Abstract
Mental imitation, perhaps a precursor to motor imitation, involves visual perspective-taking and motor imagery. Research on mental imitation in autism has been rather limited compared to that on motor imitation. The main objective of this fMRI study is to determine the differences in brain responses underlying mirroring and mentalizing networks during mental imitation in children and adolescents with ASD. Thirteen high-functioning children and adolescents with ASD and 15 age-and- IQ-matched typically developing (TD) control participants took part in this fMRI study. In the MRI scanner, participants were shown cartoon pictures of people performing everyday actions (Transitive actions: e.g., ironing clothes but with the hand missing; and Intransitive actions: e.g., clapping hands with the palms missing) and were asked to identify which hand or palm orientation would best fit the gap. The main findings are: 1) both groups performed equally while processing transitive and intransitive actions; 2) both tasks yielded activation in the bilateral inferior frontal gyrus (IFG) and inferior parietal lobule (IPL) in ASD and TD groups; 3) Increased activation was seen in ASD children, relative to TD, in left ventral premotor and right middle temporal gyrus during intransitive actions; and 4) autism symptom severity positively correlated with activation in left parietal, right middle temporal, and right premotor regions across all subjects. Overall, our findings suggest that regions mediating mirroring may be recruiting more brain resources in ASD and may have implications for understanding social movement through modeling.
Keywords: imitation, mental imitation, autism, mirror neuron system, functional MRI
1. INTRODUCTION
Imitation plays a crucial role in development, and has important implications for social development through modeling and the understanding of social movement (Pfeifer, Iacoboni, Mazziotta, & Dapretto, 2008). Imitation is a necessary precursor to symbolic functioning (Piaget, 1962) and provides a child with information about the actions and intentions of the social world, and a foundation for social development. People with autism spectrum disorders (ASD) have been found to struggle in imitating actions, gestures, and action sequences. Behaviorally, it has been shown that not all forms of imitation are equally impaired in ASD, but specific subsets of individuals may be more affected as opposed to the entire spectrum (Edwards, 2014). Rogers, Bennetto, McEvoy, and Pennington (1996) found improved performance in meaningful imitation compared to meaningless imitation in adolescents with autism. Similarly, Hamilton, Brindley, and Frith (2007) found intact goal-state imitation and motor planning in children with autism. Lower rates of spontaneous imitative behavior of actions on objects and gestures in children with ASD have been reported widely (Colombi et al., 2009; Ingersoll, 2008; Knott, Lewis, & Williams, 2007). Neuroimaging studies have also provided evidence of altered recruitment of regions underlying imitation in children and adults with ASD (Williams, 2008). For example, in a meta-analysis of 13 neuroimaging studies of action observation and action imitation in individuals with ASD, Yang and Hofmann (2016) altered recruitment of several regions associated with imitation, such as the dorsolateral prefrontal cortex, anterior cingulate, and insula in ASD participants. However, it should be noted that several other studies have also provided contrary evidence as to intact brain response and imitation skills in individuals with ASD (Dinstein et al., 2010; Marsh & Hamilton, 2011; Pokorny et al., 2015). The inconsistency in the nature of imitation investigated and the differences in findings across studies underscore the need for further investigating imitation at behavioral and at neural levels in ASD.
The Mirror Neuron System (MNS) has been suggested to play an instrumental role in action simulation and action execution (Gallese, 2009). Core regions of the MNS include the inferior frontal gyrus (IFG)/ventral pre-motor cortex (PMv) and the inferior parietal lobe (IPL). These regions communicate closely with the superior temporal sulcus (STS) to produce action understanding and action simulation (Van Overwalle & Baetens, 2009). Successful imitation likely relies not only on these MNS regions but also on their communication with other neural networks (Kana, Wadsworth, & Travers, 2011) including interactions with limbic regions (Carr, Iacoboni, Dubeau, Mazziotta, & Lenzi, 2003; Wicker et al., 2003) and with regions associated with processing theory-of-mind (ToM) (Van Overwalle & Baetens, 2009). It has been previously suggested that individuals with ASD who have deficits in imitation may also have an unusual MNS response (Dapretto et al., 2006; Williams, 2008). However, other studies have revealed intact activation and even increased activation in ASD participants in the MNS during tasks of imitation compared to typically developing (TD) children (Dinstein et al., 2010; Marsh & Hamilton, 2011; Martineau, Andersson, Barthelemy, Cottier, & Destrieux, 2010).
Most studies in ASD have examined imitation from a motor perspective (Dinstein et al., 2010) or a combination of motor and something else such as goal-directed actions (Marsh & Hamilton, 2011; Martineau et al., 2010). This could represent a problem because imitation difficulties may arise from problems related to motor planning or execution. In a study that examined different component processes of imitation in autism (Bennetto, 1999 unpublished dissertation), participants with autism performed poorly in the motor functioning and action planning aspects of imitation, but not on the spatiotemporal representation, body schema, and memory compared to TD individuals. Altered brain activity and connectivity that may lead to impaired imitation abilities in ASD may arise from aberrant action planning through motor simulation but not actual imitation deficits (Nebel, Eloyan, Barber, & Mostofsky, 2014; Nebel, Joel, et al., 2014).
