Abstract
Adolescents with single ventricle heart disease (SVHD) show autonomic, mood, and cognitive deficits, indicating aberrations in brain areas that regulate these functions. However, the gray matter integrity in autonomic, mood, and cognitive control sites is unclear. We examined regional brain gray matter changes, using high-resolution T1-weighted images (3.0-Tesla magnetic resonance scanner) with voxel based morphometry procedures, as well as mood and cognitive functions in SVHD (n = 18; age, 15.7±1.1 years; male, 10) and controls (n = 31; age, 16.0±1.1 years; male, 17). High-resolution T1-weighted images were realigned, gray matter tissue type partitioned, normalized to a common space, smoothed, and compared between groups (analysis of covariance; covariates, age and gender). The mood and cognitive scores were compared between groups using independent samples t-tests. SVHD subjects showed significantly altered mood and cognitive functions over controls. Significantly reduced gray matter emerged in multiple brain areas, including the thalamus, caudate nuclei, putamen, insula, prefrontal, post-central and precentral gyrus, occipital gyrus, para-hippocampal gyrus, temporal gyrus, and cerebellar sites in SVHD over controls. SVHD subjects show compromised gray matter integrity in autonomic, mood and cognitive control sites. The findings indicate that frequent deficits found in SVHD subjects have a brain structural basis in the condition.
Keywords: Congenital heart disease, Magnetic resonance imaging, Voxel based morphometry, Gray matter, Cognition
INTRODUCTION
Single ventricle heart disease (SVHD) is associated with neurological deficits that become more prominent during school-age and early adolescence [1–3]. Several SVHD studies have reported autonomic and neuropsychological issues, including cognitive and mood dysfunction, such as worse pragmatic language, anxiety, depression, attention deficits, impulsive behavior, and impaired executive function [4, 5]. With the increase in number of SVHD survivors [6], it is essential to identify non-cardiac sequelae that could potentially affect daily living in the condition.
Various neuroimaging studies have reported brain injury in infants with SVHD both in the preoperative and postoperative period [7–10]. Routine magnetic resonance imaging (MRI) have shown preoperative brain damage as ischemic infarcts, white matter injury, and focal or multifocal lesions in up to 50% of newborns with SVHD [7, 8, 11]. Furthermore, 30–40% may have new or increased injury seen in the postoperative period [12, 13].
Routine brain MRI, including T1-weighted imaging, does not yield enough evidence to support functional impairment in SVHD. High-resolution T1-weighted imaging based voxel-based morphometry (VBM), which is a whole brain automated technique that offers rapid unbiased assessment of brain tissue on a voxel-by-voxel basis, can show localized gray matter (GM) density integrity (i.e., the proportion of gray matter relative to other tissue types within a region) in SVHD subjects [14]. The VBM procedures have been successfully used to characterize structural differences in several brain disorders, such as obstructive sleep apnea [15], epilepsy [16], Alzheimer’s disease [17], and heart failure [18]. Also, few studies have used VBM techniques to assess gray and white matter grossly in complex congenital heart disease [19–21]. However, any underlying regional GM differences in brain areas that regulate autonomic, mood, and cognitive functions in SVHD subjects have not been examined systematically.
In this study, we aimed to examine regional GM changes in SVHD patients, who have undergone Fontan completion, compared to healthy controls using VBM procedures. We hypothesized that adolescents with SVHD will show lower GM density in autonomic, mood, and cognitive regulatory sites over healthy controls.
MATERIALS AND METHODS
Subjects
Eighteen SVHD adolescents and 31 healthy controls participated in this study. All subjects were recruited via flyers or provider referrals from the University of California Los Angeles (UCLA), Children’s Hospital Los Angeles (CHLA) pediatric cardiology clinics, and private practice cardiology groups in Southern California. SVHD subjects with age between 14–18 years and undergone surgical palliation with Fontan completion were included in this study.
All eligible healthy controls were recruited from local high schools in the Los Angeles area. Control subjects were without any history of chronic medical or psychiatric conditions, or any previous history of head injury (e.g. concussions, trauma). Exclusion criteria for SVHD and controls were claustrophobia, non-removable metal (such as braces, pacemakers), severe developmental delay precluding active study participation or ability for self-report (e.g. cerebral palsy or severe hypoxic-injury), diagnosis of depression, premature birth (< 37 weeks gestation), history of extracorporeal membrane oxygenation (ECMO) use, and cardiac arrest.
Clinical and demographic data were collected from participants and their medical records. Parental permission and assent were obtained for participants under 18 years, and informed consents were obtained from participants over 18 years. The study protocol was approved by the Institutional Review Boards at the University of California at Los Angeles and Children’s Hospital Los Angeles. All the experiments were performed in accordance with relevant guidelines and regulations.
Assessment of Depression and Anxiety
Anxiety and depressive symptoms were assessed in all subjects using two self-reported questionnaires, the Beck Anxiety Inventory (BAI) [22] and the Patient Health Questionnaire-9 (PHQ-9) [23], respectively. he BAI had 21 multiple-choice questions (each question score ranged 0–3), with score ranging from 0–63 based on symptoms severity. A subject with score > 9 is considered with anxiety symptoms [22]. The PHQ-9 is 9-item depression module, with score ranging from 0–27 (each question score ranged 0–3). A score from 5–9, 10–14, 15–19, and > 20 is considered with minimal, moderate, moderately-severe, and severe depressive symptoms, respectively [23]. These instruments have been previously used in the congenital heart disease population [24, 25].
