Abstract
Background
Extrapulmonary NEC are poorly studied and managed similar to lung NEC which may not account for differences between the two groups and also the heterogeneity within extrapulmonary NEC.
Methods
Data from the Surveillance, Epidemiology, and End Results (SEER) program between 1973 and 2012 was used to estimate the relative proportions of lung and sub groups of extrapulmonary NEC, epidemiological patterns at these sites and median- & 5-year overall survival rates.
Results
Of 162983 NEC cases, 14732 were extrapulmonary, 5509 were gastrointestinal, (37·4%), 4151 were unknown primary, (28·2%) and 5072 were of other sites (34.4%). Lung NEC had the highest proportion of small cell morphology (95.2%) and gastrointestinal NEC the least (38·7%) with the rest being other morphologies. Significant differences were noted in median age (range: 48–74); proportion of distant stage (24% – 77%) and incidence according to gender and race. Median survival of lung NEC was 7·6 months (m), gastrointestinal NEC 7·5 m (range 25.1 m at small intestine to 5.7 m at pancreas) and unknown NEC 2·5 m. 5-year survival for local stage patients ranged from 58–60% for female genital tract and small intestine to 25% for esophageal NEC. Primary site remained significant for survival even after adjusting for known prognostic variables (p<0·0001).
Conclusions
This is the largest study of NECs to date and also the first with comprehensive epidemiological data. Significant differences in incidence patterns and large variations in survival depending on anatomical site and morphological subtype were noted. A curative approach is possible for patients with non-metastatic NEC.
Keywords: small cell, large cell, neuroendocrine carcinoma, poorly differentiated, SEER
Introduction
Neuroendocrine neoplasms are grouped into well-differentiated tumors or poorly differentiated carcinomas based on morphology and markers of proliferation the latter including Ki-67 and/or mitotic index.1–3 Within the poorly differentiated neuroendocrine carcinomas, lung neuroendocrine carcinomas (lung NEC, including small cell and large cell carcinomas) are well characterized while very limited data regarding extra-pulmonary NEC exist. Such data are mainly from single institution or small database studies limited by size and/or sites evaluated.3–7 The largest of prior studies, the NORDIC NEC study included 305 extrapulmonary NEC cases limited to the gastrointestinal tract.8 Therefore, several concerns and questions regarding differences in epidemiology and clinical behavior of extrapulmonary NEC and lung NEC, implications of morphological subtypes remain unanswered.
While lung NEC are typically associated with a strong history of smoking, recent studies have suggested that the association with smoking for extrapulmonary NEC is weaker.9,10 Compared to lung NEC, the incidence of brain metastases appear to be lower in extrapulmonary NEC.11,12 Also, while some studies have suggested similar objective response rates to platinum based chemotherapies irrespective of site of origin of NEC, other studies have suggested otherwise.13,14 Thus, the clinical implications of primary site of origin of poorly differentiated NEC remains to be elucidated. Major epidemiological data concerning stage at diagnosis and survival according to different primary tumor sites and stage within gastrointestinal NEC and other extrapulmonary NEC are completely lacking, hampering present clinical decision making. While some data do not suggest differences in outcomes between large cell and small cell NEC, a recent Dutch registry study suggested better outcomes for large-cell subtypes that need to be explored further.15–18 Finally, extrapulmonary NEC of unknown primary represents 10% of all poorly differentiated cancers of unknown primary; yet very little is known about this subset.19,20
As discussed in a recent series on rare cancers, the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) program provides a rich resource to help shape treatment guidelines in such situations.21 Therefore, in the current study, we used the SEER program to comprehensively define the demographic, clinical, and prognostic features of poorly differentiated NECs.
Methods
Data were retrieved from the SEER 18 registry based on the November 2014 submission for cases with tumors of malignant behavior and known age diagnosed between the inception of the SEER program in 1973 until December 31, 2012. Poorly differentiated neuroendocrine carcinomas were identified with the ICD-O-3 morphology codes detailed in Supplementary Table 1 and classified per SEER historical staging system as localized, regional and distant. Sites of origin were classified into respiratory, gastrointestinal or unknown primary site, (ICD-O-3 topography code C80·9) with the rest being grouped into NEC of other sites.
Statistical calculations were performed using SEER*Stat software (version 8·2·1; Surveillance Research Program, National Cancer Institute, Bethesda, MD), Microsoft Excel 2013 (Microsoft Corporation, Redmond, WA), or SAS (version 9·3, SAS Institute, Cary, NC).
Demographic and tumor characteristics were compared using χ2 test. Patient characteristics were described using the entire cohort (Supplementary Table 2) and also SEER 18 cohort (Table 1) separately that is the latest registry grouping (2000–2012) to reflect the most recent trends. ANOVA (one-way analysis of variance), Kruskal-Wallis test, Mann–Whitney U test or unpaired t test were used for comparison of means or distributions of continuous variables between groups depending upon the distribution of the variable; survival was measured using Kaplan-Meier method and compared using the log-rank test. Cox proportional hazards model was used for comparing effects of prognostic variables on survival. Proportionality assumption was tested by including time dependent covariates in a separate model. All differences were considered statistically significant if two-sided p was < 0·05.
Table 1.
