Abstract
Tuberculosis is a significant problem globally for domestic animals as well as captive and free ranging wild life. Rapid point of care (POC) serology kits are well suited for the diagnosis of TB in wild animals. However, wild animals are invariably exposed to environmental non-pathogenic mycobacterium species with the development of cross reacting antibodies. In the present study, POC TB diagnosis kit was developed using a combination of pathogenic Mycobacteria specific recombinant antigens and purified protein derivatives of pathogenic and non-pathogenic Mycobacteria. To benchmark the TB antibody detection kit, particularly in respect to specificity which could not be determined in wildlife due to the lack of samples from confirmed uninfected animals, we first tested well-characterized sera from 100 M. bovis infected and 100 uninfected cattle. Then we investigated the kit’s performance using sera samples from wildlife, namely Sloth Bears (n = 74), Elephants (n = 9), Cervidae (n = 14), Felidae (n = 21), Cape buffalo (n = 2), Wild bear (n = 1) and Wild dog (n = 1).In cattle, a sensitivity of 81% and a specificity of 90% were obtained. The diagnostic sensitivity of the kit was 94% when the kit was tested using known TB positive sloth bear sera samples. 47.4% of the in-contact sloth bears turned seropositive using the rapid POC TB diagnostic kit. Seropositivity in other wild animals was 25% when the sera samples were tested using the kit. A point of care TB sero-diagnostic kit with the combination of proteins was developed and the kit was validated using the sera samples of wild animals.
Electronic supplementary material
The online version of this article (10.1007/s12088-017-0688-7) contains supplementary material, which is available to authorized users.
Keywords: Mycobacteria, POC, Rapid test, Tuberculosis, Wild TB diagnostic kit
Introduction
Tuberculosis (TB) is an emerging zoonotic disease of captive and free-ranging wildlife species with severe consequences in biodiversity and species conservation. Tuberculosis bacteria has a wide wild life host range which includes Elephants [1–3], Sloth Bears [4, 5], Arabian Oryx [6], White tailed Deer [7], Reindeer [8], European Badgers [7, 9, 10], Cervids [8], Rhinoceros [11], Lion [12], Badgers [13] and non-humane primates [2, 14]. So far 60 different wild mammal species were proven to be infected with TB [15, 16]. TB in wild animals is an emerging global concern as some of the wild species were proven to be maintenance and reservoir host for TB [17, 18]. Interspecies transmission of TB at wildlife-livestock-human interface poses public health, conservation and economic threats. Ante-mortern diagnosis of TB in wild life is difficult due to the subclinical nature of the infection and limited choice of diagnostic tests for wild life [19].
Gold standard TB diagnostic test in humans is Mycobacterial culture. However, difficulties in sample collection, long incubation period, cumbersome sample processing and sample transport methods render the test impractical for implementation in wild life. Cell mediated immune (CMI) response based diagnostic methods such as skin testing or interferon-gamma release assays are the commonly employed TB surveillance assays in domestic animals. However, CMI based assays require species specific reagents and skin testing is not practical in wild animals. In this context, sero-diagnosis of TB is an alternative in wild life with a scope for improvement in assay sensitivity and specificity [20]. Rapid pen-side sero-diagnostic kits are preferred for TB diagnosis in wild animals as they are simple, inexpensive, rapid and relatively non-invasive diagnostic methods. Moreover, the sero-diagnostic method does not require bio-containment facility, unlike the Mycobacteria detection by culture methods [21].
Multi-antigen print immunoassay (MAPIA) is being used as an appropriate tool for the identification of sero-dominant antigens of TB organism. The MAPIA methodology was adopted in various animal species such as Cattle [22, 23], Elephants [7], Reindeer [8] and European Badgers [24] for determining antigen recognition patterns of serum samples. Early Secretory Protein-6 (ESAT-6) and culture filtrate protein-10 (CFP-10) are the immuno-dominant antigenic candidates in Elephants [25]. However, the immuno-biology of TB may vary with different species and is poorly understood in wild life. Therefore, sensitivity of the assay can be compromised if only a limited number of antigens are used in the development of sero-diagnostic assays. At the same time wild animals are invariably exposed to environmental nonpathogenic Mycobacterial species [26] and various atypical mycobacterium species outside the Mycobacterium tuberculosis complex. Wild animals can mount an antibody response to environmental Mycobacterium resulting in false positivity in TB diagnosis [27].
The present study was aimed at developing a Rapid TB antibody detection kit using recombinant fusion protein of ESAT-6::CFP-10 along with purified protein derivatives (PPDs) of M. bovis for the improved diagnostic sensitivity and also with PPDs of M. avium for assessing the diagnostic specificity. To the best of our knowledge, this is the first report on the development and evaluation of a point of care kit using the above combination of antigens for the diagnosis of TB.
Materials and Methods
Immunochemicals and Reagents
Purified protein derivatives of Mycobacterium bovis (BoPPD) and Mycobacterium avium sub species avium (AvPPD) were procured from Prionics AG (Wagistrasse, Schlieren-Zurich, Switzerland). Wild animal sera samples were sourced from various zoos, rescue centers and temple elephants around India. Bovine positive and negative reference sera were from Animal and Plant Health Agency (APHA), UK.
Cloning and Expression of Recombinant ESAT-6::CFP-10 Fusion Protein
Coding sequence of ESAT-6 and CFP-10 of M. tuberculosis was synthesized as fusion gene construct (GenScript, USA). The gene construct was cloned into prokaryotic expression vector pET28a (Novagen) and the plasmid clone was used to transform chemically competent E. coli BL21 DE3 cells (Invitrogen). The protein expression was induced in the E. coli clone using 1 mM IPTG for overnight at 25 °C. The HIS6 tagged ESAT-6::CFP10 fusion protein was purified from the soluble fraction of the bacterial lysate using Ni–NTA agarose (immobilized metal affinity chromatography). Briefly, a 5 ml Ni–NTA agarose column was equilibrated with 10 column volumes of tris buffered saline (TBS) and the soluble fraction of the bacterial lysate was passed through the column and the column was washed with 20 column volumes of TBS containing 50 mM imidazole and the recombinant protein was eluted using 500 mM imidazole. The pooled protein fractions were dialyzed against PBS (pH 7.4) and purity of the protein was assessed using SDS-PAGE. The protein was identified in a western blot using anti-His antibody.
LPS Removal from the Purified TB Antigens
LPS from recombinant fusion protein ESAT-6::CFP-10 was removed using Triton X100 as per the procedure [22]. Briefly, Triton X −100 was added to the protein sample to a final concentration of 1% (v/v) and incubated at 4 °C for 1 h with continuous mixing. The sample was centrifuged at 7500 rpm for 10 min at 30 °C and the upper miceller phase was collected without disturbing the LPS rich middle and lower phase. Triton X −100 was added again to the upper phase to a final concentration of 0.5% (v/v) and the remaining steps were repeated as mentioned above. Then, the recombinant protein was analysed in SDS-PAGE and western blot.
Characterization of Recombinant Protein
The ESAT-6::CFP-10 fusion protein was characterized using reference culture positive and culture negative sera samples in immuno-blot. Identity of the protein was further established by LC–MS/MS analysis of the trypsin-treated protein sample.
