Abstract
Posttraumatic stress disorder (PTSD) is a major psychiatric disorder that is prevalent in combat veterans. Previous neuroimaging studies have found elevated amygdala activity in PTSD in response to threatening stimuli, but previous work has lacked the temporal specificity to study fast bottom-up fear responses involving the amygdala. Forty-four combat veterans, 28 with PTSD and 16 without, completed psychological testing and then a face-processing task during magnetoencephalography (MEG). The resulting MEG data were pre-processed, transformed into the time-frequency domain, and then imaged using a beamforming approach. We found that veterans with PTSD exhibited significantly stronger oscillatory activity from 50–450 ms in the left amygdala compared to veterans without PTSD while processing threatening faces. This group difference was not present while viewing neutral faces. The current study shows that amygdala hyperactivity in response to threatening cues begins quickly in PTSD, which makes theoretical sense as an adaptive bottom-up fear response.
Keywords: posttraumatic stress, magnetoencephalography, fear, bottom-up: oscillatory activity, military
1. Introduction
Neuroimaging findings in posttraumatic stress disorder (PTSD; APA, 2013) indicate elevated amygdala activity, often in conjunction with inadequate prefrontal cortex modulation of such limbic hyperactivity (Etkin & Wager, 2007; Hayes et al., 2012; Koch et al., 2016; Patel et al., 2012). However, this literature typically utilizes neuroimaging tools (e.g. fMRI, PET) that are unable to measure fronto-limbic activations with high temporal specificity, preventing strong conclusions about the underlying time course. Recent research has focused generally on fast fear pathways (Diano et al., 2017; Méndez-Bértolo et al., 2016), but research about fast amygdala reactivity in PTSD is needed.
Automatic processing of threat-related cues is linked to threat reactivity responses in PTSD (Lanius et al., 2017). Interestingly, emotionally-neutral stimuli seem to require selective attention for processing, while emotionally-laden stimuli may be less dependent on attentional resources (Vuilleumier, 2005) and more rooted in amygdala responsivity (Diano et al., 2017). Magnetoencephalography (MEG) has excellent temporal specificity, and previous MEG studies using healthy participants have found early amygdala activation in response to emotional faces (Garrido et al., 2012; Garvert et al., 2014; Luo et al., 2007, 2010), consistent with face processing studies that used intracranial recordings (Hesse et al., 2016; Mendez-Bertolo et al., 2016; Pourtois et al., 2010; Sato et al., 2011). Substantial evidence supports the capability of MEG to detect neural activity in deep brain structures (Badura-Brack et al., 2017; Cornwell et al., 2012a, 2012b, 2014; Dalal et al., 2008; McDermott et al., 2016; Proskovec et al., 2016; Pu et al., 2017; Salvadore et al., 2009, 2010; Wilson et al., 2009, 2010, 2011, 2017). One such MEG study used a seed-based functional connectivity approach found that veterans with PTSD had increased functional connectivity relative to veterans without PTSD between the amygdala and ventromedial prefrontal cortex when viewing threatening faces (Dunkley et al., 2016); however, this study did not examine the time course or amplitude or amygdala responses, and thus such data remains unavailable in patients with PTSD.
We used a face-processing paradigm involving angry and neutral faces, as faces are known to elicit strong emotional reactivity (de Gelder et al., 2006; Johnson, 2005). Threatening expressions are a key primitive threat signal, which likely provoke an evolutionary alarm response (Tamietto & de Gelder, 2010). This innate alarm system has been of interest in PTSD (Lanius et al., 2017), with the amygdala being central to an automatic response to threat (e.g. fight or flight) and symptoms of PTSD (LeDoux & Pine, 2016). Given that PTSD is associated with neurocognitive deficits including speed of information processing and attention/working memory (see Scott et al., 2015), we expected the PTSD group to have slower reaction times than veterans without PTSD. Based on previous research, we hypothesized that amygdala activity would be present and occur shortly after stimulus onset in threat trials in both groups, and specifically that amygdala activity would be significantly stronger in combat veterans with PTSD as compared to those without PTSD.
