Abstract
Introduction
It is estimated that 642 million adults worldwide will have diabetes by 2040, with 80–90% of these having type 2 diabetes mellitus (T2DM). While many new antidiabetic agents have been introduced in recent years, approximately 40% of T2DM patients still fail to achieve the recommended target HbA1c of < 7.0%. Furthermore, many patients with T2DM in Japan are treated by practitioners other than diabetes specialists; therefore, the exact treatment patterns of T2DM in Japan are difficult to quantify.
Aims
J-DISCOVER aims to address the lack of data on the management of T2DM by providing real-world data on disease management patterns and associated outcomes in a large number of Japanese T2DM patients who are initiating second-line therapy.
Design and Setting
Part of the global DISCOVER study program, J-DISCOVER will follow 2000 T2DM patients recruited from 141 sites across Japan who are aged ≥ 20 years. Recruitment began in September 2014 and follow-up will end in December 2018. The primary objective is to describe the long-term disease management patterns and clinical evolution of patients with T2DM inadequately controlled with a first-line antidiabetic therapy who initiate a second-line antidiabetic treatment. We will assess the associations between treatment patterns, including the line of antidiabetic medication used, as well as clinical and patient-reported outcomes. The primary endpoint is the mean change in HbA1c between baseline and at 6, 12, 24, and 36 months in the overall population and for patients receiving each class of second-line antidiabetic treatment.
Planned Outputs
A peer-reviewed publication reporting real-world results and implications for clinical practice.
Conclusion
By enrolling and following a large number of patients with T2DM across Japan, J-DISCOVER is expected to provide important real-world clinical data for the development of future T2DM treatment guidelines.
Funding
AstraZeneca K.K. and Ono Pharmaceutical Co., Ltd., Osaka Japan.
Clinical Trial Registration
Electronic supplementary material
The online version of this article (10.1007/s13300-017-0351-7) contains supplementary material, which is available to authorized users.
Keywords: Diabetes mellitus, Type 2, Cohort study, Japanese population
Introduction
In 220 countries and territories, the number of adults living with diabetes was estimated to be 415 million in 2015. Given the rising prevalence of diabetes, 642 million adults worldwide are expected to have diabetes by 2040 [1].
The National Health and Nutrition Survey in Japan conducted by the Ministry of Health, Labour and Welfare in 2012 reported that the number of individuals strongly suspected of having diabetes (SSD) and the number of individuals in whom diabetes cannot be ruled out (possible diabetes, PD) were estimated as approximately 9.5 and 11.0 million, respectively [2] (definitions for SSD and PD are provided in the Appendix in the Electronic supplementary material, ESM). Compared with the last survey in 2007, the estimated number of PD cases was found to have decreased from 13.2 million to 11.0 million. Conversely, the estimated number of SSD cases increased from 8.9 million to 9.5 million [2, 3]. In 2012, 65.2% of the SSD cases reported that they were currently receiving diabetes treatment. The majority (~ 85–95%) of people living with diabetes worldwide, including those in Japan, have type 2 diabetes (T2DM) [4]. The number of treatment options for T2DM has increased in recent years. A general improvement in glucose control has been reported, but the proportion of patients who achieve their target glucose levels remains low. A cross-sectional study of 53,796 T2DM patients from 73 sites across Japan conducted by the Diabetes Clinical Data Management Study Group reported that the average HbA1c of T2DM patients decreased from 7.41% in 2001 to 6.96% in 2013. However, approximately 40% of T2DM patients could not achieve the recommended target HbA1c of < 7.0% [5]. The optimal treatment target should be decided by an investigator based on the clinical condition of the patient [6]. The target values for HbA1c, lipids, and blood pressure relating to this study are shown in the Appendix in the ESM. However, the introduction of new diabetes treatments has not improved the control of diabetes as much as had been hoped, and treatments must be further improved to aid with glycemic control and reduce the complications associated with T2DM.
