Abstract
Bacillus velezensis 157 was isolated from the bark of Eucommia ulmoides, and exhibited antagonistic activity against a broad spectrum of pathogenic bacteria and fungi. Moreover, B. velezensis 157 also showed various lignocellulolytic activities including cellulase, xylanase, α-amylase, and pectinase, which had the ability of using the agro-industrial waste (soybean meal, wheat bran, sugarcane bagasse, wheat straw, rice husk, maize flour and maize straw) under solid-state fermentation and obtained several industrially valuable enzymes. Soybean meal appeared to be the most efficient substrate for the single fermentation of B. velezensis 157. Highest yield of pectinase (19.15 ± 2.66 U g−1), cellulase (46.69 ± 1.19 U g−1) and amylase (2097.18 ± 15.28 U g−1) was achieved on untreated soybean meal. Highest yield of xylanase (22.35 ± 2.24 U g−1) was obtained on untreated wheat bran. Here, we report the complete genome sequence of the B. velezensis 157, composed of a circular 4,013,317 bp chromosome with 3789 coding genes and a G + C content of 46.41%, one circular 8439 bp plasmid and a G + C content of 40.32%. The genome contained a total of 8 candidate gene clusters (bacillaene, difficidin, macrolactin, butirosin, bacillibactin, bacilysin, fengycin and surfactin), and dedicates over 15.8% of the whole genome to synthesize secondary metabolite biosynthesis. In addition, the genes encoding enzymes involved in degradation of cellulose, xylan, lignin, starch, mannan, galactoside and arabinan were found in the B. velezensis 157 genome. Thus, the study of B. velezensis 157 broadened that B. velezensis can not only be used as biocontrol agents, but also has potentially a wide range of applications in lignocellulosic biomass conversion.
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1125-2) contains supplementary material, which is available to authorized users.
Keywords: Bacillus velezensis 157, Complete genome sequencing, Secondary metabolites, Lignocellulolytic enzyme activities, Eucommia ulmoides, Agro-industrial waste
Introduction
Bacillus spp. are extensively studied biocontrol agents due to extremely strong resistance, antagonistic activities and excellent environmental adaptability (Cawoy et al. 2014; Jin et al. 2017). Mainly, Bacillus subtilis, B. amyloliquefaciens, B. cereus, B. megaterium and B. velezensis act as biocontrol agents. Recently, B. velezensis has been re-classified as a synonym of B. amyloliquefaciens subsp. plantarum, B. methylogrophicus and B. oryzicola (Dunlap et al. 2015), has been reported increasingly in recent years due to their significant application in agriculture and biotechnology such as B. velezensis GH1-13 (Kim et al. 2017b), B. velezensis S3-1 (Jin et al. 2017), B. velezensis FZB42 (Chen et al. 2007), B. velezensis M75 (Kim et al. 2017a), B. velezensis LS69 (Liu et al. 2017), B. velezensis 9912D (Pan et al. 2017), B. velezensis S499 (Molinatto et al. 2016). These kinds of bacteria have been widely used as biocontrol agents because of promoting plant growth through the production of phytohormones and producing a vast array of secondary metabolites that suppress competitive plant pathogenic microbes (Ryu et al. 2004; Lugtenberg and Kamilova 2009). However, few studies were done to explore the potential capability of B. velezensis in degrading lignocellulose components and transform lignocelluloses into useful chemicals and fuels. Solid-state fermentation (SSF) has attracted more attention to be a significant technique using agro-industrial wastes and by-products as substrates for microbial growth (Moftah et al. 2012; Salim et al. 2017), and produce industrially useful enzymes in low-cost processes, reduce costs and broaden the industrial application (Lizardi-Jiménez and Hernández-Martínez 2017; Hashemi et al. 2011; Elshishtawy et al. 2015).
In this study, we screened the microorganism producing α-amylase, cellulase, pectinase and xylanase, and cultivated by the SSF on various agro-industrial waste for enzyme production. Meanwhile, the antimicrobial assays against several zoonotic pathogenic bacteria, aquatic pathogenic bacteria and plant pathogenic fungus were also tested. Therefore, to provide a better understanding of the mechanisms involved in synthesizing secondary metabolite of Bacillus velezensis 157 and latent ability in degrading lignocelluloses, we carried out the complete genome sequencing and analyzing of B. velezensis 157.
Materials and methods
The selection and microorganism
The various endophytic bacteria collected in this study were isolated from bark of Eucommia ulmoides, Taian City, China during the 2015 growing season (July). Selective agar plates were used for screening enzymatic activities. The bacteria were maintained on LB agar slants containing (g L−1): peptone 10, yeast extract 5, NaCl 10 and agar 15. The following selective media were evaluated to screen bacteria that are capable of producing multienzyme complex (MEC) (Salim et al. 2017). Carboxymethyl cellulose (CMC) agar plates (per liter: CMC 10 g, yeast extract 3 g, K2HPO4 3 g, KH2PO4 1 g, MgSO4·7H2O 0.5 g, and agar 20 g). Starch agar plate (per liter: soluble starch 10 g, yeast extract 3 g and agar 20 g. Xylan agar plate (per liter: birch-wood xylan 10 g, yeast extract 3 g and agar 20 g). Pectin agar plate (per liter: apple pectin 10 g, yeast extract 3 g and agar 20 g). The various endophytic bacteria preserved from LB agar slants were inoculated and placed in the center of the selective agar plate at 37 °C for 48 h. The crude supernatant of candidate strains was prepared following the conventional method (Meng et al. 2015). After cultivation, starch agar plate was detected by iodine staining solution (g L−1) (KI 15 and I215 in distilled water). Carboxymethyl cellulose agar plate, xylan agar plate and pectin agar plate were soaked with Congo red. An endophytic bacteria of significant halo zone to colony ratio appeared on each selective medium was selected as the potential producing bacteria, which indicates instinct of α-amylase/cellulase/xylanase/pectinase (Lu et al. 2004).
The identification of selected endophytic bacteria
The biochemical and morphological analysis of strain 157 was performed using traditional approaches according to the Bergey’s Manual of Systematic Bacteriology, and molecular identification was obtained via 16S rRNA gene sequence analysis followed by phylogenetic studies (Paudel and Qin 2015), which was generated by neighbor-joining (NJ) method using MEGA 7.0 software (Kumar et al. 2016).
Antimicrobial assays
The test pathogenic bacteria and fungi were obtained from the China Veterinary Culture Collection Center (CVCC), China Center of Industrial Culture Collection (CICC), American Type Culture Collection (ATCC) and laboratory isolation. The antimicrobial activity of the crude extract against the test pathogenic bacteria was measured using the agar well diffusion method (Zhang et al. 2012). Briefly, the test bacteria was spread on LB agar plates with sterilized Oxford cup and 200 μL of the crude extract was added in the midpoint of the hole; equivalent amounts of culture medium were used as controls. The antimicrobial circle diameter was measured after 24-h incubation at 37 °C. Visualization of the colony confrontation assays using Bacillus velezensis 157 and plant pathogenic fungi was presented. All the PDA plates were incubated for 5 days after inoculation (Su et al. 2017).
