Abstract
Chemokines are proteins which induce chemotaxis, promote differentiation of immune cells, and cause tissue extravasation. Given these properties, their role in anti-tumor immune response in the cancer environment is of great interest. Although immunotherapy has shown clinical benefit for some cancer patients, other patients do not respond. One of the mechanisms of resistance to checkpoint inhibitors may be chemokine signaling. The CXCL9, -10, -11/CXCR3 axis regulates immune cell migration, differentiation, and activation, leading to tumor suppression (paracrine axis). However, there are some reports that show involvements of this axis in tumor growth and metastasis (autocrine axis). Thus, a better understanding of CXCL9, -10, -11/CXCR3 axis is necessary to develop effective cancer control. In this article, we summarize recent evidence regarding CXCL9, CXCL10, CXCL11/CXCR3 axis in the immune system and discuss their potential role in cancer treatment.
Keywords: CXCL9, CXCL10, CXCL11, CXCR3, cancer, immunotherapy
Introduction
Chemokines are small proteins (8–15 kD) which interact with a subset of G protein-coupled receptors. They play key roles to induce chemotaxis, promote differentiation and multiplication of leukocytes, and cause tissue extravasation.[1] In 1987, Yoshimura et al. first reported about CXCL8 (IL-8), which regulates neutrophil trafficking.[2] Since then, much attention has been devoted to understanding the functions and role of chemokines in immune response. The CXCL9, -10, -11/CXCR3 axis has been a major focus of research, since it regulates differentiation of naive T cells to T helper 1 (Th1) cells and leads migration of immune cells to their focal sites.[3] Due to this pivotal role, this axis is essential for immune system on command. Recent data has suggested its clinical significance, but little is known about clinical outcomes in patients with cancer.
The CXCL9, -10, -11/CXCR3 axis mainly regulates immune cell migration, differentiation, and activation. Immune reactivity occurs through this axis by recruitment of immune cells, such as cytotoxic lymphocytes (CTLs), natural killer (NK) cells, NKT cells, and macrophages. Furthermore, Th1 polarization by this axis also activates the immune cells in response to IFN-γ.[4] Tumor-infiltrating lymphocytes are a key for good clinical outcomes and prediction of the response to existing checkpoint inhibitors.[5, 6] However, in vivo studies suggest the axis plays a tumorigenic role as well by increasing tumor proliferation and metastasis.[7, 8] Thus, a better understanding of this axis in the tumor environment is necessary to discover its role as a potential target for immunotherapy or as a predictive indicator for existing cancer treatments.
In this review, we discuss the current evidence about the role of the CXCL9, -10, -11/CXCR3 axis in tumor environment (TME) and immune response, and discuss the opportunities for novel therapies.
The expression and implication of CXCL9, CXCL10, CXCL11 and CXCR3
Immune cells are regulated by many different cytokines (including chemokines) not only for differentiation, but also for promptly infiltrating focal tissues through chemotactic gradients. The selection of immune cells that respond to chemotaxes is based on their surface receptors. Therefore, discrimination of the chemotactic gradients must be affected by the complicated interactions between cytokines and their receptors. CXCL9, -10, -11 are selective ligands for CXCR3. The ligands are usually expressed at low levels in homeostatic conditions, but upregulated by cytokine stimulation. CXCL9, -10, -11 are mainly secreted by monocytes, endothelial cells, fibroblasts, and cancer cells in response to IFN-γ, which are synergistically enhanced by TNF-alpha.[9, 10] CXCR3 is a receptor preferentially expressed on the surface of monocytes, T cells, NK cells, dendritic cells, and cancer cells.[11, 12] CXC chemokines are classified into two groups with and without ELR (Glu-Leu-Arg) motif.[13] Those with the ELR motif can allow neutrophils to migrate and have an angiogenic effect, whereas those without the ELR motif primarily allow lymphocytic migration and inhibit angiogenesis. CXCL9, -10, -11 are ELR-negative CXC chemokines that generally attenuate angiogenesis, leading to an anti-tumor effect. Interestingly, some reports show that CXCL9, -10, -11 increase tumor proliferation and metastases.[7] This may be due to the different effects of the ligands on the variants of CXCR3 (CXCR3A, CXCR3B and CXCR3-alt). Previous studies have shown that these ligands have different temporal and spatial patterns of expression through different regulatory elements in distinct cell types. As far as the CXCR3 receptor is concerned, there are three variants with different roles in tumorigenesis. The features of each protein are described below.
CXCL9, also known as monokine induced by gamma interferon (MIG), is located on human chromosome 4, and is induced by IFN-γ but not by IFN-α/β.[14] CXCL10 and CXCL11 are also located on human chromosome 4. CXCL9 predominantly mediates lymphocytic infiltration to the focal sites and suppresses tumor growth.[15] In vivo models by Gorbachev et al. showed that CXCL9-deficient cancer cells are more tumorigenic than cancer cells expressing both CXCL9 and CXCL10.[15] Menke et al. reported that both CXCR3 and CXCL9 deficient mice had fewer loss of kidney function than CXCL10 deficient mice, showing the mice had fewer intrarenal T cells and macrophages in immune-mediated nephritis.[16]
CXCL10, known as interferon γ-induced protein 10 (IP-10), is strongly induced by IFN-γ as well as by IFN-α/β[17] and weakly by TNFα.[10] In vitro, CXCL10 can also be induced by NF-kB, and has been shown to have an early role in hypoxia-induced inflammation.[18, 19] Activation of IFN-regulatory factor 3, toll-like receptors, retinoic acid-inducible gene (RIG)-I, and melanoma differentiation-associated gene (MDA)-5 work in synergy with IFNs for CXCL10 induction.[17, 20] Serum CXCL10 concentration, but not CXCL9, was reportedly correlated with the number of circulating lymphocytes in head and neck cancer with radiation therapy.[21] Ming-Fang et al. revealed that CXCL10-deficient mice had higher mortality rate with the dengue virus infection.[22]
CXCL11, also known as interferon-inducible T-cell alpha chemoattractant (I-TAC) or interferon-gamma-inducible protein 9 (IP-9), is induced by IFN-γ and IFN-β, and weakly by IFN-α.[23] The affinity of CXCL11 for CXCR3 is the highest of the three selective ligands, followed by CXCL10 and CXCL9.[24, 25] The binding domain of CXCL11 on CXCR3 is located at a different site from that of CXCL9 and CXCL10.[26] Furthermore, CXCL11 can bind to CXCR7, which is associated with invasiveness and reduces apoptosis of tumor cells.[27]
CXCR3, also known as G protein-coupled receptor 9 (GPR9) or CD183, is a 7 transmembrane domain G-protein coupled receptor, which was first reported in 1989.[28] Like CXCL9, -10, -11, CXCR3 is also predominantly driven by IFN-γ.[29] CXCR3 has two distinct intracellular domains for activation: one is a carboxy-terminal domain for CXCL9 and CXCL10, and another is in the third intracellular loop for CXCL11.[26] CXCR3 is heavily expressed on Th1 cells, CTLs, NK cells and NKT cells. CXCR3 is downregulated on naıve T cells, but is rapidly upregulated by antigen-presenting dendritic cells,[30] leading to Th1 polarization. After polarization, Th1 cells induce activation of CTLs, NK cells, and NKT cells through IFN-γ.[4] Biochemical studies have revealed that there are at least three CXCR3 variants; CXCR3A, CXCR3B and CXCR3-alt, with unique characteristics.[31] CXCR3A represents classical CXCR3 roles which include chemotaxis and cell proliferation in IFN-γ-inducible immune responses; CXCR3B, which is spliced at an extension of the N terminus by 52 amino acids, induces cell apoptosis and inhibits cell migration; CXCR3-alt, a 101-aminoacid-truncated version, mainly mediates CXCL11 function.[31–33] Importantly, CXCR3B can also bind to CXCL4, which is released from activated platelets during platelet aggregation, in addition to CXCL9, -10, -11.[32]
The immune response for host disorders through CXCL9, -10, -11/CXCR3 axis appears to depend on both the ligands and the variants of its CXCR3 receptor. In addition, the ligands can act as antagonists for CCR3 which stimulates Th2 polarization, and only CXCL11 can bind to CXCR7, also known as atypical chemokine receptor 3 (ACKR3), which has tumorigenic potential.