The treatment landscape for T2DM patients has changed in recent years. From 2002 to 2012, the proportion of T2DM patients treated with insulin gradually decreased from 15.4 to 8.6%. In contrast, the proportion of patients treated with oral antidiabetic drugs (OADs) increased from 51.4 to 59.6% in the same period [5]. The increased number of antidiabetic agents with different modes of action available has made more combination therapies possible, meaning patients are treated with OADs for longer than before. Furthermore, T2DM is a progressive disease and many patients undergo dual and higher combination therapies following monotherapy. The Japan Diabetes Complication and its Prevention study stratified patients with T2DM by quartiles according to the mean duration of diabetes in years, and found that the odds of using any diabetes therapy were 2.39 (95% CI 1.88–3.03), 3.85 (95% CI 2.98–4.98), and 5.72 (95% CI 4.31–7.60), respectively, for the second to fourth quartiles compared with the first quartile (P < 0.001) [7].
Unlike the American Diabetes Association and the European Association for the Study of Diabetes treatment guidelines, which specify metformin as the first-line treatment [8], the treatment guidelines of the Japan Diabetes Society (JDS) do not specify first-line or combination antidiabetic drugs [8]. The JDS guidelines largely leave the selection of antidiabetic agents to the discretion of treating physicians, especially for second-line treatments or higher. The guidelines recommend selecting glucose-lowering agents based on the disease condition of each patient, with consideration given to the pharmacological and safety profile of each glucose-lowering agent [8]. The selection criteria for a second-line oral glucose-lowering drug recommend improving glycemic control through the addition of a glucose-lowering agent with a different mechanism of action or by adding or switching to insulin therapy as required [8]. The evidence behind the guidelines is limited; data on current treatments of T2DM patients and long-term glucose control are scarce.
Large-scale cohort studies in T2DM, such as the Japan Diabetes Complication and its Prevention prospective [6, 9] and the Diabetes Clinical Data Management Study Group [5, 10] studies in Japan, are often conducted at hospitals or clinics that specialize in diabetes. This is consistent with how diabetes studies are conducted overseas, including the landmark UK Prospective Diabetes study [11]. However, Japanese T2DM patients are often treated by primary physicians or other physicians who do not specialize in diabetes. Therefore, a treatment reality study on second-line and greater antidiabetic agents involving primary care physicians would be valuable.
J-DISCOVER aims to prospectively research the treatment reality and long-term glucose control of T2DM patients who are initiating second-line diabetes treatment at sites across Japan, and to generate basic data for guideline development in future. To this end, the study will include not only diabetes specialists but also other specialists at clinics and hospitals, including cardiologists and internal medicine specialists, to understand real-world clinical practices regarding T2DM patients in the early disease stage.
The DISCOVER study program (NCT02322762) will address the lack of global data by providing real-world data on disease management patterns and the associated outcomes in > 15,000 T2DM patients who are initiating second-line therapy in 37 countries other than Japan [12]. The J-DISCOVER study (NCT02226822) is part of the multinational DISCOVER study program.
Methods
Study Design and Population
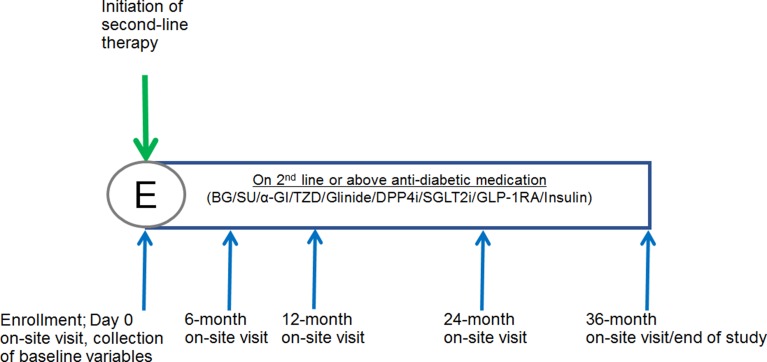
J-DISCOVER is a 3-year multicenter prospective observational longitudinal cohort study, which will be conducted at an estimated 141 sites across Japan. Enrollment and follow-up visits are outlined in Fig. 1. The enrollment period was from September 2014 to December 2015, and the entire study period stretches from September 2014 to December 2018.