2. Materials and methods
2.1. Participants
Twenty-eight male combat veterans with PTSD and 16 male combat veterans without PTSD participated in this study. All veterans served in Iraq or Afghanistan between 2003 and 2014, and were assessed using the Clinician Administered PTSD Scale (CAPS; Blake et al., 1995). The two groups of men were matched on age (PTSD: M=33.50, SD = 9.00; no-PTSD: M = 33.56, SD= 8.02), education (PTSD: M=14.71, SD = 2.21; no-PTSD: M= 14.27, SD= 1.94), race (86%; 88% Caucasian), and handedness (all right-handed). Veterans diagnosed with PTSD had significantly higher (p > 0.001) CAPS scores (M = 71.00, SD = 17.20 than those without PTSD (M = 23.5, SD = 12.90). Exclusion criteria were medical diagnoses affecting central nervous system function, brain neoplasm or lesion, significant head trauma, current substance dependence and ferromagnetic implants. Veterans on medications were not excluded, and 32% of the PTSD group and 19% of those without PTSD were taking stable dosage of an SSRI, Xanax, or mood stabilizer. Written informed consent was obtained following the ethical guidelines of the Creighton University Institutional Review Board.
2.2. Experimental paradigm
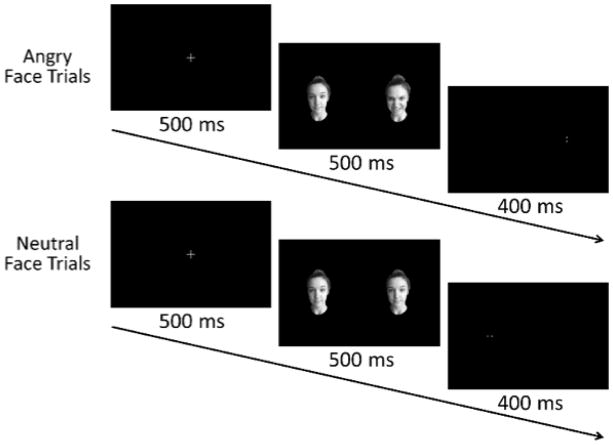
All participants completed a face-processing task (Britton et al., 2012) while seated inside the MEG chamber. Trials began with a fixation cross presented for 500 ms, followed by the presentation of a face pair for 500 ms. The stimuli varied between one angry and one neutral face (threat) or two neutral faces (neutral; Figure 1). Participants were instructed to respond with their right index or middle finger based on the location of a target that appeared in the space vacated by one of the faces. The target duration was 400 ms and a blank screen was presented between trials for an interval of 1250–1350 ms. Accuracy and response times were recorded.
Figure 1.
Facial presentation task flow.
2.3. MEG Data Acquisition, Pre-Processing & Source Reconstruction
Neuromagnetic responses were sampled continuously at 1 kHz using an acquisition bandwidth of 0.1–330 Hz and Elekta system with 306 magnetic sensors (Helsinki, Finland). All MEG data were subjected to noise reduction using the signal space separation method with a temporal extension (tSSS; Taulu and Simola, 2006), coregistered with structural MRI, and transformed into standard space after beamforming (see below).
Cardiac artifacts were removed using signal-space projection (SSP; Uusitalo and Ilmoniemi, 1997). The continuous magnetic time series was divided into epochs of 2700 ms duration (−500 to 2200 ms), with 0.0 s defined as stimulus onset (i.e., faces) and the baseline defined as the −500 to −100 ms time window. Epochs containing artifacts were rejected based on a fixed threshold method, supplemented with visual inspection. Artifact-free epochs were transformed into the time-frequency domain using complex demodulation and the resulting spectral power estimations per sensor were averaged over trials to generate time-frequency plots of mean spectral density, and then normalized using the mean power during the baseline period. This revealed a broadband (4–40 Hz) oscillatory response in many MEG sensors that started shortly after stimulus onset (50 ms) and continued through the end of the face stimulus presentation period (450 ms).
Data were then imaged (4–40 Hz, 50–450 ms) using an extension of the linearly constrained minimum variance vector beamformer (Gross et al., 2001). The resulting 3-dimensional maps of functional brain activity were 4.0 × 4.0 × 4.0 mm resolution and were statistically evaluated using a mass univariate approach based on the general linear model. All statistical maps were displayed as a function of α level, thresholded at p < 0.05, and adjusted for multiple comparisons using a spatial extent threshold (80 contiguous voxels). MEG preprocessing and imaging used the Brain Electrical Source Analysis (BESA version 6.1) software.