Incidence rates (per 100,000) of Neuroendocrine Carcinomas by Sex and Race in the SEER 18 Registry age adjusted to 2000 U.S. Standard Population(*)
| All Cases | Male | Female | White | Black | American Indian/ Alaska Native |
Asian or Pacific Islander |
|
|---|---|---|---|---|---|---|---|
| Rate (N) | Rate (N) | Rate (N) | Rate (N) | Rate (N) | Rate (N) | Rate (N) | |
| All Cases | 9.36 (99128) | 10.81 (50925) | 8.31 (48203) | 10.03 (86399) | 8.67 (8676) | 5.82 (541) | 3.87 (3354) |
| Digestive System | 0.4 (4186) | 0.49 (2305) | 0.32 (1881) | 0.39 (3392) | 0.48 (495) | 0.28 (25) | 0.29 (263) |
| Esophagus | 0.04 (433) | 0.06 (289) | 0.02 (144) | 0.04 (351) | 0.05 (53) | 0.05 (3) | 0.03 (23) |
| Stomach | 0.05 (482) | 0.07 (323) | 0.03 (159) | 0.04 (357) | 0.07 (73) | 0.02 (1) | 0.06 (50) |
| Small Intestine | 0.02 (250) | 0.03 (137) | 0.02 (113) | 0.02 (206) | 0.03 (32) | 0.01 (1) | 0.01 (10) |
| Colon | 0.11 (1106) | 0.12 (545) | 0.1 (561) | 0.11 (932) | 0.12 (115) | 0.05 (4) | 0.06 (53) |
| Rectum | 0.05 (523) | 0.06 (286) | 0.04 (237) | 0.05 (429) | 0.06 (64) | 0.02 (2) | 0.03 (27) |
| Anal Canal | 0.01 (148) | 0.01 (68) | 0.01 (80) | 0.01 (120) | 0.02 (21) | 0.01 (2) | 0 (4) |
| Pancreas | 0.07 (779) | 0.09 (438) | 0.06 (341) | 0.07 (618) | 0.1 (99) | 0.06 (7) | 0.06 (54) |
| Liver | 0.01 (135) | 0.02 (80) | 0.01 (55) | 0.01 (110) | 0.01 (11) | 0.04 (3) | 0.01 (11) |
| Gallbladder and biliary tract | 0.02 (242) | 0.02 (107) | 0.02 (135) | 0.02 (198) | 0.02 (21) | 0.02 (2) | 0.02 (21) |
| Other GI Sites | 0.01 (88) | 0.01 (32) | 0.01 (56) | 0.01 (71) | 0.01 (6) | 0 (0) | 0.01 (10) |
| Respiratory System | 8.36 (88582) | 9.55 (45142) | 7.48 (43440) | 9 (77567) | 7.64 (7627) | 5.19 (483) | 3.23 (2780) |
| Other | 0.36 (3842) | 0.46 (2052) | 0.31 (1790) | 0.38 (3235) | 0.35 (356) | 0.22 (22) | 0.24 (217) |
| Urinary Bladder | 0.14 (1430) | 0.26 (1121) | 0.05 (309) | 0.15 (1285) | 0.1 (93) | 0.04 (3) | 0.06 (48) |