Cognition Assessment
The Montreal Cognitive Assessment (MoCA) test was performed in both SVHD and control subjects. This test measures various cognitive functions, including attention and concentration, executive functions, language, memory, visuo-constructional skills, conceptual thinking, calculations and orientation [26]. The memory score on MoCA ranges from 0–30, and a score < 26 is considered abnormal. This instrument has been previously used and validated in the adolescent congenital heart disease population [27].
The Wide Range Assessment of Memory and Learning, 2nd Edition (WRAML2) was all administered in SVHD and controls for assessment of memory and learning functions. The WRAML2 measures various domains of memory, including the verbal and visual memory, attention/concentration, working memory, and visual and verbal recognition. The core battery consists of six subtests: story memory, verbal learning, design memory, picture memory, short-term memory of a visual sequential pattern, and numbers/letters that combined to yield a general memory index (GMI) score. Additionally, the other subtests include working memory and memory recognition yield the general memory recognition index (GRI) score. A score of <85 in either measures is considered impaired [28].
Magnetic Resonance Imaging
Brain MRI scans were acquired using a 3.0-Tesla MR scanner (Siemens, Magnetom Tim-Trio and Prisma, Erlangen, Germany) while participants lay supine. Foam pads on either side of the head were used to minimize head movement. Two high-resolution T1-weighted images were collected using a magnetization prepared rapid acquisition gradient-echo (MPRAGE) sequence (TR = 2200 ms; TE = 2.4 ms; inversion time = 900 ms; FA = 9°; matrix size = 320×320; FOV = 230×230 mm; slice thickness = 0.9 mm; number of slices = 192). roton density (PD) and T2 weighted images [repetition time (TR) = 10,000 ms; echo-time (TE1, TE2) = 17, 134 ms; flip angle (FA) = 130°] were also acquired simultaneously using a dual-echo turbo spin-echo sequence in the axial plane, with a 256×256 matrix size, 230×230 mm field of view (FOV), 4.0 mm slice thickness, and no inter-slice gap.
Data Processing
We used the statistical parametric mapping package SPM12 (Wellcome Department of Cognitive Neurology, UK), and MATLAB-based (The MathWorks Inc, Natick, MA) custom software to process images. Data processing steps were followed as described earlier [29]. We used Diffeomorphic Anatomic Registration Through Exponentiated Lie algebra algorithm (DARTEL) toolbox to improve inter-subject image registration [30]. Firstly, we realigned both high-resolution T1-weighted scans to remove any potential variations between scans and averaged. The averaged images were segmented into GM, white matter (WM), and cerebrospinal fluid (CSF) tissue types using the ‘new-segment’ option in the DARTEL toolbox, and then flow fields and a series of template images were generated. Finally, the flow fields and final template image created in the previous step were used to normalize GM maps (unmodulated, re-sliced to 0.7×0.7×0.7 mm) and smoothed with a Gaussian filter (10-mm full width at half maximum).
Statistical Analyses
The IBM statistical package for the social sciences (IBM SPSS, v 24, Chicago, IL) was used for data analyses. Demographic and clinical characteristics were assessed with independent samples t-tests, and categorical characteristics with the Chi-square. A p<0.05 value was considered statistically significant.
For regional GM density differences between groups, the smoothed whole-brain GM maps of SVHD and controls were compared using analysis of covariance (ANCOVA), with age and gender as covariates (SPM12; p<0.001, uncorrected; minimum extended cluster size, 10 voxels). The statistical parametric maps showing brain sites with significant GM density differences between groups were superimposed onto the mean anatomical image for anatomical identification.
To identify any associations between GM density and cognitive and mood functions, we performed partial correlations between GM density and MoCA, PHQ-9, and BAI scores in SVHD subjects (covariates, age and gender).
RESULTS
Demographic and clinical characteristics
Demographic and clinical variables of SVHD and control subjects are summarized in Table 1. No significant differences in age (p = 0.34), gender (p = 0.96), body mass index (BMI) (p = 0.28), handedness (p = 0.90), or ethnicity (p = 0.90) appeared between groups. SVHD subjects showed significantly higher PHQ-9 and BAI scores compared to control subjects (PHQ-9, p<0.001; BAI, p = 0.002). Global MoCA scores and their majority of sub-scales were significantly lower in SVHD over control subjects (p <0.05). The GMI and G scores were significantly reduced in SVHD compared to controls (p <0.001).
Table 1.
Demographic, mood, cognitive, and clinical characteristics of SVHD and controls.
