Abstract
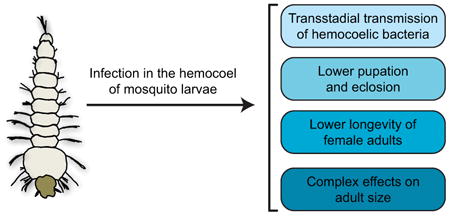
During all life stages, mosquitoes are exposed to pathogens, and employ an immune system to resist or limit infection. Although much attention has been paid to how adult mosquitoes fight infection, little is known about how an infection during the larval stage affects the biology of the resultant adult. In this study, we investigated whether a bacterial infection in the hemocoel of the African malaria mosquito, Anopheles gambiae, is transstadially transmitted from larvae to adults (both females and males), and whether immune stimulation in the hemocoel as a larva alters development or biological traits of the adult. Specifically, larvae were injected in the hemocoel with either fluorescent microspheres or Escherichia coli, and the following traits were examined: transstadial transmission, larval development to adulthood, adult survival, and adult body size. Our results show that transstadial transmission of hemocoel contents occurs from larvae to pupae and from pupae to adults, but that bacterial prevalence and intensity varies with age. Injury, immune stimulation or infection decreases the proportion of larvae that undergo pupation and eclosion, infection decreases the longevity of adult females, and treatment has complex effects on the body size of the resultant adults. The present study adds larval hemocoelic infection to the known non-genetic factors that reduce overall fitness by negatively affecting development and adult biological traits that influence mosquito vector competence.
Keywords: Culicidae, hemocoel, immunology, insect, life history, metamorphosis
Graphical abstract
1. Introduction
The holometabolous life cycle of mosquitoes includes a larval stage that inhabits aquatic environments and an adult stage that inhabits terrestrial and aerial environments, with only the adult stage directly responsible for transmitting disease-causing pathogens to vertebrate animals, including humans. Multiple stress-based experiments have shown that larval environmental factors such as temperature, larval density and food quantity can have carryover effects on adult life history traits, including effects on their susceptibility to infection (Alto, 2011; Araújo et al., 2012; Breaux et al., 2014; Briegel, 1990; Dominic Amalraj and Das, 1996; Dominic Amalraj et al., 2005; Grimstad and Walker, 1991; Kang et al., 2017; Lefèvre et al., 2013; Merritt et al., 1992; Moller-Jacobs et al., 2014; Mourya et al., 2004; Muturi et al., 2011; Roux et al., 2015; Shapiro et al., 2016; Takken et al., 1998; Tun-Lin et al., 2000; Vantaux et al., 2016; Wallace and Merritt, 1999; Yadav et al., 2005). For example, alterations in larval temperature affect the susceptibility of adults to Chikungunya, Dengue and Sindbis viruses; larval competition decreases adult survival; and larval nutritional stress influences developmental timelines, adult body size, fecundity, adult survival, and the rate of Plasmodium parasite development. Additionally, exposure of larvae to sub-lethal doses of bacteria augments the immune responses of the resultant adult. For example, a larval infection with Bacillus sp. has a negative effect on the development of filarial nematodes and Plasmodium parasites that are acquired after eclosion (Kala and Gunasekaran, 1999; Mahapatra et al., 1999; Paily et al., 2012), and inhabiting a larval environment containing Escherichia coli enhances the antimicrobial responses of adults (Moreno-García et al., 2015). The mechanisms responsible for the effect that the larval experience has on subsequent infections as adults are unknown, but for culicine mosquitoes it has been suggested that transstadial transmission (passage across molts) of a pathogen could increase immune alertness (Paily et al., 2012).
In mosquitoes, several viruses are both vertically and transstadially transmitted (Becker et al., 2003). It has also been reported that gut bacteria – including bacterial endotoxins – are transstadially passaged (Chavshin et al., 2015; Jadin et al., 1966; Paily et al., 2012; Pumpuni et al., 1996), although others have argued that adults reacquire the bacteria from the water following ecdysis (Lindh et al., 2008). Many aquatic microorganisms (e.g., nematodes, fungi, ciliates, viruses, and bacteria) are able to invade the hemocoel (body cavity) of larval mosquitoes by penetrating the cuticle or the intestinal epithelium (Granados, 1980; Kalucy and Daniel, 1972; Petersen et al., 1968; Sweeney et al., 1983; Washburn et al., 1988; Yassine et al., 2012); however, it remains unknown whether a larval-acquired infection in the hemocoel is transstadially transmitted to the hemocoel of an adult. This is a major oversight, as a hemocoelic infection during the larval stages may alter the susceptibility of an infection acquired as an adult, such as an infection with Plasmodium parasites (Imwong et al., 2011; Paul et al., 2002). Furthermore, although mosquito larvae mount powerful cellular and humoral immune responses against microorganisms in their hemocoel (Biron et al., 2005; Brey et al., 1988; Dimopoulos et al., 1997; Duncan et al., 2012; Kalucy and Daniel, 1972; League et al., 2017; League and Hillyer, 2016; Meredith et al., 2008; Richman et al., 1996; Shin et al., 2005), little is known about how immune stimulation during the larval stage affects development or the biology of the resultant female and male adults (Moreno-García et al., 2015).
In this study, we investigated whether an infection present in the hemocoel of the African malaria mosquito, Anopheles gambiae, is transstadially transmitted from larvae to adults, and whether immune stimulation in the hemocoel as a larva alters the development or biological traits of the adult, including survival and body size. Our results show that transstadial transmission of a hemocoelic infection occurs from larvae to adults, but that infection prevalence declines with each molt, and with adult age.
Additionally, bacterial intensity among infected individuals increases with each molt, decreases in the days following eclosion, and then increases as adults age further. Injury, immune stimulation or infection decreases the proportion of larvae that reach adulthood, infection decreases the longevity of adult females, and treatment has complex effects on the body size of female and male adults. Taken together, these findings show that transstadial transmission of a larval-acquired hemocoel infection occurs in mosquitoes, and that a larval infection reduces overall fitness by negatively impacting life history traits.
2. Materials and methods
2.1. Mosquito rearing and maintenance
Anopheles gambiae (G3 strain) were reared and maintained in an environmental chamber as previously described (Coggins et al., 2012). Briefly, eggs were collected and placed in plastic containers with deionized water, and hatched larvae were fed daily a mixture of koi fish food and baker's yeast. During the course of this study, all treatments were initiated in early 4th instar larvae. Larvae for each treatment were separated, and after pupation and subsequent eclosion, adult mosquitoes were fed a 10% sucrose solution ad libitum.
2.2. Mosquito injection, inoculation of immune elicitor, and bacterial infection
Early 4th instar larvae were injected in their hemocoel at the mesothorax using a Nanoject III Auto-Nanoliter Injector (Drummond Scientific Company, Broomall, PA, USA). Larvae received 69 nl of one of the following: (1) 0.2% solids 1 μm diameter green fluorescent (505/515) carboxylate-modified microspheres (Invitrogen, Carlsbad, CA, USA) in phosphate-buffered saline (PBS); (2) PBS alone (injury control); (3) tetracycline resistant, GFP-expressing Escherichia coli (modified DH5α) in Luria-Bertani's rich nutrient medium (LB); or (4) LB medium alone (injury control). For bacterial infections, E. coli were grown overnight in a shaking incubator at 37°C in LB broth. Infection doses were estimated prior to larval injections by measuring the optical density (OD600 = 5) of bacterial cultures using a BioPhotometer™ plus spectrophotometer (Eppendorf AG, Hamburg, Germany). Absolute doses were determined by spreading a 1:1000 dilution of the bacterial culture on an LB agar plate, incubating the plate overnight at 37°C, and then counting the resultant colony forming units (CFUs).
2.3. Hemocoel transstadial transmission
Larval mosquitoes were injected in their hemocoel with either fluorescent microspheres or E. coli, and each subsequent life stage (late 4th instar larvae, pupae, 1-day-old adults, 5-day-old adults, and 10-day-old adults) was assessed for the presence of the challenge agent. Due to the brightness of the long-lasting fluorescent microspheres – phagocytosed microspheres remain fluorescent for >2 weeks – the hemocoel of microsphere-injected mosquitoes from each life stage was visualized by imaging through the translucent cuticle of live individuals using bright field and fluorescence illumination on a Nikon SMZ1500 stereomicroscope (Nikon, Tokyo, Japan) connected to a Hamamatsu ORCA-Flash 2.8 digital CMOS camera (Hamamatsu Photonics, Hamamatsu, Japan) and Nikon Advanced Research NIS-Elements software. Additionally, the exuviae from treated individuals were collected following adult emergence, mounted between a microscope slide and a coverslip using Aqua Poly/Mount (Polysciences, Warrington, PA, USA), and visualized using light and fluorescence illumination on a Nikon 90i compound microscope (Nikon Corp., Tokyo, Japan) equipped with a Nikon Intensilight C-HGFI fluorescence illumination unit, a Nikon DS-Qi1Mc CCD camera, and Nikon Advanced Research NIS-Elements software. The detection of microspheres within the hemocoel of individual mosquitoes was used to determine the rate of transstadial passage of inanimate particles, and the presence in exuviae was used to indicate loss or partial loss during a molt. A total of 50 individuals were examined for each life stage and sex over the course of 10 independent trials.
In order to quantify transstadial transmission of tetracycline resistant, GFP-expressing E. coli, mosquitoes at each life stage that originated from infected larvae were homogenized in PBS and spread on LB agar plates containing tetracycline. Plates were incubated overnight at 37°C, the CFUs were counted, and the number of CFUs was then used to assess infection. The state of infection was analyzed using two descriptors: prevalence (the percentage of mosquitoes infected with E. coli) and intensity (the mean number of CFUs in each mosquito that remained infected with E. coli). In order to further confirm that all colonies originated from the E. coli inoculums, plates were also viewed by fluorescence microscopy to confirm the expression of GFP. A total of 30 individuals were assessed at each life stage and sex over the course of 7 independent trials.