Fig. 1.
Study design. E enrollment, BG biguanide, SU sulfonylurea, α-GI alpha-glucosidase inhibitor, TZD thiazolidine, DPP4i dipeptidyl-peptidase 4 inhibitor, SGLT2i sodium–glucose cotransporter 2 inhibitor, GLP-1RA glucagon-like peptide-1 receptor agonist
The primary objective is to describe the disease management patterns and long-term clinical evolution of T2DM patients inadequately controlled with a first-line antidiabetic therapy, according to the treating physician’s judgment, who are initiating a second-line antidiabetic treatment (either adding a second therapy to their initial therapy or switching to a therapy of a different medication class). In general, a diagnosis of diabetes is made if the results of a second diagnostic test performed on another day confirm a judgment of “diabetic type”. Diabetes type is indicated by the results of diagnostic tests corresponding to any of the following, as specified by the Treatment Guide for Diabetes [6]: (1) early-morning fasting glucose level ≥ 126 mg/dL, (2) 2-h plasma glucose level after 75 g glucose loading ≥ 200 mg/dL, (3) random (casual) plasma glucose measurements ≥ 200 mg/dL, and (4) HbA1c ≥ 6.5%. A full explanation of categories of the state of glycemia and decision criteria and how T2DM was diagnosed is provided in the ESM.
The study also aims to assess the associations between treatment patterns, including the first, second, and above lines of antidiabetic medications used, and clinical outcomes, including glycemic control, body weight, blood pressure, lipid profile, incidences of complications (microvascular, macrovascular, hypoglycemic and hyperglycemic events), and patient-reported outcomes. Retinopathy, nephropathy, and neuropathy are classified as microangiopathies, and coronary arteriopathy, cerebrovascular disease, and peripheral arterial disease are forms of macroangiopathy. Diabetic foot lesions are also included in diabetic complications. See the ESM for more information on how the complications of diabetes are being assessed.
This is an observational study and all treatment decisions are made by treating physicians, as in usual care.
Patients in the early disease stage often receive diabetes care at a site that does not specialize in diabetes. Therefore, patients will be recruited from sites with different specialties from diabetes, including cardiology, internal medicines, and others, to reflect real-world diabetes treatment.
The specific site and the specialties of the treating physicians may influence their clinical management of diabetes and the profile of the patients attending each site, so patients were recruited from clinics and hospitals across Japan to obtain a representative sample of T2DM patients.
Compliance with Ethics Guidelines
All procedures followed are in accordance with the the International Conference on Harmonization of Good Clinical Practice, the Ethical Guidelines for Epidemiological Research of Japan, the ethical standards of the responsible committee on human experimentation (institutional and national), and the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients included in the study. This study has been approved by the relevant ethical committees of the participating sites and informed consent was obtained from all patients at each site. All patients participate voluntarily and are free to discontinue their participation at any point.