| Variables | SVHD n = 18 (Mean ± SD) |
Controls n = 31 (Mean ± SD) |
P values |
|---|---|---|---|
|
| |||
| Age (years) | 15.7±1.1 | 16.0±1.1 | 0.34 |
|
| |||
| Gender [male] (%) | 11 (55%) | 19 (54%) | 0.96 |
|
| |||
| Ethnicity (%) | White, 9 (50%); Hispanic, 7 (39%); Other, 2 (11%) | White, 17 (55%); Hispanic, 12 (39%); Other, 2 (6%) | 0.62 |
|
| |||
| BMI (kg/m2) | 20.9±3.0 | 21.9±6.9 (n=30) | 0.58 |
|
| |||
| Handedness [right] (%) | 17 (94%) | 29 (94%) | 0.90 |
|
| |||
| Ventricle type [right] (%) | 9 (50%) | N/A | N/A |
|
| |||
| Extracardiac Fontan (%) | 16 (88%) | N/A | N/A |
|
| |||
| Fenestration (%) | 4 (22%) | N/A | N/A |
|
| |||
| * Residual Cyanosis (%) | 7 (39%) | N/A | N/A |
|
| |||
| PHQ-9 | 7.8±5.8 | 3.2±2.0 | <0.001 |
|
| |||
| BAI | 18.3±11.8 | 9.2±7.2 | 0.002 |
|
| |||
| WRAML2 GMI | 82.1±12.5 | 111.1±7.5 | <0.001 |
| WRAML2: Verbal Memory | 87.3±9.4 | 108.0±9.1 | <0.001 |
| WRAML2: Visual Memory | 95.7±10.8 | 107.8±8.0 | <0.001 |
| WRAML2: Attention | 84.6±10.1 | 111.5±7.7 | <0.001 |
|
| |||
| WRAML2 GRI | 92.4±11.0 | 111.6±8.1 (n=30) | <0.001 |
| WRAML2: Working Recognition | 88.9±8.6 | 114.2±12.9 | <0.001 |
| WRAML2: Verbal Recognition | 91.5±9.9 | 107.1±11.7 (n=30) | <0.001 |
| WRAML2: Visual Recognition | 96.0±14.0 | 109.1±11.4 | 0.001 |
|
| |||
| Total MoCA scores | 22.3±3.1 | 28.8±1.5 | <0.001 |
| MoCA: Visuospatial | 3.7±1.0 | 4.9±0.3 | <0.001 |
| MoCA: Naming | 2.8±0.4 | 3.0±0.0 | 0.02 |
| MoCA: Attention | 4.3±1.1 | 5.7±0.8 | <0.001 |
| MoCA: Language | 1.5±0.8 | 2.6±0.6 | <0.001 |
| MoCA: Abstraction | 1.3±0.6 | 1.9±0.3 | <0.001 |
| MoCA: Delayed Recall | 1.7±1.3 | 4.1±0.6 | <0.001 |
| MoCA: Orientation | 5.9±0.3 | 5.9±0.3 | 0.58 |
SD = standard deviation; N/A = not applicable; BMI = body mass index; PHQ-9= Patient Health Questionnaire-9; BAI= Beck Anxiety Inventory; WRAML2 = Wide Range Assessment of Memory and Learning, 2nd Edition; GMI = General Memory Index; GRI = General Memory Recognition Index; MoCA= Montreal Cognitive Assessment;
Pulse oximetry < 93%.
Regional Gray Matter Density Differences between SVHD Patients and Healthy Controls
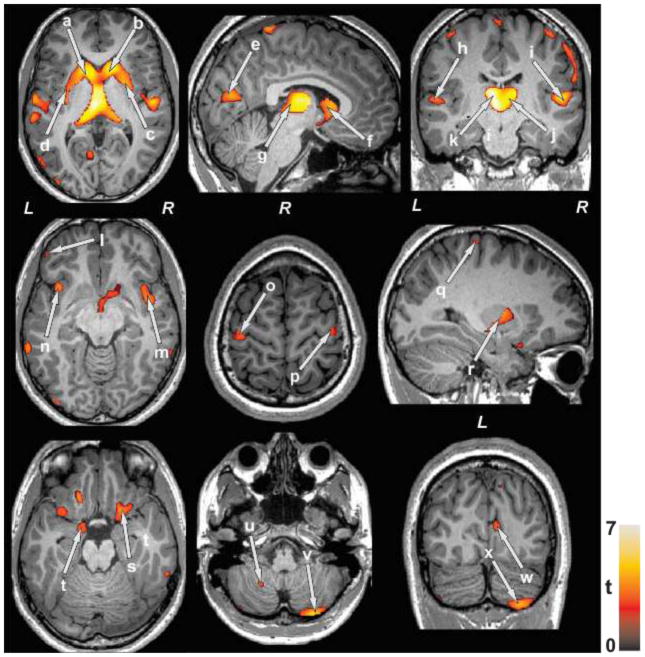
After controlling for age and gender, several brain regions showed decreased GM density in SVHD subjects compared to healthy controls (Table 2). No brain sites showed increased GM density in SVHD subjects over controls. Brain regions with decreased GM density in SVHD subjects emerged in the bilateral anterior, mid and posterior thalamus, caudate nuclei, putamen, anterior and posterior insular cortices, prefrontal cortices, post-central and pre-central gyrus, inferior and middle occipital gyri, para-hippocampal gyrus, inferior, middle, and superior temporal gyri, and anterior and posterior cerebellar cortices, in SVHD subjects compared to controls (Figure 1).
Table 2.
