Abstract
Immunoglobulin 4 (IgG4) is one of four human IgG subclasses and has several unique functional characteristics. It exhibits low affinity for complement and for most Fc receptors. It furthermore has generally high affinity for its antigen, with binding occurring in a monovalent fashion, as IgG4 can exchange Fab-arms with other IgG4 molecules. Because of these characteristics, IgG4 is believed to block its targets and prevent inflammation, which, depending on the setting, can have a protective or pathogenic effect. One example of IgG4 pathogenicity is muscle-specific kinase (MuSK) myasthenia gravis (MG), in which patients develop IgG4 MuSK autoantibodies, resulting in muscle weakness. As a consequence of the distinct IgG4 characteristics, the pathomechanism of MuSK MG is very different from IgG1-and IgG3-mediated autoimmune diseases, such as acetylcholine receptor MG. In recent years, new autoantibodies in a spectrum of autoimmune diseases have been discovered. Interestingly, some were found to be predominantly IgG4. These IgG4-mediated autoimmune diseases share many pathomechanistic aspects with MuSK MG, suggesting that IgG4-mediated autoimmunity forms a separate niche among the antibody-mediated disorders. In this review, we summarize the group of IgG4-mediated autoimmune diseases, discuss the role of IgG4 in MuSK MG, and highlight interesting future research questions for IgG4-mediated autoimmunity.
Keywords: IgG4, myasthenia gravis, MuSK, autoimmunity, neuromuscular junction
Introduction to IgG4
Antibody responses are an effective strategy of the immune system to protect against pathogens, but, when they go awry, they can also cause disease. Human antibody responses can consist of immature, low-affinity immunoglobulin (Ig) IgM and IgD responses or more mature IgE and IgG responses. IgG is further subdivided into subclasses on the basis of their morphological and functional characteristics. Structural determinants in the constant, crystallizable fragment (Fc) of the antibody dictate whether an immunoglobulin G (IgG) molecule is able to activate complement, bind and activate or inhibit Fc-receptors and immune cell–mediated cytotoxicity, or interact with other IgGs.1 On the basis of these characteristics and the type of antigen, human IgG was classified into four subclasses. IgG4 is the least prevalent IgG in healthy adults and makes up approximately 5% of the total IgG pool. Despite approximately 90% amino acid sequence homology with other IgG subclasses, IgG4 is unique, as it is functionally monovalent and causes little to no inflammation.2 IgGs normally appear as homodimers, but two residues (serine 228 and arginine 409) in IgG4 Fc facilitate continuous exchange of monomers, a process termed Fab-arm exchange.3,4 This results in bispecific antibodies and functionally monovalent binding. In other words, each Fab-arm of an IgG4 molecule will bind a different antigen, which disables its crosslinking capacity and inhibits immune complex formation. The lack of immune complex formation together with reduced binding affinity for complement factor C1q renders IgG4 a poor complement activator.5 Furthermore, interaction with Fc receptors on immune cells is dependent on the individual receptor and specific residues in the CH2 region of the Fc of an IgG molecule. For IgG4, these Fc determinants result in preferred binding to inhibitory Fc receptors.6 Lastly, when solid-phase immobilization is used, IgG4 Fc can bind to other Fc from all human IgG subclasses.7 Together, these distinct functional characteristics led IgG4 to be deemed as an anti-inflammatory antibody. For a comprehensive review on IgG4 morphology and function and the regulation of IgG4 responses, see Lighaam et al.1
For each of the unique features of IgG4, the (patho-)physiological relevance is largely unclear. Depending on the setting, an IgG4 response can be protective or pathogenic. For example, IgG4 is often considered a protective blocking antibody, as it can inhibit or prevent inflammation by competing for antigen binding with inflammatory IgG subclasses or IgE. Alternatively, IgG4 can cause severe disease in a subset of autoimmune diseases, which is be discussed below.
IgG4-mediated autoimmune diseases
The majority of known antibody-mediated autoimmune diseases are caused by IgG1 and IgG3 autoantibodies. However, in the 1980s, pemphigus, a skin-blistering disease, was recognized as the first autoimmune disease that is hallmarked by IgG4 autoantibody predominance.8 In recent years, autoimmunity research has focused, for both diagnostic and treatment purposes, on identifying new antigens in a variety of autoimmune diseases. Identification of these new antigens created an opportunity to characterize the predominant antibody subclass and disease mechanisms. To our knowledge, IgG4 plays a prominent role in the pathogenesis of at least 13 autoimmune diseases. For an extensive review on these diseases, see Huijbers et al.9 The idea that IgG4-mediated autoimmune diseases constitute a separate niche among the antibody-mediated autoimmune diseases is based on several observations (Table 1). The differences between IgG1-, IgG3- and IgG4-mediated autoimmune diseases mostly relate to the functional characteristics of the different IgG subclasses. Where IgG1 and IgG3 autoantibodies cause disease by inducing complement-dependent tissue damage, immune cell–mediated cytotoxicity, and crosslinking and internalization of the antigen, those IgG4 autoantibodies for which the disease mechanism is largely resolved simply block the function of its target antigen. It is furthermore interesting that IgG4-mediated autoimmune diseases are rarely associated with tumors.10
Table 1.
The differences between IgG1−, IgG3−, and IgG4-mediated autoimmune disease.
| IgG1 and IgG3 | IgG4 |
|---|---|
| Antigens are receptors, ion channels, or multisubunit proteins | Antigens are typically not receptors, ion channels, or multisubunit proteins |
| Pathomechanism requires complement and immune cell–mediated cytotoxicity and inflammation | Pathomechanism is blocking of essential protein–protein interactions |
| Structural damage to target tissue | No structural damage to target tissue |
| Crosslinking and internalization of the antigen | Monovalent antigen binding, no crosslinking |
| Sometimes associated with paraneoplastic events | No clear tumor association |
| Result from TH1-related cytokine expression | Result from TH2-related cytokine expression |
Since our first review on the group of IgG4-mediated autoimmune diseases, one disease entity can be added: neurofascin140/186 antibodies in patients with chronic inflammatory demyelinating polyneuropathy (CIDP).9,11 For several of the other IgG4-mediated autoimmune diseases, more evidence has been presented for a pathophysiological relationship between the IgG4 autoantibodies and disease. An overview of the main characteristics of the IgG4-mediated autoimmune diseases is given in Table 2. For five out of the 13 listed diseases, passive transfer of human total IgG or IgG4 into experimental animals has been shown to induce the symptoms of the respective disease. For the eight remaining diseases, IgG4-mediated autoimmunity is suggested by the observation that serum-derived, antigen-specific autoantibodies are predominantly of the IgG4 subclass and that their titers correlate with disease severity. In vitro assays have furthermore elucidated the pathomechanism by which these (IgG4) autoantibodies cause disease in 12 of the 13 listed diseases.
Table 2.
