Abstract
Introduction
Improved biomass and advanced fuel cookstoves can lower household air pollution (HAP), but levels of fine particulate matter (PM2.5) often remain above the World Health Organization (WHO) recommended interim target of 35 ug/m3.
Methods
Based on existing literature, we first estimate a range of likely levels of personal PM2.5 before and after a liquefied petroleum gas (LPG) intervention. Using simulations reflecting uncertainty in both the exposure estimates and exposure-response coefficients, we estimate corresponding expected health benefits for systolic blood pressure (SBP) in adults, birthweight, and pneumonia incidence among children <2 years old. We also estimate potential avoided premature mortality among those exposed.
Results
Our best estimate is that an LPG stove intervention would decrease personal PM2.5 exposure from approximately 270 ug/m3 to approximately 70 ug/m3, due to likely continued use of traditional open-fire stoves. We estimate that this decrease would lead to a 5.5 mmHg lower SBP among women over age 50, a 338 gram higher birthweight, and a 37% lower incidence of severe childhood pneumonia. We estimate that decreased SBP, if sustained, would result in a 5%–10% decrease in mortality for women over age 50. We estimate that higher birthweight would reduce infant mortality by 4 to 11 deaths per 1000 births; for comparison, the current global infant mortality rate is 32/1000 live births. Reduced exposure is estimated to prevent approximately 29 cases of severe pneumonia per year per 1000 children under 2, avoiding approximately 2–3 deaths/ 1000 per year. However, there are large uncertainties around all these estimates due to uncertainty in both exposure estimates and in exposure-response coefficients; all health effect estimates include the null value of no benefit.
Conclusions
An LPG stove intervention, while not likely to lower exposure to the WHO interim target level, is still likely to offer important health benefits.
Keywords: gas stove, intervention, PM2.5, blood pressure, birthweight, pneumonia
Introduction
Observational studies suggest potential health benefits from lower levels of household air pollution (HAP) associated with clean cookstoves; the strongest evidence is for lung cancer, chronic obstructive pulmonary disease (COPD), and childhood pneumonia (Bruce et al. 2015). However, the reduction in exposure with improved biomass stoves has not been in general sufficient to reduce levels to the WHO annual interim fine particulate matter (PM2.5) target (IT-1) level of 35 ug/m3(Bruce et al. 2015, Clark et al. 2013a, Balakrishnan et al. 2014, Pope et al. 2017). In a 2014 literature review, Rehfuess et al. (2014) estimated that the impact of an intervention with improved biomass cookstoves with chimneys vs. traditional (open-fire) stoves reduces personal PM2.5 exposure among women from 270 ug/m3 to 120 ug/m3, based on five studies with data on personal exposure. Possible reasons for less-than-desired reductions in exposure include poor stove performance (design and maintenance), stove stacking (use of traditional stoves along with new stoves), and possible other sources of PM2.5 beyond cooking (Clark et al. 2013a). Increasing evidence from combustion studies have raised questions about whether any stoves that rely on biomass (wood, dung, agricultural waste, coal, charcoal) can achieve targeted reductions in PM2.5 without other interventions such as improved ventilation (Yip et al. 2017). This less-than-desired performance of improved biomass cookstoves has motivated recent trials of stoves using non-biomass fuels, such as liquid petroleum gas (LPG).
There have been relatively few randomized intervention trials of cookstoves that have measured health effects. Smith et al. 2011 focused on childhood pneumonia in Guatemala, Thompson et al. (2011) focused on birth outcomes in Guatemala, Smith-Sivertsen et al. (2009) focused on respiratory function in Guatemala, McCracken et al. (2007) focused on blood pressure in Guatemala, Mortimer et al. (2016) focused on childhood pneumonia in Malawi, and Romieu et al. (2009) focused on adult lung function in Mexico. There is also a recently completed trial in Nigeria (Alexander et al. 2017). While the Mortimer et al. trial did not observe a protective effect for pneumonia, the other trials have shown some health benefits of improved stoves. Perhaps the best known evidence of protective effects is for childhood pneumonia in the RESPIRE trial (Smith et al. 2011). There have also been beneficial effects on blood pressure in older women (McCracken et al. 2007) and in pregnant women (Alexander et al. 2017), beneficial effects on birthweight (Thompson et al. 2011), and mixed results on lung function (Romieu et al. 2009, Smith-Silvertsen et al. 2009).
The WHO target level for an annual average PM2.5 exposure is 10 ug/m3, but given the difficulties in achieving this target level, WHO has set an annual interim target (IT-1) level of 35 ug/m3 (WHO 2014). Because of the steep slopes of estimated exposure-response curves at low levels of PM2.5 for many health outcomes, combined with a plateau of the curve at higher levels (Burnett et al. 2014), Bruce et al. (2015) have argued that substantial health gains are likely to be observed only if personal exposure levels fall below the WHO interim target level of 35 ug/m3, particularly for childhood pneumonia, ischemic heart disease (IHD), and stroke. However, Bruce et al. (2015) also noted that interventions resulting in lower levels of exposure (from 200–300 ug/m3 to 35–80 ug/m3, without necessarily attaining the 35 ug/m3 target level), nonetheless can be expected to result in 20% to 50% decreases in risk of several outcomes (COPD, lung cancer, infant pneumonia, birth outcomes). This conclusion is consistent with the WHO Guidelines for Indoor Air Quality (WHO 2014), which also notes that lowering personal exposures from 200–300 ug/m3 to 35–80 ug/m3 is likely to result in reductions in risk of 20–50% for several outcomes (WHO 2014).
Liquified petroleum gas (LPG) interventions are likely to result in lower exposures than have been observed with interventions with improved biomass cookstoves (Balakrishnan et al. 2014, Rehfeuss et al. 2014). Absent any additional exposure from traditional stoves (stove stacking) for cooking or heating, and absent any important ambient air contribution (eg, trash burning, traffic), it’s likely that LPG stove would lead to indoor levels of PM2.5 at or near the WHO target of 10 ug/m3. Even with stove stacking and elevated ambient levels, we believe that use of LPG stoves will result in substantially reduced concentrations compared to traditional or improved biomass stoves. Consequently, it is likely that LPG stove interventions can show appreciable health benefits for several outcomes, with more expected improvement than from improved biomass stoves. Interventions with LPG are becoming more feasible as the price of LPG has dropped considerably worldwide in the past 5 years (BP Global 2017). Two trials using LPG as an intervention have recently been completed, although results have not been published (Tielsch et al. 2014, Jack et al. 2015), and there is an ongoing trial using an ethanol-burning stove (HAP 2017). The large, multi-centric trial focused exclusively on cooking with LPG stoves – the four-country HAPIN (Household Air Pollution Intervention) study – has just begun (HAPIN 2017). The present authors are part of the team conducting that study.
Randomized trials are typically short-term and cannot easily evaluate long-term effects on chronic disease like lung cancer, stroke, and heart disease. However, such trials can show benefits for outcomes that can be measured in the short term, such as childhood pneumonia, birth outcomes, and adult blood pressure. These short-term outcomes can either show immediate direct benefits, such as pneumonia and low birth weight (both of which can affect infant mortality risk), or potential longer term benefits (blood pressure, which if lowered consistently results in lower rates of chronic disease).
There are relatively few studies of HAP and health effects that provide exposure-response data based on measured personal exposures (Quansah 2017). However, there are exposure-response data for the short-term outcomes mentioned above – childhood pneumonia, low birth weight, and blood pressure – based on personal exposure. In addition, Burnett et al. (2014) have developed integrated exposure-response curves (IERs) for PM2.5 in relation to chronic diseases (lung cancer, stroke, COPD, heart disease) as well as infant pneumonia, by using PM2.5 data from studies of HAP, mainstream tobacco smoke, second-hand smoke, and ambient air pollution.
Using published exposure-response data, we estimate the health benefits gained with exposure reductions anticipated to result from LPG interventions, for three outcomes that can be measured in a short-term trial: blood pressure in adult women (>age 50), birth weight, and pneumonia incidence among young children.
Methods
1. Review of the literature
Our estimates of the effects of HAP on health are drawn from published literature. First, we examined the studies included in several recent systematic reviews, including Balakrishnan et al. (2014), Rehfuess et al. (2014), Pope et al. (2017), Quansah et al. (2017), Bruce et al. (2013), and Bruce et al. (2015). We updated the results of these reviews via a PubMed search using keywords ‘PM2.5’, ‘biomass’ ‘air pollution’, ‘cookstoves’, ‘ household air pollution’, ‘blood pressure’, ‘birthweight’, ‘premature birth’, ‘pre-term birth’, ‘gas stove’ and ‘LPG’ and ‘respiratory infection’ and ‘pneumonia’. Finally, we queried investigators in the field regarding their knowledge of studies relevant to this investigation.
