in the ER cisternae in the form of swelling. C) Pancreas of ethanol-fed ADH− deer mice showing marked dilation of granular ER (black arrows). D) Pancreas of ethanol-fed ADH+ deer mice showing less marked disintegration of granular ER and formation of multiple small vesicles (black arrows).
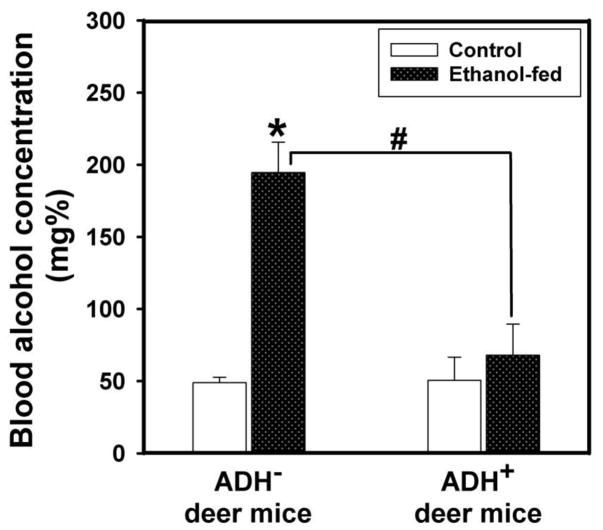
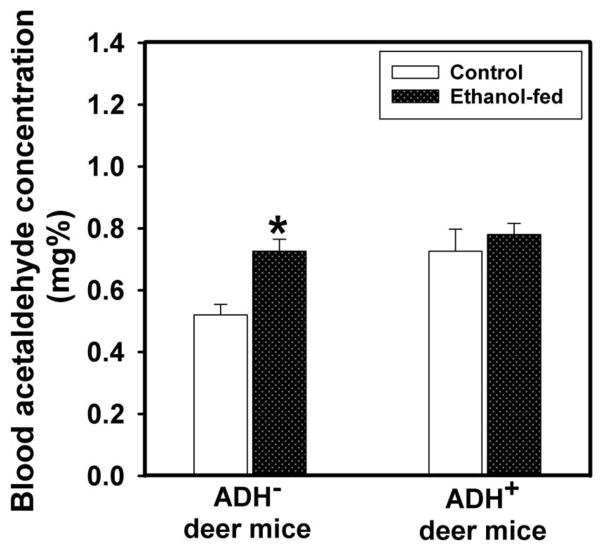
Average blood alcohol concentration (BAC) was increased by ~3 fold in ethanol-fed ADH− vs. ADH+ deer mice; average being 195 vs. 68 mg %, respectively (Fig. 4). The blood acetaldehyde levels in ethanol-fed ADH− deer mice were also significantly higher than its pair-fed controls; average being 0.73 vs. 0.52 mg%, respectively (Fig. 5). However, the blood acetaldehyde levels in ethanol-fed ADH− deer mice were comparable to those in ethanol-fed ADH+ deer mice. Neither BAC nor blood acetaldehyde levels were significantly different between ethanol-fed and pair-fed ADH+ deer mice. These findings suggest that BAC is influenced by hepatic ADH deficiency; an important metabolic condition responsible for an increased body burden of ethanol.
Fig. 4.
BAC in ADH− and ADH+ deer mice fed 3.5% ethanol daily for 4 months. Values are mean ± SEM. *P ≤ 0.05 for ethanol-fed ADH− vs. pair-fed control ADH− deer mice. #P ≤ 0.05 for ethanol-fed ADH− vs. ethanol-fed ADH+ deer mice (n=10 mice/group).
Fig. 5.
Blood acetaldehyde levels in ADH− and ADH+ deer mice fed 3.5% ethanol daily for 4 months. Values are mean ± SEM. *P ≤ 0.05 for ethanol-fed ADH− vs. pair-fed control ADH− deer mice (n=10 mice/group).
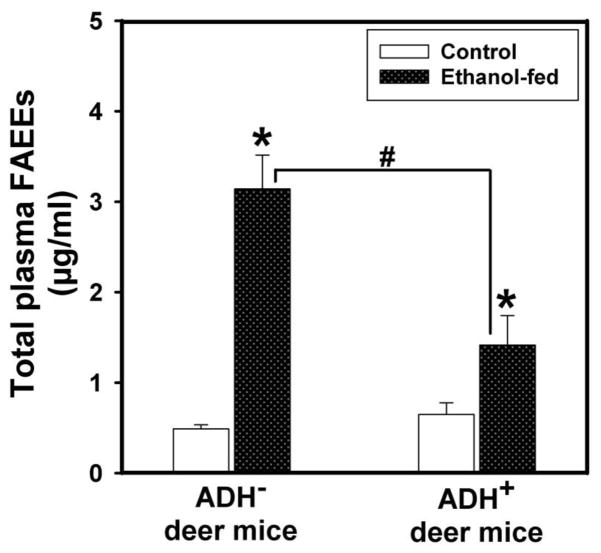
There was a significant increase for the plasma FAEEs in ethanol-fed ADH− deer mice vs. ethanol-fed ADH+ deer mice; averages being 3.14 vs. 1.42 μg/ml, respectively; palmitic (16:0) and oleic (18:1) FAEEs being the major esters found in the plasma of both strains (Fig. 6). FAEE levels in ethanol-fed ADH− and ADH+ deer mice were approximately ~6- and 2 fold greater than those in their respective pair-fed controls, suggesting that hepatic ADH deficiency significantly increases nonoxidative metabolism of ethanol.