CXCL9, CXCL10, CXCL11/CXCR3 axis for immune response
This axis works primarily for immune cell migration, differentiation, and activation. Immune reactivity for each disorder is dependent on the types of leukocytes infiltrating the focal sites. Therefore, it is critical to understand which immune cells are involved in migration, differentiation, and activation through this axis. The axis also acts directly on cancer cells and promotes cancer cell proliferation and metastasis. (Figure 1)
Figure 1.
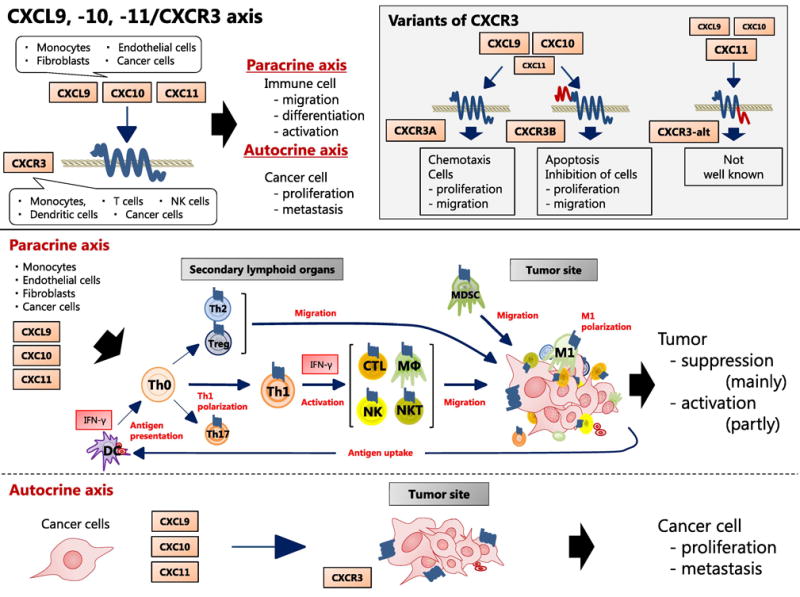
CXCL9, -10, -11/CXCR3 axis in the tumor environment. CXCL9, -10, and -11 are mainly secreted by monocytes, endothelial cells, fibroblasts, and cancer cells in response to IFN-γ. The work of CXCL9, -10, -11/CXCR3 axis is mainly divided into two directions; paracrine signaling for immune activation and autocrine signaling for proliferation and metastasis of cancer cells. As for paracrine signal, this axis works primarily for immune cell migration, differentiation, and activation. Immune reactivity is occurred with recruitment of CTLs, NK cells, NKT cells, and macrophages through this axis, and Th1 polarization by this axis also activate the immune cells in response to IFN-γ. On the contrary, as for autocrine signal, cancer cells have a propensity to metastasize due to the tumor-derived ligands activity mainly through CXCR3A. Tumor-derived chemokines are also responsible for recruitment of Th2 cells, Tregs, and MDSCs, which play the role of creating a pro-tumoral microenvironment. Abbreviations: CTLs, cytotoxic lymphocytes; NK, natural killer; NKT, natural killer T; MΦ, macrophage; MDSCs, myeloid derived suppressor cells; Th0, naive T; Th1, T helper 1; Th2, T helper 2; Th17, T helper 17; Tregs, regulatory T cell
For immune cell migration, each of the CXCR3 ligands are equally effective on activated Th1 cells, CTLs, and NK cells in vivo models of cell recruitment. [34, 35] All three variants of CXCR3 are expressed on T cells, where CXCL9, -10, -11 collectively stimulate the loss of surface CXCR3 expression and elicit directional migration responses to the focal sites.[36] Chheda et al. demonstrated a critical role of CXCR3 for CTLs migration using CXCR3 knock-out mice in a syngeneic murine model of B16 melanoma, which revealed clear tumor growth and reduced survival.[37] Furthermore, CXCL9, -10, -11 attrac Th1 cells, and block the migration of Th2 cells in response to CCR3 ligands due to their ability to serve as antagonists for CCR3.[38] On the other hand, NK cell subsets, the anti-tumor effectors that express CXCR3, are also recruited to the site in a CXCR3-dependent manner.[35] Wende et al. reported that tumor-infiltrating NK cells significantly decreased in CXCR3 knock-out mice, where the CXCL10-controlled NK cell recruitment was not only correlated with tumor cell suppression, but with a good prognosis as well.[39] Furthermore, the accumulation of γδT cells, which shows an autoimmune response to infections or cancers, is reportedly governed by CXCL9/CXCR3 axis-dependent mechanisms.[40] Interestingly, although CXCL4 induces apoptotic signals through CXCL4/CXCR3B axis,[32] Korniejewska et al. showed that CXCL4 could not elicit T cell migration in spite of intracellular calcium mobilization as well as phosphorylation of Akt and ERK. It means CXCL4 may have other roles in T cell function.[36] Experimental studies in various disease models indicate that deficiency of the three ligands for CXCR3 significantly impairs cell-mediated immunity.[34, 35, 37, 39] However, some reports conversely show that the axis regulates immune suppression by inducing Treg migration to the focal sites.[41, 42]
For immune cell differentiation, some reports show that CXCL9, -10, -11 all lead to Th1 polarization through CXCR3, whereas other reports present different functions.[41, 43, 44] In vivo model by Zohar et al[44] showed that CXCL10, like CXCL9, drove increased transcription of T-bet and RORγ, leading to the polarization of Foxp3− type 1 regulatory (Tr1) cells or T helper 17 (Th17) from naive T cells via STAT1, STAT4, and STAT5 phosphorylation. In contrast, CXCL11 decreased transcription of RORγ, but not T-bet, leading to Tr1 or Th2 cells polarization from naive T cells via p70 kinase/mTOR pathways, similar to the mechanism involving TGFβ and IL-27.[45, 46] Unfortunately, these studies did not investigate variants of CXCR3. However, considering that CXCL11 has high affinity for CXCR3 and has such functions, CXCL11 might work to stimulate cancer growth. Several studies have shown that tumor associated macrophages (TAMs) play modulatory activities in the TME, and the CXCL9, -10, -11/CXCR3 axis impacts TAMs polarization. The TAMs have opposite effects; M1 for anti-tumor activities, and M2 for pro-tumor activities. Interestingly, Oghumu et al clarified that CXCR3 deficient mice displayed increased IL-4 production and M2 polarization in a murine breast cancer model, and decreased innate and immune cell-mediated anti-tumor responses.[47] However, on the contrary, Liu et al. reported that CXCR3-positive B cells infiltrated to tumor site and operated in immunoglobulin G–dependent pathways to induce M2 polarization in hepatocellular carcinoma. This difference might be explained by the difference in the tissue background, the degree of inflammation, and the induced immune cells depending on organs and cancer types.
For immune cell activation, CXCL9, -10, -11 stimulate immune cells through Th1 polarization and activation. Th1 cells produce IFN-γ, TNF-α, IL-2 and enhance anti-tumor immunity by stimulating CTLs, NK cells, NKT cells, and macrophages.[4, 48] Furthermore, the IFN-γ-dependent immune activation loop also promotes CXCL9, -10, -11 release. Importantly, NK cells can display immune activity by modulating dendritic cell function, and also provide an early source for IFN-γ production.[35]
Naturally, immune cells, mainly Th1, CTLs, NK cells, and NKT cells, show anti-tumor effect against cancer cells through paracrine CXCL9, -10, -11/CXCR3 axis in tumor models.[15, 49, 50] However, the autocrine CXCL9, -10, -11/CXCR3 signaling in cancer cells increases cancer cell proliferation, angiogenesis, and metastasis. Past reports have already shown the possibility that cancer cells with CXCR3 have a propensity to metastasize due to autocrine signaling from the pre-metastatic niche in vitro and in vivo.[7, 8] The axis for metastases facilitates the migration of CXCR3 expressing cancer cells to ligand rich metastatic sites. As CXCR3-A plays a key role in metastasis,[51] treatment targeting only CXCR3A, not CXCR3B and CXCR3-alt, in the CXCL9, -10, -11/CXCR3 axis could be effective in metastatic disease.