2. Personal PM2.5 data
We focus on PM2.5 rather than carbon monoxide (CO), or other exposure metrics, because the bulk of the literature on health effects in relation to measured air pollution (ambient and household) refers to PM2.5. We also focus on personal rather than area (kitchen) exposures, as personal exposures are most relevant when considering health effects.
Rehfuess et al. (2014) reviewed the literature for cookstove intervention studies as part of a WHO document. Data based on five studies with either 24 or 48 hours sampling indicates that an intervention with improved biomass cookstoves with chimneys would lower personal PM2.5 exposure among women from 270 ug/m3 to about 120 ug/m3, a 56% reduction (Figure 6, Rehfeuss et al. 2014). The reduction in personal exposure due to the interventions in Rehfeuss et al. (2014) of 60%–64% corresponded well with the reduction observed in kitchen concentration.
Balakrishnan et al. (2014) conducted an analogous WHO review of the cookstove literature based on studies which were not interventions. These authors estimated personal exposure levels from traditional stoves of 267 ug/m3 of PM2.5 based on 8 studies, very similar to the estimate of 270 ug/m3 for traditional stoves in the intervention studies reviewed by Rehfeuss et. al. (2014).
The potential effect of an intervention with LPG stoves on personal PM2.5 exposure is not known. The only LPG intervention study cited by Rehfuess et al. (2014) was conducted in Sudan, and measured 24-hour kitchen, rather than personal, concentrations (Bates et al. 2005, see Annex 18). Furthermore, the data presented are for all respirable PM, and are not directly translatable to PM2.5. There is also one non-intervention study with data on exposure from LPG stoves cited by Balakrishnan et al. (2014)(Figure 9), where the personal exposure of women to PM2.5 was approximately 70 ug/m3.
For our best estimate of personal exposure from LPG stoves, we take the estimate of 70 ug/m3 from Balakrishnan et al. (2014).
The recent articles by Pope et al. (2017) and Quansah et al. (2017) reviewed much of the same literature as Balakrishnan et al. (2014) and Rehfuess et al. (2014), but revealed only one additional study (Hartinger et al. 2013) with personal PM2.5 exposure over a 48-hour period. This study found somewhat lower personal PM2.5 levels from traditional stoves (mean 145 ug/m3, n=28). We also found one more recent report from China (Hu et al. 2014), not included in the reviews cited here, which had higher levels of personal PM2.5 exposures for unvented stoves/firepits using wood or plant-based biomass (mean 406 ug/m3, geometric mean 335 ug/m3), based on 17 households. On the other hand, kitchen exposures were lower compared to personal exposures (30%–40%), unlike virtually all of the other literature. These differences from the other literature may reflect that in China there were significant sources outside the home.
Given the relatively small numbers of samples in the studies by Hartinger et al. (2013) and Hu et al. (2014), and the unusual findings in the study by Hu et al. (2014), we have chosen to rely on Rehfuess et al.’s estimate of 270 ug/m3 as our best estimate of personal PM2.5 exposure from traditional stoves.
We focus on arithmetic rather than geometric means, because these are generally what are reported in the literature. Many papers do not provide the geometric mean, and hence we don’t have a good estimate of what the literature overall indicates for the geometric mean for different types of stoves (eg, Balakrishnan et al. 2014, Table 3). The arithmetic mean, while influenced more by outliers, is comprehensible to policy makers and others who may not be familiar with geometric means, and some standards and guidelines are set in terms of annual arithmetic averages.
3. Blood pressure
Exposure-response data for blood pressure (BP) are taken from a panel study by Baumgartner et al. (2011). There were 280 women enrolled in this study (mean age 52), with multiple personal PM2.5 measurements and BP measurements. Ever-smokers and pregnant women were excluded. The geometric mean of 24-hour personal PM2.5 was 55 ug/m3 in the summer and 117 ug/m3 in the winter. Using mixed models the authors found an increase in systolic blood pressure (SBP) of 2.2 (95% CI 0.8–3.7) mmHg for each unit of log PM2.5 among all women, and an increase of 4.1 mmHg (95% CI 1.5–6.6) for each unit of log PM2.5 for women over 50. There was little effect on SBP in women aged 25–50.
There are no other comparable published studies of non-pregnant women with personal exposure to PM2.5 from HAP and with exposure-response data for BP. However, there are several supporting studies. McCracken et al. (2007) studied 120 women of age>38 years, comparing SBP among those exposed after an intervention, with controls. Personal PM2.5 averaged 264 ug/m3 in the control group, and 102 ug/m3 in the intervention group. SBP was 3.7 mmHg lower (95% CI −0.6–8.1) in the intervention arm, and diastolic BP was 3.0 mmHg lower (95% CI 0.4–5.7). The results from Baumgartner et al. (2011) are also consistent with results for women over the age of 40 years in Clark et al. (2013b), which compared SBP before and after an intervention with an improved cookstove (without a control group) among 74 Nicaraguan non-pregnant women (27 over the age of 40 years). Area PM2.5 (48-hour) was reduced from a geometric mean of 1172 ug/m3 to 208 ug/m3 in a subset of women (n=25) with HAP data (personal PM2.5 not measured). SBP dropped an average of 5.9 mmHg (95% confidence interval 0.411.3) in women age 40 years and older, after the intervention, with little effect on women under that age. Finally, similar observations were reported in a 2016 abstract regarding (non-pregnant) women and SBP in relation to HAP by Young et al. (https://epiresearch.org/wp-content/uploads/2016/07/Abstract-Book-Final-070416.pdf). These authors studied 104 women cross-sectionally, of whom 35 were older than 40 years. Among the women 40 years and older, SBP was 4.6 (−0.7, 9.9) mmHg higher per increased unit of log PM2.5, a finding remarkably similar to Baumgartner et al. (2011)(personal communication Jennifer Peel, March 12, 2017).
It should be noted that there are two studies of HAP exposure and pregnant women (Quinn et al. 2016), Alexander et al. 2017). Both studies found associations between higher exposure and higher blood pressure. These studies are not directly relevant for non-pregnant women, as blood pressure is known to vary during pregnancy, falling initially and rising in the third trimester (Hermida et al. 2000).
Other supporting data come from studies of ambient air pollution and blood pressure. Liang et al. (2014) conducted a meta-analysis of 25 cross-sectional and panel studies and found an increase of 1.39 (0.87–1.91) mmHg per 10 ug/m3 increase in PM2.5. Most of these studies used PM2.5 levels measured at the time of or slightly before BP measurements. A more recent large study from China (Lin et al. 2017) found a similar increase of 1.33 (0.04–3.56) mmHg for SBP, using an estimated prior 3-year average of PM2.5 levels. These studies show a similar effect as Baumgartner et al. (2011) in the low-dose region. For example, Baumgartner et al. (2014) would estimate a 4.6 mmHg increase in SBP going from 10 to 40 ug/m3, while using an average of the results from Liang et al. (2014) and Lin et al. (2017), would result in an estimated 4 mm/Hg increase in SBP.
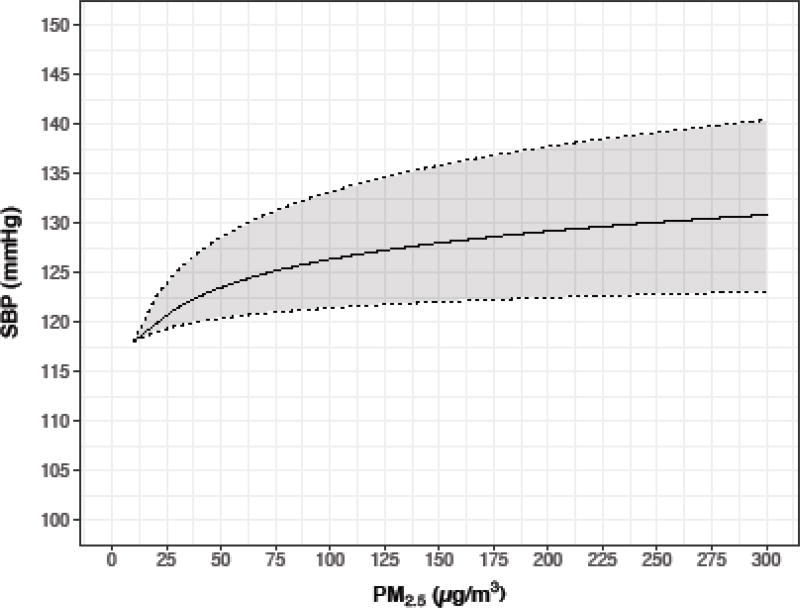
Here we focus on the effects of HAP on SBP among older women. Outside of pregnancy, older women are in general those most likely to experience environmental effects on BP, given that BP changes little with age until age 30, when it increases steadily (Pinto 2007). We therefore use the data from older women (over age 50) in Baumgartner et al. (2011) for our calculations. Given the similarity of the data from ambient air pollution studies in the low dose region, and the lack of relevant data from ambient air pollution studies at higher levels, we have not combined Baumgartner et al. (2014) with those results. From Baumgartner et al. (2011), we used as the estimated non-exposed blood pressure, the lowest SBP of approximately 118 mmHg, as per Figure 1 in that publication. We calculated expected SBP assuming a 4.1 mmHg increase (1.5–6.6) for each increase in one unit of log PM2.5.
