Abstract
The HIV-1 accessory protein Nef controls multiple aspects of the viral life cycle and host immune response, making it an attractive therapeutic target. Previous X-ray crystal structures of Nef in complex with key host cell binding partners have shed light on protein-protein interactions critical to Nef function. Crystal structures of Nef in complex with either the SH3 or tandem SH3-SH2 domains of Src-family kinases reveal distinct dimer conformations of Nef. However, the existence of these Nef dimer complexes in solution has not been established. Here we used hydrogen exchange mass spectrometry (HX MS) to compare the solution conformation of Nef alone and in complexes with the SH3 or the SH3-SH2 domains of the Src-family kinase Hck. HX MS revealed that interaction with the Hck SH3 or tandem SH3-SH2 domains induces protection of the Nef αB-helix from deuterium uptake, consistent with a role for αB in dimer formation. HX MS analysis of a Nef mutant (position Asp123, a site buried in the Nef:SH3 dimer but surface-exposed in the Nef:SH3-SH2 complex), showed a Hck-induced conformational change in Nef relative to wild type Nef. These results support a model in which Src-family kinase binding induces conformational changes in Nef to expose residues critical for interaction with the μ1 subunit of AP-1 and the MHC-I tail, and subsequent MHC-I downregulation and immune escape of HIV-infected cells required for functional interactions with downstream binding partners.
Keywords: Hydrogen exchange mass spectrometry, Host-pathogen interactions, Conformational change, HIV-1 accessory factors, Src-homology domains
Graphical Abstract
Introduction
Nef is a small HIV-1 accessory protein that enhances viral infectivity, replication, and immune escape of infected cells. Expression of Nef alone in the CD4+ cell compartment of transgenic mice is sufficient to drive an AIDS-like syndrome with 100% penetrance [1]. Conversely, high-titer replication of HIV-1 and CD4+ T-cell loss in humanized mice requires an intact Nef gene [2, 3]. Rhesus macaques infected with Nef-defective SIV also exhibit low levels of viral replication and fail to progress to simian AIDS [4]. Consistent with these animal studies, a subset of patients infected with Nef-defective HIV-1 have remained asymptomatic in the absence of antiretroviral therapy for 10 years or more [5–9]. Taken together, these data strongly support a necessary role for Nef in HIV-1 pathogenesis, and identify Nef as a promising target for antiretroviral drug development [10].
Nef has no intrinsic biochemical activity, and functions instead through interactions with host cell proteins to hijack multiple signal transduction and protein trafficking pathways to enhance viral replication and promote immune escape [11]. For example, Nef-induced downregulation of CD4 from the host cell surface prevents viral superinfection, promotes release of mature virions [12], and may reduce clearance of infected cells via ADCC [13, 14]. Nef also interferes with cell-surface display of MHC-1/HIV antigen complexes on infected cells, thereby evading HIV-specific cytotoxic CD8+ T-cells [15, 16]. Nef binds and activates several non-receptor protein kinases, including members of Src and Tec/Btk families as well as Pak2 [17–20]. Selective inhibitors and siRNA knockdown of these Nef-activated kinases have been shown to suppress Nef-dependent enhancement of HIV-1 infectivity and replication, suggesting that these kinases activate downstream signals that promote the viral life cycle [20, 21]. Src-family members expressed in HIV-1 target cells, including Hck and Lyn, are also required for Nef-dependent MHC-1 downregulation [22]. While these studies show that Nef-mediated Src-family kinase (SFK) activation and downregulation of MHC-I are functionally linked, the specific molecular mechanism connecting these pathways is not clear.
While Nef has been the subject of multiple structural studies, a complete structure of full-length Nef remains elusive due to multiple flexible regions within the protein. Models of full-length Nef have been generated by combining NMR and X-ray crystallography data of smaller fragments of Nef (Fig. 1a) [23]. The first ~60 amino acids of Nef is largely unstructured, with an N-terminal myristoyl group and a poly-basic patch responsible for membrane attachment [24, 25]. The N-terminal anchor domain is followed by a folded core that has been the focus of most structural studies to date. The Nef polyproline type II (PPII) helix, located near the N-terminal end of the core, has a conserved PxxPxR sequence responsible for Src-family kinase SH3 domain engagement [26, 27]. The SH3 domain is displaced from its negative regulatory site on the back of the SFK kinase domain when bound to Nef, resulting in kinase activation [19, 28]. The Nef core also contains several helical regions, with the αB helix forming a homodimerization interface in crystal complexes with Src-family kinase SH3 and SH3-SH2 regulatory domains (Fig. 1b,c) [26, 29]. A flexible loop near the C-terminal end of the core makes direct contacts with the AP-2 trafficking adaptor protein, an interaction essential for downregulation of CD4 [30].
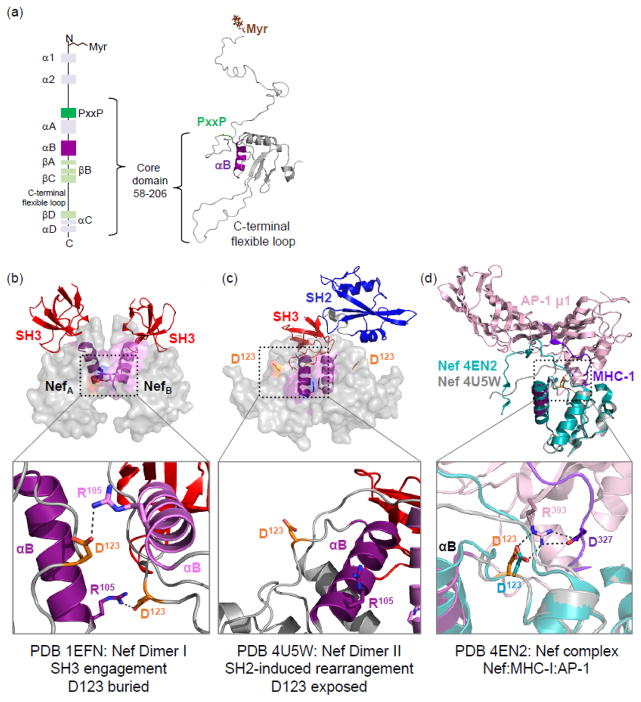
Figure 1.