Lateral Flow Assay (LFA) Design and Manufacture
LFA Design and Principle
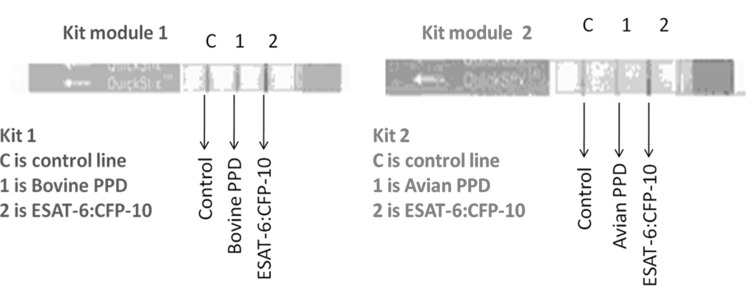
Immuno-chromatographic strip test was designed as a two module system using the combination of recombinant fusion protein, BoPPD and AvPPD. BoPPD was used in the test line 1 and the recombinant fusion protein was used in the test line 2of test module 1. Similarly, AvPPD and recombinant fusion protein was used in the test lines 1 and 2, respectively, of module 2. The conjugate pad of the assay kits contained gold conjugated protein G and streptavidin. The protein G could bind to antibodies from wide range of animals. The immune complex migrated along the membrane due to the capillary activity and the TB specific antibodies were trapped by the proteins in the test lines 1 and 2. Streptavidin gold was trapped by the biotin in the control line. Colour development in the test line 1 of the module 1 or test line 2 (either of the modules) indicated the presence of antibodies against pathogenic Mycobacteria whereas the development of colour in test line 1 of the module 2 indicated the presence of antibodies against environmental non-pathogenic Mycobacteria (Fig. 1).
Fig. 1.
LFA kit modules. In kit module 1, the test line 1 was printed with BoPPD and the test line 2 was printed with recombinant ESAT6::CFP10. Whereas in kit module 2, the test line 1 was printed with AvPPD and the test line 2 was printed with recombinant ESAT6::CFP10
Gold Conjugation of Protein G Antibodies and Streptavidin
Gold conjugation of the protein G and streptavidin was performed using gold chloride (Sigma Aldrich, USA) as per the method described [28]. Briefly, the proteins were mixed with 50-nm gold solution and pH of the solution was adjusted to 7.2 with 50 mM potassium carbonate (pH 9.6) to achieve a final concentration of 15 μg/ml. The unbound protein G from the gold conjugate solution was removed by washing with 15% bovine serum albumin solution. The gold coupled proteins were resuspended in 2% BSA in 10 mM sodium carbonate (pH 9.6) and stored in a refrigerator until further use. The Protein G-coupled gold particle was diluted in dye dilution buffer containing 1% casein and 100 mM sodium phosphate (pH 7). The diluted gold solution was spread onto conjugation pad presoaked in pretreatment buffer containing 1% NP-40, 20mMEDTA, 0.25% L-7600, 1% polyvinylpyrrolidone, 10 mM sodium phosphate and 0.1% sodium azide (pH 7.0); dried in a lyophilizer; stored in a low-humidity room until further use.
LFA Lamination Assembly and Manufacture
The assay membrane and pads were cut as 4.2 mm wide and 60 mm long composite strips using MDI strip cutter Model- M 70 to fit in a plastic assay device that provides the metrics for even flow of analytes and reagent buffer. The strips were laminated on a 300 mm × 60 mm plastic backing consists of a nitrocellulose detection strip in the middle, flanked at one end by a sample pad followed by conjugation pad and at the other end by an absorption pad. The completely laminated strips were cut into 4.2 mm-wide strips and were housed manually into plastic casing and packed in dehumidified room in an aluminum-plastic pouch with a desiccant to ensure the longevity of the product.
Validation of the Assay Kits
Diagnostic Sensitivity and Specificity of the Assay
17 samples from Sloth Bears with confirmed TB based on the postmortem lesions and acid fast staining which were tuberculosis specific were used for the study. Diagnostic sensitivity of the assay was determined using these known positive sera samples of Sloth Bears (N = 17).
Considering the difficulty in determining the TB infection status in wild animals using other tests such as skin test or IGRA, TB positive and negative bovine sera samples were used to estimate the diagnostic sensitivity and specificity of the assay. For this purpose, 200 cattle sera were obtained from APHA, UK (100 from skin-test positive cattle with culture confirmed M. bovis infection and 100 from TB-free animals). The cattle sera samples were designated negative on the basis of IFN γ release assay (IGRA) and skin test results.
Additional Sera Samples from Sloth Bears and Other Wild Animals
Sloth Bear sera samples (n = 74, including the 17 from animals with confirmed TB, referred to in the previous paragraph) were from Wildlife SOS, India and 48 sera samples from other wild animals such as Elephants (n = 9), Felidae (n = 21), Cervidae (n = 14), Cape Buffaloes(n = 2), Wild dog (n = 1) and Wild Bear (n = 1) were collected from captive animals housed in various zoological parks and temples in India. 17 of those Sloth Bears died later due to TB as described above. The remaining 57Sloth Bear sera samples were from in-contact animals with unknown disease status. The other wild animals were with unknown status for TB infection.
Screening of the Study Sera Samples and Interpretation
Sera samples and the LFA kits were brought to room temperature and a drop of sera sample (approximately 5 µl) was drawn using a disposable dropper. The sample was placed onto the sample pad (middle of sample well ‘S’) by holding the dropper vertically and avoiding air bubbles. Additionally, two drops of sample buffer (PBS) was added to the sample well to enable sample flow, through the membrane. The results were read in the results window after 10 min.
Sera samples with positive line in either of the test lines (T1 and T2) of test kit 1 or both the test lines in test kit 1 was considered as seropositive against tuberculosis. Positive line for the recombinant fusion protein was the indication of sero-positivity against pathogenic Mycobacteria. Development of positive line only for the AvPPD (Kit 2—T1) was interpreted as sero-reactivity against non-tuberculous mycobacteria. However, for the routine testing positive line only for T1 of kit 1 (BoPPD) warrants the use of kit 2.
Batch Quality Control for Assembled LFA
Every batch of the kit was checked for its performance using known positive and negative sera.
Batch Signal Strength
The signal strength of the device was considered sufficient when apparently visible lines developed with 5 µl of known positive sera.
Batch Reactivity
Aliquots of positive and negative reference sera samples of Sloth Bear (n = 4), Elephant sera samples (n = 4) and bovine sera samples (n = 4) were stored in −20° C and each batch of the LFA kits were tested with these reference sera samples.
Batch Specificity
Cross reactivity of every batch of LFA kits were tested with reference sera samples of other animal diseases such as Infectious Bovine Rhinotrachitis, Foot and Mouth Disease, Leptospirosis and PPR.
Ethics Statement
The study did not involve any experimental animal usage. The study was conducted using the sera samples available in the repository of Wildlife SOS, India. Testing of bovine sera samples were performed at APHA, UK using the samples available in their repository.
Results
Cloning and Expression of Recombinant Protein
The genetically fused coding sequences of ESAT6 and CFP 10 were cloned into prokaryotic expression vector and the ESAT6::CFP10 fusion protein was expressed as double His6 tagged protein and the positive clones were selected based on the identification of 28 kDa protein band in the immuno-blot using anti-His antibody. The recombinant protein was purified using Ni–NTA agarose column and the purified protein was verified by SDS-PAGE followed by staining with Coomassie brilliant blue. Protein estimation was done using BCA kit and the protein was aliquoted and stored at −80 °C. The protein yield was approximately 20 mg/lt of shake flask culture.