The expression level of CXCR3 in clinical cancer samples is associated with metastatic potential and patients’ prognosis.[52, 53] Hence, it is feasible to use this axis as a predictor for treatment efficacy or as a prognostic indicator. Although Wightman et al. identified the critical role of CXCL10/CXCR3 co-expression in increasing metastatic potential,[54] the relationship between the expression levels of three ligands and metastasis or prognosis are still controversial. The reduction of not only CXCR3, but also of CXCL9 and CXCL10 could suppress cancer metastatic frequencies in melanoma,[55] colon cancer,[7, 52, 56] and breast cancer models[57, 58]. There is a consensus among some groups about the association between CXCL9 [59, 60] and CXCL10 [54, 61] expression and poor prognosis or negative response to existing therapy, whereas others report that CXCL9 [62–64] and CXCL10 [65, 66] are related to opposite results. These differences in reports may be due to complex relationship between each ligand depending on the cancer types. Weisi et al. reported the interesting strategy which systematically made a score using the expression levels of CXCL9, -10, -11 to predict the patients outcome.[61] In the future, we may have to consider the expression levels of CXCL9, -10, -11 to predict patient prognosis.
CXCL9, CXCL10, CXCL11/CXCR3 axis, a target for cancer treatment
The CXCL9, -10, -11/CXCR3 axis is a promising target for drug development by activating the paracrine axis, and inhibiting the autocrine axis. Agents that augment paracrine CXCL9, -10, -11 expression, and deactivate CXCR3 expression on cancer cells have shown anti-tumor activity in several tumor models. (Table 1)
Table 1.
Cancer treatment approaches using CXCL9, -10, -11./CXCR3 axis based on pre-clinical models
| Target | Approach | Type of cancer | Works for the axis (in TME) | In vitro or in vivo | Outcome | Ref. |
|---|---|---|---|---|---|---|
| Immune activation | ||||||
| CXCL9 | Systemic plasmid-borne CXCL9 and low-dose cisplatin | Colon, lung | Induction of CTLs | in vivo | Reduction in tumor microvessel density and increasing apoptosis | [67] |
| Intratumor recombinant CXCL9 and systemic IL-2 | Kidney (RCC) | Induction of mononuclear cells | in vivo | Reduction in tumor growth and angiogenesis | [68] | |
| CXCL10 | Intratumor recombinant CXCL10 | Lung (NSCLC) | – | in vivo | Reduction in tumor growth and angiogenesis, and inhibition of metastasis | [69] |
| Retroviral CXCL10 gene transduction to tumor cells | Melanoma | (CXCL10 protein secretion) | in vitro and in vivo | Reduction in tumor microvessel density and tumor growth | [70] | |
| Retroviral CXCL10 gene transduction to tumor cells | Sarcoma, lung(LLC) | Macrophage infiltration and elevated IL-12 | in vitro and in vivo | Reduction in tumor angiogenesis, mitotic activity, and tumor growth | [71] | |
| Recombinant CXCL10, CXCL10-Ig fusion protein | Myeloma | Induction of CTLs and NK cels | in vitro and in vivo | Reduction in tumor growth | [72] | |
| CXCL10-egfrviii fusion protein | Glioma | Induction of CTLs | in vivo | Reduction in tumor growth and improving prognosis | [73, 74] | |
| CXCL11 | CXCL11-Armed oncolytic poxvirus | Mesothelioma | Induction of CTLs (not only in TME but also in the spleen and lymph organs) and NK cells | in vivo | Reduction in tumor growth and improving prognosis | [75] |
| (CXCL11-Ig fusion protein | Autoimmune encephalomyelitis | Reduction in T cell migration, and induction of regulatory T cells polarization | in vivo | ) | [44] | |
| Direct inhibitinon | ||||||
| CXCR3 | AMG487 (an antagonist of CXCR3) | Colon | – | in vitro and in vivo | Inhibition of lung metastasis, but not liver metastasis | [7] |
| Breast | Induction of Th1 and CTLs in the peripheral blood | in vitro and in vivo | Inhibition of lung metastasis, but not local cancer growth | [8] | ||
| Breast | – | in vitro and in vivo | Inhibition of lung metastasis, but not local cancer growth | [57] | ||
| osteosarcoma | – | in vitro and in vivo | Inhibition of lung metastasis (in vivo), and cancer cell growth (in vitro) | [76] | ||
Abrbreviations: CTLs, cytotoxic T lymphocytes; LLC, Lewis lung cancer; NK cells, natural killer cells; NSCLC, non small cell lung cancer; RCC, renal cell carcinoma; TME, tumor microenvironment,
The use of ligands that attract Th1 cells, CTLs, NK cells, NKT cells, and M1 macrophages into tumor sites can serve as an effective anti-tumor strategy. Zhang et al. reported that the combination of plasmid-borne CXCL9 plus cisplatin augmented colon and lung cancer reduction and CTLs activation.[67] In renal cell carcinoma tumor model, intratumoral CXCL9 and systemic IL-2 reduced tumor growth and angiogenesis through tumor-infiltrating CXCR3+ mononuclear cells.[68] Arenberg et al. reported that administration of CXCL10 by intratumor injections induced better survival of mice inoculated with lung carcinoma cells.[69] Using retroviral CXCL10 gene transduction, the usefulness of CXCL10 overexpression to inhibit tumor growth was reported in melanoma, sarcoma, and lung carcinoma models.[70, 71] Interestingly, Barash et al. showed promising results of a CXCL10–Ig fusion protein in a myeloma mouse model. This fusion protein is likely to have a longer half-life while maintaining the features of the recombinant protein, and inducing tumor infiltrating CTLs and NK cells into tumor sites.[72] Furthermore, a novel CXCL10-EGFRvIII fusion protein (IP10-scFv) with CTLs administration succeeded to induce tumor infiltrating lymphocytes and prolong survival, using a glioma mouse model.[73, 74] Since CXCL11 contributes to inducing Treg migration or promoting Tr1 and Th2 cells polarization, CXCL11-dependent therapy may be controversial as a new target for cancer therapy. In a mesothelioma mouse model, a tumor-selective oncolytic vaccinia virus with CXCL11 reportedly enhanced tumor-infiltrating CTLs and NK cells, but not CD4+ T cells, and prolonged survival.[75] In an autoimmune encephalomyelitis mouse model, the treatment with CXCL11-Ig fusion protein induced rapid disease remission through a downregulation of T cell migration and upregulation of Treg polarization,[44] suggesting the complexity of targeting CXCL11. Although these reports have not shown the role of CXCR3 variants, they might be targets of drug development by activating paracrine signaling.
The anti-CXCR3 therapy is promising. In murine models, pharmacological antagonism of CXCR3 reduced tumor growth and the development of metastasis. An antagonist for CXCR3, named AMG487, inhibited the implantation and growth of colon cancer and osteosarcoma cells in vitro, and suppressed lung metastasis in a vivo model.[7, 76] Interestingly, AMG487 could inhibit lung metastasis, but not local growth, in vivo in breast cancer.[8, 57] Cambien et al also showed that AMG487 could not suppress liver metastasis and the growth of metastatic tumor.[7] These findings indicate that anti-CXCR3 may specifically inhibit tumor metastasis while also adversely inhibiting anti-tumoral host response through paracrine CXCL9, -10, -11/CXCR3 axis. AMG 487 targets all variants of CXCR3, so suppression of paracrine axis may have a pro-tumor effect. Therefore, administration of the combination of ligands for immune activation and pharmacological inhibition of CXCR3A to prevent metastasis may be a promising new approach.
CXCL9, CXCL10, CXCL11/CXCR3 axis, an enhancer for other immune pathways
Although the clinical relevance of the IFN-γ/CXCL9, -10, -11/CXCR3 axis is getting established, it is critical to understand how this pathway crosslinks with other immune consistent pathways. (Table 2)
Table 2.
