Abstract
Age-related macular degeneration (AMD) is a retinal disease associated with significant vision loss among older adults. Previous large-scale behavioral studies indicate that people with AMD are at increased risk of cognitive deficits in language processing, particularly in verbal fluency tasks. The neural underpinnings of any relationship between AMD and higher cognitive functions, such as language processing, remain unclear. This study aims to address this issue using independent component analysis (ICA) of spontaneous brain activity at rest. In two components associated with visual processing, we observed weaker functional connectivity in the primary visual cortex and lateral occipital cortices in AMD patients compared to healthy controls, indicating that AMD might lead to differences in the neural representation of vision. In a component related to language processing, we found that increasing connectivity within the right inferior frontal gyrus (RIFG) was associated with better verbal fluency performance across all older adults, and the verbal fluency effect was greater in AMD patients than controls in both RIFG and right posterior temporal regions. As the behavioral performance of our patients is as good as that of controls, these findings suggest that preservation of verbal fluency performance in AMD might be achieved through higher contribution from right hemisphere regions in bilateral language networks. If that is the case, there may be an opportunity to promote cognitive resilience among seniors with AMD or other forms of late-life vision loss.
Keywords: Age-related macular degeneration, functional connectivity, cognitive preservation, language processing
Introduction
The human brain tends to undergo reorganization to preserve cognitive functions in the circumstances of diseases or lesions, however, controversy remains with respect to the degree and nature of brain plasticity, especially in aging. Research on visual deprivation provides a particular angle to investigate whether visual loss from retinal lesions will ultimately change the neural systems for visual processing and higher level cognitive processes in the brain. Many studies suggested that early blindness induces functional reorganization with significant activation in visual cortex during Braille reading (Sadato N et al. 1996; Burton H et al. 2002), and in sentence-level language processing (Bedny M et al. 2011). However, there is less consensus on whether late-life partial visual loss will fundamentally change the neural systems for visual and language processes. When the central retina is damaged by macular degeneration, corresponding visual representation in the primary visual cortex (V1) is deprived of input, and therefore diminished activity in V1 was observed in response to visual stimulation of retina fovea in AMD patients (Sunness JS et al. 2004). However, stimulation of the intact peripheral retina of AMD patients generated significant activation in V1, a pattern which was surprising and could be interpreted as evidence of large-scale functional reorganization of the visual cortex (Baker CI et al. 2005; Masuda Y et al. 2008). Contrary to these findings, Baseler et al. (2011) did not observe evidence of remapping since activity in V1 in macular degeneration patients was similar to the simulated results in controls.
Here, we revisit the issue of neuroplasticity in the context of late life vision loss by investigating whether macular degeneration is associated with differences in intrinsic connectivity patterns in the visual cortex and fronto-temporal language processing network. As neural activity in the cortex could be sensitive to cognitive task demands, and task demands may differ in people with low vision (Masuda Y et al. 2008), here we take a task-free approach using resting-state fMRI (i.e., rs-fMRI which measures spontaneous neural activity without an explicit cognitive task) to examining differences in cognitive behavior and brain connectivity among people with age-related macular degeneration (AMD).
AMD is a common eye condition that affects the macula, which is the portion of retina responsible for sharp, central vision (Bressler NM et al. 1988; National Eye Institute 2011). AMD normally affects people over 50 years of age, and the annual incidence of AMD increases with age, with around 30% of Americans over age 75 having AMD (National Eye Institute 2011). AMD causes severe difficulties in daily life by affecting any activities that require differentiation of fine spatial details such as reading, face and object recognition (Stelmack JA et al. 2004; Szlyk JP et al. 2004). Behavioral studies also indicate that AMD is associated with late-life cognitive decline with higher rates of cognitive impairment (Pham TQ et al. 2006; Woo SJ et al. 2012), lower scores on cognitive tests even when tests do not involve visually mediated tasks (Wong TY et al. 2002; Clemons T et al. 2006; Baker ML et al. 2009), and higher risk of incident dementia (Klaver CC et al. 1999). Recent research by our group and others observes that AMD patients perform particularly worse than healthy controls on tasks of phonemic verbal fluency (Wong TY et al. 2002; Clemons T et al. 2006; Whitson HE et al. 2010) using tasks that do not rely on visual ability, indicating that AMD might be related to speech production deficits or difficulties in word finding processes.
However, little is known about the brain mechanisms underpinning these cognitive declines associated with AMD, since very few neuroimaging studies have been performed to address this issue. Using a semantic judgment task, Szlyk and Little (2009) found that AMD patients generated greater activation in the left prefrontal and superior and inferior parietal regions than controls during single word recognition. Increasing neural activity in these regions correlated with better behavioral performance including higher response accuracy and shorter response time, suggesting that AMD might trigger functional reorganization in the fronto-parietal control network as a means of compensating for decreased visual function. It remains unclear whether activity in the fronto-parietal regions is purely driven by word level language processing per se, or if the fronto-parietal activity reflects increased cognitive effort required to support performance as task demands increase due to sensory impairment. If so, the relationship between AMD and regional brain activity may simply be influenced by task demands (Wright P et al. 2011; Davis SW et al. 2014).
Another potentially influential variable is age. Normal aging is associated with significant declines in gray matter volumes and white matter integrity in the brain, with the prefrontal cortex and temporal regions among the most vulnerable regions (Sowell ER et al. 2003; Raz N 2005; Barrick TR et al. 2010; Fjell AM and KB Walhovd 2010). Some language functions, however, are well preserved during normal aging despite age-related brain atrophy due to functional reorganization of the neural language system (Wingfield A and M Grossman 2006; Tyler LK et al. 2009; Shafto MA and LK Tyler 2014). The compensation hypothesis is one theoretical account for this the age-related pattern (Cabeza R 2002; Wierenga CE et al. 2008; Park DC and P Reuter-Lorenz 2009; Peelle JE et al. 2010). According to this hypothesis, additional neural resources, such as right fronto-temporal regions, are often recruited in older adults to compensate for brain tissue loss in the left hemisphere and maintain language functions maximally. Supporting evidence comes from observations that the additional activation in the right hemisphere correlates with better behavioral performance (Wierenga CE et al. 2008) and decreasing grey matter volume in the left fronto-temporal regions (Tyler LK et al. 2009). In contrast, other researchers suggest that age-related neural changes might reflect a more diffuse and less efficient neural response to natural age-related decline of gray and white matter (Li S-C et al. 2001; Park DC et al. 2004), since additional activation in the right hemisphere is not always correlated with behavioral performance (Logan JM et al. 2002; Meinzer M et al. 2009; Geva S et al. 2012; Diaz MT et al. 2014).
To investigate the impact of late-life visual loss on the neural language system as well as potential reorganization of this neural system as a consequence of normal aging, we examined the relation between verbal fluency, as a representative behavioral measure of language processing, and spontaneous brain activity of the language system among patients with age-related macular degeneration and age-matched seniors with healthy vision. Spontaneous neural activity is measured by low frequency (< 0.08Hz) fluctuations (LFF) of the blood oxygen level-dependent fMRI signal in so-called resting state fMRI scans, which have been considered an effective tool in exploring intrinsic functional connectivity among brain regions (Raichle ME and DA Gusnard 2005; Raichle ME and MA Mintun 2006). We used independent component analysis (ICA) to extract sets of intrinsic neural networks, then selected language and visual processing networks based on previous research.
To identify neural language networks in this study, we took a previously established dual-system model as a framework (Bozic M et al. 2010). This model proposes that a bilateral fronto-temporal system is broadly involved in basic word-level processes (Jung-Beeman M 2005; Longworth C et al. 2005), and that a left-lateralized fronto-temporal system specially engages in high-level syntactic processing (Hagoort P 2005; Tyler LK et al. 2011). If this left-lateralized vs. bi-hemispheric separation based on experimental studies is correct, we would expect to find two fronto-temporal networks from our task-free data using ICA: one network that is bi-hemispheric and another that is left-lateralized. Furthermore, we expect to find significant correlations between verbal fluency and neural activity in some regions, for example, left and right inferior frontal gyrus (IFG)(Shafto MA and LK Tyler 2014), in the bi-hemispheric language network, but not in the left-lateralized syntactic network, as verbal fluency is not a measure of syntactic processing.