Figure 1.
Exposure-response for systolic blood pressure (SBP) in women>50 from Baumgartner et al. (2011)
4. Birthweight
Wylie et al. (2017) measured personal PM2.5 and birthweight among 239 pregnant women in Tanzania, of whom 118 had personal PM2.5 measurements. The geometric mean PM2.5 level was 41 ug/m3 (geo. std. dev. 21 ug/m3), and 87% of measurements exceeded 25 ug/m3. In linear regression using the log of PM2.5, there was a decrease in birth weight of 270 grams (95% CI 0–540) for each increase in 1 unit log PM2.5. Results were similar when the data were restricted to full term births.
Supportive evidence comes from a meta-analysis of ambient air and birthweight studies. Sun et al. (2016) found a decrease of 15.9 grams (95% CI 5.0, 26.8) birthweight for each increase of 10 ug/m3 PM2.5, across 17 studies, with PM2.5 levels averaged over the entire gestation.
Further supportive evidence comes from a study in Poland of indoor air PM2.5 in relation to birthweight (Jedrychowski et al. 2004). These authors studied 362 pregnant non-smoking women in Poland, with 48-hour personal measurements of PM2.5. PM2.5 concentrations averaged 42 ug/m3, with a range of 10 to 147, and for every increase in log PM2.5 there was a decrease of 200 grams (95% CI 15–385) in birthweight, a finding similar to Wylie et al. (2016).
Thompson et al. (2011) also provide some data on personal PM2.5 exposure and birthweight, in a study using a biomass-burning chimney stove; however, there are no exposure-response data beyond the estimated effect of two different exposures. The intervention group had ppm CO levels about 1.5 ppm lower than controls, which would be about 1.69 lower CO measured as mg/m3. Using the conversion from Northcross et al. 2010, this is equivalent to an increase of 213 ug/m3 PM2.5. The intervention group had children who weighed 89 grams more than the control group, or 328 grams more in those women who had the intervention stove in the 1st or 2nd trimester. This would mean an increase in weight of about 15 gms per 10 ug/m3 decrease in PM2.5 for women exposed in the 1st or 2nd trimester, comparable to the effect found in Sun et al. (2016), assuming a linear exposure-response (as did Sun et al.).
Other information comes from a meta-analysis of 10 HAP studies from Amegah et al. (2014), who estimated that burning biomass fuel in unimproved stoves, compared to improved stoves or using other cleaner fuel, led to a decrease in birth weight on the average of 86 grams (95% CI 55–117 grams). However, no exposure levels are provided in this meta-analysis.
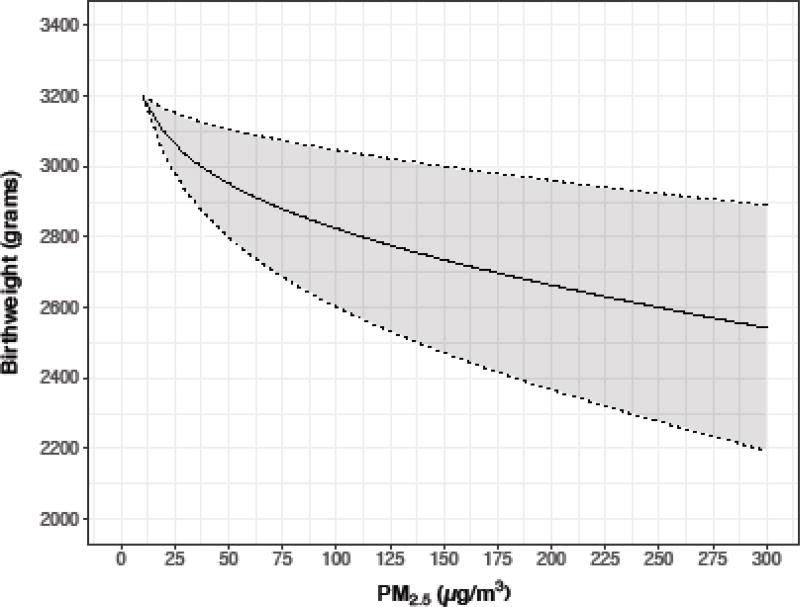
We have chosen to use a weighted average of the estimates of effects from Wylie et al. (2016), Sun et al. (2016), and Jedrychowski et al. (2014), to estimate the effect of PM2.5 on birthweight. All of these studies provide exposure-response results based on personal exposure. Two studies used log PM2.5, while one used untransformed PM2.5. For birthweight estimation considering a fixed exposure, we used the inverse of the coefficient of variation (standard deviation/mean) as our weight. We then derived the standard deviation of the weighted average, assuming independence of the three studies, and used that to construct 95% confidence intervals. We used the same weights in simulations considering exposure as a random variable (see below).
5. Childhood pneumonia
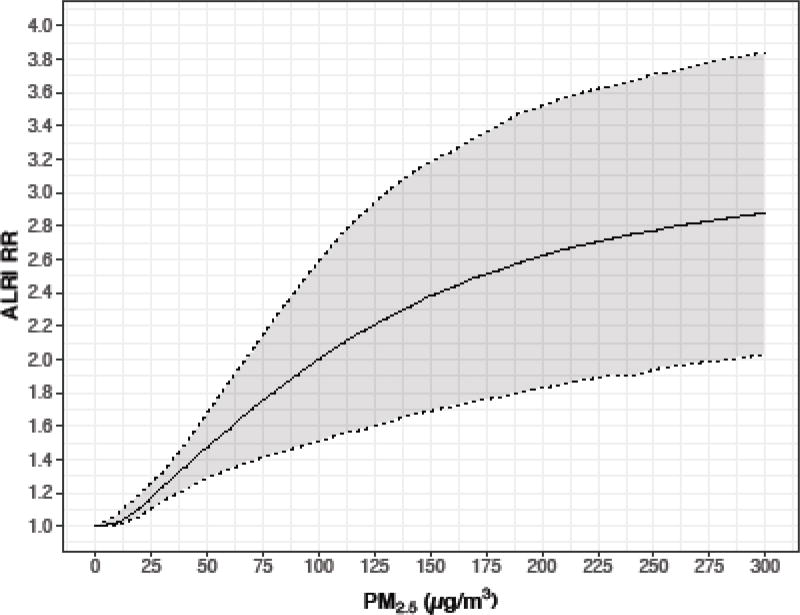
The exposure-response curve for acute lower respiratory infection (ALRI)(childhood pneumonia, <18 months) is available from Burnett et al. (2014), based on data from the RESPIRE trial as well as studies of second-hand smoke and ambient air (although the RESPIRE data provide the bulk of the data). Results are from an excess relative risk model. Supplemental detailed relative risk (RR) data from Burnett et al. (2014), for each unit of exposure from 1 to 300 ug/m3, are available (Global Health Data Exchange 2017). The exposure data in RESPIRE were based on personal measurement of the child’s carbon monoxide exposure, which was highly correlated with PM2.5 data. PM2.5 data were estimated from the CO data via a conversion equation; PM2.5 had a 0.87 correlation with CO (R-square 0.76) in these data (Northcross et al. 2010).
It should be noted that the recent randomized trial in Malawi (Mortimer et al. 2016) did not observe a reduction in childhood pneumonia with improved cookstoves, and in fact found borderline-significant higher severe pneumonia in the intervention arm; however, to date these authors have not presented exposure data, so that the impact of the intervention on exposure levels is not known. Other HAP observational study data do support the relationship observed in the RESPIRE study (eg, Ezzati and Kammen 2001), but do not provide personal PM2.5 data.