Prospective approaches related to CXCL9, -10, -11/CXCR3 axis for cancer treatment
| Treatment | Target in the axis | Type of cancer | Works for the axis (in TME) | In vitro or in vivo | Outcome (for TME and host status) | Ref. |
|---|---|---|---|---|---|---|
| Existing drugs | ||||||
| Anti-PD-1 | CXCL9, -10 | Breast, melanoma | Induction of CXCL9 and CXCL10 | in vivo | An anti-PD-1 could not reduce the tumor growth in CXCR3 knock out mice. | [37] |
| CXCL10, (IFN-γ) | Colon, melanoma | Induction of IFN-γ and CXCL10 | in vivo | Induction of Tcells and reduction in tumor growth (with ACT therapy) | [77] | |
| Anti-CTLA4 | CXCL9, -10, -11 | Melanoma | – | in vivo (human sample) | Induction of Tcells | [79] |
| Anti-DPP4 | CXCL10 | Colon, melanoma | Induction of CXCL10 | in vivo | Improving the response to immunotherapy (anti-CTLA4) | [80] |
| (CXCL10 | Patients with chronic hepatitis C | Induction of bioactive CXCL10 | in vivo (human sample) | Anti-DPP4 could presearve the bioactive form of CXCL10.) | [81] | |
| COX-inhibitor | CXCL9, -10 | Ovarian | Induction of CXCL9 and CXCL10 | in vitro | Induction of CXCL9 and CXCL10 by unselective COX inhibition, and conversely reduction of that by COX-2-spedfic inhibition | [63] |
| CXCL9, -10 | Breast | Induction of CXCL9 and CXCL10 | in vitro | Induction of CXCL9 and CXCL 10 by unselective COX inhibition, and conversely reduction of that by COX-2-spedfic inhibition | [82] | |
| COX-inhibitor with anti- PD-1 | CXCL9, -10 | Breast, melanoma | Induction of CXCL9 and CXCL10 | in vivo | In combination of celecoxib and anti-PD-1, induction of T cells and reduction of Tregs and MDSCs lead to inhibiting tumor growth | [86] |
| Lapatinib (with doxorubicin) | CXCL9, -10, -11 | Breast | Induction of CXCL9,-10,-11 through STATI | in vivo | Induction of Tcells and reduction in tumor growth | [88] |
| ATRA | CXCL9, CXCL10 | Melanoma | Induction of CXCL9 and CXCL10 | in vitro | – | [89] |
| CMF | (CXCL9) | Breast | – | in vivo (human sample) | High expression levels of CXCL9 was associated with prolonged DFS | [90] |
| Dacarbazine, temozolomide, cisplatin | CXCL9, CXCL10 | Melanoma | Induction of CXCL9 and CXCL10 | in vitro and in vivo | Induction of Tcells and improving prognosis | [91] |
| Prospective drugs | ||||||
| ACT therapy | CXCL9, -10, -11 | Melanoma | – | in vitor and in vivo | The expressions of CXCL9,-10,-11 in pretreatment tumors were associated with responsiveness to the treatment. | [92] |
| Recombinant MAGE-A3 antigen | CXCL9, -10, -11 | Melanoma | – | in vivo (human sample) | The expressions of CXCL9,-10,-11 in pretreatment tumors were associated with responsiveness to the treatment, (with an Immunostimulant, AS15 or AS02B) | [93, 94] |
| Dendritic cell vaccine therapy | CXCL9, -10, -11 | Chronic lymphocytic leukemia | Induction of CXCL9,-10,-11 | in vitro | Induction of NK cells and NKTcells | [95] |
| IL-2 | CXCL9, -10, -11 | Kidney (RCC) | Induction of CXCL9,-10,-11 | in vivo (human sample) | – | [96] |
| IL-7 (IL-7/IL-7Rα-Fc) | CXCL9, CXCL10 | Lung (LLC) | Induction of CXCL9 and CXCL10 | in vitor and in vivo | Induction of Tcells and M1 macrophage, reduction in tumor growth and improving prognosis | [97] |
| IL-7 (DC-AdIL-7) | CXCL9, CXCL10 | Bronchoalveolar cell carcinoma | Induction of CXCL9 and CXCL10 | in vivo | Reduction in tumor growth | [98] |
| miR21 | CXCL10 | Breast | Reduction of CXCL10 | in vitro | Reduction of lymphocyte migration | [99] |
NOTE: DC-AdIL-7, adenovirus vector expressing interleukin (IL)-7; IL-7/IL-7Rα-Fc, chimeric γc homeostatic cytokine; lapatinib;dual, tyrosine kinase inhibitor which interrupts HER2/neu EGFR pathways.
Abrbreviations: ACT, adoptive cell transfer; ATRA, all-trans retinoic acid; CMF, cyclophosphamide, methotrexate and 5-fluorouracile; COX, cyclooxygenase; CTLA4, cytotoxic T-lymphocyte-associated protein 4; DFS, disease free survival; DPP4, dipeptidyl peptidase-4; LLC, Lewis lung cance; MAGE-A3, melanoma-associated antigen 3; MDSCs, myeloid derived suppressor cells; NK cells, natural killer cells; NKT cells, natural killer T; PD-1, programmed death-1; RCC, renal cell carcinoma; Tregs, regulatory T cells; TME, tumor microenvironment.
The relationship between CXCL9, -10, -11/CXCR3 axis and the PDL-1/PD-1 axis is an important area of research. Programmed cell death-1 (PD-1) is heavily expressed on T cells at the tumor site than on T cells present in the peripheral blood,[77] and anti-PD-1 therapy can inhibit “immune escape” and strengthen the immune activation.[37, 77, 78] Peng et al. showed that anti-PD-1 could not only enhance T cell-mediated tumor regression but also increase the expression of IFN-γ and CXCL10, not CXCL9 and CXCL11 by bone marrow–derived cells.[77] Chheda et al. demonstrated a critical correlation between CXCR3-induced T cells homing to tumor site and anti-PD-1 treatment effect in a vivo model. Anti-PD-1 failed to shrink the tumor in CXCR3 knock out mice, suggesting that anti-PD-1 therapy is not effective without CXCL9, -10, -11/CXCR3 axis.[37] Blockade of the PDL-1/PD-1 axis in T cells may trigger a positive feedback loop at the tumor site through the CXCL9, -10/CXCR3 axis. Also using anti-CTLA4 antibody, this axis was significantly up-regulated in pretreatment melanoma lesions in patients with good clinical response after ipilimumab administration.[79] These results are in consensus and show the usefulness of tumor-infiltrating lymphocytes for anti-PD-1 therapy.
Recently, Barreira da Silva et al. showed that dipeptidyl peptidase 4, known as degradation of incretins, truncates the N-terminal of CXCL10 and limits lymphocyte migration to tumor sites. In vivo evidence showed that DPP4 inhibition enhanced tumor rejection by increasing lymphocytes homing into tumor sites through CXCL10/CXCR3 axis, which boosts the effect of immunotherapy.[80] Decalf et al. showed that DPP4 inhibition in humans can preserve the bioactive form of CXCL10, using a clinically approved DPP4 inhibitor.[81] Since DPP4 inhibitors are safe drugs with a few side effects, they are expected to be used in future therapeutic strategies.
The significance of CXCL9, -10/CXCR3 axis for cancer treatment is also further underscored by the observations that COX-inhibitors increase CXCL9, CXCL10 release from cancer cells in vitro, and promote anti-tumor effects in vivo.[63, 82] The expressions of COX2 and CXCL9 had an inverse correlation in human breast cancer tissue.[82] These reports support the important preclinical data that overexpression of both COX isoenzymes, COX-1 and COX-2, is significantly associated with a lower number of tumor-infiltrating lymphocytes and a worse prognosis in human cancers.[83–85] Furthermore, Li et al. demonstrated that the combination of COX-2 inhibitor and anti-PD1 through alginate hydrogel delivery system synergistically enhanced the presence of Th1 cells and CTLs, and increased the expression of CXCL9 and CXCL10 within the tumor.[86] Interestingly, these effects are accompanied with reduced Tregs and myeloid derived suppressor cells (MDSCs) in the tumor microenvironment. Anti-PD-1 treatment alone could not reduce Treg and MDSCs within the tumor,[77] and therefore, effective drug combinations such as anti-PD-1 and anti CTLA4,[78, 87] or anti-PD1 and a COX inhibitor, may show increased efficacies.