| Kidney and Renal Pelvis | 0.01 (87) | 0.01 (57) | 0.01 (30) | 0.01 (73) | 0.01 (9) | 0.01 (1) | 0 (4) |
| Cervix uteri | 0.05 (530) | 0 (0) | 0.1 (530) | 0.05 (392) | 0.06 (69) | 0.05 (5) | 0.06 (63) |
| Ovary | 0.02 (250) | 0 (0) | 0.04 (250) | 0.03 (212) | 0.02 (17) | 0.01 (1) | 0.02 (20) |
| Corpus and Uterus | 0.02 (178) | 0 (0) | 0.03 (178) | 0.02 (140) | 0.02 (21) | 0 (0) | 0.02 (16) |
| Prostate | 0.05 (477) | 0.11 (477) | 0 (0) | 0.05 (411) | 0.04 (40) | 0.04 (4) | 0.02 (20) |
| Unknown | 0.24 (2518) | 0.32 (1426) | 0.19 (1092) | 0.26 (2205) | 0.2 (198) | 0.13 (11) | 0.11 (94) |
Results
Patient Characteristics
In the entire cohort, 87997 (54 %) were men. Eighty eight percent (87.8 %) were white, 8.3% were black and rest were other races (Supplementary Table 2). Similar trends were noted in SEER 18 cohort as well (Table 1). Primary tumor site varied significantly according to sex (p < 0·001). Lung NEC, most gastrointestinal NEC, urinary sites and unknown primary were more common in males (Table 1; Supplementary Table 2). Racial differences were present with lung NEC and urinary bladder NEC more common in whites while gastrointestinal NEC sites were slightly more common in blacks (Table 1; Supplementary Table 2). The median age at diagnosis was 67 years (SD 10·9 m). Median age at diagnosis varied significantly by site (p < 0·0001) ranging from cervical NEC at 48 years to 74 years for bladder NEC (Table 2). In patients with known staging, significant differences were noted in stage at diagnosis according to primary tumor site (p < 0·0001). Approximately 70% of cases with lung, ovary, and pancreas NEC had distant stage at diagnosis in contrast to only 24% of those arising in the urinary bladder and 35% in the cervix (Table 2).
Table 2.
Age and Disease Stage at Diagnosis of High Grade Neuroendocrine Carcinomas by Primary Tumor Site
| Age at Diagnosis (years) |
