Abstract
Sleep deprivation (SD) causes oxidative stress in the hippocampus and subsequent memory impairment. In this study, the effect of near-infrared (NIR) photobiomodulation (PBM) on learning and memory impairment induced by acute SD was investigated. The mice were subjected to an acute SD protocol for 72 hr. Simultaneously, NIR PBM using a laser at 810 nm was delivered (once a day for 3 days) transcranially to the head to affect the entire brain of mice. The Barnes maze and the What-Where-Which task were used to assess spatial and episodic-like memories. The hippocampal levels of antioxidant enzymes and oxidative stress biomarkers were evaluated. The results showed that NIR PBM prevented cognitive impairment induced by SD. Moreover, NIR PBM therapy enhanced the antioxidant status and increased mitochondrial activity in the hippocampus of SD mice. Our findings revealed that hippocampus-related mitochondrial damage and extensive oxidative stress contribute to the occurrence of memory impairment. In contrast, NIR PBM reduced hippocampal oxidative damage, supporting the ability of 810 nm laser light to improve the antioxidant defense system and maintain mitochondrial survival. This confirms that non-invasive transcranial NIR PBM therapy ameliorates hippocampal dysfunction, which is reflected in enhanced memory function.
Keywords: Photobiomodulation, hippocampus, memory, mitochondria, ROS, antioxidant defense
1. Introduction
Sleep plays a crucial role in many biological functions and is necessary for normal physiological and mental performance (Greene and Siegel, 2004). It is well established that sleep has a strong bivariate association with memory function and may even facilitate memory consolidation (Diekelmann and Born, 2010; Maquet, 2001). Sleep deprivation is an inescapable condition that is prevalent in several walks of life, such as shift workers, military and medical personnel as well as individuals who suffers from poor sleep quality and abnormal sleep cycles (Härmä, et al., 1998; Lockley, et al., 2004). Numerous animal and clinical studies have shown a strong correlation between sleep restriction and impaired cognitive function, including memory and learning (Graves, et al., 2001; Mahmoudi, et al., 2017; Meerlo, et al., 2009). Sleep deprivation disrupts cognitive performance in individuals (Durmer and Dinges, 2005) and in animal models causes memory impairment in different cognitive tasks (Alzoubi, et al., 2017a; Azogu, et al., 2015; Smith, et al., 1998; Xie, et al., 2016). Although the precise underlying mechanisms responsible for cognitive impairment induced by sleep deprivation remain elusive, hippocampal mitochondrial dysfunction (particularly impaired complex IV activity and elevated oxidative stress) have been suggested as important factors (Chanana and Kumar, 2017; Villafuerte, et al., 2015). With respect to oxidative stress and antioxidant markers, increased hippocampal malondialdehyde (MDA) (Valvassori, et al., 2017; Zhang, et al., 2013) as well as decreased superoxide dismutase (SOD) (Zhang, et al., 2013) and glutathione peroxidase (GPx) (Arent, et al., 2015) enzyme activities following acute sleep deprivation have also been reported.
A half century ago, Endre Mester first reported evidence regarding the biological effects of a low-power ruby laser (Mester, et al., 1967). Nowadays, low-level laser/light therapy (LLLT), also known as photobiomodulation (PBM) therapy, refers to the use of low-power light (ranging between 1–500 mW) from lasers or light-emitting diodes (LEDs) in the red to near-infrared (NIR) spectrum to stimulate biological processes (Chung, et al., 2012). The bio-modulatory effects of PBM are credited to photon absorption by complex IV of the mitochondrial respiratory chain, cytochrome c oxidase (CCO), leading to enhancement of cell respiration (Karu, 2008). In this respect, light activation of CCO could increase ATP generation, modulate reactive oxygen species (ROS) and Ca2+ ions, and induce transcription factors that regulate long-lasting effects on gene expression (de Freitas and Hamblin, 2016).
Over the past decade, the application of transcranial PBM therapy as an innovative and non-invasive therapeutic method in various brain-related conditions has attracted interest by researchers in the fields of neuroscience and biophotonics (Hamblin, 2016). Moreover, there is growing evidence that NIR light could potentially improve cognitive functions such as learning and memory (Barrett and Gonzalez-Lima, 2013; Blanco, et al., 2017), sustained attention (Hwang, et al., 2016; Moghadam, et al., 2017) and executive functions (Blanco, et al., 2015) in human individuals, as well as age-related cognitive decline in animal models (Michalikova, et al., 2008; Salehpour, et al., 2017). NIR light is able to improve cerebral blood flow (Uozumi, et al., 2010) and the bioenergetic function of cortical neurons (Mochizuki-Oda, et al., 2002), reduce neuroinflammatory responses (Moreira, et al., 2009; Zhang, et al., 2014), prevent apoptosis (Salehpour, et al., 2017; Yu, et al., 2015) and modulate oxidative stress (Huang, et al., 2013). NIR light at 808 nm remarkably improved spatial learning and memory and rescued hippocampal neurons from Aβ-induced death, via suppression of superoxide production and augmentation of total antioxidant capacity (TAC) (Y. Lu, et al., 2017).
Based on the well-recognized effects of sleep restriction on human cognitive function in the modern world, investigation of novel therapies is of great interest. To the best of our knowledge, this is the first experimental study in which NIR light could potentially enhance cognitive impairment in an animal model of sleep deprivation. The current study focused on the PBM response of hippocampus-related mitochondrial function, antioxidant efficiency, and cognitive behavioral aspects in acute sleep-deprived mice.
2. Results
2.1. Barnes maze task
2.1.1. Training session
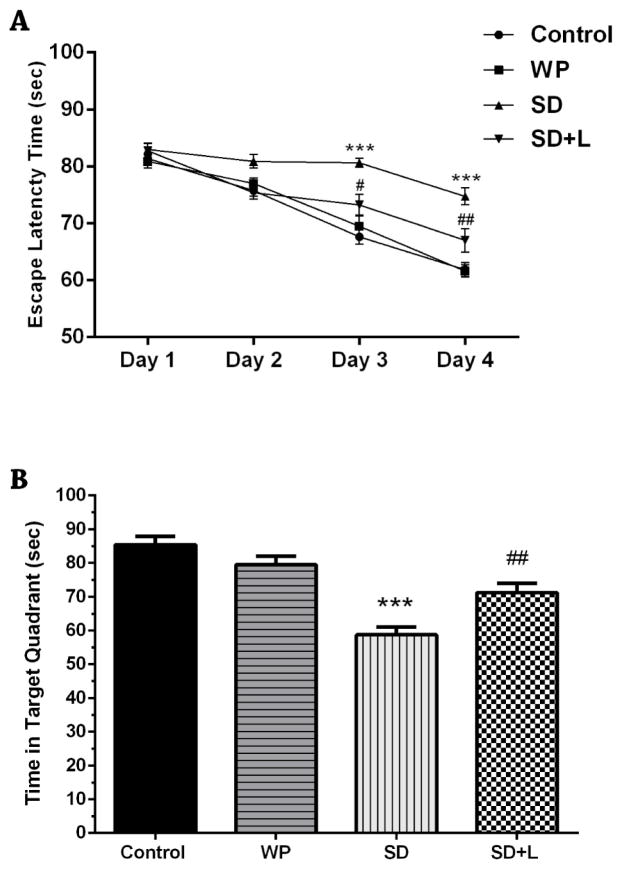
The latency times in the four trials of the training session for all four groups are shown in Figure 2A. The latency times of the SD mice were significantly longer than those of the control group (for both days p<0.001) on the 3rd and 4th days of the training session. By contrast the latency times of the NIR laser-treated SD mice were significantly shorter on the 3rd and 4th days compared with the SD animals (p<0.01 and p<0.001, respectively). There were no differences between the control and WP groups.
