Abstract
Introduction
Advanced glycation end-products (AGEs) are known to play an important role in the pathogenesis of diabetic complications. Skin autofluorescence (AF), a marker of AGE accumulation in tissue, can be measured noninvasively using a skin AF reader. The present study aimed to evaluate the relationships of skin AF with diabetic microvascular complications and carotid intima–media thickness (IMT), a surrogate marker for atherosclerosis, in Japanese subjects with type 2 diabetes (T2D).
Methods
One hundred sixty-two subjects with T2D and 42 nondiabetic control subjects attending the outpatient clinic were examined. Skin AF and carotid max-IMT were measured using an AGE Reader™ and ultrasonography, respectively. Nephropathy was classified into five stages based on the urinary albumin-to-creatinine ratio (UACR) as follows: (1) pre-nephropathy (stage 1) (UACR < 30 mg/g Cr); (2) incipient nephropathy (stage 2) (30 ≤ UACR < 300 mg/g Cr); (3) overt nephropathy (stage 3) (UACR ≥ 300 mg/g Cr); (4) kidney failure (stage 4) (estimated glomerular filtration rate (eGFR) < 30 ml/min/1.732); and (5) dialysis therapy (stage 5). Patients with kidney failure and those receiving dialysis therapy were excluded because the sample size was too small. Retinopathy was diagnosed as nondiabetic retinopathy (NDR), nonproliferative retinopathy (NPDR), or proliferative retinopathy (PDR). Diabetic peripheral neuropathy (DPN) was diagnosed if two or more of the following were present: neuropathic symptoms (decreased sensation, positive neuropathic sensory symptoms), symmetric decreased distal sensation, and unequivocally decreased or absent ankle reflexes.
Results
Skin AF values were significantly higher in subjects with T2D (2.53 ± 0.45 AU) than in nondiabetic subjects (2.19 ± 0.34 AU, p < 0.001). Skin AF significantly increased with the severity of DPN (2.39 ± 0.37 with DPN vs 2.80 ± 0.48 without DPN, p < 0.001), retinopathy (NDR 2.42 ± 0.45, mild and moderate NPDR 2.64 ± 0.42, p = 0.042, severe NPDR and PDR 2.85 ± 0.35, p < 0.001), and nephropathy (pre-nephropathy 2.42 ± 0.44, incipient nephropathy 2.62 ± 0.45, p = 0.049, overt nephropathy 2.59 ± 0.46, p = 0.80). Skin AF was an independent determinant of the presence of DPN (OR 8.49, 95% CI 2.04–44.32, p = 0.006) and retinopathy (OR 3.73, 95% CI 1.20–12.90, p = 0.028) but not of diabetic nephropathy after correcting for confounding factors. In addition, skin AF (β = 0.170, p = 0.029) was an independent determinant of max-IMT, as was age (β = 0.436, p < 0.0001), after adjusting for other risk factors.
Conclusion
Skin AF as measured using an AGE Reader is a noninvasive surrogate marker for diabetic microvascular complications and early-stage atherosclerosis.
Keywords: Atherosclerosis, Diabetic microvascular complications, Intima–media thickness, Skin autofluorescence, Type 2 diabetes
Introduction
Advanced glycation end products (AGEs) are formed via the nonenzymatic glycation of proteins or lipids and accumulate on long-lived proteins in tissues [1]. The accelerated formation and accumulation of AGEs occurs during normal aging processes, chronic hyperglycemia, chronic renal failure, and cardiovascular disease (CVD) [2–5]. In diabetes, early intensive glycemic control is an established strategy for preventing long-term diabetic complications in both type 1 diabetes (T1D) and type 2 diabetes (T2D) [6, 7]. The Diabetes Control and Complications Trial/Epidemiology and Diabetes Intervention and Complications (DCCT/EDIC) study [8, 9] demonstrated that the participants in an intensive therapy group during the DCCT continued to have a lower incidence of complications than a conventional therapy group did despite the fact that the two groups showed comparable glycemic control during the EDIC follow-up. This phenomenon was termed “metabolic memory” [8]. Furthermore, the DCCT Skin Collagen Ancillary Study showed that long-term intensive treatment resulted in lower levels of AGEs in skin biopsy, and that these skin collagen AGEs predicted the risk of developing microvascular disease and the risk of 10-year progression of microvascular disease, independent of HbA1c. Therefore, it is suggested that the accumulation of AGEs in tissue is a key factor in the pathogenesis of diabetic complications [10], which could provide a mechanism for metabolic memory [11, 12].