Genome sequencing and bioinformatic analysis
The whole genome of Bacillus velezensis 157 was sequenced using a Pacific Biosciences (PacBio) RSII Single Molecule Real-Time (SMRT) sequencing technology with a 10 Kb SMRT bell TM template library. The low-quality reads were filtered by the SMRT Analysis 2.3.0 program and the filtered reads were assembled to generate circular contig without gaps (Berlin et al. 2015). The NCBI Prokaryotic Genomes Annotation pipeline (PGAP) was used to annotate the genes of B. velezensis157. Gene clusters related to the biosynthesis of secondary metabolites were conducted based on the analysis with anti-SMASH 3.0 program (Weber et al. 2015). We used COG (Clusters of Orthologous Groups) database to predict gene functions. The Average Nucleotide Identity (ANI) between B. velezensis 157 and relative whole genomes from Bacillus species were analyzed by Jspecies software (http://www.imedea.uib.es/jspecies). Genome overview was created by Circos software to show the annotation information of B. velezensis 157 (Krzywinski et al. 2009). Core/Pan genes of the B. velezensis 157 were compared to that of the three closest known evolutionary relatives: B. velezensis FZB42 (CP000560.1), B. velezensis YAU B9601-Y2 (HE774679.1), and B. velezensis UCMB5113 (HG328254.1) by the CD-HIT rapid clustering of similar proteins software with a threshold of 50% pairwise identity and 0.7 length difference cutoffs in amino acid. Then, the Venn figure was drawn to show their relationships among the samples (Li et al. 2001, 2002; Li and Godzik 2006).
Solid-state fermentation (SSF)
In this work, the SSF was performed using soybean meal, wheat bran, sugarcane bagasse, wheat straw, rice husk, and maize flour as substrates. After dried, grinded and sieved, the particle sizes of substrates obtained were between 200 and 800 μm. The agro-industrial waste was performed in 150 mL Erlenmeyer flasks containing 5 g of dry substrate, which were sterilized at 121 °C for 20 min (0.12 MPa autoclave pressure), appropriate amount of sterile distilled water, prior inoculation to achieve a ratio of 1:0.5 (g of dry substrate: g of water), 0.5 mL of 24-h bacterial culture in LB medium (1 × 106 cells mL−1) was also considered in the moisture content. Fermentation was determined at 37 °C for 72 h. Crude enzyme was performed by mixing 1 g of fermented media with 10 mL distilled water (10:1 w/w) on a rotary shaker (180 rpm, 30 min, 37 °C). This was followed by centrifugation of the suspension at 8000 rpm for 10 min (4 °C). The supernatants were designated as a crude enzyme extract and evaluated for secreted cellulase, α-amylase, xylanase and pectinase activities (Elshishtawy et al. 2015; Kazeem et al. 2017).
Enzymatic assays
A modified microplate-based assay using 3,5-dinitro salicylic acid (DNS) method was used to measure the CMCase activity (Miller 1959). For this, 50 μL of crude enzyme extract was mixed with 100 μL of 1% (w/v) CMC prepared in 0.05 M sodium acetate buffer, pH 5 at 50 °C for 30 min. The reactions were terminated by adding 200 μL of DNS reagent, and all the mixture was boiled for 5 min for color development, cooled and then optical density was measured at 540 nm. One unit (U) of the cellulase activity was defined as the amount of enzyme that released 1 μmol of reducing sugars equivalent to glucose per minute during the reaction using a calibration curve for glucose.
The α-amylase activity was measured using 1% soluble starch as a substrate according to the method described by Hashemi et al. (2011). Briefly, the reaction of 50 μL crude enzyme extract with 100 μL of substrate (0.02 M phosphate buffer at pH 6.9) at 55 °C for 30 min, and then optical density was measured at 540 nm. One unit of α-amylase activity was defined as the liberation of reducing sugars equivalent to 1 μmol of d-glucose/min under the assay condition estimated according to the previously described DNS method.
Pectinase activity assays were performed by incubating 50 μL of crude enzyme extract with 100 μL of 1% apple pectin with citrate buffer (0.1 M, pH 5.8) and incubated 30 min at 50 °C. The release of reducing sugars was analyzed using the DNS method using a standard curve of d-galacturonic acid (Biz et al. 2016). One unit (U) of pectinase activity corresponds to the release of 1 μmol of d-galacturonic acid equivalents per minute. Activities are expressed on the basis of the mass of dry substrate (i.e. U g−1).
Substrate used was birch-wood xylan for xylanase. The reaction mixture contained 100 μL 1% substrate 0.05 M sodium acetate buffer and 50 μL of crude enzyme extract, pH 5.5 at 50 °C for 30 min. The absorbance was measured at 540 nm. One unit of xylanase activity was defined as the liberation of reducing sugars equivalent to 1 μmol of xylose/min under the assay condition estimated according to the previously described DNS method (Hero et al. 2017).
Nucleotide sequence accession number
The whole genome sequence of Bacillus velezensis 157 has been deposited in NCBI under the GenBank accession number CP022341 (chromosome), CP022342 and CP022343 (two DNA fragments of B. velezensis 157 plasmid).
Results and discussion
Screening of lignocellulolytic enzymes production by bacteria
In this study, the endophytic bacteria are isolated from the inner bark of Eucommia ulmoides, which is the traditional Chinese herb because of its medicinal importance (Hussain et al. 2016), and there were no reports on the isolation of Bacillus velezensis from E. ulmoides. On this basis, we examined different natural isolates to produce extracellular cellulase, α-amylase, xylanase and pectinase using selective agar medium plates. The agar plates contain CMC, starch, pectin, xylan as one of the exclusive nutrients, if the strain can hydrolyze and utilize the nutrients from the agar medium plates, it can grow well and easily visually detected by the appearance of the clear zones surrounding grown microbial colonies. Salim et al. selected a newly isolated Bacillus sp. TMF-1 with cellulase, pectinase, α-amylase and protease by the similar method (Salim et al. 2017). Of them, only strain 157 showed cellulase, α-amylase, xylanase and pectinase activities with its maximum hydrolysis zone among the natural isolates using selective agar medium plates (Fig S1). Literature survey highlighted that Bacillus spp. have the potential of secreting abundant extracellular enzyme. Bacillus coagulans CGMCC 9951 isolated from healthy piglet feces showed the capacity to hydrolyze protein, starch and cellulose (Gu et al. 2015). Bacillus coagulans CMB3 also showed the ability to produce more extracellular enzyme, including α-amylase, protease, pectinase, chitinase and lecithinase (Mishra et al. 2009). Thus, the rich extracellular enzyme of strain 157 implied that the isolate may point out to be a prospective source of desired enzymes in industrial applications.
