Abstract
Alcohol (ethanol) and methamphetamine (METH) co-abuse is a major public health issue. Ethanol or METH exposure has been associated with changes in neurotransmitter levels in several central brain regions. However, little is known about the effect of sequential exposure to ethanol and METH on glutamate, dopamine and serotonin tissue content in striatum and hippocampus. In this study, we investigated the effects of sequential exposure to ethanol and METH on tissue content of these neurotransmitters. Male Wistar rats were orally gavaged with either ethanol (6 g/kg) or water for seven days. Rats were administered with high dose of METH (10 mg/kg, i.p. every 2 h × 4) or saline on Day 8 and euthanized 48 hours of last METH or saline i.p. injection. In the striatum, sequential exposure to ethanol and METH increased glutamate tissue content while reducing dopamine and serotonin tissue content as compared to the group exposed to ethanol alone. In the hippocampus, sequential exposure to ethanol and METH decreased serotonin tissue content as compared to the group that was exposed to ethanol alone. However, this study showed that ethanol has no additive effect to METH on tissue content of dopamine and serotonin as compared to METH in the striatum and hippocampus. This study demonstrated that sequential exposure of ethanol and METH has an additive effect on tissue content of certain neurotransmitters in the brain.
Keywords: Ethanol, methamphetamine, dopamine, serotonin, glutamate, striatum
Introduction
Methamphetamine (METH) is an amphetamine type stimulant that induces drug desire, cognitive impairment and distraction (Simon et al., 2000, Meredith et al., 2005). Alcohol (ethanol) addiction is a disorder that is characterized by compulsive drug intake and seeking (Schuckit et al., 1994, McLellan et al., 2000). It has been shown that around 75% of amphetamine dependents consumed ethanol (Stinson et al., 2005). Several studies from our laboratory and others revealed that glutamatergic, serotoninergic and dopaminergic systems in the brain are altered with co-exposure to drugs of abuse, including METH and ethanol (Tata et al., 2007, Halpin et al., 2014, Das et al., 2015, Althobaiti et al., 2016a, Alshehri et al., 2017). Indeed, METH exposure (10 mg/kg i.p. every 2 hours × 4) increased extracellular glutamate in the striatum (Nash and Yamamoto, 1992, Stephans and Yamamoto, 1994, Mark et al., 2004, Halpin et al., 2014). Furthermore, METH exposure induced damage of dopaminergic terminals leading to lower levels of striatal dopamine (Mark et al., 2004). In regards to ethanol, acute exposure has been shown to mediate brain damage, enhance cognitive impairment and increase extracellular glutamate concentration in the hippocampus (Crews et al., 2000, Ward et al., 2009, Chefer et al., 2011, Kuzmin et al., 2012).
Despite the evidence of the high prevalence of ethanol and METH co-abuse, less is known about the effect of sequential exposure of ethanol and METH on the tissue content of neurotransmitters in the striatum and hippocampus. Thus, we hypothesized that sequential exposure to ethanol and METH would lead to synergistic effects in the alteration of tissue content of the studied neurotransmitters. To test this hypothesis, we used male Wistar rats, which received ethanol (6 g/kg) or water through oral gavage for seven days. Rats were administered with high dose of METH (10 mg/kg, i.p. every 2 h × 4) or saline on Day 8 and euthanized 48 hours after the last METH or saline injection. In this study, we used ethanol dose of 6 g/kg/day because of its ability to alter neurotransmission and neurogenesis in CNS (Nixon and Crews, 2002, Abulseoud et al., 2014). Moreover, ethanol exposure has been associated with increase in extracellular dopamine and serotonin concentrations in the nucleus accumbens and striatum (Wozniak et al., 1990, Wozniak et al., 1991, Yoshimoto et al., 1992). It has been shown that hippocampus, the brain region that is responsible for memory and learning, and the striatum, which controls reward and voluntary movement was affected by ethanol or METH (Bowyer and Ali, 2006, Rau et al., 2006, Muskens et al., 2012, Saint-Preux et al., 2013). Therefore, we investigated the effect of repeated high-dose METH exposure, administered to ethanol-exposed rats, on tissue content of several neurotransmitters such as glutamate, dopamine and serotonin in the striatum and the hippocampus.
Materials and methods
Subjects
Male Wistar rats weighing 200–300 g were used in this study. Rats were purchased from Envigo RMS, Inc. (Indianapolis, IN). Rats were single-housed and kept in a 12:12 light-dark cycle, controlled temperature at 21°C, and humidity (30%). Rats were handled and habituated prior to conducting the experiments. They had a free access to food and water during the experimental procedure; and they were deprived two hours, to make sure that they received the full dose, before oral gavage of either water or ethanol (6g/kg, made from 40% v/v). Animal housing and experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Toledo. These were in agreement with the guidelines of the Institutional Animal Care and Use Committee of the National Institutes of Health and the Guide for the Care and Use of Laboratory Animals.
Drugs
(+) - METH hydrochloride was purchased from Sigma-Aldrich (St. Louis, MO). Saline solution (0.9% NaCl) was used to dissolve METH. Ethanol (95%; Decon Labs, Inc.) was diluted in water.