2.4. Mosquito life history traits
Using the same protocol as above, larvae were injected into their hemocoel with one of the following: fluorescent microspheres (immune elicitor), PBS (injury control for immune elicitor), E. coli (bacterial challenge), or LB (injury control for bacterial challenge). An additional group did not receive an injection (naïve). For the different treatments, mosquito development was measured by recording the proportion of larvae that ecdysed into pupae, and the proportion of larvae that reached adulthood (i.e., eclosion). Due to the trauma associated with an injection, larvae that died within 24 hours of injection were excluded from analysis. For those mosquitoes that reached adulthood, their survival was recorded for the first 24 days following eclosion. Three independent trials were conducted, with each trial starting with 120 larvae per treatment, and the data were combined for analysis.
The body size of the eclosed adults was quantified for both sexes using three complementary measurements: length of the abdomen, length of the wing, and length of the hind tibia. Adult mosquitoes were anesthetized on ice and restrained dorsal-side-up on Sylgard 184 silicone plates (Dow Corning Corp, Midland, MI, USA) by placing a 0.15 mm diameter pin through the thorax. A wing and a hind leg were removed at the base with forceps, placed on the silicone plate next to the mosquito abdomen, and all three structures were photographed individually using the Nikon SMZ1500 stereomicroscope. The lengths of all three structures were then measured using the length feature of NIS Elements. The length of the abdomen was defined as the distance between the most posterior region of the postnotum and the posterior of the eighth abdominal segment (excluding the cerci). The length of the wing was defined as the distance between the axillary incision (located near the proximal region of the ambient costa) and the junction between Radius 2 (or R2, excluding the fringe) and the ambient costa (Chintapalli and Hillyer, 2016; McCann et al., 2009). The length of the hind tibia was defined as the distance between the distal end of the femur and the proximal end of the tarsus. For each treatment, these three features were measured in 60 adults (30 females and 30 males).
2.5. Statistical analyses
All statistical analyses were performed using GraphPad Prism version 7 (GraphPad Software, La Jolla, CA, USA), and differences were considered significant at P ≤ 0.05, unless otherwise noted. Data on bacterial intensity, length of the abdomen, length of the wing, and length of the hind tibia were separated by treatment group and sex, and then tested for normality using the D'Agostino-Pearson normality test. Data on bacterial intensity did not assume a normal distribution and were analyzed by the Kruskal-Wallis test followed by Dunn's multiple comparisons post-hoc test.
Data on the length of the abdomen, wing, and hind tibia assumed a normal distribution and were analyzed by repeated measures two-way ANOVA. Although this test is often used to analyze measurements of a single variable at different time points, the measurements can also be repeated at different places instead of different times (McDonald, 2014). In our case, the repeated measures are the three anatomical structures of the body (abdomen, wing, and hind tibia) of an individual mosquito. Thus, the test used treatment (naïve, injury, and either microspheres or E. coli) and feature (abdomen, wing, and hind tibia) as the variables, and was followed by Šidák's multiple comparisons post-hoc tests. The repeated-measures two-way ANOVA p-value denotes whether there are differences between the three treatments (taking the three features into account), and the Šidák's p-values denote the results of pair-wise comparisons that inform on differences between the treatments (for example, comparing naïve vs. injured, naïve vs. infected, and injured vs. infected). Adult survival was analyzed using the Log-Rank test.
For data on larval development, a series of 2x2 contingency tables were constructed to compare the proportion of larvae that pupated and the proportion of larvae that eclosed and became adults in each treatment group. Because there are three possible pairwise comparisons for each experiment (naïve vs. PBS, naïve vs. microspheres, and PBS vs. microspheres for the immune elicitor experiment, and naïve vs. LB, naïve vs. bacteria, and LB vs. bacteria for the infection experiment) we did a Fisher's exact test on each of the three pairings and then applied the Bonferroni correction for multiple tests (i.e., with three pairwise comparisons, the P value must be less than 0.05 ÷ 3, or 0.016, to be significant at the P ≤ 0.05 level) (MacDonald and Gardner, 2000; McDonald, 2014). Life history trait experiments, with the exception of survival experiments, were conducted concurrently, but were analyzed by treatment group independently. That is, the PBS/microspheres data were analyzed separately from the LB/E. coli data. As such, the naïve group is the same in both analyses.
3. Results
3.1. Transstadial transmission of hemocoel infections occurs from larvae to adults
To determine whether transstadial transmission of hemocoel infections occurs in mosquitoes, early 4th instar larvae were injected with either fluorescent microspheres or GFP-E. coli, and several phases of ontogeny (late 4th instar larvae, pupae, 1-day-old adults, 5-day-old adults, and 10-day-old adults) were assayed by fluorescence microscopy or by plating on selective media, respectively. Following injection of microspheres, fluorescence imaging of mosquito whole bodies revealed that 100% of late 4th instar larvae contained fluorescent microspheres in the hemocoel, and that the prevalence of fluorescent microspheres in the hemocoel of each subsequent phase of ontogeny was also 100%. Thus, the detection of inanimate particles within the hemocoel of adult mosquitoes suggested that an infection could also be passaged from larvae to adults.
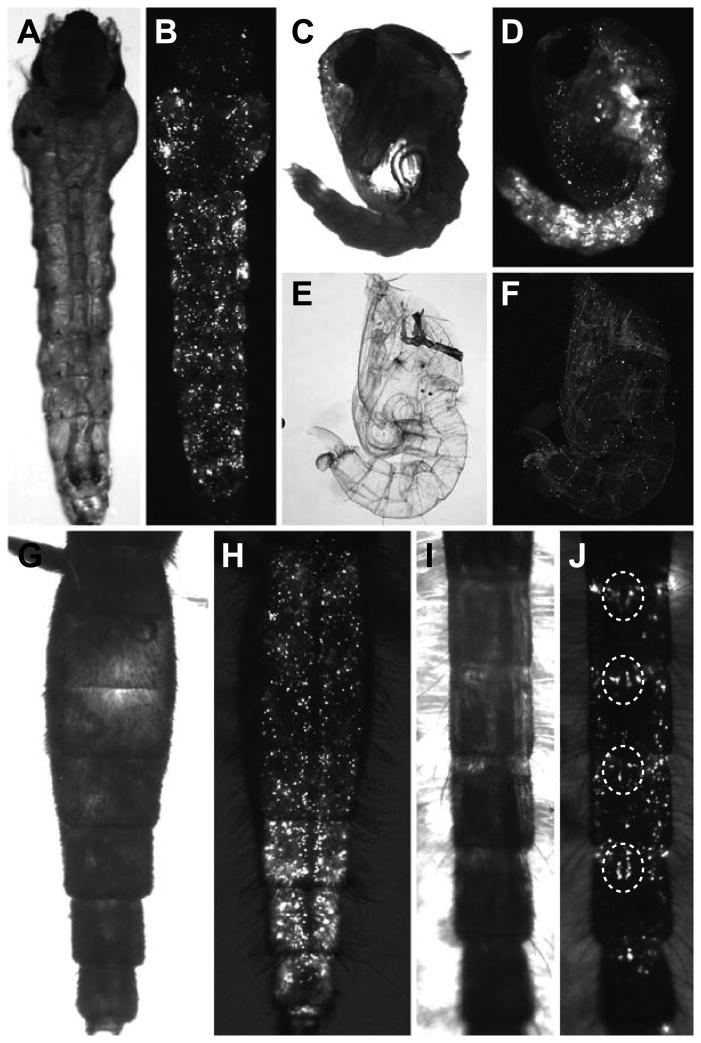
Qualitative observation of the distribution of the fluorescent microspheres revealed that they disseminate evenly throughout the hemocoel of larvae (Fig. 1A, B), and that this distribution continues after the molts that result in pupae (Fig. 1C, D) and newly eclosed adults (Fig. 1G-J). Furthermore, although transstadial passage was significant, microspheres were also released into the environment with the exuviae, indicating that not all microspheres are passaged from one stage to another (Fig. 1E, F). As adults aged, the distribution of fluorescent microspheres changed. Rather than being evenly distributed, as was observed in larvae and pupae, aging resulted in the preferential aggregation of microspheres in the periostial regions of the mid-abdominal segments (Fig. 1J). Specifically, aggregation of microspheres at the periostial regions was observed in 20%, 33%, and 87% of 1-, 5-, and 10-day-old female mosquitoes, respectively, and in 0%, 13%, and 33% of 1-, 5-, and 10-day-old male mosquitoes, respectively. These observations are consistent with earlier reports showing the phagocytosis and sequestration of foreign materials by periostial hemocytes, which are immune cells that flank the ostia (valves) of the adult heart, and thus, reside in areas of the body that receive high hemolymph flow (King and Hillyer, 2012; Sigle and Hillyer, 2016).
Figure 1.
Transstadial transmission of fluorescent microspheres present in the hemocoel of mosquitoes. A-J. Ealy stage 4th instar larvae were intrathoracically injected with fluorescent microspheres (an immune elicitor), and each life stage (including exuviae) was visualized by imaging through the cuticle using bright field (A, C, E, G, I) and fluorescence (B, D, F, H, J) illumination. Specimens pictured are a late stage 4th instar larva (A, B), a pupa (C, D), a pupal exuvium (E, F), a female 1-day-old adult (G, H), and a male 5-day-old adult (I, J). In the male adult, the aggregation of microspheres at the periostial regions of the heart is clearly observed (dotted circles).