Invasive skin biopsy methods are not applicable for measuring tissue AGEs in routine clinical practice. Since several AGEs have a characteristic fluorescence [13], a new noninvasive device, a skin autofluorescence (AF) reader or AGE reader, has been developed to quantify skin AF, which has been shown to correlate with the accumulation of skin AGEs as assessed by skin biopsy [14]. Skin AF is elevated in diabetes [13] and end-stage renal disease [15], and is associated with diabetic microvascular complications [16, 17] and cardiovascular mortality [18, 19]. Although most studies of skin AF have been performed in Caucasian subjects, there are a few that focus on Japanese subjects with T1D [20, 21]. It is also reported that skin AF values appear to depend on ethnicity [22]. However, studies of skin AF in Japanese patients with T2D are scarce. The aim of the current study was therefore to evaluate the relationships between skin AF, diabetic microvascular complications, and carotid intima–media thickness (IMT)—which is a useful marker of the presence and progression of atherosclerosis [23, 24] and an excellent predictor of future cardiovascular events [25, 26]—in Japanese subjects with T2D.
Methods
Subjects
A cross-sectional study of 166 patients with T2D attending the outpatient diabetes clinic was performed during the period from October 2016 to May 2017. The inclusion criteria were age at diagnosis of T2D ≥ 30 years and known duration of diabetes ≥ 3 years. Patients were excluded if they had anemia (hemoglobin < 10 g/dl), were pregnant, were receiving steroid therapy, or were extremely sunburnt. Of the 166 subjects with T2D who were enrolled, 162 subjects were eventually analyzed: three subjects who had kidney failure (estimated glomerular filtration rate (eGFR) < 30 ml/min/1.732) and one subject who received dialysis therapy were excluded from the analysis because of the consequent very small sample size. Forty-two nondiabetic subjects were also enrolled. Clinical assessments included a medical interview, a physical examination, and laboratory tests. Smoking habit was classified as either current smoker or not.
HbA1c was measured using high-performance liquid chromatography (ARKRAY, Kyoto, Japan). Serum creatinine, total cholesterol, low-density cholesterol (LDL-C), high-density cholesterol (HDL-C), and triglyceride (TG) were measured by SRL Inc. (Tokyo, Japan). Plasma pentosidine concentrations were also measured by SRL Inc. using an enzyme-linked immunosorbent assay (ELISA). Urine albumin levels sampled from random voided urines were measured by immunoturbidimetric assay and expressed as the urinary albumin-to-creatinine ratio (UACR). Based on the Classification of Diabetic Nephropathy 2014 in Japan [27], nephropathy was classified into five stages based on the urinary albumin-to-creatinine ratio (UACR) as follows: (1) pre-nephropathy (stage 1) (UACR < 30 mg/g Cr); (2) incipient nephropathy (stage 2) (30 ≤ UACR < 300 mg/g Cr); (3) overt nephropathy (stage 3) (UACR ≥ 300 mg/g Cr); (4) kidney failure (stage 4) (eGFR < 30 ml/min/1.732); and (5) dialysis therapy (stage 5), although patients at stage 4 and 5 were excluded from this study. Diabetic retinopathy (DR) was diagnosed by independent ophthalmologists according to a position statement by the American Diabetes Association [28]. DR was classified into mild, moderate, and severe nonproliferative retinopathy (NPDR), and proliferative retinopathy (PDR) was classified according to disease severity level. Patients without any abnormalities were denoted non-DR (NDR). Diabetic peripheral neuropathy (DPN) was diagnosed according to the recent minimal criteria for typical DPN [29], which classifies cases into possible, probable, confirmed, and subclinical diabetic sensorimotor polyneuropathy (DSPN). In the current study, DPN was diagnosed in subjects with probable DSPN. DPN was considered to occur if two or more of the following neuropathic symptoms were observed: decreased sensation, positive neuropathic sensory symptoms, symmetric decreased distal sensation, and unequivocally decreased or absent ankle reflexes. This is because nerve conduction (NC) testing and autonomic nerve testing were not performed.
Measurements of Skin AF and Carotid IMT
Skin AF was measured using an AGE Reader™ (DiagnOptics Technologies BV, Groningen, Netherlands), a fully automated noninvasive device that uses the characteristic fluorescence properties of certain AGEs to estimate the level of AGE accumulation in the skin. Technical and optical details for this device have been provided extensively elsewhere [14]. In brief, the AGE Reader illuminated a 1 cm2 area of the skin using an excitation light source with a peak excitation of 370 nm. The skin AF was determined from the ratio between the emission fluorescence in the wavelength range between 420 and 600 nm and the reflected excitation light with a wavelength of between 300 and 420 nm, which was measured using a spectrometer and software. Skin AF measurements were performed on the ventral side of the forearm in the sitting position at room temperature. A series of three consecutive measurements were carried out at three different skin sites on the same forearm, taking less than a minute of time. The mean skin AF was calculated from these three consecutive measurements and used in the analyses. The method had an intrapatient coefficient of variation of 5% [14].