Overview of the characteristics and experimental evidence for the IgG4-mediated autoimmune diseases.
| Antigen | Disease | Symptoms | Prevalence | HLA association | Target organ | Main immunogenic region |
Passive transfer confirmation in experimental animals |
Disease mechanism | VDJ gene usage |
Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| ADAMTS13 | Thrombotic thrombocytopenic purpura | Thrombocytopenia, microangiopathic hemolytic anemia resulting in organ failure | 13 per million | HLA-DRB1*11 | Vasculature | Cysteine-rich spacer domain | N/A | Inhibition of ADAMTS13-dependent von Willebrand factor cleavage | VH1-3, VH1-69, VH3-30, VH4,28 | 74, 79, 84 |
| CASPR1 | Chronic inflammatory polyneuropathy | Progressive weakness, sensory disturbances, neuropathic pain | 3 cases | N/A | Paranode of Ranvier on motor neurons | N/A | N/A | Blocking of CNTN1–Caspr1–NF155 complex | N/A | 76, 82 |
| CASPR2 | Limbic encephalitis, neuromyotonia and Morvan syndrome | Impaired cognition, seizures, fasciculations and cramps, sometimes autonomic dysfunction and insomnia | ~ 200 patients have been described thus far | N/A | Juxtaparanode of Ranvier on motor neurons and inhibitory neurons in the CNS | Discoidin domain, but with many epitopes all over the extracellular domain; glycosylation independent | N/A | Inhibition of Caspr2–TAG1 interaction altering gephyrin clustering | N/A | 72, 89 |
| Contactin1 | Chronic inflammatory polyneuroathy | Severe symmetric sensory and motor polyradiculoneuropathy, poor IV Ig response | 3–7% of CIDP patients | N/A | Paranode of Ranvier on motor neurons | Ig-like domain dependent on N-glycans | Yes, IgG4 and IgG1 | Inhibition of CNTN1–CASPR1 interaction disrupting node of Ranvier | N/A | 83 |
| Desmoglein1 | Pemphigus foliaceus | Acantholysis (skin blistering) | 2–10 per million in central Europe, with higher incidence among specific ethnic groups | HLA-DRB1*4, DRB1*14 Different HLAs associated with specific ethnic groups and geography |
Skin keratinocyte desmosomes | N-terminal cadherin-like domain 1 and 2; glycosylation independent | Yes | Inhibition of trans-adhesion | VH1, VH3 | 75 |
| Desmoglein3 | Pemphigus vulgaris | Acantholysis (skin blistering) and blisters on mucosal membranes | 2–10 per million in central Europe, with higher incidence among specific ethnic groups | HLA-DRB1*4, HLA-DRB1*08, HLA-DRB1*14 HLA-DQB1*5 Different HLAs associated with specific ethnic groups and geography |
Skin keratinocyte desmosomes and mucous membranes | N-terminal cadherin-like domain 1 and 2; glycosylation independent | Yes | Inhibition of trans-adhesion between desmoglein3 receptors, signaling inhibition and desmosome shrinkage |
VH1-46 VH1, VH3, VH4 |
75 |
| IgLON5 | Non-REM and REM parasomnia with sleep breathing dysfunction and a tauopathy | Disordered sleep and ventilation, sometimes with brain stem, gait, cognitive, or movement disorders | At least 30 patients have been identified | HLA-DQB1*0501 HAL-DRB1*1001 | Brain neuropil | Ig-like 2 domain; glycosylation independent | N/A | IgG1 caused IgLON5 cluster internalization; IgG4 has unknown effects | N/A | 77 |
| LGI1 | Limbic encephalitis | Memory, behavioural, and orientation deficits; faciabrachial dystonic seizures, often with hyponatremia | ~ 300 patients have been described | HLA-DR7 HLA-DRB4 | CNS predominantly in hippocampus and temporal cortex | EPTP repeat and leucine-rich repeat domain | Yes | Inhibition of Lgi1-ADAM interaction and AMPAR clustering | N/A | 12, 86 |
| MuSK | Myasthenia gravis | Fatigable muscle weakness | 2–9 per million | HLA-DR14-DQ5 | Muscle/neuromuscular junction | N-terminal Ig-like domain; glycosylation independent | Yes, IgGtotal and IgG4 | Inhibition of MuSK–LRP4 interaction and AChR clustering | N/A | 81 |
| Neurofascin140/186 | Chronic inflammatory polyneuroathy | Severe symmetric sensory and motor polyradiculoneuropathy. | 4 patients described thus far | N/A | Motor neurons, (para)node of Ranvier | Ig-like domains; fibronectin V domain | N/A | N/A | N/A | 11 |
| Neurofascin155 | Chronic inflammatory polyneuroathy | Aggressive, distal, sensorimotor neuropathy, poor response to IV Ig | ~ 3–7 % of CIDP patients | N/A | Motor neurons, (para)node of Ranvier | N-terminal Ig-like domain; fibronectin III,IV domain | N/A | Inhibition of cell adhesion at paranodal junction | N/A | 83 |
| PLA2R1 | Membranous nephropathy | Proteinuria, nephritis | 70% of membranous nephropathy patients | HLA-DQA1 HLA-DRB1 |
Kidney, podocytes | N-terminal Cys-R domain; fibronectin II domain and CTLD1 domain | No, as antigen is not expressed on podocytes in rodents | Perhaps inhibition of PLA2R binding to collagen | N/A | 73, 85, 87 |
| THSDA7A | Membranous nephropathy | Proteinuria, nephritis | 5% of membranous nephropathy patients | N/A | Kidney, podocytes | N/A | Yes | Binding to THSDA7A alters cytoskeletal organization through unknown mechanism | N/A | 87 |
Interestingly, there is a second group of diseases hallmarked by IgG4 autoantibody predominance, but in this group the role of IgG4 in the pathophysiology is unclear. For example, IgG4 autoantibodies dominate the response in bullous pemphigoid patients with BP180 and BP230 autoantibodies, in a subset of patients with Goodpasture disease with collagen IV autoantibodies, and in patients with encephalitis and dipeptidyl-peptidase–like protein 6 autoantibodies (DPPX).12–15 However, IgG1-related effector functions dictate the pathophysiology in these diseases. This is likely due to the co-occurrence of IgG1 autoantibodies and calls into question the role of IgG4 autoantibodies in the pathomechanism of these diseases. Lastly, anti-cyclic citrullinated protein (ACPA) antibodies are associated with rheumatoid arthritis and are often of the IgG1 and IgG4 subclasses.16 The role of ACPA antibodies in rheumatoid arthritis pathophysiology remains enigmatic. Since it is not clear whether diseases in this group are truly members of the IgG4-mediated autoimmune diseases niche, they have been summarized separately (Table 3). Passive transfer studies with purified IgG4 from these patients might help to clarify this issue.
Table 3.