To address the question whether AMD may trigger functional reorganization of the intrinsic neural language system, we compared the brain connectivity patterns associated with better verbal fluency performance among non-demented older adults with and without AMD. We expect to observe a significant interaction between age group and verbal fluency, with the relationship between verbal fluency and fronto-temporal brain connectivity stronger in AMD patients than in controls. Finally, to investigate the influence of AMD on neural connectivity in visual cortices, we identified the V1 and lateral occipital cortex (LOC) networks from the ICA analysis based on previous research, and then compared brain connectivity patterns between groups in these two visual networks. We hypothesized that there would be weaker connection in AMD patients than in controls in both visual networks, as a consequence of long-term visual deprivation.
Methods
Participants
Twenty seven AMD patients and 27 healthy controls participated in this study (9 males, ages 59–89, mean age = 72.8, for AMD patients; 12 males, ages 56–85, mean age = 72.4, for controls). All participants were community-dwelling, right-handed (Edinburgh Handedness Inventory, Oldfield RC (1971), fluent speakers of American English. AMD patients were referred from local ophthalmology clinics; all had visual impairment attributable to AMD (though a range of visual acuity was included from 0.1 to 1.25 per the Logarithm of the Minimal Angle of Resolution (LogMAR)(Kaiser PK 2009), and no other significant eye disease. All healthy controls underwent examination by an ophthalmologist to confirm they had normal vision without any significant eye diseases. All participants provided informed consent and were compensated for their time. All experimental procedures were approved by the Duke University Medical Center Institutional Review Board.
Behavioral tests
All participants received a battery of cognitive tests chosen to be suitable for individuals with vision impairment in that no tasks involved visual cueing or relied on visual ability (i.e., no reading, drawing, image recognition, etc.). Additionally, all of the tests were validated for use with auditory cues. These tests included the Controlled Oral Word Association Test with letters F, A, S (COWAT-FAS) (Spreen O and AL Benton 1977), Animal Naming (Monsch AU et al. 1992; Sager MA et al. 2006), the Fuld Object Memory Evaluation (FOME) (Fuld PA et al. 1990), the Brief Test of Adult Cognition by Telephone (BTACT, administered in person) (Tun PA and ME Lachman 2006), the Digit Span subtest from the Wechsler Adult Intelligence Scale Version III (WAIS-III) and the Logical Memory Test from the Wechsler Memory Scale Version III (WMS-III) (Iverson GL 2001). As tasks from several tests might reflect the same cognitive process such as memory or verbal fluency, we applied a factor analysis to test data. First, we performed an analysis on a set of verbal fluency-related measures, including a summative measure of phonemic verbal fluency from the COWAT (summed score for letters F, A, and S) and 4 categorical verbal fluency measures, drawn from the FOME and the Animal Naming tests, that included the semantic categories: foods, “things that make people happy,” boys’ or girls’ names, and animals. We extracted out a single factor as a representative measure of verbal fluency processes. The new factor correlated highly with each of the five input measures, with Pearson r correlation coefficients of 0.86, 0.84, 0.83, 0.74, and 0.86, respectively. We also extracted a factor of memory based on the input measures of BTACT 15-word item recall, WMS-III Logical Memory immediate and delayed recall, and total item recall from the FOME, which is based on a selective reminding procedure. The correlations between the new factor and the four input measures were 0.45, 0.94, 0.97, and 0.64, respectively. Each participant also underwent visual acuity and audiometric testing. All the cognitive tests were performed right after the MRI scans on the same day or a few days later to avoid any potential influence of cognitive test content on the functional activity in resting state scans. The duration of these cognitive tests was approximately 3 h for each participant.
MRI acquisition and imaging analysis
Scanning was performed on a 3.0 Tesla GE MR 750 whole-body 60 cm bore human scanner equipped with 40 mT/m gradients and a 150 T/m/s slew rate. An eight-channel head coil was used for radio frequency (RF) reception (General Electric, Milwaukee Wisconsin, USA). Sagittal T-1 weighted localizer images were acquired and used to define a volume for data collection and high order shimming. A semi-automated high-order shimming program was used to ensure global field homogeneity. High-resolution structural images were acquired using a 3D fSPGR pulse sequence (Repetition time [TR] = 8.156 ms; echo time [TE] = 3.18 ms; TI = 450 ms; field of view [FOV] = 25.6 cm2; flip angle =12°; voxel size = 1 × 1 × 1 mm; 166 contiguous slices, sense factor = 2). Two runs of functional images were acquired during resting state using a Gradient Echo sequence (TR = 2 s, TE = 27 ms, flip angle = 77°, FOV = 24 cm2, SENSE factor = 1, voxel size = 3.75 × 3.75 × 4 mm, 34 contiguous oblique axial slices, parallel to the AC-PC line, interleaved acquisition). In each resting state run, four initial RF excitations were performed to achieve steady state equilibrium and were subsequently discarded. Each resting state run consisted of the acquisition of a time series of 180 brain volumes (images), which lasted for 6 minutes.
The resting state data were preprocessed using an in-house developed pipeline (https://wiki.biac.duke.edu/biac:analysis:resting_pipeline) based on tools from the Oxford Centre for Functional MRI of the Brain’s Software Library (FSL version 4.0, www.fmrib.ox.ac.uk/fsl). The first four images were removed for each resting state run to achieve steady state equilibrium. All remaining functional images were aligned with respect to the first image within each run to correct for head movements during data acquisition. A bandpass filter was applied to filter the functional data in the time dimension so that frequencies were retained between 0.001 Hz and 0.08 Hz. The aligned and filtered images were normalized to the MNI space using a 12 degree of freedom affine transformation implemented in the FSL Linear Image Registration Tool. We further removed constant offsets and linear drift over each run (Fair DA et al. 2007), and regressed out the six rigid body head motion parameters, the signal averaged over the white matter and the signal averaged over the cerebrospinal fluid regions to reduce non-neuronal influence to BOLD corrections (Van Dijk KR et al. 2010). The normalized images were smoothed using an isotropic Gaussian kernel of 5 mm FWHM.
The ICA analysis was used to identify distinct cognitive networks based on functional data during resting state. We used the Group ICA toolbox (GIFT 2.0a; icatb.sourceforge.net) (Calhoun V et al. 2001; Calhoun VD et al. 2004; Calhoun VD et al. 2009) to analyze the resting state data of all participants and reduced them into 20 independent components (ICs) using principal component analysis. The Infomax ICA algorithm (Bell AJ and TJ Sejnowski 1995) was used to calculate the ICs. The group ICs were then back-reconstructed into subject-specific spatial maps, which were converted into Z-scores for the purpose of comparisons among individuals. To identify and select the primary visual processing networks from the 20 ICs, we took well-defined visual processing networks from a previous study (Laird AR et al. 2011) as spatial templates to sort the 20 ICs, and chose the best-fit component which showed the most similar spatial pattern to the templates. The same spatial template selection criterion was used to identify the language processing components based on a pre-defined language network in Laird et al. (2011). There were three visual processing networks reported in Laird et al. (2011), with the first one (IC no. 10) in the middle temporal visual association area (MT, MST, V5; BA 37/39), and the other two networks (IC nos. 11 & 12) in the primary, secondary, and tertiary visual cortices (V1, V2, V3; BA 17/18/19). The language network in Laird et al. (2011) was composed of strongly left-lateralized regions such as IFG (BA 44/45), anterior superior temporal gyrus (STG) and inferior parietal regions (BA 22/39/40).
