Abstract
This study adopted a person-centered approach to identify preadolescent salivary cortisol (sC) and alpha-amylase (sAA) co-activation response patterns and examine links to behavioral functioning and coping. Children (N=151, 51.7% male) were exposed to the Trier Social Stress Test (TSST) and one of two randomly-assigned, post-TSST coping conditions: distraction or avoidance. Multi-trajectory modeling yielded four child subgroups. Child internalizing and externalizing positively predicted High–sC High sAA relative to Low sC–Low sAA and Low sC–High sAA relative to High sC–Low sAA subgroup membership, respectively. Low sC–Low sAA children demonstrated more efficient sC recovery when primed with distraction and more protracted sC recovery when primed with avoidance. For High sC–High sAA, internalizing children, the opposite was true. Findings illustrate adjustment-linked variability in preadolescent sC–sAA co-activation response patterns that further articulates for whom effortful coping works to effectively manage stressor-induced neuroendocrine activation.
Keywords: coping, preadolescence, cortisol, alpha-amylase, trajectories
Introduction
Hypothalamic-pituitary-adrenal axis (HPA) and sympathetic-adrenomedullary system (SAM) co-activation has been implicated in the development of psychopathology (Bauer, Quas, & Boyce, 2002; Hastings et al., 2011), leading to the need to understand individual differences in HPA–SAM co-activation in childhood and adolescence. Theorists have posited that both symmetrical (e.g., high HPA–high SAM, low HPA–low SAM) and asymmetrical (e.g., low HPA–high SAM, high HPA–low SAM) activity is potentially reflective of neuroendocrine response dysregulation linked to children’s internalizing and externalizing behavior (Bauer et al., 2002). When assessed via HPA (salivary costisol, sC) and SAM (salivary alpha-amylase, sAA) indices in response to laboratory stressor paradigms, early adolescent studies have found mixed support for each proposition. Thus, there remains a lack of consensus on what constitutes well-functioning as well as dysregulated HPA-SAM co-activation response patterns.
The evidence supporting these views is based on variable-centered approaches that assume preadolescents exhibit more or less the same pattern of HPA–SAM co-activation. However, theory suggests that preadolescence is a period of substantive reformation to the HPA and SAM, when children are exposed to novel psychosocial stressors that shape their development (Spear, 2000; Steinberg, 2014). If so, then variable-centered approaches may overlook the possibility that certain subgroups of children evidence qualitatively distinct patterns of HPA–SAM co-activation that may be uniquely linked to their behavioral adjustment. Person-centered approaches that incorporate multiple, theory-driven neuroendocrine response indices (e.g., sC, sAA) have the potential to identify these subgroups. Such an approach may also provide a more precise approximation of theoretical cross-system activation patterns (Bauer et al., 2002) that index healthy functioning or signal risk for psychopathological development (Rutter, 2007).
Children’s coping, defined as effortful emotional, cognitive, and behavioral attempts to manage a stressor or children’s emotional/cognitive/behavioral reactions to it, is thought to buffer against such psychopathological development by mitigating against one of the mechanisms of risk; neuroendocrine response dysregulation (Wadsworth, 2015). Recent evidence suggests that children’s coping does in fact get “underneath the skin” (Foland-Ross, Kircanski, & Gotlib, 2014; Sladek, Doane, & Stroud, 2017), supporting quick, efficient recovery of stressor-induced neuroendocrine activation (Stewart, Mazurka, Bond, Wynne-Edwards, & Harkness, 2013). This research points to putative mechanisms of change to be leveraged in the design of coping interventions (Davidson & McEwen, 2012). However, reliance on variable-centered designs has limited understanding about for whom certain types of coping combat the potentially damaging effects of stress. If subgroups of children with distinct, adjustment-linked HPA-SAM co-activation patterns exist whose post-stressor neuroendocrine recovery is differentially influenced by specific forms of coping, then variable-centered approaches may obscure these differences (von Eye & Bogat, 2006). Person-centered analysis of this sort may bolster inference about patient-centered means of tailoring intervention content towards children’s coping strengths and weaknesses. Thus, using a community sample of preadolescent boys and girls exposed to the Trier Social Stress Test (TSST-C; Buske-Kirschbaum et al., 1997), the current study adopted a novel person-centered approach to identify HPA–SAM co-activation profiles based children’s sC–sAA response patterns, examine links to children’s internalizing and externalizing behavior, and test if two coping skills differentially contributed to sC–sAA recovery efficiency for the identified subgroups.
Models of HPA-SAM Co-activation and Links to Behavioral Functioning
Current theory posits that the sympathetic nervous system (SNS), of which the SAM is a part, and HPA respond in a coordinated fashion to support adaptation both during and following a stressor (Bauer et al., 2002; Hastings et al., 2011). Initial changes that occur in support of this adaptation take place in the SNS and involve a taxing mobilization of cardiovascular, immunologic, and central nervous system resources to quickly neutralize immediate threat. A positive feedback loop between the SNS and HPA stimulates subsequent glucocorticoid production, mobilizing longer-term resources to support adaptation to more prolonged threat exposure (Chrousos & Gold, 1992). Cortisol produced by the SNS-innervated HPA helps suppress the initial SNS response, protecting the body from damage that may result from protracted, energy-depleting SNS activity. Well-functioning HPA-SNS co-activation might involve a brief SNS response to quickly neutralize threat that does not necessitate a more dramatic HPA response or stimulates cortisol production in the event that immediate threat is not neutralized (Sapolsky, Romero, & Munck, 2000). Alternatively, HPA-SNS dysregulation might involve elevated SNS activity that fails to innervate the HPA or elevated cortisol mobilization that fails to terminate SNS activity (e.g., Koss et al., 2014).
Consensus on what constitutes neuroendocrine response dysregulation as it pertains to HPA–SNS co-activation has yet to be reached. Bauer and colleagues (2002) proposed two models of HPA and SNS (in particular the SAM system) co-activation that may manifest as symptoms of psychopathology. The Additive-Symmetry Model predicts that symmetric activation of the HPA and SNS would indicate risk and be associated with symptomatic functioning. The alternative Interactive-Asymmetry Model proposes that asymmetric activation would indicate risk for poor behavioral functioning. The available early adolescent findings lack coherence to support either model as each study has used varied methods for examining HPA–SAM co-activation in both non-clinical and at-risk children. Support for the Additive-Symmetry model has been found when examining sC-sAA basal levels to internalizing associations (El-Sheikh et al., 2008) and combinations of sC-sAA basal and reactivity level (e.g., area-under-curve with respect to ground; AUCg) to externalizing links (Gordis et al., 2006). Support for the Interactive-Asymmetry model has also been found when examining sC-sAA basal level to externalizing associations (Chen, Raine, & Granger, 2015) and sC-sAA percent increase to problem behavior links (Allwood et al., 2011). Still others have found support for both models within the same study, noting specific directions of symmetry (high HPA–high SAM) and asymmetry (low HPA–high SAM) in predicting children’s behavioral functioning (Koss et al., 2014).
To better understand individual differences in HPA–SAM co-activation to behavioral functioning linkages, three issues must be addressed. First, the inconsistent use of basal levels (e.g., pre-TSST levels, average of pre- and post-TSST levels) or reactivity indices (e.g., area-under-the-curve increase, AUCi; percent increase) within and across the HPA and SAM has contributed to discrepant operationalizations of “high” and “low” neuroendocrine activity, limiting consensus about how HPA–SAM co-activation relates to behavior. As noted by Quas et al. (2014), there is a need to model various aspects of the full cross-system response, as both well-functioning and dysregulated neuroendocrine activation involve basal levels and response organization; i.e., change patterns. Second, studies that have modeled these aspects often utilize variable-centered methods (e.g., growth curves), which are critical to understanding normative HPA–SAM co-activation while also restricted in their articulation of diverse neuroendocrine response organization. As theory posits multiple well-functioning and dysregulated neuroendocrine response profiles that may bear little semblance to one another (Bauer et al., 2002; Del Giudice, Ellis, & Shirtcliff, 2011; Quas et al., 2014), person-centered methods may more appropriately capture heterogeneity in HPA–SAM co-activation, especially for studies where variability is implicit to the developmental period examined. Third, person-centered studies have usually not modeled HPA and SAM activity concurrently (Gunnar, Frenn, Wewerka, & Van Ryzin, 2009; Ji, Negriff, Kim, & Susman, 2016) or used modeling of both level and concurrent change (i.e., response trajectories) in each system (Del Giudice, Benjamin Hinnant, Ellis, & El-Sheikh, 2012; Quas et al., 2014).