Overview of autoimmune diseases hallmarked by high levels of IgG4 autoantibodies, but with poorly defined roles for IgG4 in the disease.
| Antigen | Disease | Symptoms | Prevalence | HLA association | Target organ | Main immunogenic region |
Passive transfer confirmation in experimental animals |
Disease mechanism |
VDJ gene usage |
Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| ACPA | Rheumatoid arthritis | Stiff hands and feet joints due to chronic inflammation | ~ 60% of all rheumatoid arthristis patients | DRB1*01:01,01:02 DBR1*04:01,*04:04,*04:05,*04:08 DRB1*09:01 DRB1*10:01 |
Joints | Different citrullinated proteins carry the epitope: fibrin, fibrinogen, enolase, collagen, vimentin, and EBNA | Yes, partially. Passive transfer can exacerbate disease | Mixed effects of both IgG1 and IgG4 autoantibodies. Inflammation plays a key role. | Varying dependent on antigen | 71, 88 |
| BP180 (type XVII collagen) | Bullous pemphigoid | Acantholysis (skin blistering) | 10 per million | HLA-DQB1*0301 HLA-DRB1*04, HLA-DRB1*1101, HLA-DQB1*0302 |
Skin hemidesmosomes | Non-collagenous 16A domain | Yes | Mixed effects of both IgG1 and IgG4 autoantibodies. Complement is likely involved. | VH4 | 12, 80 |
| BP230 (dystonin-e) | Bullous pemphigoid | Acantholysis (skin blistering) | 10 per million | HLA-DQB1*0301 | Skin hemidesmosomes | Broad response, epitopes throughout the antigen, but most against collagen B and C subdomains | N/A | Mixed effects of both IgG1 and IgG4 autoantibodies. Complement is likely involved | N/A | 12, 86 |
| Collagen IV | Goodpasture disease | Alveolar hemorrhage and glomerulonephritis | 1 per million | HLA-DRB1*1501 | Kidney | α3(IV)NCI-domain | Yes | Unclear if involved in pathogenesis, mostly IgG1–3 |
V1-8 V3-48 V3-21 V-3-23 V1-69 Derived from humanized model |
78 |
| DPPX | Encephalitis | Memory and cognitive dysfunction, seizures, hyperekplexia, tremor, diarrhea | 39 patients described | N/A | CNS, hippocampus | N/A | N/A | Decreased expression of DPPX and Kv4.2. Increased excitability and action potential frequency | N/A | 13 |
The first IgG4-mediated autoimmune diseases to be described were mainly neurological disorders.9 However, it is clear that IgG4-mediated autoimmunity can also affect other organ systems. In fact, five out of 13 IgG4 diseases do not affect the nervous system. It can be expected that this niche will grow in the coming years as more antigenic targets are discovered.
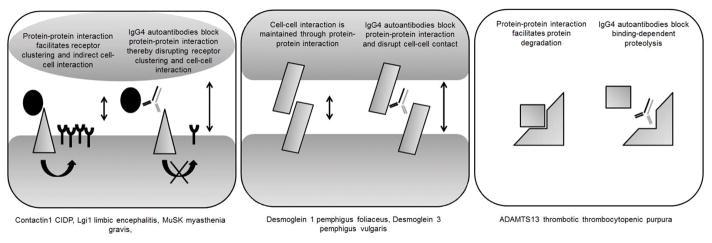
The identification of a new niche warrants further investigation on the commonalities and differences between these diseases, as it might shed light on the cause and shared potential therapeutic opportunities. A summary of commonalities and differences between the IgG4-mediated autoimmune diseases is given in Table 4. Many of the antigens that are involved in IgG1- and IgG3-mediated autoimmune diseases are multisubunit receptors or ion channels,10 as exemplified by acetylcholine receptor (AChR) myasthenia gravis (MG). Notably, the IgG4 autoantibodies described thus far do not seem to target multisubunit receptors or ion channels, but rather bind proteins associated with them.9 These antigens are often involved in stabilizing the ion channels or receptors or are themselves important for maintaining cell–cell interaction. For example, MuSK is essential in establishing and maintaining the neuromuscular junction and induces AChR clustering, while desmogleins maintain keratinocyte cell–cell interactions. Thus, the antigens function either as bridging proteins themselves or participate in a signalling cascade that facilitates cell–cell interactions. The IgG4 autoantibodies (physically) interfere in the (signalling) function of the antigens and hamper the bridging effects.9 Lastly, in thrombotic thrombocytopenic purpura with ADAMTS13 IgG4 autoantibodies, the interaction between ADAMTS13 and its substrate von Willebrand factor is obstructed, preventing the degradation of the substrate.17 In conclusion, the blocking nature of IgG4 can result in pathology through obstruction of three different protein functions (Fig. 1).
Table 4.
An overview of the commonalities and differences between the several specific IgG4-mediated autoimmune diseases.
Commonalities
|
Figure 1.
A graphic representation of the three main pathological blocking effects of IgG4 autoantibodies. The diseases, for which the pathomechanism is well characterized, are grouped below the associated IgG4 effects.
Initially, few human leukocyte antigen (HLA) associations were reported for the IgG4-mediated autoimmune diseases, and it was striking that three of them showed an HLA-DQ5 association (MuSK MG, IgLON5 non-REM and REM parasomnia, and pemphigus vulgaris in Jewish patients).9 However, Tables 2 and 3 show that IgG4 autoimmunity is not restricted to HLA-DQ5. HLA association studies are further limited for some of these diseases owing to the small number of patients described.
Immunosuppression generally forms the first-line treatment for all of these diseases, but not all patients respond well to these therapies. The observation that rituximab seems particularly effective in IgG4-mediated autoimmune diseases is therefore of great clinical value. Rituximab is a CD20 antibody that depletes all CD20-expressing B cells. Whether IgG4-producing B cells are particularly sensitive to this treatment, and why, is not known. The level of CD20 does not seem to differ between IgG4 and IgG1 memory B cells, and rituximab lowers all IgG subclass levels in bullous pemphigoid.18,19 Moreover, the numbers of total B cells, as well as naive, memory, plasmablast, and transitional B cell subsets, are normal in MuSK MG and pemphigus patients.20,21 The rapid and sustained reduction in IgG4 autoantibody titers therefore suggests that IgG4 responses might not be dominated by long-lived plasma cells and that IgG4 plasma cells express CD20.22,23 It will be exciting to learn why rituximab treatment is particularly effective in these diseases.
