Abstract
Objectives
To evaluate effects of somatosensory stimulation in the form of repetitive peripheral nerve sensory stimulation (RPSS) in combination with transcranial direct current stimulation (tDCS), tDCS alone, RPSS alone or sham RPSS+tDCS as add-on interventions to training of wrist extension with functional electrical stimulation (FES), in chronic stroke patients with moderate to severe upper limb impairments in a crossover design. We hypothesized that the combination of RPSS and tDCS would enhance the effects of FES on active range of movement (ROM) of the paretic wrist to a greater extent than RPSS alone, tDCS alone or sham RPSS+tDCS.
Materials and methods
The primary outcome was the active ROM of extension of the paretic wrist. Secondary outcomes were ROM of wrist flexion, grasp and pinch strength of the paretic and nonparetic upper limbs, and ROM of wrist extension of the nonparetic wrist. Outcomes were blindly evaluated before and after each intervention. Analysis of variance with repeated measures with factors “session” and “time” was performed.
Results
After screening 2499 subjects, 22 were included. Data from 20 subjects were analyzed. There were significant effects of “time” for grasp force of the paretic limb and for ROM of wrist extension of the nonparetic limb, but no effects of “session” or interaction “session × time”. There were no significant effects of “session”, “time” or interaction “session × time” regarding other outcomes.
Conclusions
Single sessions of PSS+tDCS, tDCS alone or RPSS alone did not improve training effects in chronic stroke patients with moderate to severe impairment.
Keywords: Stroke rehabilitation, transcutaneous electric nerve stimulation, sensory function, motor skills, transcranial direct current stimulation, electric stimulation therapy
Introduction
There are no universally accepted treatments to decrease hand impairments for patients with moderate to severe upper limb paresis in the chronic phase after stroke. Neuromodulation techniques such as repetitive transcranial magnetic stimulation (rTMS) (1–4), transcranial direct current stimulation (tDCS) (5–7) and somatosensory stimulation in the form of repetitive peripheral nerve sensory stimulation (RPSS) (8–11) have emerged as potential powerful tools to enhance motor performance or increase effects of training in stroke victims.
A critical barrier for the utilization of rTMS, tDCS and RPSS to enhance effects of training in moderately to severely affected patients with stroke is that these patients, due to paralysis, typically cannot sustain voluntary movements or practice motor tasks. This leads to a gap in applicability of neuromodulation for those in deepest need of rehabilitation options.
Functional electrical stimulation (FES) can improve upper extremity function in patients with stroke and spinal cord injury with moderate to severe motor impairments who cannot train well without external assistance (12,13). Effects of FES are enhanced by high-frequency rTMS of the motor cortex of the affected hemisphere in patients in the chronic phase with limited ability to voluntarily extend the paretic wrist (12). Statistically significant benefits in active range of movement (ROM) of wrist extension and grip power were reported after the combination of active rTMS and motor training with FES, but not after sham rTMS combined with FES or after active rTMS without training, in nine patients.
Anodal tDCS, similarly to high-frequency rTMS, can increase motor cortex excitability. In patients with mild hand impairments, the combination of RPSS of the paretic upper limb and anodal tDCS of the ipsilesional motor cortex led to one to two times greater improvements in performance of finger sequences after training than either intervention alone, and four times more than sham stimulation (11). These results suggested that central and peripheral neuromodulation interventions might have synergistic effects on enhancement of motor learning in stroke and hence lead to improved motor outcomes in patients with mild motor impairment. However, until now, tDCS and RPSS had been exclusively applied to patients with mild upper limb motor impairments. These promising results called for investigations of effects of tDCS and RPSS in patients with more severe hand impairments, who have fewer therapeutic options available.
In order to close this gap we studied effects of tDCS and RPSS with FES that assists movement initiated by the patient, thus making training with the paretic hand possible. Patients participated in four interventions in a blind, crossover study design: active RPSS+active tDCS, active RPSS+sham tDCS, sham RPSS+active tDCS and sham RPSS+sham tDCS as add-on interventions to training of wrist extension with FES.
In this proof-of-principle study, our main hypothesis was that neuromodulation would enhance the beneficial effects of FES on active ROM of the paretic wrist to a greater extent than sham, and that the combination of RPSS and tDCS would further improve this outcome compared to either RPSS or tDCS alone. In addition, we explored the effects of neuromodulation on secondary outcomes: ROM of wrist flexion, grasp and pinch strength in the paretic and nonparetic (control) upper limbs.
Materials and methods
Study design
In this randomized, controlled and double-blind proof-of-principle trial, we compared the effects of four different interventions delivered prior to training of wrist extension, on motor performance of patients with moderate to severe hand paresis in the chronic phase after stroke.
Patients > 6 months post-stroke underwent four different interventions, in a crossover design: repetitive training of wrist extension of the paretic arm for 25 minutes with FES preceded by either active RPSS+sham tDCS, sham RPSS+active tDCS, sham RPSS+sham tDCS or active RPSS+sham tDCS. RPSS was administered for 2 hours to the median, ulnar and radial nerves. Anodal tDCS was administered to the ipsilesional motor cortex within the last 20 minutes of RPSS as previously done in patients with mild deficits (11).
Participants
Patients aged ≥ 18 years with first-ever symptomatic ischemic or hemorrhagic stroke confirmed by CT or MRI at least 6 months earlier, leading to moderate to severe impairment of the upper limb defined by a score between 7 and 50 in the Fugl-Meyer Assessment of Sensorimotor Recovery after stroke, motor performance (FMA, range 0–66) (14) were eligible for the study.
