Abstract
In the present study, a method for easy and rapid synthesis of lipase nanohybrids was evaluated using cobalt chloride as an encapsulating agent. The synthesized nanohybrids exhibited higher activity (181%) compared to free lipase and improved catalytic properties at higher temperature and in harsh conditions. The nanohybrids retained 84% of their residual activity at 25 °C after 10 days. In addition, these nanohybrids also exhibited high storage stability and reusability. Collectively, the synthesis of carrier-free immobilized biocatalysts was performed rapidly within 24 h at 4 °C. Their high reusability and catalytic activities highlight the broad applicability of this method for catalysis in organic and aqueous media.
Keywords: Immobilization, Lipase, Protein-inorganic nanohybrids, Reusability, Stability
The immobilization of commercially available enzymes is a prolific area of research, particularly with the recent advances in carrier free immobilization techniques. Biocatalysis in aqueous and organic media has been readily performed using immobilized enzymes [1–3]. The protein can be self-assembled or aggregated into various types of nanostructures, which are easy to handle and can be reused multiple times. The transition metals, calcium, zinc, manganese, iron, and copper, have been widely used for the synthesis of nanohybrid-like structures of various enzymes. However, a very few studies have reported on the synthesis of protein inorganic nanohybrids using cobalt (Co) as the precipitating agent. Lipase is an industrially potent enzyme with wide range of applications [4]. To date, inorganic-protein nanohybrid structures of lipase have been primarily synthesized using calcium [5], zinc [6] and copper [7]. Nanohybrids-like structures of bovine serum albumin were synthesized using cobalt by Kim et al. [8]. Also, nanohybrids of alcohol dehydrogenase have been formed by using mineralized Co and copper as encapsulating agents [9]. This study indicated that Co protein nanohybrids showed higher activities compared to copper nanohybrids. The decreased activity in copper nanohybrids is due to nonspecific interactions between the