Fig. 6.
Total FAEE concentration in the plasma of ADH− and ADH+ deer mice fed 3.5% ethanol daily for 4 months. Values are mean ± SEM. *P ≤ 0.05 for ethanol-fed ADH− or ADH+ deer mice vs. their respective pair-fed controls. #P ≤ 0.05 for ethanol-fed ADH− vs. ethanol-fed ADH+ deer mice (n=10 mice/group).
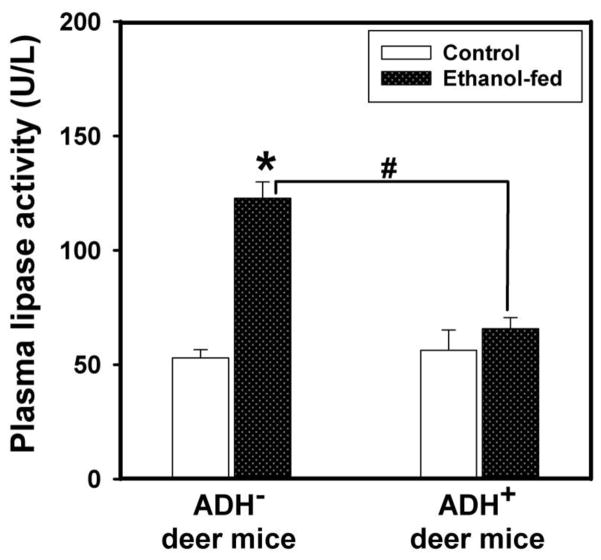
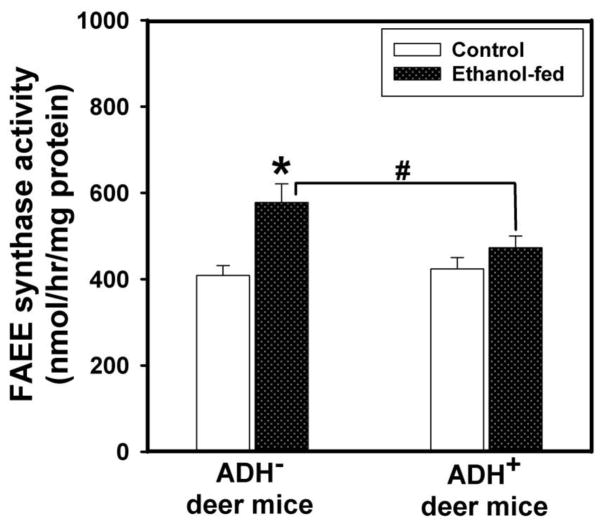
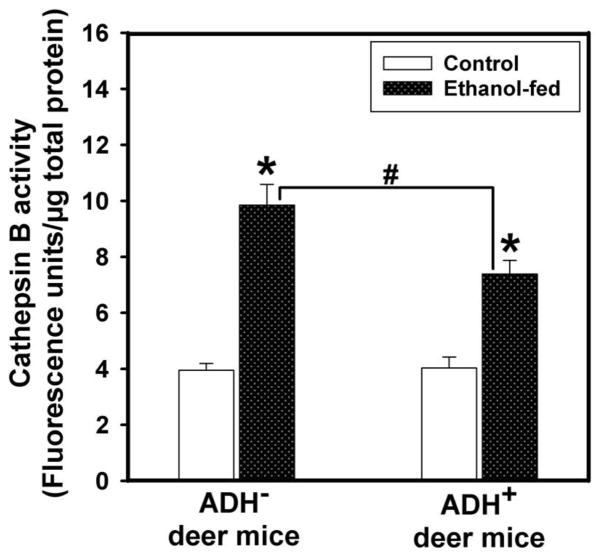
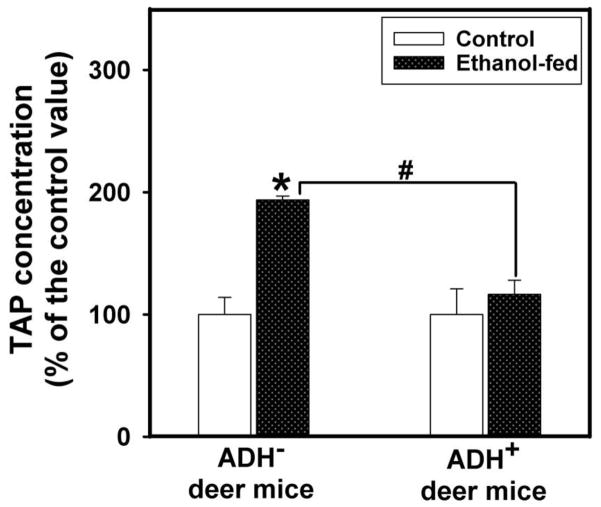
Plasma lipase activity in ethanol-fed ADH− deer mice was ~2 fold greater than those found in pair-fed ADH− deer mice and ethanol-fed ADH+ deer mice (Fig. 7). Pancreatic FAEE synthase activity was increased by ~1.5 fold in ethanol-fed vs. pair-fed control ADH− deer mice (Fig. 8). However, similar changes were not found between ethanol-fed and pair-fed ADH+ deer mice. Pancreatic cathepsin B activity in ethanol-fed ADH− and ADH+ deer mice was increased significantly; ~2.5 and 1.5 fold greater than those in their respective pair-fed controls, respectively (Fig. 9). On the other hand, pancreatic TAP levels were increased ~2 fold in ethanol-fed vs. pair-fed ADH− deer mice. Similar changes were not found for ethanol-fed ADH+ deer mice (Fig. 10). Although ethanol feeding increases plasma FAEEs and pancreatic cathepsin B in ethanol-fed vs. pair-fed ADH+ deer mice, the extent of increases were much smaller than those found for ethanol-fed vs. pair-fed ADH− deer mice. Collectively, plasma lipase, and pancreatic FAEE synthase, TAP and cathepsin B, indicate significant pancreatic injury in ethanol-fed ADH− vs. ADH+ deer mice.
Fig. 7.
Lipase activity in the plasma of ADH− and ADH+ deer mice fed 3.5% ethanol daily for 4 months. Values are mean ± SEM. *P ≤ 0.05 for ethanol-fed ADH− vs. pair-fed control ADH− deer mice. #P ≤ 0.05 for ethanol-fed ADH− vs. ethanol-fed ADH+ deer mice (n=8 each).
Fig. 8.
Pancreatic FAEE synthase activity in ADH− and ADH+ deer mice fed 3.5% ethanol daily for 4 months. Values are mean ± SEM. *P ≤ 0.05 for ethanol-fed ADH− vs. pair-fed control ADH− deer mice. #P ≤ 0.05 for ethanol-fed ADH− vs. ethanol-fed ADH+ deer mice (n=5 mice/group).