Inclusion and Exclusion Criteria
Patients were included in this study if they were female or male aged ≥ 20 years, were diagnosed with T2DM, were initiating a second oral or parenteral antidiabetic therapy added to oral antidiabetic monotherapy or were switching from the monotherapy to another monotherapy with a different class of drug, and if they provided written informed consent. There were no strict inclusion criteria based on HbA1c in this study, because all patients were already taking an OAD. However, as previously mentioned, in Japan, a diagnosis of TD2M is generally based on the following: early morning fasting plasma glucose (PG) ≥ 126 mg/dL, 2-h PG after 75 g glucose load ≥ 200 mg/dL, or casual plasma glucose ≥ 200 mg/dL [6]. The details of the inclusion and exclusion criteria used are shown in Table 1.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria |
|
Subjects in this study must fulfill all of the following criteria: 1. Provide written informed consent for study participation 2. Female or male aged 20 years or over 3. Diagnosed with type 2 diabetes mellitus 4. Initiating a second oral or parenteral antidiabetic therapy added to oral antidiabetic monotherapy or switching from the monotherapy to another monotherapy with a different drug class |
| Exclusion criteria |
|
Patients will not be eligible to participate if any of the following exclusion criteria are present: 1. Diagnosis of type 1 diabetes mellitus 2. Current pregnancy 3. Current treatment for any cancer 4. Current dialysis treatment or renal transplantation 5. Current treatment with any oral steroids 6. Participation in any randomized control trials 7. Presence of any condition/circumstance that, in the opinion of the investigator, could significantly limit the complete follow-up of the patient (e.g., tourist, non-native speaker who does not understand the local language where an interpreter is not available, psychiatric disturbances, alcohol or drug abuse). |
The study protocol was reviewed and approved by the ethics committee or the institutional review board of each participating site. Written informed consent was obtained from all participants at each participating institution in accordance with the Ethical Guidelines for Epidemiological Research of the Japanese Ministry of Health, Labour and Welfare, as well as the Declaration of Helsinki. The study is registered in clinicaltrial.gov (NCT02226822).
Data Collection
During the study period, patients will visit the study sites for routine visits. Baseline data will be collected after informed consent, and follow-up data will be obtained from patient records at 6-, 12-, 24-, and 36-month time points with a ± 2-month buffer period (Fig. 1).
This is an observational study. Regardless of any change in diabetes treatment during study period, patients will be followed, and relevant data will be collected at the study sites until the end of the study wherever possible.
Data will be entered into an electronic data capture system and checked by the data management team. The team will issue queries to investigators through the electronic data capture system. Following the manual provided, investigators will enter data and electronically sign off the electronic case report form. A schedule showing the measurements that will be performed at each visit is shown in Table 2.
Table 2.
Study plan
| Data collection | Enrollment | Year 1 | Year 2 | Year 3 | |
|---|---|---|---|---|---|
| Visit number | 1 | 2 | 3 | 4 | 5 |
| Study month | Day 0 | 6 | 12 | 24 | 36 |
| Written informed consent | Xa | ||||
| Site and physician characteristics | X | ||||
| Demographicsb | X | ||||
| Risk factors (smoking status, etc.) | X | ||||
| Changes in demographics and risk factors | X | X | |||
| Diabetes medical and treatment history | X | ||||
| Medical history (comorbidities) | X | ||||
| Concomitant medications | X | ||||
| New comorbidities, concomitant medications, and interventions (including chemotherapy, steroids, dialysis) | X | X | X | X | |
| Physical examination (including height, weight, waist circumference, heart rate, blood pressure, etc.) | X | X | X | X | X |
| Laboratory testsc | X | X | X | X | X |
| HbA1c and glucose at the time of diabetes treatment change | X | X | X | X | X |
| Diabetes medication changes from baseline | X | X | X | X | |
| QOL questionnaires (SF-36, DTSQ) | X | X | X | X | X |
| BDHQ | X | X | X | X | |
| Physical activity questionnaires (IPAQ-SV) | X | X | X | X | X |
| Minor hypoglycemic events in last 1 month | X | X | X | X | X |
| Major hypoglycemic events in last 12 months/last visit | X | X | X | X | X |
| Hyperglycemia hospitalization since the last visit | X | X | X | X | X |
| Incidence and progression of microvascular complications since last visit | X | X | X | X | |
| Incidence of macrovascular complications since last visit | X | X | X | X | |
BDHQ Brief-Type Self-Administration Diet History Questionnaire, DTSQ Diabetes Treatment Satisfaction Questionnaire, HbA1c glycated hemoglobin, IPAQ-SV International Physical Activity Questionnaire Short Version, SF-36 36-Item Short-Form Health Survey
aInformed consent must be obtained before data collection is started
bDemographics: gender, date of birth, race, living arrangement status (living alone, living with someone, etc.), education level (no education or primary school, etc.), employment status (full-time worker, part-time worker, etc.), smoking status, alcohol intake, family history of diabetes, bed time (day or night), average sleeping hours per day in usual week, menstrual status (women only)
cLaboratory tests: aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyltranspeptidase (γ-GTP), alkaline phosphatase (ALP), serum creatinine, serum albumin, fasting serum insulin (IRI), hematocrit, white blood cell, hemoglobin, platelet, serum C-peptide, high-sensitivity C-reactive protein (CRP), serum amylase, serum ketone body, serum uric acid, total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, urinary test data (if routine urinary test data are available). Urinary test data include: urinary glucose (qualitative), urine protein (qualitative), urine protein (quantitative), urine albumin (quantitative), urinary ketone (qualitative), urinary ketone (quantitative), urinary albumin to creatinine ratio, urine protein to creatinine ratio
Objectives
Primary Objectives
The primary objective is to describe the long-term disease management patterns (type and number of medicines prescribed, and other management approaches used) and clinical evolution (clinical course, such as glycemic control, including HbA1c and hypoglycemic frequencies; degree of progression and prognosis of complications; and changes in the quality of life) of patients with T2DM inadequately controlled with a first-line antidiabetic therapy who initiate a second-line antidiabetic treatment (either adding a second therapy to the first therapy or switching the first therapy to a second therapy).
