Abstract
The ability of smoking to reduce body weight serves as motivation for continued smoking. It is unclear to what extent non-nicotine constituents in cigarettes are contributing to the weight-reducing effect of smoking. The purpose of the current study was to examine the effects of nicotine and four minor tobacco alkaloids (nornicotine, cotinine, anatabine, and anabasine) on food intake, one of the key regulators of body weight. In addition, a smokeless tobacco extract (STE) and e-cigarette (EC) refill liquid were used to model the effects of actual tobacco product exposure on food intake. Male Holztman rats were trained to lever press for food pellets during daily 2h sessions in operant chambers. In Experiment 1, the effects of subcutaneous injections of saline, nicotine (0.25 – 1.00 mg/kg), nornicotine (0.50 – 6.00 mg/kg), cotinine (1.00 – 100.00 mg/kg), anatabine (0.25 – 3.00 mg/kg), and anabasine (0.50 – 4.00 mg/kg) were assessed. In Experiment 2, rats from Experiment 1 were used to examine the effects of nicotine, STE, and EC liquid. All alkaloids, except cotinine, produced a dose-dependent reduction in overall food intake. The highest doses of all drugs significantly reduced latency and response rate to obtain the first pellet. At some doses, nicotine, anatabine, and nornicotine reduced food intake within the first 45 minutes without compensatory increases in intake later in the session. STE and EC liquid produced dose dependent decreases in food intake similar to nicotine alone. These data suggest that minor tobacco alkaloids have appetite suppressant effects and warrant further investigation into their effects on body weight, energy intake, and energy expenditure under free-feeding conditions. However, findings with STE and EC liquid suggest that nicotine is the primary constituent in these products to effect food intake, whereas levels of minor alkaloids in these products may be too low to influence food intake.
Keywords: Nicotine, Minor tobacco alkaloids, Food intake, Meal patterns, Smokeless Tobacco, E-cigarettes
1. Introduction
Cigarette smoking is associated with reduced weight gain and quitting smoking results in increased weight gain (1–5). The relationship between smoking status and body weight serves as a primary motivation for continued smoking (6). Weight loss is often given as a rationale for starting to smoke, while weight gain is often cited as a deterrent for quitting (7, 8). The average weight gain among smokers is estimated between 4 – 5 kg, with a significant proportion of the population gaining well above this average (>15 kg in 13 – 33% of ex-smokers), making weight gain a major obstacle to smoking cessation (1, 2, 5).
Nicotine has been the main focus of previous research investigating smoking’s effects on body weight (9–11). Nicotine has been shown to increase metabolic rate and physical activity while decreasing food intake in smokers to produce weight loss. On the other hand, withdrawal from nicotine leads to significant weight gain, with some studies showing up to 70% of which is due to increases in energy intake (9, 12). Animal models of nicotine’s effects on body weight have shown similar changes in energy expenditure (13–16) or food intake (17–22) following nicotine administration and withdrawal from nicotine. However, nicotine replacement therapy (NRT) has produced variable effects on post-cessation weight gain, ranging from no effect (23–27) to relatively modest reductions (28–32). These findings suggest that other tobacco constituents may also affect body weight.
It is unclear to what extent other constituents in cigarettes are contributing to the reduced weigh gain in smokers. In light of their behavioral and neuropharmacological similarities to nicotine, minor tobacco alkaloids (i.e., nornicotine, cotinine, anabasine, anatabine, and myosmine) are prime candidates for contributing to these effects. Because minor tobacco alkaloids act at nicotinic acetylcholine receptors, which may be necessary for facilitating nicotine’s effects on weight loss (33), these compounds could also produce effects on body weight. Some of these compounds may also be self-administered (34, 35) and can substitute for nicotine in drug discrimination tasks (35–37). Although cotinine, nornicotine and anatabine (as anatabloc®) have all been proposed in patent applications as treatments for weight-loss (38, 39), no empirical studies of their effects on body weight per se have been published. This research gap represents a significant limitation in our knowledge of the underlying factors involved in smoking’s effects on body weight. To the extent that minor tobacco alkaloids can reduce food intake, they might have potential as pharmacotherapies for the treatment of post cessation weight gain, as well as obesity. Because obesity is a leading cause of preventable death in the United States (40), and FDA approved drugs for the treatment of obesity have had limited efficacy and undesirable side effects (41), the development of new drugs for weight loss is a continued research priority.
As the nature of tobacco use continues to change with the evolution and emergence of new tobacco products, it is important to examine how these products impact food intake and body weight. Relative to research on cigarette smokers, little research has been conducted examining the effects of smokeless tobacco products on body weight and food intake in human and animal models. In addition, e-cigarettes, which have become increasingly popular over the last several years, vary considerably between products with respect to their levels of nicotine and other constituents (e.g., minor tobacco alkaloids; (42)). Given that e-cigarettes are now regulated as tobacco products, it is important to assess the extent to which nicotine and minor alkaloids in these products might facilitate use by influencing body weight and food intake. Although the current levels of minor tobacco alkaloids in tobacco products is generally low compared to nicotine, their interaction with nicotine and thousands of other constituents could nonetheless contribute to the effects of tobacco product use on body weight and food intake. Recent human and animal studies have produced conflicting results regarding the effects of e-cigarettes on weight gain. While some studies have shown that e-cigarette vapor produces increases in physical activity and delayed weight gain (43, 44), others have shown that e-cigarette vapor has no effect on weight gain or food intake (45, 46).
The purpose of the current study was to examine the effects of four minor tobacco alkaloids (nornicotine, cotinine, anatabine, and anabasine) and nicotine alone on food intake. In this initial study, animals were allowed to lever-press for food pellets (2hr/day) in operant conditioning chambers to investigate the acute effects of a range of doses of nicotine and these alkaloids on deprivation-induced food intake. Following the assessment of isolated alkaloids, the effects of nicotine alone were compared to tobacco product formulations containing equivalent nicotine doses. For this purpose, an aqueous extract of Kodiak Wintergreen smokeless tobacco and an EC liquid were used. Because these products contain non-nicotine constituents that are known to enhance the behavioral effects of nicotine, we hypothesized that they would have a greater effect on food intake than nicotine alone.
2. Materials and methods
2.1. Animals
Sixteen male Holtzman rats (Harlan, Indianapolis, IN) weighing 300–350 g at arrival were used in this study. This strain was chosen to extend our previous study examining changes in food intake during nicotine self-administration (17). Upon arrival, all rats were individually housed in a temperature- and humidity- controlled colony room under a reversed 12 h light/dark cycle (lights off at 09:00 h) for approximately one week. Rats were maintained under a restricted feeding regimen (18 g/day), with ad libitum access to water. After one week, rats began training during 2 h operant sessions (see section 2.4.). Rats were fed approximately 30 – 45 minutes after the session termination, in their home cages. Protocols were approved by the Institutional Animal Care and Use Committee of the Minneapolis Medical Research Foundation in accordance with the 2013 NIH guide for the Care and Use of Mammals in Neuroscience and Behavioral Research.
2.2. Apparatus
Each operant conditioning chamber (29 cm × 26 cm × 33 cm; Coulbourn Instruments, Allentown, PA) was made of aluminum and Plexiglas walls, a Plexiglas ceiling, and a stainless steel grid floor. Two response levers (ENV-110RM, Med Associates) were located on the front wall 7 cm above the chamber floor. Standard grain pellets (45 mg; total Kcal/g: 3.303; breakdown: 0.796 protein, 0.345 fat, and 2.162 carbohydrate) were dispensed via a feeder (ENV-203M-45, Med Associates) into a food receptacle on the front wall located between the two levers. Each response on the right lever produced a single food pellet. Responses on the left lever were recorded, but had no programmed consequences. Water was continuously available via a spout mounted on the back wall of the chamber. Each chamber was placed inside a sound-attenuating cubicle equipped with an exhaust fan that provided masking noise. Med-PC IV (Med Associated, St Albans, VT) software was used for operating the apparatus and recording data.
2.3. Drugs
(−)-Nicotine bitartrate, (+/−)nornicotine, (+/−)cotinine, (+/−) and (+/−)anabasine, were obtained from Sigma Chemical Co. (St. Louis, MO). (+/−)Anatabine was obtained from Toronto Research Chemicals, Inc. (Ontario, Canada). All drugs were dissolved into sterile saline. The pH of all solutions was adjusted to 7.4 using dilute NaOH. All drugs were administered subcutaneously (s.c.) in a volume of 1ml/kg. All drug doses are expressed as the base. The doses selected were based on those used for these alkaloids in a prior study of intracranial self-stimulation (ICSS) behavior (47), in order to make direct comparisons between the ICSS and food intake assays.
Smokeless tobacco extract was selected for use because these products more accurately reflect non-nicotine constituent exposure in humans, while also allowing for the control of nicotine dose (48). Aqueous tobacco extract was prepared from Kodiak Wintergreen smokeless tobacco product (purchased in the Minneapolis area between January 2013 and January 2014) using general procedures described elsewhere (49). Briefly, Kodiak Wintergreen was mixed with saline at concentrations of 400 mg/ml for 18 h using a tube tipper. Saline extract was chosen because it produces a similar alkaloid extraction profile as artificial saliva and simplifies extract preparation while avoiding toxicity (49). The resulting solution was filtered through gauze, centrifuged, and the supernate was filtered. The nicotine concentration was determined by gas chromatography with nitrogen phosphorus detection, according to standard protocol in our laboratory (50), and extract was diluted to the nicotine concentrations required for the current studies.