Fig. 9.
Pancreatic cathepsin B activity in ADH− and ADH+ deer mice fed 3.5% ethanol daily for 4 months. Values are mean ± SEM. *P ≤ 0.05 for ethanol-fed ADH− or ADH+ deer mice vs. their respective pair-fed controls. #P ≤ 0.05 for ethanol-fed ADH− vs. ethanol-fed ADH+ deer mice (n=5 mice/group).
Fig. 10.
Pancreatic TAP activity in the plasma of ADH− and ADH+ deer mice fed daily 3.5% ethanol for 4 months. Values are mean ± SEM. *P 0.05 for ethanol-fed ADH− vs. pair-fed control ADH− deer mice. #P ≤ 0.05 for ethanol-fed ADH− vs. ethanol-fed ADH+ deer mice (n=5 mice/group).
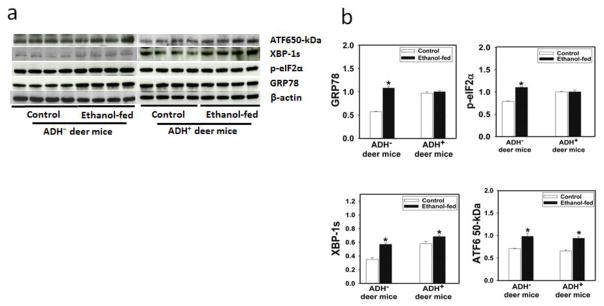
Greater expression of GRP78 (a prominent ER membrane stress marker) was found only in the pancreas of ethanol-fed ADH− deer mice. However, the significantly increased expression of XBP-1s and ATF 50kDa of IRE1α and ATF6 pathways of unfolded protein response (UPR), respectively, in both strains of deer mice fed ethanol suggest their lack of specificity as marker of ER stress. Although the data for PERK, IRE 1α, and ATF6 (three branches of UPR) were inconclusive and observed no significant changes for CHOP (data not shown), an increased expression of phosphorylated eIF2α of PERK pathway of UPR in the pancreas of ethanol-fed ADH− deer mice only is remarkable (Fig. 11). These results collectively indicate specificity of phosphorylated eIF2α as marker of ER stress and a target UPR molecule associated with metabolic basis of pancreatic toxicity of ethanol in deer mouse model.
Fig. 11.
ER stress and key UPR signaling molecules as determined by Western blot analysis using antibodies against GRP78, phosphorylated-eIf2α, XBP-1s, and ATF6 (cleaved active form) in the pancreas of ADH− and ADH+ deer mice fed 3.5% ethanol daily for 4 months. *P ≤ 0.05 for ethanol-fed ADH− or ADH+ deer mice vs. their respective pair-fed controls. a) Western blot analysis, (b) Relative intensities of bands using densitometry analysis.