Molecular identification of bacteria
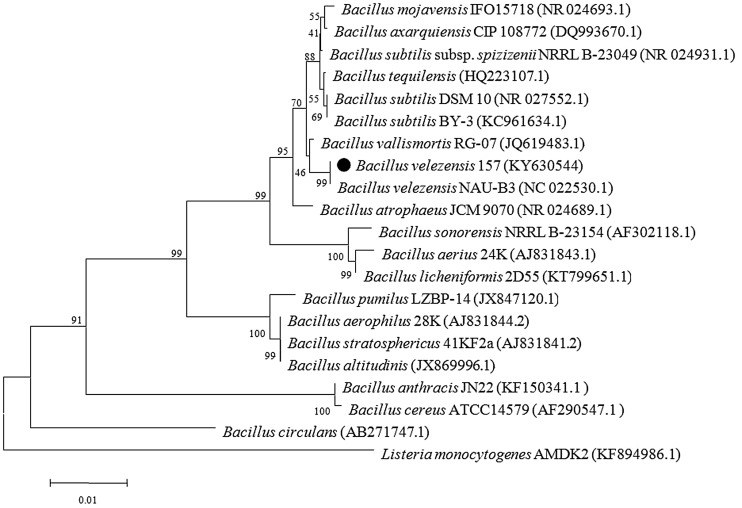
The biochemical and morphological characterization results of strain 157 are shown in (Table S1), suggested that it is probably one of the members of Bacillus spp. The genomic DNA of strain 157 was successfully extracted. The PCR primers successfully amplified 16S rDNA fragments. 1% agarose gel showed the clear bands of about 1500 bp, the 16S rRNA gene sequence of strain 157 was submitted to NCBI GenBank under the accession number: KY630544 for constructing phylogenetic tree (Fig. 1) using the neighbor-joining method. This 16S rRNA gene sequence of the strain 157 showed a 99% similarity with B. velezensis NAU-B3 (NC_022530.1). Bacillus velezensis NAU-B3 is a plant-associated bacterium, and nine giant gene clusters are dedicated to the nonribosomal synthesis of antimicrobial lipopeptides and polyketides (Wu et al. 2013). Additionally, the characteristic of average nucleotide identity (ANI) based on the relative whole genome of Bacillus species sequences available on the NCBI website was calculated using the software of JSpecies. Among all the sequenced and accessible strains, the ANI values between B. velezensis NAU-B3 and strain 157 were homology to 99.88%, and shows lower values with B. amyloliquefaciens (93%) and B. subtilis (76%) (Table S2). Literature surveyed ANI offers more accurately resolution and universally applicable technique at the subspecies level and genome-wide comparison (Chan et al. 2012). Based on these results the strain 157 was named as B. velezensis 157.
Fig. 1.
Phylogenetic tree based on 16S rRNA gene sequences showing the relationship between Bacillus velezensis 157 and closely related Bacillus species. Listeria monocytogenes AMDK2 was used as an out-group. Bootstrap values (%) based on 1000 replications are given at nodes
Antimicrobial assays
The crude extract of Bacillus velezensis 157 showed a broad inhibitory spectrum against several animal pathogenic bacteria, aquatic pathogenic bacteria and fungal plant pathogens (Table 1). No antibacterial activity was observed in the control group. In the Antimicrobial assays, B. velezensis 157 exhibited antifungal activity against Botrytis cinerea and Fusarium oxysporum. Researchers have found Tomato wilt caused by the fungus F. oxysporum is an alarming disease, causing yield losses up to 25%, and Botrytis cinerea is the most severe disease after tomato harvest, and hence prevention and cure of Botrytis cinerea is a key approach for the protection and development of tomato products worldwide (Shi and Sun 2017; Fatima and Anjum 2017). Bacillus velezensis 157 had potential for inhibiting the growth of plant pathogenic microorganisms and considered to be one of the most important methods for replacing chemicides. Moreover, B. velezensis 157 exhibited antibacterial activity against Escherichia coli and Salmonella spp, which are pathogenic to humans and lead to severe food-borne disease (Field et al. 2016). Bacillus velezensis 157 also has the ability to inhibit against Aeromonas hydrophila and A. veronii, which is a very promising discovery and of great significance to aquaculture industry. Guo X et al. hypothesized that the antibacterial mechanism of B. subtilis GC-21 and GC-22, due to preventing the colonization of pathogenic bacteria after adhering to the intestinal mucosal surface, and producing some substances or metabolites with antimicrobial properties (Guo et al. 2016). Literature surveys that A. hydrophila has increasingly been implicated as a virulent and antibiotic-resistant etiologic agent in various human diseases (Grim et al. 2013). Aeromonas veronii is a kind of opportunistic pathogen to fish and humans, significantly impending aquaculture production (Sun et al. 2016). Results of this experiment indicate that B. velezensis 157 not only has a wide inhibitory spectrum against pathogenic bacteria and fungi, but also brings a more capacious future for the application on livestock and aquatic animal.
Table 1.