Whole Tobacco Alkaloid (WTA) EC refill liquid (Dark Honey Tobacco flavor in 10 ml vials) was obtained from Aroma E-Juice (http://www.aromaejuice.com, Scottsdale, AZ). The label indicated the liquid contained 80% vegetable glycerine (VG) and 20% propylene glycol (PG), and had a nicotine concentration of 24 mg/ml. The nicotine concentration was determined in each 10 ml vial of EC liquid used, allowing dilution in saline to the nicotine concentrations required for the current studies. The pH of all solutions was adjusted to 7.4 using dilute NaOH, and administered s.c. in a volume of 1ml/kg.
2.4. Experimental procedure
2.4.1. Training
Rats were placed in operant chambers at 09:00 h, at the onset of the dark cycle. The house light remained off during the operant sessions. Stimulus lights were located 2 cm above the levels; lever presses on the active lever resulted in the delivery of food pellets and the illumination of the cue light above this lever for 2 s. This was done in order to facilitate training and discrimination between the active and inactive levers. The sessions lasted for 2 h. The FR for the first pellet was gradually increased from FR 1 to FR10 over the first week, while the FR for all other pellets remained at FR1. This mixed FR 10:FR 1 schedule remained in effect for the remainder of the study. Use of a mixed FR schedule allowed for separate measurement of motivation to initiate (FR 10) versus maintain food intake (FR 1, (51, 52)). Differential effects on these measures can help ascertain whether drugs have specific effects on satiety (reduce maintenance without affecting initiation) or more general motivational effects (reduce both measures).
After one week of training under the mixed FR schedule, s.c. injections of saline were administered twice a week (Mondays and Thursdays) for at least two weeks, 15 minutes before the session, until behavior stabilized. Behavior was considered stable when the coefficient of variance for total food intake was less than 15% across 4 consecutive saline sessions. Mean intake during these saline sessions then served as the baseline from which each drug was compared. To account for potential changes in baseline food intake over time, animals were re-baselined before a new drug was administered. No limit was placed on total food intake during the operant sessions. If intake within the session was less than 18g (i.e., 400 pellets), animals were supplemented with food in their home cages 30 minutes after the end of their session. Therefore, each rat received at least 18g of food per day, with the possibility to earn more. Any pellets left uneaten in the food hopper or that dropped into the bedding were subtracted from the total pellets.
2.4.2. Experiment 1: Effects of nicotine and minor alkaloids
Once food intake was stable following saline injections, each animal received s.c. injections of nicotine or one of the four minor tobacco alkaloids, 15 minutes before the session, twice a week (Tuesdays and Fridays). Injections of saline continued to be administered on Mondays and Thursdays. Animals continued to run on the weekends, however no drugs were administered during this time. The doses of each drug were administered in ascending order for nicotine (0, 0.25, 0.50, 0.75, or 1.00 mg/kg), nornicotine (0, 0.50, 1.00, 3.00, or 6.00 mg/kg), cotinine (0, 1.00, 6.00, 10.00, or 100.00 mg/kg), anatabine (0, 0.25, 0.50, 1.00, or 3.00 mg/kg), and anabasine (0, 0.50, 1.00, 3.00, or 4.00 mg/kg). These doses were selected based on their effects in other behavioral models (35, 37, 47, 53). Myosmine was not investigated in the present experiments, because previous research has shown either no behavioral effects (54) or only aversive effects of this alkaloid (47). If food intake had not returned to baseline levels the day prior to a drug test session, the treatment drug was not administered and another saline session was run. This only occurred after the highest doses of each drug were tested, and the number of sessions required to recover baseline did not differ between drugs. Once all four doses of a drug were administered, animals were re-baselined. Following at least two weeks of continued saline injections twice a week and stability over four consecutive saline sessions, the next drug was administered. Stability criteria remained the same. The order of drug administration was counterbalanced between animals using a Latin-Square design. This procedure continued until all animals had received nicotine (n=15; one animal was removed from the study prior to receiving nicotine due to sores from injections) and three out of four minor tobacco alkaloids (n = 11–12 per alkaloid)
2.4.3 Experiment 2: Effects of tobacco product exposure
Fourteen of the sixteen rats from Experiment 1 were used in this experiment (another rat was removed from the study due to sores from injections). Following another re-baseline period of at least two weeks of saline injections, nicotine, Kodiak, and EC liquid were administered. Based on the results from Experiment 1, three doses of nicotine (0.25, 0.50, and 1.00 mg/kg) were tested. Each product was separately compared to nicotine alone using equivalent doses. The order of product testing (nicotine v. Kodiak extract or nicotine v. EC liquid) and the order of nicotine doses was counterbalanced using a Latin-Square design. Food intake and weight gain was re-baselined between products.
2.5. Data Analysis
2.5.1. Experiment 1
Mean total food intake (pellets/session), food intake during consecutive 10-min intervals (12 per session), latency (sec) to obtain the first pellet, and running rate to obtain the first pellet (10/(latency to first pellet – latency to first response, i.e. rate of responding from emission of the first response to delivery of the first pellet) were the primary dependent measures. The latter two measures served as indices of motivation to initiate feeding. For each drug, a one-way repeated measures ANOVA was used to analyze changes in total food intake, latency to first pellet, and running rate to first pellet, followed by Dunnet post-hoc tests. Within-session analysis of food intake was analyzed using two-way ANOVAs with dose and interval as factors, followed by Dunnet post-hoc tests to evaluate changes in intake in each of the 12 ten-minute intervals across doses. This interval size provided a good description of the satiation process, showing a typical pattern involving an initial high rate of food intake in the first hour, followed by a lower rate of intake for the remainder of the session. These two rates of intake were demarcated by a distinct pause in feeding. This pattern was further analyzed by dividing total pellets into two bouts of feeding, defined as the number of pellets obtained prior to and following a pause in food intake of at least 10-minutes. This pause duration has been previously used to define a meal for rats (18, 19). In the event there were no pellets obtained within the first 10 minutes of the session, the first bout of food intake was recorded as zero, and all subsequent food intake categorized as falling under the second bout of food intake. This criterion was selected in order to examine changes in food intake within the first 30 minutes following drug injection. Food intake during the first and second bouts was analyzed for each drug using repeated measures two-way ANOVA followed by Sidak post-hoc tests to compare bouts at each dose and Dunnet post-hoc tests to compare each dose to saline within each bout. All statistical analyses were preformed using GraphPad Prism 7 (GraphPad Software, Inc.)
2.5.1. Experiment 2
The same measures and analyses were used as in Experiment 1, except the bout analysis. Also, only the two highest doses administered for nicotine alone, Kodiak extract, and EC liquid were analyzed for the bin data analyses because their time course of effects on food intake was the most consistent, whereas all three doses were evaluated for producing changes in the other three parameters. All statistical analyses were preformed using GraphPad Prism 7 (GraphPad Software, Inc.)
3. Results
3.1. Experiment 1
3.1.1. Total food intake
Figure 1 shows the mean number of pellets consumed for each dose of nicotine (panel A), nornicotine (panel B), anatabine (panel C) and anabasine (panel D) during the entire 2 h session. There was a significant effect of nicotine on mean total food intake (F(3.228, 45.2) = 17.32; p < 0.001), with a significant reduction in food intake for all four doses of nicotine compared with saline. There was a significant effect of nornicotine (F(2.77, 27.7) = 8.57; p < 0.001), with a decrease in food intake following injections of 3.00 mg/kg and 6.00 mg/kg nornicotine compared to saline. There was a significant effect of anatabine (F(2.33, 23.3) = 28.72; p < 0.001), with a significant decrease in food intake at the lowest (0.25 mg/kg) and highest dose (3.00 mg/kg) compared to saline. The reduction in food intake at the other doses approached significance (p < 0.1). There was a significant effect of anabasine (F(2.6, 26) = 27.62; p < 0.001), with a decrease in total food intake at the two highest doses (3.00 and 4.00 mg/kg) compared with saline. There was no effect of cotinine on total food intake (see Supplementary Figure 1, panel A). It should be noted that animals consumed less than 18g of food following injections of nicotine, nornicotine, cotinine, anabasine, and anatabine 89%, 65%, 19%, 75% and 54% of the time, respectively, resulting in supplemental food intake (1g to 12g) 15 minutes following the session end. All supplementary food was consumed 100% of the time.
Fig. 1.