Exclusion criteria were: absence of any active wrist extension; anesthesia of the paretic hand; strokes involving the entire hand knob area of the motor cortex (15); strokes affecting the cerebellum or cerebellar pathways in the brain stem; severe spasticity at the paretic elbow, wrist or fingers, defined as a score of >3 on the Modified (MAS) Spasticity Scale (16); history of seizures; cardiac pacemaker, other neurologic diseases such as Parkinson’s disease and uncontrolled chronic diseases, such as congestive heart failure or cancer; disabling shoulder pain or deformity of the joint of the paretic upper limb; pregnancy; a history of neurological or psychiatric disease, previous surgery involving intracranial metallic implants, aphasia or serious cognitive deficits that limited comprehension of the experimental protocol; scores in the Minimental State Examination (MMSE) below 23/30 points in patients with at least one year of education or 19/30 in those with no education (17); inability to provide informed consent; inability to adhere to the schedule of interventions and protocol assessments. These criteria were similar to those of other neuromodulation trials in stroke (10,18–21).
The protocol (number 0546/11) was approved by the institutional (Comissão de Ética para Análise de Projetos de Pesquisa do Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo) and federal (Comissão Nacional de Ética em Pesquisa) ethics committee. The study was therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All patients provided informed consent to participate. The protocol was preregistered in clinicaltrials.gov (NCT 01907737).
Baseline measures
The following characteristics were evaluated at baseline: age, gender, time after stroke, type of stroke (ischemic/hemorrhagic), lesion side/location, National Institutes of Health stroke scale (NIHSS) (22), Modified Rankin Scale (MRS) (22), Mini Mental State Examination (MMSE) (17), handedness according to the Oldfield inventory (23), wrist and finger spasticity according to the MAS (16). Lesions were classified as corticosubcortical or subcortical on 1.5 or 3T MRI in DWI, T2-weighted, FLAIR and susceptibility-weighted images by a neurologist.
Randomization, allocation concealment and blinding
Patients were randomly assigned in blocks by the principal investigator with a basic random number computerized generator in a 1:1 ratio to receive four different interventions: active RPSS+sham tDCS, active tDCS+sham PSS, sham RPSS+tDCS, or active RPSS+tDCS. Patients were blinded to the experimental hypothesis and were not allowed to interact with other patients or to discuss the interventions with the researcher who was trained to evaluate the outcomes. The randomization table was concealed in a locked cabinet accessed only by researchers performing stimulation procedures.
Interventions
After a familiarization session, patients received one of each of the four interventions in experimental sessions separated by at least 10 days to avoid carry-over effects (Figure 1, Supplementary Table 1). Patients were comfortably seated during the interventions that were always administered during the morning.
Figure 1.
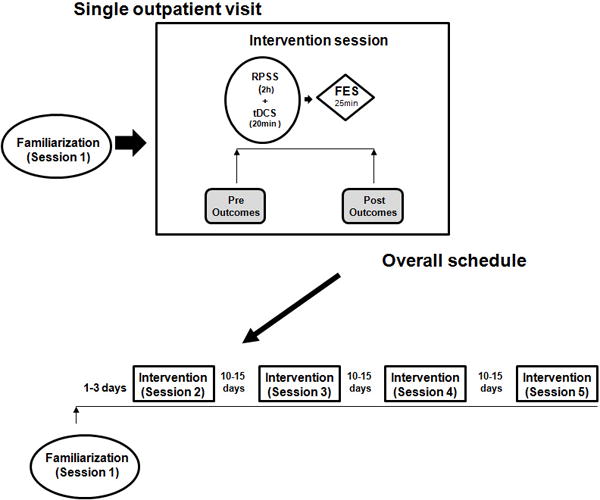
Experimental paradigm. RPSS, repetitive peripheral nerve sensory stimulation. FES, functional electrical stimulation.
In the familiarization session, patients practiced grip and pinch strength until they reached a stable motor performance, defined as a difference <20% between 3 consecutives measurements. In the same session, patients were exposed to tDCS, RPSS and FES. Each intervention was administered for 5 minutes.
RPSS
Active RPSS was applied by 3 pairs of surface electrodes (cathodes proximally placed) to stimulate the median, ulnar and radial nerves of the paretic arm. Trains of 5 electrical pulses (1ms of duration for each) at a frequency of 10Hz were delivered at 1Hz (Square Pulse Stimulator; Grass Instrument Division of Astro-med, Inc, Braintree, MA) as previously described (10,11,24). A customized device (Alfamedic Ltda, São Paulo) was used to provide independent outputs to the three nerves. Maximum output voltage was 130 Volts.
The median nerve was stimulated between the tendons of the flexor carpi radialis and palmaris longus with the cathode positioned 2–3 cm proximal to the wrist. The ulnar nerve was stimulated radially to the flexor carpi ulnaris tendon, with the cathode 3–4 cm proximal to the wrist. The radial nerve was stimulated 4–6 cm proximally to the ulnar styloid process (25). The threshold and maximum intensity of stimulation to produce paresthesias in the absence of visible muscle contractions was determined. Intensity of stimulation was then further increased until the patients reported paresthesias in the hand, in the absence of pain or visible forearm or finger movements.
Sham RPSS was applied by 3 pairs of surface electrode optimally placed to stimulated the fibular, tibial and sural nerves of the paretic leg. The fibular nerve was stimulated medially and about 12 cm proximally to the lateral malleolus. The tibial nerve was stimulated posteriorly and proximally o the medial malleolus. The sural nerve was stimulated about 12 cm proximally to the medial malleolus (25). Intensity of stimulation was then further increased until the patients reported paresthesias in the hand, in the absence of pain or visible movements.