The current functional MRI study examines the role of action simulation, and the neurobiological mechanisms underlying action simulation, in imitation independent of actual motor production in children with ASD. This action simulation or “mental imitation’ paradigm has been defined as visual perceptive-taking and motor imagery (Goldman, 2005; Jeannerod, 1994). The importance of examining mental imitation is emphasized by the simulation theory, which proposes that we gain insight into the mental workings of others by covertly or mentally simulating the actions ourselves without actually performing them (Umilta et al., 2001). Another important aspect of examining mental imitation is embodied cognition, which helps explain whether conceptual features that are engaged during fMRI imitation studies may actually apply to the real world when these same features are directly experienced (Mahon & Caramazza, 2008). In addition, two recent studies of embodied cognition in autism examined mental simulation. In Conson et al. (2015), mental simulation of one’s own body motion was examined in order to take another person’s perspective. They found that individuals with ASD solved the tasks of simulation by relying on a non-embodied strategy compared to TD controls who adopted an embodied strategy. In Conson et al. (2016), the impact of bodily information on simulation skills of adolescents with ASD was tested. They found that while both ASD and TD groups were successful in mentally simulating actions, that ability was constrained by body posture more in ASD than in TD participants. These findings are of particular interest in the context of the embodied cognition framework which connects cognition with the world via sensory and motor processes (Rugg & Thompson-Schill, 2013), which shows impairment in individuals with ASD (Thye, Bednarz, Herringshaw, Sartin, & Kana, 2017). Thus, action simulation may play an important role in better understanding imitation and in assessing social functioning and embodied cognition in ASD. More specifically, the current study plans to isolate imitation independent of actual motor production to better assess behavioral and neural correlates of simulation.
Thus, the aim of this study is to determine the activation patterns observed in children with ASD when presented with a mental imitation task involving transitive actions (actions involving an object) and intransitive actions (actions not involving an object and are more communicative). Studying transitive and intransitive actions allows for the investigation of the communicative (intransitive) and non-communicative (transitive) aspects of imitation, which serves a specific purpose when studying individuals with ASD given their socio-communicative deficits. Given that healthy individuals perform better in intransitive than transitive actions (Carmo & Rumiati, 2009), we predicted the same outcome in the TD and ASD groups; however, the ASD group will have worse performance than the TD group in both actions. This is based on previous findings of impaired action planning, a skill mediated primarily by the frontal component of the MNS–the PMv, in children with ASD. We also predicted that participants with ASD would show decreased levels of activation in MNS areas and frontal areas, but similar levels of activation in the parietal regions (e.g., IPL) during intransitive actions, but not during transitive actions. This hypothesis is based on previous results from a meta-analysis of action observation and imitation in ASD, where frontal areas showed decreased activation in the ASD group compared to TD, but no differences were found in parietal areas (Yang & Hofmann, 2016). Symptom severity and social communication would be a predictor of brain activation for both actions. The findings of this study will provide important insights into resolving imitation-derived activation and the difference in activation in children and adolescents with ASD during non-motor mental imitation. The findings will also add to the non-canonical MNS studies in ASD as the paradigm we used examines a combination of both imitation and communicative aspects of action understanding.
2. MATERIAL & METHODS
2.1 Participants
Thirteen high-functioning children and adolescents with ASD and 15 age-and-IQ-matched TD control participants took part in this fMRI study (age range: 8 to 17 years; minimum Full Scale and Non-Verbal IQ: 75, measured using the Wechsler Abbreviated Scales of Intelligence [WASI]; See Table 1). All participants with ASD were diagnosed using the autism diagnostic observation schedule (ADOS) and autism diagnostic interview (ADI), and were recruited from the autism center at our university and from local clinics and special schools. Current and past ASD symptoms were also assessed using the Autism-Spectrum Quotient (AQ) (Baron-Cohen, Wheelwright, Skinner, Martin, & Clubley, 2001) and the Social Communication Scale (SCQ) (Rutter, Bailey, & Lord, 2003). The parents/guardians completed all questionnaires. TD participants were recruited using flyers and advertisements posted at our university campus, and in local community centers (e.g., libraries, YMCAs). Participants were not included in the study if they indicated having worked with metal or having metal implanted in their bodies (either surgically or accidentally) or if they had a history of psychiatric disorders. No participants indicated having a cognitive disorder, anxiety disorder, schizophrenia, or obsessive compulsive disorder. Before participating in the study, study procedures were explained to all participants and informed consent was obtained. The study protocol and consent form were approved by the ethics committee of the UAB Institutional Review Board for human subjects research.
Table 1.
Demographic information
| TD (n = 15; 4F) | ASD (n = 13; 2F) | p-value | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Mean | S.D. | Range | Mean | S.D. | Range | ||
|
| |||||||
| Age | 12 | 1.63 | 9–15 | 12 | 2.99 | 8–17 | 0.69 |
| FSIQ | 102 | 16.19 | 83–130 | 109 | 17.27 | 80–126 | 0.32 |
| VIQ | 98 | 17.6 | 84–134 | 109 | 18.2 | 74–128 | 0.22 |
| PIQ | 99 | 9.57 | 84–119 | 108 | 15.82 | 74–124 | 0.15 |
| AQ | 35 | 27.29 | 5–80 | 72 | 34.36 | 32–135 | 0.01 |
| SCQ | 4 | 3.73 | 0–10 | 18 | 8.32 | 1–31 | <0.001 |
| RMSD | 0.26 | 0.11 | 0.11–0.45 | 0.26 | 0.15 | 0.05–0.50 | 0.98 |
Values are presented as mean, standard deviation and range. The p value is independent-t tests for differences between groups. TD, typically developing; ASD, autism spectrum disorder, F female, FSIQ, Full-scale IQ; VIQ, Verbal IQ; PIQ, performance IQ; AQ, autism quotient; SCQ, social communication questionnaire; and RMSD, root mean square deviation.