Stage at Diagnosis (%) | ||||
|---|---|---|---|---|---|
| Site (N) | Median Age at Diagnosis |
Known Stage | Unknown or Unstaged |
||
| Localized | Regional | Distant | |||
| All Cases (99128) | 67 | 8.0 | 22.7 | 69.3 | 23.4 |
| Digestive System (4186) | 68 | 12.5 | 26.7 | 60.8 | 12.8 |
| Esophagus (433) | 68 | 19.4 | 21.2 | 59.4 | 14.6 |
| Stomach (482) | 69 | 14.5 | 31.3 | 54.3 | 9.4 |
| Small Intestine (250) | 67 | 17.4 | 33.2 | 49.4 | 4.2 |
| Colon (1106) | 70 | 7.5 | 31.3 | 61.2 | 1.9 |
| Rectum (523) | 64 | 17.0 | 25.5 | 57.5 | 6.5 |
| Anal Canal (148) | 62 | 29.1 | 31.8 | 39.2 | 11.9 |
| Pancreas (779) | 67 | 6.5 | 17.9 | 75.6 | 5.6 |
| Liver (135) | 65 | 29.9 | 33.1 | 37.1 | 22.0 |
| Gallbladder and Biliary Tract (242) | 70 | 9.8 | 34.5 | 55.7 | 80.6 |
| Other GI Sites (88) | 70 | 0.0 | 23.5 | 76.5 | 88.5 |
| Respiratory System (88582) | 68 | 6.8 | 22.2 | 71.0 | 21.9 |
| Other (3842) | 67 | 32.7 | 31.4 | 36.0 | 14.9 |
| Urinary Bladder (1430) | 74 | 48.2 | 27.6 | 24.2 | 4.6 |
| Kidney and Renal Pelvis (87) | 72 | 8.0 | 33.1 | 58.9 | 5.1 |
| Cervix uteri (530) | 48 | 28.1 | 37.0 | 34.9 | 4.3 |
| Ovary (250) | 51 | 9.6 | 21.4 | 69.0 | 3.4 |
| Corpus and Uterus (178) | 65 | 16.7 | 37.1 | 46.2 | 3.7 |
| Prostate (477) | 72 | 16.5 | 20.5 | 63.0 | 23.6 |
| Unknown (2518) | 71 | 0 | 0 | 0 | 100 |
Relative Proportions per Site and Morphological subtype
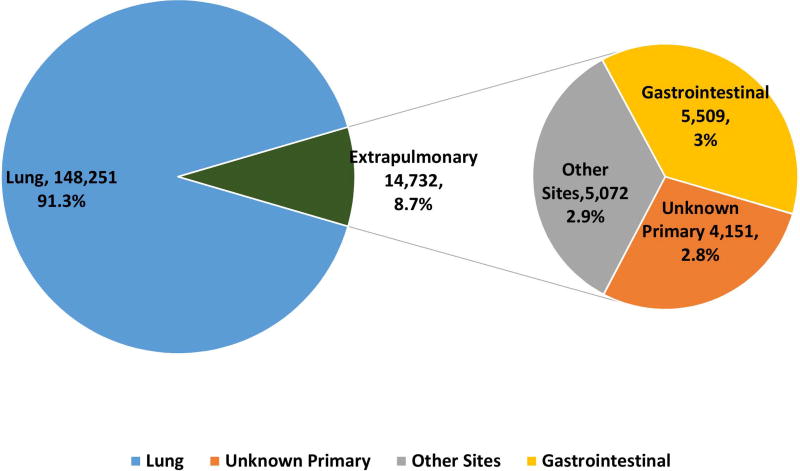
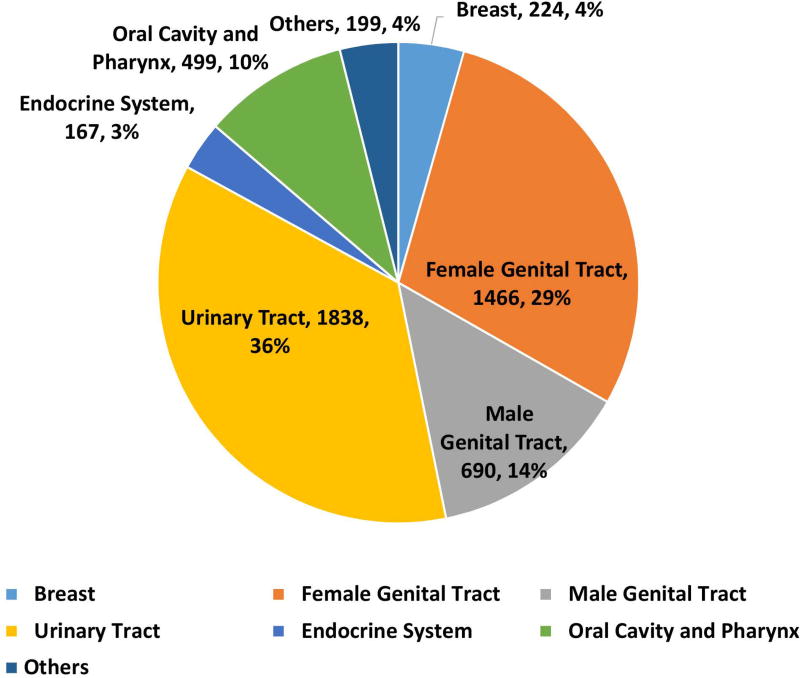
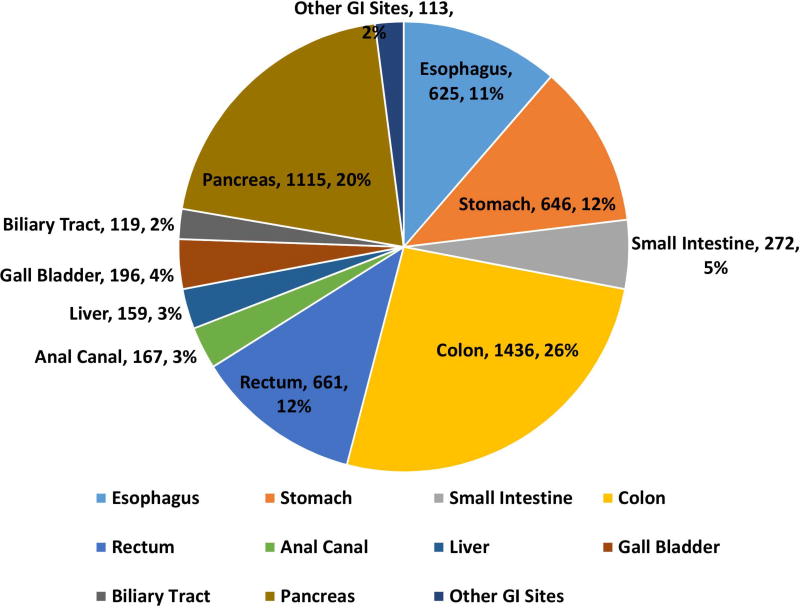
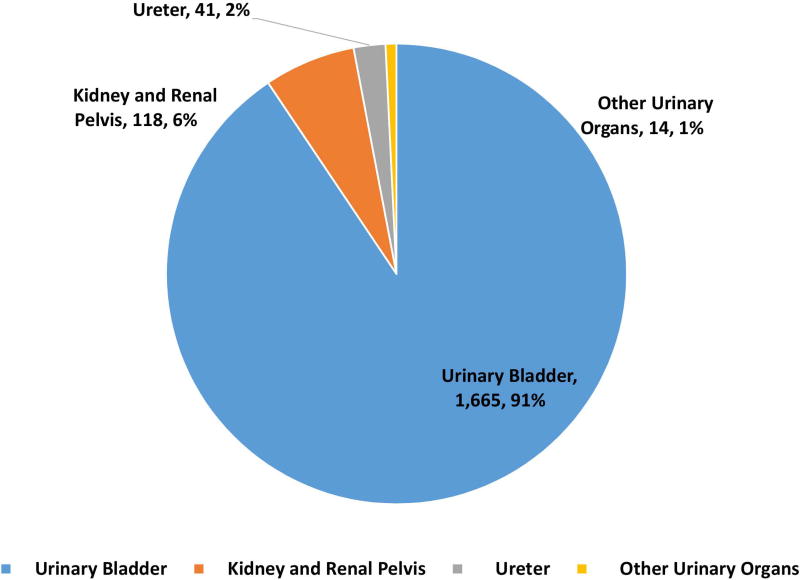
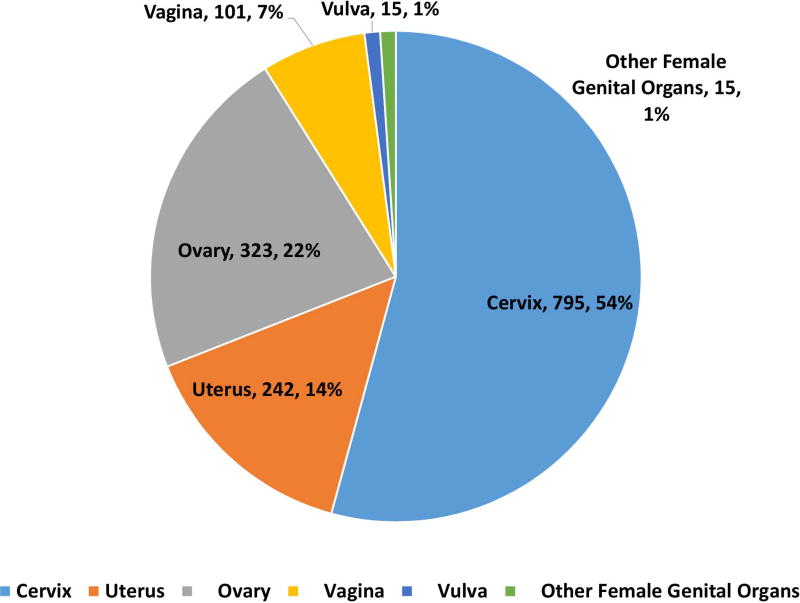
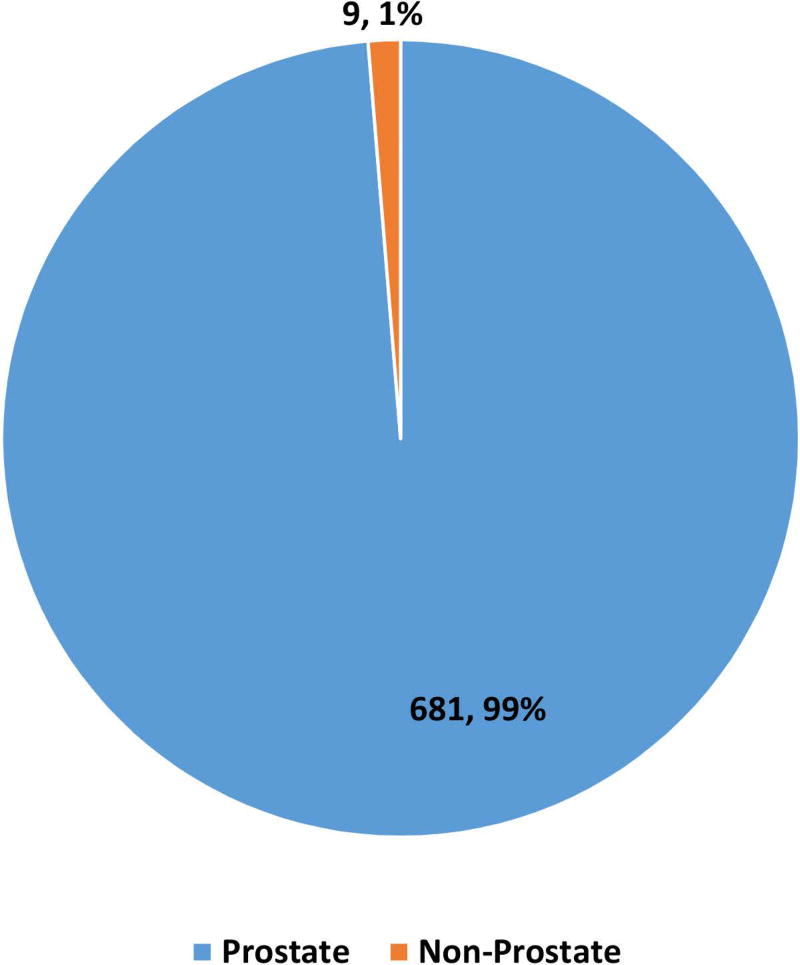
162983 patients with poorly differentiated NEC were identified with 148251 (91·3%) being lung and 14732 (8·7%) being extrapulmonary NEC (Fig 1A, 1B). Within gastrointestinal NEC, the lower gastrointestinal tract including the colon, rectum and anal canal accounted for 41%, the upper gastrointestinal tract and the