At the end of each session of treatment, patients were asked: Do you think nerve stimulation was performed?
tDCS
Anodal tDCS was applied with the anode positioned over the ipsilesional primary motor cortex (M1) within the last 20 minutes of RPSS. Two sponge electrodes embedded in a saline-soaked solution were placed on the scalp. The anode was positioned on M1 at C3 and the cathode was placed over the contralateral supraorbital area (11,26). The stimulator was kept out of the patient’s visual field. Patients were told that they might feel tingling under the electrodes or no sensations during the procedure. Active tDCS was delivered at an intensity of 1mA, fade in and fade out times of 60 seconds (Neurocom tDCS DC-Stimulator). Sham stimulation was delivered with the same parameters used for active tDCS, except for stimulus duration (2 minutes, 30 seconds of fade in and out), a method shown to achieve a good level of blinding (26).
At the end of each session of treatment, patients were asked: Do you think brain stimulation was performed?
Motor Training – Functional Electrical Stimulation (FES)
Motor training with FES was delivered immediately after each of the 2-hour RPSS/tDCS interventions with a stimulator FESMED IV (CARCI, São Paulo, Brazil) applied to the extensor digitorum communis of the paretic arm with self-adhesive (3 × 5 cm) electrodes. Stimulation parameters for FES were: 40 Hz trains of 250 μs biphasic, square constant-current pulses for 1 second. During motor training, patients were comfortably seated on a chair and their trunk and upper limb were stabilized. Patients were instructed to actively extend the wrist every time they heard the auditory signal (recording of a human voice saying “now”) to keep wrist extensor movements at a pace of 0.4 Hz. They were instructed to try to reach at least 2/3 of the total range of movement of wrist extension and then relax (12). A goniometer was used for visual reference of the target range of movement. In all sessions, training was performed for 25 minutes.
Outcome Measures
Before and after each session, the following outcomes were blindly evaluated in the paretic upper limb: active ROM of wrist extension in the paretic upper limb (primary outcome) (12); active ROM of wrist extension in the nonparetic control upper limb (secondary outcome); active ROM of wrist flexion, grasp, pinch strength in the paretic and nonparetic upper limb (secondary outcomes). We did not expect changes in outcomes evaluated in the nonparetic limb that was tested as a control for possible drifts in attention. The order of measurements (paretic or nonparetic limb first) was randomized across subjects.
Active ROM of wrist extension was chosen as the primary outcome, similarly to the study of Koganemaru et al (12), because it is frequently impaired in hemiparetic patients with stroke and is relevant for performance of most daily tasks (27–29). In addition, this outcome can be reliably measured in severely affected patients (30). Other endpoints commonly chosen in crossover studies in which single sessions of active and sham interventions are delivered, such as the Jebsen-Taylor test (31) or reaction times are tailored for patients with mild motor impairments, excluded from the present study. The Wolf Motor Function Test (32) can be performed in patients with moderate to severe upper limb weakness, but is a lengthy test and its performance before and after an intervention that lasted for about two and a half hours might be associated with fatigue.
Active ROM of wrist extension and wrist flexion were measured with an analogic goniometer (ISP® Instituto São Paulo, São Paulo, Brazil), known to provide reliable measurements for this purpose (30).
Grip strength was measured with a Jamar dynamometer (Saehan Jamar, Changwong Korea) and pinch strength, with a digital dynamometer (Kratos, São Paulo, Brazil) while the arm was stabilized and the wrist was kept in a neutral position (33). Averages of five trials of grip and pinch strength, and three trials of ROM of wrist extension and flexion, performed before and after RPSS/tDCS + FES, were calculated.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Sample size calculation
Based on the results of Koganemaru et al (12), we estimated that 16 stroke patients should be included in the study for an alpha (type I error) of 0.05 and 1-beta (power) of 0.8 to detect an improvement in 10 degrees in active ROM of wrist extension after active tDCS + active RPSS compared to sham tDCS + sham RPSS, considering a 1-sample t-test and a standard deviation of 13. This was an initial estimate based on the standard deviation of the baseline data of wrist extension in the paper (26.1), because the mean difference between the active and sham groups were not provided. We expected the standard deviation to be smaller in our sample, because in contrast to Koganemaru et al, we excluded subjects with severe spasticity. A revised calculation based on the collected data was performed after including 10 patients and the sample size was increased to 22.
Statistical analysis
Data are presented as mean (±standard deviation) or as median (range). Normality was tested with the Shapiro-Wilk test. For normally distributed data, means and standard deviations are shown, otherwise medians and ranges are presented.
For evaluation of comparability of within-subject baseline measurements across sessions, repeated-measures analysis of variance with factor “session” was performed for normally distributed data. Otherwise, Friedman’s ANOVA was performed. Wilcoxon tests were used to compare outcomes in paretic and nonparetic upper limbs at baseline.
For evaluation of effects of interventions on outcomes, repeated-measures analysis of variance with factors “session” (active RPSS+active tDCS, active RPSS+sham tDCS, sham RPSS+active tDCS, sham RPSS+sham tDCS) and “time” (before and after treatment) was performed. Analysis was performed separately for the paretic and the nonparetic upper limb. P values ≤0.05 were considered statistically significant. SPSS (version 13.1 for Windows) software was used for statistical analysis.
Cohen’s D were calculated to estimate effect sizes and for post-hoc determination of sample sizes, considering improvement in outcome in a given session compared to the sham RPSS+sham tDCS session.