Secondary Objectives
The secondary objectives include describing the overall and medication class-specific treatment response in terms of change from baseline in HbA1c, blood glucose (fasting plasma glucose [FPG], casual plasma glucose [CPG], or postprandial plasma glucose [PPG]), body weight, blood pressure and lipid profile, and the achievement rates of HbA1c and blood glucose target goals. Furthermore, we aim to describe treatment changes, including the proportion of patients who initiate with a second, third, fourth or higher level of antidiabetic medication at each follow-up visit, the proportion that initiate insulin therapy and the dose of insulin, the proportion of patients who switch antidiabetic medication, and the changes in dose for each treatment.
We also aim to describe the diabetes-associated complications in terms of microvascular complications, incidence of chronic nephropathy, dialysis, diabetic retinopathy, retinal photocoagulation, amputation of lower extremity, diabetic foot disease, peripheral nerve disorders, autonomic nervous system disorders, and erectile dysfunction in the whole population and by second-line antidiabetic medication class.
Further secondary objectives include describing the incidence of macrovascular complications (heart failure, myocardial infarction, and stroke), hypoglycemic events and hyperglycemia hospitalizations in the whole population and by second-line antidiabetic medication class; describing in the whole population and in second-line antidiabetic medication class subgroups the patient-reported quality of life, diet, and level of physical activity; identifying risk factors associated with poorer clinical outcomes during follow-up, such as patient characteristics at baseline (age, gender, duration of diabetes, and presence of comorbidities); and identifying the determinants of treatment choice at baseline.
Endpoints
Primary Endpoint
The primary endpoint is the mean change in HbA1c between baseline and at 6, 12, 24, and 36 months in the overall population and by second-line antidiabetic medication class.
Secondary Endpoints
The secondary endpoints are the mean changes in blood glucose, body weight, body mass index, and lipid profiles between baseline and 6, 12, 24, and 36 months in the overall population and by second-line antidiabetic medication class.
Exploratory Endpoints
The exploratory endpoints are the changes in antidiabetic medications, microvascular and macrovascular events, patient reported outcomes, and lifestyle variables, including sleeping behaviors, in the overall population and by second-line antidiabetic medication. Patient-reported outcomes will be measured using the standard 36-Item Short-Form Health Survey (SF-36) [13, 14], the Diabetes Treatment Satisfaction Questionnaire (DTSQ) [15], the International Physical Activity Questionnaire (IPAQ-SV) [16, 17] and the Brief-Type Self-Administered Diet History Questionnaire (BDHQ) [18–20], which are to be completed by patients at the allocated visits. Lifestyle variables will be estimated using the SF-36, DTSQ, IPAQ-SV, and BDHQ as relevant.