For each selected IC, the spatial maps for all participants were analyzed using the multiple regression method in a group random effects model in SPM8 (Wellcome Institute of Cognitive Neurology, London, UK. www.fil.ion.ucl.ac.uk), under MATLAB (Mathworks Inc., Natick, MA, USA). As age and gender might affect neural activity of our variables of interests, we included them as extraneous variables in all random-effects analysis models. For the two primary visual processing networks (the V1 and LOC ICs), the design matrix consisted of four modulators (regressors): age, gender, visual acuity scores, and group. The first modulator consisted of continuous data of actual age of each participant, and the second modulator of gender (coded as 0 or 1). The third modulator was composed of continuous data of binocular visual acuity (the mean LogMAR score value of left and right eyes) for each participant, and the fourth modulator (group) consisted of categorical data of AMD patient vs. healthy control (coded as 0 or 1). In this model, age, gender and visual acuity were taken as three extraneous variables so that any potential group difference between patients and controls, measured by the fourth modulator, would not be confounded with individual differences in the extraneous variables.
For the two language processing networks (the left-lateralized and bilateral fronto-temporal ICs), the design matrix consisted of five modulators: age, gender, verbal fluency (factor score), group, and the interaction between verbal fluency and group. The modulators of age, gender, and group were the same measures that were used in the analysis of the visual processing networks. The interaction measure was calculated by multiplying the mean-corrected verbal fluency scores and the mean-corrected group values. The correlations among these three modulators of interest were very low, r = −0.17, p > 0.1, between verbal fluency and group; r = −0.11, p > 0.1, between verbal fluency and the interaction; r < 0.001, p > 0.1, between group and the interaction.
Because the brain atrophies in healthy aging, varying brain volume sizes across older adults might affect our neuroimaging results. To evaluate the influence of brain atrophy, we measured the global brain volume of each participant from the T1 structural image, then calculated the mean brain volume size of each group (Table 1). We further performed a series of test models by including the brain volume size as an extraneous variable in each of the original models described above, and found the same effects of interest as in the original models. Because the test models indicated that main findings were not affected by varying brain volume sizes across participants, we only reported effects of interest from the original analysis models in the Results section.
Table 1.
Participant demographics2
| AMD | control | |
|---|---|---|
| N | 27 | 27 |
| Age | 72.81 (8.39) | 72.41 (7.90) |
| Gender (female proportion) | 66.67% | 55.56% |
| Education | 15.30 (2.96) | 16.04 (2.71) |
| Brain volume (native space) | 1431.46 (138.81) | 1443.22 (132.76) |
| Brain volume (normalized) | 1957.72 (29.89) | 1961.90 (44.95) |
| Pure tone average threshold, decibels (right ear) | 31.20 (17.14) | 30.41 (16.69) |
| Pure tone average threshold, decibels (left ear) | 33.44 (17.06) | 36.84 (17.86) |
| Visual acuity (OS) | 0.52 (0.38) | 0.07 (0.09) |
| Visual acuity (OD) | 0.46 (0.33) | 0.05 (0.08) |
| Visual acuity (mean)* | 0.49 (0.30) | 0.06 (0.08) |
| Verbal fluency (phonemic) | 32.74 (13.33) | 37.04 (16.39) |
| Verbal fluency (categorical) | 16.78 (4.62) | 19.74 (6.78) |
| Wechsler (initial) | 24 (6.39) | 26.19 (8.00) |
| Wechsler (delayed) | 19.93 (7.46) | 21.96 (9.24) |
| BTACT | 5.93 (2.46) | 6.04 (1.74) |
| Digit span | 4 (1.41) | 4.19 (1.52) |
| FULD Cued Item Recall | 24.56 (4.94) | 24.74 (4.52) |
| FULD Fluency - Names | 20.78 (7.33) | 20.67 (5.63) |
| FULD Fluency - Food | 21.48 (6.33) | 22.67 (6.50) |
| FULD Fluency - Happy | 13.96 (4.69) | 15.81 (5.32) |
Values provided are means, with standard deviation in parentheses.
A mask of each IC network was made based on the significant connectivity map of each IC, then applied to subsequent correlational analyses with cognitive performance to confine significant effects within each network. Significant results were identified using a two-stage process. First, connectivity strength was thresholded at p < 0.001, uncorrected, at the voxel level. Second, multiple comparisons were performed to select significant clusters only when they survive p < 0.05, at the cluster level, unless otherwise stated. SPM coordinates are reported in MNI space. Coordinates of significant clusters peaks and sub-peaks for all analyses were reported in Tables 2 & 3 in MNI space. The extents of connectivity strength are further verbally characterized in the text. Regions were identified by using the AAL atlas (Tzourio-Mazoyer N et al. 2002) and Brodmann templates as implemented in MRIcron (http://www.MRicro.com/MRicron).
Table 2.
Areas of activity for the visual processing networks and group effects in these networks
| Cluster regions | BA | size (voxels) | Max Z | MNI Coord (mm) | ||
|---|---|---|---|---|---|---|
|
| ||||||
| x | y | z | ||||
| The V1 IC | ||||||
| L/R calcarine, lingual gyri, fusiform, IOG | 17, 18, 19, 37 | 37072 | >20 | −2 | −70 | 4 |
| L/R ACC | 24, 32 | 2612 | 6.12 | 6 | 18 | 48 |
| L AG, IPL | 39 | 235 | 5.16 | −38 | −58 | 42 |
| L SFG, MFG | 9, 46 | 476 | 5.03 | −30 | 56 | 18 |
| R AG | 39 | 237 | 4.77 | 56 | −58 | 36 |
| controls minus AMD patients in the V1 network | ||||||
| L calcarine | 17 | 79 | 3.91 | −10 | −86 | 6 |
| The LOC IC | ||||||
| L/R IOG, MOG, SOG, fusiform, MTG, ITG | 18, 19, 37, 20 | 26603 | >20 | −40 | −84 | 2 |
| L IFG | 47 | 126 | 5.77 | −34 | 36 | −20 |
| R temporal pole, fusiform, parahippocampus | 20, 36, 38 | 867 | 5.22 | 20 | −2 | −28 |
| L/R SMFG, AG | 10, 32 | 980 | 5.21 | 8 | 66 | 16 |
| L/R AG | 24 | 125 | 4.39 | −2 | 10 | 20 |
| controls minus AMD patients in the LOC network | ||||||
| L MOG, MTG | 37 | 134 | 4.68 | −46 | −66 | 2 |
| R MOG, MTG, ITG | 19, 37 | 264 | 4.17 | 50 | −52 | 2 |
BA: Brodmann area, IC: independent component, L: left, R: right, AG: angular gyrus, IFG: inferior frontal gyrus, IOG: inferior occipital gyrus, IPL: inferior parietal lobule, ITG: inferior temporal gyrus, MFG: middle frontal gyrus, MOG: middle occipital gyrus, MTG: middle temporal gyrus, SFG: superior frontal gyrus, SOG: superior occipital gyrus
Table 3.