Regions of decreased gray matter density in single ventricle heart disease and controls.
| Brain region | SVHD (mean ± SD) n = 18; unit = mm3/voxel |
Control (mean ± SD) n = 31; unit = mm3/voxel |
Cluster Size (voxels) | P value |
|---|---|---|---|---|
| R Thalamus | 2.16 ± 0.19 | 2.42 ± 0.12 | 12939 | < 0.001 |
| L Thalamus | 2.41 ± 0.19 | 2.72 ± 0.12 | 13922 | < 0.001 |
| R Putamen | 1.92 ± 0.14 | 2.08 ± 0.11 | 6016 | < 0.001 |
| L Putamen | 1.61 ± 0.12 | 1.75 ± 0.10 | 5285 | < 0.001 |
| R Caudate Nucleus | 2.24 ± 0.20 | 2.50 ± 0.13 | 10073 | < 0.001 |
| L Caudate Nucleus | 2.32 ± 0.17 | 2.55 ± 0.11 | 9612 | < 0.001 |
| R Insula | 2.48 ± 0.13 | 2.62 ± 0.10 | 1999 | < 0.001 |
| L Insula | 2.17 ± 0.16 | 2.31 ± 0.11 | 1400 | < 0.001 |
| R Precentral Gyrus | 1.79 ± 0.11 | 1.92 ± 0.06 | 3005 | < 0.001 |
| L Precentral Gyrus | 1.28 ± 0.09 | 1.41 ± 0.08 | 1214 | < 0.001 |
| R Postcentral Gyrus | 1.96 ± 0.13 | 2.12 ± 0.08 | 7178 | < 0.001 |
| L Postcentral Gyrus | 1.35 ± 0.09 | 1.45 ± 0.06 | 1026 | < 0.001 |
| R Prefrontal Cortices | 0.49 ± 0.02 | 0.36 ± 0.05 | 25 | 0.001 |
| L Prefrontal Cortices | 0.37 ± 0.02 | 0.34 ± 0.04 | 19 | 0.001 |
| R Superior Temporal Gyrus | 1.93 ± 0.13 | 2.04 ± 0.11 | 101 | 0.001 |
| L Superior Temporal Gyrus | 2.25 ± 0.09 | 2.36 ± 0.08 | 1166 | < 0.001 |
| R Middle Temporal Gyrus | 2.18 ± 0.16 | 2.31 ± 0.09 | 393 | < 0.001 |
| L Middle Temporal Gyrus | 1.96 ± 0.13 | 2.08 ± 0.08 | 3001 | < 0.001 |
| R Inferior Temporal Gyrus | 2.16 ± 0.17 | 2.29 ± 0.10 | 255 | < 0.001 |
| L Inferior Temporal Gyrus | 2.19 ± 0.10 | 2.30 ± 0.07 | 168 | < 0.001 |
| R Parahippocampal Gyrus | 1.75 ± 0.11 | 1.86 ± 0.10 | 14 | 0.001 |
| L Parahippocampal Gyrus | 1.83 ± 0.07 | 1.92 ± 0.06 | 62 | < 0.001 |
| R Cerebellum | 1.96 ± 0.15 | 2.09 ± 0.10 | 2089 | < 0.001 |
| L Cerebellum | 1.65 ± 0.13 | 1.77 ± 0.09 | 819 | < 0.001 |
| L Middle Occipital Gyrus | 1.54 ± 0.16 | 1.66 ± 0.08 | 192 | 0.001 |
| L Inferior Occipital Gyrus | 1.43 ± 0.15 | 1.55 ± 0.08 | 1328 | < 0.001 |
SVHD, Single ventricle heart disease; SD, Standard Deviation; L, Left; R, Right.
Figure 1.
Brain sites with regional lower gray matter density in SVHD compared to control subjects. Brain regions with reduced gray matter density were observed in the bilateral caudate nuclei (a, b, f), putamen (c, d, r), occipital cortices (e, w), thalamus (g, j, k), temporal gyrus (h, i), prefrontal cortices (l), insular cortices (m, n), precentral gyrus (o, p), post-central gyrus (q), para-hippocampal gyrus (s, t), and cerebellar peduncles (u, v, x), in SVHD over controls. All images are in neurological convention (L = Left; = Right). Color bar indicates t-statistic values, with black/red color shows less significance and yellow color indicates higher significance in gray matter density differences between SVHD and control subjects.
Correlations between Gray Matter Density and Mood and Cognitive scores
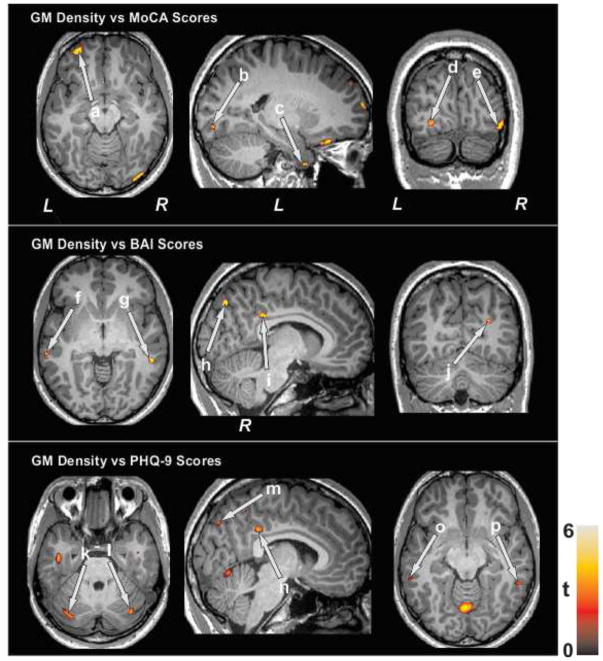
Although small sample size, the global MoCA scores showed positive correlations with GM density of the prefrontal, occipital, and temporal regions in SVHD subjects (Figure 2). Both BAI and PHQ-9 values showed negative correlations with GM density of the temporal, occipital, cingulate, and cerebellar areas in SVHD subjects (Figure 2).