Discussion
Alcoholic pancreatitis is regarded as being a chronic disease; a symptomatic pancreatitis usually exhibits underlying elements of chronic pancreatitis (Brunner et al., 2004). A small proportion of alcoholic individuals ever develop chronic pancreatitis may suggests a role of cofactor(s) such as impaired hepatic ADH (a major ethanol oxidizing enzyme) commonly found in alcoholics (Kaphalia, 2011). Earlier, we have established dose-dependent increases for total body burden of ethanol and its fatty acid conjugates, fatty acid ethyl esters (FAEEs), in plasma and pancreas, and pancreatic injury in ethanol-fed ADH− deer mice (Bhopale et al., 2006; Kaphalia et al., 2010). Therefore, studies conducted in hepatic ADH− deer mouse model are relevant to understand the metabolic basis of alcoholic pancreatitis.
Rodents are generally efficient in metabolizing ethanol as shown by the blood alcohol concentration (BAC) in ADH+ deer mice. However, hepatic ADH deficiency appears to be a key factor for impaired ethanol oxidative metabolism, a major pathway of ethanol disposition, and is supported by ~3 fold greater levels of BAC in ethanol-fed ADH− vs. ADH+ deer mice in the present study, and also by our previous reports including in alcoholic patients (Kaphalia et al., 2004; Bhopale et al., 2006; Kaphalia et al., 2010). Alternately, hepatic cytochrome P450 (CYP) 2E1, known to be induced in experimental animals after chronic ethanol consumption also oxidizes ethanol to acetaldehyde (Cederbaum, 2010). Contrary, ADH− deer mice are reported to metabolize ethanol in vivo at a rate half of that found in ethanol-fed ADH+ deer mice despite of its hepatic CYP 2E1 induction (Shigeta et al., 1984; Kaphalia et al., 2010). Therefore, very high BAC in ethanol-fed ADH− vs. ADH+ deer mice along with comparable acetaldehyde levels rules out a significant role of CYP2E1 in ethanol oxidation in our model. Therefore, hepatic ADH deficiency appears to be a major metabolic condition for increased body burden of ethanol irrespective of CYP2E1 induction during chronic ethanol feeding. Contrary to high BAC, comparable, but low blood acetaldehyde levels observed in ethanol-fed ADH− and ADH+ deer mice could be associated with rapid metabolism of acetaldehyde to acetate by mitochondrial aldehyde dehydrogenase (ALDH) 2 at the same rate it is formed (Rognstead and Grunnet, 1979). Comparable but elevated amounts of BAC as found in both control groups, however, could be attributed to endogenous production of ethanol via fermentation of dextrose/maltose by the gut bacteria (Mezey et al., 1975; Kaphalia et al., 2010). Therefore, the role of gut microbiome in the production of ethanol via fermentation of sugars, major constituents of the Lieber-DeCarli liquid diet, need further evaluation, particularly in a setting of chronic alcohol consumption.
Pancreas possesses a negligible metabolic capacity to oxidize ethanol (Laposata and Lange, 1986). Significantly increased plasma FAEEs in ethanol-fed ADH− deer mice suggests that hepatic ADH deficiency markedly facilitates disposition of ethanol via a nonoxidative metabolism of ethanol to FAEEs. Our findings are parallel to the FAEEs in the pancreas and/or plasma of ethanol-fed experimental animals that are pretreated with hepatic ADH inhibitor, 4-methylpyrazole (Manautou and Carlson, 1991; Werner et al., 2002). Since average total FAEEs are reported to be ~1 and 50 μg/g in the plasma and pancreas of ADH− deer mice fed ethanol for 2 months, respectively (Kaphalia et al., 2010), we computed pancreatic levels of FAEEs in the range of 150 μg/g based upon estimated current levels of ~3 μg total FAEEs/ml plasma. Therefore, increasing the duration of ethanol feeding in the ADH− deer mouse model may substantially increase pancreatic FAEEs and cause pancreatic injury as shown by the histological examination. The pancreatic FAEE levels projected herein are likely due to an induction of pancreatic FAEE synthase in ethanol-fed ADH− deer mice as reported in other animal models after long-term ethanol exposure (Huang and Hui, 1994; Pfützer et al., 2002).