Characterization of Recombinant Protein
Each batch of the ESAT6::CFP10 recombinant protein was verified in SDS-PAGE for purity and the protein was also subjected to immune-blot using anti-His antibody, TB positive cattle sera and TB negative cattle sera. The immuno-blots using anti-His antibody and TB positive reference sera produced a protein band of ~ 28 kDa as shown in Supplementary Fig. I and II whereas the TB negative reference sera did not react with the recombinant protein. In the MALDI TOF analysis, the fusion protein had 100% amino acid identity with the available ESAT-6 and CFP-10 amino acid sequences of M. tuberculosis and M. bovis.
Kit assembly and Batch Release Criteria
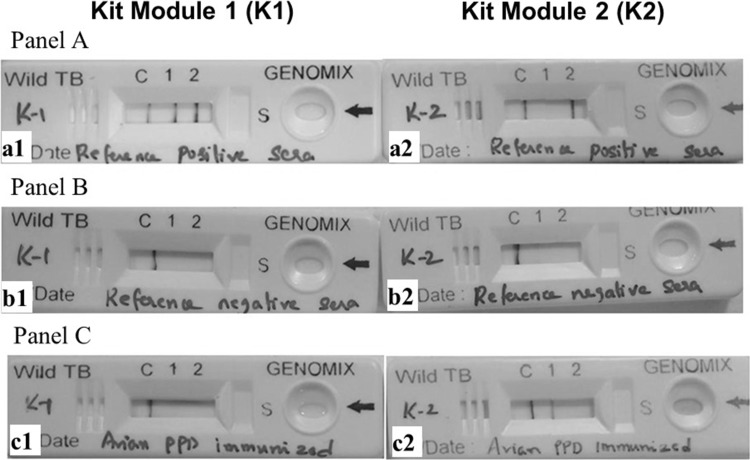
Line drawing and LFA kit assembly was carried out as mentioned in the methods section. Batch quality of the kits was assessed using reference positive and negative sera samples. The following parameters were assessed for batch clearance: 1. Reference positive sera developed lines with the recombinant fusion protein and BoPPD (Fig. 2 Panel A); 2. Reference culture negative sera developed only the control lines (Fig. 2 Panel B); 3. Rabbit hyper-immune sera against PPD of M. avium developed specific line with AvPPD (Fig. 2 Panel C). Additionally a visible control line should appear in all the tests. All the batches of the LFA kits were cleared based on the above mentioned batch release criteria.
Fig. 2.
Panel A: a1: Reference positive sera in Kit1 developed positive lines for Fusion protein and BoPPD. a2: Reference positive sera in Kit2 developed positive line only in Fusion protein. b1 and b2: Reference negative sera developed only control lines. c1 and c2: Avian PPD immunized sera developed only control lines in Kit1 and developed line for Avian PPD in Kit2
Interpretation of Result Using the LFA Kits
Reactivity of the kit was verified against sera samples from various wild life species as mentioned in the materials and methods sections. Antibody responses against the recombinant fusion protein, BoPPD and AvPPD were assessed using the kit. Reactivity of the sera samples against the recombinant fusion protein or BoPPD or both was considered positive test result in the LFA. Reactivity against AvPPD alone in the absence of any positive lines against ESAT6::CFP10 and BoPPD was classified as antibody response against non-pathogenic Mycobacteria.
Application of the Kit for Cattle Samples
To benchmark test performance, particularly in respect to specificity, which is very challenging to determine in wildlife species without samples from confirmed uninfected animals, we tested well characterized samples from GB cattle (100 skin test positive cattle with culture-confirmed bovine TB and 100 from TB-free cattle). The results of these experiments are presented in Tables 1 and 2. Applying the interpretation criteria as stipulated above, we estimated test sensitivity and specificity to be 81% (71.93–88.16% at 95% CI) and 90% (82.38–95.10% at 95% CI), respectively in cattle (Tables 1 and 2).
Table 1.
Interpretation of results and result summary of known positive cattle sera using the LFA kit
| S. no. | Fusion protein | BoPPD | AvPPD | Interpretation | Number of reactive sera/total TB positive cattle sera | Conclusion based on the LFA kit |
|---|---|---|---|---|---|---|
| 1 | Positive line | Positive line | Positive line | TB sero-positive | 15 out of 100 | TB seropositive (81 out of 100 samples) |
| 2 | Positive line | Positive line | Negative line | 15 out of 100 | ||
| 3 | Negative line | Positive line | Negative line | 41 out of 100 | ||
| 4 | Positive line | Negative line | Negative line | 0 out of 100 | ||
| 5 | Positive line | Negative line | Positive line | 2 out of 100 | ||
| 6 | Negative line | Positive line | Positive line | TB sero-positive/inconclusive | 8 out of 100 | |
| 7 | Negative line | Negative line | Positive line | TB sero-negative (positive for environmental mycobacteria) | 3 out of 100 | TB seronegative (19 out of 100 samples) |
| 8 | Negative line | Negative line | Negative line | TB sero-negative | 16 out of 100 |
The sera samples (n = 100) were from cattle which turned positive in skin test and IGRA. The estimated sensitivity of the LFA kit was 81% (71.93–88.16% at 95% CI) in cattle
Table 2.