Fig. 2.
A) Mean escape latency time of the four groups of mice during the four days of training sessions and B) mean time spent in the target quadrant in the probe session. Values represent Mean±SEM. ***p<0.001 compared with the control. #p<0.05 and ##p<0.01 compared with the SD. (WP, wide platform; SD, sleep deprived; L, laser).
2.1.2. Probe session
As shown in Figure 2B, in the probe test, SD mice spent significantly shorter times in the target quadrant compared to the control mice (p<0.001). The NIR laser-treated SD mice spent significantly longer times in the target quadrant as compared to SD mice (p<0.01). The mean value of time spent in the target quadrant for the WP group was not significantly different from the control group.
2.2. What-Where-Which task
There were no significant differences in the total observation time (total time spend exploring the novel and familiar objects) and amount of locomotor activity among the experimental groups (data not shown). As shown in Figure 3, acute sleep deprivation for a period of 72 hr significantly decreased the DI value (−0.15±0.05) compared to control mice (+0.32±0.06) (p<0.001), indicating marked impairment in episodic-like memory. However, NIR laser treatment of SD mice significantly improved the episodic-like memory performance (DI value of +0.18±0.06) compared to untreated SD group (p<0.01). Moreover, the DI value of the WP group (+0.31±0.04) was not significantly different from that of the control group.
Fig. 3.
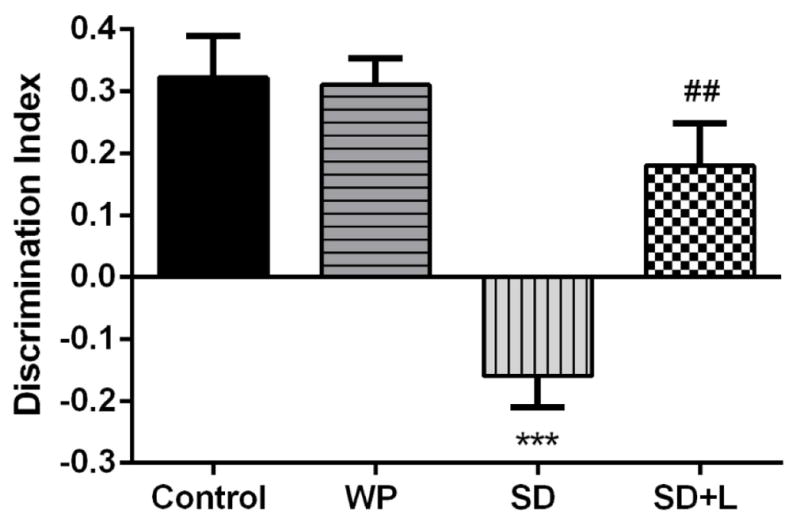
Mean discrimination index (DI) in the four groups of mice in the WWWhich task test session. Each bar represents the mean±SEM. ***p<0.001 compared with the control. ##p<0.01 compared with the SD. (WP, wide platform; SD, sleep deprived; L, laser).
2.3. Mitochondria levels
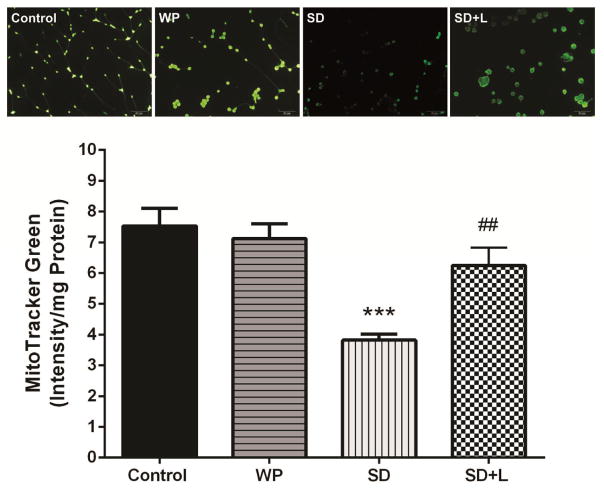
Representative microscopic images and the quantification of the MTG fluorescence are shown in Figure 4. There were significantly higher levels of hippocampal vital mitochondria in the control group than the SD group (p<0.001). The NIR laser treatment of SD mice significantly increased the vital mitochondria compared to the untreated SD group (p<0.01). Moreover, the concentration of vital mitochondria in the WP group was similar to that in the control group.
Fig. 4.
The MitoTracker Green (MTG) staining (upper panel) and mean values of MTG fluorescence intensity/mg protein (vital mitochondria index) (lower panel) in different groups. Each bar represents the mean±SEM. ***p<0.001 compared with control. ##p<0.01 compared with the SD. (WP, wide platform; SD, sleep deprived; L, laser).
2.4. ROS levels
Figure 5 shows that acute sleep deprivation caused a significant increase of ROS levels in the hippocampus of SD mice, compared with the control group (p<0.01). On the other hand, after 3 days of NIR laser treatment of SD mice the intracellular ROS levels were somewhat decreased (almost back to baseline) in comparison to untreated SD animals (p<0.05). No significant difference in ROS levels was found between the control group and the WP group.
Fig. 5.
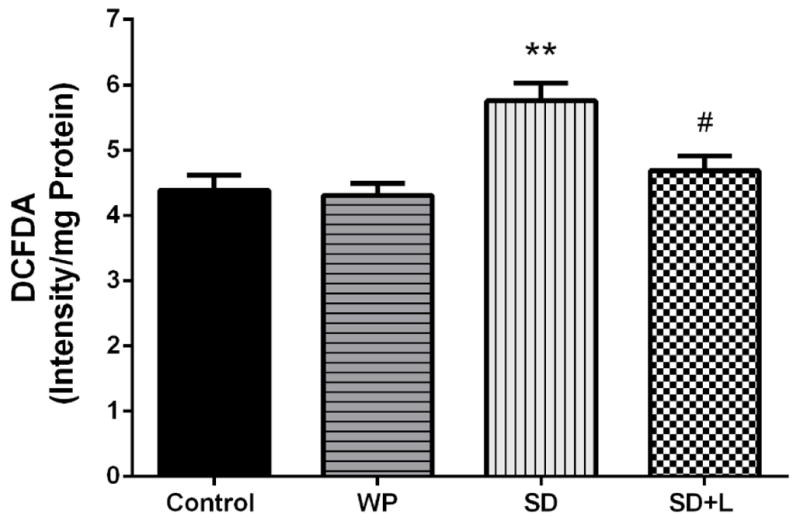
Mean of reactive oxygen species (ROS) levels in hippocampus from the four groups of mice. Each bar represents the mean±SEM. **p<0.01 compared with the control. #p<0.05 compared with the SD. (WP, wide platform; SD, sleep deprived; L, laser).