Results
Characteristics of the patients
Between July, 2013 and March, 2016, after screening 2499 patients, 22 subjects were included in the study (14 men). Two patients were excluded (one dropped out and one received botulinum toxin treatment during the study). Therefore, data from 20 patients were included in the analysis (13 men). Figure 2 shows the flow of patients through the study.
Figure 2.
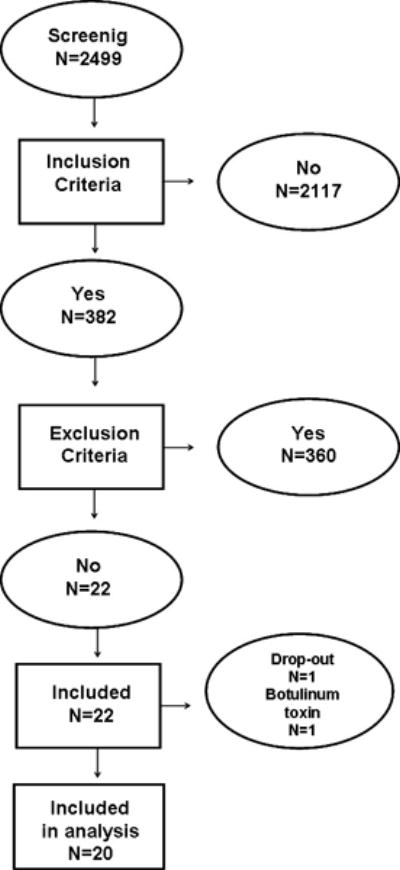
Flow of patients through the study.
The mean age (± standard deviation) was 56.6±12.3 years and the mean time from stroke, 5.7±5.7 years. The mean FMA motor score for the paretic upper limb was 38±7.5 (range, 19–49). Median MRS score was 3 (range, 1–4), median NIHSS was 3 (range, 1–8) and median MMSE score was 26 (19–30). Eighteen subjects were right-handed and two were left-handed according to the Oldfield inventory. MAS for wrist movements were 0 in 60%, 1 in 35% and +1 in 5% of the patients. Scores for finger movements were 0 in 70%, 1 in 25% and +1 in 5% of the patients.
Sixteen patients had ischemic and four, hemorrhagic strokes. Eight patients had right-sided lesions and thirteen, left-sided lesions (one patient had a bilateral pontine lesion but only the right-sided lesion was symptomatic). The lesions had corticosubcortical locations in 11 patients and were exclusively subcortical in nine (Supplementary Figure 1).
Intensities of stimulation in relation to sensory thresholds for each nerve are shown in Supplementary Table 2. Areas in which patients reported paresthesias are shown in Supplementary Figure 2. All patients received sham tDCS but 85% reported that brain stimulation had been performed in the active RPSS+sham tDCS session and 90%, in the sham RPSS+sham tDCS session. In the sham RPSS+active tDCS and active RPSS+active tDCS sessions, all patients received active tDCS and 90% believed brain stimulation had been performed in both sessions. In active RPSS+sham tDCS sessions and sham RPSS+sham tDCS, 95% of the patients reported that nerve stimulation had been performed. In the sham RPSS+active tDCS sessions and active RPSS+active tDCS sessions, 100% of the patients reported that nerve stimulation had been performed.
Primary outcome: ROM of paretic wrist extension
Results are shown in Tables 1, 2 and in Figure 3. There were no significant within-subject differences in ROM of wrist extension at baseline. There were no significant effects of “time”, “session” or interaction “session × time”. ROM of wrist extension slightly worsened in all sessions, except for the RPSS session (Table 1). The powers to analyze these effects were 8.4% for “session”, 42.6% for “time” and 16.7% for the interaction “session × time”. For the only session in which the primary outcome improved after treatment, active RPSS+sham tDCS, the effect size was 0.25 and we estimated that it would be required to include 126 subjects in order to obtain a significant difference compared to the sham RPSS+sham tDCS session, with a paired t-test.
Table 1.
Absolute values of range of movement for wrist extension, wrist flexion, grasp and pinch force in the paretic and nonparetic upper limbs, before and after each intervention. RPSS=repetitive peripheral nerve sensory stimulation. tDCS=transcranial direct current stimulation. Means±standard deviations are shown.
| Outcome | Pre active RPSS sham tDCS | Post active RPSS sham tDCS | Pre sham RPSS active tDCS | Post sham RPSS active tDCS | Pre sham RPSS sham tDCS | Post sham RPSS sham tDCS | Pre active RPSS active tDCS | Post active RPSS active tDCS |
|---|---|---|---|---|---|---|---|---|
|
Paretic upper limb
| ||||||||
| Wrist extension (degrees) | 37.2 ± 13.9 | 37.4 ± 15.1 | 38.0 ± 14.2 | 36.2 ± 13.7 | 37.7 ± 13.2 | 34.3 ± 14.0 | 37.8 ± 11.6 | 36.5 ± 13.2 |
| Wrist flexion (degrees) | 32.8 ± 15.6 | 31.3 ± 17.0 | 20.1 ± 15.5 | 29.4 ± 16.0 | 29.7 ± 12.9 | 29.0 ± 14.6 | 29.4 ± 15.7 | 29.1 ± 16.3 |
| Grasp strength (Newtons) | 80.8 ± 57.4 | 95.8 ± 64.6 | 81.0 ± 58.8 | 85.1 ± 60.4 | 83.4 ± 54.3 | 89.2 ± 56.0 | 84.3 ± 59.5 | 91.7 ± 59.3 |
| Pinch strength (Newtons) | 37.9 ± 16.1 | 38.1 ± 16.7 | 34.6 ± 17.0 | 36.4 ± 17.3 | 34.8 ± 17.4 | 36.6 ± 16.1 | 32.8 ± 16.1 | 35.8 ± 17.0 |
|
Nonparetic upper limb | ||||||||
| Wrist extension (degrees) | 62.4 ± 10.1 | 65.6 ± 7.5 | 63.1 ± 11.9 | 64.4 ± 9.4 | 59.9 ± 12.2 | 62.7 ± 8.6 | 62.4 ± 12.1 | 64.2 ± 9.9 |
| Wrist flexion (degrees) | 50.4 ± 10.3 | 50.5 ± 7.1 | 50.0 ± 9.5 | 48.9 ± 7.8 | 49.7 ± 8.7 | 51.0 ± 8.5 | 49.8 ± 7.7 | 52.2 ± 8.6 |
| Grasp strength (Newtons) | 284.3 ± 90.4 | 287.2 ± 90.6 | 285.0 ± 98.0 | 280.6 ± 86.6 | 295.2 ± 93.3 | 297.5 ± 94.7 | 279.4 ± 86.8 | 294.0 ± 91.5 |
| Pinch strength (Newtons) | 78.8 ± 24.8 | 80.1 ± 22.3 | 77.5 ± 23.7 | 70.3 ± 25.2 | 78.9 ± 27.7 | 79.4 ± 27.4 | 76.3 ± 24.5 | 77.6 ± 21.4 |
Table 2.
