Abstract
Acute hepatopancreatic necrosis disease (AHPND) caused by Vibrio parahaemolyticus has been one of the most problematic diseases in marine shrimp aquaculture throughout Southeast Asia and Latin America. To evaluate the effectiveness of a bacteriophage (phage) treatment for AHPND, a series of bioassays were carried out in a marine shrimp (Penaeus vannamei) model using an AHPND-V. parahaemolyticus strain that is highly pathogenic to shrimp. We monitored the mortality and histopathological changes during phage treatment. Shrimps treated with phage prophylaxis and phage therapy displayed significant protection from AHPND and survived a lethal bacterial challenge.
Keywords: Acute hepatopancreatic necrosis disease (AHPND), Vibrio parahaemolyticus, Phage prophylaxis, Phage therapy
Introduction
Vibriosis is a major shrimp disease caused by Vibrio species and some Vibrio parahaemolyticus strains cause acute hepatopancreatic necrosis disease (AHPND), resulting in up to 100% of mortality in marine shrimp aquaculture [1–4]. It has become a serious issue in shrimp aquaculture around the world, especially in China, Thailand, Vietnam, Malaysia, and Mexico [5–7]. Although antibiotics have been commonly used for prophylaxis or therapy for AHPND, the increasing incidence of antibiotic resistance has limited the effectiveness of antibiotics [8, 9].
Phages have been proposed as a control for infectious diseases in humans and animals [10]. The use of a phage as a therapeutic agent (phage therapy) is advantageous as it is natural and relatively inexpensive, without serious or irreversible side effects reported to date [10, 11]. There have been only few attempts to use phages to control bacterial infections in shrimps [12]. There is no feasible remedy reported for AHPND, and we previously noted that the development of effective treatment methods is needed [13].
In our previous study, phage pVp-1 induced effective bacteriolysis of AHPND-V. parahaemolyticus strains from diverse regions [13]. Our aim was to determine whether this phage could be suitable for prophylactic or/and therapeutic use against AHPND-V. parahaemolyticus in the Penaeus vannamei model.
Materials and Methods
Experiment Preparations
Virulent Siphoviridae phage pVp-1, infecting AHPND-V. parahaemolyticus strains, was used [13–15]. In our previous study [13], pVp-1 infected 90.9% (20 of 22 strains) of the AHPND-V. parahaemolyticus strains tested. Vibrio parahaemolyticus 13-028/A3 is known to be highly pathogenic, causing 100% mortality of shrimps within 24 h post-infection, and was used for the AHPND challenge [13].
SPF (Specific Pathogen Free) juvenile marine shrimps (P. vannamei, n = 96, average weight = 1.02 g) were obtained from the West Campus SPF facility, University of Arizona, Tucson, USA. Shrimps were divided into 24 groups and kept in 3 l glass tanks at appropriate conditions (water temperature 25 °C; salinity 25‰) for at least 72 h before experiments.
Phage Treatment of Infected Shrimps
In the first bioassay, all shrimps were challenged by bath immersion with V. parahaemolyticus 13-028/A3 (5.0 × 105 CFU/ml) for 24 h, except Tanks 1 and 2. Tank 1 was designated as a negative control without bacterial challenge or phage treatment; Tank 2 was designated as a phage control with phage treatment by bath immersion (1.5 × 106 PFU/ml) and feeding (1.5 × 108 PFU/shrimp) using pellets (5% of body weight) that had been impregnated with the phage suspension, but not bacterially challenged. Tank 3 was designated as a positive control with a bacterial challenge but not phage treated. Two treatment groups (Tanks 4 and 5) were fed with pellets containing the phage 1 h after the bacterial challenge. The other two treatment groups (Tanks 6 and 7) were treated with bath immersion using the phage suspension 1 h after the bacterial challenge. In the second bioassay, all conditions were the same as the first bioassay, except that the phage treatment was applied at different time points (24, 6, and 1 h prior to the bacterial challenge). In the third bioassay, the experiment was performed as for the first and second bioassays, except that the treatment groups were fed with pellets that had been impregnated with the phage at various time points (24, 6, and 1 h prior to the bacterial challenge, and 1 h after the bacterial challenge).
In all bioassays, each group was monitored for symptoms of infection and cumulative mortality was recorded daily for 5 days after the bacterial challenge. Each experiment was performed twice, on separate occasions. All animal experiments were performed in accordance with guidelines of the Animal Ethical Committee of University of Arizona.