Areas of activity for the language processing networks and relevant cognitive effects
| Cluster regions | BA | size (voxels) | Max Z | MNI Coord (mm) | ||
|---|---|---|---|---|---|---|
|
| ||||||
| x | y | z | ||||
| The left-lateralized language IC | ||||||
| L IFG, MFG, STG, MTG, ITG, SMG, AG, IPL, ACC, SMFG | 44, 45, 47, 6, 22, 21, 20, 39, 10, 32, 37 | 41666 | >20 | −52 | 34 | 8 |
| R cerebellum | 2349 | >20 | 30 | −66 | −36 | |
| R AG, SMG, IPL, SOG | 39, 40, 7, 19 | 3111 | >20 | 34 | −64 | 34 |
| R IFG | 44, 45 | 1392 | >20 | 48 | 34 | 18 |
| L calcarine, R lingual gyrus | 17, 18 | 830 | 5.45 | −6 | −90 | −8 |
| R IFG | 47 | 185 | 4.79 | 28 | 40 | −16 |
| The bilateral la nguage IC | ||||||
| R IFG, STG, MTG, ITG, SMG, AG, anterior cingulate, SMFG, SMA | 44, 45, 47, 22, 21, 20, 39, 10, 32, 37 | 26988 | >20 | 54 | 28 | −8 |
| L IFG, STG, MTG, ITG, SMG, AG, anterior cingulate, SMFG, SMA | 44, 45, 47, 22, 21, 20, 39, 10, 32, 37 | 9715 | >20 | −54 | 20 | 14 |
| R cerebellum | 669 | 7.31 | 24 | −78 | −42 | |
| L cerebellum | 579 | 6.63 | −22 | −74 | −40 | |
| L/R precuneus | 349 | 6.29 | 10 | −54 | 38 | |
| R SPL, IPL | 40, 7 | 268 | 4.88 | 34 | −60 | 58 |
| L/R ACC | 24 | 164 | 4.41 | 4 | 22 | 12 |
| Positive fluency effect in the bilateral language network | ||||||
| R IFG | 44, 45 | 126 | 3.99 | 48 | 14 | 30 |
| The interaction between fluency and groups in the bilateral language network | ||||||
| R IFG | 47 | 211 | 4.39 | 46 | −70 | 10 |
| R MTG | 37 | 94 | 4.33 | 30 | 34 | −6 |
BA: Brodmann area, IC: independent component, L: left, R: right, AG: angular gyrus, IFG: inferior frontal gyrus, IPL: inferior parietal lobule, ITG: inferior temporal gyrus, MFG: middle frontal gyrus, MTG: middle temporal gyrus, SMA: supplementary motor area, SMFG: superior medial frontal gyrus, SMG: supramarginal gyrus, SOG: superior occipital gyrus, SPL: superior parietal lobule, STG: superior temporal gyrus
To demonstrate potential interaction effects in the imaging analyses, we took significant clusters as regions of interest (ROIs), and extracted out neural connectivity in each IC network using MarsBaR (marsbar.sourceforge.net). The extracted neural activity in each ROI in each IC network was correlated with verbal fluency across AMD patients and controls, respectively, using SAS (SAS Institute Inc., Cary, NC, USA). Further group difference were tested on whether AMD patients differed from controls in neural-behavioural correlations (http://vassarstats.net/rdiff.html).
Results
Participant demographics, behavioral cognitive task performance, and visual task performance are reported in Table 1. As expected, AMD patients had significantly lower visual acuity measures compared with controls, but no significant group differences were found in any cognitive measures or in the hearing test, indicating that cognitive abilities were well preserved in this group of AMD patients.
In the group ICA, we adopted a spatial sorting approach to map each of the 20 IC maps to a given spatial template and identify the best-fit IC map. To identify potential visual processing networks, we used each of the three visual perception networks (nos. 10, 11, and 12) from Laird et al. (2011) as a spatial template in this study, and identified two best-fit ICs. One IC map (see Table 2 & Fig. 1A) was the best match spatially to the visual template no. 12 in Laird et al. (2011), and this IC was named the primary visual cortex (or V1) network based on the brain regions. The other IC map (Table 2 & Fig. 2A) was the best spatial match to both the visual templates no. 10 and no. 11 in Laird et al. (2011) in two separate sorting analyses, and this IC was named the LOC network based on the brain regions. To identify potential language processing networks, we chose the pre-defined language processing network (no. 18) in Laird et al. (2011) as a spatial sorting template, and selected two best-fit ICs. Although Laird describes only one language processing network, according to the dual-system theory (Bozic M et al. 2010), we would expect to find two fronto-temporal language processing networks, one that is left-lateralized and another that is bilateral. Indeed, the best-fit IC in this sorting analysis was a left-lateralized fronto-temporal network (Table 3 & Fig. 3A), and the second best-fit IC was a bilateral fronto-temporal network (Table 3 & Fig. 3B).
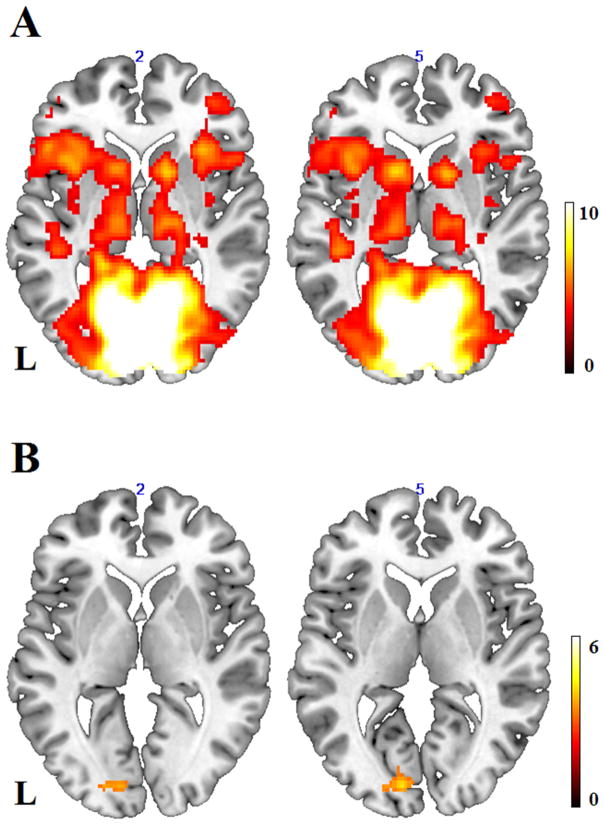
Figure 1.
(A) Significant connectivity strength pattern in the selected component of primary visual cortex at a threshold of p < 0.001, voxel-level uncorrected, and p < 0.05, cluster-level corrected. L = left hemisphere. (B) Significant connectivity strength pattern for the contrast of healthy controls minus AMD patients at a threshold of p < 0.001, voxel-level uncorrected, and p < 0.07, cluster-level corrected, indicating lower intrinsic connection strength within the primary visual network in AMD patients than controls.
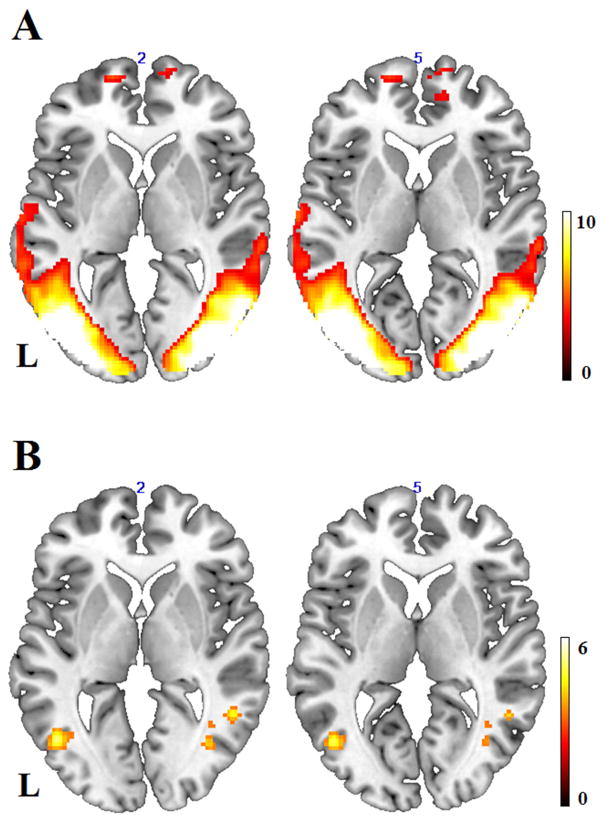
Figure 2.
(A) Significant connectivity strength pattern in the selected component of lateral occipital cortex at a threshold of p < 0.001, voxel-level uncorrected, and p < 0.05, cluster-level corrected. L = left hemisphere. (B) Significant connectivity strength pattern for the contrast of healthy controls minus AMD patients at a threshold of p < 0.001, voxel-level uncorrected, and p < 0.05, cluster-level corrected, indicating lower intrinsic connection strength within this visual network in AMD patients than controls.