Results of analysis of variance of repeated measures for outcomes in the paretic and nonparetic upper limb. F and p-values for effects of “session” (active repetitive peripheral nerve sensory stimulation [RPSS] +active transcranial direct current stimulation [tDCS], active RPSS+sham tDCS, sham RPSS+active tDCS, sham RPSS+sham tDCS), “time” (before and after treatment) and interactions “session × time” are given.
| Outcome | Effect of “session” (F, p) | Effect of “time” (F, p) | Interaction “session × time” (F, p) |
|---|---|---|---|
| Paretic upper limb | |||
| Wrist extension | F=0.87, p=0.832 | F=2.07; p=0.150 | F=1.42, p=0.702 |
| Wrist flexion | F=4.16, p=0.244 | F=0.46, p=0.498 | F=0.15, p=0.986 |
| Grasp | F=1.26, p=0.738 | F=4.71, p=0.030 | F=1.26, p=0.738 |
| Pinch | F=5.84, p=0.120 | F=2.38, p=0.123 | F=0.84, p=0.840 |
|
Nonparetic upper limb | |||
| Wrist extension | F=4.31, p=0.230 | F=4.79, p=0.029 | F=0.53, p=0.911 |
| Wrist flexion | F=1.23, p=0.747 | F=0.43, p=0.511 | F=1.56, p=0.669 |
| Grasp | F=6.01, p=0.111 | F=0.34, p=0.562 | F=1.23, p=0.746 |
| Pinch | F= 1.59, p=0.662 | F=0.66, p=0.418 | F=0.10, p=0.992 |
Figure 3.
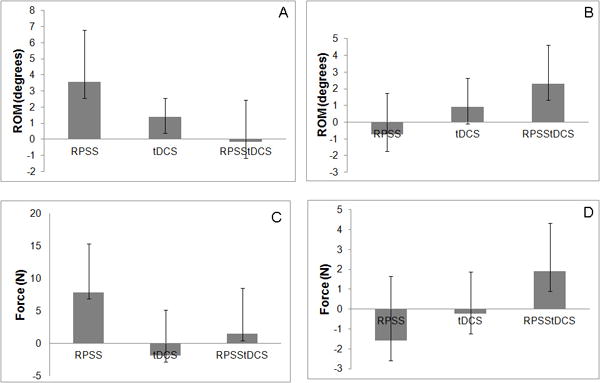
Normalized changes in the paretic upper limb, of range of movement (ROM) of wrist extension (A), wrist flexion (B), grasp (C) and pinch force (D) of the paretic limb in sessions of active repetitive peripheral nerve sensory stimulation and sham transcranial direct current stimulation (RPSS), sham repetitive peripheral nerve sensory stimulation and active transcranial direct current stimulation (tDCS), active repetitive peripheral nerve sensory stimulation and active transcranial direct current stimulation (RPSStDCS), in relation to the session of sham repetitive peripheral nerve sensory stimulation and sham transcranial direct current stimulation.
Secondary outcomes
Paretic upper limb
Results are shown in Tables 1 and 2, and in Figure 3. There were no significant differences in baseline ROM of wrist flexion across sessions (F=0.97, p=0.412). There were no significant effects of “session”, “time” or interaction “session × time” in regard to ROM of wrist flexion.
There were no significant differences in baseline grasp force across sessions (F=0.17, p=0.918). Grasp force improved after all interventions compared to baseline and to a greater extent after the RPSS session. However the effect of interaction “session × time” was not statistically significant. There was a significant effect of “time” and no effect of “session”. The effect size was 0.27. We estimated that, in order to obtain a significant difference in grasp force in the active RPSS+sham tDCS session compared to the sham RPSS+sham tDCS session, a sample size of 113 subjects would be required.
There were no significant differences in baseline pinch force across sessions (F=1.96, p=0.131). There were no significant effects of “session”, “time” or interaction “session × time” in regard to pinch force.
The intersubject variability of the results was high. Power for evaluation of all ANOVARM effects was below 50%, except for the effect of “session” for grasp force of the paretic arm.
Nonparetic upper limb
Results are shown in Tables 1 and 2, and in Figure 4. Baseline measures of ROM of wrist extension, wrist flexion, grasp and pinch strength were always greater in the non-paretic than the paretic limb (p<0.0001).