Histopathology
A separate experiment was performed as the third bioassay for histopathology by a standardized method [16]. Histopathology was examined for the severity of infection.
Results and Discussion
In our previous in vitro study, the phage pVp-1 demonstrated substantial bacteriolytic activity against three representative AHPND-V. parahaemolyticus strains (13-028/A3, 13-511/A1, and 13-306D/4) causing 100% mortality within 24 h post-infection [13]. We hypothesized that phage therapy can be useful against AHPND in shrimp. We used a marine shrimp (P. vannamei) model to evaluate the therapeutic effect of pVp-1 against AHPND-V. parahaemolyticus. Among three highly pathogenic AHPND-V. parahaemolyticus strains, 13-028/A3 was selected as a bacterial challenge strain since it induced the highest efficiency of plating value [13].
The protective effects of phage administration against experimental AHPND-V. parahaemolyticus infection are shown in Table 1. In the first bioassay, no promising result was achieved: all treatment groups showed 100% mortality without retardation of disease progression compared to the positive control group that was bacterially challenged but not treated with the phage (Table 1). We suggest that this was due to the extremely rapid progression of AHPND, so that the time delay of the phage treatment (attachment of phage to bacteria) resulted in decreased efficacy of protection.
Table 1.
Phage treatment of shrimps infected by AHPND-V. parahaemolyticus
| Bioassay | Experimental group (type*/method†) | Phage treatment (h‡) | No. of dead shrimp/no. tested | Morality (%) | |
|---|---|---|---|---|---|
| I | Tank 1 | (Negative control) | No | 0/4 | 0 |
| Tank 2 | (Phage control) | Yes | 0/4 | 0 | |
| Tank 3 | (Positive control) | No | 4/4 | 100 | |
| Tank 4 | (Therapy/feeding) | Yes (+ 1) | 4/4 | 100 | |
| Tank 5 | Yes (+ 1) | 4/4 | 100 | ||
| Tank 6 | (Therapy/immersion) | Yes (+ 1) | 4/4 | 100 | |
| Tank 7 | Yes (+ 1) | 4/4 | 100 | ||
| II | Tank 1 | (Negative control) | No | 0/4 | 0 |
| Tank 2 | (Phage control) | Yes | 0/4 | 0 | |
| Tank 3 | (Positive control) | No | 4/4 | 100 | |
| Tank 4 | (Prophylaxis/feeding) | Yes (− 24, − 6, − 1 h) | 2/4 | 50 | |
| Tank 5 | Yes (− 24, − 6, − 1 h) | 2/4 | 50 | ||
| Tank 6 | (Prophylaxis/immersion) | Yes (− 24, − 6, − 1 h) | 2/4 | 50 | |
| Tank 7 | Yes (− 24, − 6, − 1 h) | 1/4 | 25 | ||
| III | Tank 1 | (Negative control) | No | 0/4 | 0 |
| Tank 2 | (Phage control) | Yes | 0/4 | 0 | |
| Tank 3 | (Positive control) | No | 4/4 | 100 | |
| Tank 4 | (Prophylaxis + therapy/feeding) | Yes (− 24, − 6, − 1, + 1 h) | 0/4 | 0 | |
| Tank 5 | Yes (− 24, − 6, − 1, + 1 h) | 0/4 | 0 | ||
*Experimental group, type: negative control, neither bacterial challenged nor phage-treated; phage control, not challenged but treated; positive control, challenged but not treated; therapy, challenged and treated by therapeutic application; prophylaxis, treated by prophylactic application and challenged
†Experimental group, method: feeding, oral administration of phage-impregnated feed; immersion, bath immersion administration using phage suspension
‡Phage treatment, h: +, phage administration after bacterial challenge (therapeutic application); −, phage administration prior to bacterial challenge (prophylactic application)
The second bioassay was modified to evaluate the prophylactic effect of the phage. In this experiment, shrimps treated with the phage showed lower mortality rates than those in the positive control group. Cumulative mortality rates were 50% following phage treatment by phage-impregnated feeding, and 25 and 50% following phage treatment by bath immersion (Table 1).