Structural organization of HIV-1 Nef and complexes with Hck and AP-1/MHC-I. (a) The secondary structural elements of HIV-1 Nef are shown on the left in a vertical cartoon from the N- to C-terminus. Myristoylation (Myr) of the N-terminus is essential for membrane localization. Residues 58–206 (numbering based on crystal structure of Nef NL4-3; PDB 1EFN [26]) make up the structured core domain of Nef. The PxxP motif (green) interacts with SH3 domains of Hck and other Src-family kinases. Helix αB is essential for Nef dimerization. The Geyer-Peterlin model of full-length Nef [23] is shown on the right, and indicates the PxxP motif (green) and αB helix (purple). (b) Crystal structure of the HIV-1 Nef core in complex with the SH3 domain of Fyn (with Arg96 to isoleucine mutation as per Hck SH3) is modeled at the top (PDB 1EFN). Nef crystallizes as a dimer of Nef:SH3 complexes in this structure. The two Nef monomers are colored gray with the αB helices in dark purple (NefA) or light purple (NefB); the Nef dimer interface is formed by the orthogonal juxtaposition of these helices in both complexes. The side chain of key Nef residue Asp123 in each Nef monomer is rendered in orange, with the SH3 domains shown at the top in red. The Nef dimer interface is enlarged in the lower panel, to highlight the reciprocal polar contacts between Nef Asp123 and Arg105; these contacts bury Asp123 in the core of the structure. (c) Crystal structure of the HIV-1 Nef core in complex with the Hck SH3-SH2 dual domain is shown at the top (PDB 4U5W). Color scheme as per the left panel; only a single SH3-SH2 unit is shown for clarity. Note that Nef also crystallizes as a complex of Nef:SH3-SH2 dimers in this structure. However, the helical interface is completely re-oriented and much more compact, with each Nef Asp123 side chain (orange) now pointed toward the solvent (one of the Asp123 residues is enlarged in lower panel). (d) Superposition of the Nef core proteins in the complex with SH3-SH2 (PDB 4U5W; gray ribbon) and in complex with the AP-1/μ1 subunit (pink) and MHC-I tail peptide (purple); (PDB 4ENU; Nef core from this complex in cyan). The Nef core proteins from these two independently solved crystal structures adopt remarkably similar conformations. Particularly important is the nearly identical positioning of Nef Asp123 in both structures (enlarged in lower panel), which form part of a hydrogen bond network with Arg393 from AP-1 and Asp327 from MHC-I that is critical to Nef-mediated downregulation of MHC-1.
The Nef core was first crystallized in complex with the Fyn SH3 domain (PDB 1EFN [26]), which contained a point mutation (R96I) to mimic the high affinity interaction of Nef with the Hck SH3 domain (Fig. 1b, top). Nef crystallized as a 2:2 dimer of SH3 complexes in this structure, with multiple hydrophobic side chains from the αB helices interdigitating to form the dimer interface. This crystallographic dimer is stabilized by reciprocal ionic interactions involving Nef Asp123 and Arg105, which serve to ‘cap’ the dimer interface on both ends (Fig. 1b, bottom). A more recent structure (PDB 4U5W [29]) of the Nef core in complex with the tandem SH3-SH2 regulatory domains of Hck revealed a similar mechanism of interaction through the SH3 domain (Fig. 1c, top). However, in this case Nef Arg105 from each ‘half’ of the dimer complex makes a contact with Glu93 in the SH3 domain of the opposing complex. As a result, the Nef dimer interface, while still formed by the αB helix, undergoes a dramatic reorganization that exposes Nef Asp123 to the surface of the complex (Fig. 1c, bottom) [29]. In this dimer orientation, Nef Asp123 is free to interact with other proteins such as the cytoplasmic tail of MHC-1 and the AP-1 endocytic adaptor essential for its downregulation. Crystallography (PDB 4EN2 [31]) of a complex of Nef with the MHC-I cytoplasmic tail and the AP-1 μ1 subunit revealed a critical role for Nef Asp123 in the assembly of this complex, where it makes polar contacts with both partner proteins (Fig. 1d, top). Mutagenesis of Asp123 prevents both MHC-I and CD4 downregulation by Nef, and also interferes with Nef dimerization in cell-based assays [31, 32]. Together, these data suggest that the crystallographic dimers in PDB 1EFN and 4U5W are not merely crystallographic artifacts, but that in the context of SFK engagement, rearrangements occur to expose Nef Asp123 for MHC-I downregulation and trafficking functions that involve the endocytic adaptors AP-1 and AP-2. This remodeling may begin when Nef engages Hck via its SH3 domain, followed by a conformational reorganization of the Nef dimer so as to present Asp123 to the surface of the complex for MHC-I and AP-1 engagement. Indeed, the positions of the loop containing Asp123 in the crystal structures of the Nef core in complexes with the Hck SH3-SH2 domain and in the MHC-I/AP-1 complex are virtually identical (Fig. 1d, bottom).
To test the SFK-induced Nef remodeling hypothesis under solution conditions, we used hydrogen exchange mass spectrometry (HX MS) to compare changes in Nef as a function of interaction with the Hck SH3 or Hck SH3-SH2 proteins used for previous X-ray crystal structures. We prepared and purified stable complexes of each Nef-bound form and labeled them with deuterium. Our results show no evidence for protection from exchange of the Nef core protein alone in solution, consistent with a monomer. Interaction with the Hck regulatory domains, however, showed clear decreases in exchange right at the αB helix dimer interface observed in the corresponding crystal structures. HX MS analysis with a Nef Asp123 mutant previously shown to be defective for homodimerization and unable to induce receptor downregulation in cells [31, 32] provides clear evidence for two distinct Nef dimer complexes in solution. These data support the idea that Hck binding induces conformational remodeling of Nef as required for MHC-I downregulation, and are consistent with previous findings that Hck is involved in this critical Nef function [22, 33]. In this sense, Hck may serve as a structural regulator that induces a functional conformation of the Nef protein.
