Abstract
Purpose
To evaluate the relationship between pericytes and endothelial cells in retinal neovascularization through histological and immunofluorescent studies.
Methods
C57BL/6J mice were exposed to hyperoxia from postnatal day (P) 7 to P12 and were returned to room air at P12 to induce a model of oxygen-induced retinopathy (OIR). The cross sections of enucleated eyes were processed with hematoxylin and eosin. Immunofluorescent staining of pericytes, endothelial cells, and N-cadherin was performed. Microfluidic devices were fabricated out of polydimethylsiloxane using soft lithography and replica molding. Human retinal microvascular endothelial cells, human brain microvascular endothelial cells, human umbilical vein endothelial cells and human placenta pericyte were mixed and co-cultured.
Results
Unlike the three-layered vascular plexus found in retinal angiogenesis of a normal mouse, angiogenesis in the OIR model is identified by the neovascular tuft extending into the vitreous. Neovascular tufts and the three-layered vascular plexus were both covered with pericytes in the OIR model. In this pathologic vascularization, N-cadherin, known to be crucial intercellular adhesion molecule, was also present. Further evaluation using the microfluidic in vitro model, successfully developed a microvascular network of endothelial cells covered with pericytes, mimicking normal retinal angiogenesis within 6 days.
Conclusions
Pericytes covering endothelial cells were observed not only in vasculature of normal retina but also pathologic neovascularization of OIR mouse at P17. Factors involved in the endothelial cell-pericyte interaction can be evaluated as an attractive novel treatment target. These future studies can be performed using microfluidic systems, which can shorten the study time and provide three-dimensional structural evaluation.
Keywords: Endothelial cells, Microfluidics, Oxygen induced retinopathy, Pericytes, Retinal neovascularization
Angiogenesis plays an important role not only in embryonic development and cancer development, but also in retinal diseases [1]. Pathological retinal neovascularization is a well-known cause of sight-threatening diseases, including diabetic retinopathy, retinopathy of prematurity, and age-related macular degeneration [2]. Thus, in recent years many efforts have been made to understand the mechanism of angiogenesis [3,4]. The findings of different participating cell types and molecules have created the potential for multiple attractive therapeutic targets [5,6,7]. One of the first targeted treatments for retinal angiogenesis was against vascular endothelial growth factor (VEGF), and it is currently used as standard of treatment for proliferative retinal diseases [8]. However, there is still a large number of patients who do not respond well to anti-VEGF treatments, and alternative targeted treatments should be investigated.
Recently pericytes are regaining attention for their role in vascular maintenance through interaction with endothelial cells [9]. Microscopically pericytes regulate vessel permeability, endothelial cell proliferation, and vessel diameter through direct contact with endothelial cells and various paracrine signals [10,11]. However, how this interaction stabilizes vessels in normal angiogenesis and why this fails in pathologic neovascularization remains unclear. Recent studies discovered various molecular pathways, including platelet-derived growth factor (PDGF), BB/PDGF receptor β, angiopoietin-1/tie-2, and sphingosine-1-phosphate-1 pathways, to be involved in normal angiogenesis and in pathologic angiogenesis of diabetic retinopathy. Pericyte dropout and a decreased endothelial cell to pericyte ratio have been reported several times using various diabetic animal models [12,13,14,15,16].
In addition to diabetic retinopathy models developed for investigating retinal neovascularization, the oxygen-induced retinopathy (OIR) model for retinopathy of prematurity is widely used [17]. Based on this model, multiple factors, including VEGF, epidermal growth factor receptor, insulin-like growth factor binding protein-3, and nicotinamide adenine dinucleotide phospate oxidase, have been reported to be involved in retinal angiogenesis [18,19,20,21]. However, the exact mechanism of pathologic neovascularization in the OIR model is poorly understood, especially regarding the pericyte and endothelial cell relationship. There even is a previous report citing the absence of pericytes in the neovascular tuft of the OIR model [22].
It is thought that there is a different interaction between endothelial cells and pericytes in normal pathologic angiogenesis, based on the fact that there is leakage from pathologic new vessels. To identify these differences, we targeted N-cadherin, one of the two cadherins that endothelial cells express with VE-cadherin. Unlike VE-cadherin, which is essential for vascular morphogenesis, N-cadherin plays an important role in maintenance of the blood-brain barrier and maturation of endothelial sprouts through interaction with pericytes [17,18,23].