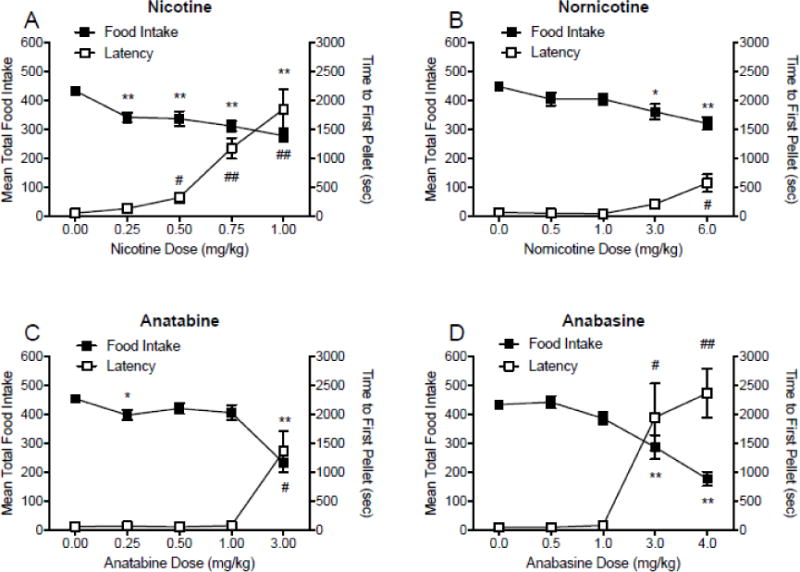
Mean (±SEM) total food intake and latency to first pellet (sec) during daily 2 h experimental sessions following injections of saline, expressed as the mean intake and latency during these injections, and four doses of nicotine (panel A), nornicotine (panel B), anatabine (panel C), and anabasine (panel D). *p < 0.05, ** p < 0.01 difference between food intake following injections of saline (0.00 mg/kg) and injections of the indicated dose. #p < 0.05, ##p < 0.01 difference between latency to first pellet following saline injections (0.00 mg/kg) and injections of the indicated dose. Effects of 0.5 and and 1.0 mg/kg anatabine approached significance (p < 0.1). Note that the dose ranges and intervals differ between panels.
3.1.2. Latency to first pellet
Figure 1 shows the mean latency to first pellet for each dose of nicotine (panel A), nornicotine (panel B), anatabine (panel C) and anabasine (panel D), during 2 h operant sessions. There was a significant effect of nicotine on latency to first pellet (F(1.667, 23.34) = 25.45; p < 0.001), with significant increases in latency at 0.50, 0.75, and 1.00 mg/kg nicotine compared with saline. There was a significant effect of nornicotine (F(1.473, 14.73) = 9.54; p < 0.01), with an increase in latency at 6.00 mg/kg nornicotine compared to saline. There was a significant effect of anatabine (F(1.01, 10.1) = 13.58; p < 0.001), with a significant decrease in response latency for the highest dose (3.00 mg/kg) compared to saline. There was a significant effect of anabasine (F(1.804, 18.04) = 13.68; p < 0.001), with an increase in response latency for the two highest doses (3.00 and 4.00 mg/kg) compared with saline. There was no effect of cotinine on response latency (see Supplementary Figure 1, panel A).
3.1.3. Changes in running rate to first pellet
Figure 2 shows the mean running rates for the first pellet following injections of each drug. There was a significant effect of nicotine on running rate (F(4,48) = 7.6; p < 0.001), with significant differences from saline at all doses tested. There was also a significant effect of nornicotine (F(4,36) = 5.9; p < 0.001), with decreases in running rate at 6.00 mg/kg nornicotine compared to saline. There was a significant effect of anatabine (F(4,32) = 2.8; p < 0.05), with decreases in running rate at 3.00 mg/kg anatabine. There was a significant effect of anabasine (F(4,40) = 6.0; p < 0.001), with decreases in running rate following injections of 3.00 and 4.00 mg/kg anabasine. There was no main effect of cotinine dose on running rate (see Supplementary Figure 1, panel B)
Fig. 2.
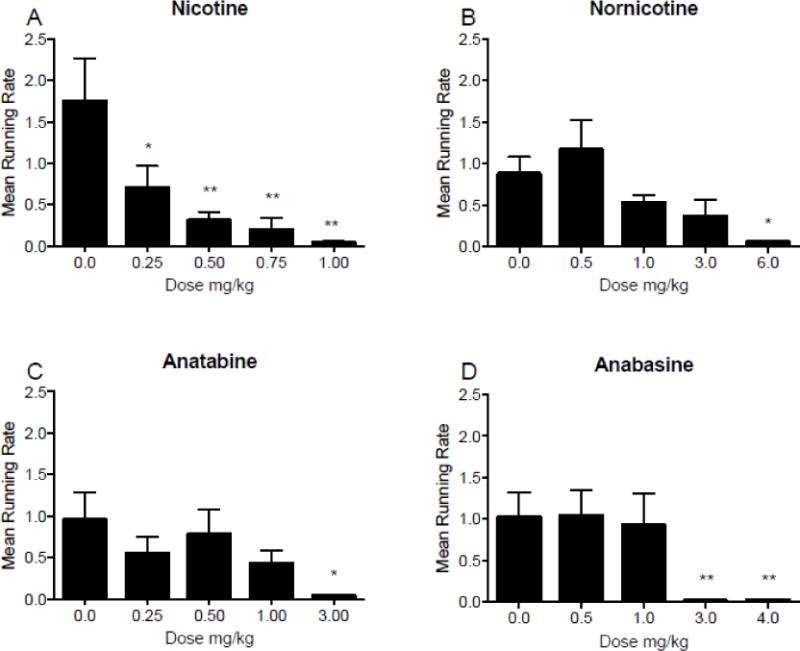
Mean (±SEM) running rates during daily 2 h experimental sessions following injections of saline, expressed as a mean running rate during these injections, and four doses of nicotine (panel A), nornicotine (panel B), anatabine (panel C), and anabasine (panel D). *p < 0.05, ** p < 0.01 difference between food intake following injections of saline (0.00 mg/kg) and the indicated dose. Note that the dose ranges and intervals differ between panels.
3.1.4. Changes in within-session rates of food intake
Figure 3 shows mean total food intake during 10 minute bins following injections of nicotine (panel A), nornicotine (panel B), anatabine (panel C), and anabasine (panel D), during two hour operant sessions. There was a main effect of bin number (F(11, 154) = 17.81; p < 0.001) and dose (F(4,56) = 17.22; p < 0.001) on mean total food intake following injections of nicotine, and a significant bin × dose interaction (F(44,616) = 12.00; p < 0.001). A significant decrease in mean total food intake was observed at all four doses of nicotine compared to saline during the first 20 min of the session, and doses 0.50, 0.75, and 1.00 mg/kg suppressed food intake up to 30 min. The 0.25 mg/kg dose also decreased intake at the end of the session (bin 12). There was a significant main effect of bin number (F(11,110) = 47.14; p < 0.001) and dose (F(4,40) = 8.92; p < 0.001) on mean total food intake following injections of nornicotine, and a significant bin × dose interaction (F(44,440) = 8.27; p < 0.001). Significant decrease in mean total food intake following injections of 3.00 and 6.00 mg/kg nornicotine in the first 30 min of the session. There was also a decrease in total food intake for 40 min following injections of 6.00 mg/kg. There was a significant main effect of bin number (F(11,110) = 34.22; p < 0.001) and dose (F(4,40) = 28.87; p < 0.001) on mean total food intake following injections of anatabine, with a significant bin × dose interaction (F(44,440) = 8.50; p < 0.001). Significant decreases were observed during the first 40 min following injections of 3.00 mg/kg anatabine. Total food intake was also decreased after injections of 0.25 mg/kg in bin 2, and after 1.00 mg/kg in bins 2 and 3 of the session. There was a significant main effect of bin number (F(11,110) = 41.11; p < 0.001) and dose (F(4,40) = 26.75; p < 0.001) on mean total food intake following injections of anabasine, with a significant bin × dose interaction (F(44,440) = 14.37; p < 0.001). Significant decreases in intake were observed for the first 40 min of the session following injections of 3.00 and 4.00 mg/kg anabasine compared to saline. Total food intake was also decreased by 1.00 mg/kg anabasine at the end of the session (bin 12). There was a significant main effect of bin number (F(11,550) = 104.8; p < 0.001), without a significant main effect of cotinine dose or a significant interaction between cotinine dose and bin number, on food intake (see Supplementary Figure 1, panel C).
Fig. 3.