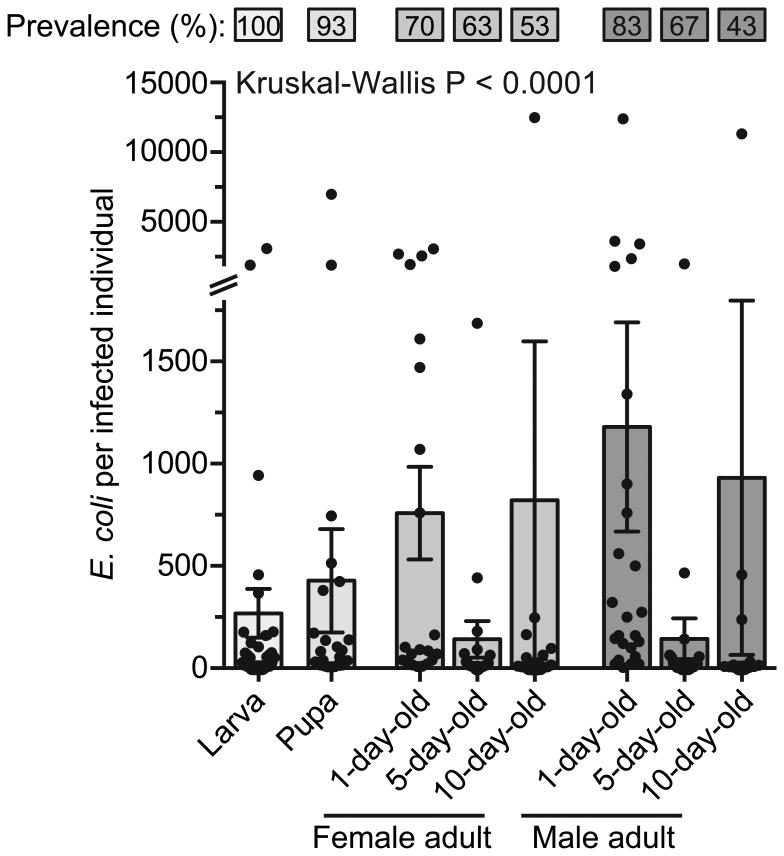
When mosquitoes were injected with E. coli instead of microspheres, transstadial transmission of this bacterium was consistently observed. Specifically, analysis of mosquito bodies by plating on selective media revealed that 100% of late 4th instar larvae remain infected with E. coli, and that prevalence of infection persists with each molt, and as adults age. Specifically, in pupae the prevalence was 93%, whereas prevalence in adults was 70%, 63%, and 53% in 1-, 5-, and 10-day-old females, respectively, and 83%, 67%, and 43% in 1-, 5-, and 10-day-old males, respectively (Fig. 2). These findings confirm that bacteria present in the mosquito hemocoel can be transstadially transmitted between molts. Furthermore, given that all late 4th instar larvae possessed E. coli, these data demonstrate that following a molt some individuals are able to clear systemic infections.
Figure 2.
Prevalence and infection intensity of individuals infected as larvae. Mosquitoes were intrathoracically infected with E. coli and the percentage of mosquitoes infected (top) and the number of E. coli in infected mosquitoes (bottom) was quantified. Column heights mark the mean, whiskers denote the S.E.M, and circles denote the values for individual mosquitoes. Kruskal-Wallis P-value compares infection intensity across stage and age.
Among mosquitoes that remained infected with E. coli (excluding mosquitoes that cleared the infection), infection intensity increased with each molt, decreased by the 5th day of adulthood, and then increased again by day 10 (Fig. 2; Kruskal-Wallis P < 0.0001). When accounting for the original inoculum of approximately 18,816 E. coli (± 2,151 S.E.M.), larvae were able to eliminate 99% of the E. coli by 24 h after the injection, indicating that the larval immune system is able to rapidly kill most of the bacteria present in their hemocoel. Following this decrease in infection intensity, the bacterial load in infected mosquitoes increased by 37.4% after the molt to pupae (Dunn's P > 0.9999), and then by 43.5% and 63.7% between the molt from pupae to 1-day-old female and male adults, respectively (Dunn's P > 0.9999 and P = 0.5333 for females and males, respectively). As adults aged, however, infection intensity decreased by 81.4% in females (Dunn's P = 0.1602) and 87.9% in males (Dunn's P < 0.001) between days 1 and 5 post-eclosion, respectively, and then increased by 82.8% in females (Dunn's P > 0.9999) and 84.6% in males (Dunn's P > 0.9999) between days 5 and 10. Taken together, these data show that as mosquitoes molt and adults age some are able to completely eliminate the infection. Furthermore, although infection intensity varies with stage and age, the infection is maintained in check, with the bacterial intensity in infected mosquitoes remaining low and never reaching the original inoculum. It is precisely because the infection intensity remained low that we were unable to visualize the anatomical distribution of the bacteria by fluorescence imaging through the cuticle.
3.2. Injury, immune stimulation or infection decreases the proportion of larvae that complete metamorphosis to adulthood
To determine whether immune stimulation in the hemocoel as a larva alters the probability of completing development, larvae were injected with fluorescent microspheres (immune elicitor), PBS (injury control for immune elicitor), E. coli (infection), LB (injury control for infection), or were not injected at all (naïve group). The efficiency of mosquito development was measured by tracking the proportion of larvae that pupated and the proportion of larvae that eclosed (reached adulthood).
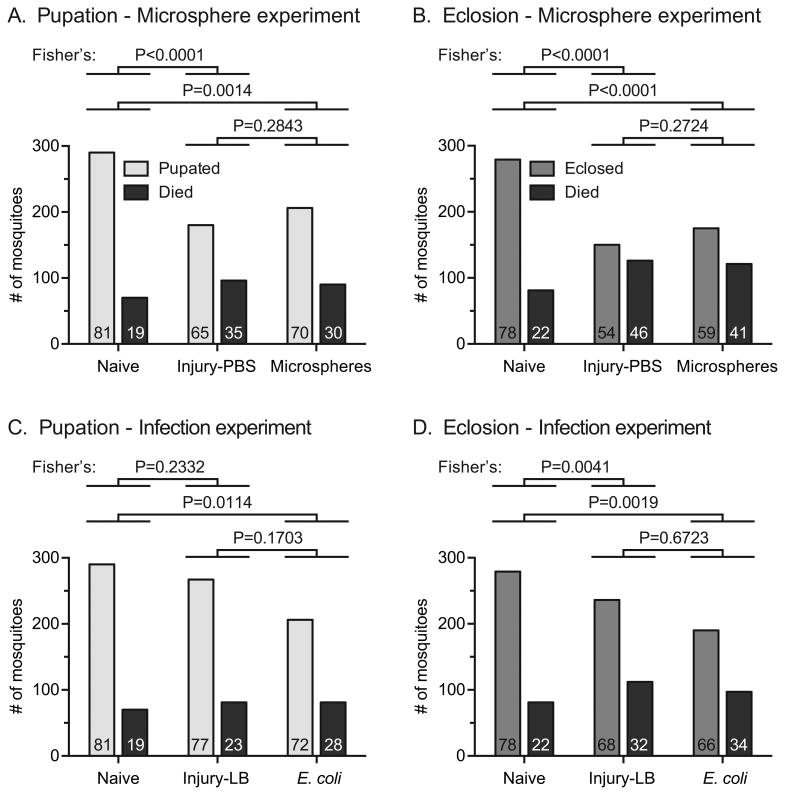
In experiments testing the effect of an immune challenge on development, the proportion of larvae that successfully pupated after an injection with PBS or microspheres decreased by 20% and 14%, respectively, relative to the proportion of naïve larvae that successfully pupated (Fig. 3A; Fisher's exact test P < 0.0001 and P = 0.0014, respectively). Although the proportion of individuals that pupated was 8% higher in the group injected with microspheres than in the group injected with PBS, this difference was not meaningful (Fisher's P = 0.2843). A similar result was obtained for the proportion of larvae that eclosed, where injection with PBS or microspheres resulted in a 31% and 24% decrease in the number of emerged adults, respectively, relative to the naïve group (Fig. 3B; Fisher's P < 0.0001 for both comparisons). Again, the 9% difference between the proportions of individuals that eclosed from PBS- and microsphere-treated larvae was not meaningful (Fisher's P = 0.2724).
Figure 3.
Number of larvae that pupated, eclosed or died. A-B. Pupation (A) and eclosion (B) of untreated individuals (naïve), individuals injured as larvae by injection of PBS, and individuals immune-stimulated as larvae by injection of microspheres. C-D. Pupation (C) and eclosion (D) of untreated individuals (naïve), individuals injured as larvae by injection of LB, and individuals infected as larvae by injection of E. coli. The numbers at the bottom of each column indicate the percentage of mosquitoes for each outcome.
Experiments testing the effect of infection on development obtained a similar result, except that the pupation of infected mosquitoes was marginally lower than that of injured mosquitoes. Specifically, relative to naïve mosquitoes, injury decreased the proportion of individuals that pupated by 5%, and infection decreased it by 11% (Fig. 3C; Fisher's P = 0.2332 and P = 0.0114, respectively). Furthermore, relative to naïve mosquitoes, injury decreased eclosion by 13%, and infection decreased it by 15% (Fig. 3D; Fisher's P = 0.0041 and P = 0.0019, respectively). Together, these data demonstrate that heightened immune activity in the larval stage – either by injury, immune stimulation or infection – has a negative effect on mosquito development.
3.3. Infection in the larval stage decreases the longevity of female adults
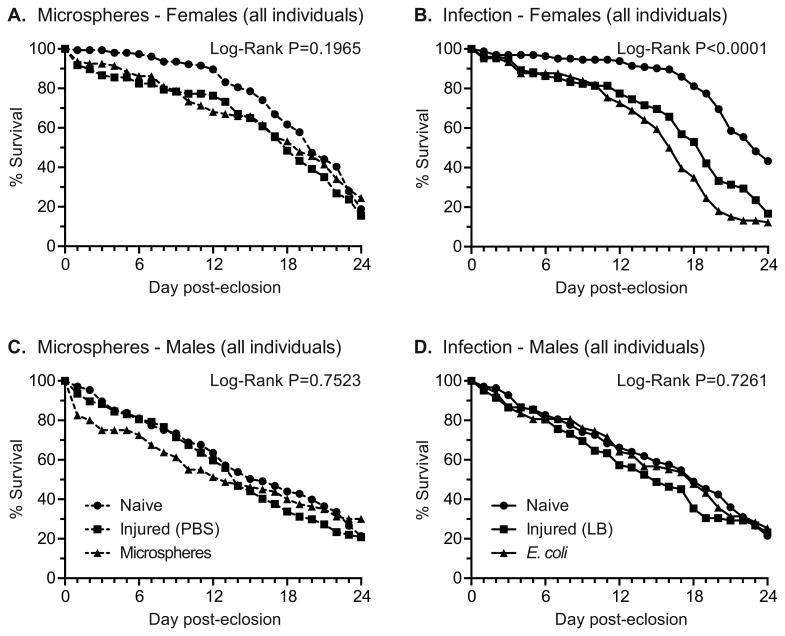
To determine whether treatment as a larva impacts the longevity of adults we tracked the survival of naïve and treated mosquitoes during the first 24 days following eclosion. For female adults, the survival rate of individuals that were naïve, injured, or injected with fluorescent microspheres as larvae was similar (Fig. 4A; Log-Rank P = 0.1965). However, when we examined survival over the first 12 days post-eclosion in order to incorporate the mean extrinsic incubation period for Plasmodium parasites (10-14 days in areas of high malaria transmission) (Charlwood et al., 1997; Killeen et al., 2000), there was a significant decrease in the rate of survivorship of females that received PBS or fluorescent microspheres, compared to naïve individuals (Log-Rank P=0.006). This decrease in the survivorship over the first 12 days of adulthood in the treated groups was evident when comparing the slopes (m) of the survival curves of naïve mosquitoes (m = -0.87, R2 = 0.93) versus those of PBS- (m = -1.61, R2 = 0.90) and microsphere-treated (m = -2.44, R2 = 0.96) mosquitoes. The opposite pattern was observed between days 12 and 24, with the survival rate of naïve mosquitoes being lower (m = -5.64, R2 = 0.98) than the survival rate of mosquitoes treated with PBS (m = -5.05, R2 = 0.99) or microspheres (m = -3.81, R2 = 0.96). Thus, by day 24 the survival of naïve (19%), injured (15%), and microsphere-treated (24%) female adults was very similar.