Carotid IMT was evaluated by an experienced physician using B-mode ultrasonography with a 7.5 MHz transducer according to the guidelines of the Japan Society of Ultrasonics [30]. In short, common carotid arteries, carotid bulbs, internal carotid arteries, and external carotid arteries were scanned bilaterally in longitudinal sections. The carotid IMT was measured as the distance from the leading edge of the first echogenic line to the leading edge of the second echogenic line. The highest IMT value was defined as the max-IMT and used in this study.
Statistical Analysis
All values are presented as the mean ± SD or the number (in parentheses) for categorical variables. A one-way ANOVA, followed by a Tukey’s post hoc or a two-tailed Student’s t test, was performed to compare mean variables between subgroups of microvascular complications. The relationships between continuous variables were examined with Pearson’s linear correlation test. A stepwise multivariate regression analysis was performed to evaluate independent variables that were significantly associated with an objective variable. For categorical variables, logistic regression analysis was used to derive the odds ratio (OR) and 95% confidence intervals (CI). A p value < 0.05 was considered to indicate statistical significance. All analyses were performed using JMP®12.2 (SAS Institute Inc., Cary, NC, USA).
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all participants before they were included in the study. The trial was not registered since it was performed in one clinic.
Results
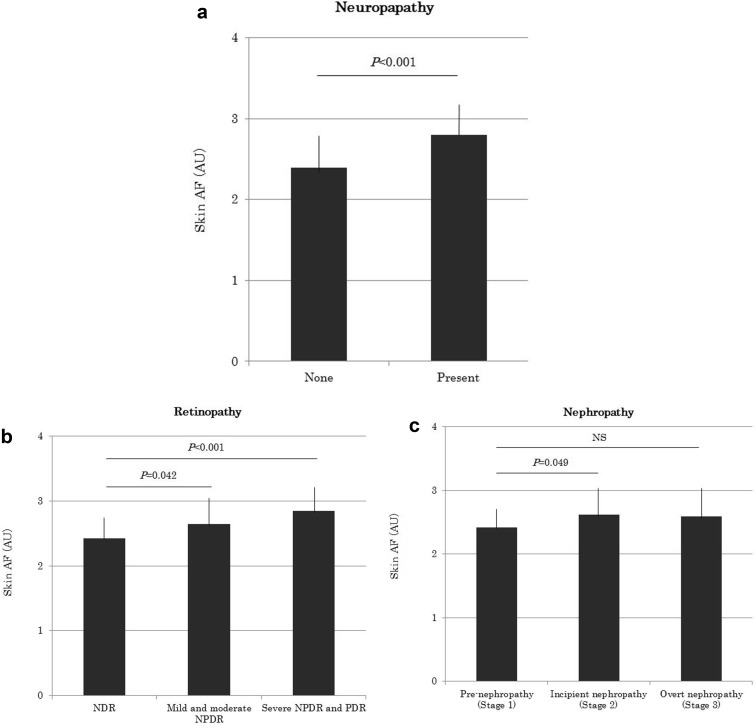
The characteristics of the study subjects are shown in Table 1. Between the nondiabetic and diabetic subjects, there were significant differences in age (53.8 ± 13 vs 61.2 ± 11.2 years), BMI (22.6 ± 4.0 vs 24.9 ± 4.0 kg/m2), systolic blood pressure (122 ± 11 vs 132 ± 11 mg), HbA1c (5.4 ± 0.3 vs 7.2 ± 0.8%), LDL-C (124.7 ± 34.9 vs 104.2 ± 27.2 mg/dl), eGFR (76.5 ± 15.7 vs 69.7 ± 18.7 ml/min/1.732), UACR (19.9 ± 10.3 vs 44.9 ± 60.4 mg/g Cr), serum pentosidine levels (0.038 ± 0.012 vs 0.046 ± 0.019 μg/ml) and max-IMT (1.10 ± 0.23 vs 1.64 ± 0.73 mm). Skin AF values were significantly higher in subjects with T2D (2.53 ± 0.45 AU) than in nondiabetic subjects (2.19 ± 0.34 AU, p < 0.001), Although diabetic and nondiabetic subjects were not matched for age, Nomoto et al. reported that there was no significant difference in skin AF between two healthy Japanese populations aged 50–60 years and 60–70 years, respectively [31]. Univariate regression analysis showed which of the factors affected skin AF values in patients with T2D. Skin AF was significantly correlated with age (r = 0.325, p = 0.007), duration of diabetes (r = 0.360, p = 0.0002), HbA1c (r = 0.203, p = 0.0038), serum pentosidine concentration (r = 0.324, p = 0.007), and eGFR (r = − 0.336, p = 0.0005). A multivariate stepwise regression analysis using variables that were significantly correlated with skin AF in the univariate analysis revealed that duration of diabetes (β = 0.35, p < 0.0001), HbA1c (β = 0.22, p = 0.006), serum pentosidine