Interpretation of results and result summary of known negative cattle sera using the LFA kit
| S. no | Fusion protein | BoPPD | AvPPD | Interpretation | Number of reactive sera/total TB negative cattle sera | Conclusion based on the LFA kit |
|---|---|---|---|---|---|---|
| 1 | Positive line | Positive line | Positive line | TB sero-positive | – | TB sero-positive (10 out of 100 samples) |
| 2 | Positive line | Positive line | Negative line | 1 out 100 | ||
| 3 | Negative line | Positive line | Negative line | 3 out 100 | ||
| 4 | Positive line | Negative line | Negative line | 3 out 100 | ||
| 5 | Positive line | Negative line | Positive line | 2 out 100 | ||
| 6 | Negative line | Positive line | Positive line | TB sero-positive/Inconclusive | 1 out 100 | |
| 7 | Negative line | Negative line | Positive line | TB sero-negative (positive for environmental mycobacteria) | 6 out of 100 | TB sero-negative (90 out 100 samples) |
| 8 | Negative line | Negative line | Negative line | TB sero-negative | 84 out of 100 |
The sera samples (n = 100) were from cattle which turned negative in skin test and IGRA. The estimated specificity of the LFA kit was 90% (82.38–95.10% at 95% CI) in cattle
Diagnostic Sensitivity in Wildlife Species
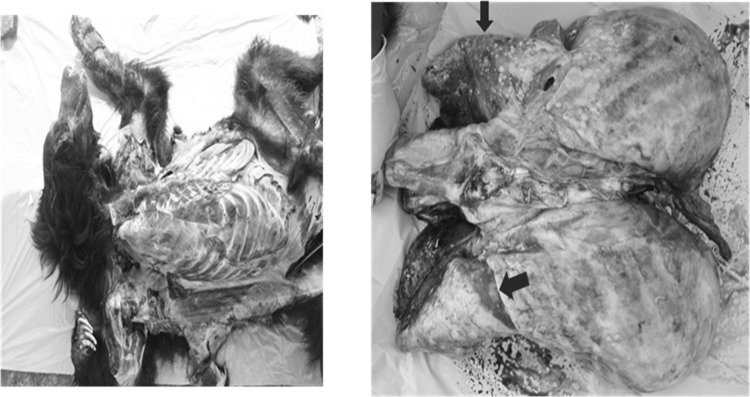
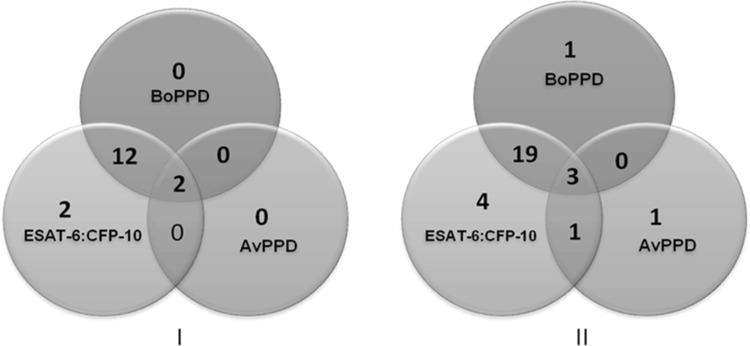
Given the constraints of working with wild life and the low number of samples typically available for any study, the present kit was evaluated using a total of 74 Sloth Bear sera samples and 48 sera samples from other wild life species. 17 out of the 74 Sloth Bears were confirmed for tuberculosis based on post mortem granuloma lesions and acid fast bacillias shown in Fig. 3. The post mortem granuloma samples were also confirmed in PCR for Mycobacteria. These 17 Sloth Bear sera were considered as known positive samples to estimate diagnostic sensitivity of the rapid wild TB kit. 16/17 of these sera samples gave positive responses using the LFA kit which indicated 94% diagnostic sensitivity (71.31–99.85% at 95% CI; Fig. 4 - I).
Fig. 3.
Postmortem confirmation of TB specific granuloma lesions in Sloth Bear lungs. Arrows indicate the pustular lesions
Fig. 4.
Venn diagram depicting the reactivity of Sloth Bear sera samples against BoPPD, ESAT-6::CFP-10 and AvPPD using the LFA kit. I: Sera from TB positive sloth bear (n = 17). The LFA kit had 94% diagnostic sensitivity (71.31–99.85% at 95% CI); II: sera from in-contact Sloth Bear (n = 57)
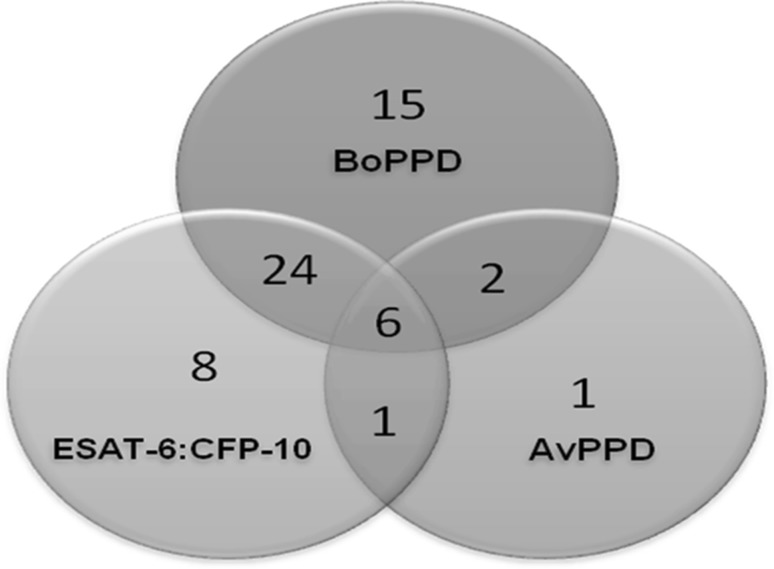
We next assessed the complete set of 122 wildlife samples including the 17 samples described in the previous paragraph. The Reactivity of these wild animal sera samples (n = 122) against recombinant fusion protein, BoPPD and AvPPD is provided in Fig. 5. Twenty seven out of the 57 live-in-contact Sloth Bear sera samples were positive using the LFA kit indicating 47.4% (Fig. 4 - II) positivity for TB. This experiment was conducted in a Bear rescue center where TB is prevalent. None of these in-contact sloth bears showed any obvious symptoms of TB. However 27 of these animals were diagnosed positive by the kit. These animals were followed subsequently and seven of these positive animals (7 out of 27) died so far. All these seven animals showed typical TB granulomas (subsequently confirmed in the lab test also) during post mortem examination indicating that the kit detected these asymptomatic TB positive animals.
Fig. 5.
Venn diagram depicting the reactivity towards BoPPD, ESAT-6::CFP-10, AvPPD using the LFA kit for all the wild animals sera samples (n = 122) of the present study
Sera samples from Elephants (n = 9), Felidae (n = 21), Cervidae (n = 14), Cape Buffaloes (n = 2), Wild Bear (n = 1) and Wild Dog (n = 1) were also tested and the seropositivity in these animals was 25%. Reactivity of individual antigens in the LFA kit for the sera samples from Sloth Bears (n = 74), Elephants (n = 9), Felidae (n = 21), Cervidae (n = 14), Cape Buffaloes (n = 2), Wild Bear (n = 1) and Wild Dog (n = 1) were presented in Table 3.
19.67% of all samples tested (n = 122) produced positive lines for the recombinant ESAT6::CFP10 fusion protein as well as BoPPD.
18.85% were reactive against one of these two proteins in the LFA.
4.91% of samples were reactive against all the three proteins in the test (ESAT6::CFP10, BoPPD and AvPPD).
One of the negative wild animal samples (negative by the kit of the present study) produced visible line for AvPPD alone (one out of 66 negative samples).
Table 3.
Interpretation of results and result summary of sera samples from wild animals (n = 122) using the LFA kit
| S. no. | Fusion protein | BoPPD | AvPPD | Interpretation | Number of reactive sera/total positive seraa (percentage within positive seraa) | Number of non-reactive sera/total negative seraa (percentage within negative seraa) | Overall percentage out of 122 samples (%) |
|---|---|---|---|---|---|---|---|
| 1 | Positive line | Positive line | Positive line | TB sero-positive | 6/56 (10.71%) | – | 4.91 |
| 2 | Positive line | Positive line | Negative line | 24/56 (42.85%) | – | 19.67 | |
| 3 | Negative line | Positive line | Negative line | 15/56 (26.78%) | – | 12.29 | |
| 4 | Positive line | Negative line | Negative line | 8/56 (14.28%) | – | 6.55 | |
| 5 | Positive line | Negative line | Positive line | 1/56 (1.78%) | – | 0.81 | |
| 6 | Negative line | Positive line | Positive line | TB sero-positive/Inconclusive | 2/56 (3.57%) | – | 1.63 |
| 7 | Negative line | Negative line | Positive line | TB sero-negtive (positive for environmental mycobacteria) | – | 1/66 (1.51%) | 0.81 |
| 8 | Negative line | Negative line | Negative line | TB sero-negtive | – | 65/66 (98.48) | 53.27 |
aThe sera samples were declared positive and negative based on the present LFA kit
Therefore, these animals were seropositive against pathogenic mycobacteria (Sr. No. 1 and 2 of the above combination), environmental mycobacteria (Sr. No. 4) and a combination of pathogenic and non-pathogenic mycobacteria (Sr. No. 3).