2.5. MDA levels
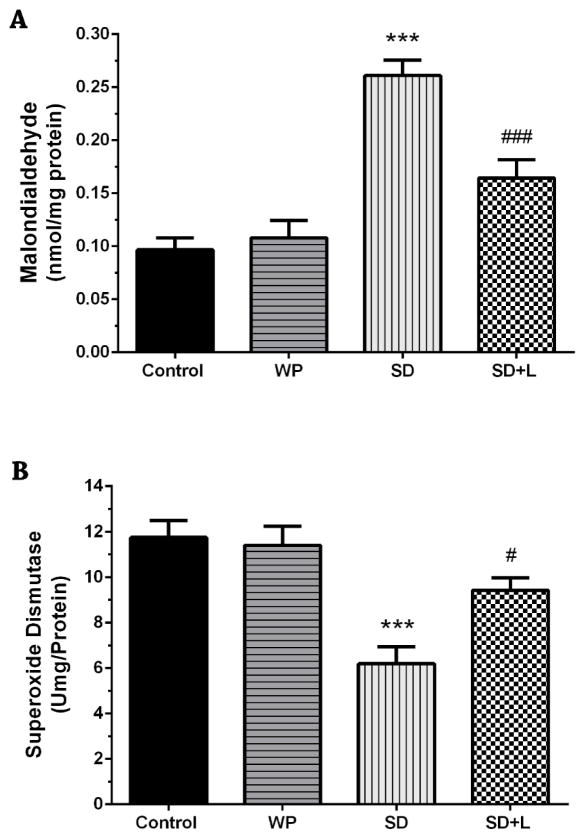
Acute sleep-deprived mice showed remarkably higher levels of MDA in the hippocampus compared to control animals as demonstrated by Figure 6A (p<0.001). With three consecutive days NIR laser treatment, mice exhibited significant partial reduction in their hippocampal MDA levels as compared to SD mice (p<0.001). No significant difference was found between control animals and WP group.
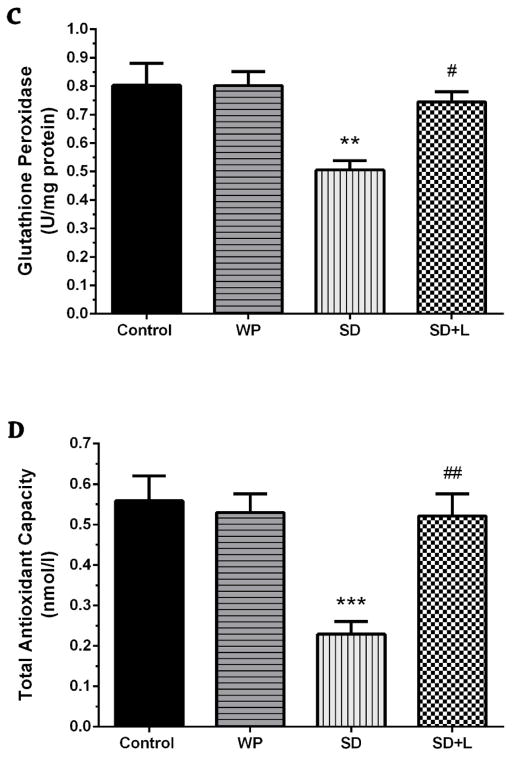
Fig. 6.
A) Mean malondialdehyde (MDA), B) superoxide dismutase (SOD), C) glutathione peroxidase (GPx), and D) total antioxidant capacity (TAC) values in the four groups of mice. Mean±SEM for each group. **p<0.01 and ***p<0.001 compared with the control. #p<0.05, ##p<0.01, and ###p<0.001 compared with the SD. (WP, wide platform; SD, sleep deprived; L, laser).
2.6. Antioxidant enzyme activities
As shown in Figure 6B, following sleep deprivation, a significant decrease in SOD enzyme activity levels were observed in SD animals when compared with those in the control animals (p<0.001). The hippocampal SOD activity was partially elevated by three days of NIR PBM therapy in SD mice compared to untreated SD mice (p<0.05).
Our results (Fig. 6C) also showed that acute sleep-deprived animals exhibited a decline in the hippocampal GPx activity (p<0.01). With NIR laser treatment of SD mice, the GPx activity was significantly increased, compared to untreated SD group (p<0.05). Moreover, both hippocampal SOD and GPx activities in the WP group were similar to those in the control group.
2.7. TAC levels
As shown in Figure 6D, the TAC levels were decreased in the hippocampus of mice subjected to acute sleep deprivation as compared to control mice (p<0.001). The transcranial NIR laser treatment of SD mice restored the hippocampal TAC levels, when compared with those in the untreated SD group (p<0.01). However, the TAC levels of the WP group were not significantly different from those of the control group.
3. Discussion
Hippocampal-dependent activities such as synaptic plasticity depending on mitochondrial activity are involved in normal memory and learning processes (Oettinghaus, et al., 2016). There is a high concentration of mitochondria in neural tissues and especially the brain, and considering the central role of mitochondrial photoacceptors in the mechanisms of PBM, NIR PBM has been suggested as a promising approach for improving brain bioenergetics and cognitive enhancement (Jack, 2017; Tian, et al., 2016). In addition, it is known that sleep deprivation induces a number of alterations in cerebral energy metabolism, and can cause cognitive impairment (Andreazza, et al., 2010; Bergmann, et al., 1989). Our data showed that acute sleep deprivation remarkably decreased vital mitochondrial levels in the hippocampus, whereas, three consecutive days of NIR laser irradiation significantly preserved the levels of active mitochondria. Mitochondria are a pivotal target for the cognitive impairment induced by sleep deprivation, where it is thought that oxidative damage adversely affects bioenergetics (Dou, et al., 2005). Cerebral respiration and subsequent ATP synthesis is correlated with the levels of vital mitochondria (Perkins and Ellisman, 2011). Dysfunction in brain bioenergetics resulting from loss of vital mitochondria is a causal event in many different diseases characterized by cognitive-decline and progressive impairment (Tarnopolsky and Beal, 2001). Recently, the neurocognitive beneficial effects of transcranial PBM have been shown in different animal models of cognitive dysfunction (Y. Lu, et al., 2017; Salehpour, et al., 2017). Recently, a report showed the neuroprotective effects of PBM in a mouse model of oxidative stress induced by administration of D-galactose (Salehpour, et al., 2017). Here an 8 J/cm2 cortical fluence of NIR laser to the mouse brain rescued the loss of active mitochondria and protected them from oxidative damage (Salehpour, et al., 2017). Mitochondria are the main intracellular source of free radicals and increasing mitochondrial Ca2+ levels also increases free radical production. Oxidative stress causes a sequence of irreversible damage to biological macromolecules including DNA, lipids, and proteins (Majdi, et al., 2016; Wang, et al., 2014). In addition, oxidative stress has a significant role in the cognitive impairment processes involved in aging (Berlett and Stadtman, 1997) as well as in brain damage resulting from traumatic brain injury (Wu, et al., 2010), and Alzheimer’s disease (Smith, et al., 2000). Furthermore, studies have shown that acute sleep deprivation also induces oxidative stress and subsequent cognitive dysfunction (Aleisa, et al., 2011; Zhang, et al., 2013). A regimen of 72 hr of sleep deprivation markedly increased the production of ROS in the hippocampus. However, three days of simultaneous therapy with NIR laser significantly attenuated these levels. This demonstrated the acute beneficial effects of NIR PBM, and is in agreement with the data from several other studies which revealed the neuroprotective effect of NIR light (Y. Lu, et al., 2017; Moro, et al., 2017; Salehpour, et al., 2017). To our knowledge, this is the first study measuring hippocampal MDA levels as a marker of oxidative stress in NIR laser exposed mice. The NIR PBM noticeably reduced oxidative stress in hippocampal neurons as measured by the marker for lipid peroxidation, which has been reported by others to be increased following acute sleep deprivation (Khadrawy, et al., 2011; Lima, et al., 2014; Silva, et al., 2004). Taken together, these findings highlight the well-known ability of NIR light to decrease oxidative damage measured by lowered MDA levels.