Results
The N-terminal arm stabilizes the Nef core protein but has no impact on SH3 binding
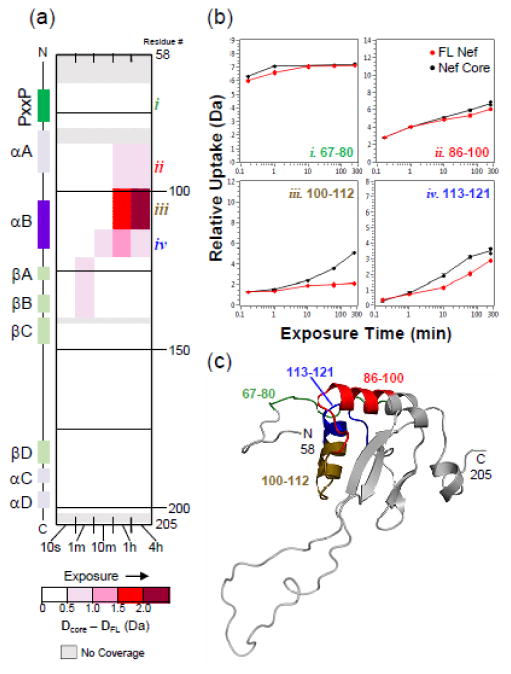
Most structural studies of Nef have focused on the folded core of the protein because the N-terminal arm (first ~60 amino acids) is very flexible, making the full-length protein difficult to crystallize or study by NMR. We have previously demonstrated that recombinant, full-length Nef proteins, produced from several laboratory and patient-derived alleles, can be studied by HX MS [34–38] and therefore applied this method to the question of Hck-induced Nef dimerization. To investigate a possible role for the N-terminal arm of Nef in dimerization, we compared deuterium incorporation of the full-length Nef protein (referred to hereafter as FL-Nef, residues 2–205, numbering as per PDB 1EFN), to the Nef core (residues 58–205). FL-Nef and Nef core were independently incubated in deuterium oxide-based buffer for time points ranging from 10 seconds to 4 hours. Exchange was quenched and the proteins digested with pepsin. Deuterium incorporation into the resulting peptic peptides (Supplementary Fig. S1a) was monitored by MS (See Materials and Methods). For this and all subsequent HX MS experiments, at least 87% of each Nef protein sequence was covered. In agreement with our previous findings [34–36, 38], peptides covering the N-terminal arm of Nef had high levels of deuteration from the earliest exchange times (Supplementary Fig. S2), consistent with a lack of structure. One exception was peptide 10–16 (sequence SAIRERM), which corresponds to a short alpha helix in a previous model of FL-Nef [23]. Exchange into FL-Nef was then compared to exchange into the Nef core and the results are summarized in Fig. 2. As indicated by the red regions in the deuterium uptake difference map, the Nef core protein incorporates more deuterium in selected regions than FL-Nef, especially at later exchange times. These results suggest that Nef core region is more stable in the context of FL-Nef due to the presence of the N-terminal arm, as previously hypothesized [35]. Specific regions of Nef that became more deuterated in the absence of the N-terminal arm localize to the αA and αB helices, with the most dramatic change observed in peptide iii from the αB helix (Fig. 2b–c). Interestingly, this region spans the crystallographic Nef dimerization interfaces (Figs. 1b–c), offering the intriguing possibility that the N-terminal arm may protect the dimerization interface during the trafficking to the plasma membrane required for Nef function.
Figure 2.
The Nef core protein is more flexible than full-length Nef (FL-Nef). (a) Comparison of deuterium uptake by FL-Nef (residues 2–205) versus the Nef core (residues 58–205) is presented in a vertical difference map of the Nef core secondary structure. Deuterium levels in the FL-Nef peptides were subtracted from those in the Nef core and the differences are color-coded as indicated in the legend. The differences found in non-overlapping peptides, displayed vertically from N- to C-terminus, are indicated for the corresponding exchange times (10 s to 4 h; shown horizontally). Greater differences are more intense red. Deuterium incorporation graphs for selected peptides (b) highlight regions where clear differences were seen (peptides i, ii, iii, iv). Uptake curves for FL-Nef peptides are shown in red and Nef core peptides are shown in black. Panel (c) maps the locations of peptides in panel (b) onto the Geyer-Peterlin model of Nef [23], where peptide i is labeled in green, peptide ii in red, peptide iii in brown, and peptide iv in blue. Deuterium incorporation graphs for all peptic peptides from this experiment are shown in Supplementary Fig. S2.
This comparison experiment also provided exchange data for the unbound PxxP motif of Nef, which must be surface-exposed in order to bind to Hck and other proteins with SH3 domains. Peptide i (Fig. 2), which includes residues 67–80 and the polyproline motif, was highly deuterated in both the full-length and core Nef proteins from the earliest labeling times. This observation suggests that the PxxP motif is solvent-exposed in both FL-Nef and in the Nef core form, and that the N-terminal arm does not impact availability of the PxxP motif for recruitment of SH3 proteins. As an independent test of this idea, we measured binding interactions of each Nef protein to the Hck SH3 and SH3-SH2 domains by SPR (Supplementary Fig S3). The kinetics and equilibrium dissociation constants for binding of Nef to both Hck proteins were the same regardless of the presence of the N-terminal arm. Taken together, the HX MS and SPR data show that the N-terminal arm does not influence SH3 domain engagement.
Hck:Nef interaction persists through purification and deuterium labeling
Having shown that the N-terminal arm offered some protection to the core, we next tested whether complexes of Nef with Hck regulatory domains alter exchange at regions predicted to be involved in dimerization. As detailed in the Introduction, previous X-ray crystal structures of Nef in complex with SFK SH3 domains or with an SH3-SH2 dual domain (as in Fig. 1b–c) revealed distinct dimers of complexes. However, the question remained as to whether these complex structures also exist in solution or are unique to the crystalline state. Nef:Hck protein complexes were generated for analysis by HX MS. In the first complex, the Hck SH3-SH2 dual domain is bound to the Nef core protein [29] (PDB 4U5W). The protein constructs used for this complex were identical to those that were crystallized. This complex purifies as a complex of dimers, as shown previously by SEC-MALS [29]. The second complex consists of the isolated Hck SH3 bound to the same Nef core protein. This complex corresponds very closely to the constructs used for the crystal structure of the Nef core in complex with a Fyn SH3 domain, modified to bind to Nef with high affinity (SH3 R96I mutant; PDB 1EFN [26]). Both Nef complexes were expressed and purified in the same way, and analytical SEC demonstrated elution profiles consistent with complex formation (Supplementary Fig. S4). HX MS was then performed on each protein complex as well as the individual protein components, and the resulting comparative regional deuterium uptake is described in detail in the following sections.
To validate the integrity of the complexes and to monitor bound Nef during the labeling period, we analyzed deuterium incorporation into the Hck SH3 and SH3-SH2 proteins before analyzing exchange into Nef. As shown in Supplementary Fig. S5a, the Hck SH3 domain was clearly protected from deuterium uptake in the presence of the Nef core, as found previously [34, 37–39]. Uptake plots for selected peptides in the SH3 domain (peptides i through iv) showed reduced deuterium incorporation upon Nef binding (Supplementary Fig. S5b). The SH3 domain within the SH3-SH2 protein was similarly protected from deuterium uptake upon Nef binding. We were surprised to observe some regions of minimal to moderate exposure in the SH2 domain in the complex of Nef with the SH3-SH2 protein, revealed as slightly higher levels of deuterium in SH2 peptides (v through viii) from the Nef-bound state. However, this phenomenon may be limited to the isolated Hck SH3-SH2 protein, as Nef binding to near-full-length Hck had no effect on the SH2 domain by HX MS [39].