Figure 4.
Percent survival of adults treated as larvae. A. Percent survival of female adults that eclosed from larvae that were untreated (naïve), injected with PBS, or injected with microspheres. B. Percent survival of female adults that eclosed from larvae that were untreated (naïve), injected with LB, or injected with E. coli. C. Percent survival of male adults that eclosed from larvae that were untreated (naïve), injected with PBS, or injected with microspheres. D. Percent survival of male adults that eclosed from larvae that were untreated (naïve), injected with LB, or injected with E. coli.
When experiments were repeated to test the effect of larval infection on adult longevity, the survivorship of female adults that had been infected with E. coli as larvae was lower than that of naïve or injured mosquitoes, and this was the case regardless of whether the data were analyzed at 24 days (Fig. 4B; Log-Rank, P<0.0001) or 12 days post-eclosion (Log-Rank, P<0.0001). In this case, the survival rate of injured (m = -1.76, R2 = 0.96) and infected (m = -1.91, R2 = 0.92) mosquitoes was fairly similar during the first 12 days of treatment, and these survival rates were significantly lower than the survival rate of naïve mosquitoes (m = -0.44, R2 = 0.90). Then, between days 12 and 22 the mortality rate of infected mosquitoes (m = -6.69, R2 = 0.98) accelerated relative to both injured mosquitoes (m = -5.41, R2 = 0.96) and naïve mosquitoes (m = -3.84, R2 = 0.87), and by days 22 and 24 there were clear differences between the survival of naïve (55% and 43%, respectively), injured (29% and 17%, respectively), and infected (13% and 12%, respectively) female adults. A closer analysis of naïve female adults showed a clear inflection point in survival, whereby these mosquitoes experienced a high rate of survival over the first 16 days of adulthood (m = -0.56, R2 = 0.94 in one experiment and m = -1.50, R2 = 0.86 in the other), and then the survival rate markedly decreased between days 16 and 24 (m = -6.15, R2 = 0.98 in one experiment and m = -6.57, R2 = 0.98 in the other).
For male mosquitoes, the survival rates of adults that were naïve, or had been injected with PBS or microspheres as larvae were similar, regardless of whether the data were analyzed over the first 24 days or over the first 12 days after eclosion (Fig. 4C; Log-Rank P = 0.7523 and P = 0.0964, respectively). Likewise, the survival rates of male adults that were naïve, had been injected with LB or had been infected with E. coli, were similar (Fig. 4D; Log-Rank P = 0.7261 for days 0-12 and P = 0.4174 for days 0-24). For both of these experiments, the mortality rate for all treatment groups was fairly linear over the first 24 days of adulthood (R2 ≥ 0.96 for all), and no clear inflection points were detected. In summary, transstadial transmission of a persistent bacterial infection decreases the longevity of female adults, which is the sex responsible for the transmission of pathogens to vertebrate hosts.
3.4. Immune stimulation as larvae has complex effects on the body size of adults
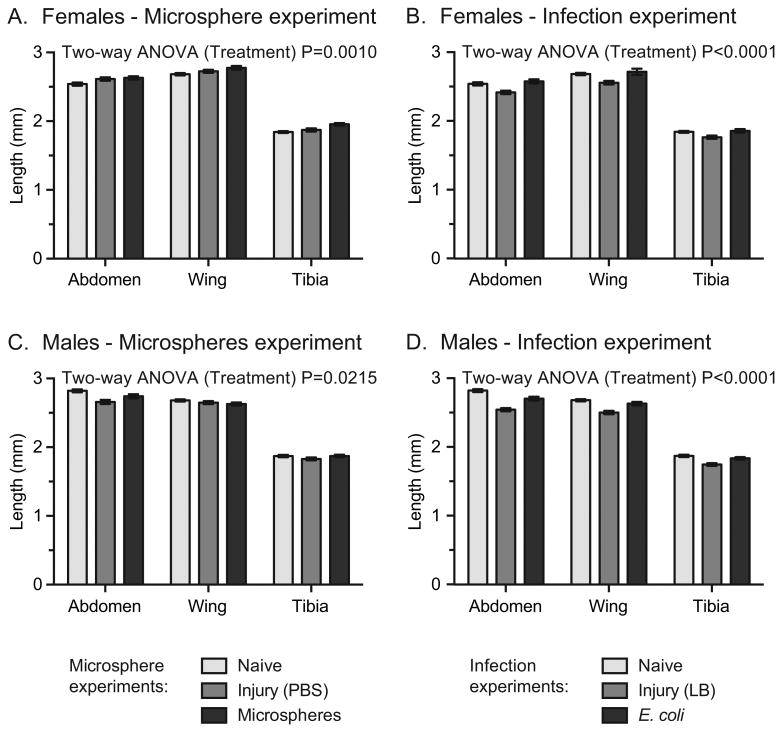
To determine whether an immune challenge as a larva impacts the size of eclosed individuals, we injected larvae with fluorescent microspheres (immune elicitor), PBS (injury control for immune elicitor), E. coli (infection), LB (injury control for infection), or did not inject them at all (naïve group), and then measured the size of the eclosed adults using three linear measurements: (1) the length of the abdomen, (2) the length of the wing, and (3) the length of the hind tibia (Fig. 5). Injection of female larvae with PBS resulted in adults with abdomens, wings and tibias that were 2.9%, 1.6% and 1.7% larger, respectively, than those of adults that eclosed from naïve larvae (Fig. 6A; Sidak's P = 0.1649, comparing main effect of treatment). These features were even larger when female larvae were treated with microspheres, where the size of the abdomens, wings and tibias were 3.6%, 3.5% and 6.1% larger, respectively, in adults that eclosed from microsphere-injected larvae than in adults that arose from naïve larvae (Fig. 6A; Sidak's P = 0.0007). A different outcome was detected when female larvae were treated with LB or E. coli. Treatment of female larvae with LB resulted in adults with abdomens, wings and tibias that were 4.9%, 4.7% and 4.2% smaller, respectively, than those of adults that eclosed from naïve larvae (Fig. 6B; Sidak's P = 0.0030), whereas infection with E. coli increased the size of these features by 1.5%, 1.3% and 0.8% relative to mosquitoes that arose from naïve larvae (Fig. 6B; Sidak's P = 0.7609).
Figure 5.
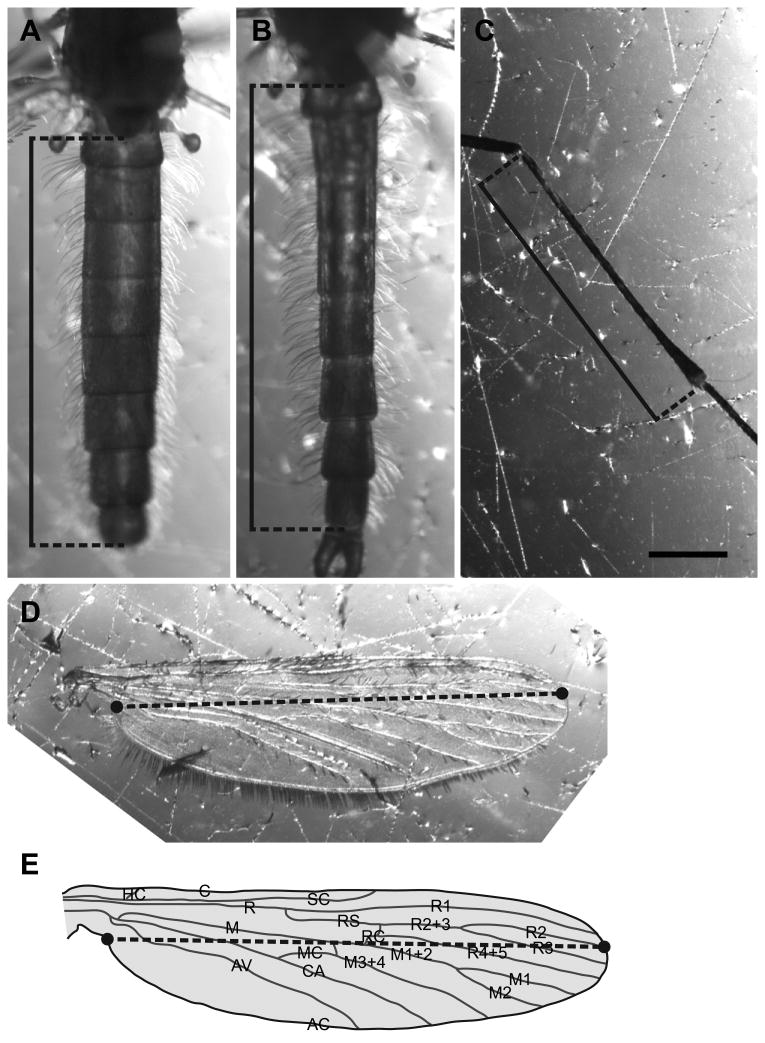
Measurement of body size features of adult Anopheles gambiae. A-B. Images of the abdomen of female (A) and male (B) adults, showing the linear measurement of the length of the abdomen (solid line). The length of the abdomen was measured as the distance between the most posterior region of the postnotum and the posterior of the eighth abdominal segment (excluding the cerci). C. Image of a portion of an adult leg, showing the linear measurement (solid line) of the tibia. D-E. Image (D) and graphical representation (E) of the adult wing. The length of the wing (dotted black line) was defined as the distance between the axillary incision (located near the proximal region of the ambient costa; on the left side of the image) and the junction between Radius 2 (R2) and the ambient costa (AC; junction on the right side of the image). The graphical representation of the wing is adapted from Chintapalli and Hillyer (Chintapalli and Hillyer, 2016). Scale bar: 0.5 mm.