Moreover, it has been shown that sleep deprivation elevates hippocampal oxidative stress by attenuating the enzyme activities of SOD (Zhang, et al., 2013) and GPx (Alzoubi, et al., 2017b). The present results that acute sleep deprivation for 72 hr caused substantial changes in hippocampal antioxidant enzymes defense systems as well as TAC levels is in agreement with these reports. Moreover, we showed that NIR laser treatment enhanced the antioxidant enzyme activities in the hippocampus, such as GPx and SOD, and improved TAC levels. These findings underline the antioxidant properties of NIR laser and confirm that 810 nm light can act as a neuroprotective modality via augmentation of the antioxidant defense capacity (Y. Lu, et al., 2017). Four weeks of administration of melatonin (tested as a neuroprotective intervention) was shown to improve hippocampal SOD and GPx activities, which had been impaired by chronic sleep deprivation (Alzoubi, et al., 2016). However, twice-daily administration of melatonin during acute sleep deprivation for 96 hr did not significantly affect hypothalamic glutathione levels (D’Almeida, et al., 2000). In comparing these data with our results, it could be speculated that brain hypothalamic region may be more susceptible to oxidative stress during acute sleep deprivation. Hence, studying the effect of NIR PBM/acute sleep deprivation on brain areas other than the hippocampus is suggested in future works.
In order to deliver a sufficient and optimum dose of light, penetration depth into the skull and the brain is a determining factor in PBM therapy. Several studies have shown superior penetration of NIR light (wavelengths above 800 nm) in comparison with red light in a transcranial light-delivery protocol (Jagdeo, et al., 2012; Sousa, et al., 2013). NIR PBM is based upon absorption of light energy by metal centers of CCO (heme and copper), which results in upregulation of electron transfer in the mitochondrial inner membrane and, consequently higher ATP production (Mochizuki-Oda, et al., 2002). At the same time, this photo-absorption event at the optimum fluence of <10 J/cm2 generates a brief burst of low-intensity mitochondrial ROS accompanied by a rise in mitochondrial membrane potential (Sharma, et al., 2011). However, much higher doses of PBM (accompanied by a drop in mitochondrial membrane potential) can produce a massive amount of ROS. These observations may explain the biphasic dose-response for PBM involving regulation of ROS levels (Huang, et al., 2009; Sharma, et al., 2011). Physiological levels of ROS have a key role in mediating signaling pathways involved in cell viability and proliferation (Sena and Chandel, 2012), while excessive and prolonged levels of ROS are highly damaging. Given this, light-induced production of brief and modest levels of ROS could stimulate neurogenesis and affect the anti-inflammatory response possibly through ROS-mediated signaling pathways (De Taboada, et al., 2011; Xuan, et al., 2014). Moreover, these mitochondrially produced low levels of ROS allow mutual communication between mitochondria and the cytosol and/or the nucleus and could alter gene expression through inducing redox-sensitive transcription factors (Zhang, et al., 2001). These signaling cascades may promote the activation of numerous enzymes and intracellular pathways linked to increased brain metabolism (de Freitas and Hamblin, 2016). In this respect, Zhang et al. (Song, et al., 2003) showed that PBM of human fibroblasts in vitro regulated the expression of genes related to mitochondrial energy metabolism and antioxidant activities. Although the complete signal transduction pathways stimulated by PBM are not completely understood, it seems that PBM improves the effectiveness of the overall antioxidant system through a transient increase in mitochondrial ROS levels (Iakymenko and Sydoryk, 2001).
To test whether NIR laser light protected against sleep deprivation-induced neurotoxicity and impaired cognitive performance, we evaluated the ability of the mice in a test of spatial memory and episodic-like memory. Since the hippocampus plays a principal role in the consolidation of memories related to spatial navigation, we used the Barnes maze as a hippocampus-dependent spatial learning and memory task. Our results showed that acute sleep deprivation significantly impaired spatial learning and memory, which is in line with previous findings (Aleisa, et al., 2011; Zhang, et al., 2013). On the other hand, once-daily transcranial NIR laser treatment for three days significantly reversed this deficiency. The potential benefit of NIR PBM therapy in enhancement of spatial memory has been shown in different animal models using Morris water maze (De Taboada, et al., 2011; Dong, et al., 2015; Xuan, et al., 2014) and Barnes maze (Y. Lu, et al., 2017; Salehpour, et al., 2017). Although these studies applied a high amount of fluence in terms of total treatment sessions, our findings surprisingly represented the neurocognitive effect of short-term NIR PBM regimen. Therefore, it seems that the acute NIR PBM therapy could beneficially affect the hippocampus and hence protect against oxidative stress damage caused by acute sleep deprivation. It is well known that the hippocampal bioenergetic capacity is linked to spatial reference memory and learning (Sadowski, et al., 2004). It could be postulated that the short-term enhancement of memory performance in the laser-treated SD animals could be due to upregulation of hippocampal energy metabolism that appears to be induced by NIR PBM, as well as to an enhancement of antioxidant capacity shown in the biochemical studies.
The WWWhich task is based on the principle of integrating the location of a specific object with particular contextual cues to create an episodic-like memory (Davis, et al., 2013). Some studies have shown that sleep loss could result in deficits in recognition and memory recall. Lu et al. (C. Lu, et al., 2017) showed that sleep deprivation of mice induced an impaired object location recognition memory. Palchykova et al. (Palchykova, et al., 2006) also reported that acute sleep loss caused a deficit in object discrimination related to recognition memory. The current study demonstrated for the first time that NIR laser treatment was able to improve the impaired episodic recognition memory induced by sleep deprivation as assessed by the WWWhich task. Our finding is in good agreement with the works of Lu et al. (Y. Lu, et al., 2017) and Salehpour et al. (Salehpour, et al., 2017) who reported the improvement of recognition memory following NIR laser treatment using 810 nm light.
In conclusion, this study has shown an improvement in cognitive performance as well as hippocampal vital mitochondria and oxidative stress defenses of sleep-deprived mice exposed to noninvasive PBM using a NIR light. Our results support the premise that the neuromodulatory effects of NIR PBM are likely to be (at least partly) due to preservation of mitochondria and improvement in antioxidant defenses in the hippocampus. Based on these findings, it could be suggested that transcranial NIR PBM, using relatively inexpensive home-use LEDs devices (which so far has been found to be totally devoid of any significant side-effects) could be recommended to workers and military personnel, likely to be adversely affected by chronic or acute sleep deprivation. However, in order to achieve this application, possible beneficial effects of the PBM therapy in other cognitive-related brain structures (e.g. cerebellum, thalamus, and cortex) should be explored in future studies.