To our knowledge, the interaction between endothelial cells and pericytes and the difference of N-cadherin expression between pathologic and normal neovascularization in the OIR model is not yet understood. We performed the present study to assess the relationship between pericytes and endothelial cells in retinal angiogenesis through histological and immunofluorescent assays.
Materials and Methods
Animals
C57BL/6J mice purchased from Central Lab Animal (Seoul, Korea) were used for the animal experiments in this study. The care, use, and treatment of all animals in this study were carried out according to the Association for Research in Vision and Ophthalmology statement for the Use of Animals in Ophthalmic and Vision Research and the guidelines established by the Seoul National University Hospital Institutional Animal Care and Use Committee.
OIR model
OIR was induced in newborn mice, as previously described in detail [22,24]. Briefly, newborn mice were exposed to hyperoxic conditions (75% oxygen) from day (P) 7 to P12 and return to normal room air conditions at P12. After the mice were sacrificed by cervical dislocation, the eyes were enucleated for the analyses at P17.
Histologic evaluation
The six enucleated eyes were fixed in 4% paraformaldehyde for 24 hours and embedded in paraffin. Then, the paraffin blocks were trimmed and sagittally sectioned at a 4-µm-thickness, parallel to the optic nerve using a rotatory microtome (Leica RM2255, Nussloch, Germany). The sections were left to float on a 45℃ water bath for 2 to 3 minutes. After the straightening of folds in the tissue, the sections were placed on adhesive coated slides and allowed to cool on a slide holder. The dried slides were deparaffinized with xylene, dehydrated with alcohol, and stained with H&E.
Immunofluorescent staining
Three representative slides from each of the six eyes were used for immunofluorescent staining. Eighteen sections were rinsed with phosphate-buffered saline (PBS) and blocked using DAKO serum-free protein block (X0909; Dako, Carpinteria, CA, USA). The samples were either incubated with primary antibodies at 4℃ overnight, followed by incubation with secondary antibodies for two hours at room temperature the next day, or they were incubated overnight at 4℃ with conjugated antibodies. The following antibodies were used for paraffin sections: Alexa Fluor 488 isolectin GS-IB4 conjugate (1 : 500 dilution; Life Technologies, Carlsbad, CA, USA), Anti-NG2 (1 : 500 dilution; Millipore, Bedford, MA, USA), anti-human CD235 (N-cadherin) (1 : 500 dilution; Biolegend, San Diego, CA, USA), Alexa Fluor 594 goat anti-rabbit IgG (1 : 200 dilution, Life Technologies) and Alexa Fluor 647 donkey anti-mouse IgG (1 : 200 dilution, Life Technologies). Nuclei were counterstained with DAPI (4′,6-diamidino-20 phenylindole). The sections were analyzed with a fluorescence microscope (Eclipse 90i; Nikon, Tokyo, Japan).
Microfluidics
Microf luidic devices were fabricated using a replica molding method [25]. Briefly, a master mold with positive relief patterns of photoresist, SU-8 (MicroChem, Iselin, NJ, USA), was prepared on a silicon wafer by photo-lithography. Microf luidic devices were fabricated out of polydimethylsiloxane (Sylgard 184; Dow Corning, Midland, MI, USA). Human umbilical vein endothelial cells (HUVECs; Lonza, Basel, Switzerland) were mixed with human placental pericytes (Promocell, Heidelberg, Germany) in a 5 : 1 ratio. Then, 5 µL of the cell suspension containing 8 × 106 cells/mL was added to the endothelial cell growth medium-2 (Lonza) channel of each microfluidic device and tilted vertically for 20 minutes for the cells to attach to the patterned fibrin on the lateral surface of the lateral micro channel. We used HUVECs that had been passaged less than four times, and the culture media was changed once on day two of culture.