Exocrine pancreas appears to be the prime target of ethanol toxicity while islets of Langerhans were intact and spared in ADH− deer mice. Electron micrographs of the pancreas of ethanol-fed ADH− deer mice showing dilation and swelling of endoplasmic reticulum (ER) and disintegration of ER cisternae, supports our histopathologic observations regarding targeted ethanol injury to the exocrine pancreatic gland. The morphological changes observed in the exocrine pancreas are consistent with elevated levels of injury markers [plasma lipase and pancreatic trypsinogen activation peptide (TAP) and cathepsin B] in ethanol-fed ADH− deer mice. Significantly greater levels of plasma lipase and pancreatic TAP in ethanol-fed ADH− vs. ADH+ deer mice support a role of hepatic ADH inhibition in ethanol-induced pancreatic injury. These results are consistent with observations of development pancreatic edema, and ultrastructural changes in acinar cells following an intra-arterial infusion of FAEEs in rats (Werner et al., 1997). Therefore, higher body burden of ethanol and associated formation of FAEEs in a chronic ethanol feeding model of ADH− deer mouse, may increase the susceptibility of the pancreas to ethanol-induced pancreatic injury. Cathepsin B (one of the lysosomal hydrolases), capable of activating trypsinogen to trypsin; a critical mediator of cell death by necrosis and inflammatory processes, may be released due to increased fragility of the lysosomal membrane by FAEEs (Haber et al., 1993; Lankisch and Banks, 1998; Halangk et al., 2000; Fortunato et al., 2006; Wang et al., 2006; Ji et al., 2009).
Pancreatic steatosis is an early stage pathologic process in alcoholic pancreatitis (Wilson et al., 1982; Simsek and Singh, 1990; López et al., 1996). How such early stage of steatosis progresses to inflammation is not well understood. We hypothesize that chronic alcohol exposure impairs hepatic ADH, and results with increased body burden of ethanol, that in turn leads to a substantial nonoxidative metabolism of ethanol to FAEEs. Therefore, a high concentration of ethanol circulating to the pancreas, the availability of free fatty acids for endogenous synthesis of FAEEs during chronic alcohol abuse may be key factor for the development of alcoholic pancreatitis. Most likely, the synthesis of FAEEs takes place in ER membranes of pancreatic acinar cells; a major site for the synthesis of pancreatic proteins and lipids, as ~5 fold greater levels of FAEEs were found in the microsomal and mitochondrial fractions, than in those of the cytosolic fraction of the pancreatic homogenate incubated with [1-14C] ethanol (unpublished data). Being lipophilic, FAEEs accumulate in lipid-rich membranes of the exocrine pancreas and may impair membrane functions and/or be transported to mitochondria along with fatty acids, causing mitochondrial toxicity such as uncoupling of oxidative phosphorylation (Lange and Sobel, 1983). FAEEs are known to consistently evoke Ca2+ release from the intracellular stores, increase the fragility of lysosomal membrane, activate trypsinogen (zymogen) and cause cell death (Haber et al., 1993; Bhopale et al., 2006; Wu et al., 2008; Huang et al., 2014). These esters moreover have been shown to activate key regulators of the inflammation and other pro-inflammatory signaling molecules (Kaphalia and Ansari, 2001; Wu et al., 2008).
ER membrane stress induces an unfolded protein response (UPR) by activating three main ER transducers (IRE 1α, PERK and ATF6) to reinstate homeostasis of the ER membrane and its functional characteristics. Aggregation of misfolded proteins in the ER lumen leads to ER stress in a number of diseases, and may induce UPR, an evolutionarily conserved mechanism whereby cells respond to stress conditions that target ER membranes (Reddy et al., 2003; Kaplowitz and Ji, 2006; Kubisch and Logsdon, 2007). Increased expression of GRP78, as found only in the pancreas of ethanol-fed ADH− deer mice, may be associated with increased endogenous biosynthesis of FAEEs in the ER membrane of the exocrine pancreas. An increased expression of phosphorylated eIF2α as found in the pancreas of ethanol-fed ADH− deer mice is important because phosphorylation of the eIF2α subunit is known to down regulate protein translation under a variety of stress conditions (Kaplowitz and Ji, 2006). IRE-1α splices XBP-1 mRNA to a spliced form (XBP-1s), a key transcriptional activator that induces the expression of many UPR responsive genes/chaperones. In ER stressed cells, ATF 90kDa (an ER membrane protein) is converted to ATF 50kDa leading to induced transcription. However, increased expression of XBP-1s and phosphorylated eIF2α in both ethanol-fed strains, suggests their lack of specificity in ethanol-induced pancreatic ER stress in our model. In addition, lack of significant changes for CHOP and apoptosis as found in both ethanol-fed groups are supportive vice versa. Overall, significant changes in ER stress and UPR signaling (phosphorylated eIF2α) as observed in the pancreas of ethanol-fed ADH− deer mice appears to be a key target pathway to understand molecular mechanism and a metabolic basis of alcoholic pancreatic injury.
In conclusion, our data suggest that altered lipid homeostasis and increased biosynthesis of FAEEs in the pancreas are closely associated with ER stress and injury observed in the pancreas of ADH− deer mice fed ethanol. A prolonged ER stress, greater biosynthesis and accumulation of FAEEs and sustained Ca2+ release from ER membranes can also induce pro-inflammatory cytokines and related cytotoxic events leading to inflammation and ultimately necrotic cell death (Huang et al., 2014). Therefore, biosynthesis and accumulation of FAEEs in the exocrine pancreatic tissue resulting in pancreatic injury and ER stress in ethanol-fed ADH− deer mice collectively suggest metabolic basis of alcoholic pancreatic injury and offer molecular targets for reversal of the disease process. Therefore, determining upstream AMPK signaling and conducting functional studies related to eIF2α phosphorylation could be important for understanding the mechanism of alcoholic chronic pancreatitis.