Apart from the above mentioned combination of results, other interesting combinations of sero-reactivity were also observed. Two of the positive samples were reactive against the BoPPD and AvPPD with no visible line for the fusion protein. Similarly, one of the positive samples produced visible lines for the fusion protein and AvPPD.
Based on the above results, it was decided that the Kit 1 (with BoPPD and recombinant protein) was to be used routinely for the sero-diagnosis of TB. The Kit 2 would be used whenever the kit 1 produced visible line against BoPPD alone which was 12.29% of our study samples.
Advantage of Using Combination of TB Specific Proteins Over Single Proteins
Using both the fusion protein and BoPPD, sensitivity of the kit had increased considerably. 41.07% of the positive sera (or 18.85% of the total wild animal sera samples tested) produced line for only one of the two antigens.
26.78% of the positive sera had produced line for PPD of M. bovis alone
14.28% of the positive sera had produced line for recombinant ESAT6::CFP10 fusion protein alone.
Specificity of the diagnostic method was assessed using AvPPD. Only two out of 124 wild animal sera generated positive lines both in BoPPD and AvPPD without any line development for the fusion protein. Cross reactivity with the environmental Mycobacteria is not ruled out in these animals. In such cases, the test may be repeated after some time using fresh sera and the diagnostic decision can be arrived based on the reactivity against ESAT6::CFP10. Additionally, the diagnostic decision can be based on the disease prevalence and local rules of the country wherein the requirement of ‘ruling out’ or ‘ruling in’ of TB in wild animals can determine the diagnostic decision.
Discussion
India is endemic for tuberculosis and the disease is prevalent in humans, domestic and wild animals. The populations of TB susceptible wild animals such as elephants, bears and boar are high in Asian and African countries. However, the data on TB epidemiology is limited in these countries with a severe effect on the management and control of TB [18, 29]. In developing countries both zoonosis and reverse zoonosis of TB are common; Mycobacterium species isolates from animals include M. tuberculosis and M. bovis in India and other developing countries [30–32]. M. tuberculosis also infects animals with similar virulence like M. bovis. The antigen complements of both organisms are almost completely identical. Thus a test detecting M. bovis will also detect M. tuberculosis, i.e. bovine PPD responses will also detect M. tuberculosis and human PPD will detect M. bovis infection. Indeed in early bovine TB control programs for cattle, human PPD was used successfully. As both M. tuberculosis and M. bovis can infect animals including wild life and cause severe pathogenesis a differential diagnosis between M. bovis and M. tuberculosis is not required nor is possible given the degree of antigen identity between these two pathogens. Wild-animals infected with either of the species are culled or segregated depending on the country’s law.
Aim of the current kit is to identify animals which are sero-positive for pathogenic TB (which includes M. tuberculosis and M. bovis) and help in the zoo or wild-life authorities to make appropriate decision or to study the TB sero-prevalence of tuberculosis in wild life.
Additionally, differentiating the pathogenic Mycobateria from environmental Mycobateria (such as M. avium sub sp. avium) is more important in the context of animal infections. Genome wide sequence search of M. avium subsp. paratuberculosis and M. avium subsp. avium revealed absence of any sequences with similarity to ESAT 6 and CFP 10 and the proteins were useful for the specific diagnosis of tuberculous Mycobacteria [33]. These proteins or their peptides had been used in IGRAs for the specific diagnosis of Tuberculosis [23]. The degree of specificity in general of these two antigens is high and they are also the mainstay of TB diagnosis both in cattle and humans [34–36]. Thus, many of the FDA and OIE approved kits such as QuantiFERON® and BOVIGAM® 2G uses ESAT6 and CFP10 peptides due to their proven ability to differentiate pathogenic TB (M. tuberculosis and M. bovis) from environmental Mycobacteria and BCG [37]. Mycobacterial species like M. marinum or M. klansasii express almost identical homologues of ESAT6 and CFP 10 [38, 39]. However, infection with these Mycobacteria is rare (both in humans and animals) and therefore, not considered to be major confounders. These two bacilli can also cause pathology and have zoonotic potential and removal of animals infected with these species will be advantageous. However, and most importantly, the homology (and therefore cross-reactivity) of M. bovis/M. tuberculosis ESAT6 and CFP10 compared to other more prevalent environmental Mycobacteria like M. avium sub sp. avium or M. avium sub sp. paratuberculosis is low.
Tuberculin skin testing and IGRA are the common TB diagnostic methods in domestic animals and humans. However, point of care serology assays are more practical for TB diagnosis in wild animals. To improve the sensitivity of detecting intracellular bacteria such as TB using sero-diagnostic kits, cocktails of antigens are required [40, 41]. At the same time, cross reactivity in antibody response due to the exposure to environmental Mycobacteria should also be taken care of while selecting the antigens for serology [42].
In the present study, BoPPD was used for increasing the sensitivity of the assay and AvPPD was used to mitigate against the cross reactivity from environmental bacteria. Tuberculosis antigen recognition pattern varies from species to species in serological assays. ESAT 6 or ESAT 6::CFP 10 fusion proteins were the earliest and most frequently recognized antigens in elephants infected with tuberculosis. The antibody response against the proteins was much earlier than the diagnosis by detecting the organism in trunk washes [2]. Though, MPB 83 and MPB 70 were the sero-dominant proteins in cattle and deer, ESAT 6 and CFP10 were also detected in majority of the tuberculosis infected animals [23, 34]. Moreover, the cross reacting antibodies from other Mycobacteria can also detect MPB 83 and MPB 70 resulting in a reduction in specificity of the assay. Therefore, a ESAT6::CFP10 fusion protein was used in the current diagnostic kit to enhance the specificity of the assay.
Performance of the point of care immuno-chromatographic strip test was assessed using Sloth Bear sera samples. 16 out of the 17 known positive sera samples turned positive in the LFA kit also. All the 16 samples were detected by the recombinant fusion protein and 27 of the in-contact Sloth Bear samples were detected by the protein. 6 out of 44 positive Sloth Bear sera samples reacted with AvPPD and all those six samples were positive for the fusion protein indicating the infection with pathogenic Mycobacteria. Therefore, the use of fusion protein aids in making the diagnostic decisions. To benchmark test performance against a larger number of animals with confirmed TB, the sensitivity and specificity of the kit was estimated using reference cattle sera (n = 100), which was lower (81%) than the estimate in sloth bears although the small sample size of our Sloth Bear assessment resulted in large confidence intervals overlapping those estimated with the cattle sera. The sero-dominant TB proteins in cattle are MPB 83 and MPB 70, and current kit did not contain these proteins as an individually expressed protein. These proteins were not included in the current kit to avoid detecting the cross reacting antibodies from environmental Mycobacteria.