Figure 4.
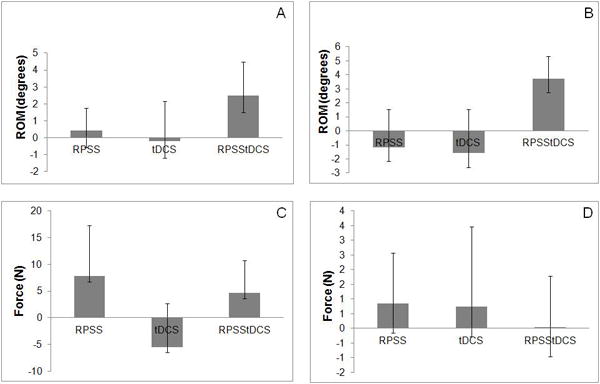
Normalized changes in the nonparetic upper limb, of range of movement (ROM) of wrist extension (A), wrist flexion (B), grasp (C) and pinch force (D) of the paretic limb in sessions of active repetitive peripheral nerve sensory stimulation and sham transcranial direct current stimulation (RPSS), sham repetitive peripheral nerve sensory stimulation and active transcranial direct current stimulation (tDCS), active repetitive peripheral nerve sensory stimulation and active transcranial direct current stimulation (RPSStDCS), in relation to the session of sham repetitive peripheral nerve sensory stimulation and sham transcranial direct current stimulation.
There were no significant differences in baseline ROM of wrist extension (F=0.84, p=0.479), flexion (F=0.04, p=0.988), grasp (F=1.93, p=0.135), or pinch force (F=0.36, p=0.707).
ROM for wrist extension improved in all sessions (Table 1). There was a significant effect of “time”, but no significant effect of “session” or interaction “session × time”.
There were no significant effects of “session”, “time” or interaction “session × time” in regard to ROM of wrist flexion, grasp or pinch force (Table 2).
Discussion
RPSS alone, tDCS alone or the combination RPSS+tDCS did not potentiate the effect of training with FES, on active ROM of wrist extension of the paretic limb in patients with moderate to severe upper limb motor deficits. ROM of wrist extension improved slightly when preceded by RPSS alone and worsened in all other sessions, possibly due to fatigue.
We have thus not found a significant effect of any of the three neuromodulation interventions on the primary outcome measure, compared to sham stimulation. We do not believe this is due to underpowering because the effect size was small and we estimated that 126 patients would be needed to attain a significant difference in ROM of wrist extension in the active RPSS+sham tDCS session, compared to the sham RPSS+sham tDCS session. Therefore under our experimental conditions this intervention, or any of the other active interventions are unlikely to result in meaningful improvements in this patient population.
The rationale for our paradigm was based on a previous publication (12) that used a slightly different technique, high-frequency rTMS, to increase excitability of the affected motor cortex and therefore boost effects of motor training, as we intended by administering anodal tDCS and RPSS in the present study. This work showed significant improvement in the same primary outcome in a single-session, crossover design in a much smaller sample and has not yet been replicated. In relation to this primary outcome measure we conclude that the experimental approach should be improved before a larger clinical trial can be successfully designed.
In regard to other secondary outcomes, training with FES did not lead to gains in active ROM of wrist flexion or pinch force of the paretic limb but was associated with enhancement of grasp force, particularly in the RPSS session compared to the other sessions. The effect size was small and the difference in improvement between the RPSS session and the other sessions was not statistically significant. Despite this, effects of active RPSS alone were greater than those of active tDCS alone or active RPSS+tDCS.
Interestingly, training of wrist extension of the paretic limb was associated with significant improvement in active ROM of wrist extension of the nonparetic limb in these patients, regardless of the neuromodulation intervention administered prior to training. Action observation may have contributed to this effect, considering that only the paretic limb received training (34,35). There were no significant improvements in performance of untrained movements (wrist flexion, grasp and pinch force).
Future studies should investigate effects of alternative RPSS+tDCS paradigms in patients with moderate to severe upper limb impairments. In mildly affected patients, anodal tDCS combined with RPSS resulted in enhancement of accuracy in performance of finger sequences, compared to sham tDCS (11). Also, RPSS alone led to smaller but significant improvements in accuracy compared to sham stimulation. In another study in which RPSS alone was applied to patients with moderate to severe motor impairments prior to 4-h modified constraint-induced therapy (MCIT) for 10 days, clinically significant improvements were reported in the Action Research Arm Test (8). Taken together with our results, these findings suggest that a single session of RPSS and tDCS may be sufficient to drive improvements in patients with mild motor deficit but not in those with moderate to severe impairments, and that several sessions of RPSS in association with intensive training may be required to enhance outcomes in these patients.
Another issue that deserves attention in future clinical trials is timing of RPSS in relation to tDCS. In a proof-of-concept study (36), four patients with moderate to severe motor impairments in the chronic phase after stroke received 10 daily sessions of anodal at the start and six patients received tDCS at the end of a 2-hour period of RPSS. Robot-aided motor training was delivered after RPSS. FMA scores improved only in the patients receiving tDCS at the start of the RPSS intervention.
This study has some limitations. Only a single session of each intervention was applied, and power was low considering the variability of the results. Still, the sample size was almost three times greater than those of previous proof-of-principle studies that combined rTMS and FES (12) or RPSS and tDCS (11).