Antimicrobial activity of the culture supernatant of Bacillus velezensis 157
| Host classification and strains | Broth mediuma | Inhibition zone (mm)a |
|---|---|---|
| Human and animals pathogenic bacteria | ||
| Staphylococcus aureus CVCC519 | LB | ++ |
| Escherichia coli CVCC233 | LB | +++ |
| E. coli CVCC236 | LB | +++ |
| E. coli CVCC196 | LB | ++ |
| E. coli CVCC205 | LB | +++ |
| E. coli BNCC125988 | LB | + |
| Salmonella typhimurium ATCC25241 | LB | ++ |
| S. choleraesuis CVCC3383 | LB | +++ |
| S. choleraesuis CVCC3775 | LB | +++ |
| S. choleraesuis CVCC79102 | LB | +++ |
| S. choleraesuis CVCC503 | LB | +++ |
| S. choleraesuis CVCC3780 | LB | +++ |
| S. enteritidis CVCC3378 | LB | ++ |
| Clostridium perfringens | LB | +++ |
| Proteus hauseri | LB | ++ |
| Aquatic pathogenic bacteria | ||
| Aeromonas hydrophila ATCC7966 | LB | ++ |
| A. veronii ATCC35624 | LB | ++ |
| A. veronii TH0426 | LB | ++ |
| A. veronii AV115 | LB | ++ |
| A. veronii AV75 | LB | ++ |
| A. caviae ATCC 15468 | LB | +++ |
| Streptococcus agalactiae | LB | ++ |
| Plant pathogenic fungib | ||
| Botrytis cinerea | PDA | + |
| Fusarium oxysporum | PDA | + |
LB Luria–bertani, PDA Potato dextrose agar
aThe antimicrobial activities were performed using the cell-free supernatant of the Bacillus velezensis 157 by Agar well diffusion assays. The diameter of the control was 9 mm, and the plus sign indicates the size of the inhibition zones. (+): the diameter of the inhibition zones is less than 10 mm; (++): the diameter of the inhibition zones is between 10 and 15 mm; (+++): diameter of the inhibition zones is more than 15 mm. Numbers of inhibitory zone diameter show an average of three replications
bThe confrontation experiments between Bacillus velezensis 157 and related fungi
General genomic features of Bacillus velezensis 157
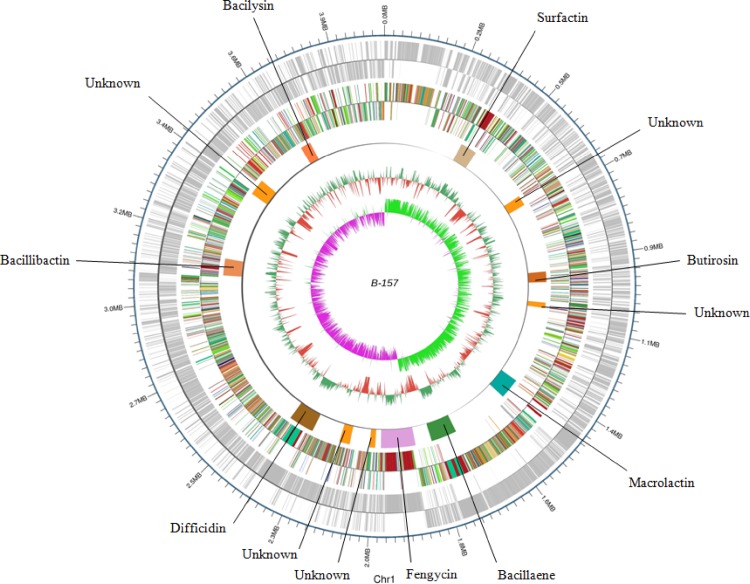
The complete genome sequence of Bacillus velezensis 157 was composed of a circular 4,013,317 bp chromosome and one circular 8439 bp plasmid. We had also extracted the plasmid of B. velezensis 157, and the gel electrophoresis demonstrated that B. velezensis 157 indeed was exist plasmid (Fig S2). GC content of the complete genome was 46.41% and that of plasmid was 40.32%, respectively. There are 3789 protein-coding sequences (CDSs) located on the chromosome, 27 rRNA genes, 86 tRNA genes, 11 sRNA genes were found in the genome (Table 2 and Fig. 2). In the genome analysis, 3369 genes were classified into the cluster of orthologous group (COG), the number of genes associated with translation, ribosomal structure and biogenesis, transcription, cell cycle control, cell division, chromosome partitioning, posttranslational modification, protein turnover, chaperones, cell motility, signal transduction mechanisms, carbohydrate transport and metabolism, coenzyme transport and metabolism, lipid transport and metabolism in B. velezensis 157 were much higher than that in compared strains (Table S3). The number of core genes in B. velezensis 157, FZB42, UCMB5113 and YAU B9601-Y2 was counted as being 3205. The pan-genome of the four strains was calculated as being 4708 CDSs. Bacillus velezensis 157 possessed 481 unique genes not found in other three B. velezensis strains (Fig. S3). The unique protein of Acyl transferase domain, which is an essential component of synthesizing polyketide synthase I (PKS I), may associate with secondary metabolites biosynthesis, transport and catabolism.
Table 2.
Genome features of Bacillus velezensis 157
| Features | Chromosome | Plasmid |
|---|---|---|
| Genome size (bp) | 4,013,317 | 8439 |
| G + C content (%) | 46.41 | 40.32 |
| Protein-coding genes (CDS) | 3789 | – |
| rRNA genes | 27 | – |
| tRNA genes | 86 | – |
Fig. 2.
The whole genome of Bacillus velezensis 157. The circular genome map consists of 7 circles. From the outer circle inward, each circle displays information about the genome of (1) forward CDS, (2) reverse CDS, (3) forward COG function classification, (4) reverse COG function classification, (5) nomenclature and locations of predictive secondary metabolite clusters, (6) G + C content and (7) GC Skew
The eight gene clusters related to the synthesis of various NRPS and antimicrobial PKS were found in the B. velezensis 157 (Table 3 and Table S4). Gene clusters direct the synthesis of the antibacterial polyketides (bacillaene, difficidin, macrolactin and butirosin, covering over 326.5 kb in all), non-ribosomal peptide synthetases (bacillibactin, bacilysin, fengycin and surfactin, covering altogether over 311.4 kb). Uniquely, we discovered that B. velezensis 157 indicates over 15.8% of the whole genome to synthesize antibiotics and siderophores by pathways not involving ribosomes, the B. velezensis FZB42 and B. velezensis LS69 only devote over 8.5% of its genome secondary metabolite production, followed by B. amyloliquefaciens DSM7 and other members of the Bacillus subtilis group contains 4–5% in synthesizing bioactive compounds (Liu et al. 2017). The operons in B. velezensis 157 encoding the biosynthetic gene cluster for the metabolites macrolactin (BGC0000181_c1), bacillaene (BGC0001089_c1), fengycin (BGC0001095_c1), difficidin (BGC0000176_c1), bacilysin (BGC0001184_c1) were found to be 100% homologous with B. velezensis FZB42, the bacillibactin (BGC0000309_c1) exhibit highly 100% identities on the amino acid level to that of B. subtilis 168, suggesting that strain 157 has strong antagonistic activities towards bacterial and fungal pathogens. However, a functional srfABCD gene cluster was attributed to the operon encoding cyclic lipopeptides surfactin (BGC000043_c1) showed 86% homologous to B. velezensis FZB42. Surfactin is a potential biosurfactant and shows antimicrobial and antiviral activities by altering membrane integrity (Peypoux et al. 1999). Interestingly, the synthesis of butirosin (BGC0000693_c1) shows only 7% similarity with the gene cluster reported in B. circulans SANK 72073, butirosin produced by B. circulans is among the clinically important 2-deoxystreptamine (DOS)-containing aminoglycoside antibiotics, the butirosin involved in B. velezensis 157 might be new in structure and resource of novel bioactive compound (Kudo et al. 2005a, b). These results also indicated that why B. velezensis 157 exhibited antagonistic activity against a broad spectrum of pathogenic bacteria and fungi in antimicrobial assays, and these gene clusters related to the synthesis of secondary metabolism with antimicrobial properties may be the main reason for inhibitory mechanism.
Table 3.