Regulatory Interference and Fit Processes
Bauer and colleagues (2002) also proposed that our understanding of HPA-SAM co-activation and related behavioral functioning may be aided by attending to children’s coping behaviors in response to stress. Stressor-induced neuroendocrine activation is functional in that it mobilizes resources to help children meet the demands of a stressful experience.1 Likewise, and consistent with Responses to Stress (RTS) theory (Connor-Smith, Compas, Wadsworth, Thomsen, & Saltzman, 2000), children’s effortful coping ensues following a stressful experience, helping them modify the source of stress or modulate their reactions to it. In so doing, coping should also contribute to recovery efficiency, or the swift termination of stressor-induced neuroendocrine activation that, left unchecked, might otherwise contribute to peripheral biological “wear and tear” and behavioral maladjustment (Brosschot & Thayer, 1998; Javaras et al., 2012). To test this proposition, immediately following the TSST-C, children were experimentally primed with either behavioral distraction or cognitive avoidance coping. Following acute stress exposure, distraction is believed to combat the potentially damaging effects of stress (i.e., terminate neuroendocrine activation, efficient recovery) by helping children consciously reengage their attention to productive or soothing activities while avoidance is thought to exacerbate these effects (i.e., prolong neuroendocrine activation, protracted recovery) by inadvertently refocusing children’s attention to the stressor. Nevertheless, different coping skills may work for different children, such that there are no universals to what constitutes effective coping (Wadsworth, 2015).
As alterations to neuroendocrine activation processes (e.g., protracted recovery) increase children’s risk for development of psychopathology (Javaras et al., 2012), it is critical to understand for whom specific forms of coping buffer against such alterations following exposure to an acute stressor. Children with higher internalizing and externalizing behaviors present with increased attention to threat, worry-related cognitions, anger-related information processing biases, perceived uncontrollability, and impulsive action (Connor-Smith et al., 2000; Grant et al. 2003). Thus, distraction for children with dysregulated, internalizing/externalizing-linked HPA-SAM profiles may contribute to protracted recovery, given that these prepotent response tendencies are thought to consume executive resources needed for effective reallocation of attention to alternate activities; i.e., regulatory interference (Bendezú, Perzow, & Wadsworth, 2016; Cole, Bendezú, Ram, & Chow, 2016). While its overuse predicts negative behavioral outcomes in the long-term, avoidance for behaviorally-maladjusted, stress-affected children can have short-term salutary effects (e.g., temporary distress alleviation, Santiago & Wadsworth, 2009; Zimmer-Gembeck & Skinner, 2011). Thus, children with dysregulated, internalizing/externalizing-linked HPA-SAM profiles may evince these benefits vis-à-vis more efficient recovery; i.e., regulatory fit (Wadsworth et al., 2016; Wadsworth, 2015).
Hypotheses
Our first aim was to identify subgroups of children with distinct HPA-SAM co-activation patterns in response to a standardized psychosocial stressor (TSST-C). As per Bauer et al. (2002), we expected to identify four subgroups: Low sC–Low sAA, High sC–Low sAA, Low sC–High sAA, and High sC–High sAA. Also, we sought to determine which aspects of the HPA-SAM co-activated neuroendocrine response (e.g., baseline levels, change patterning) were in fact distinct (e.g., “higher”, “lower”) between subgroups. Our second aim was to determine whether identified subgroups reflected well-functioning or dysregulated HPA–SAM co-activation by examining the extent to which child internalizing and externalizing behavior was associated with subgroup membership. Given the novelty of this study as well as inconsistent support for both Additive-Symmetry and Interactive-Asymmetry models, we were wary of making strong predictions. However, as children in our study more closely reflected that of El-Sheikh et al. (2008), Chen et al. (2015), and Koss et al. (2014), we expected that symmetric and asymmetric HPA–SAM profiles would be associated with internalizing and externalizing behaviors, respectively. Our final aim was to determine whether different coping skills differentially contributed to sC–sAA recovery efficiency for identified subgroups. As per regulatory interference and fit hypotheses (Compas et al., 1999; Wadsworth, 2015), we expected well-functioning HPA-SAM co-activation subgroups to display efficient recovery (i.e., more steep decline in sC-sAA levels) when primed to distraction and protracted recovery (i.e., less steep decline or increase in sC-sAA levels) when primed to avoidance. Alternatively, we expected dysregulated HPA-SAM co-activation subgroups to display protracted recovery when primed to distraction and efficient recovery when primed to avoidance.
Method
Participants
Participants were 151 fourth and fifth grade children (N=151, Mage=10.33 years, SD=1.71) and one of their parents. Children brought home recruitment flyers from suburban and semirural elementary schools that directed parents to the Preadolescent Stress and Coping (PASC) study website. Interested parents enrolled their child, provided consent, and completed questionnaires online. Mothers (85.4%) tended to be the adult respondents. Child gender was evenly distributed (51.7% male). Median annual household income was $70,500 (Min=$13,638.84; Max=500,000). The majority of participants identified as White (93.4% of caregivers and 90.7% of children).
Procedures
Once enrolled, children were scheduled to complete the 90 minute (min) experiment between the hours of 3:00 and 5:30 pm. Parents were instructed to have their children refrain from brushing their teeth, consuming a large meal, dairy, or sugary and acidic foods within an hour of their study arrival time. Saliva samples were collected throughout the experiment via passive drool into vials at six time points (T1–T6). Upon arrival, children were greeted by an experimenter who escorted the child to the “home base” data collection room. Once seated at the data collection table, children briefly rinsed their mouth with bottled water and provided their written assent. An initial saliva sample was collected afterwards (T1). Next, the experimenter administered questionnaires to the child for 40 min and then collected a second saliva sample (T2). Children walked with the experimenter to a separate room (15 sec) to complete the TSST-C, a 15 min paradigm in which participants are asked to sit and prepare (5 min), then stand and deliver (5 min) a speech and complete a mental subtraction task (5 min) in front of an unresponsive “panel of experts.” Experts were confederates unknown to participants and unaware of their randomly-assigned2 coping condition. Following completion of the TSST-C, children walked with the experimenter back to home base (15 sec) and sat down at the data collection table. A third saliva sample was taken (T3). Children then walked with the experimenter (15 sec) to one of two randomly – assigned coping condition rooms to wait while the judges scored their performance; either a distraction room (n = 78) with musical instruments, art supplies, and toys placed on a table where they were asked to sit and invited to play with the materials if they wished or in an avoidance room (n = 73) free of distractions where they were instructed to sit and simply wait and to try not to think about their TSST-C performance. After 10 min, children walked with the experimenter back to home base (15 sec) and sat down at the data collection table. A fourth saliva sample was taken (T4). Children were then interviewed for 10 min about the coping strategies they used during and after the TSST-C. A fifth saliva sample was collected (T5). Next, children engaged in a 10 min seated guided progressive muscle relaxation exercise. Then, a final sixth saliva sample was collected (T6). Experimenters debriefed children after the experiment and families received $50 for participating.
Measures
Salivary cortisol (sC) and alpha amylase (sAA)
Six saliva samples were collected via passive drool (Davis, Bruce, & Gunnar, 2002) to measure HPA and SAM responses. Saliva samples were stored in a medical grade ultra-low temperature freezer and transported on dry ice to the CORE Biomarker Lab at Penn State University (Salimetrics, LLC, State College, PA). sC levels were determined using a commercial expanded-range high-sensitivity enzyme immunosorbent assay kit (No. 1-3002/1-3012; Salimetrics, LLC, State College, PA) that detects levels in the range of 0.003 to 3.0 Kg/dL (range, 0.08Y82.77 nmol/L). sAA levels were determined using a commercial kinetic reaction assay kit (No. 1-1902; Salimetrics, LLC, State College, PA). sAA activity present in a saliva sample is proportional to the increase (over a 2 min period) in absorbance at 405 nm. Results are computed in U/mL of sAA: [Absorbance difference per min × total assay volume (328 ml) × dilution factor (200)]/[millimolar absorptivity of 2-chloro-p-nitrophenol (12.9) × sample volume (.008 ml) × light path (.97)]. sC and sAA were extracted from the same saliva samples, run in duplicate, and batched in the same order as random assignment.