Several features differ among IgG4-mediated autoimmune diseases (Tables 2–4). These include the antigen that is recognized, the different types of protein domains that form the main immunogenic region (MIR), the need for glycosylation of the antigen for autoantibody binding, the type of HLA association, and the VDJ gene usage of the autoantibodies. Given that many of these disease subsets have only recently been recognized, these details are not yet comprehensively available. For 11 IgG4-mediated autoimmune diseases, the MIR has been mapped, and for five of them the MIR is an Ig-like domain, for three a fibronectin domain, and for two a cadherin-like domain. This suggests that different types of protein domains can be involved in an IgG4 autoimmune response. With more diseases being discovered, it will be interesting to learn whether certain protein domains and structures are more prone to IgG4 responses. Epitope-mapping experiments in all these diseases further suggest that the autoantibody repertoire is oligo- or polyclonal, as epitopes outside the MIR are being recognized as well. Another important determinant of epitope structure is glycosylation. Therefore, for some antigens, the contribution of glycosylation to autoantibody–antigen binding has been studied. Thus far, contactin 1 in CIDP is the only antigen where glycosylation was proven to be essential for autoantibody binding.24 For the other IgG4-mediated autoimmune diseases, it may be possible that, while the autoimmune response develops, epitope spreading moves the immune response away from glycosylation-dependent epitopes. Given the involvement of different antigens and MIRs, it is not surprising that VDJ usage and HLA associations differ between these diseases, although it is important to realize that human monoclonal antibodies have only been isolated for three out of the 13 listed diseases. Lastly, for some IgG1- and IgG3-mediated autoimmune diseases, paraneoplastic events are associated with the onset of disease. Such associations have thus far only been only observed in a limited number of patients with IgG4 autoimmunity, which suggests that it is less relevant for IgG4.10,25,26
Evidence for IgG4 involvement in MuSK MG
Witebsky’s postulates require confirmation of autoimmunity on several levels.27 The role of MuSK autoantibodies in MG is supported by the transplacental transfer of disease, by active immunization of mice and rabbits with the MuSK antigen inducing MG, and by passive transfer of both total IgG and purified IgG4 from MuSK MG patients in mice and rabbits.28–35 The role of IgG4 in MuSK MG was first suggested by the observations that the majority of the MuSK autoantibodies are of the IgG4 subclass and that IgG4 titers correlate with disease severity.36–38 Direct evidence for the role of IgG4 in MuSK MG comes from passive transfer experiments with affinity-purified polyclonal IgG4 antibodies from MuSK MG patients.30 A pathogenic potency of monoclonal IgG4 antibodies derived from patients would provide another level of evidence, but such monoclonal antibodies have only recently been isolated and have not been functionally characterized.39 Low levels of non-IgG4 MuSK antibodies can sometimes also be detected. Whether they play a role in the pathophysiology of the disease is currently unclear, as purified MuSK IgG1–3 antibodies can inhibit AChR clustering in myotube cultures but do not inhibit the LRP4–MuSK interaction (see below).40,41 Moreover, passively transferred purified IgG1–3 patient antibodies did not bind the NMJ and did not cause MG in mice.30 In the in vitro studies, the dosing of MuSK-specific antibodies was equal for IgG1–3 and IgG4, whereas the in vivo experiments did not correct for MuSK-specific antibody dosing.30,41 This might explain these apparent discrepancies. The IgG1–3 MuSK antibody titers in the majority of patients are low or even undetectable in our experience. Furthermore, it cannot be excluded that epitope specificity might differ between MuSK antibodies of different subclasses, and this may affect their pathogenicity. Lastly, due to Fab-arm exchange, IgG4 is functionally monovalent. For polyclonal patient antibodies, it was shown that these patients carry the genetic variants that enable Fab-arm exchange, and they do so in vitro and in vivo.42 Whether the valency of anti-MuSK antibodies is relevant for their pathogenicity is not known. IgG4, likely owing to its monovalency, was shown to block the MuSK–LRP4 interaction, thereby inhibiting the trophic signalling cascade leading to AChR clustering. An important step in this cascade is dimerization and internalization of MuSK, which activates its intracellular kinase domain and transmits the clustering signal.43 It is conceivable that bivalent IgG1–IgG3 antibodies to MuSK could induce dimerization and internalization of MuSK, which would therefore strengthen the ongoing neuromuscular junction maintenance pathway and would be unlikely to result in myasthenia. The internalization might also prevent activation of complement or immune cell–mediated cytotoxicity. This hypothesis is consistent with observations that MuSK can be internalized when total IgG from patients is used in myotube cultures and can activate AChR clustering in some cases.41,44 Alternatively, the IgG1–3 antibodies might also inhibit MuSK function or activate complement. Lastly, the MuSK–ColQ interaction can be blocked by patient IgG.45 Whether the subclass of the autoantibodies is relevant for this effect is unknown. Patient-derived monoclonal antibodies might be important tools to address these mechanistic questions. In addition, it is important to emphasize that a combination of the aforementioned effects can occur in individual patients, as antibody repertoires likely vary. For MuSK MG, we have not yet encountered patients with high levels of MuSK-specific IgG1. This suggests that either the titers of IgG1–3 autoantibodies do not reach pathogenic levels or that high IgG1–3 autoantibody levels are less pathogenic in MuSK MG.
For the different forms of pemphigus, the role of autoantibody valency has been investigated extensively. In pemphigus vulgaris, active disease is associated with IgG4 autoantibodies, the switch from IgG1 to IgG4 autoantibodies is essential in developing symptoms in an endemic form of pemphigus, and passive transfer of IgG4 from patients induces the disease in mice.46–48 In contrast, some pemphigus foliaceus patients only have IgG1 autoantibodies, which can cause acantholysis in mice.49 Epitope specificity and autoantibody pathogenicity differ between autoantibodies of the IgG1 and IgG4 subclasses.50 These observations suggest that, in pemphigus, antibody isotype is not a major determinant for the pathogenic effects and that epitope specificities and autoantibody titers are more important.51,52
Other diseases associated with IgG4
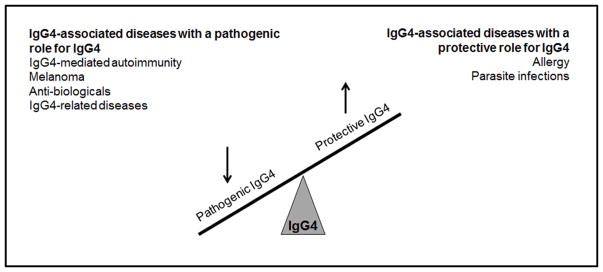
IgG4 plays a key role in a variety of other diseases. These can be distinguished as diseases where the blocking effect of IgG4 is beneficial and diseases in which the IgG4 blocking effect is pathogenic. The pathogenic effect of IgG4 is seen in the previously discussed autoimmune diseases; in melanoma, where IgG4 inhibits endogenous antitumor responses, resulting in worse disease progression and metastasis; in treatment settings with antibiologicals, where IgG4 blocks the function of the biological and renders treatment ineffective; and in a range of IgG4-related diseases where plasma cell infiltrates cause tissue damage.9,53–56 Like in IgG4-mediated autoimmunity, these IgG4-related diseases form a heterologous group of autoimmune disorders affecting a broad range of organ systems. The symptoms relate to the organ system affected. However, in contrast to IgG4-mediated autoimmunity, increased levels of IgG4 are often found in IgG4-related disease patients, but the exact role of this IgG4 and whether it recognizes a specific antigenic target is unknown. For more specific information on the pathology of IgG4-related disease, the reader is referred to recent reviews.57,58 In each of the above-mentioned settings except for the IgG4-related diseases, IgG4 antigen-specific titers correlate with disease severity, and a reduction in these titers correlates with improved health.
Interestingly, IgG4 can also have protective effects. This is seen in infections with filarial parasites and helminths, where IgG4 dampens ongoing inflammation, and in situations where prolonged inflammation or allergic responses cause serious disease.1,59 Seminal work by Aalberse and colleagues revealed that tolerance is induced in beekeepers that, after prolonged immunization, have undergone a class switch to IgG4. The IgG4 competes for binding with IgE and IgG1 to the allergen. In addition, the increase in IgG4 titer is an order of magnitude higher than IgE and IgG1, which further contributes to its competitive ability. Owing to its inability to activate neutrophils and complement, IgG4 inhibits ongoing inflammation, resulting in tolerance and reduced allergic symptoms. In these settings, the increase of antigen-specific IgG4 correlates with improved health. Figure 2 gives an overview of the effect of IgG4 in different IgG4-associated diseases.