Conclusion
In contrast to motor improvements translated into better performance of finger motor sequences in patients with mild motor impairments (11), a single session of anodal tDCS of M1 in the affected hemisphere does not enhance the effects of RPSS on motor outcomes measurable in more severely affected patients (ROM of wrist extension and flexion, grasp and pinch force). These results highlight the need to tailor neuromodulation interventions according to severity of motor impairments. Effects of repeated sessions of both interventions with different timings of tDCS in relation to RPSS, and association of task-specific training, remain to be explored in patients with moderate to severe upper limb deficits.
Supplementary Material
Supplementary Figure 1. Lesions (arrows) in magnetic resonance (T2-weighted, FLAIR or susceptibility-weighted) images of patients included in the analysis.
Supplementary Figure 2. Areas (in gray) in which each patient reported paresthesias within the territories of the median, ulnar and radial nerves. RPSS = repetitive peripheral nerve sensory stimulation. tDCS = transcranial direct current stimulation.
Acknowledgments
Source of financial support:
Funding for this study was provided by grants from the National Institutes of Health: R01NS076348-01 and D71TW009132-02.
Footnotes
Authorship statement
Adriana B. Conforto, Leonardo G. Cohen, Paul H. Peckham and André G. Machado designed the study. Adriana B. Conforto, Isabella S. Menezes, Eduardo A. Mello, Sarah M. Anjos, Inara L. Siqueira and Juliana Conti conducted the study, including patient recruitment, data collection, and data analysis. Isabella S. Menezes prepared the manuscript draft and Adriana B. Conforto wrote the final version, with important intellectual input from all of the other authors. All of the authors had complete access to the study data.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Offprints should be sent to the corresponding author.
References
- 1.Grefkes C, Fink GR. Noninvasive brain stimulation after stroke: it is time for large randomized controlled clinical trials! Curr Opin Neurol. 2016;29:714–720. doi: 10.1097/WCO.0000000000000395. [DOI] [PubMed] [Google Scholar]
- 2.Graef P, Dadalt ML, Rodrigués DA, Stein C, Pagnussat Ade S. Transcranial magnetic stimulation combined with upper-limb training for improving function after stroke: a systematic review and meta-analysis. J Neurol Sci. 2016;369:149–158. doi: 10.1016/j.jns.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 3.Smith MC, Stinear CM. Transcranial magnetic stimulation (TMS) in stroke: Ready for clinical practice? J Clin Neurosci. 2016;31:10–14. doi: 10.1016/j.jocn.2016.01.034. [DOI] [PubMed] [Google Scholar]
- 4.Lüdemann-Podubecká J, Bösl K, Nowak DA. Repetitive transcranial magnetic stimulation for motor recovery of the upper limb after stroke. Prog Brain Res. 2015;218:281–311. doi: 10.1016/bs.pbr.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Elsner B, Kugler J, Pohl M, Mehrholz J. Transcranial direct current stimulation (tDCS) for improving activities of daily living, and physical and cognitive functioning, in people after stroke. Cochrane Database Syst Rev. 2016;3:CD009645. doi: 10.1002/14651858.CD009645.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lüdemann-Podubecká J, Bösl K, Rothhardt S, Verheyden G, Nowak DA. Transcranial direct current stimulation for motor recovery of upper limb function after stroke. Neurosci Biobehav Rev. 2014;47:245–259. doi: 10.1016/j.neubiorev.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 7.Liew SL, Santarnecchi E, Buch ER, Cohen LG. Non-invasive brain stimulation in neurorehabilitation: local and distant effects for motor recovery. Front Hum Neurosci. 2014;8:378. doi: 10.3389/fnhum.2014.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrico C, Chelette KC, Westgate PM, Powell E, Nichols L, Fleischer A, et al. Nerve stimulation enhances task-oriented training in chronic, severe motor deficit after stroke: a randomized trial. Stroke. 2016;47:1879–1884. doi: 10.1161/STROKEAHA.116.012671. [DOI] [PubMed] [Google Scholar]
- 9.Fleming MK, Sorinola IO, Roberts-Lewis SF, Wolfe CD, Wellwood I, Newham DJ. The effect of combined somatosensory stimulation and task-specific training on upper limb function in chronic stroke: a double-blind randomized controlled trial. Neurorehabil Neural Repair. 2015;29:143–152. doi: 10.1177/1545968314533613. [DOI] [PubMed] [Google Scholar]
- 10.Conforto AB, Ferreiro KN, Tomasi C, dos Santos RL, Moreira VL, Marie SK, et al. Effects of somatosensory stimulation on motor function after subacute stroke. Neurorehabil Neural Repair. 2010;24:263–272. doi: 10.1177/1545968309349946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Celnik P, Paik NJ, Vandermeeren Y, Dimyan M, Cohen LG. Effects of combined peripheral nerve stimulation and brain polarization on performance of a motor sequence task after chronic stroke. Stroke. 2009;40:1764–1771. doi: 10.1161/STROKEAHA.108.540500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koganemaru S, Mima T, Thabit MN, Ikkaku T, Shimada K, Kanematsu M, et al. Recovery of upper-limb function due to enhanced use-dependent plasticity in chronic stroke patients. Brain. 2010;133:3373–3384. doi: 10.1093/brain/awq193. [DOI] [PubMed] [Google Scholar]
- 13.Quandt F, Hummel FC. The influence of functional electrical stimulation on hand motor recovery in stroke patients: a review. Exp Transl Stroke Med. 2014;6:9. doi: 10.1186/2040-7378-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michaelsen SM, Rocha AS, Knabben RJ, Rodrigues LP, Fernandes CG. Translation, adaptation and inter-rater reliability of the administration manual for the Fugl-Meyer assessment. Rev Bras Fisioter. 2011;15:80–88. [PubMed] [Google Scholar]