Results
Three-layered vascular plexus can be found in the normal development of retinal angiogenesis in mice
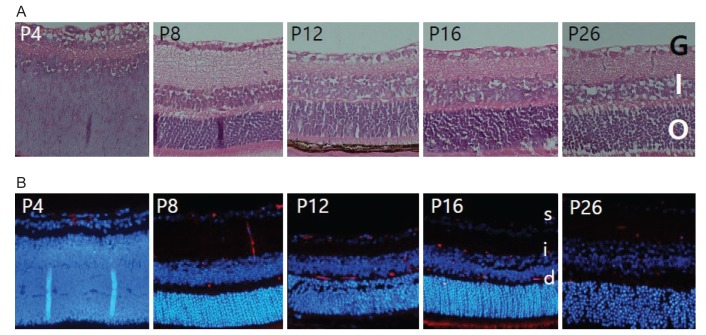
The retinal structure was evaluated on histology with H&E stains and immunof luorescence. During the first week, the superficial plexus began to develop in a radial pattern extending from the optic nerve. By P8, the superficial plexus had completely extended into the retinal periphery, and the formation of deep plexus had started. Vertical vessels ran down from the superficial plexus to the deep plexus in the outer plexiform layer. At P12, the intermediate plexus could be found in the inner plexiform layer, and a three-layered vascular plexus continued to develop (Fig. 1A, 1B.
Fig. 1. Normal development of retinal angiogenesis. (A) The retinas of normal mice at day (P) 4 to P26 were examined using H&E staining and were photographed under a microscope. (B) The retinas of normal mice at P4 to P26 were stained for endothelial cells with isolectin B4 (red) and for cell nuclei with DAPI (4′,6-diamidino-20 phenylindole, blue). G = ganglion cell layer; I = inner nuclear layer; O = outer nuclear layer; s = superficial plexus; i = intermediate plexus; d = deep plexus.
Pericytes exist in neovascular tufts
Successful development of the OIR model was confirmed with a whole-retina flat mount (Fig. 2A). To determine whether endothelial cells and pericytes were associated with any structural differences between normal and OIR mouse retinas, immunofluorescent staining was performed. Among the 18 sections, a total of 28 groups of neovascular tufts extending into the vitreous were observed at the mid-peripheral retina in the OIR model (Fig. 2B). Interestingly, all the neovascular tufts (28 / 28) and the three-layered vascular plexuses were both covered with pericytes in the OIR model (Fig. 2C).
Fig. 2. Endothelial cell and pericyte interactions in normal mice and the oxygen-induced retinopathy (OIR) model. (A) Whole retina flat mount pictures of normal mice (left) and the OIR model (right) were stained for endothelial cells with isolectin B4 (red) (scale bar 1 mm). (B) The retinas of normal mice (left) and the OIR model (right) were stained for endothelial cells with isolectin B4 (red), and for pericytes with NG2 (green). In the OIR model, pericytes were found covering the neovascular tufts that extended into the vitreous (scale bar 20 µm). (C) The retinas of normal mice (left) and the OIR model (right) were stained for endothelial cells with isolectin B4 (green), for cell nuclei with 4′,6-diamidino-20 phenylindole (DAPI, blue) and for pericytes with NG2 (red). Endothelial cells and pericytes existed both in three-layered plexuses and neovascular tufts.

N-cadherin is expressed in both intraretinal vessels and neovascular tufts
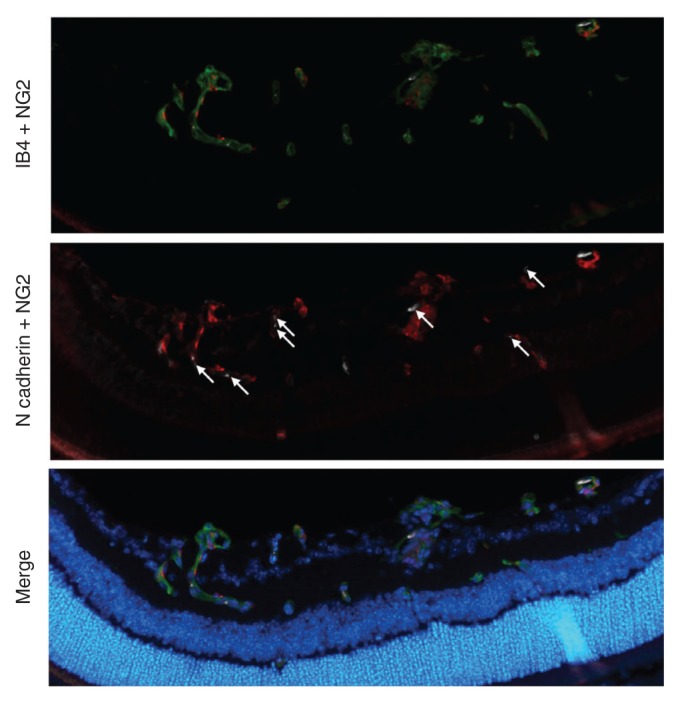
Even if pericytes are present in pathological angiogenesis, it is thought that there is a difference in the interaction between endothelial cells and pericytes in normal and pathologic angiogenesis. To identify the differences in endothelial cell-pericyte interactions between normal vessels and pathological angiogenesis, expression of N-cadherin, which is known to be crucial in endothelial-pericyte adhesion, was evaluated. Although weak, we did observe an N-cadherin signal in both intraretinal vessels and neovascular tufts (Fig. 3).