Figure 2.
The MoCA scores show positive correlations between regional brain gray mater density of the prefrontal cortices (a), occipital cortices (b, d, e), and temporal cortices (c) in SVHD subjects. The BAI values show negative correlations with brain areas including the temporal cortices (f, g), occipital cortices (h, j), and cingulate (i). The PHQ-9 scores show negative correlations with cerebellar cortices (k, l), occipital cortices (m), cingulate (n), and temporal cortices (o, p) in SVHD subjects.
DISCUSSION
SVHD subjects show deficits in autonomic, mood, and cognitive functions, which indicate impairments in brain regions that control these functions. Previous neuroimaging studies in SVHD subjects revealed the brain injury as ischemic infarcts, gray and white matter losses, and focal or multifocal abnormalities before and after surgery [11, 12, 19]. In addition, total gray matter volume loss has been shown in adolescents with SVHD and is correlated with cognitive, motor, and executive functions [12]. In this study, we showed reduced regional GM density in SVHD subjects compared to healthy controls, and these changes emerged in multiple brain regions essential for autonomic, cognitive, and mood functions, including thalamus, caudate nuclei, putamen, insular cortices, prefrontal cortices, post-central and precentral gyrus, occipital gyrus, para-hippocampal gyrus, temporal gyrus, and cerebellar cortices.
Multiple brain regions are involved in mediating cognitive functions. In our study, several brain regions that are associated with cognitive functions, including, memory, motor, and executive functions, showed decreased GM density in SVHD patients. These sites included bilateral thalamus, caudate nuclei, putamen, prefrontal, postcentral and precentral gyrus, occipital gyrus, para-hippocampal gyrus, temporal gyrus, and cerebellar cortices. Damage to para-hippocampal gyrus can lead to visuo-spatial processing and episodic memory dysfunctions, as suggested by previous animal and human based neuroimaging studies [31–33]. Both caudate nucleus and putamen has been associated with many cognitive processes, including memory and executive functions; receives inputs from several cortical and thalamic regions, which are processed further within the basal ganglia system [34, 35]. The thalamus and caudate nucleus are also part of parallel circuits that connect the basal ganglia and frontal cortex and are linked with motor and cognition regulation [36, 37], with the putamen mainly being connected to motor cortical areas [38] and the caudate nucleus being involved in cognitive frontal circuits [37]. Sensorimotor areas, i.e., precentral gyrus is associated with movement, and post-central gyrus with sensory function [39], whereas occipital gyrus is involved in visual processing functions. Cerebellum area also plays an important role in cognitive and affective processes, along with fine motor coordination as suggested by earlier functional MRI studies and clinical evidence from patients with cerebellar lesions [40, 41]. Therefore, damage to these brain regulatory areas may contribute to memory and executive function dysfunctions [42, 43], motor and behavioral problems [44–46], commonly reported in congenital heart disease.
Several sites that showed decreased GM density in SVHD subjects reported here are involved in autonomic and mood functions, deficient in the condition. Depression and anxiety symptoms are very common in patients with SVHD [47–49]. Studies have suggested brain structural deficits can account for impaired emotional processing, cognitive performance, and neurotransmission and neuroendocrine responses that are associated with mood disorders [50, 51]. Brain areas, including the thalamus, insula, para-hippocampal gyrus, occipital gyrus, temporal gyrus, and cerebellar cortices have been implicated in emotional processing in patients with anxiety and depression issues, as suggested by various neuroimaging studies [52–56]. However, insular and cerebellar cortices are also recognized to be involved in autonomic regulation [57, 58], and damage to these regions may contribute to alterations in autonomic control in SVHD, as shown here. Thus, the decreased GM density in these sites may explain clinical manifestations, such as emotional/behavioral and cardiovascular disturbances in adolescents with SVHD.
Although exact pathological processes contributing to brain injury are unclear, there are several mechanisms that may contribute to underlying GM loss in SVHD subjects. Compromised tissue volume most likely resulted from delayed brain development, due to alterations in blood circulation [59]. Another possibility includes hypoxia-ischemia condition associated with regional cerebral perfusion during cardiopulmonary bypass [11]. In addition, there are other pathological processes that may contribute to GM changes including hypotension, inflammation, and abnormal cerebral auto-regulation in SVHD subjects.
Several limitations of this study should be acknowledged, including the small sample size. Despite the smaller sample, differences appeared between groups suggesting significantly large effect sizes between in SVHD and control subjects. Although the SVHD group was fairly homogeneous, with all participants undergoing Fontan completion, the sample size prevented further subgroup analysis based on ventricle type (left vs. right), and each ventricle type may have unique brain changes or patterns of injury, as described earlier [13, 60]. Furthermore, the study participants had more optimal health [no pacemakers, prematurity or other psychosocial morbidities], and these findings may not be generalizable to all SVHD subjects. Another limitation of this study is only appearance of few correlations between GM density and mood and cognitive scores in SVHD subjects, probably due to small sample size. Thus, further studies are required to examine such correlations with large sample size.
CONCLUSIONS
In conclusion, the present study revealed significantly decreased GM density in multiple brain regions that mediate autonomic, mood, and cognitive functions in adolescents with SVHD. These brain areas included the bilateral thalamus, caudate nuclei, putamen, insular cortices, prefrontal cortices, postcentral and precentral gyrus, occipital gyrus, parahippocampal gyrus, temporal gyrus and cerebellar cortices. These findings may result from hypoxia-ischemia or developmental-induced pathological processes in the condition.