A commonly encountered limitation of testing wildlife samples is the lack of samples from animals that are confirmed TB-free. Specificity estimates are therefore difficult to ascertain. To partially overcome this limitation, we have used cattle sera from TB-free animals. Thus, the specificity of the assay was determined using known negative cattle sera from TB-free herds in TB-non-endemic areas of GB. Further, freedom of infection of individual cattle was confirmed by negative tuberculin skin and interferon-gamma test results. Using these TB-free cattle sera (n = 100) the LFA kit showed 90% specificity. This is comparable to the data by Da Silva et al. [43] reported a sensitivity and specificity of 82 and 91% for their indirect ELISA using BCG as coating antigen. Similar kind of tuberculosis antibody detection kits were tested in wild animals earlier. DPP vet TB kit was tested in white tailed deer (sensitivity 65.1 and specificity 97.8%) [44] and South American Camelids (sensitivity 74 and specificity 98%) [45]. Elephant TB STAT-PAK Kit was tested in Asian and African elephants and the reported sensitivity and specificity was 100% and 95–100%, respectively [3]. Similarly, Prima TB STAT-PAK kit was tested in Non-Human primates and the reported sensitivity and specificity was 90 and 99% respectively [46].
The major difference between the other LFA kits and the current kit is the combination of proteins present in the kit. The other kits uses MPB83 protein and ESAT6/CFP10 fusion protein. The kit which is described in the current manuscript uses ESAT6::CFP10 fusion protein and native Mycobacterial proteins (BoPPD and AvPPD). Inclusion of BoPPD in the kit had increased the sensitivity by 41% in cattle (Table 1). However, BoPPD can compromise the specificity of the kit and therefore, AvPPD was included in the kit to detect the cross reactivity (if any) from the environmental Mycobacteria. One out of 100 negative cattle (Table 2; Sr No. 6) showed BoPPD positive line which may be due to the cross reactivity of environmental mycobacteria. Considerable percentage of positive samples (41.07%) were reactive against either BoPPD or fusion protein alone. Sero-reactivity against tuberculosis varies between the species and use of multiple proteins as in MAPIA increased the sensitivity of detection. Instead, BoPPD which is a mix of Mycobacterial proteins was used in the present kit for enhancing the sensitivity. 26.78% of positive samples were reactive against BoPPD alone. Use of kit II which contained AvPPD was recommended in those circumstances to rule out any cross reactivity with antibodies against environmental Mycobacteria. Two of the total 122 samples were reactive against BoPPD and AvPPD. These animals may be tested again to confirm the TB sero-positivity.
The kit was used in wide range of wild animals including Elephants (n = 9), Felidae (n = 21), Cervidae (n = 14), Cape Buffalo (n = 2), Wild Bear (n = 1) and Wild Dogs (n = 1). Use of protein A and Protein G conjugate (instead of anti-species antibody) enabled the kit to be used universally for any of the wild species.
The results indicated that 47.4% of in-contact Sloth Bear samples which were tested using the kit turned sero-positive for tuberculosis. 25% of samples from other wild species were also positive by the test. This indicates the high prevalence of TB in Indian wild animals. The situation is further complicated by the extensive wild animal and human (animal handlers of captive wild animals and tribes in the periphery of the forest areas) as well as wild and domestic animal interactions. In India the disease is highly prevalent in domestic animals and humans. These results emphases the need for systematic TB surveillance and control programs in India for wild animals.
Sero-diagnosis of tuberculosis in wild animals was permitted in countries like USA and UK (OIE 2014), since the conventional TB diagnosis by culture/IGRA/skin testing have serious practical difficulties in implementing them in wild animals [47, 48]. For an efficient surveillance and management of Wild TB, rapid, accurate, affordable and reliable TB diagnostic kits are needed. The available commercial sero-diagnostic kit was licensed for use in few countries and the kit is for fewer species. Moreover, the cost of the kit is too high for developing countries like India. The current kit addresses some of these problems.
In the present study, a rapid TB sero-diagnostic kit which can detect antibodies due to pathogenic as well as environmental Mycobacteria infection in wild animals was developed and validated using the sera samples of wild animals. Using this kit, sero-diagnosis of TB in wild animals is enabled.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Karnataka Forests Department and Wildlife SOS for helping us with Sloth Bear sera samples. TRPVB acknowledges the DBT-BBSRC FADH funding scheme code:BT/IN/indo-uk/FADH/52/VM/2013 project titled “Development of recombinant BGG vaccine and complimentary diagnosis for TB control in cattle”.
Compliance with ethical standards
Conflict of interest
The author(s) declared no potential conflicts of interests with respect to the research, authorship, and/or publication of this article.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s12088-017-0688-7) contains supplementary material, which is available to authorized users.
Contributor Information
Martin Vordermeier, Phone: 0208 0269880, Email: martin.vordermeier@apha.gsi.gov.uk.
B. Mohana Subramanian, Phone: +91 7708002534, Email: bhaskaran.mohana@gmail.com
References
- 1.Larsen RS, Salman MD, Mikota SK, Isaza R, Montali RJ, Triantis J (2000) Evaluation of a multiple-antigen enzyme-linked immunosorbent assay for detection of Mycobacterium tuberculosis infection in captive elephants. J Zoo Wildl Med 31:291–302. 10.1638/10427260(2000)031%5b0291:EOAMAE%5d2.0.CO;2 [DOI] [PubMed]
- 2.Lyashchenko KP, Greenwald R, Esfandiari J, Olsen JH, Ball R, Dumonceaux G, Dunker F, Buckley C, Richard M, Murray S, Payeur JB, Andersen P, Pollock JM, Mikota S, Miller M, Sofranko D, Waters WR. Tuberculosis in Elephants: antibody responses to defined antigens of Mycobacterium tuberculosis, potential for early diagnosis, and monitoring of treatment. Clin Vaccine Immunol. 2006;13:722–732. doi: 10.1128/CVI.00133-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenwald R, Lyashchenko O, Esfandiari J, Miller M, Mikota S, Olsen JH, Ball R, Dumonceaux G, Schmitt D, Moller T, Payeur JB, Harris B, Sofranko D, Waters WR, Lyashchenko KP. Highly accurate antibody assays for early and rapid detection of tuberculosis in African and Asian elephants. Clin Vaccine Immunol. 2009;16:605–612. doi: 10.1128/CVI.00038-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehrotra J, Mittal A, Rastogi AK, Jaiswal AK, Bhandari NK, Sinha S (1990) Antigenic definition of plasma membrane proteins of Bacillus Calmette-Guerin, predominant activation of human T cells by low-molecular-mass integral proteins. Scand J Immunol 50:411–419. PMID:10520182 [DOI] [PubMed]