Fig. 3. N-cadherin expression in the oxygen-induced retinopathy model. The retinas of oxygen-induced retinopathy model mice were stained for endothelial cells with isolectin B4 (green), for cell nuclei with DAPI (4′,6-diamidino-20 phenylindole, blue), for pericytes with NG2 (red) and for N-cadherin (white, arrows).
A vascular network consisting of endothelial cells and pericytes was successfully created using the microfluidics system.
We created a microfluidic model that mimics in vivo neovascularization. The device was composed of a central blood vessel channel, two adjacent media channels, and an outer fibroblast channel (Fig. 4A, 4B. After six days, we successfully created vascular networks, covered by pericytes on the albumin-containing endothelial surface (Fig. 4C). Interaction between endothelial cells and pericytes was easily visualized by immunofluorescent staining using CD-31 and anti-α-smooth muscle actin, one of the pericyte cell markers (Fig. 4D) [23]. Further immunofluorescent staining revealed co-expression of N-cadherin and α-smooth muscle actin on pericytes covering the blood vessel (Fig. 4E).
Fig. 4. N-cadherin and pericyte expression in vascular networks using a microfluidic model. (A,B) Scheme of the microfluidic in vitro model that mimics retinal angiogenesis. (C) The angiogenesis model formed microvascular networks (red) was covered with pericytes (green) within 6 days. (D) Collagen IV (red) is deposited between the endothelial walls (white) and pericytes (green) at day 6. (E) N-cadherin (red) and α-smooth muscle actin (SMA, green) were co-expressed on pericytes covering the blood vessel (white) at day 6. HUVEC, human umbilical vein endothelial cell.

Discussion
Retinal neovascularization is a cardinal feature of many common diseases that lead to blindness, including diabetic retinopathy, age-related macular degeneration, and retinal vein occlusion [2,26]. The patients with these diseases can lose their independence in daily activities, and treating and caring for them creates a substantial economic burden on society [27]. To prevent blindness in these patients, it is important to understand retinal vascularization and to find a novel treatment.
Pericytes are known to be important in the formation, maturation, and stabilization of the microvasculature [28,29]. The microcirculation in the nervous system, where vascular permeability is regulated, has a higher pericyte to endothelial cell ratio than in permeable vessels. Thus, pericytes likely provide greater integrity to the retinal vasculature [30,31]. The physical contact of pericytes with endothelial cells involves the adhesion protein N-cadherin [32].
In our OIR model, we observed multiple neovascular tufts extending into the vitreous, unlike the well-organized three-layered vascular plexus in normal mouse retina. Proliferating vessels in human retina are thought to have barrier disruption and to be more permeable than normal retinal vasculature [33]. Against our expectations, not only intraretinal vasculature, but also the neovascular tufts at P17 consisted of endothelial cells surrounded by adjacent pericytes. The OIR model may also differ in endothelial cell-pericyte maturation, as in new subretinal vessels [34]. P17 of the OIR model is the peak time point for retinal neovascular tuft formation, as subretinal neovascularization is maximized with the maturation of endothelial cell-pericyte interactions at 14 days after laser photocoagulation.
Although the pathologic microvessels are known to be covered and stabilized by pericytes, barrier function attributed to endothelial cell and pericyte contact seems suboptimal in the normal retina, leading to leakage [22]. To find the difference in intercellular junctions between normal and permeable vessels, we further evaluated expression of N-cadherin. According to previous reports, N-cadherin was diffusely expressed on the cell surface, while VE-cadherin was localized at cell junctions [35]. This non-clustering nature of N-cadherin expression made examining the immunofluorescent signal difficult. Although weak, we could observe an N-cadherin signal in both intraretinal and neovascular tufts. Additional studies are needed to identify other intercellular contact molecules or paracrine factors between endothelial cells and pericytes that may attribute to vessel permeability.