4. Experimental procedures
4.1. Animals and experimental design
All experimental procedures were performed under the guidelines of the National Institutes of Health (NIH; Publication No. 85-23, revised 1985) and approved by the Higher Academic Education Institute of Rab-Rashid IACUC committee (Code Number: 96/3231/14). Forty adult male BALB/c mice with a weight range of 28–30 g were provided by the animal center of Tabriz University of Medical Sciences (TUOMS). The mice were socially housed in standard Plexiglas cages (5/cage) and kept on a 12 h light/dark cycle at a temperature of 25±2°C, with freely accessible food and water. All mice were acclimated to the animal facility for 1 week prior to the start of the experiments. The four experimental groups (10 animals per group) were designated as follows: control, wide platform (WP), sleep deprived (SD), and sleep deprived+laser (SD+L) groups. A researcher who was unaware of the identity of the experimental groups conducted all experiments including tests and analysis.
4.2. Sleep deprivation
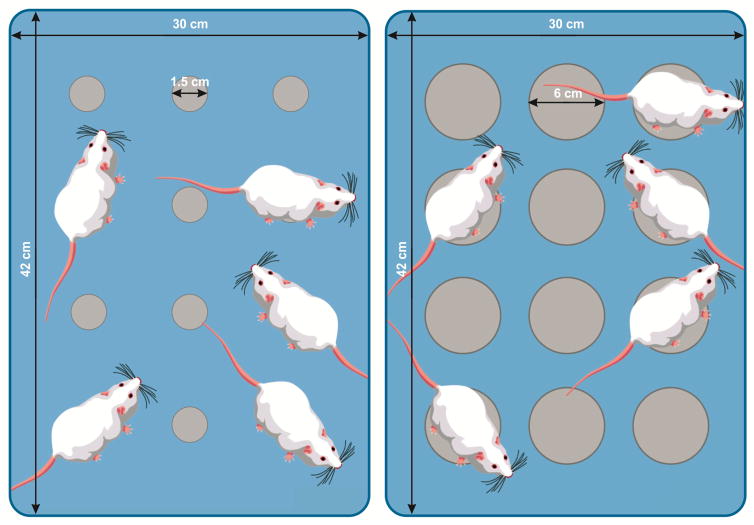
Animals were subjected to sleep deprivation for 72 hr using the modified multiple platform technique, which consists of placing five mice in a Plexiglas tank (42×30×20 cm) containing 12 circular platforms (1.5 cm diameter) with water 1 cm below the upper surface of the platforms (Wang, et al., 2017). During the sleep deprivation period, animals had free access to water and food hanging from the cage cover. Naturally, submitting animals to the novel environment as well as sleep restriction protocol cause a stressful experience (Mahmoudi, et al., 2017). Therefore, in sleep deprivation studies using modified multiple platform technique, a separate group of animals as WP group, typically is submitted to water tank containing wider platforms. In this study, the mice in WP group were submitted to the same procedure as the SD mice, except the platforms were 6 cm in diameter in order to allow animal to normal sleep without falling into water (Figure 1). For laser or sham laser treatment during the sleep deprivation protocol, animals in the WP, SD, and SD+L groups were removed from the platform and returned immediately after the therapy. The control group were maintained in their standard home cages.
Fig. 1.
Simple illustration of modified multiple platform tanks used for SD (left panel) and WP (right panel) mice.
4.3. Laser treatment
A diode GaAlAs laser (Thor Photomedicine, Chesham, UK) with 10-Hz pulsed wave (PW) mode (88% duty cycle) at 810 nm wavelength was used for transcranial irradiation. The laser was operated at an output power of 200 mW and an irradiance of 4.75 W/cm2, with spot size of 0.03 cm2. An average fluence of 8 J/cm2 per each session was delivered to the mice cortical surface for 5 sec, as previously described (Salehpour, et al., 2017). For transcranial laser treatment, the mouse was held firmly and the laser probe placed over the midline of the dorsal surface of the shaved head in region between eyes and ears. The irradiation was administered once a day (at 12:00 h) for three consecutive days. The mice in WP and SD groups underwent the same procedure as the SD+L group but the laser device was not turned on.
4.4. Barnes maze task
The spatial learning and memory test was carried out using the Barnes maze equipped with a digital video camera, as described previously (Salehpour, et al., 2017). The wooden maze, 100 cm in diameter, was elevated 50 cm above the ground. Twenty circular holes, 5.0 cm in diameter, were located 3.0 cm from the perimeter. A black plastic escape box (20×15×5 cm) was placed under the escape hole. The maze was positioned in a room where distinct spatial cues were located on the wall surrounding the maze. White noise with a sound pressure level of 80 dB was used as a negative stimulus throughout the test session. The test was conducted in sessions over five days and consisted of three sessions including adaptation, training and probe sessions. The first day consisted of one adaptation session and four trials, and the three subsequent days consisted of four trials per day, each separated by a 3-min interval. At the beginning of the adaptation session, each mouse was located in the center of the maze in a cylindrical start chamber. After 10 s the chamber was removed, the white noise was turned on, and the mouse was allowed to explore the maze for 3 min. when the animal entered the escape box, the white noise was turned off and the mouse was allowed to remain in it for 1 min. On the fifth day, which served as a probe trial, the escape box was removed and the mouse was allowed to explore the maze for 3 min. The maze and escape box were cleaned with 70% alcohol to remove any olfactory clues between trials. The following parameters were measured in each trial using a video-tracking program, Etho Vision™ (Noldus, The Netherlands). The software measured: (a) the escape latency time during the training trial sessions, and (b) the time spent in the target quadrant during the probe session.
4.5. What-Where-Which task
Episodic-like memory was tested using the What-Where-Which (WWWhich) task (Davis, et al., 2013). The apparatus consisted of two open-field square arenas made of Plexiglas (30×30×25 cm). The arenas were modified to represent two different contexts, namely contexts 1 and 2, as described previously (Salehpour, et al., 2017). The objects for the test session were assembled from LEGO® bricks. The task consisted of three sessions including habituation, exposure, and test sessions. Each mouse was habituated to each context for five minutes on one day. The next day, as an exposure session, two dissimilar objects (A and B) were first placed in the context 1 arena and the mouse was given three minutes to explore it. Then, the mouse was placed in the home cage for 30 seconds and the objects were switched (B and A) located in the context 2 arena. Next, the mouse was allowed to explore it for three minutes and then the mouse was returned to the home cage. The test session was conducted after a five-minute interval, when the mouse was presented with two identical copies of one of the objects (A or B) located in one of the two context arenas (1 or 2) for three minutes. Exposure and test sessions were conducted as one trial for two consecutive days using new objects for each day. Exploration behavior was indicated as the time spent with the nose oriented toward and within 1 cm of the object, which was measured using the video-tracking program Etho Vision (Noldus, The Netherlands). The episodic-like memory was determined by discrimination index (DI) as following formula: DI= (N−F)/(N+F), where N and F represent the exploration time on the novel and familiar objects, respectively.
4.6. Biochemical analysis
For biochemical analysis, mice were deeply anesthetized by combination of ketamine and xylazine (90 mg and 10 mg/kg, intraperitoneal, respectively) and the brains were surgically removed from the cranium and the hippocampus was isolated.