3. Results
Average accuracy in the task was 94.94% (SD: 4.61%) in the threatening condition and 95.36% (SD: 5.23%) in the neutral condition, and there were no group differences in either condition (ps > 0.23). Reaction time differed significantly on the Mann-Whitney U test between the groups for threatening (p = 0.013) and neutral (p = 0.022) conditions. Veterans with PTSD responded more slowly (neutral: M = 816.31ms, SD = 135.21; threat: M = 822.98ms, SD= 140.46) than their non-PTSD peers (neutral: M = 723.03ms, SD = 96.92; threat M = 721.62ms, SD = 101.14) in both conditions. Neither group showed a significant difference between conditions for accuracy or reaction time.
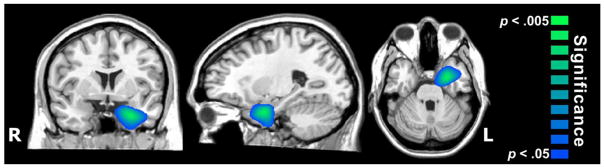
The MEG group comparisons showed that during threatening stimuli (50–450 ms), veterans with PTSD had significantly stronger oscillatory activity in the left amygdala (peak coordinate: −22, −1, −23; Tzourio-Mazoyer et al., 2002) compared to veterans without PTSD (p < .05, corrected; Figure 2). Follow-up analyses at the voxel level suggested that this amygdala response was primarily in the 6–10 Hz band from 100–300 ms. No neurophysiological differences were found between veterans with and without PTSD during neutral face processing.
Figure 2.
Veterans with PTSD exhibited stronger oscillations in the left amygdala relative to veterans without PTSD. Image has been thresholded at p < 0.05, corrected.
4. Discussion
This study utilized the excellent temporal specificity of MEG in the context of threatening visual stimuli and PTSD. The key finding was stronger left amygdala oscillations in veterans with PTSD relative to those without PTSD during the processing of threatening, but not neutral faces. The left amygdala was the only brain region to show group MEG differences during threatening faces, and there were no group differences during neutral face processing. Thus, our amygdala findings were specific to threatening faces and argue against a generalized hyperactive amygdala in PTSD.
Preferential left amygdala activation is common in response to fearful and threatening facial stimuli as opposed to neutral faces in PET (Morris et al., 1996, 1998a, 1998b) and fMRI (Breiter et al., 1996; Carlson et al., 2009 Irwin et al., 1996; Whalen et al., 1998) studies, and our findings were consistent with such findings. Behaviorally, veterans with PTSD responded more slowly to both neutral and threatening faces relative to veterans without PTSD, consistent with previous speed of processing and attention studies (See Scott, et al., 2015). Our facial processing task did not specifically direct attention toward emotional stimuli (Diano et al., 2017), and thus may nicely model the cues that provoke PTSD symptoms in daily life. Further, only face pairs that included angry expressions triggered an early amygdala response, consistent with an immediate response to threat in PTSD (Lanius et al., 2017).
To our knowledge, only three previous MEG studies have identified the timing of cortical differences in threat-cue processing in patients with PTSD compared to traumatized controls, and no MEG study has reported amygdala activation differences. These studies found that veterans without PTSD engaged the anterior cingulate more than veterans with PTSD from 90–140 ms (Todd et al., 2015), and that veterans without PTSD engaged the right ventromedial prefrontal more than those with PTSD from 400–600 ms (Khanna et al., 2017) after emotional word presentation. These MEG studies used emotional words, which are not as primitive of a threat cue as angry faces and may be why amygdala differences were not noted. Another study found that patients with PTSD recruited right prefrontal regions more than controls at 130–160 ms after affective picture presentation (Adenauer et al., 2010), but these were pictures of various items which required more processing than simply viewing neutral versus angry faces.
Importantly, our findings are consistent with MEG studies of healthy participants identifying early amygdala activation in response to emotional faces (Garrido et al., 2012; Garvert et al., 2014; Luo et al., 2007, 2010). We have extended these findings, demonstrating that amygdala activation in response to angry but not neutral faces is stronger in veterans with PTSD than in those without. Thus clarifying an early (~50 ms) and specific amygdala activation to threatening stimuli in PTSD which may reflect a bottom-up amygdala drive on cortical functioning (Liddell et al., 2004, 2005) consistent with a fear response. Future research is necessary, using primitive threatening stimuli such as emotional faces and startling sounds, but our study suggests targeting early amygdala hyperactivity in the assessment and treatment of PTSD.