Experimental design
Four groups of rats were used in this study: (1) Water-saline group was given water through oral gavage route for seven days and received four repeated intraperitoneal (i.p.) injections of saline vehicle on Day 8; (2) Water-METH group was given water through oral gavage route for seven days and received METH (10 mg/kg i.p. every 2 hours × 4) on Day 8; (3) Ethanol-saline group received ethanol (6 g/kg, made from 40% v/v) through oral gavage route for seven days and received four i.p. injections of saline vehicle on Day 8; and (4) Ethanol-METH group received ethanol (6 g/kg) through oral gavage route and METH (10 mg/kg i.p. every 2 hours × 4) on Day 8 (i.p). After 48 hours of the last METH i.p. injection, rats were immediately euthanized by CO2 inhalation and further decapitated. The brains were then extracted and quickly stored in dry ice and then kept at −80°C. The striatum and hippocampus were dissected out at −20°C using cryostat machine. Brain Stereotaxic Atlas was used to dissect out the selected brain region (Paxinos, 2007) as previously described in previous study from our laboratory (Sari and Sreemantula, 2012).
HPLC quantification of dopamine and serotonin
HPLC with electrochemical detection (EC) system was used to detect the tissue content of dopamine and serotonin in the striatum and hippocampus as previously illustrated (Althobaiti et al., 2016a). Briefly, brain regions were homogenized with pestle in 0.25N perchloric acid. Samples were sonicated for 20 min, and then centrifuged at 14000 × g for 20 min at 4°C. The supernatant was filtered through 0.22 μm filters. The filtered supernatant was injected through a C18 column (3.2 × 150mm, 3μm particle size, Thermo Scientific). The remaining pellets were re-suspended to measure protein quantity. In this study, the mobile phase consists of 54.3 mM sodium phosphate, 32 mM citric acid, 11% methanol, 0.215 mM octyl sodium sulfate, and 0.22 mM triehtylamine. The pH of the mobile phase was 4.4. CoulArray coulometric detector (model 5600A, ESA, Inc.) was used to measure the tissue content of dopamine and serotonin. The data were collected using CoulArray software. Neurotransmitter concentrations in each sample were analyzed using the area under the curve and compared with external standards in all groups.
HPLC quantification of glutamate
HPLC with electrochemical detection (EC) system was also used to analyze glutamate tissue content in the striatum and hippocampus as previously illustrated (Das et al., 2015, Althobaiti et al., 2016a). The tissue content of glutamate was determined by HPLC analysis (ESA, Inc) with electrochemical detection. Millipore water was used to lysate brain tissue and centrifuged at 14,000 rpm for 20 min. The supernatant was then filtered using 0.22 μm filter. After the samples were derivatized with O-phthalaldehyde (OPA) and sodium sulfite using a ESA Model 540 autosampler, these samples were then injected through a C18 column (3.0 × 50 mm, 2.5 μm particle size, Waters). In this experiment, the mobile phase was consisted of 0.1 M Na2HPO4, 0.1 mM EDTA and 7.5% Methanol (pH 3.0). CoulArray coulometric detection (model 5600A, ESA, Inc.) was used to analyze glutamate tissue contents. The data were collected using CoulArray software. Glutamate tissue content in each sample was analyzed using the area under the curve and compared with external standard in all groups.
Statistical analysis
Two-way ANOVA was used to analyze tissue content of studied neurotransmitters for comparison between ethanol, ethanol/METH, METH, and water control groups. When significant effects were revealed, Newman-Keuls multiple comparisons test was used to evaluate the significant difference between each drug against its corresponding control group and to assess between concentrations for each drug. Statistical significance was set at p < 0.05 and GraphPad Prism was used to assess all data.
Results
Effects of METH administered alone or with ethanol on glutamate tissue content in the striatum and hippocampus
Two-way ANOVA revealed a significant main effect of METH posttreatment in striatum [F (1, 27) = 17.95, P = 0.0002] without altering tissue content of glutamate in hippocampus [F (1, 29) = 0.2253, P = 0.6386] (Fig 1). In addition, significant main effect of oral gavage ethanol pretreatment was revealed using two-way ANOVA in striatum [F (1, 27) = 21.03, P < 0.0001], but not in hippocampus [F (1, 29) = 0.3560, P = 0.5554]. However, no significant interaction between METH posttreatment and ethanol pretreatment was found in both striatum [F (1, 27) = 0.4771, P = 0.4956] and hippocampus [F (1, 29) = 0.2662, P = 0.6098]. Newman-Keuls multiple comparisons test showed increased glutamate tissue content in the water-METH group as compared to water-saline, ethanol-saline and ethanol-METH groups in the striatum (Fig. 1A). In addition, glutamate tissue content was increased in the ethanol-METH group as compared to the ethanol-saline group (Fig. 1A). However, glutamate tissue content was decreased in the ethanol-saline group as compared to the water-saline group in the striatum (Fig. 1A). No significant changes in glutamate tissue content were found between groups in the hippocampus (Fig. 1B).
Figure 1.