Existing but imperfect pericyte and endothelial cell contacts in new retinal vessels may also be an attractive new target for treatment. Conventional anti-angiogenic therapy is limited mainly to growth factors such as VEGF, and a new method of treatment is needed. Further studies on endothelial cell and pericyte interactions may allow for therapies that selectively target pericytes that are not sufficiently interacting with endothelial cells.
As valuable as the OIR model is, preparing and staining slides is time consuming. In less than a week, we could successfully mimic retinal angiogenesis using HUVECs in the microfluidics system, a novel in vitro cell culture method [36,37]. This model has an advantage over conventional cell culture because it can generate three-dimensional structures, that more adequately represent in vivo structures [38]. With the microfluidics system, we anticipate evaluating the role of N-cadherin in vascularization more precisely, by co-culturing N-cadherin knock-out pericytes with normal endothelial cells or by co-culturing normal pericytes with N-cadherin knock-out endothelial cells.
Acknowledgements
This work was supported by the Pioneer Research Program of NRF/MEST (2012-0009544 to JHK), the Bio & Medical Technology Development Program of the National Research Foundation and MSIP (NRF-2015M3A9E6028949 to JHK).
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17:1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 2.Yoshida A, Yoshida S, Ishibashi T, Inomata H. Intraocular neovascularization. Histol Histopathol. 1999;14:1287–1294. doi: 10.14670/HH-14.1287. [DOI] [PubMed] [Google Scholar]
- 3.Ribatti D. Endogenous inhibitors of angiogenesis: a historical review. Leuk Res. 2009;33:638–644. doi: 10.1016/j.leukres.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Rezzola S, Belleri M, Gariano G, et al. In vitro and ex vivo retina angiogenesis assays. Angiogenesis. 2014;17:429–442. doi: 10.1007/s10456-013-9398-x. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida T, Gong J, Xu Z, et al. Inhibition of pathological retinal angiogenesis by the integrin αvβ3 antagonist tetraiodothyroacetic acid (tetrac) Exp Eye Res. 2012;94:41–48. doi: 10.1016/j.exer.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 7.Siemerink MJ, Klaassen I, Van Noorden CJ, Schlingemann RO. Endothelial tip cells in ocular angiogenesis: potential target for anti-angiogenesis therapy. J Histochem Cytochem. 2013;61:101–115. doi: 10.1369/0022155412467635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim LA, D'Amore PA. A brief history of anti-VEGF for the treatment of ocular angiogenesis. Am J Pathol. 2012;181:376–379. doi: 10.1016/j.ajpath.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirschi KK, D'Amore PA. Pericytes in the microvasculature. Cardiovasc Res. 1996;32:687–698. [PubMed] [Google Scholar]
- 10.Hellstrom M, Gerhardt H, Kalen M, et al. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314:15–23. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- 12.Stratman AN, Schwindt AE, Malotte KM, Davis GE. Endothelial-derived PDGF-BB and HB-EGF coordinately regulate pericyte recruitment during vasculogenic tube assembly and stabilization. Blood. 2010;116:4720–4730. doi: 10.1182/blood-2010-05-286872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antonelli-Orlidge A, Saunders KB, Smith SR, D'Amore PA. An activated form of transforming growth factor beta is produced by cocultures of endothelial cells and pericytes. Proc Natl Acad Sci U S A. 1989;86:4544–4548. doi: 10.1073/pnas.86.12.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maisonpierre PC, Suri C, Jones PF, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 15.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 16.Park SW, Yun JH, Kim JH, et al. Angiopoietin 2 induces pericyte apoptosis via α3β1 integrin signaling in diabetic retinopathy. Diabetes. 2014;63:3057–3068. doi: 10.2337/db13-1942. [DOI] [PubMed] [Google Scholar]