Although the two bioassays indicated that prophylactic application of pVp-1 is more effective than the therapeutic application, we added another bioassay to examine if the combination of these procedures can reach a higher level of protection. In the third bioassay, the protective effect of the phage was evaluated after prophylactic and therapeutic administration. In the treatment groups, the phage was administered by only one method, feeding with phage-impregnated pellets. In most aquaculture environments, the oral method is considered a cost-effective and realistic method of large scale administration, which is suitable for shrimp farms.
Shrimps in the positive control groups showed 100% cumulative mortalities within 48 h post-bacterial challenge. In addition, administration of the phage by either feeding or bath immersion (phage control) did not affect the physical condition or survival of experimental shrimps during the week of observation.
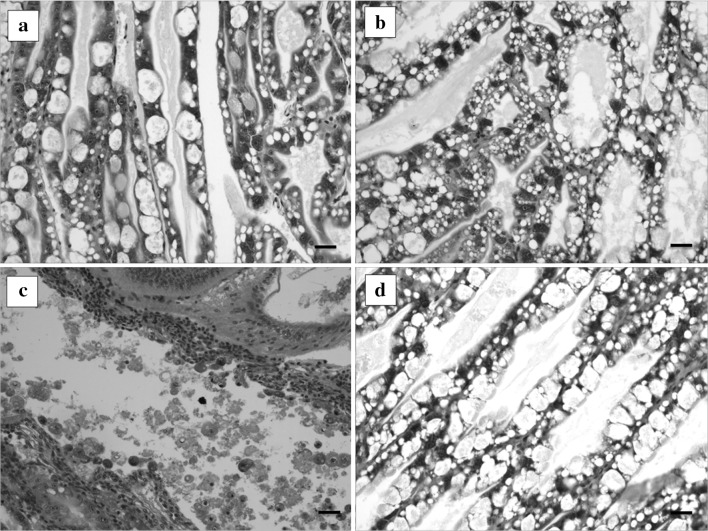
Histological analysis of the hepatopancreas from shrimps was carried out to diagnose AHPND. In the positive control group, histopathological features of shrimps showed typical AHPND signs; the acute sloughing of hepatopancreatic tubular epithelial cells is evident (Fig. 1c). However, histopathological examination of the hepatopancreas of phage-treated shrimps revealed a significant recovery of AHPND lesions following prophylactic and therapeutic treatments (Fig. 1d). Also, histopathological features of shrimps in negative control (Fig. 1a) and phage control (Fig. 1b) showed the normal appearance of the hepatopancreas.
Fig. 1.
Histopathological features of the hepatopancreas of shrimps at 48 h of phage treatment, the shrimp was challenged by AHPND-V. parahaemolyticus 13-028/A3 strain and treated with the phage pVp-1. Negative control (a) and phage control (b) showed the normal appearance of the hepatopancreas. Positive control (c), challenged but not treated, showed the acute sloughing of hepatopancreatic tubular epithelial cells. The phage-treated shrimp d demonstrated the protected morphology of the hepatopancreas. Scale bars 30 μm
As Republic of Korea is officially AHPND-free country, AHPND-related study is not permitted by law; the current study was performed in OIE reference laboratory for crustacean diseases in United States. Although the number of animals used in vivo experiment was limited, significant result of phage treatment was observed from AHPND infection. In our previous studies, the administration of pVp-1 did not cause any harm to mice or oysters during the observation period [14, 15]. In the present study, a considerable degree of mortality was observed in the phage-treated shrimps, as the progression of AHPND is extremely rapid. The timing of phage addition in relation to pathogen development is a crucial factor in the case of vibriosis [15], which may be resolved by more frequent administration of the phage.
Acknowledgements
We would like to thank all the staff at the School of Animal and Comparative Biomedical Sciences, University of Arizona, Tucson, USA, for their assistance during this study. This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2014R1A2A1A11050093 and 2017R1C1B2004616).
Footnotes
Jin Woo Jun and Jee Eun Han contributed equally to this work.