pancreas for 23% and 20% respectively (Fig 1C). About 3% were coded as liver primary although it is likely that the majority of these were metastases. In the urinary system, majority were urinary bladder (91%). (Fig 2A). In the female genital tract, 54% arose from the cervix, 14% from the uterus and another 22% from the ovaries (Fig 2B). In the male genital tract, nearly all cases were prostatic (Fig 2C).
Figure 1.
Proportion of poorly differentiated neuroendocrine carcinomas according to morphological subtype at major sites (1A), subsites including non-pulmonary, non-gastrointestinal (Other) sites (1B), gastrointestinal tract (1C).
Figure 2.
Proportion of poorly differentiated neuroendocrine carcinomas according to morphological subtype at subsites including urinary system (2A), female (2B) and male (2C) genital tracts.
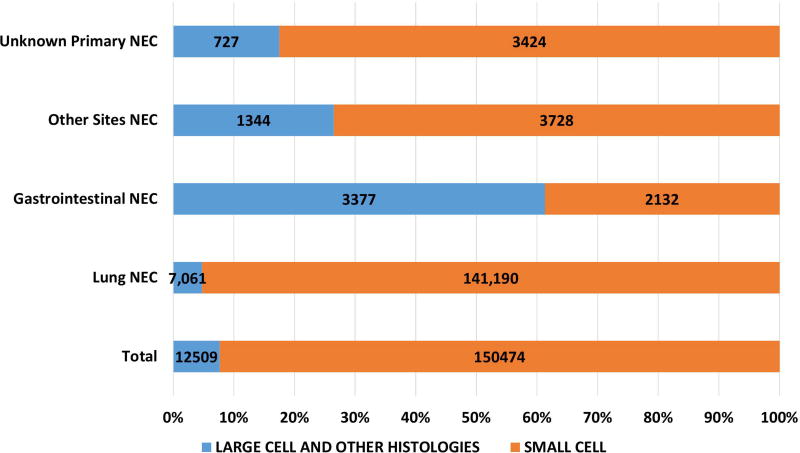
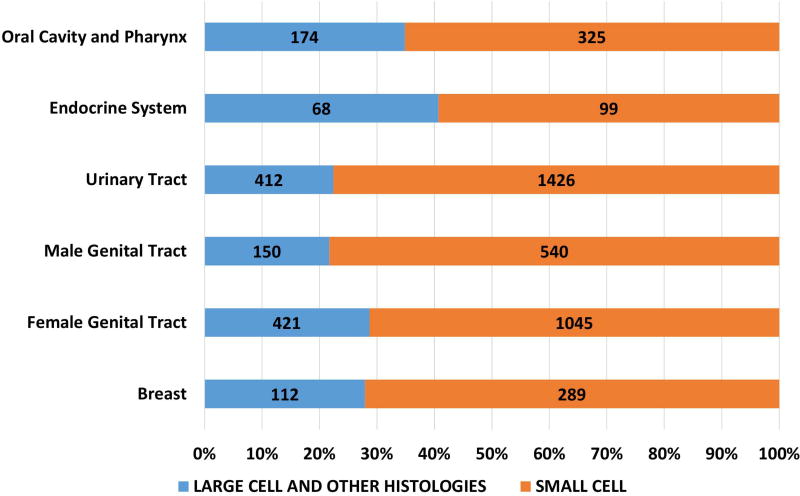
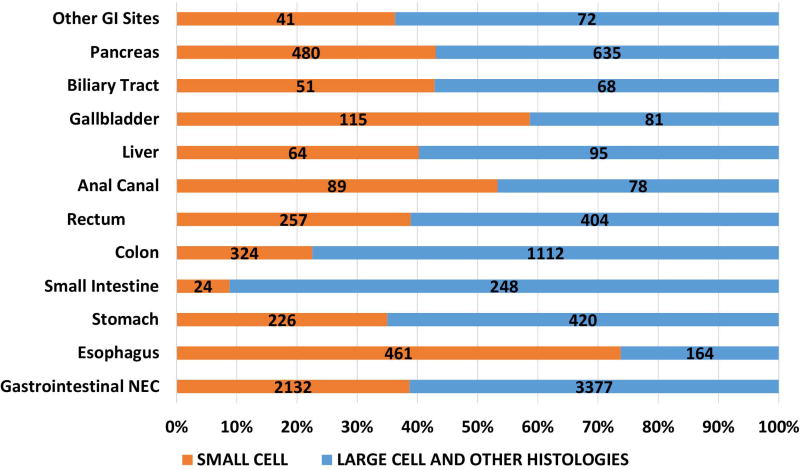
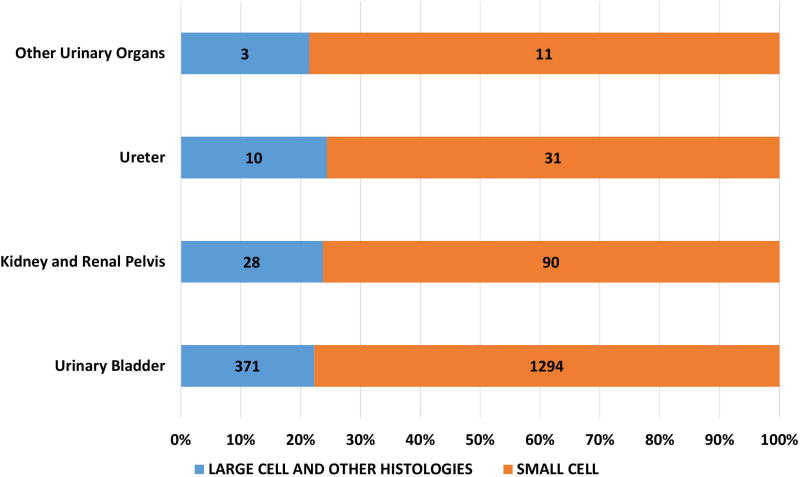
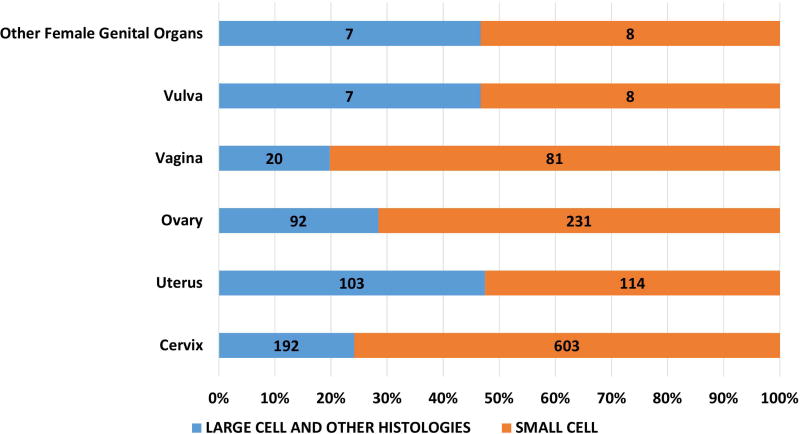
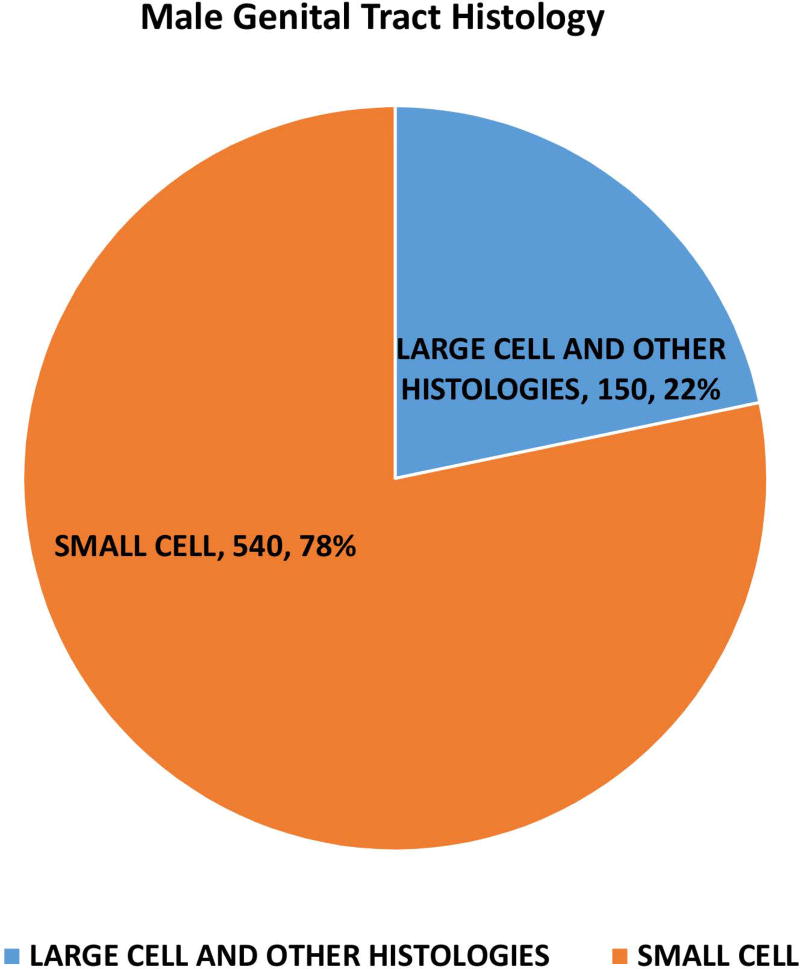
Of the 162983 cases included in the study, 150474 were small cell (92·4%), 12509 were large cell and other morphologies (7·6%). Of the total cases, 93·8% of small cell and 56.4% of large cell cases and other morphologies were lung in origin (Figure 1A). Of the large cell and other morphologies, the majority of cases (8219, 65.7%) were coded as grade 3 or 4 neuroendocrine carcinoma (ICD03 code: 8246/3) without further morphological details and 30.7% (3844) were coded as large cell carcinoma. Significant variations were noted in morphological subtypes at major anatomical areas (p < 0·0001) – with lung NEC having the highest proportion of small cell morphology (95.2%) and gastrointestinal NEC having the least (38·7%) (Fig 1A). Small cell morphology was dominant in esophageal, anal, gallbladder and genitourinary primary sites; Figures 1B, 1C).