Hck regulatory domain binding induces Nef dimer formation
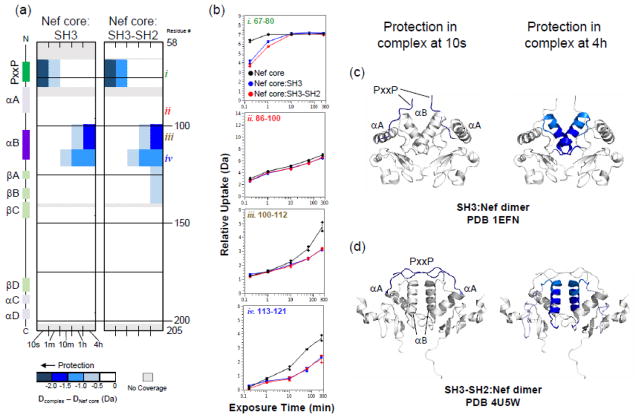
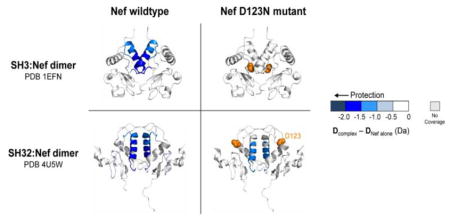
Confident that Nef was stably bound to both the SH3 and SH3-SH2 proteins, we next compared HX of Nef in each complex to that of the unbound Nef core (Fig. 3). At early labeling times, protection was observed in the PxxP region of Nef (peptide i, Fig. 3), consistent with the role of this motif in SH3 domain engagement. At later exchange times, protection was observed in the αB helix (peptides iii and iv, Fig. 3), the region of Nef implicated by the crystal structures as central to dimer formation. The protected regions were mapped onto the corresponding crystal structures of these Nef:Hck complexes (Fig. 3, panels c and d) and immediately show that the regions protected from exchange are at the dimer interface in the crystal structures of both complexes, irrespective of the distinct orientation of the αB helix in each complex. By extension, these results also allow us to conclude that Nef is a monomer in solution, and that binding to the Hck SH3 domain is sufficient to induce Nef dimerization via the αB helix.
Figure 3.
The Nef core is protected from exchange when bound to Hck SH3 or SH3-SH2. (a) Comparison of deuterium uptake by Nef core alone versus the core complex with Hck SH3 (left) or SH3-SH2 (right) is presented in a vertical difference map of the Nef core secondary structure. The deuterium level in each peptide from the Nef core alone was subtracted from those recorded from each complex and the differences are color-coded as indicated in the legend. The differences in non-overlapping peptides, displayed vertically from N- to C-terminus, are indicated for the corresponding exchange times (10 s to 4 h; shown horizontally). Greater protection is represented as a more intense shade of blue. (b) Deuterium incorporation graphs for selected peptides i, ii, iii, iv (the same peptides highlighted in Fig. 2). Uptake curves from Nef core peptides are shown in black, Nef core:SH3 complex peptides are shown in blue, and Nef core:SH3-SH2 complex peptides are shown in red. (c,d) The protection observed in Nef when bound to Hck SH3 (c) or Hck SH3-SH2 (d) after 10 s (left) or 4 h (right) of deuterium exchange is mapped onto the corresponding crystal structures as indicated. Deuterium incorporation graphs for all peptic peptides from this experiment are shown in Supplementary Fig. S7.
Characterization of a Nef dimerization-deficient mutant by HX MS
The HX MS results presented in Figure 3 support a key role for the Nef αB helix in dimerization following SH3 domain engagement in solution, consistent with previous X-ray crystal structures. However, those HX MS data cannot distinguish between the unique Nef dimer conformations present in the SH3 vs. SH3-SH2 crystal complexes, because the same Nef helical region is involved in both dimerization interfaces. We reasoned that by incorporating a Nef mutation unique to one of the two dimer conformations, we may be able to determine whether one dimer conformation is preferred over the other. For these studies, we changed Nef Asp123 to asparagine (Nef-D123N). This Nef residue is highly conserved, and substitution with asparagine prevents dimerization in cell-based fluorescence complementation assays and blocks important Nef functions related to infectivity, replication, as well as CD4 and MHC-I downregulation [31, 32]. Interestingly, in the context of the crystal complex of Nef with SH3 only (PDB 1EFN), Asp123 forms a critical salt bridge with Arg105 to stabilize the dimer interface. However, interaction of Nef with the Hck SH3-SH2 dual domain (PDB 4U5W) releases the Arg105 contacts (it makes a new contact with the SH3 domain) and exposes the Asp123 residue (compare Figs. 1b and 1c), where it is free to interact with other proteins [31]. We note that solvent accessibility calculations for Nef Asp123 using the program GETAREA show that in the smaller Nef:SH3 complex (1EFN), Asp123 has solvent accessibility of 39/42 Å2 (the two values reflect the accessibility for each Nef monomer in the complex) while the larger complex of Nef with SH3-SH2 produced values of 66/76 Å2. These observations suggested that while recombinant Nef-D123N should interact with both the isolated Hck SH3 and tandem SH3-SH2 domains, the αB helix in Nef-D123N will be protected from hydrogen exchange when bound to SH3-SH2 but not SH3 alone. The reason for this is that the Nef D123-R105 salt bridge is found in only in the SH3 complex (PDB 1EFN), and mutation of D123 should not affect Nef dimer formation when bound to Hck SH3-SH2.
Recombinant Nef-D123N core protein was expressed and purified either alone or in complex with the Hck SH3 or SH3-SH2 proteins using the same procedure for the wild-type Nef core. Analytical SEC elution profiles for the mutant protein alone and in the complexes are included in Supplementary Fig. S4. The elution profile for Nef-D123N in complex with the Hck SH3-SH2 domain was no different from that of the wild-type Nef complex. However, the complex of Nef-D123N mutant with isolated SH3 domain eluted as a doublet, suggesting that the D123N mutation may destabilize the complex of Nef with SH3 but not SH3-SH2, consistent with the crystallography. As an additional control, we also tested the interaction of Nef-D123N with SH3 and SH3-SH2 by SPR (Supplementary Fig. S3). The mutant can bind just as well as the wild-type: the mutation had little impact on the kinetics or affinity of SH3 domain interaction, suggesting that the D123N mutation does not influence the conformation of Nef to inhibit binding. To show that the D123N mutation did not cause global destabilization of the Nef protein itself, we acquired HX data for the mutant protein alone for comparison to wild-type Nef. The results are summarized in Supplementary Figs. S8 and S9 with a vertical difference map (Fig S8) comparing exchange in all peptides from the wild-type and mutant proteins. Minimal changes were only observed in areas surrounding Asp123, demonstrating that the D123N mutation does not have a global impact on Nef folding or flexibility, consistent with the SPR data.