Safety Reporting
Due to the noninterventional nature of this study, no proactive safety data collection will take place. Adverse drug reactions (ADRs) will be reported to health authorities as stated in local regulations and, if the investigator considers it appropriate, the sponsor (in the case of ADRs to an AstraZeneca product) or the corresponding marketing authorization holder of the drug will be notified.
Sample Size
The J-DISCOVER study aims to recruit 2000 patients. This sample size was calculated to provide sufficiently precise and meaningful data for the primary objective at a compound class level. The sample size was estimated to be 2000 and intended to have at least 200 patients for any specific compound class. Any qualitative variable at a frequency of 5–95% with a sample size of 200 patients should ensure a precision range of 3.0–6.9% at a 95% confidence limit.
Statistical Analyses
Statistical analysis will be conducted with SAS (version 9.2; SAS Institute Inc., Cary, NC, USA).
An interim analysis will be performed after the first and second years following the enrollment of the last patient, and the final analysis of all three years will be reported. The study population for analysis will consist of all patients enrolled in the study. Demographic variables, patient characteristics, and treatment patterns will be summarized using descriptive statistics. Descriptive statistics will include n, mean, median, standard deviation, minimum and maximum for continuous variables, and frequency for categorical variables. Inferential statistics will present data using two-sided 95% confidence intervals when considered relevant. Identification of predictors of treatment choice at baseline using baseline characteristics will be attempted using multivariable regression models. Changes in HbA1c, blood glucose, lipid profile, body weight, and blood pressure will be summarized with descriptive statistics. Stratifications will be made by antidiabetic class at baseline, and regression models will be used to see if antidiabetic class at baseline is a predictor of these outcomes.
Proportions of patients switching and/or adding treatments and/or changing doses of treatments will be summarized with descriptive statistics.
Kaplan–Meier estimates of the cumulative incidence of the following events will be calculated and plotted: switching of second-line treatment; initiation of insulin therapy; initiation of the third or above antidiabetic therapy. Multivariable Cox models will be applied to analyze time-to-event data to assess the association of treatment class at baseline and other baseline variables with clinical outcome variables.
New and/or progression of diabetic nephropathy or neuropathy; retinal laser and/or intraocular treatment due to the development of and/or a deterioration in diabetic retinopathy; nontraumatic amputation; minor and major hypoglycemia and/or hospitalization for hypoglycemia; and cardiovascular events will be summarized descriptively. Stratifications will be made by antidiabetic therapy class at baseline, and logistic regression or Cox model, depending on whether the outcome is binary or time-to-event, will be used to determine if antidiabetic therapy class at baseline is a predictor of these outcomes. Further information relating to the assessment of progression of complications is provided in the ESM.
Quality of life results will be summarized descriptively. Stratifications will be made by antidiabetic therapy class at baseline, and regression models will be used to determine if antidiabetic therapy class at baseline is a predictor of these outcomes.
Conclusion
In summary, J-DISCOVER is an ongoing single-country, multicenter, observational, prospective, longitudinal cohort study, as part of the global DISCOVER program. It is estimated that approximately 2000 patients will be enrolled and followed-up for 3 years. The study will be expected to provide basic data for the development of future guidelines for T2DM.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
J-DISCOVER is funded by AstraZeneca K.K. and Ono Pharmaceutical Co., Ltd., Osaka Japan. Because it is a noninterventional study, no drugs are supplied or funded. The J-DISCOVER Scientific Committee comprises five scientists (I.S., H.W., N.K., T.M., and M.T.). The sponsor has also provided funding for the page processing charges of the journal.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval of the version to be published. The authors thank all investigators and staff supporting this study and patients participating in the J-DISCOVER study.
Medical Writing, Editorial, and Other Assistance
Medical writing assistance in the preparation of this manuscript was provided by Marion Barnett of Edanz Medical Writing on behalf of Springer Healthcare Communications. Support for this assistance was funded by AstraZeneca K.K. and Ono Pharmaceutical Co., Ltd.