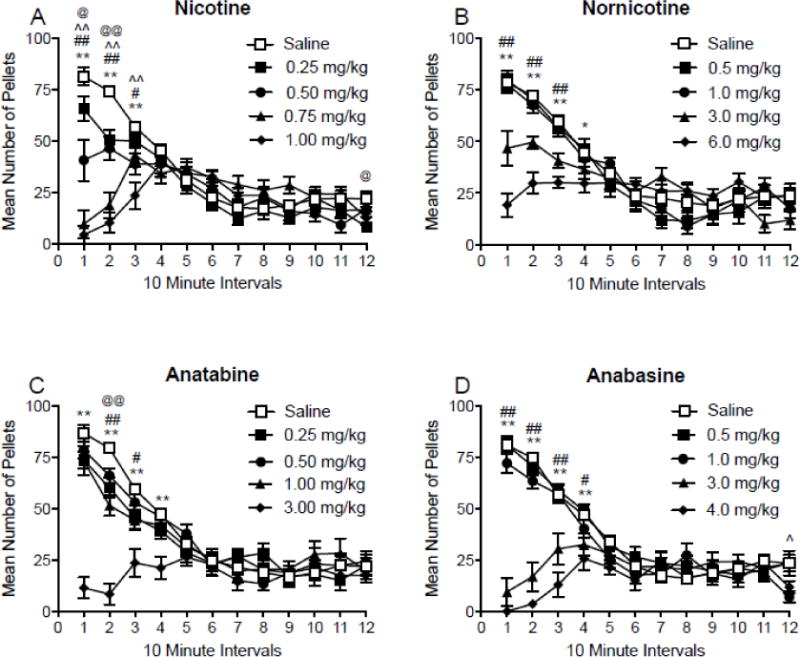
Mean (±SEM) total number of pellets consumed during 10-minute intervals throughout daily 2 h experimental sessions, following injections of saline, expressed as the mean intake during these injections, and four doses of nicotine (panel A), nornicotine (panel B), anatabine (panel C), and anabasine (panel D). @p < 0.05, @@p < 0.01 difference between saline and the lowest dose tested; ^p < 0.05, ^^p < 0.01 difference between saline and the second lowest dose tested; #p < 0.05, ##p < 0.01 difference between saline and the second highest dose tested; *p < 0.05, ** p < 0.01 difference between saline and the highest dose tested. Note that the dose ranges and intervals differ between panels.
Analysis of event records of pellet deliveries revealed that animals consumed either a single big bout or two bouts of food under baseline conditions, with the majority of intake occurring early during the session in bout one (68%). Nicotine and the minor tobacco alkaloids altered this pattern of intake. To convey drug effects at this more molar level of analysis, the session was separated into two bouts of food intake. The first bout began at the start of the session and ended when there was a 10 minute break without food intake. Some animals didn’t consume anything for the first 10 minutes or more, resulting in zero intake for bout one. Other rats began eating immediately, and consumed all of the pellets for the session in bout 1. In this case bout two intake was considered zero (occurred ~30% of the time). Three or more bouts were rare (~10% of the time). In this case, the final two bouts were combined and analyzed as the second bout.
Figure 4 shows mean food intake following saline injections and all four doses of nicotine, nornicotine, anatabine, and anabasine, during each bout of feeding before or after a pause in feeding of at least 10-minutes. There was a significant main effect of nicotine dose (F(4,56) = 18.83; p < 0.001), but not bout, and a significant bout × dose interaction (F(4,56) = 23.42; p < 0.001) on mean total food intake. A significant decrease in intake was observed at 0.50, 0.75, and 1.00 mg/kg nicotine during the first bout, and an increase in intake at 0.75 and 1.00 mg/kg nicotine during the second bout, compared to saline. Mean total intake differed at all doses between the first and second bouts of intake. There was a significant main effect of nornicotine dose (F(4,40) = 8.03; p < 0.001) and bout (F(1,10) = 56.73; p < 0.001), but no bout × dose interaction, on mean total food intake. A significant decrease in intake was observed at 6.00 mg/kg nornicotine during the first bout of intake. Mean total food intake differed at all doses, except 6.00 mg/kg nornicotine, between the first and second bouts of intake. There was a significant main effect of anatabine dose (F(4,40) = 30.66; p < 0.001) and bout (F(1,10) = 78.35; p < 0.001), and a significant bout × dose interaction (F(4,40) = 9.78; p < 0.001), on mean total food intake. A significant decrease in intake was observed during the first bout at 3.00 mg/kg anatabine. Mean total food intake differed between bouts at all doses, except for 3.00 mg/kg anatabine. There was a significant main effect of anabasine dose (F(4,40) = 25.97; p < 0.001) and bout (F(1,10) = 62.77; p < 0.001), and a significant bout × dose interaction (F(4,40) = 31.22; p < 0.001), on mean total food intake. Significant decreases in intake were observed at 3.00 and 4.00 mg/kg anabasine during the first and second bouts of intake. Mean total food intake differed at all doses between the first and second bouts of intake.
Fig. 4.
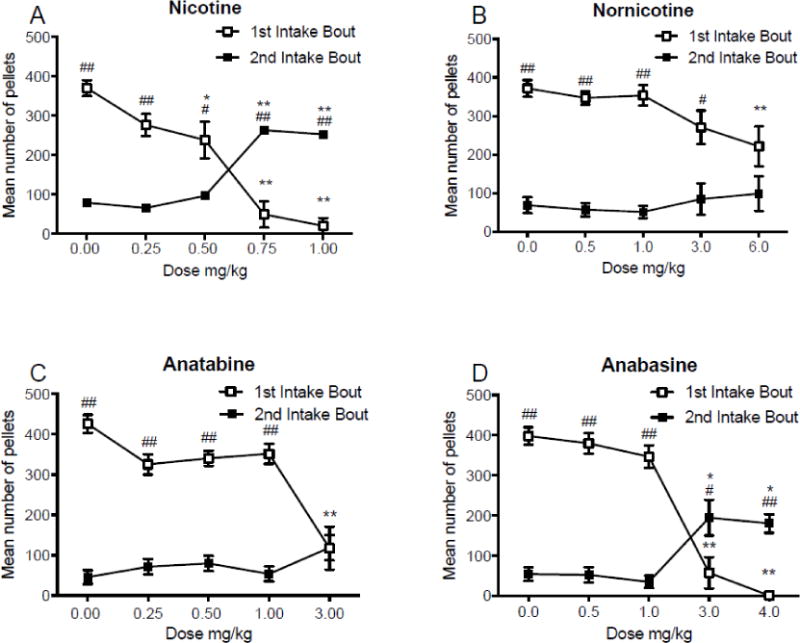
Panels A-D: Mean (±SEM) total food intake before and after a 10-minute inter-meal interval, following injections of saline, expressed as the mean intake during these injections, and 4 doses of nicotine (panel A), nornicotine (panel B), anatabine (panel C), and anabasine (panel D). *p < 0.05, **p < 0.01 difference between saline and drug at the indicated dose. #p<0.05, ##p<0.01 difference between the first and second bout of food intake. Note that the dose ranges and intervals differ between panels.
3.2. Experiment 2
3.2.1. Nicotine versus Kodiak extract
Figure 5 (panel A) shows the mean total food intake for each dose of nicotine alone or Kodiak. There was a significant main effect of dose (F(3,18) = 13.93; p < 0.001), but no effect of drug or a drug × dose interaction. Significant reductions in food intake occurred at 0.50 and 1.00 mg/kg nicotine and all doses of Kodiak extract. Figure 5 (panel B) shows the mean latency to first pellet for each dose of nicotine and Kodiak extract. There was a significant main effect of dose (F(3,18) = 19.84; p < 0.001), but no significant main effect of drug or a drug × dose interaction. Significant increases in latency occurred at 1.00 mg/kg nicotine only. Figure 5 (panel C) shows the mean running rates following injections of nicotine or Kodiak extract. There was no effect of drug or dose, or a drug × dose interaction, although there was a trend (p = 0.055) for a significant main effect for dose. There was a main effect of dose (F(3,15) = 23.66; p<0.001) on running rates, such that all doses of nicotine and Kodiak extract were decreased relative to saline. Figure 5 (panel D) shows mean total food intake during 10-minute bins following injections of nicotine and Kodiak extract. There was a main effect of bin number (F(11, 66) = 18.89; p < 0.001) and drug (F(4,24) = 6.54; p = 0.001), and a significant bin × drug interaction (F(44,264) = 4.05; p < 0.001). Significant decrease in intake occurred at both doses of nicotine and Kodiak extract compared to saline during the first 20 mins of the session. There was also a significant decrease in intake following 0.50 mg/kg nicotine during the final 30 minutes of the session.
Fig. 5.