Internalizing and externalizing symptoms
The Behavior Assessment System for Children, Second Edition (BASC-2), was used to assess child symptoms. The BASC-2 (Reynolds & Kamphaus, 2004) is a multidimensional assessment system that measures both clinical and adaptive aspects of behavior and personality. Children complete the Self Report of Personality - Child (SRP-C, ages 8 - 11) or the Self Report of Personality-Adolescent (SRP-A, ages 12–21) and parents complete the Parent Rating Scales (PRS). On the SRP-C and SRP-A, initial items are rated on a 2-point True or False scale and subsequent items are rated in a 4-point scale from 0 (Never) to 4 (Always). The SRP and PRS are reliable and valid measures of psychopathology and problem behavior with internal consistency, α, ranging from .83 to .90, and .81 to .93, respectively, for the child, and adolescent forms and .76 to .95 for the PRS forms. The SRP lacks an Externalizing broadband scale and some research suggests that adolescents may be better reporters of internal states and parents better reporters of externalizing behavior (Jensen et al., 1999; Kolko & Kazdin, 1993). In a concerted effort to use multiple informants and maintain model parsimony, the SRP Internalizing3 and PRS Externalizing scales were used to capture internalizing and externalizing symptoms, respectively. The SRP Internalizing scale assesses atypicality, locus of control, social stress, anxiety, feelings of inadequacy, and depression. The PRS Externalizing scale assesses hyperactivity, aggression, and conduct problems. T-scores with combined gender norms were used to ensure the integrity of the broadband score results.
Coping condition manipulation check
Immediately after the coping manipulation, experimenters administered a semistructred interview during which children were prompted to describe in their own words what they did in their respective coping rooms to make the situation better or themselves feel better, even if they thought it did not work. Using the Responses to Stress Coding Manual for In Vivo Coping (Wadsworth, 2013), these free responses were coded (yes/no) for distraction (e.g., “I drew a tiger and colored”, “I played with the keyboard and Legos”) as well as avoidance (and/or associated intrusive thoughts) strategies (e.g., “Tried to get the speech out my head”, “I was trying not to think about how I did but I kind of did anyways”). There was a significant relationship between children’s assigned coping condition and their reported strategies, χ2 (1, 150) =77.27, p<.001 suggesting that children complied with prompts in the distraction (91%) and avoidance (81%) conditions.
Covariates
Covariates include child gender, puberty, and medication use. Child gender was coded 0 (boy) and 1 (girl). Pubertal timing was used as an index of child puberty and computed as per recommendations outlined in Dorn, Susman, & Ponirakis (2003). First, pubertal stage was assessed using the Pubertal Development Scale (Petersen, Crockett, Richards, & Boxer, 1998), indexed by menarche, height, body hair growth, and breast growth for girls, and by voice changes, height, body hair growth, and facial hair growth for boys. Next, pubertal stage was regressed on child chronological age (days) separately for girls and boys to calculate a residual for each participant. Positive and negative residuals indicate earlier and later timing, respectively. As recommended (Granger, Hibel, Fortunato, & Kapelewski, 2009; Rohleder & Nater, 2009), medication use was also coded and controlled for.
Analytic Plan
Data reduction and preprocessing
Participants whose age was atypical for fourth and fifth grade (n=1) and who did not participate in the 90m experiment (n=1) were excluded from analyses (N=151). Cortisol and sAA values were positively skewed and a fourth-root transformation normalized those data (Miller & Plessow, 2013).
Bivariate associations
Fisher’s Z-adjusted correlations were used to examine bivariate associations. As research has demonstrated an association between gender, pubertal status, and neuroendocrine responses (Stroud et al., 2011), partial correlations controlling for these variables in addition to medication use were conducted.
MTM specification and adequacy evaluation
Multi-trajectory modeling (MTM; Nagin, Jones, Lima Passos, & Tremblay, 2016) was adopted to achieve our first aim of examining HPA-SAM co-activation profiles. MTM identifies subgroups in a population that follow similar patterns (i.e., intercepts, rates of change) of development across multiple indicators of a specific construct. In this study, MTM was used to identify subgroups of preadolescent children with similar HPA–SAM co-activation patterns based on the extent to which they exhibited similar sC and sAA indicator trajectories (i.e., T1 baselines, response patterns). The PROC TRAJ procedure (SAS 9.3; Jones, Nagin, & Roeder, 2001) with the MULTGROUPS option employed was specified to operate on a censored norm distribution model. PROC TRAJ handles missing data by utilizing Full-Information-Maximum likelihood (FIML) when estimating model parameters, which is suitable when data are assumed to be missing at random (MAR) or missing completely at random (MCAR). Little’s (1988) missing completely at random (MCAR) test was conducted for all key demographic, covariate and study variables and found to be non-significant; χ 2 (207) = 167.18, p > .250. Thus, the data could be MCAR. First, we identified the optimal number of subgroups the polynomial function that best described the shape of each group’s sC and sAA trajectory. To specify the best fitting model, 4th order polynomial functions were modeled for the initial single and more complex multi-group solutions. At each step, non-significant polynomial functions were eliminated. The log Bayes factor approximation [2loge(B10)] was utilized as a fit index at each step during model specification (Jones et al., 2001). A log Bayes factor greater than 10 is said to be strong evidence for the superior fit of the more complex model. Given our sample size (N = 151), modeling recommendations (N > 100; Nagin, 2005), and HPA-SAM co-activation theory (Bauer et al., 2002), we limited model specification to four groups. After specification, we evaluated model adequacy by calculating average posterior probability (AvePPj), odds of correct classification (OCCj), and the ratio of the probability of group assignment to the proportion of children assigned to subgroups (Nagin, 2005).
sC–sAA trajectory distinction
Because MTM models sC and sAA full response trajectories, follow-up analyses can also be used to distinguish which aspects of these trajectories (i.e., baseline levels, response patterns) are comparatively “higher” and “lower” for specific children. Thus, following MTM specification and adequacy evaluation, trajectory distinction analyses were performed with a series of Wald tests comparing T1 intercept and polynomial (i.e., change patterning) coefficient estimates for each identified subgroup’s sC and sAA trajectories.
Predictors of sC–sAA response trajectories
To achieve our second aim, multinomial logistic regression (with listwise deletion to handle missing predictor data; 1.9%) was conducted to predict identified subgroup membership using PROC CATMOD (SAS 9.3). Covariates and predictors were entered together in a single step.
sC–sAA response to coping condition
For our final aim, children’s randomly-assigned coping condition was examined as a time-varying covariate (Nagin, 2005), testing the effect of our manipulation as a turning-point event (i.e., “fork in the road”) along each subgroup’s unique sC and sAA trajectories. That is, we tested whether each subgroup’s sC and sAA trajectories were altered (more efficient or protracted recovery) by their coping condition in expected ways. As sAA activity can be observed within moments of stress exposure whereas the sC response is typically delayed (De Kloet, Joëls, & Holsboer, 2005), T3–T6 sAA and T4–T6 sC recovery-phase sample time likely indexed coping condition effects and contributions to sC and sAA recovery efficiency. Efficient recovery was operationalized as a more dramatic decrease in recovery-phase sC and sAA levels. Protracted recovery was operationalized as less dramatic decrease or continued increase in recovery-phase sC and sAA levels.
Results
Descriptive and bivariate statistics are shown in Table 1. sC and sAA values for children who were and were not taking medication did not significantly differ (all p>.25). Medication use was still controlled for in all bivariate and predictor analyses. sC levels during the experiment were highly correlated, as were sAA levels.
Table 1.