Other existing treatments, such as lapatinib with doxorubicin,[88] all-trans retinoic acid (ATRA), [89] and existing chemotherapies[90, 91] have been reported to exert therapeutic effects through the CXCL9, -10, -11/CXCR3 axis, suggesting that activation of CXCL9, -10, -11/CXCR3 axis may increase efficacies of cytotoxic and targeted therapies. (Table 2)
CXCL9, -10, -11, and CCL5 have also been identified as candidate biomarkers of adoptive T cell transfer therapy in metastatic melanoma.[92] For MAGE-A3 cancer immunotherapy, Ulloa-Montoya et al. have suggested that the pretreatment expression of CXCL9, -10 reflects the clinical response of patients with melanoma or non-small cell lung cancer through gene expression signature analysis.[93, 94] In addition, the association of this axis with immunotherapy, such as dendritic cell vaccine therapy,[95] IL-2,[96] or IL-7[97, 98] administration therapy was reported and showed the importance of CXCL9, -10, -11/CXCR3 axis in the efficacy of immunotherapy. Although there are few reports showing epigenetic involvements in this axis, miR21, an oncogenic miRNA, was reported to be a regulator of CCL5 and CXCL10 in breast cancer cells.[99] (Table 2)
These new approaches targeting CXCL9, -10, -11/CXCR3 axis treatment highlight the role of synergy in cancer treatment. Further understanding of this pathway is warranted.
Concluding remarks
The current review paid attention to exploring the role of CXCL9, -10, -11/CXCR3 axis in TME and immune response. This axis plays a critical role in immune activation through paracrine signaling, impacting efficacy of cancer treatments. Based on pre-clinical data, the combination of pharmacological ligands and inhibition of CXCR3A may lead to new opportunities for more efficient immune therapies, and enhance the effectiveness of existing chemotherapies. Further understanding of the regulation of this pathway could provide a gateway to more effective strategies in the treatment of cancer.
Highlights.
Chemokines induce chemotaxis, promote differentiation of immune cells, and cause tissue extravasation.
The CXCL9, -10, -11/CXCR3 axis regulates immune cell migration, differentiation, and activation through paracrine axis.
The axis induces tumor growth and metastasis through autocrine axis.
Preclinical researches are defining the axis as a promising target for cancer treatment.
Other immune consistent pathways strongly crosslink with this axis.
Acknowledgments
Funding: R Tokunaga was supported by the Uehara Memorial Foundation. Martin D. Berger received a grant from the Swiss Cancer League (BIL KLS-333402 2014) and the Werner and Hedy Berger-Janser Foundation for cancer research. H.-J. Lenz was supported by the NIH (P30CA014089-27S1), the Gloria Borges Wunderglo Project, the Dhont Family Foundation, and the Daniel Butler Research Fund.
Abbreviations
- Th1
T helper 1
- CTLs
cytotoxic lymphocytes
- NK
natural killer
- TME
tumor environment
- Tregs
regulatory T cells
- Th2
T helper 2
- Th17
T helper 17
- Tr1
Foxp3− type 1 regulatory
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential Conflicts of Interest Statement: H.-J. Lenz is a consultant/advisory board member for Bayer, Boehringer Ingelheim, Celgene, Merck Serono, and Roche. No potential conflicts of interest were disclosed by the other authors.
Presentation: We have not presented this review anywhere.
References
- 1.Franciszkiewicz K, Boissonnas A, Boutet M, Combadiere C, Mami-Chouaib F. Role of chemokines and chemokine receptors in shaping the effector phase of the antitumor immune response. Cancer Res. 2012;72:6325–6332. doi: 10.1158/0008-5472.CAN-12-2027. [DOI] [PubMed] [Google Scholar]
- 2.Yoshimura T, Matsushima K, Tanaka S, Robinson EA, Appella E, Oppenheim JJ, et al. Purification of a Human Monocyte-Derived Neutrophil Chemotactic Factor That Has Peptide Sequence Similarity to Other Host Defense Cytokines. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:9233–9237. doi: 10.1073/pnas.84.24.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tannenbaum CS, Tubbs R, Armstrong D, Finke JH, Bukowski RM, Hamilton TA. The CXC chemokines IP-10 and Mig are necessary for IL-12-mediated regression of the mouse RENCA tumor. J Immunol. 1998;161:927–932. [PubMed] [Google Scholar]
- 4.Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007;96:41–101. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- 5.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez-Poma SM, Salas-Benito D, Lozano T, Casares N, Riezu-Boj JI, Mancheno U, et al. Expansion of Tumor-Infiltrating CD8+ T cells Expressing PD-1 Improves the Efficacy of Adoptive T-cell Therapy. Cancer Res. 2017;77:3672–3684. doi: 10.1158/0008-5472.CAN-17-0236. [DOI] [PubMed] [Google Scholar]
- 7.Cambien B, Karimdjee BF, Richard-Fiardo P, Bziouech H, Barthel R, Millet MA, et al. Organ-specific inhibition of metastatic colon carcinoma by CXCR3 antagonism. Br J Cancer. 2009;100:1755–1764. doi: 10.1038/sj.bjc.6605078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu G, Yan HH, Pang Y, Jian J, Achyut BR, Liang X, et al. CXCR3 as a molecular target in breast cancer metastasis: inhibition of tumor cell migration and promotion of host anti-tumor immunity. Oncotarget. 2015;6:43408–43419. doi: 10.18632/oncotarget.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohmori Y, Schreiber RD, Hamilton TA. Synergy between interferon-gamma and tumor necrosis factor-alpha in transcriptional activation is mediated by cooperation between signal transducer and activator of transcription 1 and nuclear factor kappaB. J Biol Chem. 1997;272:14899–14907. doi: 10.1074/jbc.272.23.14899. [DOI] [PubMed] [Google Scholar]
- 10.Ohmori Y, Wyner L, Narumi S, Armstrong D, Stoler M, Hamilton TA. Tumor necrosis factor-alpha induces cell type and tissue-specific expression of chemoattractant cytokines in vivo. Am J Pathol. 1993;142:861–870. [PMC free article] [PubMed] [Google Scholar]
- 11.Muehlinghaus G, Cigliano L, Huehn S, Peddinghaus A, Leyendeckers H, Hauser AE, et al. Regulation of CXCR3 and CXCR4 expression during terminal differentiation of memory B cells into plasma cells. Blood. 2005;105:3965–3971. doi: 10.1182/blood-2004-08-2992. [DOI] [PubMed] [Google Scholar]
- 12.Brightling CE, Ammit AJ, Kaur D, Black JL, Wardlaw AJ, Hughes JM, et al. The CXCL10/CXCR3 axis mediates human lung mast cell migration to asthmatic airway smooth muscle. Am J Respir Crit Care Med. 2005;171:1103–1108. doi: 10.1164/rccm.200409-1220OC. [DOI] [PubMed] [Google Scholar]
- 13.Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. 1995;270:27348–27357. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- 14.Farber JM. Mig and IP-10: CXC chemokines that target lymphocytes. J Leukoc Biol. 1997;61:246–257. [PubMed] [Google Scholar]
- 15.Gorbachev AV, Kobayashi H, Kudo D, Tannenbaum CS, Finke JH, Shu S, et al. CXC chemokine ligand 9/monokine induced by IFN-gamma production by tumor cells is critical for T cell-mediated suppression of cutaneous tumors. J Immunol. 2007;178:2278–2286. doi: 10.4049/jimmunol.178.4.2278. [DOI] [PubMed] [Google Scholar]