Figure 6.
Body size of adults treated as larvae. A. Length of the abdomen, wing, and hind tibia of female adults that eclosed from larvae that were untreated (naïve), injected with PBS, or injected with microspheres. B. Length of the abdomen, wing, and hind tibia of female adults that eclosed from larvae that were untreated (naïve), injected with LB, or injected with E. coli. C. Length of the abdomen, wing, and hind tibia of male adults that eclosed from larvae that were untreated (naïve), injected with PBS, or injected with microspheres. D. Length of the abdomen, wing, and hind tibia of male adults that eclosed from larvae that were untreated (naïve), injected with LB, or injected with E. coli. Column heights mark the mean and whiskers denote the S.E.M
In males, injection of larvae with PBS resulted in adults with abdomens, wings and tibias that were 5.7%, 1.2% and 2.3% smaller, respectively, than those of adults that eclosed from untreated larvae (Fig. 6C; Sidak's P = 0.0175). The decrease in size was reduced when male larvae were treated with microspheres, where the size of these adult features were 2.8%, 1.9% and 0.0% smaller in adults that eclosed from microsphere-treated larvae than in adults that eclosed from untreated larvae (Fig. 6C; Sidak's P = 0.3025). This trend was more pronounced when the larvae were injected with LB or E. coli, where adults that eclosed from LB-treated larvae possessed abdomens, wings and tibias that were 9.8%, 6.7% and 6.8% smaller, respectively, than those of adults that eclosed from untreated larvae (Fig. 6D; Sidak's P < 0.0001). Infection reduced this change, with adults that arose from E. coli-treated larvae having features that were 4.1%, 1.9% and 2.0% smaller than those from untreated mosquitoes (Fig. 6D; Sidak's P = 0.0310). Overall, these results suggest that treatment has complex effects on mosquito size, with the effect being dictated by the nature of the treatment.
4. Discussion
Although significant progress has been made toward understanding how mosquitoes respond to infection in the adult life stage, little attention has been paid to how a larval infection affects mosquito development and other life history traits. This is a significant omission, as in recent years it has become clear that other aspects of the larval experience have a significant influence on how adult mosquitoes defend themselves from a future infection (Breaux et al., 2014; Grimstad and Walker, 1991; Kang et al., 2017; Moller-Jacobs et al., 2014; Mourya et al., 2004; Muturi et al., 2011; Shapiro et al., 2016; Vantaux et al., 2016; Yadav et al., 2005). Here, we present direct evidence of transstadial transmission of bacteria and inanimate particles from the hemocoel of larvae to the hemocoel of adults, and show the negative effects of a persistent hemocoelic infection on larval development and adult female survival.
Our initial experiments assessing the occurrence of transstadial transmission of foreign contents present in the mosquito hemocoel employed fluorescent microspheres because we have observed that these are long lasting and retain their fluorescence for over two weeks after being introduced into the hemocoel. We found that 100% of the adults that eclosed from larvae injected with microspheres contained these inanimate particles in their hemocoel, and their distribution throughout the body changed with adult age. Three important conclusions can be made from these observations. First, when using a system that functions independent of immune degradation, transstadial transmission of contents present in the mosquito hemocoel is highly prevalent during the molts from larvae to pupae, and from pupae to adults. Second, the dynamic changes in the distribution of microspheres in the days following eclosion, from dispersal throughout the hemocoel to aggregation at the periostial regions of the heart, suggest that the adult immune system actively responds to the presence of foreign material present in the hemocoel, even in the absence of an injury at the adult stage. Third, the aggregation of microspheres on the surface of the heart once again highlights the functional integration of the mosquito circulatory and immune systems, as the periostial hemocytes of adult mosquitoes rapidly phagocytose pathogens in areas of high hemolymph flow, and the periostial regions are the only locations where circulating pathogens aggregate and sessile hemocytes increase in number in response to infection (Hillyer, 2015; King and Hillyer, 2012, 2013; Sigle and Hillyer, 2016).
Similar to our findings using fluorescent microspheres, transstadial transmission of bacteria present in the hemocoel occurs frequently during the transitions from larva to pupa and from pupa to adult; however, the number of bacteria available to be passaged is limited by the larval immune system. In mosquitoes, bacterial infections induce the transcriptional upregulation of immune genes, the mitosis of hemocytes, and an increase in the lytic and melanization potential of the hemolymph (Bartholomay et al., 2010; Baton et al., 2009; Coggins et al., 2012; King and Hillyer, 2013; League et al., 2017; Pinto et al., 2009). Immune mechanisms are especially strong in the larval stage (League et al., 2017; League and Hillyer, 2016), which explains our observation that E. coli infection intensity decreased by 99% within hours of infection. This reduction is in line with the 94% reduction we observed in our earlier study (League et al., 2017). Furthermore, when we tracked the intensity of infection across molts we found that intensity increases from larvae all the way to 1-day-old adults, decreases by 5 days of adulthood, and then increases again as adults age. We hypothesize that this oscillating pattern is due to the robustness of the larval immune system, the trade-off between immune activity and the resources needed to molt (pupa and 1-day-old adults), the maturation of the adult immune system (5-day-old adults), and the waning of the immune system that typically occurs with age, i.e. immune senescence (Christensen et al., 1986; Styer et al., 2007). Again, these data are in agreement with our recent report showing that immunity is strongest in larvae and declines after metamorphosis and with adult age (League et al., 2017).
An interesting observation was that the prevalence of infection decreases with each molt and as adults age, indicating that some mosquitoes are able to cure themselves from a larval-acquired infection. Previous studies that assessed bacterial prevalence in the hemocoel of adults following an infection that was initiated earlier in the adult stage found few or no instances of hemocoelic infections being fully cleared (Gorman and Paskewitz, 2000; Hillyer et al., 2005). Taken together, these findings on prevalence and intensity of infection demonstrate that transstadial transmission of a hemocoelic infection occurs from larvae to adults, and that for some mosquitoes the infection persists whereas for others the infection is eliminated and the adults become cured. Given that by day 10 post-eclosion approximately half of the surviving mosquitoes had been able to clear the infection, we hypothesize that a larval infection may influence the ability of adult mosquitoes to combat infections acquired after eclosion.
Experiments on larval development and adult survival showed that showed that heightened immune activity in the larval stage – either by injury, immune stimulation or infection – results in decreased pupation, eclosion, and female survival, which indicates that there are tangible costs associated with continued immune activity and pathogen-associated damage. These findings are consistent with other studies that have documented trade-offs between immune alertness and biological traits in multiple insect orders. For example, in honeybees and fruit flies, selection of strains with increased immune activity resulted in populations that experience higher larval mortality, slower growth, and reduced competitive ability (Fellowes et al., 1998; Kraaijeveld and Godfray, 1997; Rothenbuhler and Thompson, 1956; Sutter et al., 1968). Furthermore, given our finding that in infected mosquitoes bacterial intensity increased as adults aged, it was not surprising that this was accompanied by a decrease in mosquito survival relative to naïve and injured mosquitoes. Mosquito longevity is particularly important for Plasmodium sp. because malaria parasites have a lengthy extrinsic incubation period (usually 10-14 days) (Charlwood et al., 1997; Killeen et al., 2000), meaning that transmission to a vertebrate host usually does not begin until mosquitoes are well into their second week of adulthood. Thus, only a small proportion of mosquitoes survive long enough to transmit the pathogen (Dawes et al., 2009). Because any reduction in vector survival would reduce the probability of transmission, and conversely, any increase in survival would considerably favor pathogen transmission (Vantaux et al., 2016), our finding that transstadial transmission of a systemic infection decreases the survival of female mosquitoes suggests that such infections are also likely to reduce mosquito-borne pathogen transmission.
Multiple reports on mosquitoes have demonstrated the impact of larval environmental stressors (e.g., temperature, larval density, food quantity) on the size of the resulting adult (Araújo et al., 2012; Gimnig et al., 2002; Heuvel, 1963; Lyimo et al., 1992). For mosquitoes, body size is a fundamental fitness trait; it is linked to survival (Araújo et al., 2012; Hawley, 1985; Landry et al., 1988), blood-feeding behavior (Araújo et al., 2012; Kitthawee et al., 1992; Nasci, 1986), reproductive success (Briegel, 1990; Packer and Corbet, 1989) and vector competence (Koella and Lyimo, 1996; Moller-Jacobs et al., 2014; Shapiro et al., 2016; Vantaux et al., 2016). Contrary to our initial expectations, treatment had complex effects on body size, with some treatments decreasing and others increasing the size of the abdomen, wing and hind tibia. Specifically, injuring female larvae by injecting PBS resulted in a nominal increase in size, and treatment with microspheres resulted in a further increase in size. Injuring females by injecting LB decreased body size, but infecting them with E. coli (grown in LB) rescued the reduction seen with injury. Earlier in the study we showed that treatment with PBS, microspheres and E. coli has a negative effect on pupation, whereas treatment with LB does not. The lack of an LB-associated effect on pupation could be because the nutrients obtained from the LB offset the negative effects of injury, whereas the negative effects seen after the other three treatments are likely due to the cost of an injury, immune stimulation, or pathogen-associated damage. As pertains to body size, injury with PBS or microspheres may result in only the most fit mosquitoes being capable of completing metamorphosis, thus resulting in larger adults. The nutrients infused into the larvae by injecting LB, however, may enhance the ability of all larvae, regardless of vigor, to complete development, but we hypothesize that the toll of having been injured as a larva results in smaller adults. We also hypothesize that treatment with E. coli, much like treatment with microspheres, selects for the most fit individuals, and thus, rescues the effects associated with injection of LB. The finding that E. coli increases body size relative to injury are in line with the increase in size seen in Aedes aegypti adults that eclose from larvae that had been treated with a sublethal dose of Bacillus thuringiensis ssp. israelensis (Bti), although different from our findings, in that case treatment had no effect on the survival of female adults (Alto and Lord, 2016).