Highlights.
Ethanol-induced pancreatic injury was studied in hepatic ADH deficient deer mice.
A key factor in ethanol-induced pancreatic injury was hepatic ADH deficiency.
Ethanol induced pancreatic injury could be initiated by FAEEs and ER stress.
Phosphorylated eIF2α could be a therapeutic target in ethanol-induced pancreatitis.
Acknowledgments
This work is supported by grants AA24699 and AA25850 funded by National Institutes on Alcohol Abuse and Alcoholism, NIH. The analysis of FAEEs was conducted in the Exposure Assessment and Biomarker Development laboratory supported by Sealy Center of Environmental Health & Medicine and NIEHS Center grant P30ES06676. Authors also acknowledge the assistance of Research Histopathology Core, UTMB. Samir Amer was supported by Egyptian Cultural and Educational Bureau (Egypt) (grant No. J2832).
Footnotes
The authors declare no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bhopale KK, Wu H, Boor PJ, Popov VL, Ansari GAS, Kaphalia BS. Metabolic basis of ethanol-induced hepatic and pancreatic injury in hepatic alcohol dehydrogenase deficient deer mice. Alcohol. 2006;39:179–188. doi: 10.1016/j.alcohol.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Brunner R, Xie J, Banks S. Does acute alcoholic pancreatitis exists with preexisting chronic pancreatitis? Journal of Clinical Gastroenterology. 2004;38:201–202. doi: 10.1097/00004836-200403000-00001. [DOI] [PubMed] [Google Scholar]
- Cederbaum AI. Role of CYP2E1 in ethanol-induced oxidant stress, fatty liver and hepatotoxicity. Digestive Diseases. 2010;28:802–811. doi: 10.1159/000324289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens DL, Mahan KJ. Alcoholic pancreatitis: Lessons from the liver. World Journal of Gastroenterology. 2010;16:1314–1320. doi: 10.3748/wjg.v16.i11.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortunato F, Deng X, Gates LK, McClain CJ, Bimmler D, Graf R, Whitcomb DC. Pancreatic response to endotoxin after chronic alcohol exposure: switch from apoptosis to necrosis? American Journal of Physiology. Gastrointestinal and Liver Physiology. 2006;290:G232–241. doi: 10.1152/ajpgi.00040.2005. [DOI] [PubMed] [Google Scholar]
- Haber PS, Wilson JS, Apte MV, Pirola RC. Fatty acid ethyl esters increase rat pancreatic lysosomal fragility. The Journal of Laboratory and Clinical Medicine. 1993;121:759–764. [PubMed] [Google Scholar]
- Halangk W, Lerch MM, Brandt-Nedelev B, Roth W, Ruthenbuerger M, Reinheckel T, Domschke W, Lippert H, Peters C, Deussing J. Role of cathepsin B in intracellular trypsinogen activation and the onset of acute pancreatitis. The Journal of Clinical Investigation. 2000;106:773–781. doi: 10.1172/JCI9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herreros-Villanueva M, Hijona E, Bañales JM, Cosme A, Bujanda L. Alcohol consumption on pancreatic diseases. World Journal of Gastroenterology. 2013;19:638–647. doi: 10.3748/wjg.v19.i5.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Booth DM, Cane MC, Chvanov M, Javed MA, Elliott VL, Armstrong JA, Dingsdale H, Cash N, Li Y, Greenhalf W, Mukherjee R, Kaphalia BS, Jaffar M, Petersen OH, Tepikin AV, Sutton R, Criddle DN. Fatty acid ethyl ester synthase inhibition ameliorates ethanol-induced Ca2+-dependent mitochondrial dysfunction and acute pancreatitis. Gut. 2014;63:1313–1324. doi: 10.1136/gutjnl-2012-304058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Hui DY. Increased cholesterol esterase level by cholesterol loading of rat pancreatoma cells. Biochimica et Biophysica Acta. 1994;1214:317–322. doi: 10.1016/0005-2760(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Ji B, Gaiser S, Chen X, Ernst SA, Logsdon CD. Intracellular trypsin induces pancreatic acinar cell death but not NF-kappaB activation. The Journal of Biological Chemistry. 2009;284:17488–17498. doi: 10.1074/jbc.M109.005520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaphalia BS. Pancreatic Toxicology. In: Nriagu JO, editor. Encyclopedia of Environmental Health. Elsevier; Burlington: 2011. pp. 315–324. [Google Scholar]