Descriptives and Partial Correlations for Child Behavior Indices, Coping Condition, and Stress Hormone Concentrations
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | (11) | (12) | (13) | (14) | (15) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (1) IP | — | ||||||||||||||
| (2) EP | .18* | — | |||||||||||||
| (3) Conditiona | .08 | −.03 | — | ||||||||||||
| (4) sC–T1 | −.16 | −.12 | −.02 | — | |||||||||||
| (5) sC–T2 | −.23* | −.14 | −.01 | .69* | — | ||||||||||
| (6) sC–T3 | −.14 | −.07 | .10 | .51* | .83* | — | |||||||||
| (7) sC–T4 | .04 | .04 | .11 | .17 | .38* | .64* | — | ||||||||
| (8) sC–T5 | .01 | .11 | .11 | .12 | .31* | .58* | .94* | — | |||||||
| (9) sC–T6 | −.06 | .12 | .14 | .15 | .35* | .62* | .89* | .96* | — | ||||||
| (10) sAA–T1 | .17 | .10 | .08 | −.01 | −.08 | −.06 | −.06 | −.01 | −.02 | — | |||||
| (11) sAA–T2 | .07 | .17 | .06 | −.01 | −.02 | .12 | .35* | .45* | .44* | .62* | — | ||||
| (12) sAA–T3 | .17 | .02 | .06 | −.09 | −.01 | .13 | .27* | .32* | .31* | .66* | .88* | — | |||
| (13) sAA–T4 | .13 | .14 | .12 | −.11 | −.04 | .12 | .32* | .38* | .38* | .67* | .81* | .84* | — | ||
| (14) sAA–T5 | .11 | .11 | .07 | −.08 | −.07 | .10 | .28* | .36* | .36* | .62* | .81* | .84* | .89* | — | |
| (15) sAA–T6 | .15 | .18* | .05 | −.01 | .01 | .15 | .33* | .40* | .41* | .57* | .87* | .81* | .87* | .85* | — |
|
| |||||||||||||||
| M | 45.56 | 51.74 | 0.52 | 0.08 | 0.08 | 0.09 | 0.14 | 0.14 | 0.11 | 83.46 | 81.59 | 119.03 | 93.91 | 91.18 | 83.38 |
| SD | 7.16 | 9.63 | 0.50 | 0.06 | 0.07 | 0.07 | 0.12 | 0.17 | 0.15 | 74.65 | 77.36 | 94.01 | 86.93 | 84.94 | 71.13 |
| Min | 36.00 | 36.00 | 0.00 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 2.00 | 2.00 | 2.30 | 2.30 | 6.40 | 2.80 |
| Max | 81.00 | 88.00 | 1.00 | 0.36 | 0.44 | 0.50 | 1.02 | 1.60 | 1.40 | 413.83 | 604.70 | 596.40 | 603.90 | 619.10 | 571.20 |
Note. N = 151;
= Spearman’s rho; IP = Internalizing Problems; EP = Externalizing Problems; sC = salivary cortisol; sAA = salivary alpha amylase; T = Time. Condition coded 0 for cognitive avoidance and 1 for behavioral distraction.
p < .05.
MTM specification and adequacy evaluation
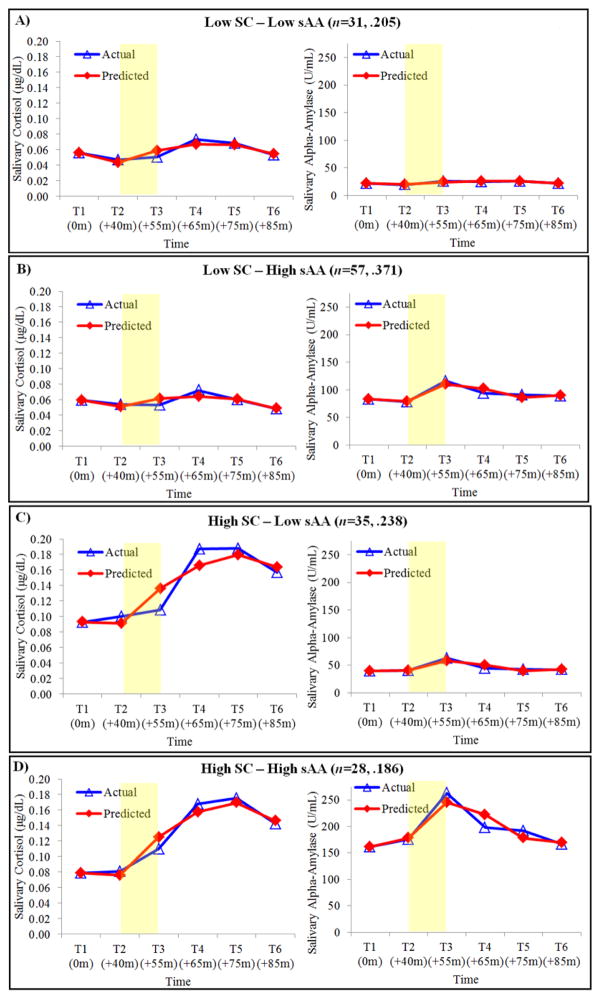
MTM results supported a four-group solution (see Figure 1). Specifically, the log Bayes factor comparing the two- to one-group model [2loge(B10) 445.74], the three- to two-group model [2loge(B10) 208.00], and the four- to three-group model [2loge(B10) 119.38] provided strong evidence for the four-group solution (Jones et al., 2001). Trajectory parameter estimates and model adequacy indices for each subgroup are displayed in Table 2. As per Nagin (2005), model adequacy indices suggested that the final model fit the data well. The Low sC–Low sAA group included 31 children (20.5%) and was characterized by relatively low sC and sAA baseline levels and less pronounced, albeit significant, sC and sAA change patterning. The Low sC–High sAA group included 57 children (37.7%) and was characterized by relatively low sC baseline levels and change patterning and concomitant relatively high sAA baseline levels as well as more pronounced change patterning. The High sC–Low sAA group included 35 children (23.2%) and was characterized by relatively high sC baseline levels and more pronounced change patterning and concomitant relatively low sAA baseline levels and change patterning. The High sC–High sAA group included 28 children (18.5%) and was characterized by relatively high sC and sAA baseline levels and more pronounced sC and sAA change patterning.
Figure 1.
Actual versus predicted salivary cortisol (sC) and alpha amylase (sAA) trajectories for the final four-group multi-trajectory model solution. Shaded region denotes when the TSST-C occurred. Reverse fourth root transformed values presented for visual clarity and ease of interpretation. Title values in parentheses reflect the number of children assigned to each group and probabilities of group membership.
Table 2.
Parameter Estimates (Standard Errors) and Model Adequacy Indices for Final Multi-Trajectory Four-Group Solution
| Salivary Cortisol (sC) | Salivary Alpha Amylase (sAA) | AvePPj | OCCj | Probj | Propj | Ratio | |
|---|---|---|---|---|---|---|---|
| Low sCA,a–Low sAAA (n = 31) | .941 | 47.847 | .205 | .205 | 1.000 | ||
| Intercept (T1) | 0.487* (0.017) | 2.177* (0.071) | |||||
| Linear | −0.006* (0.002) | −0.017 (0.011) | |||||
| Quadratic | 0.001* (0.001) | 0.001* (0.001) | |||||
| Cubic | −0.001* (0.001) | −0.001* (0.001) | |||||
| Quartic | - | - | |||||
| Low sCA,a–High sAAB,a (n = 57) | .941 | 47.847 | .371 | .377 | 0.984 | ||
| Intercept (T1) | 0.494* (0.012) | 3.022* (0.055) | |||||
| Linear | −0.004* (0.002) | −0.127* (0.035) | |||||
| Quadratic | 0.001* (0.001) | 0.006* (0.002) | |||||
| Cubic | −0.001* (0.001) | −0.001* (0.001) | |||||
| Quartic | - | 0.001* (0.001) | |||||
| High sCB,b–Low sAAC,a (n = 35) | .948 | 54.692 | .238 | .232 | 1.026 | ||
| Intercept (T1) | 0.552* (0.016) | 2.514* (0.064) | |||||
| Linear | −0.006* (0.002) | −0.131* (0.046) | |||||
| Quadratic | 0.001* (0.001) | 0.007* (0.002) | |||||
| Cubic | −0.001* (0.001) | −0.001* (0.001) | |||||
| Quartic | - | 0.001* (0.001) | |||||
| High sCB,b–High sAAD,a (n = 28) | .958 | 68.429 | .186 | .185 | 1.005 | ||
| Intercept (T1) | 0.530* (0.017) | 3.566* (0.071) | |||||
| Linear | −0.008* (0.003) | −0.144* (0.053) | |||||
| Quadratic | 0.001* (0.001) | 0.007* (0.003) | |||||
| Cubic | −0.001* (0.001) | −0.001* (0.001) | |||||
| Quartic | - | 0.001* (0.001) |
Note. AvePPj = Average posterior probability; OCCj = Odds of correct classification; Ratio = Ratio of probability of group assignment to proportion of children assigned to each group. Upper case letter-superscripts denote significant differences in intercept parameters estimates within the same physiologic marker. Lower case letter-superscripts denote significant differences in polynomial parameter estimates within the same physiologic marker.