The present study shows the deleterious effects of a pathogenic hemocoelic infection during the larval stage on mosquito development and other life history traits. Although these findings are clear, it is important to acknowledge that other microbes play beneficial, and even essential roles, in development and other traits. For example, bacteria are essential for development, as axenic larvae fail to molt (Coon et al., 2017; Coon et al., 2014). Furthermore, some gut bacteria impact the ability of pathogens to colonize mosquito hosts (Angleró-Rodríguez et al., 2016; Boissière et al., 2012; Cirimotich et al., 2011), and more recently – published after the initial submission of this manuscript – genetic modification of symbiotic bacteria associated with the gut and reproductive tract of mosquitoes has been used to inhibit the development of malaria parasites (Wang et al., 2017).
Despite ongoing control efforts, mosquito-borne diseases cause enormous health and economic burdens to people living in tropical and subtropical regions of the world. Although mosquitoes are vulnerable to a wide variety of pathogens, they possess a highly efficient immune system (Clayton et al., 2014; Hillyer, 2010); however, in natural habitats, mosquitoes are routinely challenged with biotic and abiotic pressures that affect their immune system and subsequent vector competence. Larval environmental factors, including food availability, temperature, population density, competition, chemical insecticide exposure, and biological (bacterial and fungal) pesticide exposure, have all been shown to impact the adult immune system (Alto, 2011; Bukhari et al., 2011; Capone et al., 2013; Federici et al., 2003; Federici et al., 2007; Kala and Gunasekaran, 1999; Kim and Muturi, 2013; Mahapatra et al., 1999; Moller-Jacobs et al., 2014; Mourya et al., 2004; Murdock et al., 2012; Muturi, 2013; Muturi et al., 2011; Otieno-Ayayo et al., 2008; Paily et al., 2012; Telang et al., 2012; Yadav et al., 2005). The present study adds another component – larval systemic infection – to the non-genetic factors that negatively affect both development and adult biological traits that are known to influence mosquito vector competence.
Supplementary Material
Highlights.
Larval hemocoelic infections are transstadially transmitted to adult mosquitoes
Prevalence and intensity of transstadially-passaged infections vary with stage and age
Larval injury, immune stimulation or infection decrease pupation and eclosion
Larval hemocoelic infections decrease adult female longevity
Larval hemocoelic infections have complex effects on body size
Acknowledgments
We thank Dr. Lillian Shapiro, Leah T. Sigle, Yan Yan, and Bryan Joosse for commenting on this manuscript. This research was funded by the US National Institutes of Health (NIH) grant R21-AI119596 to JFH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of this manuscript.
Footnotes
Competing interests: All authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alto BW. Interspecific larval competition between invasive Aedes japonicus and native Aedes triseriatus (Diptera: Culicidae) and adult longevity. J Med Entomol. 2011;48:232–242. doi: 10.1603/me09252. [DOI] [PubMed] [Google Scholar]
- Alto BW, Lord CC. Transstadial Effects of Bti on Traits of Aedes aegypti and Infection with Dengue Virus. PLoS Negl Trop Dis. 2016;10:e0004370. doi: 10.1371/journal.pntd.0004370. (10.1371/journal.pntd.0004370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angleró-Rodríguez YI, Blumberg BJ, Dong Y, Sandiford SL, Pike A, Clayton AM, Dimopoulos G. A natural Anopheles-associated Penicillium chrysogenum enhances mosquito susceptibility to Plasmodium infection. Sci Rep. 2016;6:34084. doi: 10.1038/srep34084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo Md, Gil LH, e-Silva Ad. Larval food quantity affects development time, survival and adult biological traits that influence the vectorial capacity of Anopheles darlingi under laboratory conditions. Malar J. 2012;11:261. doi: 10.1186/1475-2875-11-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomay LC, Waterhouse RM, Mayhew GF, Campbell CL, Michel K, Zou Z, Ramirez JL, Das S, Alvarez K, Arensburger P. Pathogenomics of Culex quinquefasciatus and meta-analysis of infection responses to diverse pathogens. Science. 2010;330:88–90. doi: 10.1126/science.1193162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baton LA, Robertson A, Warr E, Strand MR, Dimopoulos G. Genome-wide transcriptomic profiling of Anopheles gambiae hemocytes reveals pathogen-specific signatures upon bacterial challenge and Plasmodium berghei infection. BMC Genomics. 2009;10:257. doi: 10.1186/1471-2164-10-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker N, Petrić D, Boase C, Lane J, Zgomba M, Dahl C, Kaiser A. Mosquitoes and their control. Springer; New York: 2003. [Google Scholar]
- Biron DG, Agnew P, Marche L, Renault L, Sidobre C, Michalakis Y. Proteome of Aedes aegypti larvae in response to infection by the intracellular parasite Vavraia culicis. Int J Parasitol. 2005;35:1385–1397. doi: 10.1016/j.ijpara.2005.05.015. (10.1016/j.ijpara.2005.05.015) [DOI] [PubMed] [Google Scholar]
- Boissière A, Tchioffo MT, Bachar D, Abate L, Marie A, Nsango SE, Shahbazkia HR, Awono-Ambene PH, Levashina EA, Christen R, Morlais I. Midgut microbiota of the malaria mosquito vector Anopheles gambiae and interactions with Plasmodium falciparum infection. PLoS Pathog. 2012;8:e1002742. doi: 10.1371/journal.ppat.1002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breaux JA, Schumacher MK, Juliano SA. What does not kill them makes them stronger: larval environment and infectious dose alter mosquito potential to transmit filarial worms. Proc Biol Sci. 2014;281:20140459. doi: 10.1098/rspb.2014.0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brey PT, Lebrun RA, Papierok B, Ohayon H, Vennavalli S, Hafez J. Defense reactions by larvae of Aedes aegypti during infection by the aquatic fungus Lagenidium giganteum (Oomycete) Cell Tissue Res. 1988;253:245–250. doi: 10.1007/BF00221760. [DOI] [PubMed] [Google Scholar]
- Briegel H. Fecundity, metabolism, and body size in Anopheles (Diptera: Culicidae), vectors of malaria. J Med Entomol. 1990;27:839–850. doi: 10.1093/jmedent/27.5.839. [DOI] [PubMed] [Google Scholar]
- Bukhari T, Takken W, Koenraadt CJ. Development of Metarhizium anisopliae and Beauveria bassiana formulations for control of malaria mosquito larvae. Parasit Vectors. 2011;4:23. doi: 10.1186/1756-3305-4-23. (10.1186/1756-3305-4-23) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone A, Ricci I, Damiani C, Mosca M, Rossi P, Scuppa P, Crotti E, Epis S, Angeletti M, Valzano M, Sacchi L, Bandi C, Daffonchio D, Mandrioli M, Favia G. Interactions between Asaia, Plasmodium and Anopheles: new insights into mosquito symbiosis and implications in Malaria Symbiotic Control. Parasit Vectors. 2013;6:182. doi: 10.1186/1756-3305-6-182. (10.1186/1756-3305-6-182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlwood JD, Smith T, Billingsley PF, Takken W, Lyimo EO, Meuwissen JH. Survival and infection probabilities of anthropophagic anophelines from an area of high prevalence of Plasmodium falciparum in humans. Bull Entomol Res. 1997;87:445–453. [Google Scholar]
- Chavshin AR, Oshaghi MA, Vatandoost H, Yakhchali B, Zarenejad F, Terenius O. Malpighian tubules are important determinants of Pseudomonas transstadial transmission and longtime persistence in Anopheles stephensi. Parasit Vectors. 2015;8 doi: 10.1186/s13071-015-0635-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintapalli RTV, Hillyer JF. Hemolymph circulation in insect flight appendages: physiology of the wing heart and circulatory flow in the wings of the mosquito Anopheles gambiae. J Exp Biol. 2016;219:3945–3951. doi: 10.1242/jeb.148254. (10.1242/jeb.148254) [DOI] [PubMed] [Google Scholar]
- Christensen BM, LaFond MM, Christensen LA. Defense reactions of mosquitoes to filarial worms: Effect of host age on the immune response to Dirofilaria immitis microfilariae. J Parasitol. 1986:212–215. [PubMed] [Google Scholar]
- Cirimotich CM, Dong Y, Clayton AM, Sandiford SL, Souza-Neto JA, Mulenga M, Dimopoulos G. Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science. 2011;332:855–858. doi: 10.1126/science.1201618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton AM, Dong Y, Dimopoulos G. The Anopheles innate immune system in the defense against malaria infection. J Innate Immun. 2014;6:169–181. doi: 10.1159/000353602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggins SA, Estévez-Lao TY, Hillyer JF. Increased survivorship following bacterial infection by the mosquito Aedes aegypti as compared to Anopheles gambiae correlates with increased transcriptional induction of antimicrobial peptides. Dev Comp Immunol. 2012;37:390–401. doi: 10.1016/j.dci.2012.01.005. (10.1016/j.dci.2012.01.005) [DOI] [PubMed] [Google Scholar]