D123N mutation prevents Nef core dimer formation when bound to Hck SH3 but not Hck SH3-SH2
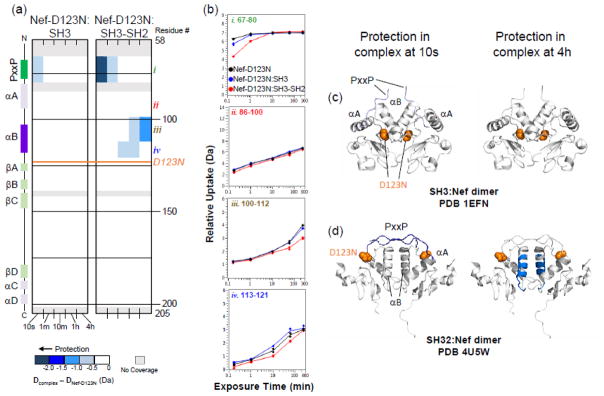
As for wild-type Nef, we checked by HX MS that the Hck SH3 domain was bound to Nef in each of the complexes. Supplementary Figures S10 and S11 show that the SH3 domain was protected from exchange in each complex, consistent with the SPR data and confirming that the D123N mutation had no discernible impact on SH3 binding to Nef. Hydrogen exchange in the Nef-D123N protein was then determined for each complex, and the results are shown in Fig. 4. Peptides derived from the PxxP motif of Nef-D123N were protected from exchange when bound to either Hck SH3 or SH3-SH2, although the magnitude of protection with SH3 was somewhat less than that observed with the wild-type Nef complexes (compare peptide i in Fig. 4b with 3b). Protection of the PxxP motif is consistent with SH3 engagement in both complexes by Nef-D123N, as per the wild-type Nef core. However, a striking difference was observed in deuterium uptake by the αB helix between the two Nef-D123N complexes. While the Nef-D123N αB helix was protected from exchange when bound to Hck SH3-SH2 (Fig. 4biii and d), no protection was observed in that region when bound to the SH3 domain alone (Fig. 4biii and c). These results are entirely consistent with the crystal structures of these two complexes. When Nef is bound to SH3 alone, each Asp123 plays a critical role in dimer stabilization by forming a reciprocal salt bridge with Arg105 in the other Nef monomer. On the other hand, Asp123 is solvent-exposed when bound to Hck SH3-SH2, and plays no apparent role in dimer stabilization. Instead, this Nef conformation is virtually identical to the one observed in the crystal complex with the MHC-I tail and the AP-1 μ1 subunit, where Nef Asp123 serves as a lynchpin to anchor the three proteins together (as in Fig. 1d). The location of the Nef D123N mutation is modeled on the crystal structures of the Nef:SH3 (PDB 1EFN) and Nef:SH3-SH2 (PDB 4U5W) complexes in Fig. 4c and 4d, respectively. Taken all together, our HX MS data demonstrate that both Nef dimer complexes exist in solution. However, the presence of the SH2 domain, and by extension full-length Hck, may dictate the formation of a Nef core dimer conformation essential for engagement of AP-1 and downregulation of MHC-I (see Discussion).
Figure 4.
D123N mutation prevents Nef core dimer formation when bound to Hck SH3 but not Hck SH3-SH2. (a) Comparison of deuterium uptake by the Nef-D123N core alone versus Nef-D123N in complex with Hck SH3 (left) or Hck SH3-SH2 (right) is presented in a vertical difference map of the Nef core secondary structure. The deuterium level of each peptide from Nef-D123N alone was subtracted from that in Nef-D123N when part of a complex and the differences are color-coded as indicated in the legend. The differences in non-overlapping peptides are displayed vertically from N- to C-terminus for each exchange time point (10 s to 4 h; shown horizontally). Greater protection is represented as a more intense shade of blue. The location of the D123N mutation is indicated in orange on the difference maps and the structures in panel (d). (b) Deuterium incorporation graphs for selected peptides i, ii, iii, iv (the same peptides highlighted in Figs. 2 and 3). Uptake curves from Nef-D123N core peptides are shown in black, Nef-D123N core:SH3 complex peptides are in blue, and Nef-D123N core:SH3-SH2 complex peptides are shown in red. (c, d) The protection observed in Nef-D123N when bound to Hck SH3 (c) or Hck SH3-SH2 (d) after 10 s (left) or 4 h (right) of deuterium exchange is plotted onto the corresponding crystal structures as indicated. Protection in the Nef-D123N αB helix is only observed in the complex with Hck SH3-SH2. Deuterium incorporation graphs for all peptic peptides from this experiment are shown in Supplementary Fig. S12.
Discussion
While mutagenesis studies have suggested that dimerization is essential for many Nef functions related to HIV replication and immune escape [32, 40], the events that control Nef dimerization are less clear. We demonstrate that in solution, the Nef core protein exists as a monomer, suggesting that the protein does not readily dimerize on its own. Analytical size exclusion chromatography shows that both the full-length and core Nef proteins elute as monomers at the low micromolar working concentrations used for HX MS, consistent with this view (Supplementary Fig. S4 and data not shown). HX MS data also demonstrate that the N-terminal arm of FL-Nef protects the core domain to some extent, perhaps to maintain the protein as an inactive monomer during transport to the membrane where Hck or other host cell factors induce dimerization. Previous HX MS studies [35], combined with neutron diffraction data [41], demonstrate that the Nef core is exposed when the myristoylated N-terminal arm interacts with experimental membranes and no evidence for reduced exchange in the Nef αB helix could be found in the absence of other proteins. Thus, membrane targeting and the accompanying conformational rearrangements of the N-terminal arm alone do not appear to be sufficient to induce stable dimer formation.
Here we show that interaction of the Nef core protein with the Hck SH3 domain, either alone or in the context of a dual SH3-SH2 protein, is consistent with formation of a dimer in solution. The hydrogen exchange protection found in Nef when part of a complex in solution is exactly in the regions shown by crystallography to be protected in a dimer. Furthermore, HX MS data obtained with the Nef-D123N mutant confirms the existence of two distinct Nef dimer conformations in solution, consistent with previous X-ray crystal structures of the Nef dimers present in complexes with SH3 [26] and SH3-SH2 [29]. Which dimer conformation is adopted in solution appears linked, at least for SFKs, to whether or not the SH2 domain is present. While the Nef αB helix forms an interface in both dimer conformations, our finding that Nef-D123N disrupts dimer formation when bound to Hck SH3 but not SH3-SH2 supports the existence of two distinct dimer states over which SH2 presides. The two structures may represent subsequent stages of conformational change. The initial binding of Nef to the Hck SH3 domain may trigger formation of a Nef dimer, which then can immediately reorganize to a modified dimer conformation if the SH2 domain is present. While this sequence would occur very rapidly in vivo, the use of constructs containing a different compliment of domains allowed us to monitor these distinct events. The conformation brought about when SH2 is present is compatible with additional effector protein interactions necessary for Nef function, as demonstrated by the high similarity of the position of Asp123 in the SH3-SH2:Nef and the Nef:MHC-I:AP-1 co-crystal structures. An alignment of both structures is presented in Fig. 1d, which suggests a possible role for Hck binding in the assembly of the Nef/AP-1 complex involved in MHC-I downregulation. In both cases, Nef Asp123 is optimally positioned to make polar contacts with Arg393 from AP-1 μ1, which in turn makes an extended polar contact with Asp327 from the MHC-I C-terminal tail peptide. This remarkable observation supports the possibility that Hck engagement induces this functional conformation of Nef. This idea is supported by previous experimental data, where suppression of Hck recruitment with Nef-binding small molecules, as well as mutagenesis of the Nef PxxP motif essential for Hck SH3 engagement, both interfere with Nef-dependent downregulation of MHC-I [22, 33]. Note that the Nef dimer need not persist after association with AP-1 and MHC-I downregulation; indeed, Nef exists as a monomer in the crystal structure of the Nef/MHC-I/AP-I μ1 complex [31]. Consequently, the Nef dimers observed in the crystal complexes with Hck may represent a transient interaction in the cell, which is then ‘handed off’ to AP-1/MHC-I once the appropriate conformation is adopted. However, in contrast to MHC-I downregulation, Hck activation by Nef may be dependent upon the transient dimer. Dimerization-defective mutants of Nef fail to activate Hck in vitro [21], while enhanced dimerization of Nef results in constitutive Hck activation in cell-based assays [42]. Thus, the initial interaction of Nef with Hck may have two important consequences: activation of the kinase, which has been shown to promote viral replication [43], and conformational remodeling of the Nef structure for subsequent interaction with the endocytic machinery essential for immune escape.