concentration (β = 034, p < 0.0001), and eGFR (β = − 0.33, p < 0.0001) were independent determinants of skin AF. Regarding associations between skin AF and diabetic microvascular complications, skin AF was significantly higher in patients with diabetic peripheral neuropathy (DPN) than in those without it (2.80 ± 0.48 vs 2.39 ± 0.37, respectively, p < 0.001) (Fig. 1a). A multiple logistic regression analysis showed that duration of diabetes (OR 1.09, 95% CI 1.03–1.17, p = 0.006), LDL-C (OR 0.97, 95% CI 0.95–0.99, p = 0.017), and skin AF (OR 8.49, 95% CI 2.04–44.3, p = 0.006) were still independent determinants of the presence of DPN after correcting for confounding risk factors (Table 2). Concerning retinopathy, skin AF significantly increased with severity of retinopathy (NDR 2.42 ± 0.45, mild and moderate NPDR 2.64 ± 0.42, severe NPDR and PDR 2.85 ± 0.35, p < 0.001) (Fig. 1b), and a multiple logistic regression analysis demonstrated that skin AF (OR 3.73, 95% CI 1.20–12.90, p = 0.028) was an independent determinant of the presence of diabetic retinopathy after correcting for risk variables (Table 2). With regard to nephropathy, skin AF in patients with incipient nephropathy (stage 2) (2.62 ± 0.45 AU) but not overt nephropathy (stage 3) (2.59 ± 0.46 AU) was significant higher than that in pre-nephropathy (stage 1) patients (2.42 ± 0.42 AU) (Fig. 1c). However, skin AF was no longer an independent factor for diabetic nephropathy according to multiple logistic regression analysis (Table 2).
Table 1.
Clinical characteristics of the study subjects
| Diabetic subjects | Nondiabetic subjects | p | |
|---|---|---|---|
| N (male/female) | 162 (89/73) | 42 (20/22) | 0.173 |
| Age (years) | 61.2 ± 11.2 | 53.8 ± 13.0 | 0.0003 |
| Smoking n (%) | 44 (27.2) | 11 (26.2) | 0.534 |
| BMI (kg/m2) | 24.9 ± 4.0 | 22.6 ± 4.0 | 0.001 |
| Duration of diabetes (years) | 14.6 ± 10.0 | – | – |
| Systolic blood pressure (mmHg) | 132 ± 11 | 122 ± 11 | < 0.010 |
| Diastolic blood pressure (mmHg) | 81 ± 9 | 81 ± 11 | 0.855 |
| HbA1c (%) | 7.2 ± 0.8 | 5.4 ± 0.3 | < 0.001 |
| Triglyceride (mg/dl) | 117.6 ± 56.5 | 127.0 ± 66.1 | 0.364 |
| HDL-C (mg/dl) | 63.1 ± 18.8 | 67.6 ± 15.5 | 0.169 |
| LDL-C (mg/dl) | 104.2 ± 27.2 | 124.7 ± 34.9 | 0.0001 |
| eGFR (ml/min/1.73 m2) | 69.7 ± 18.7 | 76.5 ± 15.7 | 0.039 |
| UACR (mg/g Cr) | 44.9 ± 60.4 | 19.9 ± 10.3 | 0.008 |
| Pentosidine (μg/ml) | 0.046 ± 0.019 | 0.038 ± 0.012 | 0.027 |
| Skin AF (AU) | 2.53 ± 0.45 | 2.19 ± 0.34 | < 0.001 |
| Max-IMT (mm) | 1.64 ± 0.73 | 1.10 ± 0.23 | < 0.001 |
| Neuropathy (none/present) | 106/56 | – | – |
| Retinopathy (NDR/mild and moderate NPDR/severe NPDR and PDR) | 104/32/26 | – | – |
| Nephropathy (stage 1/2/3) | 90/54/18 | – | – |
Data are presented as the mean ± SD
HbA1c glycated hemoglobin A1c, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, eGFR estimated glomerular filtration rate, UACR urinary albumin to creatinine ratio, AF autofluorescence, Max-IMT maximum intima–media thickness in the carotid artery, NDR nondiabetic retinopathy, NPDR nonproliferative retinopathy, PDR proliferative retinopathy
Fig. 1a–c.