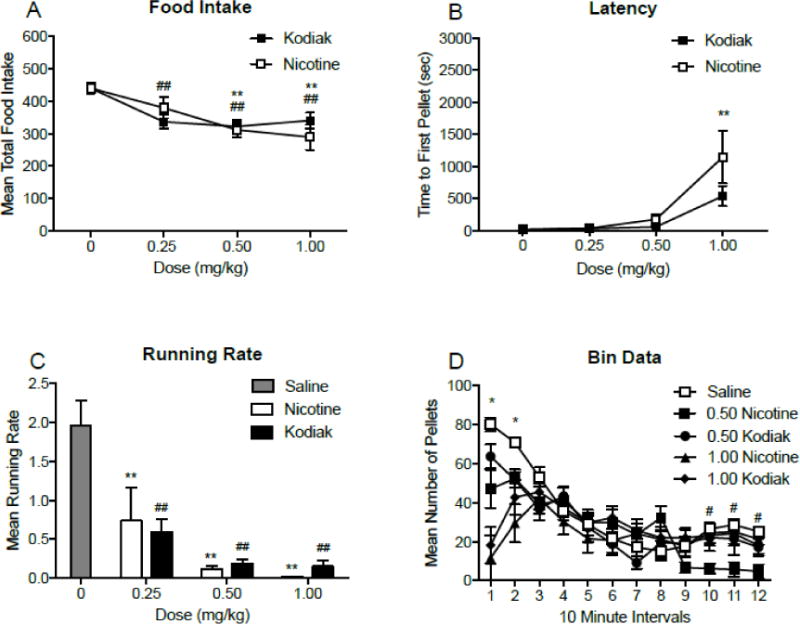
Panels A-B: Mean (±SEM) total food intake (panel A), time to first pellet (sec; panel B), throughout daily 2 h experimental sessions, following injections of three doses of nicotine and Kodiak extract. **p < 0.01 difference between saline and nicotine at the indicated dose. ##p < 0.01 difference between saline and Kodiak extract at the indicated dose. Panel C: Mean (±SEM) running rates, following injections of saline, nicotine, and Kodiak extract. **p < 0.01 difference between saline and nicotine at the indicated dose. ##p < 0.01 difference between saline and Kodiak extract at the indicated dose. Panel D: Mean (±SEM) total number of pellets consumed during 10 minute intervals throughout daily 2 h experimental sessions, following injections of two doses of nicotine and Kodiak extract. *p < 0.05 difference from saline at all drugs and doses tested. #p < 0.05 difference between saline and 0.50 mg/kg nicotine.
3.2.2. Nicotine versus EC liquid
Figure 6 (panel A) shows the mean total food intake for each dose of nicotine or EC liquid. There was a significant main effect of dose (F(3,18) = 12.2; p < 0.001), but no effect of drug or a drug × dose interaction. Significant reductions in food intake occurred at 0.50 and 1.00 mg/kg nicotine and EC liquid. Figure 6 (panel B) shows the mean latency to first pellet for each dose of nicotine and E-Juice liquid. There was a significant main effect of dose (F(3,18) = 18.6; p < 0.001), but no main effect of drug or a drug × dose interaction. Significant increases in latency occurred at 1.00 mg/kg nicotine and EC Liquid. Figure 6 (panel C) shows the mean running rates following injections of nicotine or EC liquid. There was a main effect of dose (F(2,10) = 15.77; p < 0.001), but no effect of drug or drug × dose interaction. There was a main effect of dose (F(3,15) = 27.46; p<0.001) on running rates, such that all doses of nicotine and Kodiak extract (with the exception of 0.25mg/kg Kodiak extract) were decreased relative to saline. Figure 6 (panel D) shows mean food intake during 10-minute bins for nicotine and EC liquid. There was a main effect of bin number (F(11, 66) = 8.08; p < 0.001) and drug (F(4,24) = 6.74; p = 0.001), and a significant bin × drug interaction (F(44,264) = 5.72; p < 0.001). Significant decreases in food intake occurred at both doses of nicotine and EC liquid compared to saline during the first 20 min. The 1.00 mg/kg nicotine dose decreased intake during the first 30 (bin 12).
Fig. 6.
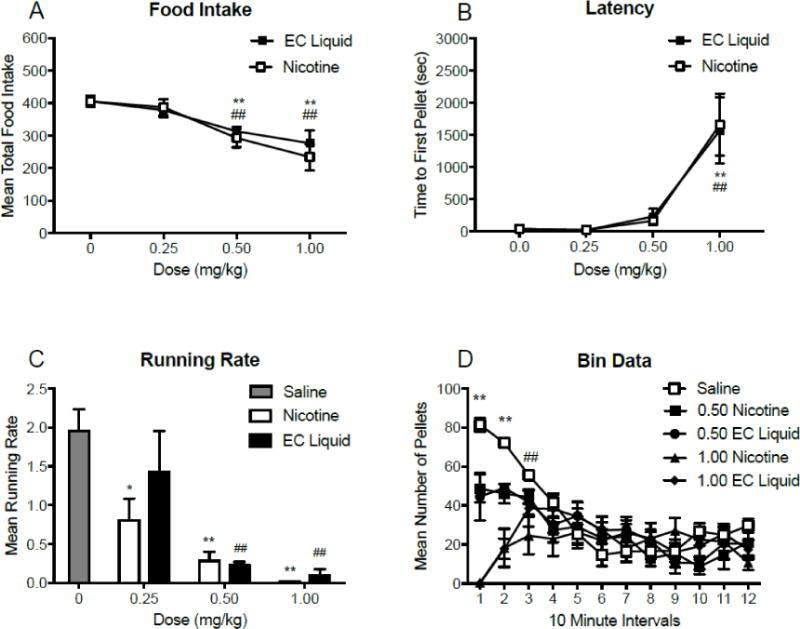
Panels A-B: Mean (±SEM) total food intake (panel A), time to first pellet (sec; panel B), throughout daily 2 h experimental sessions, following injections of three doses of nicotine and EC liquid. *p < 0.05, ** p < 0.01 difference between saline and nicotine at the indicated dose. #p < 0.05, ##p < 0.01 difference between saline and EC liquid at the indicated dose. Panel C: Mean (±SEM) running rates, following injections of saline, nicotine, and EC liquid. **p < 0.01 difference between saline and nicotine at the indicated dose. ##p < 0.01 difference between saline and EC liquid at the indicated dose. Panel D: mean (±SEM) total number of pellets consumed during 10-minute intervals throughout daily 2 h experimental sessions, following injections of two doses of nicotine and EC liquid. *p < 0.05 difference from saline for all drugs and doses tested. ##p < 0.01 difference from saline for 1.00 mg/kg nicotine only.
4. Discussion
The primary finding of the present study was that nicotine and all minor alkaloids, except for cotinine, produced a dose-dependent decrease in food intake. However, whereas nicotine and anatabine significantly reduced food intake across a wide range of doses, the other minor alkaloids only reduced food intake at doses more than tenfold higher than the lowest effective nicotine dose. In addition, latency to initiate feeding was increased at doses that produced decreases in food intake, with the exception of 0.25 and 0.50 mg/kg nicotine, 0.25 mg/kg anatabine, and 3.00 mg/kg nornicotine. These results indicate that, similar to nicotine, the minor tobacco alkaloids nornicotine, anabasine, and anatabine can all reduce food intake and motivation to initiate food intake, but with significantly lower potency than nicotine in the case of nornicotine and anabasine. Consistent with these findings, a smokeless tobacco extract and EC liquid produced decreases in food intake that were similar to nicotine alone at equivalent nicotine doses. These findings have important implications for understanding the appetite-suppressant effects of tobacco product use in humans.
4.1. Effects of Isolated Alkaloids
In the present study, nicotine reduced food intake at all doses tested. These results are similar to what has been seen previously in several other noncontingent administration studies (16, 21, 55). The differences in potency between nicotine and nornicotine and anabasine observed in the present study are consistent with their different potencies for other behavioral effects. The highest dose of nornicotine reduced food intake by 18%. Considering the difference in dose needed to achieve a similar reduction in food intake (e.g. 0.75 vs 6.0 mg/kg), nicotine appears to be around 8-fold more potent than nornicotine. Although complete dose-response curves were not obtained in the present study, this is consistent with the relative potencies reported in other behavioral and neuropharmacological studies (35, 53, 56, 57). Similar decreases in food intake were also seen with anabasine at 3.00 mg/kg, indicating an approximate order-of-magnitude lower potency compared to nicotine. These findings are consistent with a study of minor alkaloid effects on food-maintained responding in mice by Caine et al. (2014) (33). However, the similar potency of anatabine and nicotine in the present study contrasts with the Caine et al. study, which reported a 13-fold lower potency for anatabine. This discrepancy is possibly due to the use of different species between studies. Cotinine on the other hand, had no effect on any measures of food intake at the doses tested, which is consistent with other studies showing little or no effect of cotinine on operant behavior in this dose range (47). The differences in potency between nicotine and the minor alkaloids may be due to differences in their neural mechanisms of action (56) or pharmacokinetics (58), or both. Some studies suggest the reduced penetrability of minor alkaloids through the blood brain barrier compared to nicotine may be involved (53). As such, the relatively low levels of these alkaloids in smokeless tobacco or e-cigarettes do not likely contribute to any appetite suppressant effects of these products. However, despite their lower potency, the minor tobacco alkaloids could serve as parent compounds to develop new, more potent forms.
The other minor alkaloids and higher doses of nicotine only reduced overall food intake at doses that also decreased running rate and latency to first pellet. This may reflect disruptive motoric or other side effects. Alternatively, it may simply indicate a general effect on food motivation for several reasons. First, reducing food deprivation produces a similar reduction in both food intake and latency to feeding under a similar mixed schedule of food reinforcement (51). Second, none of the doses of minor alkaloids in the present study had a significant effect on response rates in an operant food-maintained nicotine discrimination assay (35). Finally, these doses of nicotine and minor tobacco alkaloids have no effect on response latencies in an intracranial self-stimulation (ICSS) model (47). It is more likely that the reduction in food intake at high doses of these alkaloids was due to general motivational effects. It should be noted that the highest dose of nicotine produced seizures in some of the rats. These seizures resolved and rats were able to move freely around the cage without any apparent motor disruption by the time they were placed in the chambers. Any other motoric effects (e.g., unsteady gate, freezing) also appeared to resolve prior to starting the session.