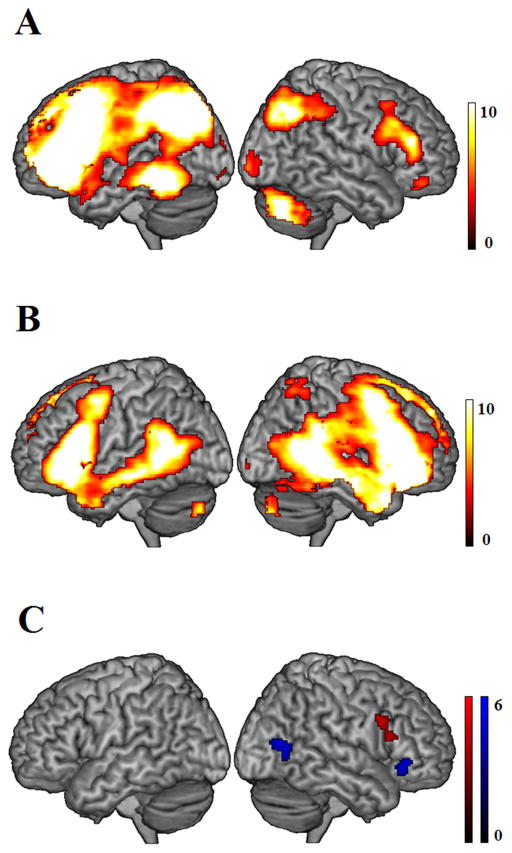
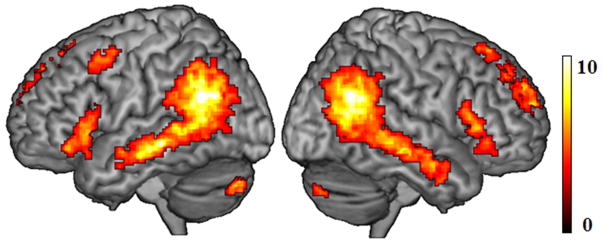
Figure 3.
Significant connectivity strength pattern in the selected component of left-lateralized language network (A) and in the component of the bilateral language network (B) for all participants. (C) Significant connectivity strength pattern for the main effect of verbal fluency across all participants (red), and significant connectivity strength pattern for the interaction between verbal fluency and group (blue), with a greater verbal fluency effect in AMD patients than that in controls. All results were thresholded at p < 0.001, voxel-level uncorrected, and p < 0.05, cluster-level corrected.
To test whether visual loss was associated with weaker intrinsic functional connectivity within visual cortex, we compared the V1 network maps of AMD patients and those of controls, after partialling out individual difference in visual acuity scores. Compared to controls, AMD patients exhibited marginally significant (p = 0.063) weaker functional connectivity strength in the L calcarine (BA 17), within the domain of primary visual cortex (Table 2 & Fig. 1B). In terms of the LOC network, we performed a similar analysis between these two groups and found significantly weaker connectivity strength for AMD patients compared with controls in bilateral LOC regions such as middle occipital gyri (MOG) and posterior middle temporal gyri (pMTG, BA 19, 37), and right posterior inferior temporal gyri (ITG, BA 37, see Table 2 & Fig. 2B).
To address the issue of whether visual loss might also be related to intrinsic functional connectivity in higher level cognitive systems such as language processing networks, we performed an ANOVA on the two selected language networks, respectively. For the bilateral fronto-temporal language network, we directly compared AMD patient with controls in the condition of group, but did not find significant difference. We further correlated each individual’s IC map to his or her verbal fluency scores, and observed a significant effect in the RIFG (BA 44, 45, see Table 3 & Fig. 3C) with increasing connectivity strength for higher verbal fluency scores. There was no significant difference between AMD patients and controls in a direct comparison of connectivity strength within the bilateral fronto-temporal language network. However, there was a significant interaction between group (AMD patients vs. controls) and verbal fluency in the RIFG (BA 47) and right pMTG (BA 37), showing greater connectivity strength for the verbal fluency effect in the AMD patients than in controls (Table 3 & Fig. 3C). In other words, the positive relationship between this network’s right-sided functional connectivity and verbal fluency performance was stronger in AMD patients, as compared to controls. For the left-lateralized language network, we performed the same analyses, but did not find any significant correlation with verbal fluency, group difference, or interaction.
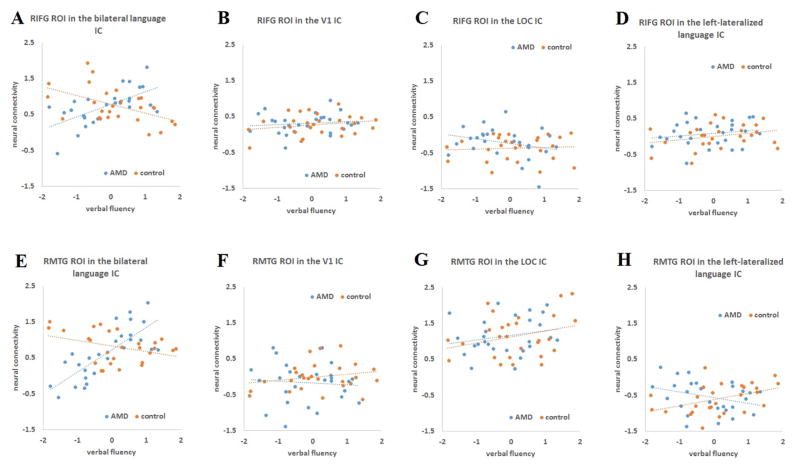
To visualize the relationship between verbal fluency performance and neural connectivity, we extracted the two significant clusters from the interaction effect (Fig. 3C) as ROIs using MarsBaR, then applied these two ROIs in each of the four ICs (V1, LOC, left-lateralized and bilateral language networks) and extracted out mean connection strength within each ROI. The mean connectivity of each ROI was plotted against verbal fluency performance for AMD patients and controls, respectively, in each IC (Figure 4). An interaction pattern was demonstrated in Fig. 4A and Fig. 4E in the bilateral language IC, with significant positive correlations between verbal fluency and neural connectivity in AMD patients (r = 0.63, for the RIFG ROI; r = 0.78, for the right pMTG ROI), while the same correlations became negative in healthy controls (r = −0.51, for the RIFG ROI; r = −0.36, for the right pMTG ROI). In contrast, no similar interactions were observed in the same correlations in the other three ICs. To test whether this interaction pattern was unique to the bilateral language network, we compared the correlational difference between AMD patients and controls in the bilateral language IC with the same correlational difference between the two groups in each of the other three ICs. For both ROIs, the group difference (the correlation in AMD patients minus that in controls) in the bilateral language IC was significantly larger than the same group difference in all other three ICs (p < 0.001, for all comparisons, http://vassarstats.net/rdiff.html), indicating that the significant interaction effect was unique to the bilateral language network.
Figure 4.
Plots of correlations between verbal fluency performance and neural connectivity based on the two significant clusters (RIFG and right posterior temporal regions) in Figure 3D. The top panel shows correlations between verbal fluency and neural connectivity extraction within the RIFG ROI across all four ICs. The bottom panel shows correlations between verbal fluency and neural connectiviy extraction within the ROI of right posterior temporal regions across all four ICs.
To further test whether the significant verbal fluency effect and the interaction were unique to the bilateral language network or not, we correlated the same three modulators (verbal fluency, group, interaction) to the two visual processing networks, respectively, but did not find any significant effect in the visual cortex. We also took an independent measure of memory scores, extracted from the factor analysis, as a control variable to explore the function of these visual and language processing networks. We correlated this memory measure to each of the two visual and two language networks, but did not find any regions with significant relationships between the memory score and connectivity strength, indicating that these networks were not sensitive to memory processing.
The bi-hemispheric IC map shows a tendency of greater activity in the right hemisphere than the left, and a potential driving force might be normal aging since all participants in this study are older adults. To test this hypothesis, we performed the same ICA on available data from 34 younger adults from a different study1, and found that the bi-hemisphere language network was bilaterally-balanced in connectivity strength pattern (Figure 5), consistent with our hypothesis that greater right-lateralization of the bi-hemispheric language network may be due to the advanced age of our participants.
Figure 5.
Significant connectivity strength pattern in the selected component of the bilateral language network in 34 younger adults in an independent study at a threshold of p < 0.001, voxel-level uncorrected, and p < 0.05, cluster-level corrected.