- 17.Connor KM, Krah NM, Dennison RJ, et al. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc. 2009;4:1565–1573. doi: 10.1038/nprot.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hewing NJ, Weskamp G, Vermaat J, et al. Intravitreal injection of TIMP3 or the EGFR inhibitor erlotinib offers protection from oxygen-induced retinopathy in mice. Invest Ophthalmol Vis Sci. 2013;54:864–870. doi: 10.1167/iovs.12-10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kielczewski JL, Hu P, Shaw LC, et al. Novel protective properties of IGFBP-3 result in enhanced pericyte ensheathment, reduced microglial activation, increased microglial apoptosis, and neuronal protection after ischemic retinal injury. Am J Pathol. 2011;178:1517–1528. doi: 10.1016/j.ajpath.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilkinson-Berka JL, Deliyanti D, Rana I, et al. NADPH oxidase, NOX1, mediates vascular injury in ischemic retinopathy. Antioxid Redox Signal. 2014;20:2726–2740. doi: 10.1089/ars.2013.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao M, Shi X, Liang J, et al. Expression of pro- and anti-angiogenic isoforms of VEGF in the mouse model of oxygen-induced retinopathy. Exp Eye Res. 2011;93:921–926. doi: 10.1016/j.exer.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Smith LE, Wesolowski E, McLellan A, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 23.Mendel TA, Clabough EB, Kao DS, et al. Pericytes derived from adipose-derived stem cells protect against retinal vasculopathy. PLoS One. 2013;8:e65691. doi: 10.1371/journal.pone.0065691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park SW, Kim JH, Kim KE, et al. Beta-lapachone inhibits pathological retinal neovascularization in oxygen-induced retinopathy via regulation of HIF-1α. J Cell Mol Med. 2014;18:875–884. doi: 10.1111/jcmm.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim J, Chung M, Kim S, et al. Engineering of a biomimetic pericyte-covered 3D microvascular network. PLoS One. 2015;10:e0133880. doi: 10.1371/journal.pone.0133880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buch H, Vinding T, Nielsen NV. Prevalence and causes of visual impairment according to World Health Organization and United States criteria in an aged, urban Scandinavian population: the Copenhagen City Eye Study. Ophthalmology. 2001;108:2347–2357. doi: 10.1016/s0161-6420(01)00823-5. [DOI] [PubMed] [Google Scholar]
- 27.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–e116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 28.Feng Y, vom Hagen F, Pfister F, et al. Impaired pericyte recruitment and abnormal retinal angiogenesis as a result of angiopoietin-2 overexpression. Thromb Haemost. 2007;97:99–108. [PubMed] [Google Scholar]
- 29.Hughes S, Chan-Ling T. Characterization of smooth muscle cell and pericyte differentiation in the rat retina in vivo. Invest Ophthalmol Vis Sci. 2004;45:2795–2806. doi: 10.1167/iovs.03-1312. [DOI] [PubMed] [Google Scholar]
- 30.Sims DE. Diversity within pericytes. Clin Exp Pharmacol Physiol. 2000;27:842–846. doi: 10.1046/j.1440-1681.2000.03343.x. [DOI] [PubMed] [Google Scholar]
- 31.Frank RN, Turczyn TJ, Das A. Pericyte coverage of retinal and cerebral capillaries. Invest Ophthalmol Vis Sci. 1990;31:999–1007. [PubMed] [Google Scholar]
- 32.Gerhardt H, Wolburg H, Redies C. N-cadherin mediates pericytic-endothelial interaction during brain angiogenesis in the chicken. Dev Dyn. 2000;218:472–479. doi: 10.1002/1097-0177(200007)218:3<472::AID-DVDY1008>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 33.Patz A. Clinical and experimental studies on retinal neovascularization: XXXIX Edward Jackson memorial lecture. Am J Ophthalmol. 1982;94:715–743. doi: 10.1016/0002-9394(82)90297-5. [DOI] [PubMed] [Google Scholar]
- 34.Ishibashi T, Inomata H, Sakamoto T, Ryan SJ. Pericytes of newly formed vessels in experimental subretinal neovascularization. Arch Ophthalmol. 1995;113:227–231. doi: 10.1001/archopht.1995.01100020111041. [DOI] [PubMed] [Google Scholar]
- 35.Salomon D, Ayalon O, Patel-King R, et al. Extrajunctional distribution of N-cadherin in cultured human endothelial cells. J Cell Sci. 1992;102(Pt 1):7–17. doi: 10.1242/jcs.102.1.7. [DOI] [PubMed] [Google Scholar]
- 36.Barbulovic-Nad I, Au SH, Wheeler AR. A microfluidic platform for complete mammalian cell culture. Lab Chip. 2010;10:1536–1542. doi: 10.1039/c002147d. [DOI] [PubMed] [Google Scholar]
- 37.Kim S, Lee H, Chung M, Jeon NL. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip. 2013;13:1489–1500. doi: 10.1039/c3lc41320a. [DOI] [PubMed] [Google Scholar]
- 38.Huh D, Hamilton GA, Ingber DE. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011;21:745–754. doi: 10.1016/j.tcb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]