Skin AF levels in the presence of or during the development of neuropathy (a), retinopathy (b), and nephropathy (c) in subjects with T2D. Data are displayed as the mean ± SD. NS not significant, AF autofluorescence, NDR nondiabetic retinopathy, NPDR nonproliferative retinopathy, PDR proliferative retinopathy
Table 2.
Variables related to the presence of diabetic neuropathy, retinopathy, and nephropathy in subjects with T2D according to multivariate logistic regression analysis
| Variables | Neuropathy | Retinopathy | Nephropathy | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Age | 1.05 (0.98–1.13) | 0.136 | 0.97 (0.92–1.02) | 0.192 | 1.00 (0.85–1.21) | 0.983 |
| Diabetes duration | 1.09 (1.03–1.17) | 0.006 | 1.03 (0.98–1.08) | 0.220 | 0.89 (0.72–1.05) | 0.231 |
| Smoking | 1.58 (0.48–5.71) | 0.478 | 0.75 (0.28–2.35) | 0.751 | 11.7 (0.83–611.7) | 0.127 |
| BMI | 1.00 (0.86–1.17) | 0.936 | 0.95 (0.82–1.08) | 0.424 | 0.99 (0.72–1.39) | 0.982 |
| SBP | 0.96 (0.91–1.01) | 0.128 | 1.01 (0.97–1.06) | 0.609 | 1.08 (0.97–1.25) | 0.195 |
| HbA1c | 2.08 (0.99–4.59) | 0.057 | 1.31 (0.72–2.41) | 0.371 | 1.39 (0.30–7.27) | 0.667 |
| LDL-C | 0.97 (0.95–0.99) | 0.017 | 0.99 (0.97–1.01) | 0.265 | 0.97 (0.92–1.01) | 0.159 |
| HDL-C | 0.99 (0.95–1.03) | 0.614 | 1.00 (0.97–1.03) | 0.870 | 0.96 (0.87–1.04) | 0.317 |
| Triglyceride | 1.00 (0.99–1.01) | 0.825 | 1.04 (0.99–1.01) | 0.439 | 1.02 (0.99–1.06) | 0.290 |
| eGFR | 0.98 (0.94–1.02) | 0.250 | 1.01 (0.98–1.04) | 0.473 | 1.04 (0.86–1.03) | 0.238 |
| UACR | 0.98 (1.01–1.01) | 0.750 | 1.00 (0.99–1.01) | 0.769 | 1.23 (1.12–1.46) | 0.001 |
| Pentosidine | 0.98 (0.94–1.02) | 0.269 | 1.02 (0.99–1.05) | 0.108 | 1.09 (0.99–1.23) | 0.118 |
| Skin AF | 8.49 (2.04–44.3) | 0.006 | 3.73 (1.20–12.9) | 0.028 | 3.52 (0.18–15.8) | 0.450 |
Bold values indicate p <0.05
BMI body mass index, SBP systolic blood pressure, HbA1c glycated hemoglobin A1c, LDL-C low-density lipoprotein cholesterol, HDL-C high-density lipoprotein cholesterol, eGFR estimated glomerular filtration rate, UACR urinary albumin to creatinine ratio, AF autofluorescence
Furthermore, the associations between carotid max-IMT and clinical variables such as skin AF were evaluated. Table 3 shows that the results of a multivariate stepwise regression analysis indicate that skin AF (β = 0.170, p = 0.029) and age (β = 0.436, p < 0.0001) were independent determinants of max-IMT after adjusting for eGFR, another risk factor.
Table 3.