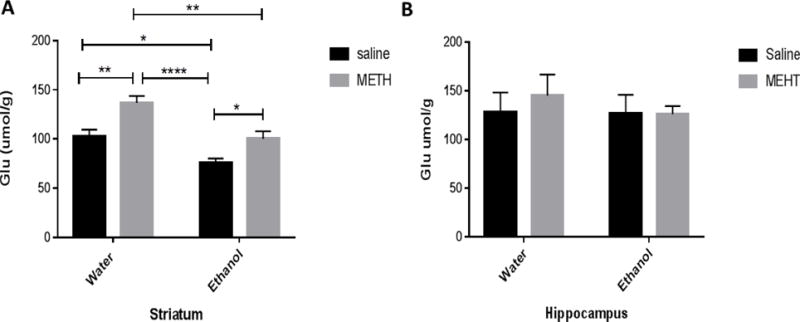
Effects of sequential exposure to ethanol (6 g/kg) and METH (10 mg/kg i.p. every 2 hours × 4) on glutamate tissue content in the striatum (A) and hippocampus (B). A) Statistical analysis revealed that glutamate tissue content in the water-METH group was significantly higher as compared to the water-saline group, ethanol-saline group and ethanol-METH group. Glutamate tissue content was significantly elevated in the ethanol-METH group as compared to ethanol-saline group. Alternatively, glutamate tissue content was significantly lower in ethanol-saline group as compared to water-saline group. B) Quantitative analysis revealed no significant change in hippocampal glutamate tissue content between groups. Values are shown as means ± S.E.M (*p < 0.05, **p < 0.01, ****p<0.0001). (n = 7–10 for each group).
Effects of METH administered alone or with ethanol on dopamine tissue content in the striatum and hippocampus
Two-way ANOVA revealed a significant main effect of METH posttreatment in striatum [F (1, 28) = 89.15, P = 0.0001] (Fig 2A) and hippocampus [F (1, 27) = 7.775, P = 0.0096] (Fig 2B). Significant effect of orally gavaged ethanol pretreatment group was found in both striatum [F (1, 28) = 35.77, P < 0.0001] and hippocampus [F (1, 27) = 5.669, P=0.0246]. Two-way ANOVA test also revealed significant interaction between METH posttreatment group and oral gavage ethanol pretreatment group in the striatum [F (1, 28) = 61.35, P < 0.0001], but not in hippocampus [F (1, 27) = 1.070, P = 0.3102]. Newman-Keuls multiple comparisons test showed no significant changes in dopamine tissue content between the water-saline group and the water-METH group in the striatum (Fig. 2A). Importantly, sequential exposure to ethanol and METH did not induce synergistic effect in the depletion of dopamine as compared to water-METH group (Fig. 2A). Also, sequential exposure to ethanol and METH induced significant depletion of dopamine in ethanol-METH group as compared to the ethanol-saline group (Fig. 2A). Additionally, dopamine tissue content was elevated in the ethanol-saline group as compared to the water-saline group (Fig. 2A) in the striatum. Furthermore, hippocampal dopamine tissue content was significantly lower in water-METH, ethanol-saline and ethanol-METH groups as compared to water-saline group (Fig 2B). No significant difference was found in hippocampal dopamine tissue content between the ethanol-saline group and the ethanol-METH group (Fig. 2B).
Figure 2.
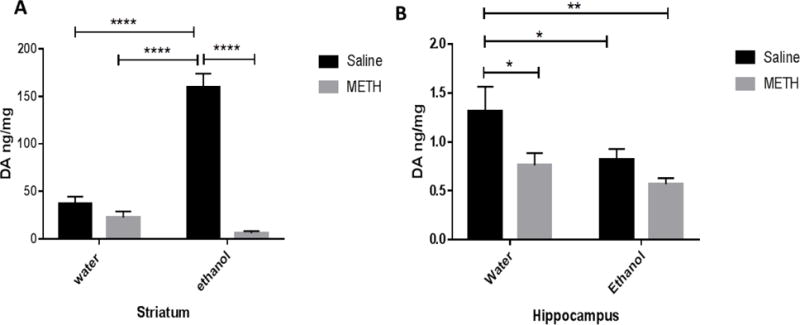
Effects of sequential exposure to ethanol (6 g/kg once a day for 7 days) and METH (10 mg/kg i.p. every 2 hours × 4) on dopamine tissue content in striatum (A) and hippocampus (B). A) No significant change in dopamine tissue content was found between the water-saline group and water-METH group. Dopamine tissue content was also not significantly changed in the ethanol-METH group compared to the water-METH group in the striatum. However, dopamine tissue content in the water-METH and ethanol-METH groups were significantly lower as compared to the ethanol-saline group. In contrast, statistical analysis revealed that dopamine tissue content was significantly elevated in the ethanol-saline group as compared to the water-saline group. B) Quantitative analysis revealed significant decrease in hippocampal dopamine tissue content in the water-METH, ethanol-saline, ethanol-METH groups as compared to the water-saline group. Values are shown as means ± S.E.M (*p < 0.05, **p < 0.01, ****p < 0.0001). (n = 7–9 for each group).
Effects of METH administered alone or with ethanol on serotonin tissue content in the striatum and hippocampus
Two-way ANOVA revealed a significant effect of METH posttreatment in striatum [F (1, 28) = 117, P < 0.0001] and hippocampus [F (1, 29) = 32.80, P < 0.0001]. Significant effect of oral gavage of ethanol pretreatment was observed in striatum [F (1, 28) = 36.31, P < 0.0001], but not in hippocampus [F (1, 29) = 0.05634 P = 0.8140]. Moreover, significant interaction between METH posttreatment and oral gavage of ethanol pretreatment in the striatum [F (1, 28) = 56.01, P < 0.0001], but not in hippocampus [F (1, 29) = 0.1826, P = 0.6723]. Newman-Keuls multiple comparisons test revealed that serotonin tissue content in the water-METH and ethanol-METH groups was significantly lower as compared to the water-saline group in the striatum (Fig. 3A). Importantly, sequential exposure to ethanol and METH did not show any synergistic effect in the reduction of striatal tissue content of serotonin in ethanol-METH group as compared to the water-METH group (Fig. 3A). Sequential exposure to ethanol and METH induced significant reduction in serotonin tissue content as compared to the ethanol-saline group in the striatum (Fig. 3A). Moreover, serotonin tissue content was significantly higher in the ethanol-saline group as compared to the water-saline group in the striatum (Fig. 3A).