Figure 2.
An overview of the effect of IgG4 in different IgG4-associated diseases and potential therapeutic strategies.
Thus, depending on the setting, IgG4 can have beneficial or detrimental effects. It is tempting to speculate that specific modulation of IgG4 production could be a promising therapeutic strategy for all of the above-mentioned IgG4-associated diseases. It is possible that these treatments would be better than general immunosuppression, as they might not affect other useful immune responses. Furthermore, low levels of IgG4 are generally well tolerated and are therefore expected to result in fewer side effects. Given the clinical relevance, a range of studies have investigated the regulation of IgG4 responses. Although many aspects of the induction, maintenance, and inhibition of IgG4 responses are still unknown, several factors have been described to contribute to IgG4 production: (1) prolonged exposure to an allergen;60 (2) TH2-related cytokines interleukin (IL)-4 and IL-13 inducing a class switch to IgG4 and IL-21 and IL-4 stimulating IgG4 production by plasma cells;61–63 (3) IL-10 derived from regulatory T and B cells;64–66 and (4) growth hormone and growth factor.67
How (and if) these factors contribute specifically to the onset and progression of IgG4-mediated autoimmune diseases is thus far not known. A small number of studies has investigated whether these factors are dysregulated in MuSK MG. These studies are all hampered by the limited number of patients included and the heterogeneous treatment regimens they received. Plasma levels of TH1−, TH2−, and TH17-related cytokines do not differ between MuSK MG patients and healthy individuals.68In vitro production of IL-4, IL-6, IL-13, and TNF-α was normal in CD40+ and nonspecific B cell receptor–stimulated MuSK MG immune cells.68,69 Transcriptomic analysis and MuSK-specific stimulation did not show altered cytokine expression compared with controls. Interestingly, interferon-γ, IL-10, IL-17A, and IL-21 production was increased in these cultures.68 Other studies have also suggested that B cell–activating factor, a factor that is secreted by dendritic cells and myeloid cells to promote B cell survival, is increased in MuSK MG patients.20,70 Furthermore, regulatory B10 cells are reduced in MuSK MG. Each of these observations could contribute to the breakdown of tolerance in MuSK MG and suggest a role for TH1 and TH17 immune regulation. The latter is particularly striking, as IgG4 production usually is related to a TH2 response. The increased production of IL-10 in immune cell cultures matches its described role as a potent IgG4 stimulator. Higher-powered studies, which also separate on the basis of treatment regimen, could shed more light on the immune status of MuSK MG patients during the disease.
Conclusions
IgG4 is an enigmatic antibody with unique characteristics that is associated with a range of (autoimmune) diseases. Depending on the setting, IgG4 can have protective or pathogenic effects. There is strong evidence that IgG4 is pathogenic in MuSK MG and other IgG4-mediated autoimmune diseases. The blocking effect of IgG4 is a pathomechanistic feature thus far shared by these diseases, but mostly different from other IgG1- and IgG3-mediated autoimmune diseases. Therefore, IgG4-mediated autoimmune diseases constitute a newly recognized and exciting niche among the antibody-mediated autoimmune diseases. Many aspects of the role and development of the IgG4 immune response in MuSK MG and other newly identified IgG4-mediated autoimmune diseases are still unknown and form interesting lines of research for the future (Table 5).
Table 5.
An overview of the accepted knowledge and unresolved questions about MuSK MG pathophysiology and the involvement of IgG4.
Accepted knowledge
|
Acknowledgments
We would like to thank the Prinses Beatrix Spierfonds (W.OR09-19) and NIH-NINDS (R21NS088723) for their financial support. We receive royalties from a patent on a MuSK ELISA and perform contract research with ArgenX. JJV was involved in the NIH-thymectomy trial, and in a FP7 European grant on MG. The LUMC has received fees from Alexion and ArgenX because of consultancies by JJV.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Lighaam LC, Rispens T. The Immunobiology of Immunoglobulin G4. Semin Liver Dis. 2016;36:200–215. doi: 10.1055/s-0036-1584322. [DOI] [PubMed] [Google Scholar]
- 2.van der Neut KM, Schuurman J, Losen M, et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science. 2007;317:1554–1557. doi: 10.1126/science.1144603. [DOI] [PubMed] [Google Scholar]
- 3.Labrijn AF, Rispens T, Meesters J, et al. Species-specific determinants in the IgG CH3 domain enable Fab-arm exchange by affecting the noncovalent CH3-CH3 interaction strength. J Immunol. 2011;187:3238–3246. doi: 10.4049/jimmunol.1003336. [DOI] [PubMed] [Google Scholar]
- 4.Rispens T, Davies AM, Ooijevaar-de HP, et al. Dynamics of inter-heavy chain interactions in human immunoglobulin G (IgG) subclasses studied by kinetic Fab arm exchange. J Biol Chem. 2014;289:6098–6109. doi: 10.1074/jbc.M113.541813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Zee JS, van SP, Aalberse RC. Inhibition of complement activation by IgG4 antibodies. Clin Exp Immunol. 1986;64:415–422. [PMC free article] [PubMed] [Google Scholar]
- 6.Bruhns P, Iannascoli B, England P, et al. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113:3716–3725. doi: 10.1182/blood-2008-09-179754. [DOI] [PubMed] [Google Scholar]
- 7.Rispens T, Ooievaar-De HP, Vermeulen E, Schuurman J, van der Neut KM, Aalberse RC. Human IgG4 binds to IgG4 and conformationally altered IgG1 via Fc-Fc interactions. J Immunol. 2009;182:4275–4281. doi: 10.4049/jimmunol.0804338. [DOI] [PubMed] [Google Scholar]
- 8.Rock B, Martins CR, Theofilopoulos AN, et al. The pathogenic effect of IgG4 autoantibodies in endemic pemphigus foliaceus (fogo selvagem) N Engl J Med. 1989;320:1463–1469. doi: 10.1056/NEJM198906013202206. [DOI] [PubMed] [Google Scholar]
- 9.Huijbers MG, Querol LA, Niks EH, et al. The expanding field of IgG4-mediated neurological autoimmune disorders. Eur J Neurol. 2015;22:1151–1161. doi: 10.1111/ene.12758. [DOI] [PubMed] [Google Scholar]
- 10.Dalmau J, Geis C, Graus F. Autoantibodies to Synaptic Receptors and Neuronal Cell Surface Proteins in Autoimmune Diseases of the Central Nervous System. Physiol Rev. 2017;97:839–887. doi: 10.1152/physrev.00010.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delmont E, Manso C, Querol L, et al. Autoantibodies to nodal isoforms of neurofascin in chronic inflammatory demyelinating polyneuropathy. Brain. 2017;140:1851–1858. doi: 10.1093/brain/awx124. [DOI] [PubMed] [Google Scholar]