Highlights.
The study demonstrates gray matter changes in single ventricle heart disease subjects using voxel based morphometry procedure.
Single ventricle heart disease showed decreased gray matter in multiple brain sites over control subjects.
Study provides structural basis for the mood and cognitive deficits found in single ventricle heart disease.
Acknowledgments
Authors would like to thank Ruchi Vig, Luke Ehlert, Ariana Dideban, and Patty Chung for assistance with data collection. This research was supported by the National Institutes of Health R01-NR013930 and R01-NR016463.
Footnotes
Conflicts of Interest:
All authors have no conflicts of interest relevant to this article to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bellinger DC, Wypij D, Rivkin MJ, DeMaso DR, Robertson RL, Jr, Dunbar-Masterson C, Rappaport LA, Wernovsky G, Jonas RA, Newburger JW. Adolescents with d-transposition of the great arteries corrected with the arterial switch procedure: neuropsychological assessment and structural brain imaging. Circulation. 2011;124:1361–9. doi: 10.1161/CIRCULATIONAHA.111.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Rhein M, Scheer I, Loenneker T, Huber R, Knirsch W, Latal B. Structural brain lesions in adolescents with congenital heart disease. J Pediatr. 2011;158:984–9. doi: 10.1016/j.jpeds.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 3.Bellinger DC, Wypij D, duPlessis AJ, Rappaport LA, Jonas RA, Wernovsky G, Newburger JW. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: The Boston Circulatory Arrest Trial. Journal of Thoracic and Cardiovascular Surgery. 2003;126:1385–1396. doi: 10.1016/s0022-5223(03)00711-6. [DOI] [PubMed] [Google Scholar]
- 4.Wernovsky G. Current insights regarding neurological and developmental abnormalities in children and young adults with complex congenital cardiac disease. Cardiology in the Young. 2006;16:92–104. doi: 10.1017/S1047951105002398. [DOI] [PubMed] [Google Scholar]
- 5.Marelli A, Miller SP, Marino BS, Jefferson AL, Newburger JW. Brain in Congenital Heart Disease Across the Lifespan: The Cumulative Burden of Injury. Circulation. 2016;133:1951–62. doi: 10.1161/CIRCULATIONAHA.115.019881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilboa SM, Devine OJ, Kucik JE, Oster ME, Riehle-Colarusso T, Nembhard WN, Xu P, Correa A, Jenkins K, Marelli AJ. Congenital Heart Defects in the United States: Estimating the Magnitude of the Affected Population in 2010. Circulation. 2016;134:101–9. doi: 10.1161/CIRCULATIONAHA.115.019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mulkey SB, Ou X, Ramakrishnaiah RH, Glasier CM, Swearingen CJ, Melguizo MS, Yap VL, Schmitz ML, Bhutta AT. White matter injury in newborns with congenital heart disease: a diffusion tensor imaging study. Pediatr Neurol. 2014;51:377–83. doi: 10.1016/j.pediatrneurol.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andropoulos DB, Hunter JV, Nelson DP, Stayer SA, Stark AR, McKenzie ED, Heinle JS, Graves DE, Fraser CD. Brain immaturity is associated with brain injury before and after neonatal cardiac surgery with high-flow bypass and cerebral oxygenation monitoring. Journal of Thoracic and Cardiovascular Surgery. 2010;139:543–556. doi: 10.1016/j.jtcvs.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Licht DJ, Shera DM, Clancy RR, Wernovsky G, Montenegro LM, Nicolson SC, Zimmerman RA, Spray TL, Gaynor JW, Vossough A. Brain maturation is delayed in infants with complex congenital heart defects. Journal of Thoracic and Cardiovascular Surgery. 2009;137:529–537. doi: 10.1016/j.jtcvs.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galli KK, Zimmerman RA, Jarvik GP, Wernovsky G, Kuypers MK, Clancy RR, Montenegro LM, Mahle WT, Newman MF, Saunders AM, Nicolson SC, Spray TL, Gaynor JW. Periventricular leukomalacia is common after neonatal cardiac surgery. Journal of Thoracic and Cardiovascular Surgery. 2004;127:692–704. doi: 10.1016/j.jtcvs.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 11.Miller SP, McQuillen PS, Hamrick S, Xu D, Glidden DV, Charlton N, Karl T, Azakie A, Ferriero DM, Barkovich AJ, Vigneron DB. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357:1928–38. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- 12.Rollins CK, Watson CG, Asaro LA, Wypij D, Vajapeyam S, Bellinger DC, DeMaso DR, Robertson RL, Jr, Newburger JW, Rivkin MJ. White matter microstructure and cognition in adolescents with congenital heart disease. J Pediatr. 2014;165:936–44e1. 2. doi: 10.1016/j.jpeds.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahle WT, Tavani F, Zimmerman RA, Nicolson SC, Galli KK, Gaynor JW, Clancy RR, Montenegro LM, Spray TL, Chiavacci RM, Wernovsky G, Kurth CD. An MRI study of neurological injury before and after congenital heart surgery. Circulation. 2002;106:I109–I114. [PubMed] [Google Scholar]
- 14.Ashburner J, Friston KJ. Voxel-based morphometry - The methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 15.Morrell MJ, Jackson ML, Twigg GL, Ghiassi R, McRobbie DW, Quest RA, Pardoe H, Pell GS, Abbott DF, Rochford PD, Jackson GD, Pierce RJ, O’Donoghue FJ, Corfield DR. Changes in brain morphology in patients with obstructive sleep apnoea. Thorax. 2010;65:908–914. doi: 10.1136/thx.2009.126730. [DOI] [PubMed] [Google Scholar]