Acknowledgments
Grant Support: This research was supported by a grant from the nonprofit organization At Ease, USA (ABB), grant R01-MH103220 from the National Institutes of Health (TWW), grant #1539067 from the National Science Foundation, and support from the Intramural Research Program of the National Institute of Mental Health (DSP). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Disclosures: Dr. Badura-Brack, Mr. McDermott, Ms. Ryan, and Drs. Heinrichs-Graham, Khanna, Pine, Bar-Haim, and Wilson report no competing financial or other interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adenauer H, Pinösch S, Catani C, Gola H, Keil J, Kissler J, Neuner F. Early processing of threat cues in posttraumatic stress disorder-evidence for a cortical vigilance-avoidance reaction. Biological psychiatry. 2010;68(5):451–8. doi: 10.1016/j.biopsych.2010.05.015. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. The diagnostic and statistical manual of mental disorders. 5. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- Badura-Brack AS, Heinrichs-Graham E, McDermott TJ, Becker KM, Ryan TJ, Khanna MM, Wilson TW. Resting-state neurophysiological abnormalities in posttraumatic stress disorder: A magnetoencephalography study. Froniers in Human Neuroscience. 2017 doi: 10.3389/fnhum.2017.00205. https://doi.org/10.3389/fnhum.2017.00205. [DOI] [PMC free article] [PubMed]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. Journal of traumatic stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, … Rosen BR. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Britton JC, Bar-Haim Y, Carver FW, Holroyd T, Norcross MA, Detloff A, … Pine DS. Isolating neural components of threat bias in pediatric anxiety. Journal of child psychology and psychiatry, and allied disciplines. 2012;53(6):678–86. doi: 10.1111/j.1469-7610.2011.02503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JM, Reinke KS, Habib R. A left amygdala mediated network for rapid orienting to masked fearful faces. Neuropsychologia. 2009;47(5):1386–9. doi: 10.1016/j.neuropsychologia.2009.01.026. [DOI] [PubMed] [Google Scholar]
- Cornwell BR, Arkin N, Overstreet C, Carver FW, Grillon C. Distinct contributions of human hippocampal theta to spatial cognition and anxiety. Hippocampus. 2012;22(9):1848–59. doi: 10.1002/hipo.22019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell BR, Overstreet C, Grillon C. Spontaneous fast gamma activity in the septal hippocampal region correlates with spatial learning in humans. Behavioural brain research. 2014;261:258–64. doi: 10.1016/j.bbr.2013.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell BR, Salvadore G, Furey M, Marquardt CA, Brutsche NE, Grillon C, Zarate CA. Synaptic potentiation is critical for rapid antidepressant response to ketamine in treatment-resistant major depression. Biological psychiatry. 2012;72(7):555–61. doi: 10.1016/j.biopsych.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal SS, Guggisberg AG, Edwards E, Sekihara K, Findlay AM, Canolty RT, … Nagarajan SS. Five-dimensional neuroimaging: localization of the time-frequency dynamics of cortical activity. NeuroImage. 2008;40(4):1686–700. doi: 10.1016/j.neuroimage.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diano M, Celeghin A, Bagnis A, Tamietto M. Amygdala Response to Emotional Stimuli without Awareness: Facts and Interpretations. Frontiers in Psychology. 2017;7 doi: 10.3389/fpsyg.2016.02029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley BT, Pang EW, Sedge PA, Jetly R, Doesburg SM, Taylor MJ. Threatening faces induce fear circuitry hypersynchrony in soldiers with post-traumatic stress disorder. Heliyon. 2016;2(1):e00063. doi: 10.1016/j.heliyon.2015.e00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. The American journal of psychiatry. 2007;164(10):1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido MI, Barnes GR, Sahani M, Dolan RJ. Functional evidence for a dual route to amygdala. Current biology: CB. 2012;22(2):129–34. doi: 10.1016/j.cub.2011.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvert MM, Friston KJ, Dolan RJ, Garrido MI. Subcortical amygdala pathways enable rapid face processing. NeuroImage. 2014;102(Pt 2):309–16. doi: 10.1016/j.neuroimage.2014.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gelder B, Meeren HK, Righart R, van den Stock J, van de Riet WA, Tamietto M. Beyond the face: exploring rapid influences of context on face processing. Progress in brain research. 2006;155:37–48. doi: 10.1016/S0079-6123(06)55003-4. [DOI] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A, Salmelin R. Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proceedings of the National Academy of Sciences. 2001;98:694–9. doi: 10.1073/pnas.98.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JP, Hayes SM, Mikedis AM. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biology of mood & anxiety disorders. 2012;2:9. doi: 10.1186/2045-5380-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse E, Mikulan E, Decety J, Sigman M, Garcia MC, Silva W, et al. Early detection of intentional harm in the human amygdala. Brain. 2016;139:54–61. doi: 10.1093/brain/awv336. [DOI] [PubMed] [Google Scholar]