6. Simulations
To incorporate uncertainty in both the exposure-response function and the exposure estimates at specific levels of interest, we used Monte Carlo simulations by drawing realizations for the exposure-response parameters and the exposures of interest from normal distributions. For the exposure-response parameters for birthweight and blood pressure, we assumed they were normally distributed with mean and standard deviation as reported in the literature. For the personal exposure level, the data in both Balakrishnan et al. (2014) and Rehfeuss et al. (2014) indicate that in most exposure studies the standard deviation is approximately equal to the mean, and hence we drew from normal distributions under these assumptions. We focused on personal exposures at 270 ug/m3, 120 ug/m3, 70 ug/m3, and 35 ug/m3, and 10 ug/m3 corresponding to plausible levels for unimproved stoves, improved stoves, gas stoves, the WHO interim target level, and the WHO recommended level (the referent).. About 15% of simulated exposure levels were below zero and discarded; hence in effect the exposures were drawn from a left-truncated normal distribution at zero. We conducted 10,000 simulations for blood pressure and birthweight. Simulations for pneumonia, in contrast, were based on the publicly available data from Burnett et al. (2014)(Global Health Exchange 2017), where 1000 Monte Carlo simulations of the three parameters in the IER model are provided. For each of these 1000 sets of parameter values, we drew an exposure as noted above, and then calculated the corresponding relative risk. We report the median, the 5th percentile, and the 95% percentile from our simulations for all three outcomes.
Results
1. Exposure-response estimates
Figures 1–3 show the estimated exposure-response curves for our three outcomes, based on data in the published literature.
Figure 3.
Exposure-response for childhood pneumonia from Burnett et al. (2014)
Table 1 provides simulation results for different exposure using the exposure-response coefficients from the literature, for selected exposure levels for all three outcomes, including our best estimates of traditional stove exposure (270 ug/m3), of an LPG intervention (70 ug/m3), as well as 120 ug/m3 (improved biomass cookstove). In Supplemental Table 1 we also provide results assuming a fixed exposure level for every 10 unit increment from 10 to 300 ug/m3.
Table 1.
Simulation results: expected outcomes at different levels of exposure for physician-diagnosed childhood pneumonia, systolic blood pressure, and birthweight
| PM2.5 ug/m3 | log PM2.5 | RR childhood pneumonia*, median, 2.5th and 97.5th percentile |
SBP (mm) women>50**, median, 2.5th and 97.5th percentile |
birthweight (grams)***, median, 2.5th and 97.5th percentile |
|---|---|---|---|---|
| 270 | 5.6 | 2.81 (1.23 –3.91) | 131.4 (120.4–142.6) | 2543 (1874, 3102 |
| 120 | 4.8 | 2.24 (1.06 – 3.51) | 128.1 (117.9–137.9) | 2770 (2306,3206) |
| 70 | 4.2 | 1.78 (1.05– 3.14) | 125.8 (115.9–134.7) | 2880 (2507, 3264) |
| 35 | 3.6 | 1.41 (1.01 –2.24) | 123.3 (113.2–130.4) | 3003 (2722, 3367) |
| 10 | 2.3 | 1.02 (1 – 1.07) | 118 | 3200 |
Burnett et al. 2014; reference group was exposed at 6 ug/m3
from Baumgartner et al. 2011, reference level of 118 mmHg taken from lower confidence level of mean SBP
from Wylie et al. 2016, Sun et al. 2016, Jedrychowski et al. 2004,, reference weight of 3200 grams taken from mean birthweight in Wylie et al.2017
As a sensitivity analysis for the birthweight data, we excluded the data from the meta-analysis of Sun et al. (2016), on the grounds that the data come from a meta-analysis of ambient air studies rather than indoor air studies, and were based on a model using untransformed PM2.5, rather than log PM2.5, used in the other two studies. The simulation results indicated somewhat lower median birthweights at each exposure level than when Sun et al. (2016) was included (Table 1). For example, results for 270 ug/m3 were a median birthweight of 2448 (5th% 1832, 95th% 2993), and for 70 ug/m3 were 2756 (5th% 2304, 95th% 3186). But the estimated benefit of a decrease of exposure from 270 ugm3 to 70 ug/m3 did not differ greatly. With Sun et al. in the analysis, the benefit was an increase of 338 grams, while without Sun et al. the increase was 308 grams.
2. Potential impacts on health
a) Blood pressure
Current evidence supports a causal link between PM2.5 and cardiovascular disease and stroke (Hoek et al. 2013), which in turn are also known to be related to higher blood pressure (James et al. 2014). PM2.5 effects on cardiovascular and cerebrovascular disease might be mediated by changes in blood pressure. To date, however, there is limited evidence of an effect of HAP on cardiovascular disease (Fatmi and Coggon 2016, Mitter et al. 2016, Weichenthal et al. 2017).
Based on our simulations, estimated PM2.5 exposures from biomass stoves (270 ug/m3) and LPG stoves (70 ug/m3) would result in estimated SBP among women >50 years of age of 131.4 and 126.0 mmHg, respectively (median difference 5.5, 2.5th% −8.6, 97.5th% 20.8). While the estimated decrease in SBP associated with lower levels of PM2.5 may appear modest, on a population level (assuming such reductions were sustainable) it would be expected to result in important estimated reductions in risks for ischemic heart disease and stroke. However, the 95% simulation interval is wide and includes the null value.
A recent randomized trial of blood pressure medication among older US adults at 102 clinics across the US and Puerto Rico resulted in a decrease in all-cause mortality of about 1.82% per mmHg reduction in SBP, so that gas stoves compared to traditional stoves might – in the long term and assuming the point estimate of a reduction of 5.5 is accurate – result in a 10% reduction in mortality (SPRINT group, 2015). Longitudinal analyses of NHANES data suggest an approximate 10% increase in overall mortality risk for every 10 mmHg increase in SBP for those over age 50, which would suggest about a 5.5% reduction in mortality for users of LPG stoves vs. traditional stoves over age 50 years (Taylor et al. 2011). In comparison, the IER curve for ischemic heart disease death from Burnett et al. (2014), indicates that for 70 year olds the RR for heart disease death would drop approximately 9% with a change in exposure from 270 ug/m3 to 70 ug/m3, a finding not dissimilar from the above estimates which were based on extrapolating from blood pressure to heart disease mortality.
It should be noted that the benefits of lowered SBP are likely to be seen even in populations with relative low SBP at baseline. In the SPRINT study referenced above, the protective effect of treatment was strongest in the treatment group which had the lowest BP at baseline (<132 mmHg, RR=0.70).
Increased heart disease is likely to due to PM2.5 from HAP in men as well as women. However, we lack personal PM2.5 measurements from HAP among men, as they generally do not cook and most data comes from women. Men would be expected to be exposed to less PM2.5 due to biomass burning than women, but still have appreciable exposures well above WHO recommended levels.
b) Birthweight
The combined data from Wylie et al. (2016), Sun et al. (2016), and Jedrychowski et al. (2004), indicate a median decrease in birth weight of those exposed to 70 ug/m3 (typical of LPG stoves), vs. 270 ug/m3 (typical of unimproved stoves), of 343 grams (2.5th% −344, 97.5th% 1114). Again, however the simulation interval includes the null value of 0.
Reduced birthweight is very strongly associated with high infant mortality, although the mechanisms are not well understood, and the association might not be causal (Wilcox 2001, Hernandez-Diaz et al. 2008). Reduced birthweight has also been associated with disease later in life (Gluckman et al. 2008). There is also some evidence of a direct effect of air pollution on infant mortality (Romieu et al. 2012, Bruce et al. 2013), although the data are sparse and it is not clear if that effect might be mediated by maternal factors and reduced birth weight. A direct causal effect of birthweight on infant mortality is made more plausible assuming some of those with reduced birthweight are presumably pre-term birth, given that there is evidence that both ambient PM2.5 (Malley et al. 2017) and HAP (Amegah et al. 2014), are associated with pre-term birth, and pre-term birth is strongly related to infant mortality. WHO estimates that about 12% of births in developing countries are pre-term and estimates that about 50% of infant mortality worldwide is due to pre-term birth (WHO 2012).
If we assume a causal effect of low birth weight on infant mortality, given that there is a drop of one unit (per 1000) in infant mortality for each 30 grams of birthweight (Wilcox, 2001), an improvement of 343 grams (LPG stoves vs traditional stoves, assuming the point estimate is accurate) would translate into an approximate drop in infant mortality of 11/1000 live births. To put this in perspective, the worldwide infant mortality rate in 2015 was 32/1000 (World Bank 2017).
A more conservative estimate of effect on infant mortality might consider that among low birthweight babies, only pre-term births affect infant mortality. Sun et al. (2015) have estimated the exposureresponse curve for PM2.5 and pre-term birth in a meta-analysis of 13 ambient air studies. They found a 1.13 increased odds of a pre-term birth for every 10 ug/m3 increment of PM2.5. While this assumption of a linear increase in log odds may hold true for the relatively low levels of PM2.5 in ambient air, it may not hold for higher levels of PM2.5, as noted by Malley et al. (2017), given the high exposure-response slopes at low levels followed by a plateau seen for the odds ratio for a variety of outcomes in relation to PM2.5. We have estimated a modified exposure-response curve from Sun et al. (2015) using log-transformed PM2.5, such that it crosses the original curve from Sun et al. at the 50 ug/m3 point, and then starts to trail off (an odds ratio of 2.00 for each log 10 units of PM2.5.)(see Supplemental Figure 1).