soluble metal ions (Cu2+) and the protein domains or the active site whereas Co tends to interact with only histidine tags. Cobalt has been reported to enhance the activity of lipase in the low millimolar range. During the synthesis of protein inorganic nanoflowers, protein components interact with the primary metals to initiate nucleation, which is followed by aggregation and anisotropic growth of platelets-like structures that assemble into round oval flower-like shapes. The lipase immobilization as Lip-Co3(PO4)2 nanohybrids were synthesized by the precipitation method using a protocol described by Li et al. [10] with minor modifications. CoCl2 (2 mM) has been incubated for 24 h with the different concentrations of protein (0.2–0.5 mg/mL) at 4 °C in 4 mL of phosphate buffer saline (PBS). The precipitated proteins were separated by centrifugation at 10,000×g for 5 min. The synthesized nanohybrids were lyophilized and stored at 4 °C until use in experiments. The relative activities (RAs) and encapsulation yields (EYs) were calculated as follows: RA (%) = 100 × total activity of the nanohybrids/total activity of the free enzyme; EY (%) = 100 × protein concentration of nanohybrids/initial protein concentration (mg/mL) [11].
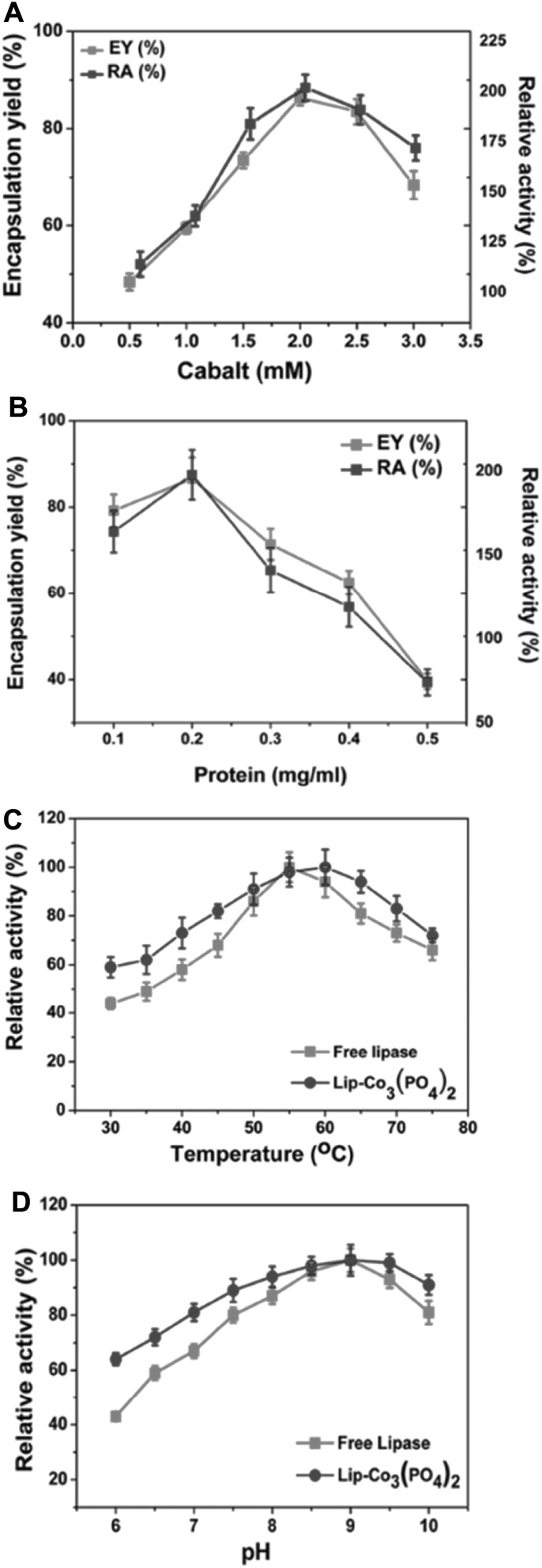
The immobilized enzymes were characterized by scanning electron microscopy (SEM), confocal microscopy, and energy dispersive x-ray spectroscopy (EDX). The biochemical characterizations of free enzyme and the synthesized nanohybrids were performed to compare their properties. Interestingly, the synthesized nanohybrids exhibited 181% higher activity in comparison to the free enzyme. Lipase immobilization onto various nanoparticles and matrices to improve catalytic performance has been studied [12–14]. Lip-Co3(PO4)2 nanohybrids showed excellent operational stability, indicating their potential applications in industry. The concentration of Co was varied from 0.5 to 3.0 mM, and their maximum EY and RA were recorded using a concentration of 2.0 mM (Fig. 1). In a recent study, the synthesis of lipase nanohybrids using CuSO4 (2 mM) was reported after 3 days of incubation [15]. The concentration