- 16.Menke J, Zeller GC, Kikawada E, Means TK, Huang XR, Lan HY, et al. CXCL9, but not CXCL10, promotes CXCR3-dependent immune-mediated kidney disease. J Am Soc Nephrol. 2008;19:1177–1189. doi: 10.1681/ASN.2007111179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qian C, An H, Yu Y, Liu S, Cao X. TLR agonists induce regulatory dendritic cells to recruit Th1 cells via preferential IP-10 secretion and inhibit Th1 proliferation. Blood. 2007;109:3308–3315. doi: 10.1182/blood-2006-08-040337. [DOI] [PubMed] [Google Scholar]
- 18.Schmid H, Boucherot A, Yasuda Y, Henger A, Brunner B, Eichinger F, et al. Modular activation of nuclear factor-kappaB transcriptional programs in human diabetic nephropathy. Diabetes. 2006;55:2993–3003. doi: 10.2337/db06-0477. [DOI] [PubMed] [Google Scholar]
- 19.Xia JB, Liu GH, Chen ZY, Mao CZ, Zhou DC, Wu HY, et al. Hypoxia/ischemia promotes CXCL10 expression in cardiac microvascular endothelial cells by NFkB activation. Cytokine. 2016;81:63–70. doi: 10.1016/j.cyto.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Wang Q, Nagarkar DR, Bowman ER, Schneider D, Gosangi B, Lei J, et al. Role of double-stranded RNA pattern recognition receptors in rhinovirus-induced airway epithelial cell responses. J Immunol. 2009;183:6989–6997. doi: 10.4049/jimmunol.0901386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sridharan V, Margalit DN, Lynch SA, Severgnini M, Zhou J, Chau NG, et al. Definitive chemoradiation alters the immunologic landscape and immune checkpoints in head and neck cancer. Br J Cancer. 2016;115:252–260. doi: 10.1038/bjc.2016.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsieh MF, Lai SL, Chen JP, Sung JM, Lin YL, Wu-Hsieh BA, et al. Both CXCR3 and CXCL10/IFN-inducible protein 10 are required for resistance to primary infection by dengue virus. J Immunol. 2006;177:1855–1863. doi: 10.4049/jimmunol.177.3.1855. [DOI] [PubMed] [Google Scholar]
- 23.Rani MR, Foster GR, Leung S, Leaman D, Stark GR, Ransohoff RM. Characterization of beta-R1, a gene that is selectively induced by interferon beta (IFN-beta) compared with IFN-alpha. J Biol Chem. 1996;271:22878–22884. doi: 10.1074/jbc.271.37.22878. [DOI] [PubMed] [Google Scholar]
- 24.Weng Y, Siciliano SJ, Waldburger KE, Sirotina-Meisher A, Staruch MJ, Daugherty BL, et al. Binding and functional properties of recombinant and endogenous CXCR3 chemokine receptors. J Biol Chem. 1998;273:18288–18291. doi: 10.1074/jbc.273.29.18288. [DOI] [PubMed] [Google Scholar]
- 25.Cole KE, Strick CA, Paradis TJ, Ogborne KT, Loetscher M, Gladue RP, et al. Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J Exp Med. 1998;187:2009–2021. doi: 10.1084/jem.187.12.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colvin RA, Campanella GS, Sun J, Luster AD. Intracellular domains of CXCR3 that mediate CXCL9, CXCL10, and CXCL11 function. J Biol Chem. 2004;279:30219–30227. doi: 10.1074/jbc.M403595200. [DOI] [PubMed] [Google Scholar]
- 27.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, et al. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184:963–969. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakajima C, Mukai T, Yamaguchi N, Morimoto Y, Park WR, Iwasaki M, et al. Induction of the chemokine receptor CXCR3 on TCR-stimulated T cells: dependence on the release from persistent TCR-triggering and requirement for IFN-gamma stimulation. Eur J Immunol. 2002;32:1792–1801. doi: 10.1002/1521-4141(200206)32:6<1792::AID-IMMU1792>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 30.Kim CH, Rott L, Kunkel EJ, Genovese MC, Andrew DP, Wu LJ, et al. Rules of chemokine receptor association with T cell polarization in vivo. Journal of Clinical Investigation. 2001;108:1331–1339. doi: 10.1172/JCI13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu B, Humbles A, Bota D, Gerard C, Moser B, Soler D, et al. Structure and function of the murine chemokine receptor CXCR3. Eur J Immunol. 1999;29:3804–3812. doi: 10.1002/(SICI)1521-4141(199911)29:11<3804::AID-IMMU3804>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 32.Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L, et al. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med. 2003;197:1537–1549. doi: 10.1084/jem.20021897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ehlert JE, Addison CA, Burdick MD, Kunkel SL, Strieter RM. Identification and partial characterization of a variant of human CXCR3 generated by posttranscriptional exon skipping. J Immunol. 2004;173:6234–6240. doi: 10.4049/jimmunol.173.10.6234. [DOI] [PubMed] [Google Scholar]
- 34.Campanella GS, Medoff BD, Manice LA, Colvin RA, Luster AD. Development of a novel chemokine-mediated in vivo T cell recruitment assay. J Immunol Methods. 2008;331:127–139. doi: 10.1016/j.jim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, et al. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5:1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 36.Korniejewska A, McKnight AJ, Johnson Z, Watson ML, Ward SG. Expression and agonist responsiveness of CXCR3 variants in human T lymphocytes. Immunology. 2011;132:503–515. doi: 10.1111/j.1365-2567.2010.03384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chheda ZS, Sharma RK, Jala VR, Luster AD, Haribabu B. Chemoattractant Receptors BLT1 and CXCR3 Regulate Antitumor Immunity by Facilitating CD8+ T Cell Migration into Tumors. J Immunol. 2016;197:2016–2026. doi: 10.4049/jimmunol.1502376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xanthou G, Duchesnes CE, Williams TJ, Pease JE. CCR3 functional responses are regulated by both CXCR3 and its ligands CXCL9, CXCL10 and CXCL11. Eur J Immunol. 2003;33:2241–2250. doi: 10.1002/eji.200323787. [DOI] [PubMed] [Google Scholar]
- 39.Wendel M, Galani IE, Suri-Payer E, Cerwenka A. Natural killer cell accumulation in tumors is dependent on IFN-gamma and CXCR3 ligands. Cancer Res. 2008;68:8437–8445. doi: 10.1158/0008-5472.CAN-08-1440. [DOI] [PubMed] [Google Scholar]
- 40.Patil RS, Shah SU, Shrikhande SV, Goel M, Dikshit RP, Chiplunkar SV. IL17 producing gammadeltaT cells induce angiogenesis and are associated with poor survival in gallbladder cancer patients. Int J Cancer. 2016;139:869–881. doi: 10.1002/ijc.30134. [DOI] [PubMed] [Google Scholar]
- 41.Yang S, Wang B, Guan C, Wu B, Cai C, Wang M, et al. Foxp3+IL−17+ T cells promote development of cancer-initiating cells in colorectal cancer. J Leukoc Biol. 2011;89:85–91. doi: 10.1189/jlb.0910506. [DOI] [PubMed] [Google Scholar]
- 42.Mulligan AM, Raitman I, Feeley L, Pinnaduwage D, Nguyen LT, O’Malley FP, et al. Tumoral lymphocytic infiltration and expression of the chemokine CXCL10 in breast cancers from the Ontario Familial Breast Cancer Registry. Clin Cancer Res. 2013;19:336–346. doi: 10.1158/1078-0432.CCR-11-3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wildbaum G, Netzer N, Karin N. Plasmid DNA encoding IFN-gamma-inducible protein 10 redirects antigen-specific T cell polarization and suppresses experimental autoimmune encephalomyelitis. J Immunol. 2002;168:5885–5892. doi: 10.4049/jimmunol.168.11.5885. [DOI] [PubMed] [Google Scholar]