Figure 3.
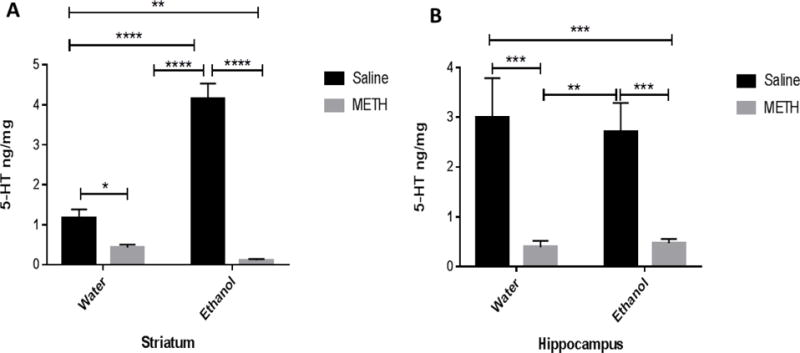
Effects of sequential exposure to ethanol (6 g/kg once a day for 7 days) and METH (10 mg/kg i.p. every 2 hours × 4) on serotonin tissue content in the striatum (A) and hippocampus (B). A) Statistical analysis revealed lower serotonin tissue content in the water-METH group as compared to water-saline group. However, serotonin tissue content was not changed in the ethanol-METH group as compared to the water-METH group. Serotonin tissue content was significantly diminished in the water-METH and ethanol-METH groups as compared to ethanol-saline group. However, tissue content of serotonin was significantly higher in the ethanol-saline group as compared to water-saline group. B) Statistical analysis revealed serotonin tissue content was significantly diminished in the water-METH and ethanol-METH groups as compared to water-saline group. In addition, serotonin tissue content was significantly reduced in the water-METH and ethanol-METH groups as compared to the ethanol-saline group. Values are shown as means ± S.E.M (**p < 0.01, ***p < 0.001, ****p < 0.0001) (n = 7–10 for each group).
Regarding to the hippocampus, tissue content of serotonin was decreased in the water-METH, and ethanol-METH groups as compared to the water-saline group (Fig. 3B). There were no synergistic effects with sequential exposure to ethanol and METH in the reduction of serotonin tissue content as compared to water-METH group (Fig. 3B). Sequential exposure to ethanol and METH induced reduction in serotonin tissue content as compared to the ethanol-saline group in the hippocampus (Fig. 3B). Nevertheless, serotonin tissue content was not changed in the ethanol-saline group as compared to the water-saline group (Fig. 3B).
Discussion
In the present study, we report that repeated high-dose METH alone increased striatal tissue content of glutamate, compared to saline-treated control group, with no effect on hippocampal glutamate content. These findings demonstrate that repeated high-dose METH might differentially affect the striatal and hippocampal glutamate content. The increase in striatal glutamate tissue content is well expected since METH acutely increased extracellular glutamate concentration in striatum (Halpin et al., 2014). Although METH is known to acutely increases the extracellular glutamate concentration in ventral hippocampus (Rocher and Gardier, 2001, Raudensky and Yamamoto, 2007, Qi et al., 2012), we did not find any significant effect of METH in hippocampal glutamate tissue content. This discrepancy might arise from the following facts- firstly, we determined glutamate tissue content in whole hippocampus whereas previous reports detected extracellular glutamate in ventral hippocampus. Secondly, previous reports showed an increase in extracellular glutamate within eight hours of METH injection whereas in the present study we collected brain tissue after 48 hours of the last METH injection. Thirdly, we report here the tissue content of glutamate, which is the sum of both extra- and intracellular glutamate. The intra-cellular glutamate is present in millimolar range whereas extracellular glutamate is present in micromolar range (Nedergaard et al., 2002). Besides, a fraction of glutamate concentration serves in neurotransmission as the majority of glutamate is utilized in other purposes such as glutamine synthesis and energy production through metabolism (Nedergaard et al., 2002). However, our present finding of the METH in hippocampal glutamate tissue content is in agreement with a most recent study demonstrated no difference in hippocampal glutamate tissue content following chronic or binge METH exposure (Kesby et al., 2017).
In this report, we showed that seven-day gavage of ethanol decreased tissue content of glutamate in striatum with no effect on tissue content of glutamate in hippocampus. Striatal tissue content of glutamate in rats exposed to ethanol and METH was significantly higher compared to rats exposed to ethanol alone, but significantly lower compared to rats exposed to METH alone. These findings are quite surprising since repeated ethanol exposure is associated with elevated extracellular glutamate concentration in the nucleus accumbens (Melendez et al., 2005), striatum (Murphy et al., 1985) and hippocampus (Chefer et al., 2011). One possible explanation for this dissimilarity might be attributed to binge ethanol exposure followed by 48 hours of withdrawal. Neurotoxicity might be another reason behind this decreased striatal glutamate content since binge ethanol has been shown to impart damages in corticolimbic brain regions (Crews et al., 2000, Obernier et al., 2002). Disrupted glutamate-glutamine cycle, including decreased glutamate content has also been shown in patients with alcohol use disorders (Thoma et al., 2011). Our current result is also in concert with the recent finding from our laboratory that showed decreased glutamate tissue content in the nucleus accumbens following binge ethanol-withdrawal (Das et al., 2016).