of protein is a crucial parameter during nucleation. Thus, the protein concentration was varied from 0.1 to 0.5 mg/mL in the reaction mixture. The EY of nanohybrids was highest using 0.2 mg/mL of protein and RA was increased to 181%. The physicochemical properties of the Lip-Co3(PO4)2 nanohybrids were changed. The nanohybrids showed highest activity at 60 °C, while the optimum temperature for free enzyme was 55 °C. On the other hand, the optimum pH for both the free and nanohybrid-immobilized lipase was recorded to be 9.0.
Fig. 1.
Different concentrations of CoCl2 were used to optimize encapsulation yield and relative activity of lipase (0.5–3.0 mM) in phosphate buffer (a). The enzyme concentration was varied from 0.1 to 0.5 mg/mL (b). The temperature and pH optima for free lipase and the synthesized nanohybrids of Lip-Co3(PO4)2 were determined under standard assay conditions (c, d)
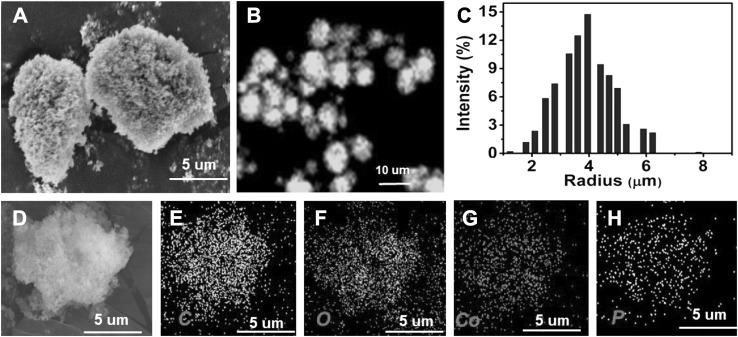
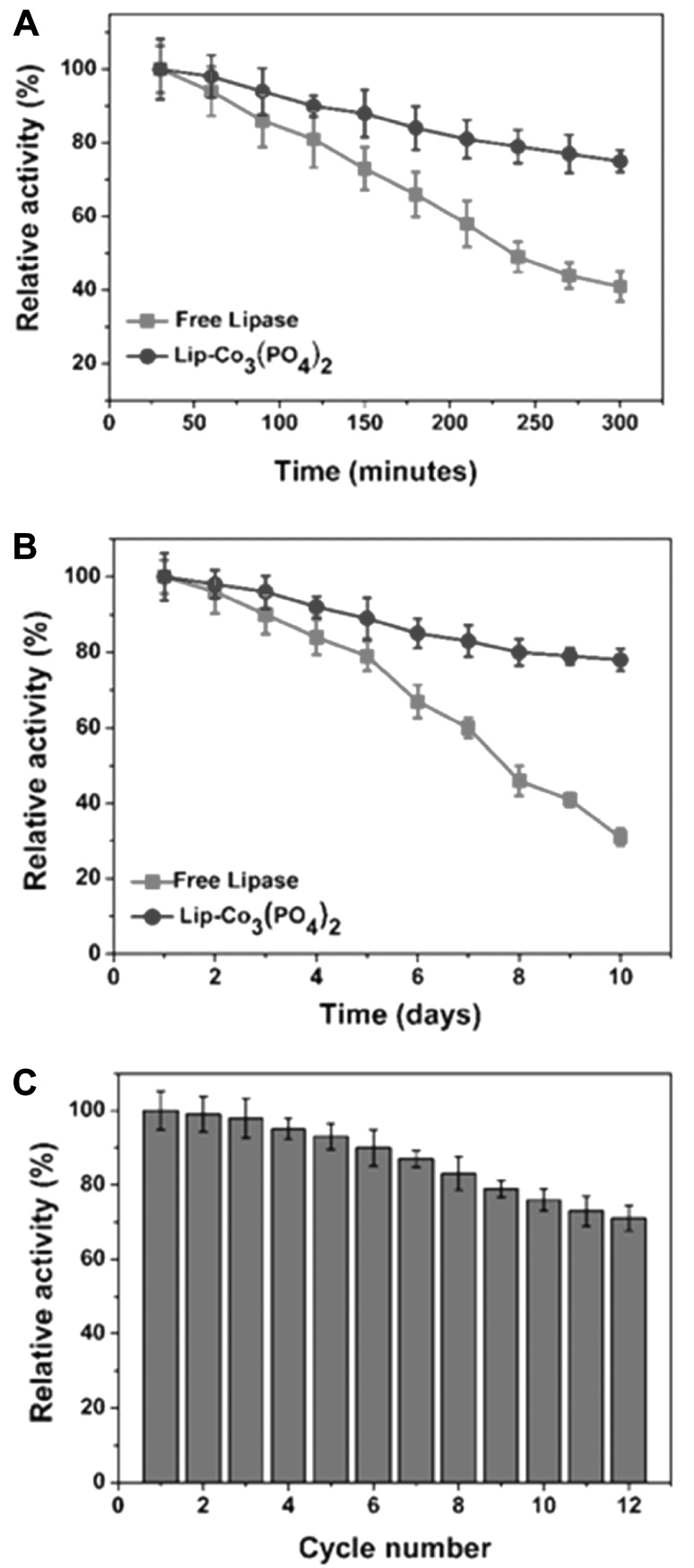
The morphologies of Lip-Co3(PO4)2 nanohybrids synthesized with 0.2 mg/mL lipase was confirmed by SEM analysis (Fig. 2). The SEM imaging of nanohybrids synthesized using CoCl2 and lipase protein showed a diameter of 6–8 µm. DLS spectra indicated the average particle size was 8 µm. The encapsulation of lipase inside the nanohybrids was confirmed by the presence of FITC-labelled lipase. Lipase labeling was performed using the method described by Patel et al. [16]. Elemental analyses of nanohybrids confirmed the presence of Co and oxygen content, which indicates metal-embedded protein. The hydrolytic activities of the free and lipase nanohybrids were determined using p-nitrophenyl palmitate (p-NPP) as a substrate. The thermal stabilities of the Lip-Co3(PO4)2 nanohybrids was tested at 70 °C (Fig. 3). The free enzyme showed 39% of their residual activity after 300 min of incubation at 70 °C, whereas the synthesized nanohybrids retained 83% of their original activity after 300 min. Thus, the temperature stability of the synthesized nanohybrids was increased by 2.2-fold, which indicated the suitability of this method for performing biocatalytic reactions at higher temperatures.