Relative risk factors for max-IMT in subjects with T2D
| Univariate | Multivariate | |||
|---|---|---|---|---|
| r | p | β | p | |
| Age | 0.471 | < 0.0001 | 0.436 | < 0.0001 |
| Diabetes duration | 0.100 | 0.264 | ||
| Smoking | 0.070 | 0.435 | ||
| BMI | − 0.117 | 0.191 | ||
| SBP | 0.167 | 0.062 | ||
| HbA1c | − 0.042 | 0.642 | ||
| LDL-C | − 0.082 | 0.360 | ||
| HDL-C | − 0.041 | 0.646 | ||
| Triglyceride | − 0.070 | 0.439 | ||
| eGFR | − 0.225 | 0.011 | 0.042 | 0.618 |
| UACR | 0.079 | 0.378 | ||
| Pentosidine | 0.090 | 0.318 | ||
| Skin AF | 0.284 | 0.001 | 0.170 | 0.029 |
| R 2 | 0.242 | |||
Bold values indicate p <0.05
BMI body mass index, SBP systolic blood pressure, HbA1c glycated hemoglobin A1c, LDL-C low-density lipoprotein cholesterol, HDL-C high-density lipoprotein cholesterol, eGFR estimated glomerular filtration rate, UACR urinary albumin to creatinine ratio, AF autofluorescence, β partial regression coefficient
Discussion
The present study demonstrated that skin AF was higher in patients with T2DM than in non-DM subjects. Skin AF was associated with age, HbA1c, serum pentosidine concentration, and eGFR according to a multivariate stepwise regression analysis, as reported previously [14, 15, 17]. Since pathologic changes in diabetic complications occur gradually over a long period, tissue AGEs that accumulate on long-lived proteins in tissues have been implicated in the pathology of diabetic complications, while short-lived glycated proteins such as HbA1c or glycated albumin are not necessarily the best predictors of these complications. The DCCT/EDIC study clearly demonstrated that the intensive glycemic control maintained during the DCCT period continued to have a beneficial effect on diabetic complications during the EDIC follow-on study even though glycemic control worsened during the EDIC period [8, 9]. Previously high HbA1c levels may result in the formation of AGEs such as furosine, pentosidine, and carboxymethyl-lysine (CML), which could explain a subsequent enhanced risk of diabetic microvascular complications even when HbA1c levels are currently well controlled [9, 11].
This study found that skin AF increased with the severity of diabetic microvascular complications, and was an independent determinant of retinopathy and neuropathy. Although serum pentosidine, a well-known fluorescent AGE, was significantly correlated with skin AF, levels of this AGE did not correlate with diabetic microvascular complications after adjusting for known risk factors (e.g., age, duration of DM, HbA1c). Circulating AGE levels do not necessarily reflect AGE levels in tissues because tissue AGEs have a slower turnover than serum AGEs [32]. Most studies of skin AF have been performed in Caucasian subjects with T1D and T2D [16–18], although a few reports have focused on Japanese subjects with T1D [20, 21], but studies of skin AF in Japanese patients with T2D are scarce. In cases of diabetic nephropathy, albuminuria is an early symptom that increases in severity from pre-nephropathy to incipient nephropathy to overt nephropathy. Skin AF was higher in subjects with incipient nephropathy but not overt nephropathy compared to pre-nephropathy subjects, and was significantly associated with eGFR according to the univariate analysis. These findings are similar to those reported previously for diabetic patients [14, 16, 18]. However, the multiple regression analysis revealed that skin AF was not an independent determinant of nephropathy, even though skin AF has been reported to be an independent determinant of the presence and progression of nephropathy as assessed by chronic kidney disease (CKD) stage [15]. Because the nephropathy stage classification was performed based on the UACR according to the Classification of Diabetic Nephropathy 2014 in Japan [27] in the current study, the study population included heterogeneous subjects with eGFR ≥ 30 ml/min/1.732. In addition, subjects with kidney failure and those who were on dialysis therapy were excluded from the analysis because of the very small sample size involved (n = 4). These could explain the differences between the results of the eGFR-based classification performed in the previous study [15] and the results of the albuminuria-based classification performed in the current study.
Carotid IMT is a useful surrogate marker for the presence and progression of atherosclerosis [23, 24], and it is an excellent predictor of future cardiovascular events [25, 26], while skin AF has been demonstrated to be associated with cardiovascular disease [33, 34]. The present study found an association between skin AF and carotid IMT, although only max-IMT was evaluated in this study because max-IMT is a better indicator of atherosclerosis in patients with T2D [35]. Among the various studies of the association between skin AF and carotid IMT that have been published, there has been one on Japanese subjects with T1D [21] but very few on Japanese subjects with T2D, for whom skin AF values appear to be significantly different from those observed in corresponding Caucasian populations [22].
This study has some limitations. First, because the sample size is small and because it is a cross-sectional study without any longitudinal assessment, the results do not necessarily indicate a causal relationship between skin AF and diabetic complications. Second, regarding diabetic neuropathy, autonomic neuropathy was not assessed by specific tests. Also, this study may not reflect subjects with kidney failure because such cases were excluded from the analysis due to their scarcity. Third, nonfluorescent skin AGEs such as CML cannot be measured using the AGE Reader. Nonetheless, levels of nonfluorescent skin AGEs were reported to be significantly associated with skin AF [13]. Therefore, a further large prospective study is needed to validate the findings of the current study.
Conclusions
The current study demonstrates that skin AF is elevated in subjects with T2D in comparison with nondiabetic subjects, and is associated with diabetic microvascular complications and carotid max-IMT. Therefore, skin AF, as measured using an AGE Reader, is an easy and noninvasive surrogate marker for evaluating diabetic microvascular complications and early-stage atherosclerosis.