2.2 Stimuli and Experimental Paradigm
The fMRI experiment consisted of an action simulation task designed in an event-related format. This experiment was aimed at measuring mental imitation ability, requiring subjects to perform all the necessary components of imitation except for the motor execution aspect. In other words, this task involved imagining the imitative act, which is usually a precursor to the motor action. This part also comprised planning the imitative act, a step which may prove critical in determining the ultimate outcome. The stimuli for this experiment were based on a paradigm developed by Mozaz and colleagues (Mozaz, Rothi, Anderson, Crucian, & Heilman, 2002). During this experiment, participants were shown cartoon pictures of people performing everyday actions (e.g., ironing clothes) but with the hand missing. Ten of these stimuli showed Transitive acts (which require an object) and ten stimuli showed Intransitive acts (which do not require an object and are generally communicative in nature). For each item, there were 3 options of hand grasps presented as high-quality images beneath the cartoon picture. The participants were asked to identify which hand (via button press) would best fill in the gap for a series of picture stimuli (see Figure 1). In order to control for potential practice effects, the order of presentation of stimuli within the experiment was randomized across participants. Participants were also presented with a fixation (baseline) condition for a total of 5 baseline periods, each lasting 24 seconds. During baseline, participants were shown a white cross centered on a black background and instructed to relax and wait for the next image to appear (Figure 1). This experiment not only targeted visuospatial ability (by requiring an individual to mentally rotate hands to fill in the gap correctly), but also action planning (by requiring the individual to plan and simulate the action in their mind).
Figure 1.
Examples of experimental stimuli depicting examples of transitive and instructive actions.
2.3 Image Acquisition
All fMRI scans were acquired using the Siemens 3.0 Tesla Allegra head-only scanner (Siemens Medical Inc., Erlangen, Germany) located at the UAB Civitan International Research Center (CIRC). For structural imaging, initial high resolution T1-weighted scans were acquired using a 160-slice 3D MPRAGE (Magnetization Prepared Rapid Gradient Echo) volume scan with TR = 200 ms, TE = 3.34 ms, flip angle = 12°, FOV = 25.6 cm, 256 × 256 matrix size, and 1 mm slice thickness. A single-shot gradient-recalled echo-planar pulse sequence was used to acquire functional images (TR= 1000 ms, TE = 30ms, flip angle = 60 degrees, FOV = 24 cm, matrix =64 × 64). Seventeen adjacent oblique axial slices were acquired in an interleaved sequence with 5 mm slice thickness, 1 mm slice gap, a 24 × 24 cm field of view (FOV), and a 64 × 64 matrix, resulting in an in-plane resolution of 3.75 × 3.75 × 5 mm. The stimuli were rear-projected onto a translucent plastic screen and participants viewed the screen through a mirror attached to the head coil. Quality control checks were applied to the acquired data to examine the signal to noise ratio, temporal signal to noise ratio, ghosting, and motion artifacts. Data that did not meet quality standards were not included in further analyses.
2.4 fMRI Data Analyses
Functional images were processed using the Statistical Parametric Mapping (SPM12) software (Wellcome Department of Cognitive Neurology, London, UK) and Analysis of Functional NeuroImages AFNI software (Cox, 1996). Functional images were motion-corrected by registering each functional volume to the first time point of the scan, normalized to MNI space, resampled to 3mm isotropic, and a Gaussian spatial smoothing filter with a global full-width-at-half-maximum (FWHM) of 8mm was applied. Functional images were individually scaled to a mean of 100, and whole-brain statistical analyses were performed on an individual basis using a general linear model (GLM) approach using AFNI’s 3dDeconvolve with Imitation (transitive and intransitive) and baseline trials as regressors of interest. The orthogonal contrasts Transitive vs. Baseline and Intransitive vs. Baseline were computed to assess average differences in brain response. Areas of statistically significant activation differences were determined using one- and two-sample t-tests using a random-effects model via AFNI’s 3dttest++. To correct for multiple comparisons, 10,000 Monte Carlo simulations were computed to obtain a cluster-size-corrected FWE threshold of p < 0.05 for between-group effects (uncorrected voxelwise threshold of p < 0.01; minimum cluster size of 45 voxels). We ran an additional analysis using a functional mask of activation patterns derived from a large number of studies of action understanding from Neurosynthusing the term “action observation” (See Supplementary Figure 1).
2.5 Accounting for Head Motion
Because head motion can impact fMRI analysis (Satterthwaite et al., 2013; Van Dijk, Sabuncu, & Buckner, 2012), the following precautions were taken. Head motion was quantified as the Euclidean distance calculated from six rigid-body motion parameters (translation in x, y, z directions, and rotation in pitch, roll, yaw angles) for two consecutive time points. For any time point where this measure was > 1.5mm, which was considered excessive motion, that time point as well as the immediately preceding and subsequent time points were modeled out (Power, Barnes, Snyder, Schlaggar, & Petersen, 2012). Participants who retained more than 80% of their time points after calculating motion outliers were used. Average head motion over each participant’s session was defined as the root mean square of displacement (RMSD) and did not significantly differ between groups.