Disclosures
Kiyoshi Hashigami is an employee of AstraZeneca K.K. Masaru Kawashima is an employee of Ono Pharmaceutical Co., Ltd. Naoto Katakami holds an endowed chair (in the Department of Metabolism and Atherosclerosis, Graduate School of Medicine, Osaka University, Osaka, Japan) established by funds from Kowa Pharmaceutical Co., and has received honoraria from Arkray, Inc., Astellas Pharma Inc., AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo Inc., Dainippon Sumitomo Pharma, Eli Lilly, Kowa Pharmaceutical Co. Ltd., Kyowa Hakko Kirin Co. Ltd., Mitsubishi Tanabe Pharma, Merck & Co. Inc., Novartis Pharmaceuticals, Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Sanofi, Shionogi & Co. Ltd., and Takeda Pharmaceutical Co. Ltd. Mitsuyoshi Takahara has received honoraria from Astellas Pharma Inc., AstraZeneca, Boehringer Ingelheim, Eli Lilly, Kowa Pharmaceutical Co. Ltd., Merck & Co., Inc., Mitsubishi Tanabe Pharma, Novartis Pharmaceuticals, Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Sanofi, Shionogi & Co. Ltd., Taisho Toyama Pharmaceutical Co. Ltd., and Takeda Pharmaceutical Co. Ltd. Tomoya Mita has received honoraria from Astellas Pharma Inc., AstraZeneca, Daiichi Sankyo Inc., Eli Lilly, Kowa Pharmaceutical Co. Ltd., Kyowa Hakko Kirin Co. Ltd., Merck & Co. Inc, Mitsubishi Tanabe Pharma, Novartis Pharmaceuticals, Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Sanwa Kagaku Kenkyusho, and Takeda Pharmaceutical Co. Ltd. Iichiro Shimomura has received honoraria from Astellas Pharma Inc., AstraZeneca, Boehringer Ingelheim, Kowa Co. Ltd., Merck Sharp & Dohme, Mitsubishi Tanabe Pharma Co., Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co. Ltd., Sanwa Kagaku Kenkyusho Co. Ltd., and Takeda Pharmaceutical Co. Ltd., and research grants from the Astellas Pharma Inc., AstraZeneca, Daiichi Sankyo Inc., Eli Lilly, Japan Foundation for Applied Enzymology, Japan Science and Technology Agency, Kowa Co. Ltd., Kyowa Hakko Kirin Co. Ltd., Midori Health Management Center, Mitsubishi Tanabe Pharma Co., Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co. Ltd., Sanofi, Suzuken Memorial Foundation, and Takeda Pharmaceutical Co. Ltd. Hirotaka Watada has received honoraria from Boehringer Ingelheim, Daiichi Sankyo Inc., Dainippon Sumitomo Pharma Co. Ltd., Eli Lilly, Kowa Co. Ltd., Merck Sharp & Dohme, Novo Nordisk Pharma Ltd., Novartis Pharmaceuticals, Ono Pharmaceutical Co., Sanofi, Sanwa Kagaku Kenkyusho, and Takeda Pharmaceutical Co., and research funds from AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo Inc., Dainippon Sumitomo Pharma, Eli Lilly, Kissei Pharma, Merck Sharp & Dohme, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical Co., Novartis Pharmaceuticals, Novo Nordisk Pharma Ltd., Pfizer, Sanofi, Sanwa Kagaku Kenkyusho, Shionogi Pharma, Takeda Pharmaceutical Co., and Terumo Corp.
Compliance with Ethics Guidelines
All procedures followed will be in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients included in the study.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/7BFCF0607F7A928B.
References
- 1.International Diabetes Federation. IDF diabetes atlas executive summary. 7th ed. Brussels: International Diabetes Federation; 2015. p. 4.
- 2.Ministry of Health, Labour and Welfare. The National Health and Nutrition Survey in Japan. Tokyo: Ministry of Health, Labour and Welfare; 2014. p. 200.
- 3.The Ministry of Health. Labour and Welfare: The National Health and Nutrition Survey in Japan, 2007. Tokyo: Ministry of Health, Labour and Welfare; 2010. p. 56.