p < .05.
sC–sAA trajectory distinction
Wald tests revealed that sC trajectories for Low sC–Low sAA and Low sC–High sAA children were distinct from that of High sC–Low sAA and High sC–High sAA children with respect to both intercept and polynomial estimates (Table 2). That is, High sC–Low sAA and High sC–High sAA children exhibited sC response trajectories with more elevated baseline levels as well as more dramatic response patterns, compared to their Low sC–Low sAA and Low sC–High sAA counterparts. Children in the two Low sC subgroups exhibited sC trajectories that on average could not be distinguished from one another with respect to either baseline levels or response patterning. This was also the case for children in both High sC groups. Wald tests also revealed that sAA trajectories for the average child each subgroup were distinct with respect to intercept but, for the most part, not polynomial estimates. That is, the average child in each subgroup exhibited sAA trajectories that were distinct from one another with respect to baseline levels: lowest (Low sC–Low sAA), 2nd lowest (High sC–Low sAA), 2nd highest (Low sC–High sAA), and highest (High sC–High sAA) sAA baseline levels. However, with the exception of Low sC–Low sAA, sAA response patterns for the average child in each subgroup did not significantly differ. Using T2 sC and sAA as baseline indicators did not alter trajectory distinction conclusions.
Predictors of sC–sAA response trajectories
Study covariates were not significantly related to group membership. Greater internalizing symptoms, B=0.089, SE= 0.043, p<.05, increased the likelihood of High sC–High sAA group membership relative to the Low sC–Low sAA group (Table 3). That is, compared to Low sC–Low sAA children, High sC–High sAA children were more likely to report internalizing symptoms. Also, higher externalizing symptoms, B=0.058, SE= 0.028, p<.05 increased the likelihood of Low sC–High sAA group membership relative to the High sC–Low sAA group. Compared to High sC–Low sAA children, Low sC–High sAA children were more likely to report externalizing symptoms. No other significant predictions were noted.
Table 3.
Parameter Estimates (Standard Errors) for Multinomial Logistic Regressions Predicting Group Membership
| Reference Group | Low sC–Low sAA | High sC–Low sAA | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Comparison Group | vs. Low sC–High sAA | vs. High sC–Low sAA | vs. High sC–High sAA | vs. Low sC–Low sAA | vs. Low sC–High sAA | vs. High sC–High sAA |
| Intercept | −2.613 | −0.500 | −4.254 | 0.500 | −2.113 | −3.754 |
| (1.971) | (2.250) | (2.349) | (2.250) | (1.919) | (2.254) | |
| Covariates | ||||||
| Child gender | −0.233 | 0.659 | 0.528 | −0.659 | −0.892 | −0.131 |
| (0.482) | (0.551) | (0.603) | (0.551) | (0.495) | (0.607) | |
| Child puberty | 1.087 | 0.775 | 0.802 | 0.775 | 0.312 | 0.026 |
| (0.586) | (0.673) | (0.747) | (0.673) | (0.586) | (0.743) | |
| Child medication | −0.086 | −0.044 | 0.102 | 0.044 | −0.042 | 0.147 |
| (0.195) | (0.224) | (0.206) | (0.224) | (0.218) | (0.225) | |
| Predictor | ||||||
| Internalizing | 0.054 | 0.049 | 0.089* | −0.049 | 0.005 | 0.040 |
| Problems | (0.039) | (0.043) | (0.043) | (0.043) | (0.033) | (0.036) |
| Externalizing | 0.018 | −0.040 | −0.008 | 0.040 | 0.058* | 0.032 |
| Problems | (0.026) | (0.033) | (0.033) | (0.033) | (0.028) | (0.035) |
|
| ||||||
| χ2 (df) | 16.967 (15) | |||||
| Nagelkerke’s R2 | .127 | |||||
Note. Parameter estimates reflect multinomial log-odds of group membership (reference vs. comparison) for each unit increase in covariate or predictor of interest.
p < .05.
sC–sAA response to coping condition
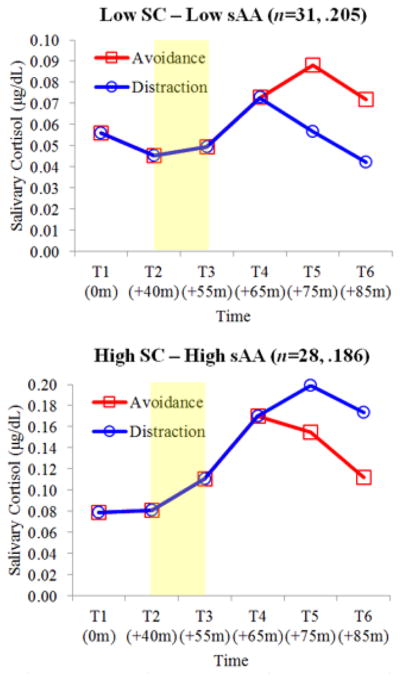
Coping condition had a significant time-varying covariate effect on sC trajectories in two of the four identified subgroups: Low sC–Low sAA, B=−0.034, SE= 0.013, p<.01; High sC–High sAA, B=0.063, SE= 0.017, p<.001 (Figure 2)4. The sC trajectories of Low sC–Low sAA children randomly assigned to distraction (58.1%) demonstrated efficient recovery, with T4-T6 sC levels that decreased in a linear fashion towards baseline. Alternatively, the sC trajectories of Low sC–Low sAA children randomly assigned to avoidance (41.9%) demonstrated protracted recovery, with sC levels that continued to increase from T4 to T5 and then began to decrease from T5 to T6. For High sC–High sAA children, the opposite was observed. sC trajectories for High sC–High sAA children randomly assigned to avoidance (57.1%) demonstrated efficient recovery while those for High sC–High sAA children randomly assigned to distraction (42.9%) displayed protracted recovery.
Figure 2.
Estimated salivary cortisol (sC) concentrations for Low sC–Low sAA and High sC–High sAA groups bycoping condition. Shaded region denotes when the TSST-C occurred. Reverse fourth root transformed values presented for visual clarity and ease of interpretation. Title values in parentheses reflect the number of childrenassigned to each group and probabilities of group membership.
Discussion
This study identified distinct profiles of preadolescent HPA-SAM co-activation patterning and linked them to behavioral functioning and coping-related neuroendocrine recovery efficiency. Using multi trajectory modeling of HPA (sC) and SAM (sAA) index activity in response to a standardized psychosocial stressor (TSST-C), four hypothesized subgroups emerged whose sC and sAA trajectories were consistent with specific cross-system co-activation theories (Bauer et al., 2002). These trajectories were distinct with respect to both baseline levels and change patterning. Additionally, identified HPA-SAM profiles related to children’s behavioral functioning in both expected and unexpected ways, suggesting that Additive-Symmetry and Interactive-Asymmetry consistent patterns of HPA-SAM co-activation may differ for children with internalizing versus externalizing symptoms. Lastly, children with different HPA-SAM co-activation profiles used different coping skills to support neuroendocrine recovery efficiency in a manner consistent with their parent – and self–reported behavioral functioning. This study provides a fine-grained depiction of meaningful variability in preadolescent HPA-SAM co-activation while also advancing support for the claim that there are no universals to what constitutes effective coping (Wadsworth, 2015).