Nicotine, nornicotine, and anabasine have all been shown to elevate ICSS thresholds at the highest doses used in the present study. To the extent that an elevation in ICSS threshold provides a measure of the function of fundamental brain pathways mediating motivation and reinforcement, this suggests that a non-specific motivational deficit (i.e. reduced efficacy of any type of reinforcer) may have contributed to their anorectic effects at these doses. Lower doses of these drugs, however, do not produce elevations in ICSS thresholds, suggesting that the reduction in running rate by nicotine, nornicotine, and anabasine reflects an attenuation of food motivation (i.e. both reduced hunger and enhanced satiation) rather than a non-specific motivational deficit. Similarly, none of the doses of anatabine that reduced food intake in the present study produce motivational or performance deficits in an ICSS model (47), suggesting their effect was due to an attenuation of food motivation per se.
The analysis of food intake across 10-min segments of the session allowed a relatively fine-grained analysis of the time course of drug effects and revealed some differences between the alkaloids. Nicotine, nornicotine, and anabasine all reduced intake early in the session at the same doses that reduced mean total intake. Other studies have shown a similar time course for the effects of nicotine and nornicotine on food-maintained responding, with levels returning to baseline within 60 minutes (57, 59). In contrast, anatabine reduced early session intake at doses that did not reduce total intake across the entire session. Differences in half-lives between these compounds don’t directly correspond to the differences in time course of effects seen between nicotine and the minor tobacco alkaloids, as their elimination half-lives range from 9–20 hours (58). A better correlation between the relative half-lives of the alkaloids and their time course for suppression of food intake may have been observed in the present study had longer sessions and different levels of food deprivation been used.
Differences between alkaloids in their effect on the within-session pattern of food intake were also apparent at a more macro level of analysis. Baseline sessions typically involved an initial bout of food intake at a high rate, then a pause in feeding, followed by a second bout of intake at a lower rate for the remainder of the session. Nicotine, nornicotine, and anatabine, but not anabasine, typically reduced food intake in the beginning of the session without compensatory increases in food intake in the second bout. At high doses of nicotine, significant decreases in food intake were observed early in the session, followed by a rebound in food intake above saline control levels later in the session. Such compensatory effects were not observed with lower nicotine doses. Significant compensation was also not seen with nornicotine or anatabine at any dose. As such, these compounds may have a therapeutic advantage for the treatment of obesity and/or post-cessation weight gain, in that they are not associated with potentially problematic rebound effects (60, 61). However, given the longer half-lives of these compounds noted above, it is necessary to examine them in extended access sessions to better evaluate this. It is also important to mention that we were unable to verify that the pellets were consumed immediately following lever pressing. However, because of the limited session duration and high number of pellets consumed, it seems unlikely that there would have been long intervals between lever pressing and pellet consumption.
4.2 Effects of Tobacco Product Extracts
The Kodiak extract and EC liquid used in the present study produced changes in food intake, latency to first pellet, and running rates that were comparable to nicotine alone. The failure to find any differences in food intake between nicotine, Kodiak extract, and EC liquid suggests that the primary driving force behind the reductions in food intake following use of these tobacco products is nicotine. These findings are consistent with a prior animal study reporting that smoking-relevant doses of minor alkaloids and other compounds present in cigarette smoke (e.g. harman, norharmane, acetaldehyde) do not moderate weight gain or energy expenditure in rats when they are self-administered as a cocktail with nicotine (14). Thus, at their present relative concentrations, minor alkaloids may not contribute to any appetite suppressant effects of tobacco products or ECs.
The results from the present study are consistent with research suggesting that smokeless tobacco use can reduce weight gain in its own right and attenuate increased weight gain during smoking cessation in a manner similar to nicotine replacement therapies (NRT). For example, one study reported that smokeless tobacco use reduced weight gain (62), and another study found an increase in weight gain in individuals who quit using smokeless tobacco relative to nonusers and those continuing to use smokeless tobacco (63). In addition, less weight gain has been reported in individuals who switched from smoking cigarettes to using smokeless tobacco (63) compared to those who quit smoking without switching. Smokeless tobacco may therefore be similar to NRT, which is also able to have some beneficial effects on body weight during smoking cessation (64). However, some studies have failed to show any weight loss with smokeless tobacco use (65). These findings suggest that while smokeless tobacco may reduce weight gain, its effects may be less robust than cigarette smoking. Whether this is due to differences in relative concentrations of nicotine and non-nicotine constituents between cigarettes and smokeless tobacco is unclear. A direct comparison of cigarette smoke extracts and smokeless tobacco extracts using methods similar to the present study would help address this issue.
Few studies have examined the effects of ECs on food intake. A recent investigation evaluating post-cessation weight gain in individuals who switched from conventional cigarettes to e-cigarettes showed an ability of EC use to mitigate post-cessation weight gain (43). In addition, there was a lower incidence of increases in hunger in individuals who had switched to ECs in that study. These findings are consistent with results from the present experiment and suggest that ECs may be an effective tool for helping individuals quit smoking without significant weight gain. In contrast to the present findings, one study in mice showed that EC aerosol had no significant effect on food intake or body weight during exposure or withdrawal, despite brain nicotine levels being comparable to those from exposure to cigarette smoke, which did reduce food intake (46, 60). This inconsistency with the present study may be due to the EC used, route of administration (vapor v. subcutaneous injection), species used (mouse v. rat), or the methods for calculating food consumption (total consumption divided by number of mice in each box v. individual measurements).
It is worth mentioning that under the Family Smoking Prevention and Tobacco Control Act, the FDA has the authority to reduce the nicotine content in tobacco products as a population-wide approach to facilitate smoking cessation. One concern is that continued presence of non-nicotine constituents like minor tobacco alkaloids might contribute to the effects of smoking on body weight and facilitate continued smoking of reduced nicotine content cigarettes among smokers concerned about weight gain. The significant effects of the minor tobacco alkaloids on food intake in Experiment #1 of the present study were found with doses considerably higher than those delivered by tobacco products. Accordingly, there was no difference between nicotine and E-juice/Kodiak extracts in Experiment 2. It is therefore unlikely that reduced-nicotine tobacco products would maintain weight loss and thereby facilitate continued use.
4.3. Limitations
There are a number of methodological limitations of the present study that need to be addressed in future studies. First, because the session only lasted 2 h, we were unable to determine the longer-term effects of acute exposure to the alkaloids. Second, drug effects were assessed in a state of high motivation to consume food. As such, the dose-response curves (i.e. potencies) for these alkaloids may differ at lower levels of food deprivation. Third, food restriction made it unfeasible to examine weight gain as a dependent measure. Fourth, the effects of chronic exposure to nicotine and minor tobacco alkaloids, as occurs in humans, were not examined. In addition, there are thousands of other constituents in tobacco products and smoke and numerous other tobacco products on the market that were not examined in the present study. It will be important to examine whether and to what extent these may also affect weight gain and food intake. Despite these limitations, the present study addresses and important knowledge gap regarding the potential role of non-nicotine constituents in moderating the effects of tobacco product use and cessation on food intake and body weight gain. Addressing its limitations will be an important next step to examine the effects of tobacco alkaloids under more clinically relevant dosing and feeding conditions.
5. Conclusions
In the present study, the minor tobacco alkaloids nornicotine, anatabine, and anabasine, all produced reductions in food intake within the first 30 minutes of injections without significant compensatory increases in food intake later in the session. These reductions in food intake produced changes in meal patterns, specific to meal size, in a manner that was similar to the effects of nicotine alone. This study provides novel preliminary evidence that individual minor tobacco alkaloids have effects on food intake and suggests that further exploration of the potential effects of these compounds on body weight and metabolism are warranted. The present study also suggests that nicotine is the primary component in EC liquid and smokeless tobacco products that affects food intake. The current levels of minor tobacco alkaloids and other non-nicotine constituents do not appear to be sufficient to contribute to any appetite suppressant effects of these products.
Supplementary Material
Highlights.