Small molecules that prevent these conformational transitions may interfere with Nef-mediated downregulation of MHC-I. Several classes of small molecules have been reported that bind directly to Nef and prevent Nef-induced MHC-I downregulation [21, 33, 44]. In addition, these compounds restore MHC-I/HIV-1 antigen complexes to the surface of infected CD4+ T cells, resulting in recognition and killing by autologous cytotoxic T-lymphocytes [45]. These observations raise the exciting possibility that drugs based on these compounds may have clinical value in strategies to reduce the latent HIV-1 reservoir through the patient’s own CTL response. The HX MS system established here will provide a powerful tool to better understand the mechanism of action of these compounds and how they may affect Nef conformational transitions. Further, our HX MS-based protein studies support the broader possibility that compounds interfering with conformational transitions in HIV-1 Nef structure have exciting potential as a new class of antiretroviral agents.
Materials and Methods
Bacterial Expression Vector Construction
The bacterial expression plasmids encoding the C-terminally His6-tagged human Hck SH3-SH2 tandem regulatory domains [residues 72–242; numbering based on the crystal structure of human c-Src (PDB 2SRC)] and both the full-length and core domain of HIV-1 Nef [SF2 allele residues 1–205 and 58–205, respectively; numbering based on the crystal structure of Nef NL4-3 (PDB 1EFN)] are described in Alvarado, et al [29]. Plasmids encoding the C-terminally His6-tagged human Hck SH3 (residues 77–140; numbering was based on the structure of human c-Src) were constructed by PCR amplification of the corresponding coding regions. The SH3 and Nef core PCR products were subcloned via Nde I and Xho I restriction sites into the bacterial expression vectors pET-21a(+) and pET-21b(+) (EMD Millipore), respectively. A fragment of the Nef-SF2 coding region of pET-14b/Nef-D123N [32] was excised with KpnI and EcoRI and subcloned into a pET-30a plasmid encoding the Nef-SF2 core sequence plus the C-terminal His6 affinity tag. The coding regions of all final bacterial expression plasmids were confirmed by DNA sequencing.
Protein Expression and Purification
Expression and purification conditions for recombinant Nef (full-length, core, D123N mutant) and Hck (SH3 and SH3-SH2) proteins in E. coli are described in detail elsewhere [29] with slight modifications. For ion-exchange and Ni-IMAC column chromatography steps, the flow rate was increased to 2 mL/min, and buffer exchange after IMAC chromatography involved dialysis against 3 × 1 L of anion-exchange buffer at 4 °C. Purification of the four protein complexes used in this study (wild-type and D123N forms of the Nef core in complex with either Hck SH3 or SH3-SH2) are also based on our previously published methods, in which cell pellets from cultures expressing the individual protein components were combined prior to lysis and column chromatography [29].
Surface Plasmon Resonance
Recombinant, purified Hck SH3 and SH3-SH2 proteins were exchanged into HBS-EP buffer (10 mM HEPES, pH 7.4, 150 mM NaCl, 3 mM EDTA, 0.05% v/v Surfactant P20) while recombinant Nef proteins were exchanged into HBS-EPD (HBS-EP buffer supplemented with 1 mM DTT) prior to analysis on a two-channel Reichert SR7500DC SPR platform. The Hck SH3 and SH3-SH2 proteins were covalently immobilized onto carboxy-methyl dextran SPR chips (Reichert) using standard EDC/NHS amine coupling chemistry. Nef proteins were then injected in triplicate over a range of concentrations (0.041 to 3.3 μM) at a rate of 30 μL/min for one min until equilibrium was reached, followed by dissociation in HBS-EBD for 3 min. Kinetic rate constants were calculated from reference-corrected sensorgrams using TraceDrawer software, and were best fit by 1:1 Langmuir (SH3) or two-state (SH3-SH2) binding models.
Analytical Size Exclusion Chromatography
Analytical size-exclusion chromatography (SEC) was conducted using a Superdex 75 10/300 GL column (GE Healthcare Life Sciences) equilibrated with running buffer (20 mM Tris-HCl, 150 mM NaCl, 10% (v/v) glycerol, 2 mM TCEP, pH 8.3). All SEC runs were conducted at a flow rate of 0.5 mL/min with each protein sample at a final concentration of 12.5 μM in a volume of 100 μL. Size-exclusion chromatography protein standards (Sigma-Aldrich) included bovine serum albumin (66 kDa), carbonic anhydrase (29 kDa), cytochrome C (12.5 kDa) and aprotinin (6.5 kDa).
Deuterium Labeling
Stock solutions of individual proteins and complexes were prepared to final concentrations of 65 μM in 20 mM Tris-HCl, pH 8.3, 100 mM NaCl, 3 mM DTT. Each protein or complex (110 pmol in 1.7 μl) was diluted 15-fold into identical buffer prepared with D2O. Labeling reactions were quenched after various times (10 s, 1 min, 10 min, 1 h, 4 h) with an equal volume of ice-cold quench buffer (100 mM K2HPO4/KH2PO4, pH 2.3). Undeuterated controls were prepared in the same way using the H2O-based buffer and quenched for digestion. One unique protein expression and purification prep was used for all protein samples, with the exceptions of the Nef core:Hck SH3 and Nef core:Hck SH3-SH2 complexes, for which two independent samples were prepared and analyzed. All labeling reactions were performed in duplicate, and a representative experiment is shown for each sample.
Online Protein Digestion and Mass Analysis
After quenching, samples were injected onto a Waters nanoAcquity with HDX technology for online pepsin digestion at 15 °C and UPLC peptide separation at 0 °C. Samples were digested on a 2.1 × 50 mm stainless steel column packed with POROS 20AL resin coupled to porcine pepsin (Sigma). Peptides were trapped and desalted on a VanGuard Pre-Column trap (2.1 × 5mm, Acquity UPLC BEH C18, 1.7 μm) for 3 min. Peptides were eluted from the trap and separated using an Acquity UPLC HSS T3 1.8 μm 1.0 × 50 mm column with a 5–35% gradient of acetonitrile over 6 min at a flow rate of 65 μl/min. Mass spectra were acquired using a Waters Synapt G2Si mass spectrometer. Peptides were identified from triplicate undeuterated samples of each protein alone and in complexes using Waters MSE and Waters Protein Lynx Global Server (PLGS) 3.0. Peptide maps were generated and deuterium incorporation analyzed using Waters DynamX 3.0 software.