- Coon KL, Valzania L, McKinney DA, Vogel KJ, Brown MR, Strand MR. Bacteria-mediated hypoxia functions as a signal for mosquito development. Proc Natl Acad Sci USA. 2017;114:E5362–E5369. doi: 10.1073/pnas.1702983114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coon KL, Vogel KJ, Brown MR, Strand MR. Mosquitoes rely on their gut microbiota for development. Mol Ecol. 2014;23:2727–2739. doi: 10.1111/mec.12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes EJ, Churcher TS, Zhuang S, Sinden RE, Basáñez MG. Anopheles mortality is both age-and Plasmodium-density dependent: implications for malaria transmission. Malar J. 2009;8:228. doi: 10.1186/1475-2875-8-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos G, Richman A, Muller HM, Kafatos FC. Molecular immune responses of the mosquito Anopheles gambiae to bacteria and malaria parasites. Proc Natl Acad Sci USA. 1997;94:11508–11513. doi: 10.1073/pnas.94.21.11508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominic Amalraj D, Das PK. Life-table characteristics of T. splendens (Diptera: Culicidae) cohorts reared under controlled found regimens. J Vector Ecol. 1996;21:136–145. [Google Scholar]
- Dominic Amalraj D, Sivagnaname N, Das PK. Effect of food on immature development, consumption rate, and relative growth rate of Toxorhynchites splendens (Diptera: Culicidae), a predator of container breeding mosquitoes. Mem Inst Oswaldo Cruz. 2005;100:893–902. doi: 10.1590/s0074-02762005000800012. [DOI] [PubMed] [Google Scholar]
- Duncan AB, Agnew P, Noel V, Demettre E, Seveno M, Brizard JP, Michalakis Y. Proteome of Aedes aegypti in response to infection and coinfection with microsporidian parasites. Ecol Evol. 2012;2:681–694. doi: 10.1002/ece3.199. (10.1002/ece3.199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federici BA, Park HW, Bideshi DK, Wirth MC, Johnson JJ. Recombinant bacteria for mosquito control. J Exp Biol. 2003;206:3877–3885. doi: 10.1242/jeb.00643. [DOI] [PubMed] [Google Scholar]
- Federici BA, Park HW, Bideshi DK, Wirth MC, Johnson JJ, Sakano Y, Tang M. Developing recombinant bacteria for control of mosquito larvae. J Am Mosq Control Assoc. 2007;23:164–175. doi: 10.2987/8756-971X(2007)23[164:DRBFCO]2.0.CO;2. (10.2987/8756-971X(2007)23[164:DRBFCO]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Fellowes MD, Kraaijeveld AR, Godfray HC. Trade-off associated with selection for increased ability to resist parasitoid attack in Drosophila melanogaster. Proc Biol Sci. 1998;265:1553–1558. doi: 10.1098/rspb.1998.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimnig JE, Ombok M, Otieno S, Kaufman MG, Vulule JM, Walker ED. Density-dependent development of Anopheles gambiae (Diptera: Culicidae) larvae in artificial habitats. J Med Entomol. 2002;39:162–172. doi: 10.1603/0022-2585-39.1.162. [DOI] [PubMed] [Google Scholar]
- Gorman MJ, Paskewitz SM. Persistence of infection in mosquitoes injected with bacteria. J Invertebr Pathol. 2000;75:296–297. doi: 10.1006/jipa.2000.4930. [DOI] [PubMed] [Google Scholar]
- Granados RR. Infectivity and mode of action of baculoviruses. Biotechnol Bioeng. 1980;22:1377–1405. [Google Scholar]
- Grimstad PR, Walker ED. Aedes triseriatus (Diptera: Culicidae) and La Crosse virus. IV. Nutritional deprivation of larvae affects the adult barriers to infection and transmission. J Med Entomol. 1991;28:378–386. doi: 10.1093/jmedent/28.3.378. [DOI] [PubMed] [Google Scholar]
- Hawley WA. The effect of larval density on adult longevity of a mosquito, Aedes sierrensis: epidemiological consequences. J Anim Ecol. 1985:955–964. [Google Scholar]
- Heuvel MJ. The effect of rearing temperature on the wing length, thorax length, leg length and ovariole number of the adult mosquito, Aedes aegypti (L.) Ecol Entomol. 1963;115:197–216. [Google Scholar]
- Hillyer JF. Mosquito immunity. Adv Exp Med Biol. 2010;708:218–238. doi: 10.1007/978-1-4419-8059-5_12. Hillyer, J.F., 2015. Integrated Immune and Cardiovascular Function in Pancrustacea: Lessons from the Insects. Integr Comp Biol, 55, 843-855. (10.1093/icb/icv021) [DOI] [PubMed] [Google Scholar]
- Hillyer JF, Schmidt SL, Fuchs JF, Boyle JP, Christensen BM. Age-associated mortality in immune challenged mosquitoes (Aedes aegypti) correlates with a decrease in haemocyte numbers. Cell Microbiol. 2005;7:39–51. doi: 10.1111/j.1462-5822.2004.00430.x. [DOI] [PubMed] [Google Scholar]
- Imwong M, Nakeesathit S, Day NP, White NJ. A review of mixed malaria species infections in anopheline mosquitoes. Malar J. 2011;10:253. doi: 10.1186/1475-2875-10-253. (10.1186/1475-2875-10-253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadin J, Vincke IH, Dunjic A, Delville JP, Wery M, Bafort J, Scheepers-Biva M. Role of Pseudomonas in the sporogenesis of the hematozoon of malaria in the mosquito. Bull Soc Pathol Exot Filiales. 1966;59:514. [PubMed] [Google Scholar]
- Kala MK, Gunasekaran K. Effect of Bacillus thuringiensis ssp. israelensis on the development of Plasmodium gallinaceum in Aedes aegypti (Diptera: Culicidae) Ann Trop Med Parasitol. 1999;93:89–95. doi: 10.1080/00034989958843. [DOI] [PubMed] [Google Scholar]
- Kalucy EC, Daniel A. The reaction of Anopheles annulipes larvae to infection by Aeromonas punctata. J Invertebr Pathol. 1972;19:189–197. doi: 10.1016/0022-2011(72)90209-1. [DOI] [PubMed] [Google Scholar]
- Kang DS, Alcalay Y, Lovin DD, Cunningham JM, Eng MW, Chadee DD, Severson DW. Larval stress alters dengue virus susceptibility in Aedes aegypti (L.) adult females. Acta Trop. 2017;174:97–101. doi: 10.1016/j.actatropica.2017.06.018. (10.1016/j.actatropica.2017.06.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen GF, McKenzie FE, Foy BD, Schieffelin C, Billingsley PF, Beier JC. A simplified model for predicting malaria entomologic inoculation rates based on entomologic and parasitologic parameters relevant to control. Am J Trop Med Hyg. 2000;62:535–544. doi: 10.4269/ajtmh.2000.62.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Muturi EJ. Effect of larval density and Sindbis virus infection on immune responses in Aedes aegypti. J Insect Physiol. 2013;59:604–610. doi: 10.1016/j.jinsphys.2013.03.010. (10.1016/j.jinsphys.2013.03.010) [DOI] [PubMed] [Google Scholar]
- King JG, Hillyer JF. Infection-induced interaction between the mosquito circulatory and immune systems. PLoS Pathog. 2012;8:e1003058. doi: 10.1371/journal.ppat.1003058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JG, Hillyer JF. Spatial and temporal in vivo analysis of circulating and sessile immune cells in mosquitoes: hemocyte mitosis following infection. BMC Biol. 2013;11:55. doi: 10.1186/1741-7007-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitthawee S, Edman JD, Upatham ES. Relationship between female Anopheles dirus (Diptera: Culicidae) body size and parity in a biting population. J Med Entomol. 1992;29:921–926. doi: 10.1093/jmedent/29.6.921. [DOI] [PubMed] [Google Scholar]
- Koella JC, Lyimo EO. Variability in the relationship between weight and wing length of Anopheles gambiae (Diptera: Culicidae) J Med Entomol. 1996;33:261–264. doi: 10.1093/jmedent/33.2.261. [DOI] [PubMed] [Google Scholar]
- Kraaijeveld AR, Godfray HCJ. Trade-off between parasitoid resistance and larval competitive ability in Drosophila melangogaster. Nature. 1997;389:278. doi: 10.1038/38483. [DOI] [PubMed] [Google Scholar]
- Landry SV, DeFoliart GR, Hogg DB. Adult body size and survivorship in a field population of Aedes triseriatus. J Am Mosq Control Assoc. 1988;4:121–128. [PubMed] [Google Scholar]
- League GP, Estévez-Lao TY, Yan Y, Garcia-Lopez VA, Hillyer JF. Anopheles gambiae larvae mount stronger immune responses against bacterial infection than adults: evidence of adaptive decoupling in mosquitoes. Parasit Vectors. 2017;10:367. doi: 10.1186/s13071-017-2302-6. (10.1186/s13071-017-2302-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- League GP, Hillyer JF. Functional integration of the circulatory, immune, and respiratory systems in mosquito larvae: pathogen killing in the hemocyte-rich tracheal tufts. BMC Biol. 2016;14:78. doi: 10.1186/s12915-016-0305-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefèvre T, Vantaux A, Dabire KR, Mouline K, Cohuet A. Non-genetic determinants of mosquito competence for malaria parasites. PLoS Pathog. 2013;9:e1003365. doi: 10.1371/journal.ppat.1003365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindh JM, Borg-Karlson AK, Faye I. Transstadial and horizontal transfer of bacteria within a colony of Anopheles gambiae (Diptera: Culicidae) and oviposition response to bacteria-containing water. Acta Trop. 2008;107:242–250. doi: 10.1016/j.actatropica.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Lyimo EO, Takken W, Koella JC. Effect of rearing temperature and larval density on larval survival, age at pupation and adult size of Anopheles gambiae. Entomol Exp Appl. 1992;63:265–271. [Google Scholar]
- MacDonald PL, Gardner RC. Type I error rate comparisons of post hoc procedures for IxJ chi-square tables. Educ Psychol Meas. 2000;60:735–754. [Google Scholar]
- Mahapatra N, Hazra RK, Rup S, Acharya AS, Dash AP. Bacillus sphaericus interferes with the development of Brugia malayi in Aedes aegypti. J Helminthol. 1999;73:279–280. [PubMed] [Google Scholar]
- McCann S, Day JF, Allan S, Lord CC. Age modifies the effect of body size on fecundity in Culex quinquefasciatus Say (Diptera: Culicidae) J Vector Ecol. 2009;34:174–181. doi: 10.1111/j.1948-7134.2009.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JH. Handbook of Biological Statistics. Sparky House Publishing; Baltimore, Maryland: 2014. [Google Scholar]