Acknowledgements
Funding
No funding or sponsorship was received for this study. Article processing charges were funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. Dr. Yoshioka is the guarantor for this article and takes responsibility for the integrityof the work as a whole.
Disclosures
Keiji Yoshioka has nothing to disclose.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all participants before they were included in the study.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/46DCF0604477A81F.
References
- 1.Brownlee M. Advanced protein glycosylation in diabetes and aging. Ann Rev Med. 1995;46:223–234. doi: 10.1146/annurev.med.46.1.223. [DOI] [PubMed] [Google Scholar]
- 2.Koetsier M, Lutgers HL, de Jonge C, Links TP, Smit AJ, Graaff R. Reference values of skin autofluorescence. Diabetes Technol Ther. 2010;12:399–403. doi: 10.1089/dia.2009.0113. [DOI] [PubMed] [Google Scholar]
- 3.Vlassara H, Palace MR. Diabetes and advanced glycation end-products. J Intern Med. 2002;251:87–101. doi: 10.1046/j.1365-2796.2002.00932.x. [DOI] [PubMed] [Google Scholar]
- 4.Miyata T, Wada Y, Cai Z, et al. Implications of an increased oxidative stress in the formation of advanced glycation end products in patients with end-stage renal failure. Kidney Int. 1997;51:1170–1181. doi: 10.1038/ki.1997.160. [DOI] [PubMed] [Google Scholar]
- 5.Bierhaus A, Hofmann MA, Ziegler R, Nawroth PP. AGEs and their interaction with AGE-receptors in vascular disease and diabetes mellitus I. Cardiovasc Res. 1998;37:586–600. doi: 10.1016/S0008-6363(97)00233-2. [DOI] [PubMed] [Google Scholar]
- 6.The Diabetes Control and Complications Trial (DCCT) Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes. N Engl J Med. 1993;329:977–86. [DOI] [PubMed]
- 7.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observation study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Writing Team for the Diabetes Control and Complications Trial/Epidermiology of Diabetes Interventions and Complications Research Groups Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA. 2002;287:2563–2569. doi: 10.1001/jama.287.19.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Writing Team for the Diabetes Control and Complications Trial/Epidermiology of Diabetes Interventions and Complications Research Groups Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidermiology of Diabetes Interventions and Complications (EDIC) study. JAMA. 2003;290:2159–2167. doi: 10.1001/jama.290.16.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamagishi S, Nakamura N, Suematsu M, Kaseda K, Matsui T. Advanced glycation end products: a molecular target for vascular complications in diabetes. Mol Med. 2015;21(Suppl. 1):S32–S40. doi: 10.2119/molmed.2015.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monnier VM, Bautista O, Kenny D, et al. Skin collagen glycation, glycoxidation, and crosslinking are lower in subjects with long-term intensive versus conventional therapy of type 1 diabetes: relevance of glycated collagen products versus HbA1c as markers of diabetic complications. Diabetes. 1999;48:870–880. doi: 10.2337/diabetes.48.4.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Genuth S, Sun W, Cleary P, et al. Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10-year progression of diabetic retinopathy and nephropathy in the Diabetes Complications Trials and Epidemiology of Diabetes Interventions and Complications participants with type 1 diabetes. Diabetes. 2005;54:3103–11. [DOI] [PMC free article] [PubMed]
- 13.Monnier VM, Vishwanah V, Frank KE, Elmets CA, Dauchor P, Kohn RR. Relation between complications of type 1 diabetes mellitus and collagen-linked fluorescence. N Engl J Med. 1986;314:403–408. doi: 10.1056/NEJM198602133140702. [DOI] [PubMed] [Google Scholar]
- 14.Meerwaldt R, Graaff R, Oomen PHN, et al. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia. 2004;47:1324–1330. doi: 10.1007/s00125-004-1451-2. [DOI] [PubMed] [Google Scholar]
- 15.Gerrits E, Smit AJ, Bilo HJG. AGEs, autofluorescence and renal function. Nephrol Dial Transplant. 2009;24:710–3. [DOI] [PubMed]
- 16.Orchard TJ, Lyons TJ, Cleary PA, et al. The association of skin intrinsic fluorescence with type 1 diabetes complications in the DCCT/EDIC study. Diabetes Care. 2013;36:3146–3153. doi: 10.2337/dc12-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerrits EG, Lutgers HL, Kleefstra N, et al. Skin autofluorescence: a tool to identify type 2 diabetic patients at risk for developing microvascular complications. Diabetes Care. 2008;31:517–521. doi: 10.2337/dc07-1755. [DOI] [PubMed] [Google Scholar]