Fig. 2.
Field emission scanning electron microscopy (FE-SEM) images of Lip-Co3(PO4)2 nanohybrids (a). FITC-labelled images of synthesized Lip-Co3(PO4)2 nanohybrids by confocal microscopy (b). Dynamic light scattering (DLS) spectra of synthesized nanohybrids to determine the average size of particles (c). The elemental analysis of Lip-Co3(PO4)2 nanohybrids using dot mapping imaging showed the presence of carbon, oxygen, cobalt, and phosphorous content (d–h)
Fig. 3.
Temperature stabilities (a), storage stabilities (b), and reusability (c) of free lipase and lipase nanohybrids. In stability experiments, the activity of free and immobilized enzymes before incubation was considered as 100%. The decrease in activity was recorded after each interval of time under standard assay conditions
The storage stabilities of free and lipase nanohybrids were tested by incubating them in tris buffer (50 mM pH 9.0) at room temperature for 10 days, and residual activities were measured daily. The storage stabilities of the lipase nanohybrids were analyzed over a period of 10 days at room temperature. Lipase nanohybrids retained high residual activities of 84.0% after 10 days of incubation. However, free lipase showed a 75% decrease compared to its original activity after 10 days of incubation. The high storage stabilities of the lipase as Lip-Co3(PO4)2 nanohybrid complexes were improved compared with previous reports. In a recent study, Zn3(PO4)2 nanoflowers of lipase had shown 74% residual activity after 20 days of incubation [6]. The lipase nanohybrids were also reused to measure hydrolysis of p-NPP under standard assay conditions. Interestingly, Lip-Co3(PO4)2 nanohybrid complexes retained 74.6% of their residual activities after 12 cycles. Consequently, the reusability Lip-Co3(PO4)2 nanohybrids was much improved compared to lactoperoxidase nanohybrids, which showed only 15% activity after 6 cycles [17].
In summary, we report that lipase was embedded in nanohybrids containing 2 mM CoCl2 in PBS (pH 7.4) at 4 °C. The method appears to be very effective for improving the catalytic properties and stabilities of industrially important enzymes. The use of Co embedded inorganic nanohybrids can be extended to other industrially important enzyme, such as laccase, cellulase, and horseradish peroxidase. Additionally, the reusability of these nanohybrids is also higher as compared to other nanohybrids in the previous reports. Thus, Co-embedded nanohybrids of one or multiple enzymes may have strong industrial potential in near future.
Acknowledgements
This work was supported by the 2017 KU Brain Pool Fellowship of Konkuk University. This research was supported by the Ministry of Science, ICT and Future Planning, Republic of Korea (NRF-2013R1A1A2012159 and NRF-2013R1A1A2007561). This work was also supported by the Energy Efficiency and Resources Core Technology Program of the KETEP, granted financial resource from the Ministry of Trade, Industry and Energy, Republic of Korea (20153010092130). This research was supported by the KU Research Professor program of Konkuk University.
Compliance with Ethical Standards
Conflict of interest
There is no conflict of interest among the authors.
Contributor Information
Sanjay K. S. Patel, Email: sanjaykspatel@gmail.com
Jung-Kul Lee, Email: jkrhee@konkuk.ac.kr.