2.6 Brain-behavior relationships
We further examined the relationship between task-related fMRI BOLD activation and the behavioral data by conducting whole-brain correlational analyses. Statistical parametric maps from the contrasts Transitive vs. Baseline and Intransitive vs. Baseline along with measures of Autism Quotient (AQ) and Social Communication Questionnaire (SCQ) were correlated at the whole brain level using AFNI’s 3dTCorr, and cluster correction was applied as described above.
3 RESULTS
3.1 Overview
This study examined the role of mirroring mechanism in mediating mental simulations of actions without actual motor movement. The main results are: 1) There were no statistically significant group differences in performance accuracy or in response time in both conditions; 2) Processing both transitive and intransitive actions when contrasted with fixation baseline yielded significant activity in IFG in both groups group; 3) Neither group demonstrated greater level of activation for transitive actions. However, the ASD group showed several areas of increased activation while processing intransitive actions;and 4) autism symptomatology significantly predicted activation during intransitive actions in left superior parietal, right middle temporal and right ventral premotor cortices.
3.2 Behavioral Results
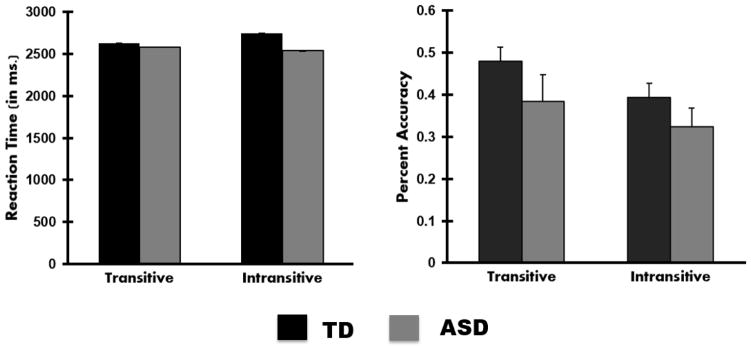
Two separate mixed-model analysis of variance (ANOVA) were conducted to explore the effect of group (ASD or TD) and task (transitive or intransitive) on reaction time and accuracy (Figure 2). The first analysis looking at reaction time revealed no main effects of group or task, and nor was there an interaction. The second analysis revealed a main effect of task F(1, 26) = 4.8, p < .05, where all participants had greater accuracy in the transitive condition (M= 43%, SD = 7%) than in the intransitive condition (M= 36%, SD = 5%), but no main effect of group or a significant interaction was found.
Figure 2.
Bar graphs depicting reaction time in ms. and accuracy. Error bars represent standard error of the mean.
3.3 Brain Activation Results
3.3.1 Within- and Between-Group Activation
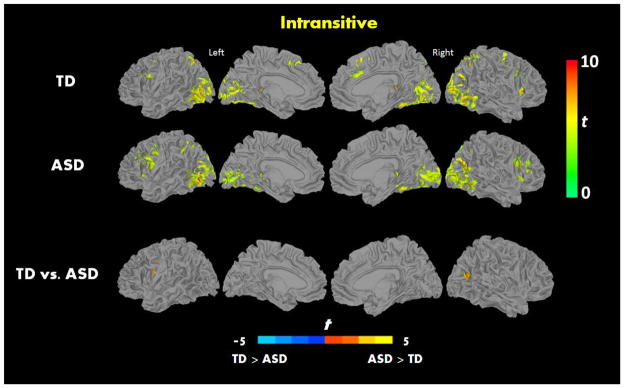
When processing transitive actions, relative to fixation baseline, both groups showed strong activation in inferior frontal, superior parietal, lateral occipital, and insular regions, and no significant differences were found between the groups (See Figure 3 and Table 2). When processing intransitive actions, relative to fixation baseline, both groups showed strong activation in IFG and insula. When comparing the two groups directly, the ASD group showed increased activation relative to the TD in left PMv and right middle temporal gyrus (See Figure 4 and Table 2). Similar patterns of activation were also seen in an ROI analysis that used a mask of regions involved in action understanding created using the Neurosynth (See Supplementary Figure 1).
Figure 3.
Within-group brain activation results for: A) typically developing and B) ASD groups for the contrast Transitive vs. Baseline (p < 0.05, FWE corrected).
Table 2.