- Kaphalia BS, Ansari GA. Fatty acid ethyl esters and ethanol-induced pancreatitis. Cellular and Molecular Biology (Noisy-Le-Grand, France) 2001;47:OL173–L179. Online. [PubMed] [Google Scholar]
- Kaphalia BS, Ansari GAS. Purification and characterization of rat pancreatic fatty acid ethyl ester synthase and its structural and functional relationship to pancreatic cholesterol esterase. Journal of Biochemical and Molecular Toxicology. 2003;17:338–345. doi: 10.1002/jbt.10097. [DOI] [PubMed] [Google Scholar]
- Kaphalia BS, Bhopale KK, Kondraganti S, Wu H, Boor PJ, Ansari GAS. Pancreatic injury in hepatic alcohol dehydrogenase-deficient deer mice after subchronic exposure to ethanol. Toxicology and Applied Pharmacology. 2010;246:154–162. doi: 10.1016/j.taap.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaphalia BS, Cai P, Khan MF, Okorodudu AO, Ansari GAS. Fatty acid ethyl esters: Markers of alcohol abuse and alcoholism. Alcohol. 2004;34:151–158. doi: 10.1016/j.alcohol.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Kaphalia BS, Ghanayem BI, Ansari GA. Nonoxidative metabolism of 2-butoxyethanol via fatty acid conjugation in Fischer 344 rats. Journal of Toxicology and Environmental Health. 1996;49:463–479. doi: 10.1080/009841096160691. [DOI] [PubMed] [Google Scholar]
- Kaplowitz N, Ji C. Unfolding new mechanisms of alcoholic liver disease in the endoplasmic reticulum. Journal of Gastroenterology and Hepatology. 2006;21(Suppl 3):S7–9. doi: 10.1111/j.1440-1746.2006.04581.x. [DOI] [PubMed] [Google Scholar]
- Kubisch CH, Logsdon CD. Secretagogues differentially activate endoplasmic reticulum stress responses in pancreatic acinar cells. American Journal of Physiology Gastrointestinal and Liver Physiology. 2007;292:G1804–1812. doi: 10.1152/ajpgi.00078.2007. [DOI] [PubMed] [Google Scholar]
- Lange LG, Sobel BE. Mitochondrial dysfunction induced by fatty acid ethyl esters, myocardial metabolites of ethanol. The Journal of Clinical Investigation. 1983;72:724–731. doi: 10.1172/JCI111022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankisch PG, Banks PA. Pancreatitis. New York, Berlin, Heidelberg: Springer-Verlag; 1998. p. 377. [Google Scholar]
- Laposata EA, Lange LG. Presence of nonoxidative ethanol metabolism in human organs commonly damaged by ethanol abuse. Science (New York, NY) 1986;231:497–499. doi: 10.1126/science.3941913. [DOI] [PubMed] [Google Scholar]
- Lieber CS. Cytochrome P-4502E1: its physiological and pathological role. Physiological Reviews. 1997;77:517–544. doi: 10.1152/physrev.1997.77.2.517. [DOI] [PubMed] [Google Scholar]
- López JM, Bombi JA, Valderrama R, Giménez A, Parés A, Caballería J, Imperial S, Navarro S. Effects of prolonged ethanol intake and malnutrition on rat pancreas. Gut. 1996;38:285–292. doi: 10.1136/gut.38.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manautou JE, Carlson GP. Ethanol-induced fatty acid ethyl ester formation in vivo and in vitro in rat lung. Toxicology. 1991;70:303–312. doi: 10.1016/0300-483x(91)90005-l. [DOI] [PubMed] [Google Scholar]
- Mezey E, Imbembo AL, Potter JJ, Rent KC, Lombardo R, Holt PR. Endogenous ethanol production and hepatic disease following jejunoileal bypass for morbid obesity. The American Journal of Clinical Nutrition. 1975;28:1277–1283. doi: 10.1093/ajcn/28.11.1277. [DOI] [PubMed] [Google Scholar]
- NIAAA (National Institute on Alcohol Abuse and Alcoholism) 10th Special Report to the U.S. Congress on Alcohol and Health. U.S. Department of Health and Human Services, NIH; 2000. Alcohol Health Services Research. [Google Scholar]
- Nuutinen H, Lindros KO, Salaspuro M. Determinants of Blood Acetaldehyde Level during Ethanol Oxidation in Chronic Alcoholics. Alcoholism: Clinical and Experimental Research. 1983;72:163–168. doi: 10.1111/j.1530-0277.1983.tb05432.x. [DOI] [PubMed] [Google Scholar]
- Panés J, Caballería J, Guitart R, Parés A, Soler X, Rodamilans M, Navasa M, Parés X, Bosch J, Rodés J. Determinants of ethanol and acetaldehyde metabolism in chronic alcoholics. Alcoholism: Clinical and Experimental Research. 1993;17:48–53. doi: 10.1111/j.1530-0277.1993.tb00725.x. [DOI] [PubMed] [Google Scholar]