- 44.Zohar Y, Wildbaum G, Novak R, Salzman AL, Thelen M, Alon R, et al. CXCL11-dependent induction of FOXP3-negative regulatory T cells suppresses autoimmune encephalomyelitis. J Clin Invest. 2014;124:2009–2022. doi: 10.1172/JCI71951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 46.Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. 2010;11:854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oghumu S, Varikuti S, Terrazas C, Kotov D, Nasser MW, Powell CA, et al. CXCR3 deficiency enhances tumor progression by promoting macrophage M2 polarization in a murine breast cancer model. Immunology. 2014;143:109–119. doi: 10.1111/imm.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hensbergen PJ, Wijnands PG, Schreurs MW, Scheper RJ, Willemze R, Tensen CP. The CXCR3 targeting chemokine CXCL11 has potent antitumor activity in vivo involving attraction of CD8+ T lymphocytes but not inhibition of angiogenesis. J Immunother. 2005;28:343–351. doi: 10.1097/01.cji.0000165355.26795.27. [DOI] [PubMed] [Google Scholar]
- 50.Yang X, Chu Y, Wang Y, Zhang R, Xiong S. Targeted in vivo expression of IFN-gamma-inducible protein 10 induces specific antitumor activity. J Leukoc Biol. 2006;80:1434–1444. doi: 10.1189/jlb.0306212. [DOI] [PubMed] [Google Scholar]
- 51.Yang C, Zheng W, Du W. CXCR3A contributes to the invasion and metastasis of gastric cancer cells. Oncol Rep. 2016;36:1686–1692. doi: 10.3892/or.2016.4953. [DOI] [PubMed] [Google Scholar]
- 52.Kawada K, Hosogi H, Sonoshita M, Sakashita H, Manabe T, Shimahara Y, et al. Chemokine receptor CXCR3 promotes colon cancer metastasis to lymph nodes. Oncogene. 2007;26:4679–4688. doi: 10.1038/sj.onc.1210267. [DOI] [PubMed] [Google Scholar]
- 53.Monteagudo C, Martin JM, Jorda E, Llombart-Bosch A. CXCR3 chemokine receptor immunoreactivity in primary cutaneous malignant melanoma: correlation with clinicopathological prognostic factors. J Clin Pathol. 2007;60:596–599. doi: 10.1136/jcp.2005.032144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wightman SC, Uppal A, Pitroda SP, Ganai S, Burnette B, Stack M, et al. Oncogenic CXCL10 signalling drives metastasis development and poor clinical outcome. Br J Cancer. 2015;113:327–335. doi: 10.1038/bjc.2015.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kawada K, Sonoshita M, Sakashita H, Takabayashi A, Yamaoka Y, Manabe T, et al. Pivotal role of CXCR3 in melanoma cell metastasis to lymph nodes. Cancer Res. 2004;64:4010–4017. doi: 10.1158/0008-5472.CAN-03-1757. [DOI] [PubMed] [Google Scholar]
- 56.Zipin-Roitman A, Meshel T, Sagi-Assif O, Shalmon B, Avivi C, Pfeffer RM, et al. CXCL10 promotes invasion-related properties in human colorectal carcinoma cells. Cancer Res. 2007;67:3396–3405. doi: 10.1158/0008-5472.CAN-06-3087. [DOI] [PubMed] [Google Scholar]
- 57.Walser TC, Rifat S, Ma X, Kundu N, Ward C, Goloubeva O, et al. Antagonism of CXCR3 inhibits lung metastasis in a murine model of metastatic breast cancer. Cancer Res. 2006;66:7701–7707. doi: 10.1158/0008-5472.CAN-06-0709. [DOI] [PubMed] [Google Scholar]
- 58.Ma X, Norsworthy K, Kundu N, Rodgers WH, Gimotty PA, Goloubeva O, et al. CXCR3 expression is associated with poor survival in breast cancer and promotes metastasis in a murine model. Mol Cancer Ther. 2009;8:490–498. doi: 10.1158/1535-7163.MCT-08-0485. [DOI] [PubMed] [Google Scholar]
- 59.Mir MA, Maurer MJ, Ziesmer SC, Slager SL, Habermann T, Macon WR, et al. Elevated serum levels of IL-2R, IL-1RA, and CXCL9 are associated with a poor prognosis in follicular lymphoma. Blood. 2015;125:992–998. doi: 10.1182/blood-2014-06-583369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blank S, Nienhuser H, Dreikhausen L, Sisic L, Heger U, Ott K, et al. Inflammatory cytokines are associated with response and prognosis in patients with esophageal cancer. Oncotarget. 2017;8:47518–47532. doi: 10.18632/oncotarget.17671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu WS, Liu YD, Fu Q, Zhou L, Chang Y, Xu L, et al. Elevated expression of IFN-inducible CXCR3 ligands predicts poor prognosis in patients with non-metastatic clear-cell renal cell carcinoma. Oncotarget. 2016;7:13976–13983. doi: 10.18632/oncotarget.7468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walser TC, Ma X, Kundu N, Dorsey R, Goloubeva O, Fulton AM. Immune-mediated modulation of breast cancer growth and metastasis by the chemokine Mig (CXCL9) in a murine model. J Immunother. 2007;30:490–498. doi: 10.1097/CJI.0b013e318031b551. [DOI] [PubMed] [Google Scholar]
- 63.Bronger H, Singer J, Windmuller C, Reuning U, Zech D, Delbridge C, et al. CXCL9 and CXCL10 predict survival and are regulated by cyclooxygenase inhibition in advanced serous ovarian cancer. Br J Cancer. 2016;115:553–563. doi: 10.1038/bjc.2016.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu Z, Huang X, Han X, Li Z, Zhu Q, Yan J, et al. The chemokine CXCL9 expression is associated with better prognosis for colorectal carcinoma patients. Biomed Pharmacother. 2016;78:8–13. doi: 10.1016/j.biopha.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 65.Bolomsky A, Schreder M, Hubl W, Zojer N, Hilbe W, Ludwig H. Monokine induced by interferon gamma (MIG/CXCL9) is an independent prognostic factor in newly diagnosed myeloma. Leuk Lymphoma. 2016;57:2516–2525. doi: 10.3109/10428194.2016.1151511. [DOI] [PubMed] [Google Scholar]
- 66.Sato Y, Motoyama S, Nanjo H, Wakita A, Yoshino K, Sasaki T, et al. CXCL10 Expression Status is Prognostic in Patients with Advanced Thoracic Esophageal Squamous Cell Carcinoma. Ann Surg Oncol. 2016;23:936–942. doi: 10.1245/s10434-015-4909-1. [DOI] [PubMed] [Google Scholar]
- 67.Zhang R, Tian L, Chen LJ, Xiao F, Hou JM, Zhao X, et al. Combination of MIG (CXCL9) chemokine gene therapy with low-dose cisplatin improves therapeutic efficacy against murine carcinoma. Gene Ther. 2006;13:1263–1271. doi: 10.1038/sj.gt.3302756. [DOI] [PubMed] [Google Scholar]
- 68.Pan J, Burdick MD, Belperio JA, Xue YY, Gerard C, Sharma S, et al. CXCR3/CXCR3 ligand biological axis impairs RENCA tumor growth by a mechanism of immunoangiostasis. J Immunol. 2006;176:1456–1464. doi: 10.4049/jimmunol.176.3.1456. [DOI] [PubMed] [Google Scholar]
- 69.Arenberg DA, White ES, Burdick MD, Strom SRB, Strieter RM. Improved survival in tumor-bearing SCID mice treated with interferon-gamma-inducible protein 10 (IP-10/CXCL10) Cancer Immunology Immunotherapy. 2001;50:533–538. doi: 10.1007/s00262-001-0231-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Feldman AL, Friedl J, Lans TE, Libutti SK, Lorang D, Miller MS, et al. Retroviral gene transfer of interferon-inducible protein 10 inhibits growth of human melanoma xenografts. Int J Cancer. 2002;99:149–153. doi: 10.1002/ijc.10292. [DOI] [PubMed] [Google Scholar]
- 71.Sun Y, Finger C, Alvarez-Vallina L, Cichutek K, Buchholz CJ. Chronic gene delivery of interferon-inducible protein 10 through replication-competent retrovirus vectors suppresses tumor growth. Cancer Gene Ther. 2005;12:900–912. doi: 10.1038/sj.cgt.7700854. [DOI] [PubMed] [Google Scholar]