Results for the contrasts Transitive vs. Baseline and Intransitive vs. Baseline
| Region | Hemi. | Cluster Vol. (in μl) | Peak coordinates (MNI) | Peak t | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| x | y | z | |||||
| Transitive vs. Baseline | |||||||
| TD | Inferior Occipital Gyrus | R | 164079 | 51 | −51 | −3 | 8.8 |
| Insula | R | 13230 | 33 | 24 | 3 | 8.1 | |
| Inferior frontal gyrus | L | 12177 | −48 | 9 | 12 | 6.4 | |
| SMA | L | 4428 | 0 | 24 | 53 | 5.7 | |
| ASD | Inferior Temporal Gyrus | R | 100818 | 50 | −48 | −9 | 9.4 |
| Precentral Gyrus | L | 19548 | −48 | 3 | 27 | 6.2 | |
| Precentral Gyrus | R | 13446 | 45 | 0 | 30 | 6.5 | |
| Cerebellum | L | 2295 | −24 | −84 | −21 | 3.7 | |
| Intransitive vs. Baseline | |||||||
| TD | Middle occipital gyrus | R | 152874 | 33 | −78 | 24 | 8.3 |
| Superior frontal gyrus | R | 7857 | 33 | −6 | 63 | 4.9 | |
| Inferior frontal gyrus | L | 7560 | −51 | 21 | 24 | 4.7 | |
| SMA | L | 5346 | 3 | 21 | 48 | 5.5 | |
| Insula | R | 2025 | 33 | 24 | 3 | 7.2 | |
| ASD | Inferior occipital | L | 149391 | −48 | −69 | −3 | 9.3 |
| Inferior frontal gyrus | L | 20358 | −48 | 9 | 15 | 7.6 | |
| Inferior frontal gyrus | R | 12960 | 54 | 15 | 18 | 5.8 | |
| Insula | R | 2106 | 36 | 24 | 0 | 5.7 | |
| ASD > TD | Precentral | L | 1755 | −48 | 3 | 42 | 4.3 |
| Middle temporal gyrus | R | 1269 | 45 | −63 | 15 | 4.7 | |
L, left; R, right; Hemi., hemisphere; TD, typically developing; ASD, autism spectrum disorder; vol. volume; MNI, Montreal Neurological Institute
Figure 4.
Within-group brain activation results for A) typically developing, B) ASD, and C) significant between-group differences for the contrast Intransitive vs. Baseline (p < 0.05, FWE corrected).
3.3.2 Brain-Behavior relationships
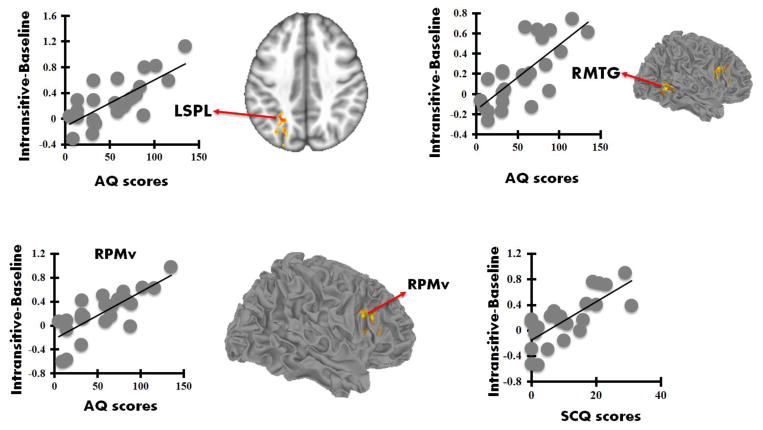
After pooling all the subjects into one single group, AQ scores for all participants were found to be significantly positively correlated with parameter estimates from RPMv (r = 0.69), RMTG (r = 0.67), and LSPL (r = 0.65) during simulation of intransitive actions, but not during simulation of transitive actions (p < 0.05 FWE corrected; Figure 5). In addition, SCQ scores for all participants were found to be significantly positively correlated with parameter estimates from RPMv (r = 0.73) during simulation of intransitive actions, but not during simulation of transitive actions (p < 0.05 FWE corrected; Figure 5).
Figure 5.
Scatterplots showing significant positive correlations between parameter estimates (Intransitive actions) with AQ and SCQ scores (p < 0.05, FWE corrected).
4. DISCUSSION
This fMRI study examined the neural bases of action simulation in ASD and TD children. Behavioral results indicated similar performance across participant groups in simulating both transitive and intransitive actions. Although the ASD group had reduced accuracy in both tasks, this was not significantly different compared to the TD group as we originally had hypothesized. Surprisingly, our findings in the TD group did not replicate previous findings involving TD individuals (Carmo & Rumiati, 2009). There could be some factors attributing to this discrepancy such as differences in age; our study recruited a younger sample of participants (average age of 12 years), whereas Carmo and colleagues average age was 27 years. Another factor is that our task was performed inside an MRI scanner with the constraints of noise, time, etc., unlike the Carmo et al study, which is a behavioral study. Yet another factor is the difference in the stimuli used between these two studies. While the Carmo et al study utilized pantomimed action scenarios in a movie format, the actions used in our study were static cartoon representations of actions.
The behavioral results of our study were accompanied by both groups showing increased activation in core MNS regions (IFG and IPL) while simulating transitive and intransitive actions. Given that our task involved action observation rather than motor performance, these findings are consistent with a meta-analysis of action observation in neurotypical individuals (Caspers, Zilles, Laird, & Eickhoff, 2010), where activation in PMv, SMA, superior parietal, and middle occipital areas overlapped with the peaks of activation in our study. However, analysis of group differences revealed significantly increased activation in ASD participants, relative to TD, in processing intransitive actions, but not during transitive actions. Intransitive actions are more communicative in nature compared to transitive actions which are more object- and goal-related (Bonivento, Rothstein, Humphreys, & Chechlacz, 2014; Carmo & Rumiati, 2009). Therefore, it is possible that participants with ASD needed more neural resources to simulate such actions and find the appropriate solution to those questions. Transitive actions depicted by the cartoons, on the other hand, represented common and familiar activities that the participants may already know from previous experience (e.g., ironing a shirt) and are more frequently used than intransitive actions (Mozaz et al., 2002). This absence of group differences during transitive actions may be related to the familiarity of the actions used since it has been previously shown that novel actions that have not been learned compared to typical actions tend to produce more activation in MNS regions (Vogt et al., 2007); therefore, our stimuli may have depicted actions that were not novel enough to both groups as there were no differences in activation. Additionally, this absence of significant differences in the transitive condition is in line with one of our previous studies where we examined the means (actions involving an object in the context of this paper) of actions and no significant group differences between TD and ASD for the means condition were found (Libero et al., 2014).