This person-centered approach to examining variability in preadolescent HPA-SAM co-activation response patterning identified four subgroups that reasonably map onto response profiles described by Bauer et al. (2002). The relatively even distribution of children among subgroups is remarkable and consonant with the claim that preadolescence is a period marked by considerable neuroendocrine response reorganization and heterogeneity with respect to HPA–SAM co-activation (Steinberg, 2014). Our findings lend new insights about how preadolescents respond to laboratory stressors. Previous studies of non-clinical, healthy children using person-centered modeling of single indicator response trajectories have found subgroups of children without significant sC changes in response to the TSST-C, concluding that children in this age range are largely hyporesponsive to this paradigm (e.g., Gunnar et al., 2009; Gunnar, Werwerka, Frenn, Long, & Griggs, 2009; Ji et al., 2016). We extend this evidence by showing that all child subgroups were responsive (significant polynomial estimates) to the TSST in their own unique HPA-SAM coordinated fashion, highlighting the advantages of using both sC and sAA in a person-centered framework.
Children’s HPA-SAM co-activation profiles were related to their behavioral functioning in theoretically meaningful ways, further validating our MTM approximation of cross-system co-activation (Bauer et al., 2002). This finding is consonant with previous studies suggesting that redundancy in HPA-SAM responding contributes to risk for internalizing problems specifically (El-Sheikh et al., 2008; Koss et al., 2014). Hyperarousal of this sort is associated with increased attention to threat, intrusive thoughts, and worry-related cognitions (Urasche & Blair, 2015; Weems, Zakem, Costa, Cannon, & Watts, 2005). High sC–High sAA patterning may reflect dysregulated HPA-SAM co-activation (Bauer et al., 2002), given that one function of sC is to terminate (i.e., down-regulate) the energy-consumptive initial SNS response to threat. In this case, it would appear that exaggerated sC activity failed to modulate costly SAM activity in response to threat. However, the Additive-Symmetry model also suggests that concurrent low levels of HPA and SAM activity may be linked to poorer behavioral functioning. Our findings may contradict this proposition: Low sC–Low sAA, relative to High sC–High sAA children, were less likely to report internalizing behavior. In other work, “blunted” cross-system activity has been linked to aggression and adjustment problems (Raine, 2005). However, the Low sC–Low sAA trajectories demonstrated significant change patterning, possibly indicating a response, albeit smaller than the High sC–High sAA group. Also, Low sC–Low sAA links to problem behavior have been shown in studies of at-risk youth (e.g., maltreated; Gordis et al., 2006). Thus, the Low sC–Low sAA group in our normative sample may not reflect the “blunted” responses found in studies of at-risk children. Rather, it is possible that this particular symmetry reflects well-regulated HPA-SAM co-activation.
We also found partial support for the Interactive-Asymmetry model. Children with greater externalizing behavior were more likely to be in the Low sC–High sAA group relative to High sC–Low sAA. This finding is consistent with previous studies suggesting that a lack of coordination between HPA and SAM responses contributes to risk for externalizing specifically (Koss et al., 2014). Indeed, these studies show that sC hypoarousal is associated with externalizing (Shoal, Giancola, & Kirillova, 2003; Shirtcliff, Granger, Booth, & Johnson, 2005). We extend this literature by proposing that Low sC–High sAA co-activation may more accurately reflect a dysregulated neuroendocrine response indexing risk for externalizing (Bauer et al., 2002). The well-functioning positive feedback loop between SNS and HPA helps stimulate cortisol production necessary to mobilize longer-term resources for managing threat and circumvent overreliance on physically-taxing initial SNS activity. In this case, Low sC–High sAA patterning may reflect a response to threat where elevated resource-depleting sAA activity fails to innervate the HPA. Interactive-Asymmetry also suggests that high HPA and low SNS activity could link to poorer behavioral functioning. Our findings do not support this proposition: High sC–Low sAA, relative to Low sC–High sAA, children were less likely to present with externalizing. Gordis et al. (2006) reported similar findings: High sC–Low sAA children presented with the lowest parent-reported aggression. A well-functioning HPA-SNS feedback loop operates such that a sufficient initial SNS response innervates the HPA in order to mobilize longer-term resources for managing threat (Sapolsky et al., 2000). Thus, it is possible that High sC–Low sAA asymmetry reflects well-regulated HPA-SAM co-activation, whereas Low sC–High sAA reflects dysregulation and risk for externalizing.
Children’s HPA-SAM co-activation response profiles were also related to their use of coping in a manner consistent with our regulatory interference and fit hypotheses (Bendezu et al., 2016; Wadsworth et al., 2016). The Responses to Stress theoretical framework suggests that effective coping involves match between child-level factors and available coping resources (Compas et al., 1999). Our previous variable-centered investigations have supported this claim, demonstrating that both distraction and avoidance contribute to neuroendocrine recovery efficiency when children’s characteristic ways of coping and involuntary stress response tendencies are in line with the condition they are assigned (Bendezu et al., 2016; Wadsworth et al., 2016). Our person-centered approach extends this line of inquiry by identifying those specific children from whom distraction and avoidance coping work in different ways. That is, children with well-functioning Low sC–Low sAA profiles and less internalizing tendencies displayed efficient sC recovery patterning when primed with distraction and protracted sC recovery patterning when primed with avoidance. This finding is consistent with our assertion that Low sC–Low sAA children demonstrated optimal HPA-SAM co-activation patterning and consonant with traditional views on the utility of these two coping skills and their regulatory effects on SRS recovery (Connor-Smith et al., 2000). However, the opposite was true for children with dysregulated High sC–High sAA profiles and greater internalizing propensity. These children displayed protracted sC recovery patterns when primed to distract and efficient sC recovery when primed with avoidance.
Three plausible explanations in support of our regulatory interference and fit consistent findings deserve mention. First, both internalizing and mutli-system hyperarousal are associated with involuntary attention to threat, intrusive thoughts, and rumination (Vasey & Daleiden, 1996; Grant et al., 2003). Thus, automatic cognitive processes for High sC–High sAA children may consume executive resources requisite for effective reallocation of attention to coping resources (e.g., distraction); regulatory interference. Second, prompting children who tend to rely on rudimentary coping strategies (e.g., avoidance) to engage in behavioral distraction that they are less accustomed to may inadvertently draw on executive resources typically allocated to the suppression of worry-related and depressogenic cognitions (Wenzlaff & Wegner, 2000). This inability to effectively engage in distraction and possible rebound of negative thoughts may increase feelings of guilt, self-blame, and a sense of failure (Compas et al., 1999; Kelly & Kahn, 1994), all of which are characteristic of internalizing. Thus, allowing children with already limited executive resources to engage in a rudimentary strategy (i.e., avoidance) they are more accustomed to may facilitate recovery; regulatory fit. Third, avoidance is thought to evince temporary benefits (e.g., momentary distress alleviation) for these children particularly in the face of uncontrollable stressors that offer little opportunity to disengage (Santiago & Wadsworth, 2009; Zimmer-Gembeck & Skinner, 2011). The TSST-C is argued to be such a stressor (Dickerson & Kemeny, 2004). Our findings provide preliminary validation that avoidance can serve as functional adaptation for some children (e.g., Del Giudice et al., 2011; Wadsworth, 2015). Furthermore, for internalizing children with dysregulated HPA-SAM co-activation, the beneficial effects of distraction may require more than mere child prompting. As the rigid enactment of avoidance in more controllable, less stressful contexts predicts long-term negative outcomes (Wadsworth, 2015), intervention may be required to help children who overly rely on avoidance develop focused distraction skills as well as the use of a broader array of engagement strategies.
Two other issues related to our coping analyses deserve mention. First, our coping manipulation did not significantly alter Low sC–High sAA or High sC–Low sAA recovery. The externalizing difficulties (or lack thereof) that characterized these groups, controlling for internalizing and presumably cognitive aspects more central to its presentation (e.g., intrusive thoughts, rumination), did not lend to regulatory interference or fit processes as expected. Second, our coping manipulation altered recovery patterning for sC but not sAA trajectories. As effortful coping is believed to manifest in the recovery phase (Connor-Smith et al., 2000), coping may evidence primarily in the second wave (HPA) of the HPA-SAM co-activation neuroendocrine response when sC and sAA are modeled simultaneously, modulating how long-term rather than immediate resources for managing acute stress are utilized.