There are several mechanisms that explain how the toxic doses of ethanol and METH affect the concentrations of neurotransmitters in the brain. METH acts as a substrate for both dopamine transporter (DAT) and serotonin transporter (SERT). Upon diffusion into axon terminal, METH acutely disrupts vesicle proton gradient and function of vesicular monoamine transporters resulting in an increase in cytoplasmic dopamine and serotonin levels (Halpin et al., 2014). Subsequently, excess of dopamine and serotonin concentrations are released into synaptic cleft and extracellular space. In long term, METH causes damages to dopaminergic and serotonergic axon terminals in both striatum and hippocampus (Halpin et al., 2014). Moreover, METH causes neuronal cell death through initiation of excitotoxicity, which further activates sequence of events, including reactive oxygen species generation, nitric oxide production and apoptotic pathways (Bruno et al., 1993, Cadet et al., 2005). Alternatively, the pharmacology of ethanol is more complex and it may affect multiple neurotransmitter systems. Acute ethanol consumption enhances GABAergic neurotransmission through the increase of the chloride conductance of GABAA receptor (Nevo and Hamon, 1995). Unlike acute ethanol, chronic ethanol consumption enhances glutamatergic neurotransmission leading to hyper-neuroexcitation. One of the causes of chronic ethanol-induced hyper-neuroexcitation might be attributed to decrease in a major glial glutamate transporter such as glutamate transporter 1 (GLT-1) in the nucleus accumbens (Sari and Sreemantula, 2012, Das et al., 2016) and striatum (Abulseoud et al., 2014). Interestingly, METH exposure also decreased the expression of GLT-1 in the nucleus accumbens, striatum and hippocampus (Althobaiti et al., 2016b, Alshehri et al., 2017). Therefore, one of the mechanisms is that METH and ethanol affect glutamate tissue content through alteration of GLT-1 expression in the brain.
In addition, high dose of METH has been shown to increase glutamate release and long-term dopamine depletion in striatum through activation of striatonigral pathway (Mark et al., 2004). METH is also known to interact with the transporters of serotonin and dopamine and affect the vesicular monoamine transporters (VMAT 2) (Fleckenstein et al., 1997, Burrows et al., 2000, Riddle et al., 2002). Furthermore, ethanol and METH are proven to increase ammonia, which is known to generate oxidative stress and induce damage to dopamine and serotonin neuronal terminals (Imam et al., 2001b, Das and Vasudevan, 2007, Halpin et al., 2014).
In this study, we demonstrated that seven-day ethanol gavage increased striatal tissue content of dopamine. This result is fairly expected as ethanol is known to increase dopamine tissue content in the nucleus accumbens (Mcbride et al., 1990). However, in this study METH alone did not significantly affect striatal dopamine tissue content. This is in contrast to finding from previous study demonstrated that repeated METH administration caused long-term depletion of striatal dopamine (Wagner et al., 1980, Halpin and Yamamoto, 2012, Northrop and Yamamoto, 2013, Blaker and Yamamoto, 2017). This dissimilarity might be attributed to the differential time-point of tissue collection (two days versus seven or more days METH post-injection) and/or euthanization method (CO2 inhalation versus live-decapitation). Interestingly, repeated high-dose of METH in ethanol-exposed rats decreased the striatal tissue content of dopamine and serotonin as compared to ethanol but not to METH alone. These findings put forth evidence that ethanol and METH co-exposure exacerbated the striatal dopaminergic depletion as compared to ethanol alone. Co-exposure to METH and ethanol was found to diminish the action of ethanol. These findings are in accordance with previous reports that revealed that exposure to METH with dose of 12.5mg/kg or 10mg/kg given i.p. every 2 hours decreased the dopamine and serotonin tissue content in the striatum (Friedman et al., 1998, Imam et al., 2001a, Raudensky and Yamamoto, 2007, Tata et al., 2007). Also, hippocampal tissue content of dopamine was decreased in rats exposed sequentially to METH and ethanol or drug alone compared to control rats. In contrast, it has been shown that dopamine concentration was not changed in the hippocampus with exposure to METH (Northrop and Yamamoto, 2013).
Finally, we determined the striatal and hippocampal tissue content of serotonin in ethanol and/or METH groups and compared with their corresponding control groups. Seven-day ethanol gavage increased tissue content of serotonin in striatum but not in hippocampus. These findings are in agreement with previous study, which has shown that exposure to a single dose of ethanol (2.5 g/kg, i.p.) increased serotonin tissue content in the nucleus accumbens (Mcbride et al., 1990). Also, it has been reported that i.p. injections of ethanol (1.0 g/kg) for five consecutive days elevated extracellular serotonin concentration in the nucleus accumbens (Smith and Weiss, 1999). Repeated METH i.p. injections alone caused significant depletion of both striatal and hippocampal serotonin while addition of ethanol did not aggravate this depletion significantly. This result provides evidence, in addition to dopamine depletion, that METH alone can lead to serotonin depletion in both striatum and hippocampus at 48 hours METH post-injection. Also, sequential exposure to ethanol and METH did affect the tissue content of dopamine and serotonin significantly as compared to exposure to METH alone.