- 12.Bagci IS, Horvath ON, Ruzicka T, Sardy M. Bullous pemphigoid. Autoimmun Rev. 2017;16:445–455. doi: 10.1016/j.autrev.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Hara M, Arino H, Petit-Pedrol M, et al. DPPX antibody-associated encephalitis: Main syndrome and antibody effects. Neurology. 2017;88:1340–1348. doi: 10.1212/WNL.0000000000003796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olaru F, Wang XP, Luo W, et al. Proteolysis breaks tolerance toward intact alpha345(IV) collagen, eliciting novel anti-glomerular basement membrane autoantibodies specific for alpha345NC1 hexamers. J Immunol. 2013;190:1424–1432. doi: 10.4049/jimmunol.1202204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao J, Yan Y, Cui Z, Yang R, Zhao MH. The immunoglobulin G subclass distribution of anti-GBM autoantibodies against rHalpha3(IV)NC1 is associated with disease severity. Hum Immunol. 2009;70:425–429. doi: 10.1016/j.humimm.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Verpoort KN, Jol-van der Zijde CM, Papendrecht-van der Voort EA, et al. Isotype distribution of anti-cyclic citrullinated peptide antibodies in undifferentiated arthritis and rheumatoid arthritis reflects an ongoing immune response. Arthritis Rheum. 2006;54:3799–3808. doi: 10.1002/art.22279. [DOI] [PubMed] [Google Scholar]
- 17.Ferrari S, Mudde GC, Rieger M, Veyradier A, Kremer Hovinga JA, Scheiflinger F. IgG subclass distribution of anti-ADAMTS13 antibodies in patients with acquired thrombotic thrombocytopenic purpura. J Thromb Haemost. 2009;7:1703–1710. doi: 10.1111/j.1538-7836.2009.03568.x. [DOI] [PubMed] [Google Scholar]
- 18.Lighaam LC, Vermeulen E, Bleker T, et al. Phenotypic differences between IgG4+ and IgG1+ B cells point to distinct regulation of the IgG4 response. J Allergy Clin Immunol. 2014;133:267–270. doi: 10.1016/j.jaci.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 19.Ronaghy A, Streilein RD, Hall RP., III Rituximab decreases without preference all subclasses of IgG anti-BP180 autoantibodies in refractory bullous pemphigoid (BP) J Dermatol Sci. 2014;74:93–94. doi: 10.1016/j.jdermsci.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 20.Guptill JT, Yi JS, Sanders DB, et al. Characterization of B cells in muscle-specific kinase antibody myasthenia gravis. Neurol Neuroimmunol Neuroinflamm. 2015;2:e77. doi: 10.1212/NXI.0000000000000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mouquet H, Musette P, Gougeon ML, et al. B-cell depletion immunotherapy in pemphigus: effects on cellular and humoral immune responses. J Invest Dermatol. 2008;128:2859–2869. doi: 10.1038/jid.2008.178. [DOI] [PubMed] [Google Scholar]
- 22.Diaz-Manera J, Martinez-Hernandez E, Querol L, et al. Long-lasting treatment effect of rituximab in MuSK myasthenia. Neurology. 2012;78:189–193. doi: 10.1212/WNL.0b013e3182407982. [DOI] [PubMed] [Google Scholar]
- 23.Joly P, Maho-Vaillant M, Prost-Squarcioni C, et al. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet. 2017;389:2031–2040. doi: 10.1016/S0140-6736(17)30070-3. [DOI] [PubMed] [Google Scholar]
- 24.Labasque M, Hivert B, Nogales-Gadea G, Querol L, Illa I, Faivre-Sarrailh C. Specific contactin N-glycans are implicated in neurofascin binding and autoimmune targeting in peripheral neuropathies. J Biol Chem. 2014;289:7907–7918. doi: 10.1074/jbc.M113.528489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito A, Sasaki R, Ii Y, Nakayama S, Motomura M, Tomimoto H. A case of thymoma-associated myasthenia gravis with anti-MuSK antibodies. Rinsho Shinkeigaku. 2013;53:372–375. doi: 10.5692/clinicalneurol.53.372. [DOI] [PubMed] [Google Scholar]
- 26.Lauriola L, Ranelletti F, Maggiano N, et al. Thymus changes in anti-MuSK-positive and -negative myasthenia gravis. Neurology. 2005;64:536–538. doi: 10.1212/01.WNL.0000150587.71497.B6. [DOI] [PubMed] [Google Scholar]
- 27.Rose NR, Bona C. Defining criteria for autoimmune diseases (Witebsky’s postulates revisited) Immunol Today. 1993;14:426–430. doi: 10.1016/0167-5699(93)90244-F. [DOI] [PubMed] [Google Scholar]
- 28.Cole RN, Reddel SW, Gervasio OL, Phillips WD. Anti-MuSK patient antibodies disrupt the mouse neuromuscular junction. Ann Neurol. 2008;63:782–789. doi: 10.1002/ana.21371. [DOI] [PubMed] [Google Scholar]
- 29.Jha S, Xu K, Maruta T, et al. Myasthenia gravis induced in mice by immunization with the recombinant extracellular domain of rat muscle-specific kinase (MuSK) J Neuroimmunol. 2006;175:107–117. doi: 10.1016/j.jneuroim.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 30.Klooster R, Plomp JJ, Huijbers MG, et al. Muscle-specific kinase myasthenia gravis IgG4 autoantibodies cause severe neuromuscular junction dysfunction in mice. Brain. 2012;135:1081–1101. doi: 10.1093/brain/aws025. [DOI] [PubMed] [Google Scholar]
- 31.Mori S, Kubo S, Akiyoshi T, et al. Antibodies against muscle-specific kinase impair both presynaptic and postsynaptic functions in a murine model of myasthenia gravis. Am J Pathol. 2012;180:798–810. doi: 10.1016/j.ajpath.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 32.Niks EH, Verrips A, Semmekrot BA, et al. A transient neonatal myasthenic syndrome with anti-musk antibodies. Neurology. 2008;70:1215–1216. doi: 10.1212/01.wnl.0000307751.20968.f1. [DOI] [PubMed] [Google Scholar]
- 33.Punga AR, Lin S, Oliveri F, Meinen S, Ruegg MA. Muscle-selective synaptic disassembly and reorganization in MuSK antibody positive MG mice. Exp Neurol. 2011;230:207–217. doi: 10.1016/j.expneurol.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 34.Shigemoto K, Kubo S, Maruyama N, et al. Induction of myasthenia by immunization against muscle-specific kinase. J Clin Invest. 2006;116:1016–1024. doi: 10.1172/JCI21545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viegas S, Jacobson L, Waters P, et al. Passive and active immunization models of MuSK-Ab positive myasthenia: electrophysiological evidence for pre and postsynaptic defects. Exp Neurol. 2012;234:506–512. doi: 10.1016/j.expneurol.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 36.Huijbers MG, Vink AF, Niks EH, et al. Longitudinal epitope mapping in MuSK myasthenia gravis: implications for disease severity. J Neuroimmunol. 2016;291:82–88. doi: 10.1016/j.jneuroim.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 37.McConville J, Farrugia ME, Beeson D, et al. Detection and characterization of MuSK antibodies in seronegative myasthenia gravis. Ann Neurol. 2004;55:580–584. doi: 10.1002/ana.20061. [DOI] [PubMed] [Google Scholar]