- 18.Lutgers HL, Graaff R, Links TP, et al. Skin autofluorescence as an noninvasive marker of vascular damage in patients with type 2 diabetes. Diabetes Care. 2006;29:2654–2659. doi: 10.2337/dc05-2173. [DOI] [PubMed] [Google Scholar]
- 19.Yamagishi S, Fukami K, Matsui T. Evaluation of tissue accumulation levels of advanced glycation end products by skin autofluorescence: a novel marker of vascular complications in high-risk patients for cardiovascular disease. Int J Cardiol. 2015;185:263–268. doi: 10.1016/j.ijcard.2015.03.167. [DOI] [PubMed] [Google Scholar]
- 20.Sugisawa E, Miura J, Iwamoto Y, Uchigata Y. Skin autofluorescence reflects integration of past long-term glycemic control in patients with type 1 diabetes. Diabetes Care. 2013;36:2339–2345. doi: 10.2337/dc12-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osawa S, Katakami N, Kuroda A, et al. Skin autofluorescence is associated with early-stage atherosclerosis in patients with type 1 diabetes. J Atherosler Thromb. 2017;24:312–326. doi: 10.5551/jat.35592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mook-Kanamori MJ, Selim MM, Takiddin AH, et al. Ethnic and gender differences in advanced glycation end products measured by skin auto-fluorescence. Dermatoendocrinol. 2013;5:325–30. [DOI] [PMC free article] [PubMed]
- 23.Salonen R, Salonen JT. Determinants of carotid intima–media thickness: a population based ultrasonography study in eastern Finnish men. J Intern Med. 1991;229:225–231. doi: 10.1111/j.1365-2796.1991.tb00336.x. [DOI] [PubMed] [Google Scholar]
- 24.Burke G, Evans GW, Riley WA, et al. Arterial wall thickness is associated with prevalent cardiovascular disease in middle-aged adults. The Atherosclerosis Risk in Communities (ARIC) Study. Stroke. 1995;26:386–391. doi: 10.1161/01.STR.26.3.386. [DOI] [PubMed] [Google Scholar]
- 25.Katakami N, Kaneto H, Shimomura I. Carotid ultrasonography: a potent tool for better clinical practice in diagnosis of atherosclerosis in diabetic patients. J Diabetes Invest. 2014;5:3–13. doi: 10.1111/jdi.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima–media thickness: a systemic review and meta-analysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 27.Haneda M, Utsunomiya K, Koya D, et al. A new classification of diabetic nephropathy 2014: a report from Joint Committee on Diabetic Nephropathy. Diabetol Int. 2014;5:207–211. doi: 10.1007/s13340-014-0197-4. [DOI] [Google Scholar]
- 28.Solomon S, Chew E, Duh E, et al. Diabetic retinopathy: a position statement by American Diabetes Association. Diabetes Care. 2017;40:412–418. doi: 10.2337/dc16-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tesfaye S, Boulton AJM, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terminology and Diagnostic Criteria Committee, Japan Society of Ultrasonics in Medicine Subcommittee for Preparing Guidelines for Ultrasound Diagnosis of Carotid Artery Standard method for ultrasound evaluation of carotid artery lesions. J Med Ultrason. 2009;36:501–518. [Google Scholar]
- 31.Nomoto K, Yagi M, Arita S, Hamada U, Yonei Y. A survey of fluorescence derived from advanced glycation end products in the skin of Japanese: differences with age and measurement location. Anti Aging Med. 2012;9:119–124. [Google Scholar]
- 32.Arso S, Graaff R, van Oeveren W, et al. Advanced glycation end-products and skin autofluorescence in end-stage renal disease: a review. Clin Chem Lab Med. 2014;52:11–20. doi: 10.1515/cclm-2012-0832. [DOI] [PubMed] [Google Scholar]
- 33.Meerwaldt R, Lutgers HL, Links TP. Skin autofluorescence is a strong predictor of cardiac mortality in diabetes. Diabetes Care. 2007;30:107–112. doi: 10.2337/dc06-1391. [DOI] [PubMed] [Google Scholar]
- 34.Lutgers HL, Gerrits EG, Graaff R, et al. Skin autofluorescence provides additional information to the UK Prospective Diabetes Study (UKPDS) risk score for the estimation of cardiovascular prognosis in type 2 diabetes mellitus. Diabetologia. 2009;52:789–97. [DOI] [PubMed]
- 35.Irie Y, Katakami N, Kaneto H, et al. Maximum carotid intima–media thickness improves the prediction ability of coronary artery stenosis in type 2 diabetic patients without history of coronary artery disease. Athrosclerosis. 2012;221:438–444. doi: 10.1016/j.atherosclerosis.2012.01.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.