In conclusion, both ethanol and METH exposure, compared to ethanol alone, increased glutamate tissue content in the striatum but not in the hippocampus. Interestingly, ethanol increased dopamine tissue content in the striatum while decreased dopamine tissue content in the hippocampus. Pre-exposure of ethanol did not have any additive effect on METH-induced depletion of dopamine and serotonin tissue content in both the striatum and hippocampus. In addition, ethanol alone increased serotonin tissue content in striatum but not in the hippocampus, however, METH alone depleted serotonin tissue content in both striatum and hippocampus. These data suggest that both glutamatergic and monoaminergic systems would be therapeutic targets for the treatment of the co-abuse of METH and ethanol.
Highlights.
-
>
Reduction of dopamine and 5-HT in ethanol/METH group in striatum/hippocampus.
-
>
Increased glutamate levels in ethanol/METH group in striatum.
-
>
Decreased glutamate levels in Ethanol/METH group compared to METH in striatum.
Acknowledgments
This work was supported in part by Award Number R01AA019458 (Y.S.) from the National Institutes on Alcohol Abuse and Alcoholism and by fund from the University of Toledo.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors declare no conflict of interest.
References
- Abulseoud OA, Camsari UM, Ruby CL, Kasasbeh A, Choi S, Choi D-S. Attenuation of ethanol withdrawal by ceftriaxone-induced upregulation of glutamate transporter EAAT2. Neuropsychopharmacology. 2014;39:1674–1684. doi: 10.1038/npp.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshehri FS, Althobaiti YS, Sari Y. Effects of Administered Ethanol and Methamphetamine on Glial Glutamate Transporters in Rat Striatum and Hippocampus. Journal of molecular neuroscience: MN. 2017;61:343–350. doi: 10.1007/s12031-016-0859-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althobaiti YS, Almalki AH, Das SC, Alshehri FS, Sari Y. Effects of repeated high-dose methamphetamine and ceftriaxone post-treatments on tissue content of dopamine and serotonin as well as glutamate and glutamine. Neuroscience Letters. 2016a;634:25–31. doi: 10.1016/j.neulet.2016.09.058. [DOI] [PubMed] [Google Scholar]
- Althobaiti YS, Alshehri FS, Almalki AH, Sari Y. Effects of Ceftriaxone on Glial Glutamate Transporters in Wistar Rats Administered Sequential Ethanol and Methamphetamine. Frontiers in neuroscience. 2016b;10:427. doi: 10.3389/fnins.2016.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaker AL, Yamamoto BK. Methamphetamine-Induced Brain Injury and Alcohol Drinking. J Neuroimmune Pharmacol. 2017 doi: 10.1007/s11481-017-9764-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowyer JF, Ali S. High doses of methamphetamine that cause disruption of the blood-brain barrier in limbic regions produce extensive neuronal degeneration in mouse hippocampus. Synapse. 2006;60:521–532. doi: 10.1002/syn.20324. [DOI] [PubMed] [Google Scholar]
- Bruno V, Scapagnini U, Canonico PL. Excitatory amino acids and neurotoxicity. Funct Neurol. 1993;8:279–292. [PubMed] [Google Scholar]
- Burrows KB, Gudelsky G, Yamamoto BK. Rapid and transient inhibition of mitochondrial function following methamphetamine or 3,4-methylenedioxymethamphetamine administration. Eur J Pharmacol. 2000;398:11–18. doi: 10.1016/s0014-2999(00)00264-8. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Jayanthi S, Deng X. Methamphetamine-induced neuronal apoptosis involves the activation of multiple death pathways. Review Neurotox Res. 2005;8:199–206. doi: 10.1007/BF03033973. [DOI] [PubMed] [Google Scholar]
- Chefer V, Meis J, Wang G, Kuzmin A, Bakalkin G, Shippenberg T. Repeated exposure to moderate doses of ethanol augments hippocampal glutamate neurotransmission by increasing release. Addiction biology. 2011;16:229–237. doi: 10.1111/j.1369-1600.2010.00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcoholism: Clinical and Experimental Research. 2000;24:1712–1723. [PubMed] [Google Scholar]
- Das SC, Althobaiti YS, Alshehri FS, Sari Y. Binge ethanol withdrawal: effects on post-withdrawal ethanol intake, glutamate–glutamine cycle and monoamine tissue content in P rat model. Behavioural brain research. 2016;303:120–125. doi: 10.1016/j.bbr.2016.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SC, Yamamoto BK, Hristov AM, Sari Y. Ceftriaxone attenuates ethanol drinking and restores extracellular glutamate concentration through normalization of GLT-1 in nucleus accumbens of male alcohol-preferring rats. Neuropharmacology. 2015;97:67–74. doi: 10.1016/j.neuropharm.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SK, Vasudevan DM. Alcohol-induced oxidative stress. Life sciences. 2007;81:177–187. doi: 10.1016/j.lfs.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Metzger RR, Wilkins DG, Gibb JW, Hanson GR. Rapid and reversible effects of methamphetamine on dopamine transporters. The Journal of pharmacology and experimental therapeutics. 1997;282:834–838. [PubMed] [Google Scholar]