- Irwin W, Davidson RJ, Lowe MJ, Mock BJ, Sorenson JA, Turski PA. Human amygdala activation detected with echo-planar functional magnetic resonance imaging. Psychiatry Research. 1996;67:135–143. doi: 10.1097/00001756-199607290-00014. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Subcortical face processing. Nature reviews Neuroscience. 2005;6(10):766–74. doi: 10.1038/nrn1766. [DOI] [PubMed] [Google Scholar]
- Khanna MM, Badura-Brack AS, McDermott TJ, Embury CM, Weismand AI, Shpeherd A, Ryan TJ, Heinrichs-Graham E, Wilson TW. Veterans with post-traumatic stress disorder exhibit altered emotional processing and attentional control during an emotional Stroop task. Psychological Medicine. 2017 doi: 10.1017/S0033291717000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch SB, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M. Aberrant resting-state brain activity in posttraumatic stress disorder: A meta-analysis and systematic review Depression and anxiety. 2016;33(7):592–605. doi: 10.1002/da.22478. [DOI] [PubMed] [Google Scholar]
- Lanius R, Rabellino D, Boyd J, Harricharan S, Frewen P, McKinnon M. The innate alarm system in PTSD: conscious and subconscious processing of threat. Current Opinion in Psychology. 2017;14:109–115. doi: 10.1016/j.copsyc.2016.11.006. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Pine DS. Using Neuroscience to Help Understand Fear and Anxiety: A Two-System Framework. The American journal of psychiatry. 2016;173(11):1083–1093. doi: 10.1176/appi.ajp.2016.16030353. [DOI] [PubMed] [Google Scholar]
- Liddell BJ, Brown KJ, Kemp AH, Barton MJ, Das P, Peduto A, … Williams LM. A direct brainstem-amygdala-cortical “alarm” system for subliminal signals of fear. NeuroImage. 2005;24(1):235–43. doi: 10.1016/j.neuroimage.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Liddell BJ, Williams LM, Rathjen J, Shevrin H, Gordon E. A temporal dissociation of subliminal versus supraliminal fear perception: an event-related potential study. Journal of cognitive neuroscience. 2004;16(3):479–486. doi: 10.1162/089892904322926809. [DOI] [PubMed] [Google Scholar]
- Luo Q, Holroyd T, Jones M, Hendler T, Blair J. Neural dynamics for facial threat processing as revealed by gamma band synchronization using MEG. NeuroImage. 2007;34(2):839–47. doi: 10.1016/j.neuroimage.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Q, Holroyd T, Majestic C, Cheng X, Schechter J, Blair RJ. Emotional automaticity is a matter of timing. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30(17):5825–9. doi: 10.1523/JNEUROSCI.BC-5668-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott TJ, Badura-Brack AS, Becker KM, Ryan TJ, Khanna MM, Heinrichs-Graham E, Wilson TW. Male veterans with PTSD exhibit aberrant neural dynamics during working memory processing: an MEG study. Journal of psychiatry & neuroscience: JPN. 2016;41(4):251–60. doi: 10.1503/jpn.150058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez-Bértolo C, Moratti S, Toledano R, Lopez-Sosa F, Martínez-Alvarez R, Mah YH, … Strange BA. A fast pathway for fear in human amygdala. Nature neuroscience. 2016;19(8):1041–9. doi: 10.1038/nn.4324. [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ. A differential neural response in the human amygdala to fearful and happy facial expression. Nature. 1996;31:812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Morris J, Friston KJ, Buchel C, Frith CD, Young AW, Calder AJ, Dolan RJ. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998a;121:47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- Morris J, Öhman A, Dolan RJ. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998b;393:425–418. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- Patel R, Spreng RN, Shin LM, Girard TA. Neurocircuitry models of posttrss disorder and beyond: a meta-analysis of functional neuroimaging studies. Neuroscience and biobehavioral reviews. 2012;36(9):2130–42. doi: 10.1016/j.neubiorev.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Spinelli L, Seeck M, Vuilleumier P. Temporal precedence of emotion over attention modulations in the lateral amygdala: Intracranial ERP evidence from a patient with temporal lobe epilepsy. Cognitive Affective and Behavioral Neuroscience. 2010;10:83–93. doi: 10.3758/CABN.10.1.83. [DOI] [PubMed] [Google Scholar]