Gene clusters involved in synthesis of biocontrol metabolites in Bacillus velezensis 157
| Cluster | Cluster categorya | Size (kb) | Position | Secondary metaboliteb | Strains (genetic similaritya) (%) |
|---|---|---|---|---|---|
| 1 | Nrps | 65.4 | 317,617–383,024 | Surfactin | 86 |
| 2 | Phosphonate | 40.8 | 625,790–666,677 | Unknown | |
| 3 | Otherks | 41.2 | 944,830–986,074 | Butirosin | 7 |
| 4 | Terpene | 20.74 | 1,068,391–1,089,131 | Unknown | |
| 5 | Transatpks | 82.2 | 1,400,648–1,482,839 | Macrolactin | 100 |
| 6 | Transatpks-Nrps | 102.7 | 1,714,900–1,817,583 | Bacillaene | 100 |
| 7 | Transatpks-Nrps | 137.8 | 1,883,269–2,021,102 | Fengycin | 100 |
| 8 | Terpene | 21.8 | 2,044,355–2,066,238 | Unknown | |
| 9 | T3pks | 41.1 | 2,146,409–2,187,515 | Unknown | |
| 10 | Transatpks | 100.4 | 2,302,503–2,402,947 | Difficidin | 100 |
| 11 | Bacteriocin-Nrps | 66.8 | 3,053,496–3,120,291 | Bacillibactin | 100 |
| 12 | Nrps | 65.3 | 3,398,874–3,464,216 | Unknown | |
| 13 | Otherc | 41.4 | 3,666,299–3,707,717 | Bacilysin | 100 |
aCluster identified according to anti-SMASH 3.0
bSecondary metabolites potentially produced based on the gene clusters
cCluster containing a secondary metabolite-related protein that does not belong to any other category
In addition, the genes related to the degradation of lignocellulose were detected in the genome of B. velezensis 157 (Table 4). Regarding cellulose degradation: endoglucanase, β-glucanase, α-glucosidase, α-amylase and pectate lyase were found in the complete genome of B. velezensis 157. Regarding hemicellulose degradation: 1,4-β-xylosidase, arabinoxylan arabinofuranohydrolase, 1,4-beta-xylanase, glucuronoxylanase, arabinanendo-1,5-α-l-arabinosidase, α-N-arabinofuranoside, β-mannosidase, endo-1,4-β-galactosidase, 6-phospho-β-galactosidase, xylose isomerase. The gene encoding deferrochelatase involved in lignin degradation was also detected in the genome of B. velezensis 157. As shown in Table 4, the related genes of degradation of lignocellulose were highly observed in all strains; the gene encoding α-glucosidase (ASK59912.1) was unique in B. velezensis 157. However, glucohydrolase and laccase genes were found in the genome of B. amyloliquefaciens DSM7T, B. velezensis FZB42 and Bacillus sp. 257, but were not detected in B. velezensis 157 (Rückert et al. 2011; Gong et al. 2017). The existence of these genes implies that strain 157 has the potential for utilizing lignocelluloses and possibly a new use for production of the lignocellulolytic enzyme.
Table 4.
Comparison of genes encoding lignocellulose-degrading enzymes in Bacillus velezensis 157 and other Bacillus strains
| Bacillus velezensis 157 | DSM7T | FZB42 | 275 | |
|---|---|---|---|---|
| Predicted function | Accession no. | |||
| Cellulose-related | ||||
| Endoglucanase | ASK58591.1 | × | O | O |
| β-glucanase | ASK60337.1 | O | O | O |
| 6-phospho-α-glucosidase | ASK57640.1 | O | O | O |
| 6-phospho-β-glucosidase | ASK60295.1 | O | O | O |
| 6-phospho-β-glucosidase | ASK58708.1 | × | × | O |
| α-glucosidase (alpha-galactosidase) | ASK59490.1 | O | O | O |
| α-glucosidase (alpha-galactosidase) | ASK59569.1 | O | O | × |
| α-glucosidase (alpha-galactosidase) | ASK59912.1 | × | × | × |
| α-glucosidase (alpha-galactosidase) | ASK57158.1 | O | O | × |
| Aryl-phospho-β-d-glucosidase | ASK57207.1 | O | O | O |
| α-amylase | ASK57175.1 | × | O | O |
| Pectate lyase | ASK60345.1 | O | O | O |
| Pectate lyase | ASK57571.1 | O | O | O |
| Hemicellulose-related | ||||
| Glycoside hydrolase 43 family protein (1,4-β-xylosidase) | ASK58518.1 | × | O | O |
| Arabinoxylan arabinofuranohydrolase | ASK60598.1 | O | O | O |
| 1,4-beta-xylanase | ASK60111.1 | × | O | O |
| Glucuronoxylanase | ASK58595.1 | O | O | O |
| Arabinan endo-1,5-α-l-arabinosidase | ASK59359.1 | O | O | O |
| Arabinan endo-1,5-α-l-arabinosidase | ASK60350.1 | O | O | × |
| α-N-arabinofuranoside | ASK59336.1 | O | O | O |
| α-N-arabinofuranoside | ASK59351.1 | O | O | O |
| β-mannosidase | ASK60312.1 | O | O | O |
| Endo-1,4-β-galactosidase | ASK57983.1 | O | O | O |
| 6-phospho-β-galactosidase (lacG) | ASK57989.1 | O | O | O |
| Xylose isomerase | ASK58520.1 | × | O | O |
| Lignin-related | ||||
| Deferrochelatase | ASK60272.1 | O | O | O |
O detected, × not detected
Lignocellulolytic enzymes production on solid-state fermentation using different agro-industrial wastes
As shown in Table 5, Bacillus velezensis 157 appeared to use all of the agro-industrial waste after 72 h of cultivation and α-amylase, cellulase, xylanase and pectinase were all detected. The most promising substrate seemed to be soybean meal, followed by wheat straw > wheat bran > Rice husk > Maize flour. Conversely, lower cellulolytic activity was performed when B. velezensis 157 was cultivated on maize straw and sugarcane bagasse. Biological fermentation can dispose the agro-waste, obtain the desired enzymes and recycle the excess of agro-industrial waste (Lizardi-Jiménez and Hernández-Martínez 2017). Similar studies have been reported that Salim et al. (2017) assessed newly isolated Bacillus sp. TMF-1 was cultivated on various types of agricultural by-products and obtained industrially valuable enzymes such as proteases, α-amylase, cellulase, pectinase. Hashemi et al. (2011) investigated the production of Ca-independent α-amylase by Bacillus sp. KR-8104 in SSF.
Table 5.