Survival
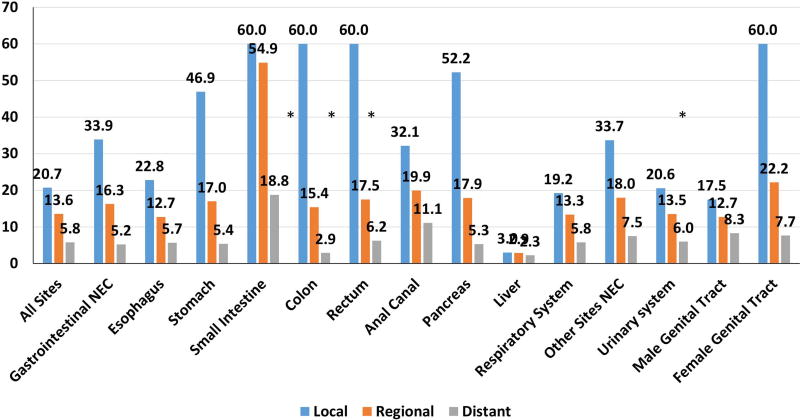
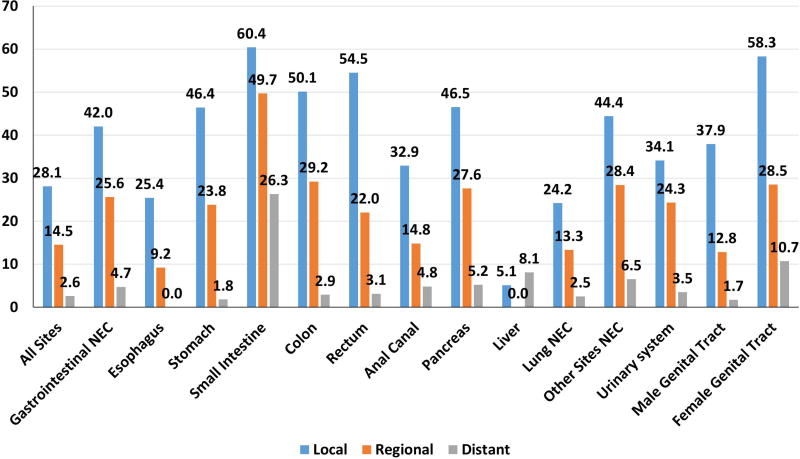
The median survival of all cases was 7·7 months (m). Significant differences in survival were noted according to stage at diagnosis, site, and morphology (all p < 0·0001) (Figures 3A, 3B). Survival was also significantly impacted by anatomical site and morphological subtype. For instance, median survival of all lung NEC was 7·6 m in contrast to that at other sites at 14·5 m (Table 3). Of note, NEC of unknown sites (2·5 m) had the shortest survival possibly due to the metastatic nature of the majority of these cases. Within gastrointestinal NEC, small intestine had the longest median survival (25·1 m) while pancreas (5·7 m) had the shortest median survival. 5-year survival was much lower for lung NEC (5.6%) and gastrointestinal NEC (13·1%) compared to other primary NEC (26.0%). Significant differences in survival were also found between different sites according to morphological subtype (p < 0·001). (Supplementary Table 3) with small cell histology being associated with worse median and 5-year survival at most sites. Similarly, localized stage in gastrointestinal primary sites with a dominance of small cell morphology (eg esophageal and anal) had a lower 5-year survival than primary sites dominated by large cell and other morphologies (eg stomach and colorectal) (Figure 3). Localized NEC had a median survival of 20.7 m, regional NEC 13.6 m and distant disease 5.8 m. Importantly, a significant proportion of patients without distant disease were alive after 5-years (Figure 3). 5-year survival for local stage patients ranged from 60% for female genital tract and 40–50% for gastric, pancreatic, and colorectal to 25% for esophageal NEC. Even with regional disease, substantial rates of 5-year survival were seen in some sites ranging from 28.5% for female genital tract to 9.2% in esophageal. Survival for distant stage was poor irrespective of site with only female genital tract having 5-year survival reaching 10%. An exception was small intestine NEC that appeared to have better survival for all stages with an overall 5-year survival of 34.8%. We performed multivariate analysis of the impact of primary site while adjusting for prognostic factors influencing survival of NEC patients using the Cox proportional hazards model. The prognostic parameters included were disease stage, anatomical site, morphological subtype, gender, race, and year of diagnosis (divided into 2 equal periods, 1973–1992; 1993–2012). The multivariate model confirmed the prognostic importance of primary site (p< 0·0001) (Table 3).
Figure 3.
Median (3A) and 5-year survival (3B) of poorly differentiated neuroendocrine carcinomas across primary sites according to stage. “*” : median survival not reached.
Table 3.