- 72.Barash U, Zohar Y, Wildbaum G, Beider K, Nagler A, Karin N, et al. Heparanase enhances myeloma progression via CXCL10 downregulation. Leukemia. 2014;28:2178–2187. doi: 10.1038/leu.2014.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang X, Lu XL, Zhao HY, Zhang FC, Jiang XB. A novel recombinant protein of IP10-EGFRvIIIscFv and CD8(+) cytotoxic T lymphocytes synergistically inhibits the growth of implanted glioma in mice. Cancer Immunol Immunother. 2013;62:1261–1272. doi: 10.1007/s00262-013-1426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang X, Zhang FC, Zhao HY, Lu XL, Sun Y, Xiong ZY, et al. Human IP10-scFv and DC-induced CTL synergistically inhibit the growth of glioma in a xenograft model. Tumour Biol. 2014;35:7781–7791. doi: 10.1007/s13277-014-1867-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Z, Ravindranathan R, Li J, Kalinski P, Guo ZS, Bartlett DL. CXCL11-Armed oncolytic poxvirus elicits potent antitumor immunity and shows enhanced therapeutic efficacy. Oncoimmunology. 2016;5:e1091554. doi: 10.1080/2162402X.2015.1091554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pradelli E, Karimdjee-Soilihi B, Michiels JF, Ricci JE, Millet MA, Vandenbos F, et al. Antagonism of chemokine receptor CXCR3 inhibits osteosarcoma metastasis to lungs. Int J Cancer. 2009;125:2586–2594. doi: 10.1002/ijc.24665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peng W, Liu C, Xu C, Lou Y, Chen J, Yang Y, et al. PD-1 blockade enhances T-cell migration to tumors by elevating IFN-gamma inducible chemokines. Cancer Res. 2012;72:5209–5218. doi: 10.1158/0008-5472.CAN-12-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ji RR, Chasalow SD, Wang L, Hamid O, Schmidt H, Cogswell J, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother. 2012;61:1019–1031. doi: 10.1007/s00262-011-1172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barreira da Silva R, Laird ME, Yatim N, Fiette L, Ingersoll MA, Albert ML. Dipeptidylpeptidase 4 inhibition enhances lymphocyte trafficking, improving both naturally occurring tumor immunity and immunotherapy. Nat Immunol. 2015;16:850–858. doi: 10.1038/ni.3201. [DOI] [PubMed] [Google Scholar]
- 81.Decalf J, Tarbell KV, Casrouge A, Price JD, Linder G, Mottez E, et al. Inhibition of DPP4 activity in humans establishes its in vivo role in CXCL10 post-translational modification: prospective placebo-controlled clinical studies. EMBO Mol Med. 2016;8:679–683. doi: 10.15252/emmm.201506145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bronger H, Kraeft S, Schwarz-Boeger U, Cerny C, Stockel A, Avril S, et al. Modulation of CXCR3 ligand secretion by prostaglandin E2 and cyclooxygenase inhibitors in human breast cancer. Breast Cancer Res. 2012;14:R30. doi: 10.1186/bcr3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu M, Matsumura N, Mandai M, Li K, Yagi H, Baba T, et al. Classification using hierarchical clustering of tumor-infiltrating immune cells identifies poor prognostic ovarian cancers with high levels of COX expression. Mod Pathol. 2009;22:373–384. doi: 10.1038/modpathol.2008.187. [DOI] [PubMed] [Google Scholar]
- 84.Prima V, Kaliberova LN, Kaliberov S, Curiel DT, Kusmartsev S. COX2/mPGES1/PGE2 pathway regulates PD-L1 expression in tumor-associated macrophages and myeloid-derived suppressor cells. Proc Natl Acad Sci U S A. 2017;114:1117–1122. doi: 10.1073/pnas.1612920114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cao Y, Nishihara R, Qian ZR, Song M, Mima K, Inamura K, et al. Regular Aspirin Use Associates With Lower Risk of Colorectal Cancers With Low Numbers of Tumor-Infiltrating Lymphocytes. Gastroenterology. 2016;151:879–892 e874. doi: 10.1053/j.gastro.2016.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li YK, Fang M, Zhang J, Wang J, Song Y, Shi J, et al. Hydrogel dual delivered celecoxib and anti-PD-1 synergistically improve antitumor immunity. Oncoimmunology. 2016;5 doi: 10.1080/2162402X.2015.1074374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Das R, Verma R, Sznol M, Boddupalli CS, Gettinger SN, Kluger H, et al. Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. J Immunol. 2015;194:950–959. doi: 10.4049/jimmunol.1401686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hannesdottir L, Tymoszuk P, Parajuli N, Wasmer MH, Philipp S, Daschil N, et al. Lapatinib and doxorubicin enhance the Stat1-dependent antitumor immune response. Eur J Immunol. 2013;43:2718–2729. doi: 10.1002/eji.201242505. [DOI] [PubMed] [Google Scholar]
- 89.Szabo A, Osman RM, Bacskai I, Kumar BV, Agod Z, Lanyi A, et al. Temporally designed treatment of melanoma cells by ATRA and polyI: C results in enhanced chemokine and IFNbeta secretion controlled differently by TLR3 and MDA5. Melanoma Res. 2012;22:351–361. doi: 10.1097/CMR.0b013e328357076c. [DOI] [PubMed] [Google Scholar]
- 90.Specht K, Harbeck N, Smida J, Annecke K, Reich U, Naehrig J, et al. Expression profiling identifies genes that predict recurrence of breast cancer after adjuvant CMF-based chemotherapy. Breast Cancer Res Treat. 2009;118:45–56. doi: 10.1007/s10549-008-0207-y. [DOI] [PubMed] [Google Scholar]
- 91.Hong M, Puaux AL, Huang C, Loumagne L, Tow C, Mackay C, et al. Chemotherapy induces intratumoral expression of chemokines in cutaneous melanoma, favoring T-cell infiltration and tumor control. Cancer Res. 2011;71:6997–7009. doi: 10.1158/0008-5472.CAN-11-1466. [DOI] [PubMed] [Google Scholar]
- 92.Bedognetti D, Spivey TL, Zhao Y, Uccellini L, Tomei S, Dudley ME, et al. CXCR3/CCR5 pathways in metastatic melanoma patients treated with adoptive therapy and interleukin-2. Br J Cancer. 2013;109:2412–2423. doi: 10.1038/bjc.2013.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ulloa-Montoya F, Louahed J, Dizier B, Gruselle O, Spiessens B, Lehmann FF, et al. Predictive gene signature in MAGE-A3 antigen-specific cancer immunotherapy. J Clin Oncol. 2013;31:2388–2395. doi: 10.1200/JCO.2012.44.3762. [DOI] [PubMed] [Google Scholar]
- 94.Wang E, Bedognetti D, Marincola FM. Prediction of response to anticancer immunotherapy using gene signatures. J Clin Oncol. 2013;31:2369–2371. doi: 10.1200/JCO.2013.49.2157. [DOI] [PubMed] [Google Scholar]
- 95.Gustafsson K, Junevik K, Werlenius O, Holmgren S, Karlsson-Parra A, Andersson PO. Tumour-loaded alpha-type 1-polarized dendritic cells from patients with chronic lymphocytic leukaemia produce a superior NK−, NKT− and CD8+ T cell-attracting chemokine profile. Scand J Immunol. 2011;74:318–326. doi: 10.1111/j.1365-3083.2011.02580.x. [DOI] [PubMed] [Google Scholar]
- 96.Reckamp KL, Figlin RA, Moldawer N, Pantuck AJ, Belldegrun AS, Burdick MD, et al. Expression of CXCR3 on mononuclear cells and CXCR3 ligands in patients with metastatic renal cell carcinoma in response to systemic IL-2 therapy. J Immunother. 2007;30:417–424. doi: 10.1097/CJI.0b013e31802e089a. [DOI] [PubMed] [Google Scholar]
- 97.Sharma S, Batra RK, Yang SC, Hillinger S, Zhu L, Atianzar K, et al. Interleukin-7 gene-modified dendritic cells reduce pulmonary tumor burden in spontaneous murine bronchoalveolar cell carcinoma. Hum Gene Ther. 2003;14:1511–1524. doi: 10.1089/104303403322495025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Andersson A, Srivastava MK, Harris-White M, Huang M, Zhu L, Elashoff D, et al. Role of CXCR3 ligands in IL-7/IL-7R alpha-Fc-mediated antitumor activity in lung cancer. Clin Cancer Res. 2011;17:3660–3672. doi: 10.1158/1078-0432.CCR-10-3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang Z, Han J, Cui Y, Zhou X, Fan K. miRNA-21 inhibition enhances RANTES and IP-10 release in MCF-7 via PIAS3 and STAT3 signalling and causes increased lymphocyte migration. Biochem Biophys Res Commun. 2013;439:384–389. doi: 10.1016/j.bbrc.2013.08.072. [DOI] [PubMed] [Google Scholar]