Lignocellulolytic enzymes production on solid state fermentation using different agro-industrial wastes by Bacillus velezensis 157 (U g−1 substrate) (after 72 h at 37 °C, 1:0.5, 0.5 mL inoculum B. velezensis 157)
| Agro-waste | Cellulase | α-amylase | Xylase | Pectinase |
|---|---|---|---|---|
| Maize straw | 9.62 ± 0.33 | 253.42 ± 0.981 | 5.92 ± 0.35 | 8.49 ± 0.15 |
| Wheat straw | 43.92 ± 5.44 | 1430.11 ± 20.24 | 13.77 ± 1.15 | 14.15 ± 1.14 |
| Wheat bran | 40.68 ± 0.51 | 1348.73 ± 30.41 | 22.35 ± 2.24 | 13.61 ± 3.74 |
| Soybean meal | 46.69 ± 1.19 | 2097.18 ± 15.28 | 13.55 ± 0.89 | 19.15 ± 2.66 |
| Rice husk | 39.03 ± 0.34 | 1080.06 ± 11.89 | 14.43 ± 1.31 | 12.73 ± 1.58 |
| Maize flour | 38.91 ± 0.28 | 178.71 ± 8.44 | 13.62 ± 2.52 | 12.92 ± 3.21 |
| Sugarcane bagasse | 7.37 ± 0.23 | 35.54 ± 12.13 | 13.62 ± 3.11 | 7.86 ± 0.95 |
Values are means of (n = 3), ± SD (vertical bars)
Among the agro-industrial waste, soybean meal has around 48% protein, 35–40% carbohydrates, 7–10% water, 5–6% minerals and less than 1% fat (3–4% of acid hydrolyzed fat), of them the carbohydrates in soybean meal consist of approximately 10% free sugars (5% sucrose, 4% stachyose and 1% raffinose) and between 20 and 30% non-starch polysaccharides, in which approximately 8% are cellulose and the remaining are 17% pectic polysaccharides (Choct et al. 2010). It was obvious that pectin is a high proportion in soybean meal, so it was significant to induce pectinase production and the highest yield of pectinase was achieved about 19.15 ± 2.66 U g−1 by B. velezensis 157, meanwhile the highest yield of cellulase and α-amylase activity reached 46.69 ± 1.19 and 2097.18 ± 15.28 U g−1, respectively. We hypothesized that the induction mechanism of cellulase in B. velezensis was not the same as that of fungal cellulase; simple sugar or polysaccharide may also play a crucial role on cellulase induction (Manfredi et al. 2016). The highest yield of xylanase (22.35 ± 2.24 U g−1) was noted in the SSF on wheat bran. It is worth noting that wheat bran is rich in cellulose (20%) and hemicellulose (50%) (Salim et al. 2017). These results suggested that B. velezensis 157 can use various agro-industrial wastes to produce four enzymes through SSF. Bacillus velezensis could be a new candidate strains for applications in various unexploited biomass.
Conclusion
In conclusion, newly isolated Bacillus velezensis 157, the strain with a broad inhibitory spectrum against pathogenic bacteria and fungi, has the huge cover rate in synthesizing secondary metabolite production on the genome. Meanwhile, it has the cellulolytic, xylanolytic, pectinic and amylatic enzyme activities and also has genes encoding various lignocellulolytic enzymes. B. velezensis 157 has the potential to utilize various agro-industrial wastes obtaining valuable enzymes. Soybean meal appeared to be the most efficient substrate for the single fermentation of B. velezensis 157. The whole genome information of B. velezensis 157 has opened up a better understanding to provide useful genomic information of B. velezensis, to understand its biocontrol mechanisms and degradation of lignocelluloses, and to expand the application of agricultural and biotechnological fields.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 Figure S1. The decoloring circles of Bacillus velezensis 157 on selective mediums: a Congo red on CMC-amended agar plate, b Pectin agar plate, c the crude extract of Bacillus velezensis 157 (200 μL) was poured into the wells in Xylan agar plate, which was made with a gel cutter d Starch agar was overlayed with KI solution. Figure S2. Gel electrophoresis of native plasmid of Bacillus velezensis 157. Figure S3. Venn diagram of the four Bacillus velezensis strains based on protein cluster analysis, comparing the shared and unique protein clusters with each other (DOC 1192 kb)
Acknowledgements
This research was supported by The platform of comprehensive utilization of straw resources in Jilin Province (2014c-1) and the National High-Tech R&D Program of China (863 program) (2013AA102806), Science and Technology Development Program of Jilin Province (20160519011JH), Special Funds for Industrial Innovation of Jilin Province (2016C063), National Key R&D program of China (2017YFD0501000).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Long Chen and Wei Gu have contributed equally to this work.
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1125-2) contains supplementary material, which is available to authorized users.
Contributor Information
Chun-Feng Wang, Phone: +86-0431- 84533425, Email: wangchunfeng@jlau.edu.cn.
Ai-Dong Qian, Phone: +86-0431-84533426, Email: qianaidong0115@163.com.
References
- Berlin K, Koren S, Chin CS, Drake JP, Landolin JM, Phillippy AM. Assembling large genomes with single-molecule sequencing and locality-sensitive hashing. Nat Biotechnol. 2015;33(6):623–630. doi: 10.1038/nbt.3238. [DOI] [PubMed] [Google Scholar]
- Biz A, Finkler ATJ, Pitol LO, Medina BS, Krieger N, Mitchell DA. Production of pectinases by solid-state fermentation of a mixture of citrus waste and sugarcane bagasse in a pilot-scale packed-bed bioreactor. Biochem Eng J. 2016;111:54–62. doi: 10.1016/j.bej.2016.03.007. [DOI] [Google Scholar]
- Cawoy H, Mariutto M, Henry G, Fisher C, Vasilyeva N, Thonart P, Dommes J, Ongena M. Plant defense stimulation by natural isolates of bacillus depends on efficient surfactin production. Mol Plant Microbe Interact. 2014;27(2):87–100. doi: 10.1094/MPMI-09-13-0262-R. [DOI] [PubMed] [Google Scholar]
- Chan JZ, Halachev MR, Loman NJ, Constantinidou C, Pallen MJ. Defining bacterial species in the genomic era: insights from the genus Acinetobacter. BMC Microbiol. 2012;12(1):302. doi: 10.1186/1471-2180-12-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XH, Koumoutsi A, Scholz R, Eisenreich A, Schneider K, Heinemeyer I, Morgenstern B, Voss B, Hess WR, Reva O, Junge H, Voigt B, Jungblut PR, Vater J, Sussmuth R, Liesegang H, Strittmatter A, Gottschalk G, Borriss R. Comparative analysis of the complete genome sequence of the plant growth-promoting bacterium Bacillus amyloliquefaciens FZB42. Nat Biotechnol. 2007;25(9):1007–1014. doi: 10.1038/nbt1325. [DOI] [PubMed] [Google Scholar]