Supplementary Material
Highlights.
Nef function requires interactions with Src kinases and endocytic adaptors
HX MS reveals Nef N-terminal arm protects core domain
Dimer interface protected in solution when bound to Hck SH3 or SH3-SH2 domains
SH3-SH2 binding exposes Nef Asp123 for interaction with downstream partners
Results support a role for Hck in conformational remodeling of Nef structure
Acknowledgments
This work was supported by National Institutes of Health grants AI102724 and AI057083 to TES and GM101135 to JRE. JRE acknowledges a research collaboration with the Waters Corporation.
Abbreviations
- ADCC
antibody-dependent cell-mediated cytotoxicity
- AP-1/AP-2
Adaptor protein 1/2
- CTL
cytotoxic T-lymphocyte
- HX MS
Hydrogen exchange mass spectrometry
- MHC-1
major histocompatibility complex-1
- SEC-MALS
size-exclusion chromatography multi-angle light scattering
- SFK
Src-family kinase
- SIV
simian immunodeficiency virus
- SPR
surface plasmon resonance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hanna Z, Kay DG, Cool M, Jothy S, Rebai N, Jolicoeur P. Transgenic mice expressing human immunodeficiency virus type 1 in immune cells develop a severe AIDS-like disease. J Virol. 1998;72:121–132. doi: 10.1128/jvi.72.1.121-132.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watkins RL, Foster JL, Garcia JV. In vivo analysis of Nef’s role in HIV-1 replication, systemic T cell activation and CD4(+) T cell loss. Retrovirology. 2015;12:61. doi: 10.1186/s12977-015-0187-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zou W, Denton PW, Watkins RL, Krisko JF, Nochi T, Foster JL, et al. Nef functions in BLT mice to enhance HIV-1 replication and deplete CD4+CD8+ thymocytes. Retrovirology. 2012;9:44. doi: 10.1186/1742-4690-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kestler HW, 3rd, Ringler DJ, Mori K, Panicali DL, Sehgal PK, Daniel MD, et al. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 5.Kirchhoff F, Greenough TC, Brettler DB, Sullivan JL, Desrosiers RC. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N Engl J Med. 1995;332:228–232. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- 6.Deacon NJ, Tsykin A, Solomon A, Smith K, Ludford-Menting M, Hooker DJ, et al. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 7.Kondo M, Shima T, Nishizawa M, Sudo K, Iwamuro S, Okabe T, et al. Identification of attenuated variants of HIV-1 circulating recombinant form 01_AE that are associated with slow disease progression due to gross genetic alterations in the nef/long terminal repeat sequences. J Infect Dis. 2005;192:56–61. doi: 10.1086/430739. [DOI] [PubMed] [Google Scholar]
- 8.Salvi R, Garbuglia AR, Di Caro A, Pulciani S, Montella F, Benedetto A. Grossly defective nef gene sequences in a human immunodeficiency virus type 1-seropositive long-term nonprogressor. J Virol. 1998;72:3646–3657. doi: 10.1128/jvi.72.5.3646-3657.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorry PR, McPhee DA, Verity E, Dyer WB, Wesselingh SL, Learmont J, et al. Pathogenicity and immunogenicity of attenuated, nef-deleted HIV-1 strains in vivo. Retrovirology. 2007;4:66. doi: 10.1186/1742-4690-4-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smithgall TE, Thomas G. Small molecule inhibitors of the HIV-1 virulence factor, Nef. Drug Discov Today Technol. 2013;10:e523–529. doi: 10.1016/j.ddtec.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pereira EA, daSilva LL. HIV-1 Nef: Taking Control of Protein Trafficking. Traffic. 2016;17:976–996. doi: 10.1111/tra.12412. [DOI] [PubMed] [Google Scholar]
- 12.Benson RE, Sanfridson A, Ottinger JS, Doyle C, Cullen BR. Downregulation of cell-surface CD4 expression by simian immunodeficiency virus Nef prevents viral super infection. J Exp Med. 1993;177:1561–1566. doi: 10.1084/jem.177.6.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veillette M, Desormeaux A, Medjahed H, Gharsallah NE, Coutu M, Baalwa J, et al. Interaction with cellular CD4 exposes HIV-1 envelope epitopes targeted by antibody-dependent cell-mediated cytotoxicity. J Virol. 2014;88:2633–2644. doi: 10.1128/JVI.03230-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pham TN, Lukhele S, Hajjar F, Routy JP, Cohen EA. HIV Nef and Vpu protect HIV-infected CD4+ T cells from antibody-mediated cell lysis through down-modulation of CD4 and BST2. Retrovirology. 2014;11:15. doi: 10.1186/1742-4690-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins KL, Chen BK, Kalams SA, Walker BD, Baltimore D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391:397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 16.Pawlak EN, Dikeakos JD. HIV-1 Nef: a master manipulator of the membrane trafficking machinery mediating immune evasion. Biochim Biophys Acta. 2015;1850:733–741. doi: 10.1016/j.bbagen.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Arora VK, Molina RP, Foster JL, Blakemore JL, Chernoff J, Fredericksen BL, et al. Lentivirus Nef specifically activates Pak2. J Virol. 2000;74:11081–11087. doi: 10.1128/jvi.74.23.11081-11087.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Renkema GH, Manninen A, Mann DA, Harris M, Saksela K. Identification of the Nef-associated kinase as p21-activated kinase 2. Curr Biol. 1999;9:1407–1410. doi: 10.1016/s0960-9822(00)80086-x. [DOI] [PubMed] [Google Scholar]
- 19.Briggs SD, Sharkey M, Stevenson M, Smithgall TE. SH3-mediated Hck tyrosine kinase activation and fibroblast transformation by the Nef protein of HIV-1. J Biol Chem. 1997;272:17899–17902. doi: 10.1074/jbc.272.29.17899. [DOI] [PubMed] [Google Scholar]
- 20.Tarafdar S, Poe JA, Smithgall TE. The Accessory Factor Nef Links HIV-1 to Tec/Btk Kinases in an SH3 Domain-dependent Manner. J Biol Chem. 2014 doi: 10.1074/jbc.M114.572099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emert-Sedlak LA, Narute P, Shu ST, Poe JA, Shi H, Yanamala N, et al. Effector kinase coupling enables high-throughput screens for direct HIV-1 Nef antagonists with antiretroviral activity. Chem Biol. 2013;20:82–91. doi: 10.1016/j.chembiol.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hung CH, Thomas L, Ruby CE, Atkins KM, Morris NP, Knight ZA, et al. HIV-1 Nef assembles a Src family kinase-ZAP-70/Syk-PI3K cascade to downregulate cell-surface MHC-I. Cell Host Microbe. 2007;1:121–133. doi: 10.1016/j.chom.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Geyer M, Peterlin BM. Domain assembly, surface accessibility and sequence conservation in full length HIV-1 Nef. FEBS Lett. 2001;496:91–95. doi: 10.1016/s0014-5793(01)02394-8. [DOI] [PubMed] [Google Scholar]