- 38.Niks EH, van LY, Leite MI, et al. Clinical fluctuations in MuSK myasthenia gravis are related to antigen-specific IgG4 instead of IgG1. J Neuroimmunol. 2008;195:151–156. doi: 10.1016/j.jneuroim.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 39.Stathopoulos P, Kumar A, Nowak RJ, O’Connor KC. Autoantibody-producing plasmablasts after B cell depletion identified in muscle-specific kinase myasthenia gravis. JCI Insight. 2017:2. doi: 10.1172/jci.insight.94263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huijbers MG, Zhang W, Klooster R, et al. MuSK IgG4 autoantibodies cause myasthenia gravis by inhibiting binding between MuSK and Lrp4. Proc Natl Acad Sci U S A. 2013;110:20783–20788. doi: 10.1073/pnas.1313944110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koneczny I, Cossins J, Waters P, Beeson D, Vincent A. MuSK myasthenia gravis IgG4 disrupts the interaction of LRP4 with MuSK but both IgG4 and IgG1–3 can disperse preformed agrin-independent AChR clusters. PLoS One. 2013;8:e80695. doi: 10.1371/journal.pone.0080695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koneczny I, Stevens JA, De RA, et al. IgG4 autoantibodies against muscle-specific kinase undergo Fab-arm exchange in myasthenia gravis patients. J Autoimmun. 2017;77:104–115. doi: 10.1016/j.jaut.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 43.Zhu D, Yang Z, Luo Z, Luo S, Xiong WC, Mei L. Muscle-specific receptor tyrosine kinase endocytosis in acetylcholine receptor clustering in response to agrin. J Neurosci. 2008;28:1688–1696. doi: 10.1523/JNEUROSCI.4130-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cole RN, Ghazanfari N, Ngo ST, Gervasio OL, Reddel SW, Phillips WD. Patient autoantibodies deplete postsynaptic muscle-specific kinase leading to disassembly of the ACh receptor scaffold and myasthenia gravis in mice. J Physiol. 2010;588:3217–3229. doi: 10.1113/jphysiol.2010.190298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kawakami Y, Ito M, Hirayama M, et al. Anti-MuSK autoantibodies block binding of collagen Q to MuSK. Neurology. 2011;77:1819–1826. doi: 10.1212/WNL.0b013e318237f660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ayatollahi M, Joubeh S, Mortazavi H, Jefferis R, Ghaderi A. IgG4 as the predominant autoantibody in sera from patients with active state of pemphigus vulgaris. J Eur Acad Dermatol Venereol. 2004;18:241–242. doi: 10.1111/j.1468-3083.2004.00708.x. [DOI] [PubMed] [Google Scholar]
- 47.Warren SJ, Arteaga LA, Rivitti EA, et al. The role of subclass switching in the pathogenesis of endemic pemphigus foliaceus. J Invest Dermatol. 2003;120:104–108. doi: 10.1046/j.1523-1747.2003.12017.x. [DOI] [PubMed] [Google Scholar]
- 48.Yeh SW, Cavacini LA, Bhol KC, et al. Pathogenic human monoclonal antibody against desmoglein 3. Clin Immunol. 2006;120:68–75. doi: 10.1016/j.clim.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 49.Hacker-Foegen MK, Janson M, Amagai M, Fairley JA, Lin MS. Pathogenicity and epitope characteristics of anti-desmoglein-1 from pemphigus foliaceus patients expressing only IgG1 autoantibodies. J Invest Dermatol. 2003;121:1373–1378. doi: 10.1111/j.1523-1747.2003.12608.x. [DOI] [PubMed] [Google Scholar]
- 50.Bhol K, Natarajan K, Nagarwalla N, Mohimen A, Aoki V, Ahmed AR. Correlation of peptide specificity and IgG subclass with pathogenic and nonpathogenic autoantibodies in pemphigus vulgaris: a model for autoimmunity. Proc Natl Acad Sci U S A. 1995;92:5239–5243. doi: 10.1073/pnas.92.11.5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Di ZG, Di LG, Corti D, et al. Pemphigus autoantibodies generated through somatic mutations target the desmoglein-3 cis-interface. J Clin Invest. 2012;122:3781–3790. doi: 10.1172/JCI64413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sitaru C, Mihai S, Zillikens D. The relevance of the IgG subclass of autoantibodies for blister induction in autoimmune bullous skin diseases. Arch Dermatol Res. 2007;299:1–8. doi: 10.1007/s00403-007-0734-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Daveau M, Pavie-Fischer J, Rivat L, et al. IgG4 subclass in malignant melanoma. J Natl Cancer Inst. 1977;58:189–192. doi: 10.1093/jnci/58.2.189. [DOI] [PubMed] [Google Scholar]
- 54.Karagiannis P, Gilbert AE, Josephs DH, et al. IgG4 subclass antibodies impair antitumor immunity in melanoma. J Clin Invest. 2013;123:1457–1474. doi: 10.1172/JCI65579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pieringer H, Parzer I, Wohrer A, Reis P, Oppl B, Zwerina J. IgG4- related disease: an orphan disease with many faces. Orphanet J Rare Dis. 2014;9:110. doi: 10.1186/s13023-014-0110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Schouwenburg PA, Krieckaert CL, Nurmohamed M, et al. IgG4 production against adalimumab during long term treatment of RA patients. J Clin Immunol. 2012;32:1000–1006. doi: 10.1007/s10875-012-9705-0. [DOI] [PubMed] [Google Scholar]
- 57.Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet. 2015;385:1460–1471. doi: 10.1016/S0140-6736(14)60720-0. [DOI] [PubMed] [Google Scholar]
- 58.Yadlapati S, Verheyen E, Efthimiou P. IgG4-related disease: a complex under-diagnosed clinical entity. Rheumatol Int. 2017 doi: 10.1007/s00296-017-3765-7. [DOI] [PubMed] [Google Scholar]
- 59.Kurniawan A, Yazdanbakhsh M, van RR, et al. Differential expression of IgE and IgG4 specific antibody responses in asymptomatic and chronic human filariasis. J Immunol. 1993;150:3941–3950. [PubMed] [Google Scholar]
- 60.Aalberse RC, van der Gaag R, Van LJ. Serologic aspects of IgG4 antibodies. I. Prolonged immunization results in an IgG4-restricted response. J Immunol. 1983;130:722–726. [PubMed] [Google Scholar]
- 61.Gascan H, Gauchat JF, Roncarolo MG, Yssel H, Spits H, de Vries JE. Human B cell clones can be induced to proliferate and to switch to IgE and IgG4 synthesis by interleukin 4 and a signal provided by activated CD4+ T cell clones. J Exp Med. 1991;173:747–750. doi: 10.1084/jem.173.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Punnonen J, Aversa G, Cocks BG, et al. Interleukin 13 induces interleukin 4-independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc Natl Acad Sci U S A. 1993;90:3730–3734. doi: 10.1073/pnas.90.8.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wood N, Bourque K, Donaldson DD, et al. IL-21 effects on human IgE production in response to IL-4 or IL-13. Cell Immunol. 2004;231:133–145. doi: 10.1016/j.cellimm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 64.Akdis CA, Blesken T, Akdis M, Wuthrich B, Blaser K. Role of interleukin 10 in specific immunotherapy. J Clin Invest. 1998;102:98–106. doi: 10.1172/JCI2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jeannin P, Lecoanet S, Delneste Y, Gauchat JF, Bonnefoy JY. IgE versus IgG4 production can be differentially regulated by IL-10. J Immunol. 1998;160:3555–3561. [PubMed] [Google Scholar]