It should be noted that we did not find hypoactivation in MNS regions in our participants with ASD. While this may be inconsistent with some studies of imitation in autism (Dapretto et al., 2006; Williams, 2008), it is in line with other studies failing to find reduced activation within the MNS in ASD (Dapretto et al., 2006; Fan, Decety, Yang, Liu, & Cheng, 2010; Press, Richardson, & Bird, 2010). One of the factors that might explain some of these inconsistencies in those previous studies is age range, since some studies have examined children and adolescents, and others have looked exclusively at young adults. Future studies should either covary for age or specifically look at only children, adolescents, or adults.
Despite both groups activating core regions of the MNS, one of the specific regions of differential activation was the left PMv, with increased activation in ASD participants during intransitive actions, which required individuals to imagine in a more “communicative” context. Increased left PMv activation in ASD is consistent with recent findings of increased activation in imitation tasks in adolescents with ASD in the MNS (Perkins, Bittar, McGillivray, Cox, & Stokes, 2015). Difficulty in intransitive actions in ASD may elicit more brain resources in the MNS. Another node of increased activation, the RMTG in ASD, may reflect problems assessing the significance of socio-communicate events (Grezes, Wicker, Berthoz, & de Gelder, 2009). This pattern of activation difference may have implications for understanding communicative intent and social development through modeling in ASD. Differences in activation in these regions may provide additional evidence that the ASD group’s recruitment of additional resources to perform the task equally with the TD group.
Brain-behavior relationship analyses revealed that children with higher AQ scores and higher SCQ scores tend to show more activation during simulation of intransitive actions. While AQ was positively correlated with activation in LSPL, RMTG, and PMv, the SCQ scores were correlated with PMv activation in ASD participants. This finding is noteworthy as this parallels our activation findings where ASD children showed hyperactivation in PMv and MTG. Similar relationships between autism symptomatology and brain functioning have been reported previously. For example, using EEG, Fan et al. (2010) found a positive correlation between ADI-R communication scores and Mu suppression in MNS areas. In TD, Mu suppression in MNS areas is indicative of sensorimotor resonance, and in ASD, Mu suppression is believed to be impaired (Oberman, Ramachandran, & Pineda, 2008). Overall, these findings provide evidence for a relationship between autism symptom severity and how individuals with autism may allocate neural resources in cognitively and socially demanding tasks.
In general, children with ASD in our study showed intact ability in mental simulation of transitive and intransitive actions, along with increased activation in frontal, but equal activation in posterior areas of the MNS relative to the TD group. However, there are a few limitations of the current study that should be taken into consideration while interpreting the findings. First, the sample size, although on par with several fMRI studies in the field, may not have enough statistical power to detect significant effect. Future studies should include more participants. Second, the structured environment of the fMRI procedure and the task may mask difficulties associated with imitation in real-world social settings including lack of attention and the presence of more motivating stimuli (Trevarthen & Aitken, 2001). The results of the current study also suggest that research should examine both integrated functioning within the MNS and also between the MNS and other related neural regions.
5. CONCLUSIONS
Overall, the findings of this study reveal the role of mirroring and perspective taking mechanisms at behavioral and neural levels in tasks of action simulation. In addition, actions that have communicative meanings may elicit more neural resources in children with autism. This increased use of resources was also found to be related to autism symptoms positively, further suggesting a relationship between symptom level and neural activity. Although more research is needed, our findings may suggest deficits in simulation (mirroring mechanism) and the embodied cognition framework (bridge between cognition and the real world via sensory processes) in participants with autism that affects their simulation and social functions. Our previous findings of altered activation patterns during motor execution in ASD (Wadsworth et al., 2017) suggested deficiencies in motor execution. Conversely, the findings of the current study may also suggest differences in activation present at the motor imagination and planning stages, which are necessary for initial motor execution. Longitudinal studies in future will be particularly important given recent findings of critical developmental shift occurring around puberty and its impact on the findings in ASD, which may explain some of the inconsistent findings in the field (Peper, van den Heuvel, Mandl, Hulshoff Pol, & van Honk, 2011; Uddin, Supekar, & Menon, 2013).
Supplementary Material
HIGHLIGHTS.
Poor imitation skills have been a characteristic feature of children with autism
Findings of fMRI studies of imitation in ASD are less consistent.
This fMRI study examines action simulation and the neural circuitry underlying it
Our findings show increased brain activation in ASD compared to control children.
Autism symptoms were positively correlated with brain activation social actions.