- 5.Rishikesavan R, Sha AA, Chandranaik BM, Basavarajappa K, Giridhar P, Renukaprasad C. Study on prevalence of Tuberculosis in rescued captive Sloth Bears (Melursus ursinus) J Vet Pub Health. 2006;6:53–54. [Google Scholar]
- 6.Rietkerk FE, Griffin FT, Wood B, Mubarak SM, Delima EC, Badri OM, Lindsay NB, Williamson D. Treatment of Bovine Tuberculosis in an Arabian Oryx (Oryx leucoryx) J Zoo Wildl Med. 1993;24:523–527. [Google Scholar]
- 7.Lyashchenko KP, Greenwald R, Esfandiari J, Chambers MA, Vicente J, Gortazar C, Santos N, Correia-Neves M, Buddle BM, Jackson R, O’Brien DJ, Schmitt S, Palmer MV, Delahay RJ, Waters WR. Animal-side serologic assay for rapid detection of Mycobacterium bovis infection in multiple species of free-ranging. Wildl Vet Microbiol. 2008;132:283–292. doi: 10.1016/j.vetmic.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 8.Waters WR, Palmer MV, Bannantine JP, Greenwald R, Esfandiari J, Andersen P, McNair J, Pollock JM, Lyashchenko KP. Antibody responses in reindeer (Rangifer tarandus) infected with Mycobacterium bovis. Clin Diagn Lab Immunol. 2005;12:727–735. doi: 10.1128/CDLI.12.6.727-735.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clifton-Hadley RS, Wilesmith JW, Stuart FA. Mycobacterium bovis in the European badger (Meles meles) Epidemiological findings in tuberculosis badgers from a naturally infected population. Epidemiol Infect. 1993;111:9–19. doi: 10.1017/S0950268800056624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chambers MA, Pressling WA, Cheeseman CL, Clifton-Hadley RS, Hewinson RG. Value of existing serological tests for identifying badgers that shed Mycobacterium bovis. Vet Microbiol. 2002;86:183–189. doi: 10.1016/S0378-1135(02)00012-3. [DOI] [PubMed] [Google Scholar]
- 11.Mann PC, Bush M, Janssen DL, Frank ES, Montali RJ (1981) Clinicopathologic correlations of tuberculosis in large zoo mammals. J Am Vet Med Assoc 179:1123–1129. PMID:7035420 [PubMed]
- 12.Cleaveland S, Mlengeya T, Kazwala RR, Michel A, Kaare MT, Jones SL, Eblate E, Shirima GM, Packer C. Tuberculosis in Tanzanian wildlife. J Wildl Dis. 2005;41:446–453. doi: 10.7589/0090-3558-41.2.446. [DOI] [PubMed] [Google Scholar]
- 13.Donnelly CA, Woodroffe R, Cox DR, Bourne FJ, Cheeseman CL, Clifton-Hadley RS, Wei G, Gettinby G, Gilks P, Jenkins H, Johnston WT, Le Fevre AM, McInerney JP, Morrison WI. Positive and negative effects of widespread badger culling on tuberculosis in cattle. Nature. 2006;439:843–846. doi: 10.1038/nature04454. [DOI] [PubMed] [Google Scholar]
- 14.Frost PA (2006) Tuberculosis in nonhuman primates with an emphasis on Mycobacterium bovis. In: Thoen CO, Steele JH, Gilsdorf MJ (eds) Mycobacterium bovis infection in Animals and Humans, 2nd edn. Blackwell Publishing Ltd, Oxford
- 15.De Lisle GW, Mackintosh CG, Bengis RG (2001) Mycobacterium bovis in free-living and captive wildlife, including farmed deer. Revue Scientifique et Technique de 1’ Office International des Epizooties 20:86–111. PMID:11288522 [DOI] [PubMed]
- 16.Thoen CO, Lobue PA, Enarson DA, Kaneene JB, De kantor IN (2009) Tuberculosis a re-emerging disease in animals and humans. Vet Ital 45:135-181. PMID: 20391396 [PubMed]
- 17.Alvarez J, Perez AM, Bezos J, Casal C, Romero B, Rodriguez-Campos S, Saez-Llorente JL, Diaz R, Carpintero J, de Juan L, Domínguez L. Eradication of bovine tuberculosis at a herd-level in Madrid, Spain study of within-herd transmission dynamics over a 12 year period. BMC Vet. 2012;8:100. doi: 10.1186/1746-6148-8-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Garine WM, Caron A, Kock R, Tschopp R, Munyeme M, Hofmeyr M, Michel A. A review of bovine tuberculosis at the wildlife-livestock human interface in sub-saharan Africa. Epidemiol Infect. 2013;141:1342–1356. doi: 10.1017/S0950268813000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mancuso JD, Tribble D, Mazurek GH, Li Y, Olsen C, Aronson NE, Geiter L, Goodwin D, Keep LW. Impact of targeted testing for latent tuberculosis infection using commercially available diagnostics. Clin Infect Dis. 2011;53:234–244. doi: 10.1093/cid/cir321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stevens JB, Thoen CO, Rohonczy EB, Tessaro S, Kelly HA, Duncan JR (1998) The immunological response of llamas (Lama glama) following experimental infection with Mycobacterium bovis. Can J Vet Res 62:102–109.PMCID:PMC1189455 [PMC free article] [PubMed]
- 21.Parsons LM, Somoskövi A, Gutierrez C, Lee E, Paramasivan CN, Abimiku A, Spector S, Roscigno G, Nkengasong J. Laboratory diagnosis of tuberculosis in resource-poor countries challenges and opportunities. Clin Microbiol Rev. 2011;24:314–350. doi: 10.1128/CMR.00059-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veerasami M, Reddy DS, Sugumar P, Naidu SS, Bahekar V, Mahesh kumar EK, Mukherjee F, Rana SK, Chandran D, Das D, Srinivasan VA. Multi-antigen print immunoassay for sero-epidemiological surveillance of bovine tuberculosis on Indian cattle farms. Vet Ital. 2012;48:253–267. [PubMed] [Google Scholar]
- 23.Waters WR, Palmer MV, Thacker TC, Bannantine JP, Vordermeier HM, Hewinson RG, Greenwald R, Esfandiari J, McNair J, Pollock JM, Andersen P, Lyashchenko KP. Early antibody responses to experimental Mycobacterium bovis infection of cattle. Clin Vaccine Immunol. 2006;13:648–654. doi: 10.1128/CVI.00061-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lesellier S, Corner L, Costello E, Sleeman P, Lyashchenko K, Greenwald R, Esfandiari J, Singh M, Hewinson RG, Chambers M, Gormley E. Antigen specific immunological responses of badgers (Meles meles) experimentally infected with Mycobacterium bovis. Vet Immunol Immunopathol. 2008;122:35–45. doi: 10.1016/j.vetimm.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Duncan AE, Lyashchenko K, Greenwald R, Miller M, Ball R. Application of Elephant TB STAT-PAK assay and MAPIA (multi-antigen print immunoassay) for detection of tuberculosis and monitoring of treatment in black rhinoceros (Diceros bicornis) J Zoo Wildl Med. 2009;40:781–785. doi: 10.1638/2009-0044.1. [DOI] [PubMed] [Google Scholar]
- 26.Vosloo W, Tippoo P, Hughes JE, Harriman N, Emms M, Beatty DW, Zappe H, Steyn LM. Characterization of a lipoprotein in Mycobacterium bovis (BCG) with sequence similarity to the secreted protein MPB70. Gene. 1997;188:123–128. doi: 10.1016/S0378-1119(96)00806-2. [DOI] [PubMed] [Google Scholar]