- Panés J, Soler X, Parés A, Caballería J, Farrés J, Rodés J, Parés X. Influence of liver disease on hepatic alcohol and aldehyde dehydrogenases. Gastroenterology. 1989;97:708–714. doi: 10.1016/0016-5085(89)90642-2. [DOI] [PubMed] [Google Scholar]
- Pfützer RH, Tadic SD, Li HS, Thompson BS, Zhang JY, Ford ME, Eagon PK, Whitcomb DC. Pancreatic cholesterol esterase, ES-10, and fatty acid ethyl ester synthase III gene expression are increased in the pancreas and liver but not in the brain or heart with long-term ethanol feeding in rats. Pancreas. 2002;25:101–106. doi: 10.1097/00006676-200207000-00021. [DOI] [PubMed] [Google Scholar]
- Pitchumoni CS, Glasser M, Saran RM, Panchacharam P, Thelmo W. Pancreatic Fibrosis in Chronic Alcoholics and Nonalcoholics without Clinical Pancreatitis. The American Journal of Gastroenterology. 1984;79:382–388. [PubMed] [Google Scholar]
- Reddy RK, Mao C, Baumeister P, Austin RC, Kaufman RJ, Lee AS. Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors. Role of ATP binding site in suppression of caspase-7 activation. Journal of Biological Chemistry. 2003;278:20915–20924. doi: 10.1074/jbc.M212328200. [DOI] [PubMed] [Google Scholar]
- Rinderknecht H. Activation of pancreatic zymogens. Normal activation, premature intrapancreatic activation, protective mechanisms against inappropriate activation. Digestive Diseases and Sciences. 1986;31:314–321. doi: 10.1007/BF01318124. [DOI] [PubMed] [Google Scholar]
- Rognstead R, Grunnet N. Enzymatic pathways of ethanol metabolism. In: Majchrowicz E, Noble EP, editors. Biochemistry and Pharmacology of Ethanol. New York, NY: Plenum Press; 1979. pp. 65–86. [Google Scholar]
- Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, Brewer RD. 2010 National and State Costs of Excessive Alcohol Consumption. American Journal of Preventive Medicine. 2015;49:e73–e79. doi: 10.1016/j.amepre.2015.05.031. [DOI] [PubMed] [Google Scholar]
- Sharkawi M. In vivo inhibition of liver alcohol dehydrogenase by ethanol administration. Life Sciences. 1984;35:2353–2357. doi: 10.1016/0024-3205(84)90527-7. [DOI] [PubMed] [Google Scholar]
- Shigeta Y, Nomura F, Iida S, Leo MA, Felder MR, Lieber CS. Ethanol metabolism in vivo by the microsomal ethanol-oxidizing system in deer mice lacking alcohol dehydrogenase (ADH) Biochemical Pharmacology. 1984;33:807–814. doi: 10.1016/0006-2952(84)90466-0. [DOI] [PubMed] [Google Scholar]
- Simsek H, Singh M. Effect of prolonged ethanol intake on pancreatic lipids in the rat pancreas. Pancreas. 1990;5:401–407. doi: 10.1097/00006676-199007000-00005. [DOI] [PubMed] [Google Scholar]
- Singh M, Simsek H. Ethanol and the pancreas. Current status. Gastroenterology. 1990;98:1051–1062. doi: 10.1016/0016-5085(90)90033-w. [DOI] [PubMed] [Google Scholar]
- Wang YL, Hu R, Lugea A, Gukovsky I, Smoot D, Gukovskaya AS, Pandol SJ. Ethanol feeding alters death signaling in the pancreas. Pancreas. 2006;32(4):351–359. doi: 10.1097/01.mpa.0000220859.93496.e1. [DOI] [PubMed] [Google Scholar]
- Werner J, Laposata M, Fernández-del Castillo C, Saghir M, Iozzo RV, Lewandrowski KB, Warshaw AL. Pancreatic injury in rats induced by fatty acid ethyl ester, a nonoxidative metabolite of alcohol. Gastroenterology. 1997;113:286–294. doi: 10.1016/s0016-5085(97)70106-9. [DOI] [PubMed] [Google Scholar]
- Werner J, Saghir M, Warshaw AL, Lewandrowski KB, Laposata M, Iozzo RV, Carter EA, Schatz RJ, Fernández-Del Castillo C. Alcoholic pancreatitis in rats: injury from nonoxidative metabolites of ethanol. American Journal of Physiology Gastrointestinal and Liver Physiology. 2002;283:G65–73. doi: 10.1152/ajpgi.00419.2001. [DOI] [PubMed] [Google Scholar]
- Wilson JS, Colley PW, Sosula L, Pirola RC, Chapman BA, Somer JB. Alcohol Causes a Fatty Pancreas. A Rat Model of Ethanol-induced Pancreatic Steatosis. Alcoholism: Clinical and Experimental Research. 1982;6:117–121. doi: 10.1111/j.1530-0277.1982.tb05389.x. [DOI] [PubMed] [Google Scholar]
- Wu H, Bhopale KK, Ansari GA, Kaphalia BS. Ethanol-induced cytotoxicity in rat pancreatic acinar AR42J cells: role of fatty acid ethyl esters. Alcohol and Alcoholism. 2008;43:1–8. doi: 10.1093/alcalc/agm044. [DOI] [PubMed] [Google Scholar]