Using this modified exposure-response curve, and assuming a baseline rate of 7% pre-term birth at 10 ug/m3 based on European countries with low pre-term birth rates (WHO 2017), we find that an exposure of 270 ug/m3 (from traditional stoves) would be estimated to have a pre-term birth rate of 18.8%, compared to an estimated rate of 12.6% at 70 ug/m3 (a change of 6.2%). Assuming that 7.3% of preterm births die as infants (WHO 2012), out of 1000 live births, we would expect 13.7 (188*.073) deaths among those exposed at 270 ug/m3, versus 9.2 deaths among those exposed at 70 ug/m3, an estimated decrease in 4.5 deaths per 1000 live births.
c) Pneumonia
From the point of view of subsequent mortality, severe infant pneumonia cases are of primary interest, compared to all pneumonia. Severe infant cases are the most likely to die, with an estimated 9% mortality rate (Walker et al. 2013). The baseline severe infant pneumonia rate (physician diagnosed) was 260 cases per 1000 child years among the control group in the 2002–2004 RESPIRE study in Guatemala. However, in recent years the pneumococcal polysaccharide vaccine (PPV) has been introduced, lowering pneumonia rates (Berical et al. 2016). More recent data in rural areas of other low/middle income countries suggest that a current baseline rate of severe pneumonia in the first 2 years of life is likely to be closer to 50 to 60 cases per 1000 children (Farooqi et al. 2015, Mackenzie et al. 2014).
We have roughly assumed that those with the highest personal exposure (300 ug/m3) have a rate of 80 cases per 1000, and then used the relative risks from Burnett et al. (2014) to estimate the rate for lower levels of exposure. Table 2 shows the number of excess cases per 1000 child years with exposures above 10 ug/m3, using the rate ratios from Table 1.
Table 2.
Pneumonia cases expected per year among 1000 children, and excess cases compared to a level of 10 ug/m3 PM2.5*
| PM2.5 | RR** | Severe cases/yr |
|---|---|---|
| 300 | 2.88 | 80 |
| 270 | 2.81 (1.23 –3.91) | 78 (34–109) |
| 120 | 2.24 (1.06 – 3.51) | 62 (29–98) |
| 70 | 1.78 (1.05– 3.14) | 49 (29–87) |
| 35 | 1.41 (1.01 –2.24) | 39 (28–62) |
| 10* | 1.02 (1 – 1.07) | 28 (28–30) |
10 ug/m3 is the WHO recommended level
RRs from simulations, as per Table 1
From Table 2 we see that the estimated decrease in cases from an exposure of 270 ug/m3 (estimated exposure for traditional stove users) to an exposure of 70 ug/m3 (estimated exposure for LPG users) is 29 cases of severe pneumonia. Given the estimated 9% mortality rate (Walker et al. 2013), an LPG intervention would be expected to avoid about 2–3 deaths from infant pneumonia among per year among 1000 children.
Discussion
This study demonstrates the hypothetical impacts of LPG use on exposure and health. LPG use is increasing worldwide as households move up the energy ladder. The feasibility of LPG being adopted, in rural areas now using biomass, is increasing as gas prices are lowered. However, LPG still is more expensive than biomass in much of the world, making price an issue in adaptation. Countries like India and Peru have begun nationwide subsidization of LPG, which should have substantial impact.
We argue that use of LPG would have important health benefits compared to biomass stoves even if PM2.5 levels are not lowered to WHO standards, due to stove stacking and other exposure via ambient air.
Despite the uncertainty around the relationship between HAP exposure and health, and in accordance with others (Bruce et al. 2015, Johnson et al. 2015), our results provide supportive evidence that appreciable health gains can be achieved at reduced PM2.5 concentrations that are still higher than WHO recommend interim levels of exposure (35 ug/m3). At 70 ug/m3, using our best estimate of the expected personal PM2.5 exposure in homes with LPG stoves (based only a single study), our simulations indicate that there is an expected reduction of approximately 37% of infant pneumonia cases (avoiding 2–3 deaths annually per 1000 infants), an expected 5.5 mmHg reduction in SBP among older women, and an expected 338 gram increase in birthweight, compared to traditional unimproved stoves (270 ug/m3). Improvements in these three outcomes can be measured in the short-term in an intervention study. The reductions in SBP and the increases in birthweight would be expected to lead to later decreases in morbidity and mortality.
We have noted that the evidence suggests that age is a potential modifier of the expected blood pressure benefits to be achieved following a clean fuel cookstove intervention, with benefits accruing primarily to older women. Evidence from the ambient air pollution field suggests that other factors may also confer increased susceptibility to the adverse health effects of higher air pollution exposures (Clark and Peel 2014). Large robust interventions, which permit sub-group analyses, are needed to understand if these same susceptibility factors may also lead to larger health benefits following reductions in household air pollution. Potential modifying factors include poor nutrition, psychosocial stressors, other environmental pollutants, and comorbid conditions, all of which have been implicated in the adverse outcomes typically associated with HAP (Clark and Peel 2014).
There are two important sources of uncertainty in our estimates, which led us to use simulations to estimate health effects in order to incorporate both of them. One is the estimate of the exposure level resulting in our estimates of personal exposure levels from traditional biomass stoves and from LPG stoves, the latter based on only one study. We reflect this uncertainty by assuming that our estimates are drawn from normal distributions in which the standard deviation equals the mean, which reflects high uncertainty, and also reflects what has been observed in exposure studies. The high observed standard deviations of personal PM2.5 measurements in any given study stem from a number of factors, one of the most important being that only one or a small number of measurements are collected per person, so that between-person variability in a given study may reflect a lot of within-person-variability. For example, if measurements are collected over a period of time in different seasons, we know there will be marked variability due to season, with higher PM2.5 being present in the winter (Ni et al. 2016, Huang et al. 2017, Carter et al. 2016), and many subjects may have measurement from only one season. Similarly, cooking practices may differ one day to the next, and sparse measurements will reflect such variability. Compliance may also vary person-to-person. Besides such within-person variation within a given study, there is a very wide range of results across studies (Balakrishnan et al. 2014, Rehfeuss et al. 2014). This is also likely to reflect these same sources of variation – eg, if one study was conducted only in winter and another was conducted in summer.
The other important source of uncertainty in our results is that our exposure-response data are based on a small number of studies. Sample sizes for these studies were reasonable and confidence intervals relatively tight, but nonetheless confidence in results is limited by the small number of studies. For SBP, we used an exposure-response curve based on only a single study, albeit with good supporting evidence from other studies. For childhood pneumonia, the exposure-response data are largely from a single HAP study, supplemented by other studies of infant pneumonia in relation to ambient air and second-hand tobacco smoke. For birthweight, the exposure-response data are based on three studies. This reliance on only a few studies is due to the paucity of studies with PM2.5 personal exposure data used in exposure-response analyses. Consistency of effect needs to be demonstrated in other studies of HAP and additional settings, before strong inferences can be made that the associations observed are causal.
Of the two sources of uncertainty in our simulations, the uncertainty in exposure is much greater than the uncertainty due to exposure-response coefficients. While we have focused on the point estimates in the expected amount of improvement in health outcomes, our expected improvements include the null value of 0 for all outcomes. For example, the expected improvement in SBP due to lowering PM2.5 levels from 270 ug/m3 to 70 ug/m3 is 5.5 mmHg, with a 90% simulation interval of −6.2 to 18.0 mmHg. Without the uncertainty in exposure, the corresponding expected improvement is again 5.5 mgHg, but the 90% simulation is 2.5 to 8.4 mgHg, excluding the null. The width of this interval increases 4-fold after incorporating uncertainty regarding exposure.
Of note is that our estimate of the improvement of birth weight (338 gram increase) due to reduced exposure to PM2.5 is greater than the increase in birthweight estimated due to mothers stopping smoking (200 grams, US Surgeon General 1990), and 4–5 times greater than the effect due to exposure to second-hand smoke (60 grams, Salmasi et al. 2010). The increase in weight due to decreasing biomass PM2.5 exposure seems disproportionate as compared to the decrease due to stopping maternal smoking, given the presumed likely amounts of exposure to the fetus. However, the components of tobacco smoke vs. biomass burning may affect the fetus differently. There is some evidence from animal studies, for example, that biomass causes a stronger inflammatory response in the lung than cigarette smoke (Mehra et al. 2012); similar seemingly disproportionate effects might be seen for the fetus.