- 24.Bentham M, Mazaleyrat S, Harris M. Role of myristoylation and N-terminal basic residues in membrane association of the human immunodeficiency virus type 1 Nef protein. J Gen Virol. 2006;87:563–571. doi: 10.1099/vir.0.81200-0. [DOI] [PubMed] [Google Scholar]
- 25.Gerlach H, Laumann V, Martens S, Becker CF, Goody RS, Geyer M. HIV-1 Nef membrane association depends on charge, curvature, composition and sequence. Nat Chem Biol. 2010;6:46–53. doi: 10.1038/nchembio.268. [DOI] [PubMed] [Google Scholar]
- 26.Lee CH, Saksela K, Mirza UA, Chait BT, Kuriyan J. Crystal structure of the conserved core of HIV-1 Nef complexed with a Src family SH3 domain. Cell. 1996;85:931–942. doi: 10.1016/s0092-8674(00)81276-3. [DOI] [PubMed] [Google Scholar]
- 27.Saksela K, Cheng G, Baltimore D. Proline-rich (PxxP) motifs in HIV-1 Nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef+ viruses but not for down-regulation of CD4. EMBO J. 1995;14:484–491. doi: 10.1002/j.1460-2075.1995.tb07024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moarefi I, LaFevre-Bernt M, Sicheri F, Huse M, Lee CH, Kuriyan J, et al. Activation of the Src-family tyrosine kinase Hck by SH3 domain displacement. Nature. 1997;385:650–653. doi: 10.1038/385650a0. [DOI] [PubMed] [Google Scholar]
- 29.Alvarado JJ, Tarafdar S, Yeh JI, Smithgall TE. Interaction with the Src homology (SH3-SH2) region of the Src-family kinase Hck structures the HIV-1 Nef dimer for kinase activation and effector recruitment. J Biol Chem. 2014;289:28539–28553. doi: 10.1074/jbc.M114.600031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren X, Park SY, Bonifacino JS, Hurley JH. How HIV-1 Nef hijacks the AP-2 clathrin adaptor to downregulate CD4. Elife. 2014;3:e01754. doi: 10.7554/eLife.01754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jia X, Singh R, Homann S, Yang H, Guatelli J, Xiong Y. Structural basis of evasion of cellular adaptive immunity by HIV-1 Nef. Nat Struct Mol Biol. 2012;19:701–706. doi: 10.1038/nsmb.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poe JA, Smithgall TE. HIV-1 Nef dimerization is required for Nef-mediated receptor downregulation and viral replication. J Mol Biol. 2009;394:329–342. doi: 10.1016/j.jmb.2009.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dikeakos JD, Atkins KM, Thomas L, Emert-Sedlak L, Byeon IJ, Jung J, et al. Small molecule inhibition of HIV-1-induced MHC-I down-regulation identifies a temporally regulated switch in Nef action. Mol Biol Cell. 2010;21:3279–3292. doi: 10.1091/mbc.E10-05-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wales TE, Poe JA, Emert-Sedlak L, Morgan CR, Smithgall TE, Engen JR. Hydrogen Exchange Mass Spectrometry of Related Proteins with Divergent Sequences: A Comparative Study of HIV-1 Nef Allelic Variants. J Am Soc Mass Spectrom. 2016;27:1048–1061. doi: 10.1007/s13361-016-1365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pirrone GF, Emert-Sedlak LA, Wales TE, Smithgall TE, Kent MS, Engen JR. Membrane-Associated Conformation of HIV-1 Nef Investigated with Hydrogen Exchange Mass Spectrometry at a Langmuir Monolayer. Anal Chem. 2015;87:7030–7035. doi: 10.1021/acs.analchem.5b01725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morgan CR, Miglionico BV, Engen JR. Effects of HIV-1 Nef on human N-myristoyltransferase 1. Biochemistry. 2011;50:3394–3403. doi: 10.1021/bi200197e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trible RP, Emert-Sedlak L, Wales TE, Ayyavoo V, Engen JR, Smithgall TE. Allosteric loss-of-function mutations in HIV-1 Nef from a long-term non-progressor. J Mol Biol. 2007;374:121–129. doi: 10.1016/j.jmb.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hochrein JM, Wales TE, Lerner EC, Schiavone AP, Smithgall TE, Engen JR. Conformational features of the full-length HIV and SIV Nef proteins determined by mass spectrometry. Biochemistry. 2006;45:7733–7739. doi: 10.1021/bi060438x. [DOI] [PubMed] [Google Scholar]
- 39.Wales TE, Hochrein JM, Morgan CR, Emert-Sedlak LA, Smithgall TE, Engen JR. Subtle Dynamic Changes Accompany Hck Activation by HIV-1 Nef and are Reversed by an Antiretroviral Kinase Inhibitor. Biochemistry. 2015;54:6382–6391. doi: 10.1021/acs.biochem.5b00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shu ST, Emert-Sedlak LA, Smithgall TE. Cell-based Fluorescence Complementation Reveals a Role for HIV-1 Nef Protein Dimerization in AP-2 Adaptor Recruitment and CD4 Co-receptor Down-regulation. J Biol Chem. 2017;292:2670–2678. doi: 10.1074/jbc.M116.770016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akgun B, Satija S, Nanda H, Pirrone GF, Shi X, Engen JR, et al. Conformational transition of membrane-associated terminally acylated HIV-1 Nef. Structure. 2013;21:1822–1833. doi: 10.1016/j.str.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ye H, Choi HJ, Poe J, Smithgall TE. Oligomerization is required for HIV-1 Nef-induced activation of the Src family protein-tyrosine kinase, Hck. Biochemistry. 2004;43:15775–15784. doi: 10.1021/bi048712f. [DOI] [PubMed] [Google Scholar]
- 43.Emert-Sedlak L, Kodama T, Lerner EC, Dai W, Foster C, Day BW, et al. Chemical library screens targeting an HIV-1 accessory factor/host cell kinase complex identify novel antiretroviral compounds. ACS Chem Biol. 2009;4:939–947. doi: 10.1021/cb900195c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trible RP, Narute P, Emert-Sedlak LA, Alvarado JJ, Atkins K, Thomas L, et al. Discovery of a diaminoquinoxaline benzenesulfonamide antagonist of HIV-1 Nef function using a yeast-based phenotypic screen. Retrovirology. 2013;10:135. doi: 10.1186/1742-4690-10-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mujib S, Saiyed A, Fadel S, Bozorgzad A, Aidarus N, Yue FY, et al. Pharmacologic HIV-1 Nef blockade promotes CD8 T cell-mediated elimination of latently HIV-1-infected cells in vitro. JCI Insight. 2017;2 doi: 10.1172/jci.insight.93684. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.