- Choct M, Dersjant-Li Y, McLeish J, Peisker M. Soy oligosaccharides and soluble non-starch polysaccharides: a review of digestion, nutritive and anti-nutritive effects in pigs and poultry. Asian Aust J Anim Sci. 2010;23(10):1386–1398. doi: 10.5713/ajas.2010.90222. [DOI] [Google Scholar]
- Dunlap CA, Kim SJ, Kwon SW, Rooney AP. Bacillus velezensis is not a later heterotypic synonym of Bacillus amyloliquefaciens; Bacillus methylotrophicus, Bacillus amyloliquefaciens subsp plantarum and ‘Bacillus oryzicola’ are later heterotypic synonyms of Bacillus velezensis based on phylogenomics. Int J Syst Evol Microbiol. 2015 doi: 10.1099/ijsem.0.000858. [DOI] [PubMed] [Google Scholar]
- Elshishtawy RM, Mohamed SA, Asiri AM, Gomaa AM, Ibrahim IH, Altalhi HA. Saccharification and hydrolytic enzyme production of alkali pre-treated wheat bran by Trichoderma virens under solid state fermentation. BMC Biotechnol. 2015;15(1):37. doi: 10.1186/s12896-015-0158-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatima S, Anjum T. Identification of a Potential ISR Determinant from Pseudomonas aeruginosa PM12 against Fusarium Wilt in Tomato. Front Plant Sci. 2017;8:848. doi: 10.3389/fpls.2017.00848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field D, O’Connor R, Cotter PD, Ross RP, Hill C. In Vitro activities of nisin and nisin derivatives alone and in combination with antibiotics against Staphylococcus biofilms. Front Microbiol. 2016;7(e46884):508. doi: 10.3389/fmicb.2016.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G, Kim S, Lee SM, Woo HM, Park TH, Um Y. Complete genome sequence of Bacillus sp. 275, producing extracellular cellulolytic, xylanolytic and ligninolytic enzymes. J Biotechnol. 2017;254:59–62. doi: 10.1016/j.jbiotec.2017.05.021. [DOI] [PubMed] [Google Scholar]
- Grim CJ, Kozlova EV, Sha J, Fitts EC, van Lier CJ, Kirtley ML, Joseph SJ, Read TD, Burd EM, Tall BD, Joseph SW, Horneman AJ, Chopra AK, Shak JR. Characterization of Aeromonas hydrophila wound pathotypes by comparative genomic and functional analyses of virulence genes. MBio. 2013;4(2):e00013–e00064. doi: 10.1128/mBio.00064-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu SB, Zhao LN, Wu Y, Li SC, Sun JR, Huang JF, Li DD. Potential probiotic attributes of a new strain of Bacillus coagulans CGMCC 9951 isolated from healthy piglet feces. World J Microbiol Biotechnol. 2015;31(6):851–863. doi: 10.1007/s11274-015-1838-x. [DOI] [PubMed] [Google Scholar]
- Guo X, Chen DD, Peng KS, Cui ZW, Zhang XJ, Li S, Zhang YA. Identification and characterization of Bacillus subtilis from grass carp (Ctenopharynodon idellus) for use as probiotic additives in aquatic feed. Fish Shellfish Immunol. 2016;52:74–84. doi: 10.1016/j.fsi.2016.03.017. [DOI] [PubMed] [Google Scholar]
- Hashemi M, Shojaosadati SA, Razavi SH, Mousavi SM. Evaluation of Ca-independent α-amylase production by Bacillus sp. KR-8104 in submerged and solid state fermentation systems. Iran J Biotechnol. 2011;9(3):188–196. doi: 10.1016/j.nbt.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Hero JS, Pisa JH, Perotti NI, Romero CM, Martínez MA. Endoglucanase and xylanase production by Bacillus sp. AR03 in co-culture glycosyl hydrolases by Bacillus sp. AR03 in co-culture. Prep Biochem. 2017 doi: 10.1080/10826068.2017.1280826. [DOI] [PubMed] [Google Scholar]
- Hussain T, Tan B, Liu G, Oladele OA, Rahu N, Tossou MC, Yin Y. Health-promoting properties of Eucommia ulmoides: a review. Evid Based Complement Alternat Med. 2016;2016:5202908. doi: 10.1155/2016/5202908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Q, Jiang Q, Zhao L, Su C, Li S, Si F, Li S, Zhou C, Mu Y, Xiao M. Complete genome sequence of Bacillus velezensis S3-1, a potential biological pesticide with plant pathogen inhibiting and plant promoting capabilities. J Biotechnol. 2017;259:199–203. doi: 10.1016/j.jbiotec.2017.07.011. [DOI] [PubMed] [Google Scholar]
- Kazeem MO, Shah UK, Baharuddin AS, AbdulRahman NA. Prospecting agro-waste cocktail: supplementation for cellulase production by a newly isolated thermophilic B. licheniformis 2D55. Appl Biochem. 2017 doi: 10.1007/s12010-017-2401-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Lee SY, Weon HY, Sang MK, Song J. Complete genome sequence of Bacillus velezensis M75, a biocontrol agent against fungal plant pathogens, isolated from cotton waste. J Biotechnol. 2017;241:112–115. doi: 10.1016/j.jbiotec.2016.11.023. [DOI] [PubMed] [Google Scholar]
- Kim SY, Song H, Sang MK, Weon HY, Song J. The complete genome sequence of Bacillus velezensis strain GH1-13 reveals agriculturally beneficial properties and a unique plasmid. J Biotechnol. 2017;259:221–227. doi: 10.1016/j.jbiotec.2017.06.1206. [DOI] [PubMed] [Google Scholar]
- Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19(9):1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo F, Kawabe K, Kuriki H, Eguchi T, Kakinuma K. A new family of glucose-1-phosphate/glucosamine-1-phosphate nucleotidylyltransferase in the biosynthetic pathways for antibiotics. J Am Chem Soc. 2005;127(6):1711–1718. doi: 10.1021/ja044921b. [DOI] [PubMed] [Google Scholar]
- Kudo F, Numakura M, Tamegai H, Yamamoto H, Eguchi T, Kakinuma K. Extended sequence and functional analysis of the butirosin biosynthetic gene cluster in Bacillus circulans SANK 72073. J Antibiot (Tokyo) 2005;58(6):373–379. doi: 10.1038/ja.2005.47. [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis Version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Godzik A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics. 2006;22(13):1658–1659. doi: 10.1093/bioinformatics/btl158. [DOI] [PubMed] [Google Scholar]
- Li W, Jaroszewski L, Godzik A. Clustering of highly homologous sequences to reduce the size of large protein databases. Bioinformatics. 2001;17(3):282–283. doi: 10.1093/bioinformatics/17.3.282. [DOI] [PubMed] [Google Scholar]
- Li W, Jaroszewski L, Godzik A. Tolerating some redundancy significantly speeds up clustering of large protein databases. Bioinformatics. 2002;18(1):77–82. doi: 10.1093/bioinformatics/18.1.77. [DOI] [PubMed] [Google Scholar]
- Liu G, Kong Y, Fan Y, Geng C, Peng D, Sun M. Whole-genome sequencing of Bacillus velezensis LS69, a strain with a broad inhibitory spectrum against pathogenic bacteria. J Biotechnol. 2017;249:20–24. doi: 10.1016/j.jbiotec.2017.03.018. [DOI] [PubMed] [Google Scholar]