- 27.Corner LA, Barrett RH, Lepper AWD, Lewis V, Pearson CW. A survey of mycobacteriosis of feral pigs in the Northern Territory. Aust Vet J. 1981;57:537–542. doi: 10.1111/j.1751-0813.1981.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 28.Beesley JE. Immuno labelling and electron microscopy in cyto chemistry. Curr Opin Immunol. 2015;2:927–931. doi: 10.1016/0952-7915(89)90180-5. [DOI] [PubMed] [Google Scholar]
- 29.Rhodes SG, Gunn-Mooore D, Boschiroli ML, Schiller I, Esfandiari JR, Greenwald R, Lyaschenko KP. Comparative study of IFN-gamma and antibody tests for feline tuberculosis. Vet Immunol Immunopathol. 2011;144:129–134. doi: 10.1016/j.vetimm.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 30.Ameni G, Tadesse K, Hailu E, Deresse Y, Medhin G, Aseffa A, Hewinson G, Vordermeier M, Berg S. Transmission of M. tuberculosis between Farmers and Cattle in Central Ethiopia. PLoS One. 2013;8:e76891. doi: 10.1371/journal.pone.0076891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y, Chao Y, Deng Q, Liu T, Xiang J, Chen J, Zhou J, Zhan Z, Kuang Y, Cai H, Chen H, Guo A. Potential challenges to the stop plan for humans in china; cattle maintain M. bovis and M. tuberculosis. Tuberculosis. 2009;89:95–100. doi: 10.1016/j.tube.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Bhanu Rekha V, Gunaseelan L, Ganesh P, Reza N, Sukumar B. Molecular detection of Mycobacterium tuberculosis from bovine milk samples. J Adv Vet Anim Res. 2015;2:80–83. doi: 10.5455/javar.2015.b44. [DOI] [Google Scholar]
- 33.Waters WR, Palmer MV, Bannantine JP, Whipple DL, Greenwald R, Esfandiari J, Andersen P, McNair J, Pollock JM, Lyashchenko KP. Antigen recognition by serum antibodies in white-tailed deer (Odocoileus virginianus) experimentally infected with Mycobacterium bovis. Clin Diagn Lab Immunol. 2004;11:849–855. doi: 10.1128/CDLI.11.5.849-855.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Renshaw PS, Lightbody KL, Veverka V, Muskett FW, Kelly G, Frenkiel TA, Gordon SV, Hewinson RG, Burke B, Norman J, Williamson RA, Carr MD. Structure and function of the complex formed by the tuberculosis virulence factors CFP-10 and ESAT-6. EMBO. 2005;24:2491–2498. doi: 10.1038/sj.emboj.7600732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guinn KM, Hickey MJ, Mathur SK, Zakel KL, Grotzke JE, Lewinsohn DM, Smith S, Sherman DR. Individual RD1-region genes are required for export of ESAT-6/CFP-10 and for virulence of Mycobacterium tuberculosis. Mol Microbiol. 2004;51:359–370. doi: 10.1046/j.1365-2958.2003.03844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harboe M, Oettinger T, Wiker HG, Rosenkrands I, Andersen P (1996) Evidence for occurrence of the ESAT‐6 protein in Mycobacterium tuberculosis and virulent Mycobacterium bovis and for its absence in Mycobacterium bovis BCG. Infect Immunol 64:16–22. PMCID:PMC173721 [DOI] [PMC free article] [PubMed]
- 37.Vordermeier HM, Gareth J, Jones Bryce M, Buddle R, Hewinson G, Villarreal-Ramos Bernardo. Bovine tuberculosis in cattle: vaccines, DIVA tests, and host biomarker discovery. Annu Rev Anim Biosci. 2016;4:87–109. doi: 10.1146/annurev-animal-021815-111311. [DOI] [PubMed] [Google Scholar]
- 38.Arend SM, de Haas P, Leyten E, Rosenkrands I, Rigouts L, Andersen P, Mijs W, van Dissel JT, van Soolingen D. ESAT-6 and CFP-10 in clinical versus environmental isolates of Mycobacterium kansasii. J infect Dis. 2005;191:1301–1310. doi: 10.1086/428950. [DOI] [PubMed] [Google Scholar]
- 39.Arend SM, Van Meijgaarden KE, De Boer K, De Palou EC, Van Soolingen D, Ottenhoff TH, Van Dissel JT. Tuberculin skin testing and in vitro T cell responses to ESAT-6 and culture filtrate protein 10 after infection with Mycobacterium marinum or M. Kansasii. J Infect Dis. 2012;186:1797–1807. doi: 10.1086/345760. [DOI] [PubMed] [Google Scholar]
- 40.Lyashchenko KP, Singh M, Colangeli R, Gennaro ML. A multi-antigen print immunoassay for the development of serological diagnosis of infectious diseases. J Immunol Methods. 2000;242:91–100. doi: 10.1016/S0022-1759(00)00241-6. [DOI] [PubMed] [Google Scholar]
- 41.Sharma AK, Dhasmana N, Dubey N, Kumar N, Gangwal A, Gupta M, Singh Y. Bacterial virulence factors: secreted for survival. Indian J Microbiol. 2017;57:1–10. doi: 10.1007/s12088-016-0625-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aurtenetxe O, Barral M, Vicente J, de la Fuente J, Gortazar C, Juste RA. Development and validation of an enzyme-linked immunosorbent assay for antibodies against Mycobacterium bovis in European wild boar. BMC Vet Res. 2008;4:43. doi: 10.1186/1746-6148-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Da Silva EB, Silva BD, Leon JR, Kipnis A, Santos IK, Junqueira-Kipnis AP. Using BCG and Ag85 as antigens in an indirect ELISA for the diagnosis of bovine tuberculosis. Vet J. 2011;187:276–278. doi: 10.1016/j.tvjl.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 44.Lyashchenko KP, Greenwald R, Esfandiari J, O’Brien DJ, Schmitt SM, Palmer MV, Waters WR. Rapid detection of serum antibody by dual-path platform VetTB assay in white-tailed deer infected with Mycobacterium bovis. Clin Vaccine Immunol. 2013;20:907–911. doi: 10.1128/CVI.00120-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lyashchenko KP, Greenwald R, Esfandiari J, Rhodes S, Dean G, de la Rua-Domenech R, Meylan M, Vordermeier HM, Zanolari P. Diagnostic value of animal-side antibody assays for rapid detection of Mycobacterium bovis or Mycobacterium microti infection in South American camelids. Clin Vaccine Immunol. 2011;18:2143–2147. doi: 10.1128/CVI.05386-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lyashchenko KP, Greenwald R, Esfandiari J, Greenwald D, Nacy CA, Gibson S, Didier PJ, Washington M, Szczerba P, Motzel S, Handt L, Pollock JM, McNair J, Andersen P, Langermans JA, Verreck F, Ervin S, Ervin F, McCombs C. PrimaTB STAT-PAK assay, a novel, rapid lateral-flow test for tuberculosis in nonhuman primates. Clin Vaccine Immunol. 2007;14:1158–1164. doi: 10.1128/CVI.00230-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cousins DV, Florisson N (2005) A review of tests available for use in the diagnosis of tuberculosis in non-bovine species. Rev Sci Technol 24:1039–1059. PMID:16642773 [PubMed]
- 48.Chambers MA, Waterhouse S, Lyashchenko K, Delahay R, Sayers R, Hewinson RG. Performance of TB immunodiagnostic tests in Eurasian badgers (Meles meles) of different ages and the influence of duration of infection on serological sensitivity. BMC Vet Res. 2009;5:42. doi: 10.1186/1746-6148-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.