- Proskovec AL, Heinrichs-Graham E, Wilson TW. Aging modulates the oscillatory dynamics underlying successful working memory encoding and maintenance. Human brain mapping. 2016;37(6):2348–61. doi: 10.1002/hbm.23178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu Y, Cornwell BR, Cheyne D, Johnson BW. The functional role of human right hippocampal/parahippocampal theta rhythm in environmental encoding during virtual spatial navigation. Human brain mapping. 2017;38(3):1347–1361. doi: 10.1002/hbm.23458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadore G, Cornwell BR, Colon-Rosario V, Coppola R, Grillon C, Zarate CA, Manji HK. Increased anterior cingulate cortical activity in response to fearful faces: a neurophysiological biomarker that predicts rapid antidepressant response to ketamine. Biological psychiatry. 2009;65(4):289–95. doi: 10.1016/j.biopsych.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadore G, Cornwell BR, Sambataro F, Latov D, Colon-Rosario V, Carver F, … Zarate CA. Anterior cingulate desynchronization and functional connectivity with the amygdala during a working memory task predict rapid antidepressant response to ketamine. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35(7):1415–22. doi: 10.1038/npp.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato W, Kochiyama T, Uono S, Matsuda K, Usui K, Inoue Y, et al. Rapid amygdala gamma oscillations in response to fearful facial expressions. Neuropsychologia. 2011;49:612–7. doi: 10.1016/j.neuropsychologia.2010.12.025. [DOI] [PubMed] [Google Scholar]
- Scott C, Matt G, Wrocklage K, Crnich C, Jordan J, Southwick S, … Schweinsburg B. A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychological Bulletin. 2015;141(1):105. doi: 10.1037/a0038039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamietto M, de Gelder B. Neural bases of the non-conscious perception of emotional signals. Nature reviews Neuroscience. 2010;11(10):697–709. doi: 10.1038/nrn2889. [DOI] [PubMed] [Google Scholar]
- Taulu S, Simola J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Physics in medicine and biology. 2006;51:1759–68. doi: 10.1088/0031-9155/51/7/008. [DOI] [PubMed] [Google Scholar]
- Todd RM, MacDonald MJ, Sedge P, Robertson A, Jetly R, Taylor MJ, Pang EW. Soldiers With Posttraumatic Stress Disorder See a World Full of Threat: Magnetoencephalography Reveals Enhanced Tuning to Combat-Related Cues. Biological psychiatry. 2015;78(12):821–9. doi: 10.1016/j.biopsych.2015.05.011. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uusitalo MA, Ilmoniemi R. Signal-space projection method for separating MEG or EEG into components. Medical and Biological Engineering and Computing. 1997;35:135–140. doi: 10.1007/BF02534144. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends in cognitive sciences. 2005;9(12):585–94. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: An event related fMRI Study. Neuron. 2001;20:829–841. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Jounal of Neuroscience. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Proskovec AL, Heinrichs-Graham E, O’Neill J, Robertson KR, Fox HS, Swindells S. Aberrant Neuronal Dynamics during Working Memory Operations in the Aging HIV-Infected Brain. Scientific reports. 2017;7:41568. doi: 10.1038/srep41568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Slason E, Asherin R, Kronberg E, Reite ML, Teale PD, Rojas DC. An extended motor network generates beta and gamma oscillatory perturbations during development. Brain and cognition. 2010;73(2):75–84. doi: 10.1016/j.bandc.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Slason E, Asherin R, Kronberg E, Teale PD, Reite ML, Rojas DC. Abnormal gamma and beta MEG activity during finger movements in early-onset psychosis. Developmental neuropsychology. 2011;36(5):596–613. doi: 10.1080/87565641.2011.555573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Slason E, Hernandez OO, Asherin R, Reite ML, Teale PD, Rojas DC. Aberrant high-frequency desynchronization of cerebellar cortices in early-onset psychosis. Psychiatry research. 2009;174(1):47–56. doi: 10.1016/j.pscychresns.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]