- Meredith JM, Hurd H, Lehane MJ, Eggleston P. The malaria vector mosquito Anopheles gambiae expresses a suite of larval-specific defensin genes. Insect Mol Biol. 2008;17:103–112. doi: 10.1111/j.1365-2583.2008.00786.x. (10.1111/j.1365-2583.2008.00786.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt RW, Dadd RH, Walker ED. Feeding behavior, natural food, and nutritional relationships of larval mosquitoes. Annu Rev Entomol. 1992;37:349–374. doi: 10.1146/annurev.en.37.010192.002025. [DOI] [PubMed] [Google Scholar]
- Moller-Jacobs LL, Murdock CC, Thomas MB. Capacity of mosquitoes to transmit malaria depends on larval environment. Parasit Vectors. 2014;7:593. doi: 10.1186/s13071-014-0593-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-García M, Vargas V, Ramírez-Bello I, Hernández-Martínez G, Lanz-Mendoza H. Bacterial exposure at the larval stage induced sexual immune dimorphism and priming in adult Aedes aegypti mosquitoes. PLoS One. 2015;10:e0133240. doi: 10.1371/journal.pone.0133240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourya DT, Yadav P, Mishra AC. Effect of temperature stress on immature stages and susceptibility of Aedes aegypti mosquitoes to chikungunya virus. Am J Trop Med Hyg. 2004;70:346–350. [PubMed] [Google Scholar]
- Murdock CC, Paaijmans KP, Bell AS, King JG, Hillyer JF, Read AF, Thomas MB. Complex effects of temperature on mosquito immune function. Proc Biol Sci. 2012;279:3357–3366. doi: 10.1098/rspb.2012.0638. (10.1098/rspb.2012.0638) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muturi EJ. Larval rearing temperature influences the effect of malathion on Aedes aegypti life history traits and immune responses. Chemosphere. 2013;92:1111–1116. doi: 10.1016/j.chemosphere.2013.01.055. (10.1016/j.chemosphere.2013.01.055) [DOI] [PubMed] [Google Scholar]
- Muturi EJ, Kim CH, Alto BW, Berenbaum MR, Schuler MA. Larval environmental stress alters Aedes aegypti competence for Sindbis virus. Trop Med Int Health. 2011;16:955–964. doi: 10.1111/j.1365-3156.2011.02796.x. [DOI] [PubMed] [Google Scholar]
- Nasci RS. The size of emerging and host-seeking Aedes aegypti and the relation of size to blood-feeding success in the field. J Am Mosq Control Assoc. 1986;2:61–62. [PubMed] [Google Scholar]
- Otieno-Ayayo ZN, Zaritsky A, Wirth MC, Manasherob R, Khasdan V, Cahan R, Ben-Dov E. Variations in the mosquito larvicidal activities of toxins from Bacillus thuringiensis ssp. israelensis. Environ Microbiol. 2008;10:2191–2199. doi: 10.1111/j.1462-2920.2008.01696.x. (10.1111/j.1462-2920.2008.01696.x) [DOI] [PubMed] [Google Scholar]
- Packer MJ, Corbet PS. Size variation and reproductive success of female Aedes punctor (Diptera: Culicidae) Ecol Entomol. 1989;14:297–309. [Google Scholar]
- Paily KP, Geetha I, Kumar BA, Balaraman K. Bacillus sphaericus in the adults of Culex quinquefasciatus mosquitoes emerged from treated larvae and its effect on development of the filarial parasite, Wuchereria bancrofti. Parasitol Res. 2012;110:2229–2235. doi: 10.1007/s00436-011-2754-0. [DOI] [PubMed] [Google Scholar]
- Paul RE, Nu VA, Krettli AU, Brey PT. Interspecific competition during transmission of two sympatric malaria parasite species to the mosquito vector. Proc Biol Sci. 2002;269:2551–2557. doi: 10.1098/rspb.2002.2171. (10.1098/rspb.2002.2171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen JJ, Chapman HC, Woodard DB. The bionomics of a mermithid nematode of larval mosquitoes in southwestern Louisiana. Mosq News. 1968;28:346–352. [Google Scholar]
- Pinto SB, Lombardo F, Koutsos AC, Waterhouse RM, McKay K, An C, Ramakrishnan C, Kafatos FC, Michel K. Discovery of Plasmodium modulators by genome-wide analysis of circulating hemocytes in Anopheles gambiae. Proc Natl Acad Sci USA. 2009;106:21270–21275. doi: 10.1073/pnas.0909463106. (10.1073/pnas.0909463106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumpuni CB, Demaio J, Kent M, Davis JR, Beier JC. Bacterial population dynamics in three anopheline species: the impact on Plasmodium sporogonic development. Am J Trop Med Hyg. 1996;54:214–218. doi: 10.4269/ajtmh.1996.54.214. [DOI] [PubMed] [Google Scholar]
- Richman AM, Bulet P, Hetru C, Barillas-Mury C, Hoffmann JA, Kafalos FC. Inducible immune factors of the vector mosquito Anopheles gambiae: biochemical purification of a defensin antibacterial peptide and molecular cloning of preprodefensin cDNA. Insect Mol Biol. 1996;5:203–210. doi: 10.1111/j.1365-2583.1996.tb00055.x. [DOI] [PubMed] [Google Scholar]
- Rothenbuhler WC, Thompson VC. Resistance to American foulbrood in honey bees. I. Differential survival of larvae of different genetic lines. J Econ Entomol. 1956;49:470–475. [Google Scholar]
- Roux O, Vantaux A, Roche B, Yameogo KB, Dabiré KR, Diabaté A, Simard F, Lefèvre T. Evidence for carry-over effects of predator exposure on pathogen transmission potential. Proc Biol Sci. 2015;282:20152430. doi: 10.1098/rspb.2015.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro LL, Murdock CC, Jacobs GR, Thomas RJ, Thomas MB. Larval food quantity affects the capacity of adult mosquitoes to transmit human malaria. Proc Biol Sci. 2016;283:20160298. doi: 10.1098/rspb.2016.0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SW, Kokoza V, Bian G, Cheon HM, Kim YJ, Raikhel AS. REL1, a homologue of Drosophila dorsal, regulates toll antifungal immune pathway in the female mosquito Aedes aegypti. J Biol Chem. 2005;280:16499–16507. doi: 10.1074/jbc.M500711200. (10.1074/jbc.M500711200) [DOI] [PubMed] [Google Scholar]
- Sigle LT, Hillyer JF. Mosquito hemocytes preferentially aggregate and phagocytose pathogens in the periostial regions of the heart that experience the most hemolymph flow. Dev Comp Immunol. 2016;55:90–101. doi: 10.1016/j.dci.2015.10.018. [DOI] [PubMed] [Google Scholar]
- Styer LM, Carey JR, Wang JL, Scott TW. Mosquitoes do senesce: departure from the paradigm of constant mortality. Am J Trop Med Hyg. 2007;76:111–117. [PMC free article] [PubMed] [Google Scholar]
- Sutter GR, Rothenbuhler WC, Raun ES. Resistance to American foulbrood in honey bees: VII. Growth of resistant and susceptible larvae. J Invertebr Pathol. 1968;12:25–28. [Google Scholar]
- Sweeney AW, Inman AO, Bland CE, Wright RG. The fine structure of Culicinomyces clavisporus invading mosquito larvae. J Invertebr Pathol. 1983;42:224–243. doi: 10.1016/0022-2011(83)90065-4. [DOI] [PubMed] [Google Scholar]
- Takken W, Klowden MJ, Chambers GM. Effect of body size on host seeking and blood meal utilization in Anopheles gambiae sensu stricto (Diptera: Culicidae): the disadvantage of being small. J Med Entomol. 1998;35:639–645. doi: 10.1093/jmedent/35.5.639. [DOI] [PubMed] [Google Scholar]
- Telang A, Qayum AA, Parker A, Sacchetta BR, Byrnes GR. Larval nutritional stress affects vector immune traits in adult yellow fever mosquito Aedes aegypti (Stegomyia aegypti) Med Vet Entomol. 2012;26:271–281. doi: 10.1111/j.1365-2915.2011.00993.x. (10.1111/j.1365-2915.2011.00993.x) [DOI] [PubMed] [Google Scholar]
- Tun-Lin W, Burkot TR, Kay BH. Effects of temperature and larval diet on development rates and survival of the dengue vector Aedes aegypti in north Queensland, Australia. Med Vet Entomol. 2000;14:31–37. doi: 10.1046/j.1365-2915.2000.00207.x. [DOI] [PubMed] [Google Scholar]
- Vantaux A, Ouattarra I, Lefèvre T, Dabiré KR. Effects of larvicidal and larval nutritional stresses on Anopheles gambiae development, survival and competence for Plasmodium falciparum. Parasit Vectors. 2016;9:226. doi: 10.1186/s13071-016-1514-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JR, Merritt RW. Influence of microclimate, food, and predation on Anopheles quadrimaculatus (Diptera: Culicidae) growth and development rates, survivorship, and adult size in a Michigan pond. Environ Entomol. 1999;28:233–239. [Google Scholar]
- Wang S, Dos-Santos ALA, Huang W, Liu KC, Oshaghi MA, Wei G, Agre P, Jacobs-Lorena M. Driving mosquito refractoriness to Plasmodium falciparum with engineered symbiotic bacteria. Science. 2017;357:1399–1402. doi: 10.1126/science.aan5478. (10.1126/science.aan5478) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn JO, Egerter DE, Anderson JR, Saunders GA. Density reduction in larval mosquito (Diptera: Culicidae) populations by interactions between a parasitic ciliate (Ciliophora: Tetrahymenidae) and an opportunistic fungal (Oömycetes: Pythiaceae) parasite. J Med Entomol. 1988;25:307–314. doi: 10.1093/jmedent/25.5.307. [DOI] [PubMed] [Google Scholar]
- Yadav P, Barde PV, Gokhale MD, Vipat V, Mishra AC, Pal JK, Mourya DT. Effect of temperature and insecticide stresses on Aedes aegypti larvae and their influence on the susceptibility of mosquitoes to dengue-2 virus. Southeast Asian J Trop Med Public Health. 2005;36:1139. [PubMed] [Google Scholar]
- Yassine H, Kamareddine L, Osta MA. The mosquito melanization response is implicated in defense against the entomopathogenic fungus Beauveria bassiana. PLoS Pathog. 2012;8:e1003029. doi: 10.1371/journal.ppat.1003029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.