Discussion
In this study we investigated whether individuals with late-life visual loss exhibited unique patterns of intrinsic functional connectivity within neural networks involved in visual and language processes. We found that AMD patients exhibited significantly lower functional connectivity within V1 and LOC networks, but showed stronger neuro-behavioral correlations with verbal fluency in right IFG and right posterior temporal regions, compared to age-matched controls. These findings suggest that the intrinsic function of visual cortex is weakened probably as a consequence of long-term visual deprivation, while the language processing capacity in AMD may be well preserved where there is stronger connectivity in the right fronto-temporal regions. Higher functional connectivity in these regions could reflect functional reorganization (plasticity) or an inherent functional advantage (cognitive reserve) in these participants.
This study expands our understanding of the potential influence of visual deprivation on neural activity in visual cortex. Similar to past descriptions in early blindness (Liu Y et al. 2007), our findings are consistent with the hypothesis that late-life partial visual loss could fundamentally change the neural substrates of visual representation by weakening intrinsic connectivity strength within the V1 and LOC regions. Further, our findings are broadly consistent with the general loss hypothesis that visual deprivation might cause malfunction of the visual cortex to some extent (Sunness JS et al. 2004; Pascual-Leone A et al. 2005; Baseler HA et al. 2011). In contrast, we did not find evidence in support of the hypothesis of large-scale functional reorganization or remapping within visual cortex (Baker CI et al. 2005), since AMD patients did not show greater connectivity strength than controls in any of the V1 and LOC regions. One notable difference between this study and most of previous studies is that we adopt a task-free approach, so further tasked-based research is needed to investigate whether functional reorganization would be uncovered by the cognitive demands of an explicit task.
There was a significant correlation between higher verbal fluency scores and increasing activity in RIFG (BA 44/45) across all participants. These right prefrontal regions might be involved in phonological processing or in resolving competition among competing word candidates as they were often reported in various phonological and competition tasks, together with their left homologous sites (Bozic M et al. 2010; Shafto MA et al. 2010; Shafto MA and LK Tyler 2014). As our participants are all older adults, this finding is in keeping with the compensation theory of aging (Cabeza R 2002; Wierenga CE et al. 2008; Park DC and P Reuter-Lorenz 2009; Peelle JE et al. 2010), although other explanations cannot be ruled out. As older adults encounter increasing difficulties in finding appropriate word candidates during speech production and consequently generate more errors in retrieving phonological information of words, the fluency of word production, measured by the verbal fluency scores, could be taken as a representative measure of word-finding difficulties or executive control processes in general. From this perspective, the RIFG might be involved in a general selection process among competing word candidates while monitoring speech errors. Activation of the RIFG has been frequently reported in previous studies on competition and selection processes (Thompson-Schill SL et al. 1997; Zhuang J et al. 2011), and the RIFG might play an equally important role in the selection processes as the LIFG in younger adults (Bozic M et al. 2010; Zhuang J et al. 2014). RIFG may play an especially important role in the older adults, as older adults tend to rely more on the right hemisphere to compensate for brain tissue loss in the LIFG. Future studies are needed to explicitly compare brain changes in older vs. younger individuals who experience vision loss.
More importantly, the correlation between verbal fluency and intrinsic FC in RIFG (BA 44/45) and right pMTG (BA 37) was significantly greater in AMD patients than in controls. As the healthy controls were matched to the AMD patients in both age and cognitive abilities and analyses controlled for age variation and gender, this is not an effect of aging, but could be evidence of functional reorganization in the right hemisphere triggered by AMD. Given that the larger population of AMD patients show clear cognitive decline, particularly in the verbal fluency task, the cognitive abilities of the selected sample of AMD patients in this study are unusual in that they did not differ from controls. This provided a unique opportunity to investigate the relationship between late-life partial visual loss and neural networks for language processing while controlling for the overall influence of general cognitive functioning. The findings in this study suggest that, although long-term visual deprivation might induce deficits in language processing as shown in previous large population studies, stronger connectivity strength within right fronto-temporal regions could help to maintain language capacities, and it raises the possibility that this reorganization was beneficial to our AMD participants. Analogous to the typical fronto-temporal language network in the left hemisphere, the right frontal and posterior temporal regions might work together as a coherent network underpinning word generation processing. Previous neuropsychological research (Longworth C et al. 2005) has demonstrated that patients with extensive lesions in the left fronto-temporal regions and intact right homologous sites can still recognize simple spoken words rapidly and efficiently with equivalent performance (measured by semantic priming effects) as controls. Accumulating neuroimaging studies have also observed age-related increasing activation in the right hemisphere that helps to maintain working efficiency of the language processing system (Wingfield A and M Grossman 2006; Tyler LK et al. 2009). The activated right pMTG (BA 37) might function similarly as the left pMTG (BA 37) in integrating multiple sources of sensory information for object recognition (Stewart L et al. 2001). Furthermore, bilateral pMTG (BA 37) is likely the interface linking language and visual-spatial processing networks (Ardila A et al. 2015).
One notable finding of this study is that there were no significant group differences in verbal fluency, or age group difference in the effect of verbal fluency in the left fronto-temporal regions within the bi-hemispheric language network. There are several considerations to understand this null effect. First, it is the right hemisphere, particularly the RIFG, which has been widely reported as the main site of functional reorganization of the language processing system. This study evaluated the brain mechanisms of functional reorganization in a potentially select group of AMD patients (i.e., relative high performers willing to participate in research), and it was our prediction to observe significant connectivity strength pattern in the right hemisphere. Second, the bi-hemispheric language network selected from the ICA was slightly right-lateralized with higher connectivity strength in the right than the left fronto-temporal regions, and this right-lateralized pattern might bias the effects of verbal fluency and interaction towards the right hemisphere. The right-lateralization of this language network is very likely due to aging, as all participants in this study are older adults who tend to recruit more neural resources in the right hemisphere than younger adults in performing the same language tasks (Geva S et al. 2012; Peelle JE et al. 2013). In contrast, when only younger adults were included in the same ICA (e.g. Figure 5), the bi-hemispheric IC became bilaterally balanced (Greicius MD et al. 2003).
Using the task-free ICA approach, we successfully revealed neuro-behavior relationships and AMD-associated neural differences in this study. The verbal fluency measure only produced significant correlations with the FC of the bi-hemispheric language network, whereas fluency was not associated with the intrinsic FC of other networks such as the two visual processing networks. As a control measure, the memory factor did not correlate with any significant region in any of the visual or language networks, suggesting that the present finding of the verbal fluency effect and interaction is specific. As no previous research has demonstrated any deficits in syntactic processing in the AMD population, we did not design any syntactic representation tests in this study; therefore, we could not assess whether the function of the left-lateralized language network selected from the ICA might have been related to syntactic representation.
The AMD patients exhibited reduced functional connectivity in visual cortex, a result which was expected, considering that visual cortex relies heavily on bottom up stimulation. In contrast, the group differences in language-specific networks were more subtle, despite a tendency toward lower verbal fluency performance in those with AMD. This finding is consistent with the notion that higher level cognitive functions, such as language processing, have greater opportunity to be supported through brain plasticity, such as by effective recruitment of supplemental regions. Specifically, we observed a relationship between verbal fluency performance and higher connectivity of right fronto-temporal regions within a language network, and this behavior-brain relationship was stronger in participants with AMD. Although we can’t determine the causality of these cross-sectional relationships, one potential explanation is that compensatory recruitment of specific brain regions plays an important role in preserving function when higher-order cognitive processes have been threatened by deleterious changes in other networks (e.g., primary sensory networks). One caveat on the verbal fluency measure used in this study is that we did not design to separate the effects of phonemic fluency from semantic or categorical fluency which highly correlate with each other. Future studies could be designed to investigate the two fluency effects in further detail.
Highlights.
AMD is associated with weaker intrinsic functional connectivity in primary visual cortex
AMD is associated with weaker functional connectivity in lateral occipital cortex
Language preservation is linked with higher right fronto-temporal connectivity in AMD patients
Acknowledgments
This research was funded by NIH grant AG 043438 to HEW and NIH grant AG 034138 to MTD. The authors declare no conflicts of interest.