- Lizardi-Jiménez MA, Hernández-Martínez R. Solid state fermentation (SSF): diversity of applications to valorize waste and biomass. 3 Biotech. 2017;7(1):44. doi: 10.1007/s13205-017-0692-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu WJ, Wang HT, Nie YF, Wang ZC, Huang DY, Qiu XY, Chen JC. Effect of inoculating flower stalks and vegetable waste with ligno-cellulolytic microorganisms on the composting process. J Environ Sci Health B. 2004;39(5–6):871–887. doi: 10.1081/LESB-200030896. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B, Kamilova F. Plant-growth-promoting rhizobacteria. Annu Rev Microbiol. 2009;63(1):541–556. doi: 10.1146/annurev.micro.62.081307.162918. [DOI] [PubMed] [Google Scholar]
- Manfredi AP, Pisa JH, Valdeon DH, Perotti NI, Martinez MA. Synergistic effect of simple sugars and carboxymethyl cellulose on the production of a cellulolytic cocktail from Bacillus sp. AR03 and enzyme activity characterization. Appl Biochem Biotechnol. 2016;179(1):16–32. doi: 10.1007/s12010-015-1976-5. [DOI] [PubMed] [Google Scholar]
- Meng F, Ma L, Ji S, Yang W, Cao B. Isolation and characterization of Bacillus subtilis strain BY-3, a thermophilic and efficient cellulase-producing bacterium on untreated plant biomass. Lett Appl Microbiol. 2015;59(3):306–312. doi: 10.1111/lam.12276. [DOI] [PubMed] [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31(3):426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Mishra RR, Rath B, Sahu HK, Thatoi HN. Characterization and extracellular enzyme activity of predominant marine Bacillus spp. isolated from sea water of Orissa coast, India. Malays J Microbiol. 2009;5(2):87–93. [Google Scholar]
- Moftah OA, Grbavčić S, Zuža M, Luković N, Bezbradica D, Knežević-Jugović Z. Adding value to the oil cake as a waste from oil processing industry: production of lipase and protease by Candida utilis in solid state fermentation. Appl Biochem Biotechnol. 2012;166(2):348–364. doi: 10.1007/s12010-011-9429-2. [DOI] [PubMed] [Google Scholar]
- Molinatto G, Puopolo G, Sonego P, Moretto M, Engelen K, Viti C, Ongena M, Pertot I. Complete genome sequence of Bacillus amyloliquefaciens subsp. plantarum S499, a rhizobacterium that triggers plant defences and inhibits fungal phytopathogens. J Biotechnol. 2016;238:56–59. doi: 10.1016/j.jbiotec.2016.09.013. [DOI] [PubMed] [Google Scholar]
- Pan HQ, Li QL, Hu JC. The complete genome sequence of Bacillus velezensis 9912D reveals its biocontrol mechanism as a novel commercial biological fungicide agent. J Biotechnol. 2017;247:25–28. doi: 10.1016/j.jbiotec.2017.02.022. [DOI] [PubMed] [Google Scholar]
- Paudel YP, Qin W. Characterization of novel cellulase-producing bacteria isolated from rotting wood samples. Appl Biochem Biotechnol. 2015;177(5):1186–1198. doi: 10.1007/s12010-015-1806-9. [DOI] [PubMed] [Google Scholar]
- Peypoux F, Bonmatin JM, Wallach J. Recent trends in the biochemistry of surfactin. Appl Microbiol Biotechnol. 1999;51(5):553–563. doi: 10.1007/s002530051432. [DOI] [PubMed] [Google Scholar]
- Rückert C, Blom J, Chen X, Reva O, Borriss R. Genome sequence of B. amyloliquefaciens type strain DSM7(T) reveals differences to plant-associated B. amyloliquefaciens FZB42. J Biotechnol. 2011;155(1):78–85. doi: 10.1016/j.jbiotec.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Ryu CM, Farag MA, Hu CH, Reddy MS, Kloepper JW, Pare PW. Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol. 2004;134(3):1017–1026. doi: 10.1104/pp.103.026583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim AA, Grbavcic S, Sekuljica N, Stefanovic A, Jakovetic Tanaskovic S, Lukovic N, Knezevic-Jugovic Z. Production of enzymes by a newly isolated Bacillus sp. TMF-1 in solid state fermentation on agricultural by-products: the evaluation of substrate pretreatment methods. Bioresour Technol. 2017;228:193–200. doi: 10.1016/j.biortech.2016.12.081. [DOI] [PubMed] [Google Scholar]
- Shi JF, Sun CQ. Isolation, identification, and biocontrol of antagonistic bacterium against Botrytis cinerea after tomato harvest. Braz J Microbiol. 2017;48(4):706–714. doi: 10.1016/j.bjm.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C, Liu Y, Yan S, Zhi L. Complete genome sequence of Serratia sp. YD25 (KCTC 42987) presenting strong antagonistic activities to various pathogenic fungi and bacteria. J Biotechnol. 2017;245:9–13. doi: 10.1016/j.jbiotec.2017.01.011. [DOI] [PubMed] [Google Scholar]
- Sun J, Zhang X, Gao X, Jiang Q, Yi W, Li L. Characterization of virulence properties of Aeromonas veronii isolated from diseased Gibel Carp (Carassiusgibelio) Int J Mol Sci. 2016;17(4):1061–1068. doi: 10.3390/ijms17040496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T, Kai B, Duddela S, Krug D, Kim HU, Bruccoleri R, Sang YL, Fischbach MA, Müller R, Wohlleben W. AntiSMASH 3.0—a comprehensive resource for the genome mining of biosynthetic gene clusters. Nucleic Acids Res. 2015;43(1):237–243. doi: 10.1093/nar/gkv437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Qiao J, Blom J, Rueckert C, Reva O, Gao X, Borriss R. The rhizobacterium Bacillus amyloliquefaciens subsp. plantarum NAU-B3 contains a large inversion within the central portion of the genome. Genome Announc. 2013 doi: 10.1128/genomeA.00941-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang Q, Feng X, Li S, Xia J, Xu H. A novel agar diffusion assay for qualitative and quantitative estimation of ε-polylysine in fermentation broths and foods. Food Res Int. 2012;48(1):49–56. doi: 10.1016/j.foodres.2012.02.017. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 Figure S1. The decoloring circles of Bacillus velezensis 157 on selective mediums: a Congo red on CMC-amended agar plate, b Pectin agar plate, c the crude extract of Bacillus velezensis 157 (200 μL) was poured into the wells in Xylan agar plate, which was made with a gel cutter d Starch agar was overlayed with KI solution. Figure S2. Gel electrophoresis of native plasmid of Bacillus velezensis 157. Figure S3. Venn diagram of the four Bacillus velezensis strains based on protein cluster analysis, comparing the shared and unique protein clusters with each other (DOC 1192 kb)