Nicotine, nornicotine, anatabine, and anabasine reduced food intake
These drugs also increased the latency to obtain food
Effects of smokeless tobacco extract and e-cigarette liquid were similar to nicotine
Appetite suppressant effect of tobacco products is primarily due to nicotine
Minor alkaloids suppress appetite at doses higher than in tobacco products
Acknowledgments
Supported by NIH/NCI grant U19-CA157345 (Hatsukami DH and Shields P, MPI; LeSage MG, PL), and a Career Development Award (MGL), postdoctoral fellowship (PEB) from the Minneapolis Medical Research Foundation and by Award T32DK083250 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; to PEB). These funding institutions had no role in the study design, data collection, data analysis, interpretation of the data, manuscript preparation, or decisions to submit the manuscript for publication. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aubin H-J, Farley A, Lycett D, Lahmek P, Aveyard P. Weight gain in smokers after quitting cigarettes: meta-analysis. BMJ. 2012;345:e4439–e4439. doi: 10.1136/bmj.e4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flegal KM, Troiano RP, Pamuk ER. The influence of smoking cessation on the prevalence of overweight in the United States. The New England Journal of Medicine. 1995;333:1165–1170. doi: 10.1056/NEJM199511023331801. [DOI] [PubMed] [Google Scholar]
- 3.Klesges RC, Meyers AW, Klesges LM, La Vasque ME. Smoking, body weight, and their effects on smoking behavior: a comprehensive review of the literature. Psychol Bull. 1989;106:204–230. doi: 10.1037/0033-2909.106.2.204. [DOI] [PubMed] [Google Scholar]
- 4.Swan GE, Jack LM, Ward MM. Subgroups of smokers with different success rates after use of transdermal nicotine. Addiction. 1997;92:207–217. [PubMed] [Google Scholar]
- 5.Williamson DF, Madans J, Anda RF, Kleinman JC, Giovino GA, Byers T. Smoking cessation and severity of weight gain in a national cohort. N Engl J Med. 1991;324:739–745. doi: 10.1056/NEJM199103143241106. [DOI] [PubMed] [Google Scholar]
- 6.Gritz ER, Klesges RC, Meyers AW. The smoking and body weight relationship: Implications for intervention and postcessation weight control. Annals of Behavioral Medicine 1989 [Google Scholar]
- 7.Bush T, Hsu C, Levine MD, Magnusson B, Miles L. Weight gain and smoking: perceptions and experiences of obese quitline participants. BMC Public Health. (2nd) 2014;14:928–9. doi: 10.1186/1471-2458-14-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pomerleau CS, Kurth CL. Willingness of female smokers to tolerate postcessation weight gain. J Subst Abuse. 1996;8:371–378. doi: 10.1016/s0899-3289(96)90215-1. [DOI] [PubMed] [Google Scholar]
- 9.Audrain-McGovern J, Benowitz NL. Cigarette smoking, nicotine, and body weight. Clin Pharmacol Ther. 2011;90:164–168. doi: 10.1038/clpt.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiolero A, Faeh D, Paccaud F, Cornuz J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr. 2008;87:801–809. doi: 10.1093/ajcn/87.4.801. [DOI] [PubMed] [Google Scholar]
- 11.Filozof C, Fernández Pinilla MC, Fernández-Cruz A. Smoking cessation and weight gain. Obes Rev. 2004;5:95–103. doi: 10.1111/j.1467-789X.2004.00131.x. [DOI] [PubMed] [Google Scholar]
- 12.Stamford BA, Matter S, Fell RD, Papanek P. Effects of smoking cessation on weight gain, metabolic rate, caloric consumption, and blood lipids. Am J Clin Nutr. 1986;43:486–494. doi: 10.1093/ajcn/43.4.486. [DOI] [PubMed] [Google Scholar]
- 13.Hellerstein MK, Benowitz NL, Neese RA, Schwartz JM, Hoh R, Jacob P, Hsieh J, Faix D. Effects of cigarette smoking and its cessation on lipid metabolism and energy expenditure in heavy smokers. Journal of Clinical Investigation. 1994;93:265–272. doi: 10.1172/JCI116955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofstetter A, Schutz Y, Jéquier E. Increased 24-hour energy expenditure in cigarette smokers. New England Journal of Medicine. 1986;314:79–82. doi: 10.1056/NEJM198601093140204. [DOI] [PubMed] [Google Scholar]
- 15.Hur Y-N, Hong G-H, Choi S-H, Shin K-H, Chun B-G. High fat diet altered the mechanism of energy homeostasis induced by nicotine and withdrawal in C57BL/6 mice. Mol Cells. 2010;30:219–226. doi: 10.1007/s10059-010-0110-3. [DOI] [PubMed] [Google Scholar]
- 16.Rupprecht LE, Smith TT, Donny EC, Sved AF. Self-Administered Nicotine Suppresses Body Weight Gain Independent of Food Intake in Male Rats. Nicotine & Tobacco Res. 2016;18:1869–1876. doi: 10.1093/ntr/ntw113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bunney PE, Burroughs D, Hernandez C, LeSage MG. The effects of nicotine self-administration and withdrawal on concurrently available chow and sucrose intake in adult male rats. Physiol Behav. 2016;154:49–59. doi: 10.1016/j.physbeh.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grebenstein PE, Thompson IE, Rowland NE. The effects of extended intravenous nicotine administration on body weight and meal patterns in male Sprague–Dawley Rats. Psychopharmacology (Berl) 2013;228:359–366. doi: 10.1007/s00213-013-3043-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grebenstein PE, Harp JL, Rowland NE. The effects of noncontingent and self-administered cytisine on body weight and meal patterns in male Sprague– Dawley rats. Pharmacol Biochem Behav. 2010;110:192–200. doi: 10.1016/j.pbb.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNair E, Bryson R. Effects of nicotine on weight change and food consumption in rats. Pharmacol Biochem Behav. 1983;18:341–344. doi: 10.1016/0091-3057(83)90451-3. [DOI] [PubMed] [Google Scholar]
- 21.Miyata G, Meguid MM, Varma M, Fetissov SO, Kim HJ. Nicotine alters the usual reciprocity between meal size and meal number in female rat. Physiol Behav. 2001;74:169–176. doi: 10.1016/s0031-9384(01)00540-6. [DOI] [PubMed] [Google Scholar]
- 22.O’Dell LE, Chen SA, Smith RT, Specio SE, Balster RL, Paterson NE, Markou A, Zorrilla EP, Koob GF. Extended access to nicotine self-administration leads to dependence: circadian measures, withdrawal measures, and extinction behavior in rats. Journal of Pharmacology and Experimental Therapeutics. 2006;320:180–193. doi: 10.1124/jpet.106.105270. [DOI] [PubMed] [Google Scholar]
- 23.Allen AM, Kleppinger A, Lando H, Oncken C. Effect of nicotine patch on energy intake and weight gain in postmenopausal women during smoking cessation. Eat Behav. 2013;14:420–423. doi: 10.1016/j.eatbeh.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Assali AR, Beigel Y, Schreibman R, Shafer Z, Fainaru M. Weight gain and insulin resistance during nicotine replacement therapy. Clin Cardiol. 1999;22:357–360. doi: 10.1002/clc.4960220512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Komiyama M, Wada H, Ura S, Yamakage H, Satoh-Asahara N, Shimatsu A, Koyama H, Kono K, Takahashi Y, Hasegawa K. Analysis of factors that determine weight gain during smoking cessation Therapy. PLoS ONE. 2013;8:e72010–6. doi: 10.1371/journal.pone.0072010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prod’hom S, Locatelli I, Giraudon K, Marques-Vidal P, Clair C, Bize R, Cornuz J. Predictors of weight change in sedentary smokers receiving a standard smoking cessation intervention. Nicotine & Tobacco Res. 2013;15:910–916. doi: 10.1093/ntr/nts217. [DOI] [PubMed] [Google Scholar]
- 27.Tønnesen P, Paoletti P, Gustavsson G, Russell MA, Saracci R, Gulsvik A, Rijcken B, Sawe U. Higher dosage nicotine patches increase one-year smoking cessation rates: results from the European CEASE trial. Collaborative European Anti-Smoking Evaluation. European Respiratory Society. European Respiratory Journal. 1999;13:238–246. doi: 10.1034/j.1399-3003.1999.13b04.x. [DOI] [PubMed] [Google Scholar]
- 28.Dale LC, Schroeder DR, Wolter TD, Croghan IT, Hurt RD, Offord KP. Weight change after smoking cessation using variable doses of transdermal nicotine replacement. J Gen Intern Med. 1998;13:9–15. doi: 10.1046/j.1525-1497.1998.00002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emont SL, Cummings KM. Weight gain following smoking cessation: a possible role for nicotine replacement in weight management. Addictive Behaviors. 1987;12:151–155. doi: 10.1016/0306-4603(87)90021-9. [DOI] [PubMed] [Google Scholar]
- 30.Farley AC, Hajek P, Lycett D, Aveyard P. Interventions for preventing weight gain after smoking cessation (Review) 2012:1–152. doi: 10.1002/14651858.CD006219.pub3. [DOI] [PubMed] [Google Scholar]
- 31.Gross J, Stitzer ML, Maldonado J. Nicotine replacement: Effects on postcessation weight gain. Journal of Consulting and Clinical Psychology. 1989;57:87–92. doi: 10.1037//0022-006x.57.1.87. [DOI] [PubMed] [Google Scholar]