Footnotes
Resting state data were collected on these healthy participants under a similar protocol as used in our study. They were instructed to perform explicit cognitive tasks on language processing after the resting state scans. Part of the task-based data were published in Diaz et al. (2014).
Disclosure Statement
All authors of this manuscript declare no conflicts of interest.
This research was funded by NIH grant AG 043438 to HEW and NIH grant AG 034138 to MTD & DJM.
This manuscript has not been published elsewhere, nor is it currently under consideration for publication elsewhere.
This research has been approved by the Duke University Medical Center Institutional Review Board.
All authors have reviewed the contents of the manuscript, approved its contents, and validated the accuracy of the data.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ardila A, Bernal B, Rosselli M. Language and visual perception associations: meta-analytic connectivity modeling of Brodmann area 37. Behavioural neurology. 2015 doi: 10.1155/2015/565871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CI, Peli E, Knouf N, Kanwisher NG. Reorganization of visual processing in macular degeneration. Journal of Neuroscience. 2005;25:614–618. doi: 10.1523/JNEUROSCI.3476-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker ML, Wang JJ, Rogers S, Klein R, Kuller LH, Larsen EK, Wong TY. Early age-related macular degeneration, cognitive function, and dementia: the Cardiovascular Health Study. Archives of ophthalmology. 2009;127:667–673. doi: 10.1001/archophthalmol.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrick TR, Charlton RA, Clark CA, Markus HS. White matter structural decline in normal ageing: a prospective longitudinal study using tract-based spatial statistics. Neuroimage. 2010;51:565–577. doi: 10.1016/j.neuroimage.2010.02.033. [DOI] [PubMed] [Google Scholar]
- Baseler HA, Gouws A, Haak KV, Racey C, Crossland MD, Tufail A, Rubin GS, Cornelissen FW, Morland AB. Large-scale remapping of visual cortex is absent in adult humans with macular degeneration. Nature Neuroscience. 2011;14:649–655. doi: 10.1038/nn.2793. [DOI] [PubMed] [Google Scholar]
- Bedny M, Pascual-Leone A, Dodell-Feder D, Fedorenko E, Saxe R. Language processing in the occipital cortex of congenitally blind adults. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4429–4434. doi: 10.1073/pnas.1014818108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural computation. 1995;7:1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- Bozic M, Tyler LK, Ives DT, Randall B, Marslen-Wilson WD. Bihemispheric foundations for human speech comprehension. Proceedings of the National Academy of Science. 2010;107:17439–17444. doi: 10.1073/pnas.1000531107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler NM, Bressler SB, Fine SL. Age-related macular degeneration. Survey of ophthalmology. 1988;32:375–413. doi: 10.1016/0039-6257(88)90052-5. [DOI] [PubMed] [Google Scholar]
- Burton H, Snyder AZ, Conturo TE, Akbudak E, Ollinger JM, Raichle ME. Adaptive changes in early and late blind: A fMRI study of Braille reading. Journal of Neurophysiology. 2002;87:589–607. doi: 10.1152/jn.00285.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychology and Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Calhoun V, Adali T, Pearlson G, Pekar J. A method for making group inferences from functional MRI data using independent component analysis. Human brain mapping. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adalı T, Pekar JJ. A method for comparing group fMRI data using independent component analysis: application to visual, motor and visuomotor tasks. Magnetic resonance imaging. 2004;22:1181–1191. doi: 10.1016/j.mri.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Liu J, Adalı T. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage. 2009;45:S163–S172. doi: 10.1016/j.neuroimage.2008.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemons T, Rankin M, McBee W. Cognitive impairment in the Age-Related Eye Disease Study: AREDS report No. 16. Archives of ophthalmology (Chicago, Ill: 1960) 2006;124:537–543. doi: 10.1001/archopht.124.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SW, Zhuang J, Wright P, Tyler LK. Age-related sensitivity to task-related modulation of language-processing networks. Neuropsychologia. 2014;63:107–115. doi: 10.1016/j.neuropsychologia.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz MT, Johnson MA, Burke DM, Madden DJ. Age-related differences in the neural bases of phonological and semantic processes. Journal of Cognitive Neuroscience. 2014:1–14. doi: 10.1162/jocn_a_00665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NU, Wenger KK, Fox MD, Snyder AZ, Raichle ME, Petersen SE. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage. 2007;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB. Structural brain changes in aging: courses, causes and cognitive consequences. Reviews in the Neurosciences. 2010;21:187–221. doi: 10.1515/revneuro.2010.21.3.187. [DOI] [PubMed] [Google Scholar]
- Fuld PA, Masur DM, Blau AD, Crystal H, Aronson MK. Object-memory evaluation for prospective detection of dementia in normal functioning elderly: predictive and normative data. Journal of Clinical and Experimental Neuropsychology. 1990;12:520–528. doi: 10.1080/01688639008400998. [DOI] [PubMed] [Google Scholar]
- Geva S, Jones PS, Crinion JT, Price CJ, Baron JC, Warburton EA. The effect of aging on the neural correlates of phonological word retrieval. Journal of Cognitive Neuroscience. 2012;24:2135–2146. doi: 10.1162/jocn_a_00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagoort P. On Broca, brain, and binding: a new framework. Trends in cognitive sciences. 2005;9:416–423. doi: 10.1016/j.tics.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Iverson GL. Interpreting change on the WAIS-III/WMS-III in clinical samples. Archives of Clinical Neuropsychology. 2001;16:183–191. [PubMed] [Google Scholar]
- Jung-Beeman M. Bilateral brain processes for comprehending natural language. Trends in Cognitive Sciences. 2005;9:512–518. doi: 10.1016/j.tics.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Kaiser PK. Prospective evaluation of visual acuity assessment: a comparison of snellen versus ETDRS charts in clinical practice (An AOS Thesis) Transactions of the American Ophthalmological Society. 2009;107:311. [PMC free article] [PubMed] [Google Scholar]
- Klaver CC, Ott A, Hofman A, Assink JJ, Breteler MM, de Jong PT. Is age-related maculopathy associated with Alzheimer’s disease?: the Rotterdam Study. American journal of epidemiology. 1999;150:963–968. doi: 10.1093/oxfordjournals.aje.a010105. [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, Glahn DC, Beckmann CF, Smith SM, Fox PT. Behavioral interpretations of intrinsic connectivity networks. Journal of cognitive neuroscience. 2011;23:4022–4037. doi: 10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S-C, Lindenberger U, Sikström S. Aging cognition: from neuromodulation to representation. Trends in cognitive sciences. 2001;5:479–486. doi: 10.1016/s1364-6613(00)01769-1. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yu C, Liang M, Li J, Tian L, Zhou Y, Qin W, Li K, Jiang T. Whole brain functional connectivity in the early blind. Brain. 2007;130:2085–2096. doi: 10.1093/brain/awm121. [DOI] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Longworth C, Marslen-Wilson WD, Randall B, Tyler LK. Getting to the meaning of the regular past tense: Evidence from neuropsychology. Journal of Cognitive Neuroscience. 2005;17:1087–1097. doi: 10.1162/0898929054475109. [DOI] [PubMed] [Google Scholar]
- Masuda Y, Dumoulin SO, Nakadomari S, Wandell BA. V1 projection zone signals in human macular degeneration depend on task, not stimulus. Cerebral Cortex. 2008;18:2483–2493. doi: 10.1093/cercor/bhm256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M, Flaisch T, Wilser L, Euitz C, Rockstroh B, Conway T, Gonzalez Rothi LJ, Crosson B. Neural signatures of semantic and phonemic fluency in young and old adults. Journal of Cognitive Neuroscience. 2009;21:2007–2018. doi: 10.1162/jocn.2009.21219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsch AU, Bondi MW, Butters N, Salmon DP, Katzman R, Thal LJ. Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Archives of Neurology. 1992;49:1253–1258. doi: 10.1001/archneur.1992.00530360051017. [DOI] [PubMed] [Google Scholar]
- National Eye Institute. Facts about age related macular degeneration. 2011 www.nei.nih.gov/health/maculardegen/armd_facts.