We have noted the high variability in estimates of personal exposure in any given study. In attempting to assess a long term average, which may be the exposure of interest for many outccomes, measurement error is inevitable in due to the few samples obtained from individuals. For some outcomes (eg., birth outcomes) we do not know the proper time window for the most relevant exposure. Such error in the measurement of personal exposure is likely to be of the classical type, would be expected to be non-differential, and would therefore be expected to bias exposure-response coefficients to the null. Beside measurement error for exposure, there may be error in measuring outcome; for example ascertainment of severe infant pneumonia is not straightforward.
Furthermore, PM2.5 may in fact not be the most appropriate summary measure of HAP’s impact on health. We need further exposure-response data in HAP settings which include assessment of other contaminants (eg, PAHs, NO2, black carbon, CO)(Naeher et al. 2007). Even if PM2.5 is the best measure of HAP exposure (most predictive in relation to health), it would be useful to have PM2.5 analyses that include data on composition and source apportionment; composition and sources of PM2.5 vary by setting, and thus toxicity, mutagenicity, and oxidative potential may also vary.
Other sources of uncertainty include possible biases in observational studies due to confounding or selection bias. One cause of concern in relation to observational studies is possible bias due to socioeconomic status, which may be associated with clean fuel use and lower PM2.5 exposure. Regarding the studies used here, however, confounding by SES seems relatively unlikely. The studies used here adjusted for SES in their analyses, often via education, although residual confounding is still possible. The studies used here for the most part included populations which were quite homogenous, e.g., all with biomass stoves (i.e., comparing effects across a range of biomass-related PM2.5 concentrations), or intervention studies where the improved or clean fuel stove was disseminated among biomass stove users; this homogeneity is another factor likely to limit the type of confounding often present in studies observationally comparing users of different stove types or fuels.
In conclusion, despite all the uncertainties noted above that are inherent in the data, existing evidence indicates that substantial exposure reductions and health gains can be expected from an LPG intervention.
Supplementary Material
Figure 2.
Exposure-response for birthweight, weighted average of Wylie et al (2017), Sun et al. (2016), and Jedrychowski et al. (2004)
Highlights.
Improved biomass and advanced fuel cookstoves can lower household air pollution
Yet levels of fine particulate matter (PM2.5) often remain above the WHO targets
We conducted simulations estimating health benefits from a gas stove intervention
We estimate marked health benefits for birthweight, blood pressure, and pneumonia
There are large uncertainties in our estimates, driven by uncertainty over exposure
Acknowledgments
We thank Kalpana Balakrishnan for her help in finding relevant literature on exposure.
Grant support: This work was supported by an NIH grant for an upcoming trial of LPG stoves (trial registration NCT02944682)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflict of interest.
References
- Alexander D, Northcross A, Wilson N, Dutta A, Pandya R, Ibigbami T, Adu D, Olamijulo J, Morhason-Bello O, Karrison T, Ojengbede O, Olopade CO. Randomized Controlled Ethanol Cookstove Intervention and Blood Pressure in Pregnant Nigerian Women. Am J Respir Crit Care Med. 2017 Jan 12; doi: 10.1164/rccm.201606-1177OC. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Amegah AK, Quansah R, Jaakkola JJ. Household air pollution from solid fuel use and risk of adverse pregnancy outcomes: a systematic review and meta-analysis of the empirical evidence. PLoS One. 2014 Dec 2;9(12):e113920. doi: 10.1371/journal.pone.0113920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan K, Mehta S, Ghosh S, Johnson M, Brauer B, Zhang J, Naeher L, Smith K. Review 5: Population levels of household air pollution and exposures, WHO Indoor Air Quality Guidelines. Household Fuel Combustion; WHO, Geneva: 2014. [Google Scholar]
- Bates L, et al. Smoke, health, and household energy. [both last accessed Sept 21. 2017];1 https://practicalaction.org/smoke-health-and-household-energy, and Annex 18, https://practicalaction.org/smoke-health-and-household-energy. [Google Scholar]
- Baumgartner J, Schauer JJ, Ezzati M, Lu L, Cheng C, Patz JA, Bautista LE. Indoor air pollution and blood pressure in adult women living in rural China. Environ Health Perspect. 2011 Oct;119(10):1390–5. doi: 10.1289/ehp.1003371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berical AC, Harris D, Dela Cruz CS, Possick JD. Pneumococcal Vaccination Strategies. An Update and Perspective. Ann Am Thorac Soc. 2016 Jun;13(6):933–44. doi: 10.1513/AnnalsATS.201511-778FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BP Global, Natural gas prices. [last accessed May 8, 2017]; http://www.bp.com/en/global/corporate/energy-economics/statistical-review-of-world-energy/natural-gas/natural-gas-prices.html.
- Brook RD, Bard RL, Burnett RT, Shin HH, Vette A, Croghan C, Phillips M, Rodes C, Thornburg J, Williams R. Differences in blood pressure and vascular responses associated with ambient fine particulate matter exposures measured at the personal versus community level. Occup Environ Med. 2011 Mar;68(3):224–30. doi: 10.1136/oem.2009.053991. [DOI] [PubMed] [Google Scholar]
- Bruce N, Pope D, Rehfuess E, Balakrishnan K, Adair-Rohani H, Dora C. WHO indoor air quality guidelines on household fuel combustion: Strategy implications of new evidence on interventions and exposure–risk functions. Atmospheric Environment. 2015;106:451–457. [Google Scholar]
- Bruce NG, Dherani MK, Das JK, Balakrishnan K, Adair-Rohani H, Bhutta ZA, Pope D. Control of household air pollution for child survival: estimates for intervention impacts. BMC Public Health. 2013;13(Suppl 3):S8. doi: 10.1186/1471-2458-13-S3-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett RT, Pope CA, 3rd, Ezzati M, Olives C, Lim SS, Mehta S, Shin HH, Singh G, Hubbell B, Brauer M, Anderson HR, Smith KR, Balmes JR, Bruce NG, Kan H, Laden F, Prüss-Ustün A, Turner MC, Gapstur SM, Diver WR, Cohen A. An integrated risk function for estimating the global burden of disease attributable to ambient fine particulate matter exposure. Environ Health Perspect. 2014 Apr;122(4):397–403. doi: 10.1289/ehp.1307049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter E, Archer-Nicholls S, Ni K, Lai AM, Niu H, Secrest MH, Sauer SM, Schauer JJ, Ezzati M, Wiedinmyer C, Yang X, Baumgartner J. Seasonal and Diurnal Air Pollution from Residential Cooking and Space Heating in the Eastern Tibetan Plateau. Environ Sci Technol. 2016 Aug 2;50(15):8353–61. doi: 10.1021/acs.est.6b00082. [DOI] [PubMed] [Google Scholar]
- Clark ML, Bazemore H, Reynolds SJ, Heiderscheidt JM, Conway S, Bachand AM, Volckens J, Peel JL. A baseline evaluation of traditional cook stove smoke exposures and indicators of cardiovascular and respiratory health among Nicaraguan women. Int J Occup Environ Health. 2011 Apr-Jun;17(2):113–21. doi: 10.1179/107735211799030942. [DOI] [PubMed] [Google Scholar]
- Clark ML, Peel JL, Balakrishnan K, Breysse PN, Chillrud SN, Naeher LP, Rodes CE, Vette AF, Balbus JM. Health and household air pollution from solid fuel use: the need for improved exposure assessment. Environ Health Perspect. 2013a Oct;121(10):1120–8. doi: 10.1289/ehp.1206429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark ML, Bachand AM, Heiderscheidt JM, Yoder SA, Luna B, Volckens J, Koehler KA, Conway S, Reynolds SJ, Peel JL. Impact of a cleaner-burning cookstove intervention on blood pressure in Nicaraguan women. Indoor Air. 2013b Apr;23(2):105–14. doi: 10.1111/ina.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M, Peel J. Perspective in household air pollution research: who will benefit from interventions. Curr Envir Health Rpt. 2014;1:25–257. [Google Scholar]
- Ezzati M, Kammen D. Indoor air pollution from biomass combustion and acute respiratory infections in Kenya: an exposure-response study. Lancet. 2001 Aug 25;358(9282):619–24. doi: 10.1016/s0140-6736(01)05777-4. [DOI] [PubMed] [Google Scholar]
- Farooqui H, Jit M, Heymann DL, Zodpey S. Burden of Severe Pneumonia, Pneumococcal Pneumonia and Pneumonia Deaths in Indian States: Modelling Based Estimates. PLoS One. 2015 Jun 18;10(6):e0129191. doi: 10.1371/journal.pone.0129191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatmi Z, Coggon D. Coronary heart disease and household air pollution from use of solid fuel: a systematic review. Br Med Bull. 2016 Jun;118(1):91–109. doi: 10.1093/bmb/ldw015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Health Data Exchange. [last accessed Sept 11, 2017]; http://ghdx.healthdata.org/record/global-burden-disease-study-2010-gbd-2010-ambient-air-pollution-risk-model-1990-2010.
- Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008 Jul 3;359(1):61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAP (Household Air Pollution and Pregnancy), trial registration NCT02394574. [last accessed May 6, 2017]; https://clinicaltrials.gov/ct2/show/NCT02394574.
- HAPIN (Household Air Pollution Intervention), trial registration NCT0294468. [last accessed May 6, 2017]; https://projectreporter.nih.gov/project_info_description.cfm?aid=9207315&icde=31240699&ddparam=&ddvalue=&ddsub=&cr=1&csb=default&cs=ASC)
- Hartinger SM, Commodore AA, Hattendorf J, Lanata CF, Gil AI, Verastegui H, Aguilar-Villalobos M, Mäusezahl D, Naeher LP. Chimney stoves modestly improved indoor air quality measurements compared with traditional open fire stoves: results from a small-scale intervention study in rural Peru. Indoor Air. 2013 Aug;23(4):342–52. doi: 10.1111/ina.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek G, Krishnan RM, Beelen R, et al. Long-term air pollution exposure and cardio- respiratory mortality: a review. Environmental Health. 2013;12:43. doi: 10.1186/1476-069X-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermida RC, Ayala DE, Mojón A, Fernández JR, Alonso I, Silva I, Ucieda R, Iglesias M. Blood pressure patterns in normal pregnancy, gestational hypertension, and preeclampsia. Hypertension. 2000 Aug;36(2):149–58. doi: 10.1161/01.hyp.36.2.149. [DOI] [PubMed] [Google Scholar]
- Hernández-Díaz S, Wilcox AJ, Schisterman EF, Hernán MA. From causal diagrams to birth weight-specific curves of infant mortality. Eur J Epidemiol. 2008;23(3):163–6. doi: 10.1007/s10654-007-9220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Downward GS, Reiss B, Xu J, Bassig BA, Hosgood HD, 3rd, Zhang L, Seow WJ, Wu G, Chapman RS, Tian L, Wei F, Vermeulen R, Lan Q. Personal and indoor PM2.5 exposure from burning solid fuels in vented and unvented stoves in a rural region of China with a high incidence of lung cancer. Environ Sci Technol. 2014;48(15):8456–64. doi: 10.1021/es502201s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Du W, Chen Y, Shen G, Su S, Lin N, Shen H, Zhu D, Yuan C, Duan Y, Liu J, Li B, Tao S. Household air pollution and personal inhalation exposure to particles (TSP/PM(2.5)/PM(1.0)/PM(0.25)) in rural Shanxi, North China. Environ Pollut. 2017 Aug 25;231(Pt 1):635–643. doi: 10.1016/j.envpol.2017.08.063. [DOI] [PubMed] [Google Scholar]
- Jack DW, Asante KP, Wylie BJ, Chillrud SN, Whyatt RM, Ae-Ngibise KA, Quinn AK, Yawson AK, Boamah EA, Agyei O, Mujtaba M, Kaali S, Kinney P, Owusu-Agyei S. Ghana randomized air pollution and health study (GRAPHS): study protocol for a randomized controlled trial. Trials. 2015 Sep 22;16:420. doi: 10.1186/s13063-015-0930-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC, Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT, Jr, Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014 Feb 5;311(5):507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- Jedrychowski W, Bendkowska I, Flak E, Penar A, Jacek R, Kaim I, Spengler JD, Camann D, Perera FP. Estimated risk for altered fetal growth resulting from exposure to fine particles during pregnancy: an epidemiologic prospective cohort study in Poland. Environ Health Perspect. 2004 Oct;112(14):1398–402. doi: 10.1289/ehp.7065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MA, Chiang RA. Quantitative Guidance for Stove Usage and Performance to Achieve Health and Environmental Targets. Environ Health Perspect. 2015 Aug;123(8):820–6. doi: 10.1289/ehp.1408681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang R, Zhang B, Zhao X, Ruan Y, Lian H, Fan Z. Effect of exposure to PM2.5 on blood pressure: a systematic review and meta-analysis. J Hypertens. 2014 Nov;32(11):2130–40. doi: 10.1097/HJH.0000000000000342. [DOI] [PubMed] [Google Scholar]
- Lin H, Guo Y, Zheng Y, Di Q, Liu T, Xiao J, Li X, Zeng W, Cummings-Vaughn LA, Howard SW, Vaughn MG, Qian ZM, Ma W, Wu F. Long-Term Effects of Ambient PM(2.5) on Hypertension and Blood Pressure and Attributable Risk Among Older Chinese Adults. Hypertension. 2017 May;69(5):806–812. doi: 10.1161/HYPERTENSIONAHA.116.08839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie GA, Bottomley C, van Hoek AJ, Jeffries D, Ota M, Zaman SM, Greenwood B, Cutts F. Efficacy of different pneumococcal conjugate vaccine schedules against pneumonia, hospitalisation, and mortality: re-analysis of a randomised trial in the Gambia. Vaccine. 2014 May 1;32(21):2493–500. doi: 10.1016/j.vaccine.2014.02.081. [DOI] [PubMed] [Google Scholar]
- Mehra D, Geraghty PM, Hardigan AA, Foronjy R. A comparison of the inflammatory and proteolytic effects of dung biomass and cigarette smoke exposure in the lung. PLoS One. 2012;7(12):e52889. doi: 10.1371/journal.pone.0052889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni K, Carter E, Schauer JJ, Ezzati M, Zhang Y, Niu H, Lai AM, Shan M, Wang Y, Yang X, Baumgartner J. Seasonal variation in outdoor, indoor, and personal air pollution exposures of women using wood stoves in the Tibetan Plateau: Baseline assessment for an energy intervention study. Environ Int. 2016 Sep;94:449–457. doi: 10.1016/j.envint.2016.05.029. [DOI] [PubMed] [Google Scholar]
- Lewis JJ, Hollingsworth JW, Chartier RT, Cooper EM, Foster WM, Gomes GL, Kussin PS, MacInnis JJ, Padhi BK, Panigrahi P, Rodes CE, Ryde IT, Singha AK, Stapleton HM, Thornburg J, Young CJ, Meyer JN, Pattanayak SK. Biogas Stoves Reduce Firewood Use, Household Air Pollution, and Hospital Visits in Odisha, India. Environ Sci Technol. 2017 Jan 3;51(1):560–569. doi: 10.1021/acs.est.6b02466. [DOI] [PubMed] [Google Scholar]
- Malley CS, Kuylenstierna JC, Vallack HW, Henze DK, Blencowe H, Ashmore MR. Preterm birth associated with maternal fine particulate matter exposure: A global, regional and national assessment. Environ Int. 2017 Feb 10; doi: 10.1016/j.envint.2017.01.023. pii: S0160-4120(16)30599-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- McCracken JP, Smith KR, Díaz A, Mittleman MA, Schwartz J. Chimney stove intervention to reduce long-term wood smoke exposure lowers blood pressure among Guatemalan women. Environ Health Perspect. 2007 Jul;115(7):996–1001. doi: 10.1289/ehp.9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitter SS, Vedanthan R, Islami F, Pourshams A, Khademi H, Kamangar F, Abnet CC, Dawsey SM, Pharoah PD, Brennan P, Fuster V, Boffetta P, Malekzadeh R. Household Fuel Use and Cardiovascular Disease Mortality: Golestan Cohort Study. Circulation. 2016 Jun 14;133(24):2360–9. doi: 10.1161/CIRCULATIONAHA.115.020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer K, Ndamala CB, Naunje AW, Malava J, Katundu C, Weston W, Havens D, Pope D, Bruce NG, Nyirenda M, Wang D, Crampin A, Grigg J, Balmes J, Gordon SB. A cleaner burning biomass-fuelled cookstove intervention to prevent pneumonia in children under 5 years old in rural Malawi (the Cooking and Pneumonia Study): a cluster randomised controlled trial. Lancet. 2016 Dec 6; doi: 10.1016/S0140-6736(16)32507-7. pii: S0140-6736(16)32507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeher LP, Brauer M, Lipsett M, Zelikoff JT, Simpson CD, Koenig JQ, Smith KR. Woodsmoke health effects: a review. Inhal Toxicol. 2007 Jan;19(1):67–106. doi: 10.1080/08958370600985875. [DOI] [PubMed] [Google Scholar]
- Northcross A, Chowdhury Z, McCracken J, Canuz E, Smith KR. Estimating personal PM2.5 exposures using CO measurements in Guatemalan households cooking with wood fuel. J Environ Monit. 2010 Apr;12(4):873–8. doi: 10.1039/b916068j. [DOI] [PubMed] [Google Scholar]