- 32.Hughes JR, Hatsukami DK. Effects of three doses of transdermal nicotine on post-cessation eating, hunger and weight. J Subst Abuse. 1997;9:151–159. doi: 10.1016/s0899-3289(97)90013-4. [DOI] [PubMed] [Google Scholar]
- 33.Mineur YS, Abizaid A, Rao Y, Salas R, DiLeone RJ, Gundisch D, Diano S, De Biasi M, Horvath TL, Gao XB, Picciotto MR. Nicotine decreases food intake through activation of POMC neurons. Science. 2011;332:1330–1332. doi: 10.1126/science.1201889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bardo MT, Green TA, Crooks PA, Dwoskin LP. Nornicotine is self-administered intravenously by rats. Psychopharmacology (Berl) 1999;146:290–296. doi: 10.1007/s002130051119. [DOI] [PubMed] [Google Scholar]
- 35.Caine SB, Collins GT, Thomsen M, Wright C, Lanier RK, Mello NK. Nicotine-like behavioral effects of the minor tobacco alkaloids nornicotine, anabasine, and anatabine in male rodents. Experimental and Clinical Psychopharmacology. 2014;22:9–22. doi: 10.1037/a0035749. [DOI] [PubMed] [Google Scholar]
- 36.Brioni JD, O’Neill AB, Kim DJ, Decker MW. Nicotinic receptor agonists exhibit anxiolytic-like effects on the elevated plus-maze test. European Journal of Pharmacology. 1993;238:1–8. doi: 10.1016/0014-2999(93)90498-7. [DOI] [PubMed] [Google Scholar]
- 37.Stolerman IP, Garcha HS, Pratt JA, Kumar R. Role of training dose in discrimination of nicotine and related compounds by rats. Psychopharmacology (Berl) 1984;84:413–419. doi: 10.1007/BF00555223. [DOI] [PubMed] [Google Scholar]
- 38.Crooks PA, Dwoskin LP, Bardo MT. Nornicotine enantiomers for use as a treatment for dopamine related conditions and disease states. 5,691,365. US Patent Office 1998
- 39.Keenan RM. Nicotine metabolites, nicotine dependence and human body weight. 5,573,774. US Patent Office 1996
- 40.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 41.Patel D. Pharmacotherapy for the management of obesity. Metabolism. 2015;64:1376–1385. doi: 10.1016/j.metabol.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 42.Han S, Chen H, Zhang X, Liu T, Fu Y. Levels of selected groups of compounds in refill solutions for electronic cigarettes. Nicotine & Tobacco Res. 2016;18:708–714. doi: 10.1093/ntr/ntv189. [DOI] [PubMed] [Google Scholar]
- 43.Russo C, Cibella F, Caponnetto P, Campagna D, Maglia M, Frazzetto E, Mondati E, Caruso M, Polosa R. Evaluation of post cessation weight gain in a 1-year randomized smoking cessation trial of electronic cigarettes. Nature Publishing Group. 2015:1–9. doi: 10.1038/srep18763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith D, Aherrera A, Lopez A, Neptune E, Winickoff JP, Klein JD, Chen G, Lazarus P, Collaco JM, McGrath-Morrow SA. Adult behavior in male mice exposed to e-cigarette nicotine vapors during late prenatal and early postnatal life. PLoS ONE. 2015;10:e0137953. doi: 10.1371/journal.pone.0137953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Golli El N, Dkhili H, Dallagi Y, Rahali D, Lasram M, Bini-Dhouib I, Lebret M, Rosa J-P, Fazaa El S, Allal-El Asmi M. Comparison between electronic cigarette refill liquid and nicotine on metabolic parameters in rats. Life Sciences. 2016;146:131–138. doi: 10.1016/j.lfs.2015.12.049. [DOI] [PubMed] [Google Scholar]
- 46.Ponzoni L, Moretti M, Sala M, Fasoli F, Mucchietto V, Lucini V, Cannazza G, Gallesi G, Castellana CN, Clementi F, Zoli M, Gotti C, Braida D. Different physiological and behavioural effects of e-cigarette vapour and cigarette smoke in mice. European Neuropsychopharmacology. 2015;25:1775–1786. doi: 10.1016/j.euroneuro.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 47.Harris AC, Tally L, Muelken P, Banal A, Schmidt CE, Cao Q, LeSage MG. Effects of nicotine and minor tobacco alkaloids on intracranial-self-stimulation in rats. Drug and Alcohol Dependence. 2015:1–5. doi: 10.1016/j.drugalcdep.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harris AC, Tally L, Schmidt CE, Muelken P, Stepanov I, Saha S, Vogel RI, LeSage MG. Animal models to assess the abuse liability of tobacco products: Effects of smokeless tobacco extracts on intracranial self-stimulation. Drug and Alcohol Dependence. 2015;147:60–67. doi: 10.1016/j.drugalcdep.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harris AC, Stepanov I, Pentel PR, LeSage MG. Delivery of nicotine in an extract of a smokeless tobacco product reduces its reinforcement-attenuating and discriminative stimulus effects in rats. Psychopharmacology (Berl) 2011;220:565–576. doi: 10.1007/s00213-011-2514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hieda Y, Keyler DE, VanDeVoort JT, Niedbala RS, Raphael DE, Ross CA, Pentel PR. Immunization of rats reduces nicotine distribution to brain. Psychopharmacology (Berl) 1999;143:150–157. doi: 10.1007/s002130050930. [DOI] [PubMed] [Google Scholar]
- 51.Rudski JM, Billington CJ, Levine AS. Naloxone’s effects on operant responding depend upon level of deprivation. Pharmacol Biochem Behav. 1994;49:377–383. doi: 10.1016/0091-3057(94)90437-5. [DOI] [PubMed] [Google Scholar]
- 52.Rudski JM, Grace M, Kuskowski MA, Billington CJ, Levine AS. Behavioral effects of naloxone on neuropeptide Y-induced feeding. Pharmacol Biochem Behav. 1996;54:771–777. doi: 10.1016/0091-3057(96)00019-6. [DOI] [PubMed] [Google Scholar]
- 53.Dwoskin LP, Crooks PA, Teng L, Green TA, Bardo MT. Acute and chronic effects of nornicotine on locomotor activity in rats: altered response to nicotine. Psychopharmacology (Berl) 1999;145:442–451. doi: 10.1007/s002130051079. [DOI] [PubMed] [Google Scholar]
- 54.Hall BJ, Wells C, Allenby C, Lin MY, Hao I, Marshall L, Rose JE, Levin ED. Differential effects of non-nicotine tobacco constituent compounds on nicotine self-administration in rats. Pharmacol Biochem Behav. 2014;120:103–108. doi: 10.1016/j.pbb.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bellinger L, Cepeda-Benito A, Wellman PJ. Meal patterns in male rats during and after intermittent nicotine administration. Pharmacol Biochem Behav. 2003;74:495–504. doi: 10.1016/s0091-3057(02)01033-x. [DOI] [PubMed] [Google Scholar]
- 56.Dwoskin LP, Teng LH, Crooks PA. Nornicotine, a nicotine metabolite and tobacco alkaloid: desensitization of nicotinic receptor-stimulated dopamine release from rat striatum. European Journal of Pharmacology. 2001;428:69–79. doi: 10.1016/s0014-2999(01)01283-3. [DOI] [PubMed] [Google Scholar]
- 57.Goldberg SR, Risner ME, Stolerman IP, Reavill C, Garcha HS. Nicotine and some related compounds: effects on schedule-controlled behaviour and discriminative properties in rats. Psychopharmacology (Berl) 1989;97:295–302. doi: 10.1007/BF00439441. [DOI] [PubMed] [Google Scholar]
- 58.Jacob P, Yu L, Shulgin AT, Benowitz NL. Minor tobacco alkaloids as biomarkers for tobacco use: Comparison of users of cigarettes, smokeless tobacco, cigars, and pipes. Am J Public Health. 1999;89:731–736. doi: 10.2105/ajph.89.5.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Risner ME, Cone EJ, Benowitz NL, Jacob P. Effects of the stereoisomers of nicotine and nornicotine on schedule-controlled responding and physiological parameters of dogs. Journal of Pharmacology and Experimental Therapeutics. 1988;244:807–813. [PubMed] [Google Scholar]
- 60.Foltin RW. Effects of anorectic drugs on the topography of feeding behavior in baboons. Journal of Pharmacology and Experimental Therapeutics. 1989;249:101–109. [PubMed] [Google Scholar]
- 61.LeSage MG, Stafford D, Glowa JR. Effects of anorectic drugs on food intake under progressive-ratio and free-access conditions in rats. Journal of the Experimental Analysis of Behavior. 2004;82:275–292. doi: 10.1901/jeab.2004.82-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hansson J, Galanti MR, Magnusson C, Hergens M-P. Weight gain and incident obesity among male snus users. BMC Public Health. 2011;11:371. doi: 10.1186/1471-2458-11-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rodu B, Stegmayr B, Nasic S, Cole P, Asplund K. The influence of smoking and smokeless tobacco use on weight amongst men. J Intern Med. 2004;255:102–107. doi: 10.1046/j.0954-6820.2003.01244.x. [DOI] [PubMed] [Google Scholar]
- 64.Allen SS, Hatsukami D, Brintnell DM, Bade T. Effect of nicotine replacement therapy on post-cessation weight gain and nutrient intake: A randomized controlled trial of postmenopausal female smokers. Addictive Behaviors. 2005;30:1273–1280. doi: 10.1016/j.addbeh.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 65.Vander Weg M, Klesges R, DeBon M. Relationship between smokeless tobacco use and body weight in young adult military recruits. Nicotine & Tobacco Res. 2005;7:301–305. doi: 10.1080/14622200500056317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