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR. Aging reduces neural specialization in ventral visual cortex. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:13091–13095. doi: 10.1073/pnas.0405148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: Aging and neurocognitive scaffolding. Annual Review of Psychology. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Amedi A, Fregni F, Merabet LB. The plastic human brain cortex. Annu Rev Neurosci. 2005;28:377–401. doi: 10.1146/annurev.neuro.27.070203.144216. [DOI] [PubMed] [Google Scholar]
- Peelle JE, Chandrasekaran K, Powers J, Smith EE, Grossman M. Age-related vulnerability in the neural systems supporting semantic processing. Frontiers in aging neuroscience. 2013;5:46. doi: 10.3389/fnagi.2013.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle JE, Troiani V, Wingfield A, Grossman M. Neural processing during older adults’ comprehension of spoken sentences: age differences in resource allocation and connectivity. Cerebral Cortex. 2010;20:773–782. doi: 10.1093/cercor/bhp142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham TQ, Kifley A, Mitchell P, Wang JJ. Relation of age-related macular degeneration and cognitive impairment in an older population. Gerontology. 2006;52:353–358. doi: 10.1159/000094984. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Gusnard DA. Intrinsic brain activity sets the stage for expression of motivated behavior. Journal of Comparative Neurology. 2005;493:167–176. doi: 10.1002/cne.20752. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- Raz N. The aging brain observed in vivo: Differential changes and their modifiers. In: Cabeza R, Nyberg L, Park D, editors. Cognitive neuroscience of aging: Linking cognitive and cerebral aging. Oxford: Oxford University Press; 2005. pp. 19–57. [Google Scholar]
- Sadato N, Pascual-Leone A, Grafman J, Ibanez V, Delber M, Dold G, Hallett M. Activation of the primary visual cortex by Braille reading in blind subjects. Nature. 1996;380:526–528. doi: 10.1038/380526a0. [DOI] [PubMed] [Google Scholar]
- Sager MA, Hermann BP, La Rue A, Woodard JL. Screening for dementia in community-based memory clinics. WMJ-MADISON- 2006;105:25. [PubMed] [Google Scholar]
- Shafto MA, Stamatakis EA, Tam PP, Tyler LK. Word retrieval failures in old age: The relationship between structure and function. Journal of Cognitive Neuroscience. 2010;22:1530–1540. doi: 10.1162/jocn.2009.21321. [DOI] [PubMed] [Google Scholar]
- Shafto MA, Tyler LK. Language in the aging brain: the network dynamics of cognitive decline and preservation. Science. 2014;346:583–587. doi: 10.1126/science.1254404. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nature Neuroscience. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Spreen O, Benton AL. Neurosensory center comprehensive examination for aphasia (NCCEA), 1977 revision: manual of instructions. Neuropsychology Laboratory, University of Victoria; 1977. [Google Scholar]
- Stelmack JA, Szlyk JP, Stelmack TR, Demers-Turco P, Williams RT, Massof RW. Psychometric properties of the veterans affairs low-vision visual functioning questionnaire. Investigative ophthalmology & visual science. 2004;45:3919–3928. doi: 10.1167/iovs.04-0208. [DOI] [PubMed] [Google Scholar]
- Stewart L, Meyer B-U, Frith U, Rothwell J. Left posterior BA37 is involved in object recognition: a TMS study. Neuropsychologia. 2001;39:1–6. doi: 10.1016/s0028-3932(00)00084-1. [DOI] [PubMed] [Google Scholar]
- Sunness JS, Liu T, Yantis S. Retinotopic mapping of the visual cortex using functional magnetic resonance imaging in a patient with central scotomas from atrophic macular degeneration. Ophthalmology. 2004;111:1595–1598. doi: 10.1016/j.ophtha.2003.12.050. [DOI] [PubMed] [Google Scholar]
- Szlyk JP, Little DM. An FMRI study of word-level recognition and processing in patients with age-related macular degeneration. Investigative ophthalmology & visual science. 2009;50:4487–4495. doi: 10.1167/iovs.08-2258. [DOI] [PubMed] [Google Scholar]
- Szlyk JP, Stelmack J, Massof RW, Stelmack TR, Demers-Turco P, Williams RT, Wright BD. Performance of the veterans affairs low vision visual functioning questionnaire. Journal of Visual Impairment and Blindness. 2004;98:261–275. [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proceedings of the National Academy of Science. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tun PA, Lachman ME. Telephone assessment of cognitive function in adulthood: the Brief Test of Adult Cognition by Telephone. Age and Ageing. 2006;35:629–632. doi: 10.1093/ageing/afl095. [DOI] [PubMed] [Google Scholar]
- Tyler LK, Marslen-Wilson WD, Randall B, Wright P, Devereux BJ, Zhuang J, Papoutsi M, Stamatakis EA. Left inferior frontal cortex and syntax: function, structure and behaviour in patients with left hemisphere damage. Brain. 2011;134:415–431. doi: 10.1093/brain/awq369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler LK, Shafto MA, Randall B, Wright P, Marslen-Wilson WD, Stamatakis EA. Preserving syntactic processing across the adult life span: the modulation of the frontotemporal language system in the context of age-related atrophy. Cerebral Cortex. 2009:bhp105. doi: 10.1093/cercor/bhp105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. Journal of neurophysiology. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitson HE, Ansah D, Whitaker D, Potter G, Cousins SW, MacDonald H, Pieper CF, Landerman L, Steffens DC, Cohen HJ. Prevalence and patterns of comorbid cognitive impairment in low vision rehabilitation for macular disease. Archives of gerontology and geriatrics. 2010;50:209–212. doi: 10.1016/j.archger.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CE, Benjamin M, Gopinath K, Perlstein WM, Leonard CM, Gonzalez Rothi LJ, Conway T, Cato MA, Briggs R, Crosson B. Age-related changes in word retrieval: Role of bilateral frontal and subcortical networks. Neurobiology of Aging. 2008;29:436–451. doi: 10.1016/j.neurobiolaging.2006.10.024. [DOI] [PubMed] [Google Scholar]
- Wingfield A, Grossman M. Language and the aging brain: patterns of neural compensation revealed by functional brain imaging. Journal of Neurophysiology. 2006;96:2830–2839. doi: 10.1152/jn.00628.2006. [DOI] [PubMed] [Google Scholar]
- Wong TY, Klein R, Nieto FJ, Moraes SA, Mosley TH, Couper DJ, Klein BE, Boland LL, Hubbard LD, Sharrett AR. Is early age-related maculopathy related to cognitive function? The Atherosclerosis Risk in Communities Study. American journal of ophthalmology. 2002;134:828–835. doi: 10.1016/s0002-9394(02)01672-0. [DOI] [PubMed] [Google Scholar]
- Woo SJ, Park KH, Ahn J, Choe JY, Jeong H, Han JW, Kim TH, Kim KW. Cognitive impairment in age-related macular degeneration and geographic atrophy. Ophthalmology. 2012;119:2094–2101. doi: 10.1016/j.ophtha.2012.04.026. [DOI] [PubMed] [Google Scholar]
- Wright P, Randall B, Marslen-Wilson WD, Tyler LK. Dissociating linguistic and task-related activity in the left inferior frontal gyrus. Journal of Cognitive Neuroscience. 2011;23:404–413. doi: 10.1162/jocn.2010.21450. [DOI] [PubMed] [Google Scholar]
- Zhuang J, Randall B, Stamatakis EA, Marslen-Wilson WD, Tyler LK. The interaction of lexical semantic and cohort competition in spoken word recognition: An fMRI study. Journal of Cognitive Neuroscience. 2011;23:3778–3790. doi: 10.1162/jocn_a_00046. [DOI] [PubMed] [Google Scholar]
- Zhuang J, Tyler LK, Randall B, Stamatakis EA, Marslen-Wilson WD. Optimally efficient neural systems for processing spoken language. Cereb Cortex. 2014;24:908–918. doi: 10.1093/cercor/bhs366. [DOI] [PMC free article] [PubMed] [Google Scholar]