Survival Analysis of High Grade Neuroendocrine Carcinomas
| Univariate | Multivariate * | |||||
|---|---|---|---|---|---|---|
| Primary Tumor Site | Median Survival (months) |
Hazard Ratio |
95% CI | Hazard Ratio |
95% CI | Multivariate p |
| Lung | 7.6 | 1.0 | - | 1.0 | < 0.0001 | |
| Esophagus | 7.2 | 0.97 | 0.89 – 1.05 | 1.09 | 1.00 – 1.19 | |
| Stomach | 8.8 | 0.77 | 0.71 – 0.84 | 1.02 | 0.93 – 1.11 | |
| Small Intestine | 25.1 | 0.42 | 0.37 – 0.48 | 0.56 | 0.49 – 0.64 | |
| Colon | 5.9 | 0.82 | 0.78 – 0.87 | 1.09 | 1.03 – 1.16 | |
| Rectum | 9.9 | 0.73 | 0.68 – 0.80 | 1.01 | 0.92 – 1.09 | |
| Anal Canal | 16.0 | 0.57 | 0.48 – 0.67 | 0.81 | 0.68 – 0.97 | |
| Pancreas | 5.7 | 0.93 | 0.87 – 0.99 | 1.10 | 1.03 – 1.18 | |
| Liver | 2.5 | 1.3 | 1.11 – 1.53 | 1.85 | 1.57 – 2.18 | |
| Gallbladder and Biliary Tract | 8.9 | 0.89 | 0.79 – 1.00 | 1.04 | 0.92 – 1.17 | |
| Other GI Sites | 3.8 | 1.04 | 0.86 – 1.27 | 1.26 | 1.03 – 1.53 | |
| Urinary Bladder | 11.8 | 0.60 | 0.57 – 0.63 | 0.82 | 0.78 – 0.87 | |
| Kidney and Renal Pelvis | 5.7 | 0.85 | 0.7 – 1.03 | 0.96 | 0.79 – 1.17 | |
| Cervix uteri | 18.2 | 0.4 | 0.36 – 0.43 | 0.62 | 0.57 – 0.68 | |
| Ovary | 10.7 | 0.57 | 0.5 – 0.64 | 0.82 | 0.72 – 0.93 | |
| Corpus and Uterus | 12.0 | 0.51 | 0.44 – 0.6 | 0.73 | 0.63 – 0.86 | |
| Prostate | 10.9 | 0.75 | 0.69 – 0.81 | 0.72 | 0.67 – 0.78 | |
| Other# | 14.5 | 0.42 | 0.39 – 0.45 | 0.5 | 0.47 – 0.53 | |
| Unknown | 2.5 | 1.22 | 1.18 – 1.26 | 1.25 | 1.21 – 1.29 | |
The model controlled for stage, histology, sex, race, age, year of diagnosis.
Other sites included miscellaneous, rare sites such as head and neck, endocrine system, eye and breast
Discussion
High quality data regarding poorly differentiated NEC are lacking due to their rare incidence outside of the lung and diverse primary sites. This is the largest study of poorly differentiated NEC to date. Of the extrapulmonary NEC, gastrointestinal NEC and NEC of unknown primary were the largest groups. Significant differences in demographics, stage at diagnosis and survival were noted based on primary site and morphological subtype. These data thus question the present extrapolation of lung NEC treatment data to extrapulmonary NEC patients. Ideally, future studies should therefore distinguish between lung NEC and extrapulmonary NEC and should consider stratifying patients according to the different primary sites. However, several challenges need to be considered: a) the rare incidence and aggressive nature of these malignancies may make timely accrual onto studies difficult b) morphological classification of NEC is often difficult. As is evident from the current study, NEC coded as neuroendocrine carcinoma without further details constitute a significant proportion of all NEC possibly reflecting the difficulty in accurate morphological classification. Such difficulties in morphological assessment of poorly differentiated NEC were also illustrated by a recent study that showed very low rates (33%) of agreement between pathologists regarding such classification.22 How do we overcome these challenges? One option would be to group sites based on proportions of morphological subtypes (eg “small-cell predominant” and “large-cell predominant”) based on our data to improve accrual. Alternatively, large scale, retrospective molecular and genomic analysis of these tumors may provide targets for basket trials that are agnostic to primary site and/or morphological subtype. For instance, the recent, early experience with rovalpituzumab tesirine, an anti-DLL3 antibody-drug conjugate that showed significant response in pre-treated small cell lung cancer patients could be extended to extra-pulmonary small cell cancers.21,23,24
In our study, a considerable proportion of patients without distant disease were alive after 5-years. Almost half the patients with localized cervix, gastric, colorectal or pancreatic primaries were still alive after 5 years. Even with regional disease, 5-year survival rates were above 20% for several primary sites. Definitive recommendations for the management of extrapulmonary NEC with resectable disease however are lacking. While some consensus guidelines recommend resection in combination with chemotherapy and/or radiation, others recommend surgical resection followed by adjuvant chemotherapy and radiation to be considered.25,26 Reflecting this recent studies have come to conflicting conclusions regarding the role of surgery in multi-modality management of localized gastrointestinal NEC27–29. It is likely that decisions regarding will need to be individualized based on site but our study clearly shows the need to have more concrete data and guidelines to improve outcomes for these patients.
Our study has several limitations. The SEER database does not classify patients according to the WHO 2010 classification nor does it have Ki67 proliferation data. Several known prognostic factors in cancer survival such as treatment, socioeconomic, performance status, and co-morbidities have not been included. Use of systemic chemotherapy is also not noted in the SEER database.
In spite of these limitations, our study has important implications. This is the first population based study describing the epidemiology and demographics of poorly differentiated NEC patients. We observed significant differences in demographics, and large variations in survival depending on primary site and morphological subtype. A curative approach is possible for substantial proportion of patients with localized or regional stage. In contrast, distant stage NEC is associated with dismal prognosis and novel therapies are required.
Supplementary Material
Acknowledgments
Funding
This work and the University of Texas MD Anderson Cancer Center was supported by the National Institutes of Health through Cancer Center Support Grant P30CA016672
Footnotes
Author Contributions: AD, HS and JY were involved in the design; all authors were involved the conduct and reporting of the study. AD responsible for the overall content as guarantor.