- 16.Keller SS, Wilke M, Wieshmann UC, Sluming VA, Roberts N. Comparison of standard and optimized voxel-based morphometry for analysis of brain changes associated with temporal lobe epilepsy. Neuroimage. 2004;23:860–868. doi: 10.1016/j.neuroimage.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 17.Karas GB, Scheltens P, Rombouts SARB, Visser PJ, van Schijndel RA, Fox NC, Barkhof F. Global and local gray matter loss in mild cognitive impairment and Alzheimer’s disease. Neuroimage. 2004;23:708–716. doi: 10.1016/j.neuroimage.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Woo MA, Macey PM, Fonarow GC, Hamilton MA, Harper RM. Regional brain gray matter loss in heart failure. Journal of Applied Physiology. 2003;95:677–684. doi: 10.1152/japplphysiol.00101.2003. [DOI] [PubMed] [Google Scholar]
- 19.von Rhein M, Buchmann A, Hagmann C, Huber R, Klaver P, Knirsch W, Latal B. Brain volumes predict neurodevelopment in adolescents after surgery for congenital heart disease. Brain. 2014;137:268–76. doi: 10.1093/brain/awt322. [DOI] [PubMed] [Google Scholar]
- 20.Cordina R, Grieve S, Barnett M, Lagopoulos J, Malitz N, Celermajer DS. Brain Volumetrics, Regional Cortical Thickness and Radiographic Findings in Adults with Cyanotic Congenital Heart Disease. Neuroimage-Clinical. 2014;4:319–325. doi: 10.1016/j.nicl.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watanabe K, Matsui M, Matsuzawa J, Tanaka C, Noguchi K, Yoshimura N, Hongo K, Ishiguro M, Wanatabe S, Hirono K, Uese K, Ichida F, Origasa H, Nakazawa J, Oshima Y, Miyawaki T, Matsuzaki T, Yagihara T, Bilker W, Gur RC. Impaired neuroanatomic development in infants with congenital heart disease. Journal of Thoracic and Cardiovascular Surgery. 2009;137:146–153. doi: 10.1016/j.jtcvs.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 22.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–7. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 23.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pike NA, Evangelista LS, Doering LV, Eastwood JA, Lewis AB, Child JS. Quality of Life, Health Status, and Depression Comparison Between Adolescents and Adults After the Fontan Procedure With Healthy Counterparts. Journal of Cardiovascular Nursing. 2012;27:539–546. doi: 10.1097/JCN.0b013e31822ce5f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bang JS, Jo S, Kim GB, Kwon BS, Bae EJ, Noh CI, Choi JY. The mental health and quality of life of adult patients with congenital heart disease. International Journal of Cardiology. 2013;170:49–53. doi: 10.1016/j.ijcard.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 27.Pike NA, Poulsen MK, Woo MA. Validity of the Montreal Cognitive Assessment Screener in Adolescents and Young Adults With and Without Congenital Heart Disease. Nurs Res. 2017;66:222–230. doi: 10.1097/NNR.0000000000000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheslow D, Adams W. Wide range assessment of memory and learning administration and technical manual. 2. Wilmington, DE: Wide Range Inc; 2003. [Google Scholar]
- 29.Ashburner J. VBM Tutorial. 2010 [Google Scholar]
- 30.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Zolamorgan S, Squire LR, Amaral DG, Suzuki WA. Lesions of Perirhinal and Parahippocampal Cortex That Spare the Amygdala and Hippocampal-Formation Produce Severe Memory Impairment. Journal of Neuroscience. 1989;9:4355–4370. doi: 10.1523/JNEUROSCI.09-12-04355.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ploner CJ, Gaymard BM, Rivaud-Pechoux S, Baulac M, Clemenceau S, Samson S, Pierrot-Deseilligny C. Lesions affecting the parahippocampal cortex yield spatial memory deficits in humans. Cerebral Cortex. 2000;10:1211–1216. doi: 10.1093/cercor/10.12.1211. [DOI] [PubMed] [Google Scholar]
- 33.Aminoff EM, Kveraga K, Bar M. The role of the parahippocampal cortex in cognition. Trends in Cognitive Sciences. 2013;17:379–390. doi: 10.1016/j.tics.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lanciego JL, Luquin N, Obeso JA. Functional Neuroanatomy of the Basal Ganglia. Cold Spring Harbor Perspectives in Medicine. 2012;2 doi: 10.1101/cshperspect.a009621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18:2735–47. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- 36.DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Archives of Neurology. 2007;64:20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- 37.Alexander GE, Delong MR, Strick PL. Parallel Organization of Functionally Segregated Circuits Linking Basal Ganglia and Cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 38.Bonelli RM, Cummings JL. Frontal-subcortical circuitry and behavior. Dialogues Clin Neurosci. 2007;9:141–51. doi: 10.31887/DCNS.2007.9.2/rbonelli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cramer SC, Moore CI, Finklestein SP, Rosen BR. A pilot study of somatotopic mapping after cortical infarct. Stroke. 2000;31:668–71. doi: 10.1161/01.str.31.3.668. [DOI] [PubMed] [Google Scholar]