- 15.Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, et al. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997;120:141–157. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]
- 16.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67:206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 17.Almeida OP. Mini mental state examination and the diagnosis of dementia in Brazil. Arq Neuropsiquiatr. 1998;56:605–612. doi: 10.1590/s0004-282x1998000400014. [DOI] [PubMed] [Google Scholar]
- 18.Hummel F, Cohen LG. Improvement of motor function with noninvasive cortical stimulation in a patient with chronic stroke. Neurorehabil Neural Repair. 2005;19:14–19. doi: 10.1177/1545968304272698. [DOI] [PubMed] [Google Scholar]
- 19.Khedr EM, Abdel-Fadeil MR, Farghali A, Qaid M. Role of 1 and 3 Hz repetitive transcranial magnetic stimulation on motor function recovery after acute ischaemic stroke. Eur J Neurol. 2009;16:1323–1330. doi: 10.1111/j.1468-1331.2009.02746.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim YH, You SH, Ko MH, Park JW, Lee KH, Jang SH, et al. Repetitive transcranial magnetic stimulation-induced corticomotor excitability and associated motor skill acquisition in chronic stroke. Stroke. 2006;37:1471–1476. doi: 10.1161/01.STR.0000221233.55497.51. [DOI] [PubMed] [Google Scholar]
- 21.Fregni F, Boggio PS, Valle AC, Rocha RR, Duarte J, Ferreira MJ, et al. A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke. 2006;37:2115–2122. doi: 10.1161/01.STR.0000231390.58967.6b. [DOI] [PubMed] [Google Scholar]
- 22.Cincura C, Pontes-Neto OM, Neville IS, Mendes HF, Menezes DF, Mariano DC, et al. Validation of the National Institutes of Health Stroke Scale, modified Rankin Scale and Barthel Index in Brazil: the role of cultural adaptation and structured interviewing. Cerebrovasc Dis. 2009;27:119–122. doi: 10.1159/000177918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 24.Sawaki L, Wu CW, Kaelin-Lang A, Cohen LG. Effects of somatosensory stimulation on use-dependent plasticity in chronic stroke. Stroke. 2006;37:246–247. doi: 10.1161/01.STR.0000195130.16843.ac. [DOI] [PubMed] [Google Scholar]
- 25.Sethi RK, Thompson LL. The electromyographer’s handbook. Boston, USA: Little, Brown and Company; 1989. [Google Scholar]
- 26.Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol. 2016;127:1031–1048. doi: 10.1016/j.clinph.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pandyan AD, Cameron M, Powell J, Stott DJ, Granat MH. Contractures in the post-stroke wrist: a pilot study of its time course of development and its association with upper limb recovery. Clin Rehabil. 2003;17:88–95. doi: 10.1191/0269215503cr587oa. [DOI] [PubMed] [Google Scholar]
- 28.Malhotra S, Cousins E, Ward A, Day C, Jones P, Roffe C, et al. An investigation into the agreement between clinical, biomechanical and neurophysiological measures of spasticity. Clin Rehabil. 2008;22:1105–1115. doi: 10.1177/0269215508095089. [DOI] [PubMed] [Google Scholar]
- 29.Smedes F, van der Salm A, Koel G, Oosterveld F. Manual mobilization of the wrist: a pilot study in rehabilitation of patients with a chronic hemiplegic hand post-stroke. J Hand Ther. 2014;27:209–215. doi: 10.1016/j.jht.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Flinn N, Trombly-Latham C, Podolski C. Occupational therapy for physical dysfunction. Baltimore, USA: Williams & Wilkins; 2008. Assessing abilities and capacities in Range of motion, strength, and endurance. [Google Scholar]
- 31.Ferreiro KN, Santos RL, Conforto AB. Psychometric properties of the portuguese version of the Jebsen-Taylor test for adults with mild hemiparesis. Rev Bras Fisioter. 2010;14:377–382. doi: 10.1590/s1413-35552010005000018. [DOI] [PubMed] [Google Scholar]
- 32.Wolf SL, Catlin PA, Ellis M, Archer AL, Morgan B, Piacentino A. Assessing Wolf motor function test as outcome measure for research in patients after stroke. Stroke. 2001;32:1635–1639. doi: 10.1161/01.str.32.7.1635. [DOI] [PubMed] [Google Scholar]
- 33.Mathiowetz V, Weber K, Volland G, Kashman N. Reliability and validity of grip and pinch strength evaluations. J Hand Surg. 1984;9:222–226. doi: 10.1016/s0363-5023(84)80146-x. [DOI] [PubMed] [Google Scholar]
- 34.Buccino G. Action observation treatment: a novel tool in neurorehabilitation. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130185. doi: 10.1098/rstb.2013.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ertelt D, Binkofski F. Action observation as a tool for neurorehabilitation to moderate motor deficits and aphasia following stroke. Neural Regen Res. 2012;15(7):2063–2074. doi: 10.3969/j.issn.1673-5374.2012.26.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Powell ES, Carrico C, Westgate PM, Chelette KC, Nichols L, Reddy L, et al. Time configuration of combined neuromodulation and motor training after stroke: A proof-of-concept study. NeuroRehabilitation. 2016;39:439–449. doi: 10.3233/NRE-161375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Lesions (arrows) in magnetic resonance (T2-weighted, FLAIR or susceptibility-weighted) images of patients included in the analysis.
Supplementary Figure 2. Areas (in gray) in which each patient reported paresthesias within the territories of the median, ulnar and radial nerves. RPSS = repetitive peripheral nerve sensory stimulation. tDCS = transcranial direct current stimulation.