- Friedman SD, Castañeda E, Hodge GK. Long-term monoamine depletion, differential recovery, and subtle behavioral impairment following methamphetamine-induced neurotoxicity. Pharmacology Biochemistry and Behavior. 1998;61:35–44. doi: 10.1016/s0091-3057(98)00066-5. [DOI] [PubMed] [Google Scholar]
- Halpin LE, Northrop NA, Yamamoto BK. Ammonia mediates methamphetamine-induced increases in glutamate and excitotoxicity. Neuropsychopharmacology. 2014;39:1031–1038. doi: 10.1038/npp.2013.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin LE, Yamamoto BK. Peripheral ammonia as a mediator of methamphetamine neurotoxicity. J Neurosci. 2012;32:13155–13163. doi: 10.1523/JNEUROSCI.2530-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imam SZ, EL-YAZAL J, Newport GD, Itzhak Y, Cadet JL, Slikker W, Ali SF. Methamphetamine-Induced Dopaminergic Neurotoxicity: Role of Peroxynitrite and Neuroprotective Role of Antioxidants and Peroxynitrite Decomposition Catalysts. Annals of the New York Academy of Sciences. 2001a;939:366–380. doi: 10.1111/j.1749-6632.2001.tb03646.x. [DOI] [PubMed] [Google Scholar]
- Imam SZ, Newport GD, Itzhak Y, Cadet JL, Islam F, Slikker W, Ali SF. Peroxynitrite plays a role in methamphetamine‐induced dopaminergic neurotoxicity: evidence from mice lacking neuronal nitric oxide synthase gene or overexpressing copper–zinc superoxide dismutase. Journal of neurochemistry. 2001b;76:745–749. doi: 10.1046/j.1471-4159.2001.00029.x. [DOI] [PubMed] [Google Scholar]
- Kesby JP, Chang A, Markou A, Semenova S. Modeling human methamphetamine use patterns in mice: chronic and binge methamphetamine exposure, reward function and neurochemistry. Addict Biol. 2017 doi: 10.1111/adb.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin A, Liljequist S, Meis J, Chefer V, Shippenberg T, Bakalkin G. Repeated moderate‐dose ethanol bouts impair cognitive function in Wistar rats. Addiction biology. 2012;17:132–140. doi: 10.1111/j.1369-1600.2010.00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark KA, Soghomonian J-J, Yamamoto BK. High-dose methamphetamine acutely activates the striatonigral pathway to increase striatal glutamate and mediate long-term dopamine toxicity. The Journal of neuroscience. 2004;24:11449–11456. doi: 10.1523/JNEUROSCI.3597-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcbride WJ, Murphy JM, Lumeng L, Li TK. Serotonin, Dopamine and Gaba Involvement in Alcohol Drinking of Selectively Bred Rats. Alcohol. 1990;7:199–205. doi: 10.1016/0741-8329(90)90005-w. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. Jama. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Hicks MP, Cagle SS, Kalivas PW. Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcoholism: Clinical and Experimental Research. 2005;29:326–333. doi: 10.1097/01.alc.0000156086.65665.4d. [DOI] [PubMed] [Google Scholar]
- Meredith CW, Jaffe C, Ang-Lee K, Saxon AJ. Implications of chronic methamphetamine use: a literature review. Harvard review of psychiatry. 2005;13:141–154. doi: 10.1080/10673220591003605. [DOI] [PubMed] [Google Scholar]
- Murphy J, Cunningham S, McBride W. Effects of 250 mg% ethanol on monoamine and amino acid release from rat striatal slices. Brain research bulletin. 1985;14:439–442. doi: 10.1016/0361-9230(85)90022-x. [DOI] [PubMed] [Google Scholar]
- Muskens J, Schellekens A, de Leeuw F, Tendolkar I, Hepark S. Damage in the dorsal striatum alleviates addictive behavior. General hospital psychiatry. 2012;34:702.e709–702.e711. doi: 10.1016/j.genhosppsych.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Nash JF, Yamamoto BK. Methamphetamine neurotoxicity and striatal glutamate release: comparison to 3, 4-methylenedioxymethamphetamine. Brain research. 1992;581:237–243. doi: 10.1016/0006-8993(92)90713-j. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Takano T, Hansen AJ. Beyond the role of glutamate as a neurotransmitter. Nat Rev Neurosci. 2002;3:748–755. doi: 10.1038/nrn916. [DOI] [PubMed] [Google Scholar]
- Nevo I, Hamon M. Neurotransmitter and neuromodulatory mechanisms involved in alcohol abuse and alcoholism. Neurochem Int. 1995;26:305–336. doi: 10.1016/0197-0186(94)00139-l. discussion 337–342. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J Neurochem. 2002;83:1087–1093. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- Northrop NA, Yamamoto BK. Cyclooxygenase activity contributes to the monoaminergic damage caused by serial exposure to stress and methamphetamine. Neuropharmacology. 2013;72:96–105. doi: 10.1016/j.neuropharm.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obernier JA, Bouldin TW, Crews FT. Binge ethanol exposure in adult rats causes necrotic cell death. Alcoholism, clinical and experimental research. 2002;26:547–557. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York, NY: Academic Press; 2007. [Google Scholar]
- Qi J, Han WY, Yang JY, Wang LH, Dong YX, Wang F, Song M, Wu CF. Oxytocin regulates changes of extracellular glutamate and GABA levels induced by methamphetamine in the mouse brain. Addict Biol. 2012;17:758–769. doi: 10.1111/j.1369-1600.2012.00439.x. [DOI] [PubMed] [Google Scholar]