- 40.Schmahmann JD. From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Hum Brain Mapp. 1996;4:174–98. doi: 10.1002/(SICI)1097-0193(1996)4:3<174::AID-HBM3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 41.Schmahmann JD, Pandya DN. Anatomic organization of the basilar pontine projections from prefrontal cortices in rhesus monkey. J Neurosci. 1997;17:438–58. doi: 10.1523/JNEUROSCI.17-01-00438.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pike NA, Woo MA, Poulsen MK, Evangelista W, Faire D, Halnon NJ, Lewis AB, Kumar R. Predictors of Memory Deficits in Adolescents and Young Adults with Congenital Heart Disease Compared to Healthy Controls. Front Pediatr. 2016;4:117. doi: 10.3389/fped.2016.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cassidy AR, White MT, DeMaso DR, Newburger JW, Bellinger DC. Executive Function in Children and Adolescents with Critical Cyanotic Congenital Heart Disease. J Int Neuropsychol Soc. 2015;21:34–49. doi: 10.1017/S1355617714001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liamlahi R, von Rhein M, Buhrer S, Valsangiacomo Buechel ER, Knirsch W, Landolt MA, Latal B. Motor dysfunction and behavioural problems frequently coexist with congenital heart disease in school-age children. Acta Paediatr. 2014;103:752–8. doi: 10.1111/apa.12639. [DOI] [PubMed] [Google Scholar]
- 45.Spijkerboer AW, Utens EM, Bogers AJ, Helbing WA, Verhulst FC. A historical comparison of long-term behavioral and emotional outcomes in children and adolescents after invasive treatment for congenital heart disease. J Pediatr Surg. 2008;43:534–9. doi: 10.1016/j.jpedsurg.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 46.Pike NA, Evangelista LS, Doering LV, Koniak-Griffin D, Lewis AB, Child JS. Clinical profile of the adolescent/adult Fontan survivor. Congenit Heart Dis. 2011;6:9–17. doi: 10.1111/j.1747-0803.2010.00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DeMaso DR, Calderon J, Taylor GA, Holland JE, Stopp C, White MT, Bellinger DC, Rivkin MJ, Wypij D, Newburger JW. Psychiatric Disorders in Adolescents With Single Ventricle Congenital Heart Disease. Pediatrics. 2017;139 doi: 10.1542/peds.2016-2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luyckx K, Rassart J, Goossens E, Apers S, Oris L, Moons P. Development and persistence of depressive symptoms in adolescents with CHD. Cardiol Young. 2016;26:1115–22. doi: 10.1017/S1047951115001882. [DOI] [PubMed] [Google Scholar]
- 49.Kovacs AH, Saidi AS, Kuhl EA, Sears SF, Silversides C, Harrison JL, Ong L, Colman J, Oechslin E, Nolan RP. Depression and anxiety in adult congenital heart disease: predictors and prevalence. Int J Cardiol. 2009;137:158–64. doi: 10.1016/j.ijcard.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 50.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin EI, Ressler KJ, Binder E, Nemeroff CB. The neurobiology of anxiety disorders: brain imaging, genetics, and psychoneuroendocrinology. Psychiatr Clin North Am. 2009;32:549–75. doi: 10.1016/j.psc.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baur V, Hanggi J, Langer N, Jancke L. Resting-State Functional and Structural Connectivity Within an Insula-Amygdala Route Specifically Index State and Trait Anxiety. Biological Psychiatry. 2013;73:85–92. doi: 10.1016/j.biopsych.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 53.Modi S, Kumar M, Kumar P, Khushu S. Aberrant functional connectivity of resting state networks associated with trait anxiety. Psychiatry Res. 2015;234:25–34. doi: 10.1016/j.pscychresns.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 54.Zeng LL, Shen H, Liu L, Wang LB, Li BJ, Fang P, Zhou ZT, Li YM, Hu DW. Identifying major depression using whole-brain functional connectivity: a multivariate pattern analysis. Brain. 2012;135:1498–1507. doi: 10.1093/brain/aws059. [DOI] [PubMed] [Google Scholar]
- 55.Liu L, Zeng LL, Li Y, Ma Q, Li B, Shen H, Hu D. Altered cerebellar functional connectivity with intrinsic connectivity networks in adults with major depressive disorder. PLoS One. 2012;7:e39516. doi: 10.1371/journal.pone.0039516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biological Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 57.Shoemaker JK, Goswami R. Forebrain neurocircuitry associated with human reflex cardiovascular control. Frontiers in Physiology. 2015;6 doi: 10.3389/fphys.2015.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harper RM, Bandler R, Spriggs D, Alger JR. Lateralized and widespread brain activation during transient blood pressure elevation revealed by magnetic resonance imaging. Journal of Comparative Neurology. 2000;417:195–204. doi: 10.1002/(sici)1096-9861(20000207)417:2<195::aid-cne5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 59.McQuillen PS, Goff DA, Licht DJ. Effects of congenital heart disease on brain development. Prog Pediatr Cardiol. 2010;29:79–85. doi: 10.1016/j.ppedcard.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Glauser TA, Rorke LB, Weinberg PM, Clancy RR. Acquired neuropathologic lesions associated with the hypoplastic left heart syndrome. Pediatrics. 1990;85:991–1000. [PubMed] [Google Scholar]