References
- 1.Burge CA, Mark Eakin C, Friedman CS, Froelich B, Hershberger PK, Hofmann EE, Petes LE, Prager KC, Weil E, Willis BL, Ford SE, Harvell CD. Climate change influences on marine infectious diseases: implications for management and society. Ann Rev Mar Sci. 2014;6:249–277. doi: 10.1146/annurev-marine-010213-135029. [DOI] [PubMed] [Google Scholar]
- 2.Lightner DV, Redman RM, Pantoja CR, Noble BL, Tran LH. Early mortality syndrome affects shrimp in Asia. Glob Aquac Advocate. 2012;15:40. [Google Scholar]
- 3.Flegel TW. Historic emergence, impact and current status of shrimp pathogens in Asia. J Invertebr Pathol. 2012;110:166–173. doi: 10.1016/j.jip.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Leaño EM, Mohan CV. Early mortality syndrome threatens Asia’s shrimp farms. Glob Aquac Advocate. 2012;2012:38–39. [Google Scholar]
- 5.NACA . Asia Pacific emergency regional consultation on the emerging shrimp disease: early mortality syndrome (EMS)/acute hepatopancreatic necrosis syndrome (AHPNS) Bangkok: Network of Aquaculture Centres in Asia-Pacific Bangkok; 2012. [Google Scholar]
- 6.Soto-Rodriguez SA, Gomez-Gill B, Lozano-Olvera R, Betancourt-Lozano M, Morales-Covarrubias MS. Field and experimental evidence of Vibrio parahaemolyticus as the causative agent of acute hepatopancreatic necrosis disease of cultured shrimp (Litopenaeus vannamei) in Northwestern Mexico. Appl Environ Microbiol. 2015;81:1689–1699. doi: 10.1128/AEM.03610-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang BC, Liu F, Bian HH, Liu J, Pan LQ, Huang J. Isolation, identification, and pathogenicity analysis of a Vibrio parahaemolyticus strain from Litopenaeus vannamei. Prog Fish Sci. 2012;33:56–62. [Google Scholar]
- 8.Han JE, Mohney LL, Tang KFJ, Pantoja CR, Lightner DV. Plasmid mediated tetracycline resistance of Vibrio parahaemolyticus associated with acute hepatopancreatic necrosis disease (AHPND) in shrimps. Aquac Rep. 2015;2:17–21. doi: 10.1016/j.aqrep.2015.04.003. [DOI] [Google Scholar]
- 9.Neela FA, Nonaka L, Suzuki S. The diversity of multi-drug resistance profiles in tetracycline-resistant Vibrio species isolated from coastal sediments and seawater. J Microbiol. 2007;45:64–68. [PubMed] [Google Scholar]
- 10.Sulakvelidze A, Kutter E. Bacteriophage therapy in humans. In: Kutter E, Sulakvelidze A, editors. Bacteriophages: biology and application. Boca Raton: CRC Press; 2005. pp. 381–436. [Google Scholar]
- 11.Gutiérrez D, Martinez B, Rodriguez A, Garcia P. Isolation and characterization of bacteriophages infecting Staphylococcus epidermidis. Curr Microbiol. 2010;61:601–608. doi: 10.1007/s00284-010-9659-5. [DOI] [PubMed] [Google Scholar]
- 12.Alagappan K, Karuppiah V, Deivasigamani B. Protective effect of phages on experimental V. parahaemolyticus infection and immune response in shrimp (Fabricius, 1798) Aquaculture. 2016;453:86–92. doi: 10.1016/j.aquaculture.2015.11.037. [DOI] [Google Scholar]
- 13.Jun JW, Han JE, Tang KFJ, Lightner DV, Kim J, Seo SW, Park SC. Potential application of bacteriophage pVp-1: agent combating Vibrio parahaemolyticus strains associated with acute hepatopancreatic necrosis disease (AHPND) in shrimp. Aquaculture. 2016;457:100–103. doi: 10.1016/j.aquaculture.2016.02.018. [DOI] [Google Scholar]
- 14.Jun JW, Kim HJ, Yun SK, Chai JY, Park SC. Eating oysters without risk of vibriosis: application of a bacteriophage against Vibrio parahaemolyticus in oysters. Int J Food Microbiol. 2014;188:31–35. doi: 10.1016/j.ijfoodmicro.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Jun JW, Shin TH, Kim JH, Shin SP, Han JE, Heo GJ, De Zoysa M, Shin GW, Chai JY, Park SC. Bacteriophage therapy of a Vibrio parahaemolyticus infection caused by a multiple antibiotic resistant O3:K6 pandemic clinical strain. J Infect Dis. 2014;210:72–78. doi: 10.1093/infdis/jiu059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lightner DV. A handbook of shrimp pathology and diagnostic procedures for diseases of cultured penaeid shrimp. Baton Rouge: World Aquaculture Society; 1996. [Google Scholar]