The current study has several limitations. First, given the possibility that our four-group solution was in part a function of sample size, cautious interpretation as well as replication of the study findings using a larger sample size is warranted. Additionally, we studied comparatively lower-risk and more ethnically homogenous preadolescent children, which limits the generalizability of the findings. MTM of HPA-SAM co-activation in future studies of at-risk and clinical preadolescent children may identify similar or additional unique subgroups. For example, a Low sC–Low sAA subgroup in an at-risk or clinical sample may be differentiated from our Low sC–Low sAA subgroup by evincing intercept limited sC–sAA trajectories (i.e., “blunted”) and an increased likelihood of presenting with externalizing. Second, we limited our investigation to two non-invasive, easily obtainable indices of the preadolescent neuroendocrine response, one of which the literature has yet to establish as a conclusive index of SAM activity (sAA; Nater & Rohleder, 2009). Thus, the current study was consequently limited to the specific cross-system co-activation theory tested (i.e., Bauer et al., 2002). As MTM permits the simultaneous modeling of multiple related but distinct variables, future studies that incorporate additional well-established markers (e.g., respiratory sinus arrhythmia, heart rate, galvanic skin response, plasma norepinephrine) may be able to identify more nuanced profiles and test alternate models; e.g., adaptive calibration model (Del Giudice et al., 2011). Third, we did not consider contextual factors known to influence HPA-SAM co-activation; e.g., environmental stress exposure, family risk. Koss et al. (2014) found similar High sC–High sAA to internalizing and Low sC–High sAA to externalizing links, though they were pronounced in the context of marital discord. As our behavioral predictor effects were small, there may be additional context-based (e.g., chronic stress) unexplained variance in HPA-SAM co-activation, internalizing and externalizing behavior links that future studies might account for.
Highlights.
Multi-trajectory modeling revealed four subgroups of HPA-SAM responses to the TSST.
Child externalizing positively predicted Low HPA-High SAM group membership.
Child internalizing positively predicted High HPA-High SAM group membership.
Low HPA-Low SAM children showed more efficient recovery when using distraction.
High HPA-High SAM children showed more efficient recovery when using avoidance.
Footnotes
Stressor-induced neuroendocrine activation does not need to be reduced for optimal well-being. Some research in adults suggests that inadequate cortisol (Duncko, Makatsori, Fickova, Selko, & Jezova, 2006) and alpha-amylase (Hlavacova, Solarikova, Marko, Brezina, & Jezova, 2017) responses are evident in persons with high trait anxiety.
Children were randomized to condition within gender and investigators were not privy to results of this process.
Child- and parent-reported internalizing symptoms were positively associated, r=.27, p<.01.
sC recovery patterning was analyzed with follow-up repeated measures ANOVA using recovery phase time as a within-subjects factor and subgroup membership (Low sC-Low sAA, High sC-High sAA) and coping condition as between-subjects factors. Verifying our time-varying covariate effects, a significant three-way interaction between subgroup membership, coping condition, and recovery-phase sample time emerged, B=0.012, SE= 0.005, p<.05.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jason Jose Bendezu, The Pennsylvania State University, Department of Psychology.
Martha E. Wadsworth, The Pennsylvania State University, Department of Psychology
References
- Allwood MA, Handwerger K, Kivlighan KT, Granger DA, Stroud LR. Direct and moderating links of salivary alpha-amylase and cortisol stress-reactivity to youth behavioral and emotional adjustment. Biological Psychology. 2011;88(1):57–64. doi: 10.1016/j.biopsycho.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer AM, Quas JA, Boyce WT. Associations between physiological reactivity and children’s behavior: Advantages of a multisystem approach. Journal of Developmental and Behavioral Pediatrics. 2002;23(2):102–113. doi: 10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Bendezú JJ, Perzow SE, Wadsworth ME. What constitutes effective coping and efficient physiologic regulation following psychosocial stress depends on involuntary stress responses. Psychoneuroendocrinology. 2016;73:42–50. doi: 10.1016/j.psyneuen.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Uchino BN, Cacioppo JT. Origins of baseline variance and the law of initial values. Psychophysiology. 1994;31:204–210. doi: 10.1111/j.1469-8986.1994.tb01042.x. [DOI] [PubMed] [Google Scholar]
- Burt KB, Obradović J. The construct of psychophysiological reactivity: Statistical and psychometric issues. Developmental Review. 2012;33:29–57. [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Wustmans A, Kirschbaum C, Rauh W, Hellhammer D. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosomatic Medicine. 1997;59:419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- Chen FR, Raine A, Granger DA. Tactics for modeling multiple salivary analyte data in relation to behavior problems: Additive, ratio, and interaction effects. Psychoneuroendocrinology. 2015;51:188–200. doi: 10.1016/j.psyneuen.2014.09.027. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders: Overview of physical and behavioral homeostasis. JAMA. 1992;267(9):1244–1252. [PubMed] [Google Scholar]
- Connor-Smith JK, Compas BE, Wadsworth ME, Thomsen AH, Saltzman H. Responses to stress in adolescence: Measurement of coping and involuntary stress responses. Journal of Consulting and Clinical Psychology. 2000;68(6):976–992. [PubMed] [Google Scholar]
- Cole PM, Bendezú JJ, Ram N, Chow SM. Dynamical systems modeling of early childhood self-regulation. Emotion. 2017;17(4):684–699. doi: 10.1037/emo0000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, McEwen BS. Social influences on neuroplasticity: Stress and interventions to promote well-being. Nature Neuroscience. 2012;15:689–695. doi: 10.1038/nn.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Bruce J, Gunnar MR. The anterior attention network: Associations with temperament and neuroendocrine activity in 6-year-old children. Developmental Psychobiology. 2002;40:43–56. doi: 10.1002/dev.10012. [DOI] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, Shirtcliff EA. The adaptive calibration model of stress responsivity. Neuroscience and Biobehavioral Reviews. 2011;35:1562–1592. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giudice M, Hinnant JB, Ellis BJ, El-Sheikh M. Adaptive patterns of stress responsivity: A preliminary investigation. Developmental Psychology. 2012;48:775–790. doi: 10.1037/a0026519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130(3):355. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dorn LD, Susman EJ, Ponirakis A. Pubertal timing and adolescent adjustment and behavior: Conclusions vary by rater. Journal of Youth and Adolescence. 2003;32:157–167. [Google Scholar]
- Duncko R, Makatsori A, Fickova E, Selko D, Jezova D. Altered coordination of the neuroendocrine response during psychosocial stress in subjects with high trait anxiety. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2006;30(6):1058–1066. doi: 10.1016/j.pnpbp.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Foland-Ross LC, Kircanski K, Gotlib IH. Coping with having a depressed mother: the role of stress and coping in hypothalamic-pituitary-adrenal axis dysfunction in girls at familial risk for major depression. Development and Psychopathology. 2014;26:1401–1409. doi: 10.1017/S0954579414001102. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Erath SA, Buckhalt JA, Granger DA, Mize J. Cortisol and children’s adjustment: The moderating role of sympathetic nervous system activity. Journal of Abnormal Child Psychology. 2008;36(4):601–611. doi: 10.1007/s10802-007-9204-6. [DOI] [PubMed] [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, Trickett PK. Asymmetry between salivary cortisol and α-amylase reactivity to stress: Relation to aggressive behavior in adolescents. Psychoneuroendocrinology. 2006;31(8):976–987. doi: 10.1016/j.psyneuen.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Granger DA, Hibel LC, Fortunato CK, Kapelewski CH. Medication effects on salivary cortisol: Tactics and strategy to minimize impact in behavioral and developmental science. Psychoneuroendocrinology. 2009;34(10):1437–1448. doi: 10.1016/j.psyneuen.