Acknowledgments
This research was supported by the NIH T-32 Training grant (T32 NS061788-01), the Eunice Kennedy Shriver Pilot Study Award (5P30HD038985), and the UAB Department of Psychology faculty start-up funds. The authors would like to thank Lauren Libero and Thomas DeRamus for their help with different aspects of this study. Finally, we would like to extend our sincerest appreciation to the participants and families who generously gave their time and courage to participate in this neuroimaging study.
Footnotes
Conflict of Interest: No conflicts declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord. 2001;31(1):5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Bonivento C, Rothstein P, Humphreys G, Chechlacz M. Neural correlates of transitive and intransitive action imitation: an investigation using voxel-based morphometry. Neuroimage Clin. 2014;6:488–497. doi: 10.1016/j.nicl.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo JC, Rumiati RI. Imitation of transitive and intransitive actions in healthy individuals. Brain Cogn. 2009;69(3):460–464. doi: 10.1016/j.bandc.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci U S A. 2003;100(9):5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers S, Zilles K, Laird AR, Eickhoff SB. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage. 2010;50(3):1148–1167. doi: 10.1016/j.neuroimage.2009.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombi C, Liebal K, Tomasello M, Young G, Warneken F, Rogers SJ. Examining correlates of cooperation in autism: Imitation, joint attention, and understanding intentions. Autism. 2009;13(2):143–163. doi: 10.1177/1362361308098514. [DOI] [PubMed] [Google Scholar]
- Conson M, Hamilton A, De Bellis F, Errico D, Improta I, Mazzarella E, … Frolli A. Body Constraints on Motor Simulation in Autism Spectrum Disorders. J Autism Dev Disord. 2016;46(3):1051–1060. doi: 10.1007/s10803-015-2652-x. [DOI] [PubMed] [Google Scholar]
- Conson M, Mazzarella E, Esposito D, Grossi D, Marino N, Massagli A, Frolli A. “Put Myself Into Your Place”: Embodied Simulation and Perspective Taking in Autism Spectrum Disorders. Autism Res. 2015;8(4):454–466. doi: 10.1002/aur.1460. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, Iacoboni M. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat Neurosci. 2006;9(1):28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I, Thomas C, Humphreys K, Minshew N, Behrmann M, Heeger DJ. Normal movement selectivity in autism. Neuron. 2010;66(3):461–469. doi: 10.1016/j.neuron.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards LA. A meta-analysis of imitation abilities in individuals with autism spectrum disorders. Autism Res. 2014;7(3):363–380. doi: 10.1002/aur.1379. [DOI] [PubMed] [Google Scholar]
- Fan YT, Decety J, Yang CY, Liu JL, Cheng Y. Unbroken mirror neurons in autism spectrum disorders. J Child Psychol Psychiatry. 2010;51(9):981–988. doi: 10.1111/j.1469-7610.2010.02269.x. [DOI] [PubMed] [Google Scholar]
- Gallese V. Mirror Neurons, Embodied Simulation, and the Neural Basis of Social Identification. Psychoanalytic Dialogues. 2009;19:519–536. [Google Scholar]
- Goldman AI. Perspective on Imitation, from Neuroscience to Social Science. Vol. 2. Cambridge: MIT Press; 2005. Imitation, mind reading, and simulation; pp. 79–93. [Google Scholar]
- Grezes J, Wicker B, Berthoz S, de Gelder B. A failure to grasp the affective meaning of actions in autism spectrum disorder subjects. Neuropsychologia. 2009;47(8–9):1816–1825. doi: 10.1016/j.neuropsychologia.2009.02.021. [DOI] [PubMed] [Google Scholar]
- Hamilton AF, Brindley RM, Frith U. Imitation and action understanding in autistic spectrum disorders: how valid is the hypothesis of a deficit in the mirror neuron system? Neuropsychologia. 2007;45(8):1859–1868. doi: 10.1016/j.neuropsychologia.2006.11.022. [DOI] [PubMed] [Google Scholar]
- Ingersoll B. The Social Role of Imitation in Autism. Implications for the Treatment of Imitation Deficits. Infants & Young Children. 2008;21(2):107–119. [Google Scholar]
- Jeannerod M. The representing brain: Neural correlates of motor intention and imagery. Behavioral and Brain Sciences. 1994;17(2):187. [Google Scholar]
- Kana RK, Wadsworth HM, Travers BG. A systems level analysis of the mirror neuron hypothesis and imitation impairments in autism spectrum disorders. Neurosci Biobehav Rev. 2011;35(3):894–902. doi: 10.1016/j.neubiorev.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Knott F, Lewis C, Williams T. Sibling interaction of children with autism: development over 12 months. J Autism Dev Disord. 2007;37(10):1987–1995. doi: 10.1007/s10803-006-0347-z. [DOI] [PubMed] [Google Scholar]
- Libero LE, Maximo JO, Deshpande HD, Klinger LG, Klinger MR, Kana RK. The role of mirroring and mentalizing networks in mediating action intentions in autism. Mol Autism. 2014;5(1):50. doi: 10.1186/2040-2392-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon BZ, Caramazza A. A critical look at the embodied cognition hypothesis and a new proposal for grounding conceptual content. J Physiol Paris. 2008;102(1–3):59–70. doi: 10.1016/j.jphysparis.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Marsh LE, Hamilton AF. Dissociation of mirroring and mentalising systems in autism. Neuroimage. 2011;56(3):1511–1519. doi: 10.1016/j.neuroimage.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Martineau J, Andersson F, Barthelemy C, Cottier JP, Destrieux C. Atypical activation of the mirror neuron system during perception of hand motion in autism. Brain Res. 2010;1320:168–175. doi: 10.1016/j.brainres.2010.01.035. [DOI] [PubMed] [Google Scholar]