- 66.Satoguina JS, Weyand E, Larbi J, Hoerauf A. T regulatory-1 cells induce IgG4 production by B cells: role of IL-10. J Immunol. 2005;174:4718–4726. doi: 10.4049/jimmunol.174.8.4718. [DOI] [PubMed] [Google Scholar]
- 67.Kimata H, Fujimoto M. Growth hormone and insulin-like growth factor I induce immunoglobulin (Ig)E and IgG4 production by human B cells. J Exp Med. 1994;180:727–732. doi: 10.1084/jem.180.2.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yilmaz V, Oflazer P, Aysal F, et al. Differential Cytokine Changes in Patients with Myasthenia Gravis with Antibodies against AChR and MuSK. PLoS One. 2015;10:e0123546. doi: 10.1371/journal.pone.0123546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yilmaz V, Oflazer P, Aysal F, et al. B cells produce less IL-10, IL-6 and TNF-alpha in myasthenia gravis. Autoimmunity. 2015;48:201–207. doi: 10.3109/08916934.2014.992517. [DOI] [PubMed] [Google Scholar]
- 70.Ragheb S, Lisak R, Lewis R, Van SG, Gonzales F, Simon K. A potential role for B-cell activating factor in the pathogenesis of autoimmune myasthenia gravis. Arch Neurol. 2008;65:1358–1362. doi: 10.1001/archneur.65.10.1358. [DOI] [PubMed] [Google Scholar]
- 71.Amara K, Steen J, Murray F, et al. Monoclonal IgG antibodies generated from joint-derived B cells of RA patients have a strong bias toward citrullinated autoantigen recognition. J Exp Med. 2013;210:445–455. doi: 10.1084/jem.20121486. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 72.Bastiaansen AEM, van SA, Titulaer MJ. Autoimmune encephalitis with anti-leucine-rich glioma-inactivated 1 or anti-contactin-associated protein-like 2 antibodies (formerly called voltage-gated potassium channel-complex antibodies) Curr Opin Neurol. 2017;30:302–309. doi: 10.1097/WCO.0000000000000444. [DOI] [PubMed] [Google Scholar]
- 73.Beck LH., Jr PLA2R and THSD7A: Disparate Paths to the Same Disease? J Am Soc Nephrol. 2017;28:2579–2589. doi: 10.1681/ASN.2017020178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Casina VC, Hu W, Mao JH, et al. High-resolution epitope mapping by HX MS reveals the pathogenic mechanism and a possible therapy for autoimmune TTP syndrome. Proc Natl Acad Sci U S A. 2015;112:9620–9625. doi: 10.1073/pnas.1512561112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Di ZG, Amber KT, Sayar BS, Muller EJ, Borradori L. Immune response in pemphigus and beyond: progresses and emerging concepts. Semin Immunopathol. 2016;38:57–74. doi: 10.1007/s00281-015-0541-1. [DOI] [PubMed] [Google Scholar]
- 76.Doppler K, Appeltshauser L, Villmann C, et al. Auto-antibodies to contactin-associated protein 1 (Caspr) in two patients with painful inflammatory neuropathy. Brain. 2016;139:2617–2630. doi: 10.1093/brain/aww189. [DOI] [PubMed] [Google Scholar]
- 77.Gaig C, Graus F, Compta Y, et al. Clinical manifestations of the anti-IgLON5 disease. Neurology. 2017;88:1736–1743. doi: 10.1212/WNL.0000000000003887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Greco A, Rizzo MI, De VA, et al. Goodpasture’s syndrome: a clinical update. Autoimmun Rev. 2015;14:246–253. doi: 10.1016/j.autrev.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 79.Mariotte E, Azoulay E, Galicier L, et al. Epidemiology and pathophysiology of adulthood-onset thrombotic microangiopathy with severe ADAMTS13 deficiency (thrombotic thrombocytopenic purpura): a cross-sectional analysis of the French national registry for thrombotic microangiopathy. Lancet Haematol. 2016;3:e237–e245. doi: 10.1016/S2352-3026(16)30018-7. [DOI] [PubMed] [Google Scholar]
- 80.Peyron E, Nicolas JF, Reano A, et al. Human monoclonal autoantibodies specific for the bullous pemphigoid antigen 1 (BPAg 1) J Immunol. 1994;153:1333–1339. [PubMed] [Google Scholar]
- 81.Phillips WD, Vincent A. Pathogenesis of myasthenia gravis: update on disease types, models, and mechanisms. F1000Res. 2016:5. doi: 10.12688/f1000research.8206.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Querol L, Nogales-Gadea G, Rojas-Garcia R, et al. Antibodies to contactin-1 in chronic inflammatory demyelinating polyneuropathy. Ann Neurol. 2013;73:370–380. doi: 10.1002/ana.23794. [DOI] [PubMed] [Google Scholar]
- 83.Querol L, Illa I. Paranodal and other autoantibodies in chronic inflammatory neuropathies. Curr Opin Neurol. 2015;28:474–479. doi: 10.1097/WCO.0000000000000233. [DOI] [PubMed] [Google Scholar]
- 84.Schaller M, Vogel M, Kentouche K, Lammle B, Kremer Hovinga JA. The splenic autoimmune response to ADAMTS13 in thrombotic thrombocytopenic purpura contains recurrent antigen-binding CDR3 motifs. Blood. 2014;124:3469–3479. doi: 10.1182/blood-2014-04-561142. [DOI] [PubMed] [Google Scholar]
- 85.Sinico RA, Mezzina N, Trezzi B, Ghiggeri GM, Radice A. Immunology of membranous nephropathy: from animal models to humans. Clin Exp Immunol. 2016;183:157–165. doi: 10.1111/cei.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Skaria M, Jaunin F, Hunziker T, et al. IgG autoantibodies from bullous pemphigoid patients recognize multiple antigenic reactive sites located predominantly within the B and C subdomains of the COOH-terminus of BP230. J Invest Dermatol. 2000;114:998–1004. doi: 10.1046/j.1523-1747.2000.00893.x. [DOI] [PubMed] [Google Scholar]
- 87.Tomas NM, Hoxha E, Reinicke AT, et al. Autoantibodies against thrombospondin type 1 domain-containing 7A induce membranous nephropathy. J Clin Invest. 2016;126:2519–2532. doi: 10.1172/JCI85265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van der Woude D, Catrina AI. HLA and anti-citrullinated protein antibodies: Building blocks in RA. Best Pract Res Clin Rheumatol. 2015;29:692–705. doi: 10.1016/j.berh.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 89.van Sonderen SA, Petit-Pedrol M, Dalmau J, Titulaer MJ. The value of LGI1, Caspr2 and voltage-gated potassium channel antibodies in encephalitis. Nat Rev Neurol. 2017;13:290–301. doi: 10.1038/nrneurol.2017.43. [DOI] [PubMed] [Google Scholar]