References
- 1.Patel SKS, Choi SH, Kang YC, Lee J-K. Large-scale aerosol-assisted synthesis of biofriendly Fe2O3 yolk-shell particles: a promising support for enzyme immobilization. Nanoscale. 2016;8:6728–6738. doi: 10.1039/C6NR00346J. [DOI] [PubMed] [Google Scholar]
- 2.Patel SKS, Singh RK, Kumar A, Jeong JH, Jeong SH, Kalia VC, Kim IW, Lee J-K. Biological methanol production by immobilized Methylocella tundrae using simulated biohythane as a feed. Bioresour Technol. 2017;241:922–927. doi: 10.1016/j.biortech.2017.05.160. [DOI] [PubMed] [Google Scholar]
- 3.Kim T-S, Patel SKS, Selvaraj C, Jung W-S, Pan C-H, Kang YC, Lee J-K. A highly efficient sorbitol dehydrogenase from Gluconobacter oxydans G624 and improvement of its stability through immobilization. Sci Rep. 2016;6:33438. doi: 10.1038/srep33438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar A, Dhar K, Kanwar SS, Arora PK. Lipase catalysis in organic solvents: advantages and applications. Biol Proc Online. 2016;18:2. doi: 10.1186/s12575-016-0033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ke C, Fan Y, Chen Y, Xu L, Yan Y. A new lipase-inorganic hybrid nanoflower with enhanced enzyme activity. RSC Adv. 2016;6:19413–19416. doi: 10.1039/C6RA01564F. [DOI] [Google Scholar]
- 6.Zhang B, Li P, Zhang H, Wang H, Li X, Tian L, Ali N, Ali Z, Zhang Q. Preparation of lipase/Zn3(PO4)2 hybrid nanoflower and its catalytic performance as an immobilized enzyme. Chem Eng J. 2016;291:287–297. doi: 10.1016/j.cej.2016.01.104. [DOI] [Google Scholar]
- 7.Cui J, Zhao Y, Liu R, Zhong C, Jia S. Surfactant-activated lipase hybrid nanoflowers with enhanced enzymatic performance. Sci Rep. 2016;6:27928. doi: 10.1038/srep27928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim KH, Jeong J-M, Lee SJ, Choi BG, Lee KG. Protein-directed assembly of cobalt phosphate hybrid nanoflowers. J Colloid Interface Sci. 2016;48:44–50. doi: 10.1016/j.jcis.2016.08.059. [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Gallego F, Yate L. Selective biomineralization of Co3(PO4)2-sponges triggered by His-tagged proteins: efficient heterogeneous biocatalysts for redox processes. Chem Commun. 2015;51:8753–8756. doi: 10.1039/C5CC00318K. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, Zhang Y, Su Y, Ouyang P, Ge J, Liu Z. Spatial co-localization of multi-enzymes by inorganic nanocrystal-protein complexes. Chem Commun. 2014;50:12465–12468. doi: 10.1039/C4CC05478D. [DOI] [PubMed] [Google Scholar]
- 11.Patel SKS, Otari SV, Kang YC, Lee JK. Protein-inorganic hybrid system for efficient his-tagged enzymes immobilization and its application in l-xylulose production. RSC Adv. 2017;7:3488–3494. doi: 10.1039/C6RA24404A. [DOI] [Google Scholar]
- 12.Anwar MZ, Kim DJ, Kumar A, Patel SKS, Otari S, Mardina P, Jeong JH, Sohn JH, Kim JH, Park JT, Lee JK. SnO2 hollow nanotubes: a novel and efficient support matrix for enzyme immobilization. Sci Rep. 2017;7:15333. doi: 10.1038/s41598-017-15550-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashok K, Shamsher SK. An innovative approach to immobilize lipase onto natural fiber and its application for the synthesis of 2-octyl ferulate in an organic medium. Curr Biotechol. 2012;1:241–248. doi: 10.2174/2211550111201030241. [DOI] [Google Scholar]
- 14.Kumar A, Zhang SW, Wu GB, Wu CC, Chen JP, Baskaran R, Liu ZD. Cellulose binding domain assisted immobilization of lipase (GSlip-CBD) onto cellulosic nanogel: characterization and application in organic medium. Colloids Surface B Biointerfaces. 2015;136:1042–1050. doi: 10.1016/j.colsurfb.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Lee HR, Chung M, Kim MI, Ha SH. Preparation of glutaraldehyde-treated lipase-inorganic hybrid nanoflowers and their catalytic performance as immobilized enzymes. Enzyme Microb Technol. 2017;105:24–29. doi: 10.1016/j.enzmictec.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Patel SKS, Choi SH, Kang YC, Lee J-K. Eco-friendly composite of Fe3O4-reduced graphene oxide particles for efficient enzyme immobilization. ACS Appl Mater Interfaces. 2017;9:2213–2222. doi: 10.1021/acsami.6b05165. [DOI] [PubMed] [Google Scholar]
- 17.Altinkaynak C, Yilmaz I, Koksal Z, Ozdemir H, Ocsoy I, Ozdemir N. Preparation of lactoperoxidase incorporated hybrid nanoflower and its excellent activity and stability. Int J Biol Macromol. 2016;84:402–409. doi: 10.1016/j.ijbiomac.2015.12.018. [DOI] [PubMed] [Google Scholar]