4.6.1 MitoTracker green staining
Mitotracker green dye (Cell Signaling Technology, USA) which diffuses across the plasma membrane was used to indicate the active mitochondria mass of the hippocampal neurons. For the staining process, samples were washed twice with phosphate buffered saline (10 mM phosphate, 137 mM NaCl, and 2.7 mM KCl). Then, hippocampal cells were obtained by enzymatic digestion (trypsin 0.1%) and mechanical dispersion (extrusion through a Pasteur pipette in DMEM culture media enriched with 10 mL/L MEM amino acids, 2 mmol/L glutamine, 5.6 mg/mL amphotericin B and 25 mg/mL gentamicin with 0.3% (w/v) bovine serum albumin) (Velardez, et al., 2003). The suspended cells were washed and centrifuged at 500 g (twice with phosphate buffered saline) and washing buffer was then replaced with DMEM. Finally, Mitotracker green dye (at a concentration range of 100–400 nM) was added into the media and incubated for 30 min at 37 °C in CO2 incubator. Fluorescence emission was read either at λex=490 nm or λem=516 nm using a fluorescent plate reader and data were presented as Mitotracker green fluorescence/mg protein) (Fig. 3).
4.6.2. Reactive oxygen species (ROS) levels
Dichlorodihydro-fluorescein diacetate (DCFDA) dye was used to determine ROS production in hippocampal neurons (Sripetchwandee, et al., 2014). Briefly, the hippocampal neurons suspension was prepared (as described above), and was incubated with 2μM DCFDA for 20 min in DMEM culture media. Fluorescence emission was read at λex=485 nm and λem=530 nm using a fluorescent plate reader. The ROS levels were presented as fluorescence intensity/mg protein (Fig. 4).
4.6.3. Antioxidant and Lipid peroxidation assays
To determine antioxidant enzyme activities (SOD and GPx), TAC and lipid peroxidation levels, brain hippocampus samples were homogenized in 1.15 % KCl solution and then centrifuged at 112 g for 10 min at 4 °C (Pourmemar, et al., 2017).
The MDA levels were determined using the thiobarbituric acid reactive substances (TBARS) method (Farajpour, et al., 2017; Khorrami, et al., 2014).
SOD activity was measured using a RANSOD (Randox Laboratories Ltd, Crumlin, United Kingdom) laboratory kit by assessment of the degree of inhibition of a tetrazolium reaction with authentic superoxide. All working solutions were prepared based on the manufacturer’s instructions and the absorbance was measured at λ=500 nm (Pourmemar, et al., 2017).
GPx activity of hippocampal neurons was measured using RANSOD (Randox Laboratories Ltd, Crumlin, United Kingdom) assay kit. The oxidized form of glutathione, in the presence of glutathione reductase (at a concentration ≥ 0.5 units/L) and 0.28 mmol/L of NADPH, is immediately converted to the reduced form with simultaneous oxidation of NADPH to produce NAD+. The decrease in absorbance at 340 nm (37 °C) was measured using a spectrophotometer, and then GPx concentration was calculated (Pourmemar, et al., 2017).
TAC was measured using a Randox total antioxidant status kit (Randox Laboratories Ltd, Crumlin, United Kingdom) in which blue-green color of the 2,2′-azidobis [3-ethylbenzothiazoline-6-sulfonic acid] radical cation (ABTS•+) was suppressed by antioxidants. The degree of this color change is proportional to anti-oxidant concentration and was measured as a change in absorbance at λ=600 nm (Farajpour, et al., 2017).
Activity values were expressed as U/mg protein, TAC and MDA levels were expressed as nmol/L and nmol/mg, respectively.
4.7. Statistical analysis
The all data were expressed as mean ± standard error of the mean (SEM). The statistical significance between values from experimental groups were analyzed by one-way ANOVA followed by Tukey’s post-hoc test using Graph Pad Prism 6.01 (Graph Pad Software Inc., La Jolla, CA, USA). A p-value <0.05 was considered statistically significant.
Highlights.
PBM improves episodic and spatial memories in sleep-deprived mice
PBM preserves vital mitochondria in the hippocampus of sleep-deprived mice
PBM attenuates oxidative stress in the hippocampus of sleep-deprived mice
Acknowledgments
We thank to Prof. Mehdi Farhoudi, Director, Neurosciences Research Center (NSRC) of TUOMS, and Prof. Soltan-Ali Mahboob, Head of the Higher Academic Education Institute of Rab-Rashid for providing support and encouragements for the work. Michael R. Hamblin was supported by US NIH grants R01AI050875 and R21AI121700.
Footnotes
Disclosure statement
The authors have no conflicts of interest to disclose.
References
- Aleisa A, et al. Acute nicotine treatment prevents rem sleep deprivation-induced learning and memory impairment in rat. Hippocampus. 2011;21:899–909. doi: 10.1002/hipo.20806. [DOI] [PubMed] [Google Scholar]
- Alzoubi KH, et al. Arbutus andrachne L. Reverses Sleep Deprivation-Induced Memory Impairments in Rats. Mol Neurobiol. 2017a:1–7. doi: 10.1007/s12035-017-0387-8. [DOI] [PubMed] [Google Scholar]
- Alzoubi KH, et al. Chronic melatonin treatment prevents memory impairment induced by chronic sleep deprivation. Mol Neurobiol. 2016;53:3439–3447. doi: 10.1007/s12035-015-9286-z. [DOI] [PubMed] [Google Scholar]
- Alzoubi KH, et al. L-carnitine Prevents Memory Impairment Induced by Chronic REM-Sleep Deprivation. Brain Res Bull. 2017b doi: 10.1016/j.brainresbull.2017.04.004. [DOI] [PubMed] [Google Scholar]
- Andreazza AC, et al. Impairment of the mitochondrial electron transport chain due to sleep deprivation in mice. J Psychiatr Res. 2010;44:775–780. doi: 10.1016/j.jpsychires.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Arent CO, et al. The effects of n-acetylcysteine and/or deferoxamine on manic-like behavior and brain oxidative damage in mice submitted to the paradoxal sleep deprivation model of mania. J Psychiatr Res. 2015;65:71–79. doi: 10.1016/j.jpsychires.2015.04.011. [DOI] [PubMed] [Google Scholar]
- Azogu I, et al. Acute sleep deprivation enhances avoidance learning and spatial memory and induces delayed alterations in neurochemical expression of GR, TH, DRD1, pCREB and Ki67 in rats. Behav Brain Res. 2015;279:177–190. doi: 10.1016/j.bbr.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Barrett D, Gonzalez-Lima F. Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans. Neuroscience. 2013;230:13–23. doi: 10.1016/j.neuroscience.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Bergmann BM, et al. Sleep deprivation in the rat: V. Energy use and mediation. Sleep. 1989;12:31–41. doi: 10.1093/sleep/12.1.31. [DOI] [PubMed] [Google Scholar]
- Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem. 1997;272:20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- Blanco NJ, et al. Improving executive function using transcranial infrared laser stimulation. J Neuropsychol. 2015 doi: 10.1111/jnp.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco NJ, et al. Transcranial infrared laser stimulation improves rule-based, but not information-integration, category learning in humans. Neurobiol Learn Mem. 2017;139:69–75. doi: 10.1016/j.nlm.2016.12.016. [DOI] [PubMed] [Google Scholar]