- Mozaz M, Rothi LJ, Anderson JM, Crucian GP, Heilman KM. Postural knowledge of transitive pantomimes and intransitive gestures. J Int Neuropsychol Soc. 2002;8(7):958–962. doi: 10.1017/s1355617702870114. [DOI] [PubMed] [Google Scholar]
- Nebel MB, Eloyan A, Barber AD, Mostofsky SH. Precentral gyrus functional connectivity signatures of autism. Front Syst Neurosci. 2014;8:80. doi: 10.3389/fnsys.2014.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebel MB, Joel SE, Muschelli J, Barber AD, Caffo BS, Pekar JJ, Mostofsky SH. Disruption of functional organization within the primary motor cortex in children with autism. Hum Brain Mapp. 2014;35(2):567–580. doi: 10.1002/hbm.22188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberman LM, Ramachandran VS, Pineda JA. Modulation of mu suppression in children with autism spectrum disorders in response to familiar or unfamiliar stimuli: the mirror neuron hypothesis. Neuropsychologia. 2008;46(5):1558–1565. doi: 10.1016/j.neuropsychologia.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Peper JS, van den Heuvel MP, Mandl RC, Hulshoff Pol HE, van Honk J. Sex steroids and connectivity in the human brain: a review of neuroimaging studies. Psychoneuroendocrinology. 2011;36(8):1101–1113. doi: 10.1016/j.psyneuen.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Perkins TJ, Bittar RG, McGillivray JA, Cox II, Stokes MA. Increased premotor cortex activation in high functioning autism during action observation. J Clin Neurosci. 2015;22(4):664–669. doi: 10.1016/j.jocn.2014.10.007. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Iacoboni M, Mazziotta JC, Dapretto M. Mirroring others’ emotions relates to empathy and interpersonal competence in children. Neuroimage. 2008;39(4):2076–2085. doi: 10.1016/j.neuroimage.2007.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piaget J. Play, dreams, and imitation in childhood. New York: W.W. Norton & Co; 1962. [Google Scholar]
- Pokorny JJ, Hatt NV, Colombi C, Vivanti G, Rogers SJ, Rivera SM. The Action Observation System when Observing Hand Actions in Autism and Typical Development. Autism Res. 2015;8(3):284–296. doi: 10.1002/aur.1445. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press C, Richardson D, Bird G. Intact imitation of emotional facial actions in autism spectrum conditions. Neuropsychologia. 2010;48(11):3291–3297. doi: 10.1016/j.neuropsychologia.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, Bennetto L, McEvoy R, Pennington BF. Imitation and pantomime in high-functioning adolescents with autism spectrum disorders. Child Dev. 1996;67(5):2060–2073. [PubMed] [Google Scholar]
- Rugg MD, Thompson-Schill SL. Moving Forward With fMRI Data. Perspect Psychol Sci. 2013;8(1):84–87. doi: 10.1177/1745691612469030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. The Social Communication Questionnaire. Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, … Wolf DH. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thye MD, Bednarz HM, Herringshaw AJ, Sartin EB, Kana RK. The impact of atypical sensory processing on social impairments in autism spectrum disorder. Dev Cogn Neurosci. 2017 doi: 10.1016/j.dcn.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevarthen C, Aitken KJ. Infant intersubjectivity: research, theory, and clinical applications. J Child Psychol Psychiatry. 2001;42(1):3–48. [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Menon V. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Front Hum Neurosci. 2013;7:458. doi: 10.3389/fnhum.2013.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umilta MA, Kohler E, Gallese V, Fogassi L, Fadiga L, Keysers C, Rizzolatti G. I know what you are doing. a neurophysiological study. Neuron. 2001;31(1):155–165. doi: 10.1016/s0896-6273(01)00337-3. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59(1):431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F, Baetens K. Understanding others’ actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage. 2009;48(3):564–584. doi: 10.1016/j.neuroimage.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Vogt S, Buccino G, Wohlschlager AM, Canessa N, Shah NJ, Zilles K, … Fink GR. Prefrontal involvement in imitation learning of hand actions: effects of practice and expertise. Neuroimage. 2007;37(4):1371–1383. doi: 10.1016/j.neuroimage.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Wadsworth HM, Maximo JO, Lemelman AR, Clayton K, Sivaraman S, Deshpande HD, … Kana RK. The Action Imitation network and motor imitation in children and adolescents with autism. Neuroscience. 2017;343:147–156. doi: 10.1016/j.neuroscience.2016.12.001. [DOI] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40(3):655–664. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Williams JH. Self-other relations in social development and autism: multiple roles for mirror neurons and other brain bases. Autism Res. 2008;1(2):73–90. doi: 10.1002/aur.15. [DOI] [PubMed] [Google Scholar]
- Yang J, Hofmann J. Action observation and imitation in autism spectrum disorders: an ALE meta-analysis of fMRI studies. Brain Imaging Behav. 2016;10(4):960–969. doi: 10.1007/s11682-015-9456-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.