- 4.World Health Organization . Global status report on noncommunicable diseases; 2010. Geneva: World Health Organization; 2014. [Google Scholar]
- 5.Japan Diabetes Clinical Data Management Study Group. Basic summary data. 2014. Available from: http://jddm.jp/data/index.html. Accessed 19 Apr 2017.
- 6.Japanese Diabetes Society (ed.) Treatment guide for diabetes 2014–2015. Tokyo: Bunkodo; 2014. Available at: http://www.fa.kyorin.co.jp/jds/uploads/Treatment_Guide_for_Diabetes_2014-2015.pdf. Accessed 8 February 2017.
- 7.Hayashino Y, Izumi K, Okamura S, et al. Duration of diabetes and types of diabetes therapy in Japanese patients with type 2 diabetes: the Japan Diabetes Complication and its Prevention Prospective Study 3 (JDCP study 3). J Diabetes Investig. 2017;8:243–9. [DOI] [PMC free article] [PubMed]
- 8.Inzuchci SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the study of diabetes. Diabetes Care. 2015;38:140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 9.Tajima N, Nishimura R, Izumi K, et al. A large-scale, observational study to investigate the current status of diabetes complications and their prevention in Japan: research outline and baseline data for type 2 diabetes: JDCP study1. J Jpn Diabetes Soc. 2015;58:346–357. doi: 10.1007/s13340-015-0248-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujihara K, Hanyu O, Heianza Y, et al. Comparison of clinical characteristics in patients with type 2 diabetes among whom different antihyperglycemic agents were prescribed as monotherapy or combination therapy by diabetes specialists. J Diabetes Investig. 2016;7:260–269. doi: 10.1111/jdi.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–53. [PubMed]
- 12.Ji L, Bonnet F, Charbonnel B, et al. Towards an improved global understanding of treatment and outcomes in people with type 2 diabetes: rationale and methods of the DISCOVER observational study program. J Diabetes Complicat. 2017;31:1188–1196. doi: 10.1016/j.jdiacomp.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Fukuhara S, Bito S, Green J, Hsiao A, Kurokawa K. Translation, adaptation, and validation of the SF-36 Health Survey for use in Japan. J Clin Epidemiol. 1998;51:1037–1044. doi: 10.1016/S0895-4356(98)00095-X. [DOI] [PubMed] [Google Scholar]
- 14.Fukuhara S, Ware JE, Kosinski M, Wada S, Gandek B. Psychometric and clinical tests of validity of the Japanese SF-36 Health Survey. J Clin Epidemiol. 1998;51:1045–1053. doi: 10.1016/S0895-4356(98)00096-1. [DOI] [PubMed] [Google Scholar]
- 15.Ishi H, Bradley C, Riazi A, Barendse S, Yamamoto T. The Japanese version of the Diabetes Treatment Satisfaction Questionnaire (DSTQ): translation and clinical evaluation. Igaku no Ayumi. 2000;192:809–814. [Google Scholar]
- 16.Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 17.Murase N, Katsumura T, Ueda C, Inoue S, Shimomitsu T. Validity and reliability of Japanese version of International Physical Activity Questionnaire. J Health Welf Statistics. 2002;49:1–9. [Google Scholar]
- 18.Sasaki S, Yanagibori R, Amano K. Self-administered diet history questionnaire developed for health education: a relative validation of the test-version by comparison with 3-day diet record in women. J Epidemiol. 1998;8:203–215. doi: 10.2188/jea.8.203. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi S, Murakami K, Sasaki S, et al. Comparison of relative validity of food group intakes estimated by comprehensive and brief-type self-administered diet history questionnaires against 16 d dietary records in Japanese adults. Public Health Nutr. 2011;14:1200–1211. doi: 10.1017/S1368980011000504. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka H. Health Labour Sciences Research Grant research on cancer prevention and health services (study about evaluation methods of nutrient and dietary program for “Healthy Japan 21”: comprehensive study report 2001–2003). Tokyo: National Institute of Health and Nutrition (Japan); 2004. p 32
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.