Presented in part at the North American Neuroendocrine Tumor Society 2016 Annual Symposium, September 30 – October 1, 2016, Jackson, Wyoming and at the14th Annual European Neuroendocrine Tumor Society Conference, March 8 – 10, 2017
Disclosures
The authors have declared no conflicts of interest
References
- 1.Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39(6):707–712. doi: 10.1097/MPA.0b013e3181ec124e. [DOI] [PubMed] [Google Scholar]
- 2.Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol. 2015;10(9):1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 3.Soto DE, Eisbruch A. Limited-stage extrapulmonary small cell carcinoma: outcomes after modern chemotherapy and radiotherapy. Cancer J. 2007;13(4):243–246. doi: 10.1097/PPO.0b013e31813ffe7c. [DOI] [PubMed] [Google Scholar]
- 4.Ochsenreither S, Marnitz-Schultze S, Schneider A, et al. Extrapulmonary small cell carcinoma (EPSCC): 10 years' multi-disciplinary experience at Charite. Anticancer Res. 2009;29(8):3411–3415. [PubMed] [Google Scholar]
- 5.Kim JH, Lee SH, Park J, et al. Extrapulmonary small-cell carcinoma: a single-institution experience. Jpn J Clin Oncol. 2004;34(5):250–254. doi: 10.1093/jjco/hyh052. [DOI] [PubMed] [Google Scholar]
- 6.Sengoz M, Abacioglu U, Salepci T, Eren F, Yumuk F, Turhal S. Extrapulmonary small cell carcinoma: multimodality treatment results. Tumori. 2003;89(3):274–277. doi: 10.1177/030089160308900308. [DOI] [PubMed] [Google Scholar]
- 7.Sorbye H, Strosberg J, Baudin E, Klimstra DS, Yao JC. Gastroenteropancreatic high-grade neuroendocrine carcinoma. Cancer. 2014;120(18):2814–2823. doi: 10.1002/cncr.28721. [DOI] [PubMed] [Google Scholar]
- 8.Sorbye H, Welin S, Langer SW, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol. 2013;24(1):152–160. doi: 10.1093/annonc/mds276. [DOI] [PubMed] [Google Scholar]
- 9.Ettinger DS, Aisner J. Changing face of small-cell lung cancer: real and artifact. J Clin Oncol. 2006;24(28):4526–4527. doi: 10.1200/JCO.2006.07.3841. [DOI] [PubMed] [Google Scholar]
- 10.Conte B, George B, Overman M, et al. High-Grade Neuroendocrine Colorectal Carcinomas: A Retrospective Study of 100 Patients. Clin Colorectal Cancer. 2015 doi: 10.1016/j.clcc.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cicin I, Karagol H, Uzunoglu S, et al. Extrapulmonary small-cell carcinoma compared with small-cell lung carcinoma: a retrospective single-center study. Cancer. 2007;110(5):1068–1076. doi: 10.1002/cncr.22887. [DOI] [PubMed] [Google Scholar]
- 12.Joyce EA, Kavanagh J, Sheehy N, Beddy P, O'Keeffe SA. Imaging features of extrapulmonary small cell carcinoma. Clin Radiol. 2013;68(9):953–961. doi: 10.1016/j.crad.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Terashima T, Morizane C, Hiraoka N, et al. Comparison of chemotherapeutic treatment outcomes of advanced extrapulmonary neuroendocrine carcinomas and advanced small-cell lung carcinoma. Neuroendocrinology. 2012;96(4):324–332. doi: 10.1159/000338794. [DOI] [PubMed] [Google Scholar]
- 14.Brennan SM, Gregory DL, Stillie A, Herschtal A, Mac Manus M, Ball DL. Should extrapulmonary small cell cancer be managed like small cell lung cancer? Cancer. 2010;116(4):888–895. doi: 10.1002/cncr.24858. [DOI] [PubMed] [Google Scholar]
- 15.Shia J, Tang LH, Weiser MR, et al. Is nonsmall cell type high-grade neuroendocrine carcinoma of the tubular gastrointestinal tract a distinct disease entity? Am J Surg Pathol. 2008;32(5):719–731. doi: 10.1097/PAS.0b013e318159371c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basturk O, Tang L, Hruban RH, et al. Poorly differentiated neuroendocrine carcinomas of the pancreas: a clinicopathologic analysis of 44 cases. Am J Surg Pathol. 2014;38(4):437–447. doi: 10.1097/PAS.0000000000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volante M, Birocco N, Gatti G, et al. Extrapulmonary neuroendocrine small and large cell carcinomas: a review of controversial diagnostic and therapeutic issues. Hum Pathol. 2014;45(4):665–673. doi: 10.1016/j.humpath.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Matsui K, Jin XM, Kitagawa M, Miwa A. Clinicopathologic features of neuroendocrine carcinomas of the stomach: appraisal of small cell and large cell variants. Arch Pathol Lab Med. 1998;122(11):1010–1017. [PubMed] [Google Scholar]
- 19.Spigel DR, Hainsworth JD, Greco FA. Neuroendocrine carcinoma of unknown primary site. Semin Oncol. 2009;36(1):52–59. doi: 10.1053/j.seminoncol.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Hainsworth JD, Johnson DH, Greco FA. Poorly differentiated neuroendocrine carcinoma of unknown primary site. A newly recognized clinicopathologic entity. Ann Intern Med. 1988;109(5):364–371. doi: 10.7326/0003-4819-109-5-364. [DOI] [PubMed] [Google Scholar]
- 21.Saunders LR, Bankovich AJ, Anderson WC, et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci Transl Med. 2015;7(302):302ra136. doi: 10.1126/scitranslmed.aac9459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang LH, Basturk O, Sue JJ, Klimstra DS. A Practical Approach to the Classification of WHO Grade 3 (G3) Well-differentiated Neuroendocrine Tumor (WD-NET) and Poorly Differentiated Neuroendocrine Carcinoma (PD-NEC) of the Pancreas. Am J Surg Pathol. 2016;40(9):1192–1202. doi: 10.1097/PAS.0000000000000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudin CMPC, Bauer TM, et al. Safety and efficacy of single-agent rovalpituzumab tesirine (SC16LD6.5), a delta-like protein 3 (DLL3)-targeted antibody-drug conjugate (ADC) in recurrent or refractory small cell lung cancer (SCLC). Paper presented at: American Society of Clinical Oncology Annual Symposium; 2016; Chicago, IL. [Google Scholar]
- 24.Rudin CM, Pietanza MC, Bauer TM, et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol. 2017;18(1):42–51. doi: 10.1016/S1470-2045(16)30565-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kulke MH, Shah MH, Benson AB, 3rd, et al. Neuroendocrine tumors, version 1.2015. J Natl Compr Canc Netw. 2015;13(1):78–108. doi: 10.6004/jnccn.2015.0011. [DOI] [PubMed] [Google Scholar]
- 26.Kunz PL, Reidy-Lagunes D, Anthony LB, et al. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas. 2013;42(4):557–577. doi: 10.1097/MPA.0b013e31828e34a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crippa S, Partelli S, Bassi C, et al. Long-term outcomes and prognostic factors in neuroendocrine carcinomas of the pancreas: Morphology matters. Surgery. 2016;159(3):862–871. doi: 10.1016/j.surg.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 28.Haugvik SP, Janson ET, Osterlund P, et al. Surgical Treatment as a Principle for Patients with High-Grade Pancreatic Neuroendocrine Carcinoma: A Nordic Multicenter Comparative Study. Ann Surg Oncol. 2016;23(5):1721–1728. doi: 10.1245/s10434-015-5013-2. [DOI] [PubMed] [Google Scholar]
- 29.Smith JD, Reidy DL, Goodman KA, Shia J, Nash GM. A retrospective review of 126 high-grade neuroendocrine carcinomas of the colon and rectum. Ann Surg Oncol. 2014;21(9):2956–2962. doi: 10.1245/s10434-014-3725-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.