- Pinto E. Blood pressure and ageing. Postgrad Med J. 2007 Feb;83(976):109–14. doi: 10.1136/pgmj.2006.048371. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA, 3rd, Burnett RT, Turner MC, Cohen A, Krewski D, Jerrett M, Gapstur SM, Thun MJ. Lung cancer and cardiovascular disease mortality associated with ambient air pollution and cigarette smoke: shape of the exposure-response relationships. Environ Health Perspect. 2011 Nov;119(11):1616–21. doi: 10.1289/ehp.1103639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope D, Bruce N, Dherani M, Jogoe K, Rehfuess E. Real-life effectiveness of ‘improved’ stoves and clean fuels in reducing PM2.5 and CO; systematic review and meta-analysis. Env Int. 2017;101:7–18. doi: 10.1016/j.envint.2017.01.012. [DOI] [PubMed] [Google Scholar]
- Quansah R, Semple S, Ochieng CA, Juvekar S, Armah FA, Luginaah I, Emina J. Effectiveness of interventions to reduce household air pollution and/or improve health in homes using solid fuel in low-and-middle income countries: A systematic review and meta-analysis. Environ Int. 2017 Mar 21; doi: 10.1016/j.envint.2017.03.010. pii: S0160-4120(17)30470-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Quinn AK, Ae-Ngibise KA, Jack DW, Boamah EA, Enuameh Y, Mujtaba MN, Chillrud SN, Wylie BJ, Owusu-Agyei S, Kinney PL, Asante KP. Association of Carbon Monoxide exposure with blood pressure among pregnant women in rural Ghana: Evidence from GRAPHS. Int J Hyg Environ Health. 2016 Mar;219(2):176–83. doi: 10.1016/j.ijheh.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfuess E, Pope D, Bruce N, Dherani M, Jagoe K, Naeher L, Noonan C. WHO Indoor Air Quality Guidelines: Household Fuel Combustion. WHO; Geneva: 2014. Review 6: Impacts of interventions on household air pollution concentrations and personal exposure. [Google Scholar]
- Romieu I, Riojas-Rodríguez H, Marrón-Mares AT, Schilmann A, Perez-Padilla R, Masera O. Improved biomass stove intervention in rural Mexico: impact on the respiratory health of women. Am J Respir Crit Care Med. 2009 Oct 1;180(7):649–56. doi: 10.1164/rccm.200810-1556OC. [DOI] [PubMed] [Google Scholar]
- Romieu I, Gouveia N, Cifuentes LA, de Leon AP, Junger W, Vera J, Strappa V, Hurtado-Díaz M, Miranda-Soberanis V, Rojas-Bracho L, Carbajal-Arroyo L, Tzintzun-Cervantes G. HEI Health Review Committee.. Multicity study of air pollution and mortality in Latin America (the ESCALA study) Res Rep Health Eff Inst. 2012 Oct;(171):5–86. [PubMed] [Google Scholar]
- Salmasi G, Grady R, Jones J, McDonald SD Knowledge Synthesis Group. Environmental tobacco smoke exposure and perinatal outcomes: a systematic review and meta-analyses. Acta Obstet Gynecol Scand. 2010;89(4):423–41. doi: 10.3109/00016340903505748. [DOI] [PubMed] [Google Scholar]
- Smith KR, Peel JL. Mind the gap. Environ Health Perspect. 2010 Dec;118(12):1643–5. doi: 10.1289/ehp.1002517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, McCracken JP, Weber MW, Hubbard A, Jenny A, Thompson LM, Balmes J, Diaz A, Arana B, Bruce N. Effect of reduction in household air pollution on childhood pneumonia in Guatemala (RESPIRE): a randomised controlled trial. Lancet. 2011 Nov 12;378(9804):1717–26. doi: 10.1016/S0140-6736(11)60921-5. [DOI] [PubMed] [Google Scholar]
- Smith-Sivertsen T, Díaz E, Pope D, Lie RT, Díaz A, McCracken J, Bakke P, Arana B, Smith KR, Bruce N. Effect of reducing indoor air pollution on women's respiratory symptoms and lung function: the RESPIRE Randomized Trial, Guatemala. Am J Epidemiol. 2009 Jul 15;170(2):211–20. doi: 10.1093/aje/kwp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPRINT Research Group. Wright JT, Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC, Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015 Nov 26;373(22):2103–16. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Luo X, Zhao C, Chung NgRW, Lim CE, Zhang B, Liu T. The association between fine particulate matter exposure during pregnancy and preterm birth: a meta-analysis. BMC Pregnancy Childbirth. 2015 Nov 18;15:300. doi: 10.1186/s12884-015-0738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Luo X, Zhao C, Zhang B, Tao J, Yang Z, Ma W, Liu T. The associations between birth weight and exposure to fine particulate matter (PM2.5) and its chemical constituents during pregnancy: A meta-analysis. Environ Pollut. 2016 Apr;211:38–47. doi: 10.1016/j.envpol.2015.12.022. [DOI] [PubMed] [Google Scholar]
- US Surgeon General. The Health Benefits of Smoking Cessation. US Health and Human Serivice; Rockville, Maryland: 1990. pp. 367–423. [Google Scholar]
- Taylor BC, Wilt TJ, Welch HG. Impact of diastolic and systolic blood pressure on mortality: implications for the definition of "normal". J Gen Intern Med. 2011 Jul;26(7):685–90. doi: 10.1007/s11606-011-1660-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LM, Bruce N, Eskenazi B, Diaz A, Pope D, Smith KR. Impact of reduced maternal exposures to wood smoke from an introduced chimney stove on newborn birth weight in rural Guatemala. Environ Health Perspect. 2011 Oct;119(10):1489–94. doi: 10.1289/ehp.1002928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tielsch JM, Katz J, Zeger SL, Khatry SK, Shrestha L, Breysse P, Checkley W, Mullany LC, LeClerq SC. Designs of two randomized, community-based trials to assess the impact of alternative cookstove installation on respiratory illness among young children and reproductive outcomes in rural Nepal. BMC Public Health. 2014 Dec 15;14:1271. doi: 10.1186/1471-2458-14-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CL, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, O'Brien KL, Campbell H, Black RE. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013 Apr 20;381(9875):1405–16. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichenthal S, Kulka R, Lavigne E, van Rijswijk D, Brauer M, Villeneuve PJ, Stieb D, Joseph L, Burnett RT. Biomass Burning as a Source of Ambient Fine Particulate Air Pollution and Acute Myocardial Infarction. Epidemiology. 2017 May;28(3):329–337. doi: 10.1097/EDE.0000000000000636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox AJ. On the importance--and the unimportance--of birthweight. Int J Epidemiol. 2001 Dec;30(6):1233–41. doi: 10.1093/ije/30.6.1233. [DOI] [PubMed] [Google Scholar]
- WHO. Guidelines for indoor air quality: household fuel combustion. Geneva: 2014. [PubMed] [Google Scholar]
- WHO. Born too soon: the Global Action Report on Pre-term Birth. Geneva: 2012. [Google Scholar]
- WHO. [last accessed May 6, 2017];2017 http://www.euro.who.int/__data/assets/pdf_file/0004/277735/Born-too-soon_preterm-birth-in-Europe-trends,-causes-and-prevention.pdf?ua=1.
- World Bank. [last accessed May 6, 2017];2017 http://data.worldbank.org/indicator/SP.DYN.IMRT.IN.
- Wylie BJ, Kishashu Y, Matechi E, Zhou Z, Coull B, Abioye AI, Dionisio KL, Mugusi F, Premji Z, Fawzi W, Hauser R, Ezzati M. Maternal exposure to carbon monoxide and fine particulate matter during pregnancy in an urban Tanzanian cohort. Indoor Air. 2017 Jan;27(1):136–146. doi: 10.1111/ina.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip F, Christensen B, Sircar K, Naeher L, Bruce N, Pennise D, Lozier M, Pilishvili T, Loo Farrar J, Stanistreet D, Nyagol R, Muoki J, de Beer L, Sage M, Kapil V. Assessment of traditional and improved stove use on household air pollution and personal exposures in rural western Kenya. Environ Int. 2017 Feb;99:185–191. doi: 10.1016/j.envint.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitlin J, Szamotulska K, Drewniak N, Mohangoo AD, Chalmers J, Sakkeus L, Irgens L, Gatt M, Gissler M, Blondel B Euro-Peristat Preterm Study Group. Preterm birth time trends in Europe: a study of 19 countries. BJOG. 2013 Oct;120(11):1356–65. doi: 10.1111/1471-0528.12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.