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Grant KE, Compas BE, Stuhlmacher AF, Thurm AE, McMahon SD, Halpert JA. Stressors and child and adolescent psychopathology: Moving from markers to mechanisms of risk. Psychological Bulletin. 2003;129(3):447–466. doi: 10.1037/0033-2909.129.3.447. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamus–pituitary–adrenal activity over the transition to adolescence: Normative changes and associations with puberty. Development and Psychopathology. 2009;21(01):69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson PE, Janlert U, Theorell T, Hammarström A. Life-course socioeconomic trajectories and diurnal cortisol regulation in adulthood. Psychoneuroendocrinology. 2010;35(4):613–623. doi: 10.1016/j.psyneuen.2009.09.019. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Badanes LS, Abela JR, Watamura SE. Hypothalamic–pituitary–adrenal axis dysregulation in dysphoric children and adolescents: Cortisol reactivity to psychosocial stress from preschool through middle adolescence. Biological Psychiatry. 2010;68(5):484–490. doi: 10.1016/j.biopsych.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings PD, Shirtcliff EA, Klimes-Dougan B, Allison AL, Derose L, Kendziora KT, … Zahn-Waxler C. Allostasis and the development of internalizing and externalizing problems: Changing relations with physiological systems across adolescence. Developmental Psychopathology. 2011;23(4):1149–1165. doi: 10.1017/S0954579411000538. [DOI] [PubMed] [Google Scholar]
- Hlavacova N, Solarikova P, Marko M, Brezina I, Jezova D. Blunted cortisol response to psychosocial stress in atopic patients is associated with decreasein salivary alpha-amylase and aldosterone: Focus on sex and menstrual cycle phase. Psychoneuroendocrinology. 2017;78:31–38. doi: 10.1016/j.psyneuen.2017.01.007. [DOI] [PubMed] [Google Scholar]
- Jensen PS, Rubio-Stipec M, Canino G, Bird HR, Dulcan MK, Schwab-Stone ME, Lahey BB. Parent and child contributions to diagnosis of mental disorder: Are both informants always necessary? Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38:1569–1579. doi: 10.1097/00004583-199912000-00019. [DOI] [PubMed] [Google Scholar]
- Ji J, Negriff S, Kim H, Susman EJ. A study of cortisol reactivity and recovery among young adolescents: Heterogeneity and longitudinal stability and change. Developmental Psychobiology. 2016;58(3):283–302. doi: 10.1002/dev.21369. [DOI] [PubMed] [Google Scholar]
- Jones BL, Nagin DS. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociological Methods & Research. 2007;35(4):542–571. [Google Scholar]
- Jones BL, Nagin D, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methods & Research. 2001;29(3):374–393. [Google Scholar]
- Kelly AE, Kahn JH. Effects of suppression of personal intrusive thoughts. Journal of Personality and Social Psychology. 1994;66(6):998–1006. [Google Scholar]
- Kolko DJ, Kazdin AE. Emotional/behavioral problems in clinic and nonclinic children: Correspondence among child, parent, and teacher reports. Journal of Child Psychology & Psychiatry. 1993;34:991–1006. doi: 10.1111/j.1469-7610.1993.tb01103.x. [DOI] [PubMed] [Google Scholar]
- Koss KJ, George MRW, Cummings EM, Davies PT, El-Sheikh M, Cicchetti D. Asymmetry in children’s salivary cortisol and alpha-amylase in the context of marital conflict: Links to children’s emotional security and adjustment. Developmental Psychobiology. 2014;56(4):836–849. doi: 10.1002/dev.21156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little RJ. A test of missing completely at random for multivariate data with missing values. Journal of the American Statistical Association. 1988;83:1198–1202. [Google Scholar]
- McEwen BS. The neurobiology of stress: From serendipity to clinical relevance. Brain Research. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- Nagin DS. Group-based modeling of development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- Nagin DS, Jones BL, Lima Passos V, Tremblay RE. Group-based multi-trajectory modeling. Statistical Methods in Medical Research. 2016;0(0):1–9. doi: 10.1177/0962280216673085. [DOI] [PubMed] [Google Scholar]
- Nater UM, Rohleder N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: Current state of research. Psychoneuroendocrinology. 2009;34(4):486–496. doi: 10.1016/j.psyneuen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17(2):117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Quas JA, Yim IS, Oberlander TF, Nordstokke D, Essex MJ, Boyce WT. The symphonic structure of childhood stress reactivity: Patterns of sympathetic, parasympathetic, and adrenocortical responses to psychological challenge. Development and Psychopathology. 2014;26:963–982. doi: 10.1017/S0954579414000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A. The interaction of biological and social measures in the explanation of antisocial and violent behavior. In: Stoff D, Susman E, editors. Developmental psychobiology of aggression. New York: Cambridge University Press; 2005. pp. 13–42. [Google Scholar]
- Reynolds C, Kamphaus R. BASC-2: Behavior assessment system for children. 2004 doi: 10.1080/21622965.2021.1929232. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Nater UM. Determinants of salivary α-amylase in humans and methodological considerations. Psychoneuroendocrinology. 2009;34(4):469–485. doi: 10.1016/j.psyneuen.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Rutter M. Psychopathological development across adolescence. Journal of Youth and Adolescence. 2007;36:101– 110. [Google Scholar]
- Santiago CD, Wadsworth ME. Coping with family conflict: What’s helpful and what’s not for low- income adolescents. Journal of Child and Family Studies. 2009;18(2):192. [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Sladek MR, Doane LD, Stroud CB. Individual and day-to-day differences in active coping predict diurnal cortisol patterns among early adolescent girls. Journal of Youth and Adolescence. 2017;46(1):121–135. doi: 10.1007/s10964-016-0591-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Booth A, Johnson D. Low salivary cortisol levels and externalizing behavior problems in youth. Development and Psychopathology. 2005;17:167–184. doi: 10.1017/s0954579405050091. [DOI] [PubMed] [Google Scholar]
- Shoal GD, Giancola PR, Kirillova GP. Salivary cortisol, personality, and aggressive behavior in adolescent boys: A 5-year longitudinal study. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42:1101–1107. doi: 10.1097/01.CHI.0000070246.24125.6D. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Age of opportunity: Lessons from the new science of adolescence. Boston, MA: Houghton Mifflin Harcourt; 2014. [Google Scholar]
- Stewart JG, Mazurka R, Bond L, Wynne-Edwards KE, Harkness KL. Rumination and impaired cortisol recovery following a social stressor in adolescent depression. Journal of Abnormal Child Psychology. 2013;41(7):1015–1026. doi: 10.1007/s10802-013-9740-1. [DOI] [PubMed] [Google Scholar]
- Ursache A, Blair C. Children’s cortisol and salivary alpha-amylase interact to predict attention bias to threatening stimuli. Physiology and Behavior. 2015;138:266–272. doi: 10.1016/j.physbeh.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Eye A, Bogat GA. Person-oriented and variable-oriented research: Concepts, results, and development. Merrill-Palmer Quarterly. 2006;52(3):390–420. [Google Scholar]
- Vasey MW, Daleiden EL. Information-processing pathways to cognitive interference in childhood. In: Sarason IG, Pierce G, Sarason B, editors. Cognitive interference: Theory, methods, and findings. Hillsdale NJ: Erlbaum; 1996. pp. 117–138. [Google Scholar]
- Wadsworth ME. Development of maladaptive coping: A functional adaptation to chronic, uncontrollable stress. Child Development Perspectives. 2015;9(2):96–100. doi: 10.1111/cdep.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth ME, Bendezu JJ, Loughlin-Presnal J, Ahlkvist JA, Tilghman-Osborne E, Bianco H, … Hurwich-Reiss E. Unlocking the black box: A multi-level analysis of preadolescent children’s coping. Journal of Clinical Child and Adolescent Psychology. 2016;0:1–15. doi: 10.1080/15374416.2016.1141356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weems CF, Zakem A, Costa NM, Cannon MF, Watts SE. Physiological response and childhood anxiety: Association with symptoms of anxiety disorders and cognitive bias. Journal of Clinical Child and Adolescent Psychology. 2005;34:712–723. doi: 10.1207/s15374424jccp3404_13. [DOI] [PubMed] [Google Scholar]
- Zimmer-Gembeck MJ, Skinner EA. The development of coping across childhood and adolescence: An integrative review and critique of research. International Journal of Behavioral Development. 2011;35(1):1–17. [Google Scholar]