- Chanana P, Kumar A. An Insight into Mechanisms underlying Sleep Deprivation Induced Cognitive Dysfunction. J Sleep Disord Ther. 2017;5 2167-0277.1000258. [Google Scholar]
- Chung H, et al. The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng. 2012;40:516–533. doi: 10.1007/s10439-011-0454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Almeida V, et al. Melatonin treatment does not prevent decreases in brain glutathione levels induced by sleep deprivation. Eur J Pharmacol. 2000;390:299–302. doi: 10.1016/s0014-2999(99)00924-3. [DOI] [PubMed] [Google Scholar]
- Davis KE, et al. Episodic-like memory for what-where-which occasion is selectively impaired in the 3xTgAD mouse model of Alzheimer’s disease. J Alzheimers Dis. 2013;33:681–698. doi: 10.3233/JAD-2012-121543. [DOI] [PubMed] [Google Scholar]
- de Freitas LF, Hamblin MR. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron. 2016;22:348–364. doi: 10.1109/JSTQE.2016.2561201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Taboada L, et al. Transcranial laser therapy attenuates amyloid-β peptide neuropathology in amyloid-β protein precursor transgenic mice. J Alzheimers Dis. 2011;23:521–535. doi: 10.3233/JAD-2010-100894. [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Dong T, et al. Low-level light in combination with metabolic modulators for effective therapy of injured brain. J Cereb Blood Flow Metab. 2015;35:1435–1444. doi: 10.1038/jcbfm.2015.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou W, et al. The Effect of sleep deprivation on cognitive and cerebral mitochondrial respiratory function in rats. Neurosci Bull. 2005;21:204. [Google Scholar]
- Durmer JS, Dinges DF. Semin Neurol. 2005. Neurocognitive consequences of sleep deprivation; pp. 117–129. Copyright© 2005 by Thieme Medical Publishers, Inc., 333 Seventh Avenue, New York, NY 10001, USA. [DOI] [PubMed] [Google Scholar]
- Farajpour R, et al. Chronic Administration of Rosa canina Hydro-Alcoholic Extract Attenuates Depressive-Like Behavior and Recognition Memory Impairment in Diabetic Mice: A Possible Role of Oxidative Stress. Med Princ Pract. 2017;26:245–250. doi: 10.1159/000464364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves L, et al. Sleep and memory: a molecular perspective. Trends Neurosci. 2001;24:237–243. doi: 10.1016/s0166-2236(00)01744-6. [DOI] [PubMed] [Google Scholar]
- Greene R, Siegel J. Sleep. Neuromolecular Med. 2004;5:59–68. doi: 10.1385/NMM:5:1:059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblin MR. Shining light on the head: photobiomodulation for brain disorders. BBA clinical. 2016;6:113–124. doi: 10.1016/j.bbacli.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härmä M, et al. Combined effects of shift work and life-style on the prevalence of insomnia, sleep deprivation and daytime sleepiness. Scand J Work Environ Health. 1998:300–307. doi: 10.5271/sjweh.324. [DOI] [PubMed] [Google Scholar]
- Huang Y-Y, et al. Biphasic dose response in low level light therapy. Dose-Response. 7, dose-response. 09–027. Hamblin. 2009 doi: 10.2203/dose-response.09-027.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, et al. Low-level laser therapy (LLLT) reduces oxidative stress in primary cortical neurons in vitro. J Biophotonics. 2013;6:829–838. doi: 10.1002/jbio.201200157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J, et al. Cognitive enhancement by transcranial laser stimulation and acute aerobic exercise. Lasers Med Sci. 2016;31:1151–1160. doi: 10.1007/s10103-016-1962-3. [DOI] [PubMed] [Google Scholar]
- Iakymenko I, Sydoryk E. Regulatory role of low-intensity laser radiation on the status of the antioxidant system. Ukrains’ kyi biokhimichnyi zhurnal (1999) 2001;73:16–23. [PubMed] [Google Scholar]
- Jack C. Treating cognitive impairment with transcranial low level laser therapy. J Photochem Photobiol B, Biol. 2017;168:149–155. doi: 10.1016/j.jphotobiol.2017.02.008. [DOI] [PubMed] [Google Scholar]
- Jagdeo JR, et al. Transcranial red and near infrared light transmission in a cadaveric model. PloS One. 2012;7:e47460. doi: 10.1371/journal.pone.0047460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karu TI. Mitochondrial signaling in mammalian cells activated by red and near-IR radiation. Photochem Photobiol. 2008;84:1091–1099. doi: 10.1111/j.1751-1097.2008.00394.x. [DOI] [PubMed] [Google Scholar]
- Khadrawy YA, et al. Effect of oxidative stress induced by paradoxical sleep deprivation on the activities of Na+, K+-ATPase and acetylcholinesterase in the cortex and hippocampus of rat. Transl Res. 2011;157:100–107. doi: 10.1016/j.trsl.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Khorrami A, et al. Investigation of the memory impairment in rats fed with oxidized-cholesterol-rich diet employing passive avoidance test. Drug Res. 2014 doi: 10.1055/s-0034-1370950. [DOI] [PubMed] [Google Scholar]
- Lima AMA, et al. Differential effects of paradoxical sleep deprivation on memory and oxidative stress. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:399–406. doi: 10.1007/s00210-013-0955-z. [DOI] [PubMed] [Google Scholar]
- Lockley SW, et al. Effect of reducing interns’ weekly work hours on sleep and attentional failures. N Engl J Med. 2004;351:1829–1837. doi: 10.1056/NEJMoa041404. [DOI] [PubMed] [Google Scholar]
- Lu C, et al. Exploring the Effect of Ginsenoside Rh1 in a Sleep Deprivation-Induced Mouse Memory Impairment Model. Phytother Res. 2017;31:763–770. doi: 10.1002/ptr.5797. [DOI] [PubMed] [Google Scholar]
- Lu Y, et al. Low-level laser therapy for beta amyloid toxicity in rat hippocampus. Neurobiol Aging. 2017;49:165–182. doi: 10.1016/j.neurobiolaging.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi J, et al. A Protocol for Conventional Sleep Deprivation Methods in Rats. J Exp Clin Neurosci. 2017;4:1–4. [Google Scholar]
- Majdi A, et al. Permissive role of cytosolic pH acidification in neurodegeneration: a closer look at its causes and consequences. J Neurosci Res. 2016;94:879–887. doi: 10.1002/jnr.23757. [DOI] [PubMed] [Google Scholar]
- Maquet P. The role of sleep in learning and memory. Science. 2001;294:1048–1052. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- Meerlo P, et al. New neurons in the adult brain: the role of sleep and consequences of sleep loss. Sleep Med Rev. 2009;13:187–194. doi: 10.1016/j.smrv.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mester E, et al. Effect of laser on hair growth of mice. Kiserl Orvostud. 1967;19:628–631. [Google Scholar]
- Michalikova S, et al. Emotional responses and memory performance of middle-aged CD1 mice in a 3D maze: effects of low infrared light. Neurobiol Learn Mem. 2008;89:480–488. doi: 10.1016/j.nlm.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Mochizuki-Oda N, et al. Effects of near-infra-red laser irradiation on adenosine triphosphate and adenosine diphosphate contents of rat brain tissue. Neurosci Lett. 2002;323:207–210. doi: 10.1016/s0304-3940(02)00159-3. [DOI] [PubMed] [Google Scholar]
- Moghadam HS, et al. Beneficial Effects of Transcranial Light Emitting Diode (LED) Therapy on Attentional Performance: An Experimental Design. Iran Red Crescent Med J 2017 [Google Scholar]