- Rau KS, Birdsall E, Volz TJ, Riordan JA, Baucum AJ, 2nd, Adair BP, Bitter R, Gibb JW, Hanson GR, Fleckenstein AE. Methamphetamine administration reduces hippocampal vesicular monoamine transporter-2 uptake. The Journal of pharmacology and experimental therapeutics. 2006;318:676–682. doi: 10.1124/jpet.105.099200. [DOI] [PubMed] [Google Scholar]
- Raudensky J, Yamamoto BK. Effects of chronic unpredictable stress and methamphetamine on hippocampal glutamate function. Brain research. 2007;1135:129–135. doi: 10.1016/j.brainres.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle EL, Topham MK, Haycock JW, Hanson GR, Fleckenstein AE. Differential trafficking of the vesicular monoamine transporter-2 by methamphetamine and cocaine. Eur J Pharmacol. 2002;449:71–74. doi: 10.1016/s0014-2999(02)01985-4. [DOI] [PubMed] [Google Scholar]
- Rocher C, Gardier AM. Effects of repeated systemic administration of d-Fenfluramine on serotonin and glutamate release in rat ventral hippocampus: comparison with methamphetamine using in vivo microdialysis. NAUNYN SCHMIEDEBURGS ARCHIVES OF PHARMACOLOGY. 2001;363:422–428. doi: 10.1007/s002100000381. [DOI] [PubMed] [Google Scholar]
- Saint-Preux F, Bores LR, Tulloch I, Ladenheim B, Kim R, Thanos PK, Volkow ND, Cadet JL. Chronic co-administration of nicotine and methamphetamine causes differential expression of immediate early genes in the dorsal striatum and nucleus accumbens of rats. Neuroscience. 2013;243:89–96. doi: 10.1016/j.neuroscience.2013.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Sreemantula S. Neuroimmunophilin GPI-1046 reduces ethanol consumption in part through activation of GLT1 in alcohol-preferring rats. Neuroscience. 2012;227:327–335. doi: 10.1016/j.neuroscience.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Hesselbrock V, Tipp J, Anthenelli R, Bucholz K, Radziminski S. A comparison of DSM-III-R, DSM-IV and ICD-10 substance use disorders diagnoses in 1922 men and women subjects in the COGA study. Collaborative Study on the Genetics of Alcoholism. Addiction. 1994;89:1629–1638. doi: 10.1111/j.1360-0443.1994.tb03764.x. [DOI] [PubMed] [Google Scholar]
- Simon SL, Domier C, Carnell J, Brethen P, Rawson R, Ling W. Cognitive impairment in individuals currently using methamphetamine. The American journal on addictions. 2000;9:222–231. doi: 10.1080/10550490050148053. [DOI] [PubMed] [Google Scholar]
- Smith AD, Weiss F. Ethanol exposure differentially alters central monoamine neurotransmission in alcohol-preferring versus-nonpreferring rats. Journal of Pharmacology and Experimental Therapeutics. 1999;288:1223–1228. [PubMed] [Google Scholar]
- Stephans SE, Yamamoto BK. Methamphetamine‐induced neurotoxicity: Roles for glutamate and dopamine efflux. Synapse. 1994;17:203–209. doi: 10.1002/syn.890170310. [DOI] [PubMed] [Google Scholar]
- Stinson FS, Grant BF, Dawson DA, Ruan WJ, Huang B, Saha T. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2005;80:105–116. doi: 10.1016/j.drugalcdep.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Tata DA, Raudensky J, Yamamoto BK. Augmentation of methamphetamine-induced toxicity in the rat striatum by unpredictable stress: contribution of enhanced hyperthermia. Eur J Neurosci. 2007;26:739–748. doi: 10.1111/j.1460-9568.2007.05688.x. [DOI] [PubMed] [Google Scholar]
- Thoma R, Mullins P, Ruhl D, Monnig M, Yeo RA, Caprihan A, Bogenschutz M, Lysne P, Tonigan S, Kalyanam R, Gasparovic C. Perturbation of the glutamate-glutamine system in alcohol dependence and remission. Neuropsychopharmacology. 2011;36:1359–1365. doi: 10.1038/npp.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GC, Ricaurte GA, Seiden LS, Schuster CR, Miller RJ, Westley J. Long-lasting depletions of striatal dopamine and loss of dopamine uptake sites following repeated administration of methamphetamine. Brain Res. 1980;181:151–160. doi: 10.1016/0006-8993(80)91265-2. [DOI] [PubMed] [Google Scholar]
- Ward RJ, Colivicchi MA, Allen R, Schol F, Lallemand F, De Witte P, Ballini C, Corte LD, Dexter D. Neuro‐inflammation induced in the hippocampus of ‘binge drinking’rats may be mediated by elevated extracellular glutamate content. Journal of neurochemistry. 2009;111:1119–1128. doi: 10.1111/j.1471-4159.2009.06389.x. [DOI] [PubMed] [Google Scholar]
- Wozniak KM, Pert A, Linnoila M. Antagonism of 5-HT3 receptors attenuates the effects of ethanol on extracellular dopamine. European journal of pharmacology. 1990;187:287–289. doi: 10.1016/0014-2999(90)90015-x. [DOI] [PubMed] [Google Scholar]
- Wozniak KM, Pert A, Mele A, Linnoila M. Focal application of alcohols elevates extracellular dopamine in rat brain: a microdialysis study. Brain research. 1991;540:31–40. doi: 10.1016/0006-8993(91)90489-i. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, McBride W, Lumeng L, Li TK. Ethanol enhances the release of dopamine and serotonin in the nucleus accumbens of HAD and LAD lines of rats. Alcoholism: Clinical and Experimental Research. 1992;16:781–785. doi: 10.1111/j.1530-0277.1992.tb00678.x. [DOI] [PubMed] [Google Scholar]