- Moreira MS, et al. Effect of phototherapy with low intensity laser on local and systemic immunomodulation following focal brain damage in rat. J Photochem Photobiol B, Biol. 2009;97:145–151. doi: 10.1016/j.jphotobiol.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Moro C, et al. No evidence for toxicity after long-term photobiomodulation in normal non-human primates. Exp Brain Res. 2017;235:3081–3092. doi: 10.1007/s00221-017-5048-7. [DOI] [PubMed] [Google Scholar]
- Oettinghaus B, et al. Synaptic dysfunction, memory deficits and hippocampal atrophy due to ablation of mitochondrial fission in adult forebrain neurons. Cell Death Differ. 2016;23:18. doi: 10.1038/cdd.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palchykova S, et al. Sleep deprivation impairs object recognition in mice. Neurobiol Learn Mem. 2006;85:263–271. doi: 10.1016/j.nlm.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Perkins GA, Ellisman MH. Mitochondrial configurations in peripheral nerve suggest differential ATP production. J Struct Biol. 2011;173:117–127. doi: 10.1016/j.jsb.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourmemar E, et al. Intranasal cerebrolysin attenuates learning and memory impairments in D-galactose-induced senescence in mice. Exp Gerontol. 2017;87:16–22. doi: 10.1016/j.exger.2016.11.011. [DOI] [PubMed] [Google Scholar]
- Sadowski M, et al. Amyloid-β deposition is associated with decreased hippocampal glucose metabolism and spatial memory impairment in APP/PS1 mice. J Neuropathol Exp Neurol. 2004;63:418–428. doi: 10.1093/jnen/63.5.418. [DOI] [PubMed] [Google Scholar]
- Salehpour F, et al. Transcranial low-level laser therapy improves brain mitochondrial function and cognitive impairment in D-galactose-induced aging mice. Neurobiol Aging. 2017 doi: 10.1016/j.neurobiolaging.2017.06.025. [DOI] [PubMed] [Google Scholar]
- Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 2012;48:158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SK, et al. Dose response effects of 810 nm laser light on mouse primary cortical neurons. Lasers Surg Med. 2011;43:851–859. doi: 10.1002/lsm.21100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva R, et al. Role of hippocampal oxidative stress in memory deficits induced by sleep deprivation in mice. Neuropharmacology. 2004;46:895–903. doi: 10.1016/j.neuropharm.2003.11.032. [DOI] [PubMed] [Google Scholar]
- Smith CT, et al. Brief paradoxical sleep deprivation impairs reference, but not working, memory in the radial arm maze task. Neurobiol Learn Mem. 1998;69:211–217. doi: 10.1006/nlme.1997.3809. [DOI] [PubMed] [Google Scholar]
- Smith MA, et al. Oxidative stress in Alzheimer’s disease. Biochim Biophys Acta, Mol Basis Dis. 2000;1502:139–144. doi: 10.1016/s0925-4439(00)00040-5. [DOI] [PubMed] [Google Scholar]
- Song S, et al. cDNA microarray analysis of gene expression profiles in human fibroblast cells irradiated with red light. J Invest Dermatol. 2003;120:849–857. doi: 10.1046/j.1523-1747.2003.12133.x. [DOI] [PubMed] [Google Scholar]
- Sousa MVPd, et al. Inhomogeneity in optical properties of rat brain: a study for LLLT dosimetry. Mechanisms for Low-Light Therapy VIII 2013 [Google Scholar]
- Sripetchwandee J, et al. Combined therapy of iron chelator and antioxidant completely restores brain dysfunction induced by iron toxicity. PLoS One. 2014;9:e85115. doi: 10.1371/journal.pone.0085115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnopolsky MA, Beal MF. Potential for creatine and other therapies targeting cellular energy dysfunction in neurological disorders. Ann Neurol. 2001;49:561–574. [PubMed] [Google Scholar]
- Tian F, et al. Transcranial laser stimulation improves human cerebral oxygenation. Lasers Surg Med. 2016 doi: 10.1002/lsm.22471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uozumi Y, et al. Targeted increase in cerebral blood flow by transcranial near-infrared laser irradiation. Lasers Surg Med. 2010;42:566–576. doi: 10.1002/lsm.20938. [DOI] [PubMed] [Google Scholar]
- Valvassori SS, et al. Lithium ameliorates sleep deprivation-induced mania-like behavior, hypothalamic-pituitary-adrenal (HPA) axis alterations, oxidative stress and elevations of cytokine concentrations in the brain and serum of mice. Bipolar Disord. 2017;19:246–258. doi: 10.1111/bdi.12503. [DOI] [PubMed] [Google Scholar]
- Velardez MO, et al. Nitric oxide decreases the production of inositol phosphates stimulated by angiotensin II and thyrotropin-releasing hormone in anterior pituitary cells. Eur J Endocrinol. 2003;148:89–97. doi: 10.1530/eje.0.1480089. [DOI] [PubMed] [Google Scholar]
- Villafuerte G, et al. Sleep deprivation and oxidative stress in animal models: a systematic review. Oxid Med Cell Longev. 2015 doi: 10.1155/2015/234952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, et al. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim Biophys Acta, Mol Basis Dis. 2014;1842:1240–1247. doi: 10.1016/j.bbadis.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, et al. Paradoxical sleep deprivation modulates depressive-like behaviors by regulating the MAOA levels in the amygdala and hippocampus. Brain Res. 2017;1664:17–24. doi: 10.1016/j.brainres.2017.03.022. [DOI] [PubMed] [Google Scholar]
- Wu A, et al. Vitamin E protects against oxidative damage and learning disability after mild traumatic brain injury in rats. Neurorehabil Neural Repair. 2010;24:290–298. doi: 10.1177/1545968309348318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie M, et al. Short-term sleep deprivation disrupts the molecular composition of ionotropic glutamate receptors in entorhinal cortex and impairs the rat spatial reference memory. Behav Brain Res. 2016;300:70–76. doi: 10.1016/j.bbr.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Xuan W, et al. Transcranial low-level laser therapy enhances learning, memory, and neuroprogenitor cells after traumatic brain injury in mice. J Biomed Opt. 2014;19:108003–108003. doi: 10.1117/1.JBO.19.10.108003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, et al. Near infrared radiation protects against oxygen-glucose deprivation-induced neurotoxicity by down-regulating neuronal nitric oxide synthase (nNOS) activity in vitro. Metab Brain Dis. 2015;30:829–837. doi: 10.1007/s11011-015-9663-3. [DOI] [PubMed] [Google Scholar]
- Zhang L, et al. Melatonin ameliorates cognitive impairment induced by sleep deprivation in rats: role of oxidative stress, BDNF and CaMKII. Behav Brain Res. 2013;256:72–81. doi: 10.1016/j.bbr.2013.07.051. [DOI] [PubMed] [Google Scholar]
- Zhang Q, et al. Low-level laser therapy effectively prevents secondary brain injury induced by immediate early responsive gene X-1 deficiency. J Cereb Blood Flow Metab. 2014;34:1391–1401. doi: 10.1038/jcbfm.2014.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, et al. Reactive oxygen species mediate tumor necrosis factor alpha-converting, enzyme-dependent ectodomain shedding induced by phorbol myristate acetate. FASEB J. 2001;15:303–305. doi: 10.1096/fj.00-0371fje. [DOI] [PubMed] [Google Scholar]