Abstract
Introduction
The risk of rheumatoid arthritis (RA) associated with dipeptidyl peptidase-4 inhibitor (DPP-4i) use is unclear. This study assesses the RA risk associated with DPP-4i use among a diabetic cohort initiating second-line therapy.
Methods
This was a nested case–control study, using the adult diabetic population starting second-line antidiabetic therapy from IMS LifeLink Plus® database (2006–2015). Cases were those with two or more RA diagnosis, at least one prescription, and 180 days enrollment prior to the event date (earliest of the two: first RA diagnosis, first RA prescription). Controls were drawn from the nest after matching (1:15) with cases on index date (± 90 days), age (± 5 years), sex, and event date (imputed to have the same time difference between cohort entry and event date as the matched case). Exposure and covariate information was gathered from the 180-day period prior to event date. Conditional logistic regression was used to assess exposure among cases and controls. Adjusted analysis was carried out after controlling for important medications and comorbidities.
Results
The final sample consists of 790 cases and 11,850 controls; of these, 151 cases (19.11%) and 2177 controls (18.37%) had DPP-4i claims during the exposure assessment period. DPP-4i therapy was not significantly associated with the development of RA after adjusting for covariates (OR = 1.156, 95% CI 0.936–1.429). Changing the exposure definition or exposure window to 1 year and subgroup analyses yielded similar results except for the non-insulin-using subgroup (OR = 1.299, 95% CI 1.001–1.985) which showed a significant positive association.
Conclusion
DPP-4i were not significantly associated with the risk of RA compared with other second-line antidiabetic therapies.
Keywords: DPP-4 inhibitors, Rheumatoid arthritis, Adverse effects, Type 2 diabetes mellitus, Claims data, Nested case–control study
Introduction
Diabetes is one of the most prevalent chronic diseases in the USA [1]. Among diabetics, 95% of the patients suffer from type 2 diabetes mellitus (T2DM), which carries with it a significant comorbidity burden, affecting patient’s healthcare cost [2, 3] burden and quality of life [4]. The current recommendations for treatment of T2DM involve oral hypoglycemic therapy to lower blood glucose levels (HbA1c). Biguanides are the most commonly initiated oral agents used to treat T2DM therapy [5]. Over time, biguanides become less effective at controlling HbA1c levels and the addition of second-line therapies is often necessary [6]. The commonly used second-line therapies include sulfonylureas, thiazolidinedione, dipeptidyl peptidase-4 inhibitors (DPP-4i), and incretin mimetics [7].
DPP-4i are a relatively new class of oral antidiabetics with the first agent (sitagliptin) being approved in 2006 [8]. The approval was based on trial data demonstrating efficacy in controlling HbA1c along with other benefits such as not increasing weight and decreasing rates of hypoglycemic events [9]. The trial data also reported some significant adverse events, namely increased rates of upper respiratory tract infections, urinary tract infections, and hypersensitivity reactions [10]. Randomized controlled trials often lack statistical power to detect less frequent adverse events associated with drug use [11]. Epidemiological studies serve as useful tools to study specific rare adverse events. One such adverse event was brought to light by a recently published report from the Food and Drug Administration (FDA). The report highlighted the risk of arthralgia and joint pain symptoms associated with the use of DPP-4i including one case of suspected rheumatoid arthritis (RA) [12].
DPP-4i act by blocking the transmembrane glycoprotein DPP-4, present in gut epithelia [13]. Preclinical studies have shown that DPP-4 is also present throughout the body, including joint fluids and the immune cells specifically fibroblasts, T cells, and macrophages [14, 15]. This enzyme is an inflammatory mediator, hence the inhibition of DPP-4 at off-target sites is purported to be responsible for its protective effects in inflammation [16]. It is possible that this anti-inflammatory mechanism also reduces the risk of RA, an autoimmune disorder characterized by pain, inflammation, and gradual deterioration of joints [17].
Clinical studies have provided mixed results. Previous reports have found a link between DPP-4i and risk of acute pancreatitis [18, 19]. Similarly, evidence from case reports suggests that DPP-4i may be associated with increased joint pain [20–23]. These reports may be confounded by the presence of various co-morbidities. Additionally, the studies reported joint pain symptoms as an adverse event but did not report on RA. An epidemiological cohort study assessed the risk of various autoimmune disorders such as systemic lupus erythematosus (SLE) and RA [24]. The study reported that the use of DPP-4i was associated with significantly reduced risk of RA; however, the effect was not significant in subsequent subgroup analysis. Although the study results are consistent with preclinical data, they contradict the findings of multiple case reports. The ambiguity in the current literature requires a thorough evaluation of the risk of RA among DPP-4i therapy users. This study aims to assess the risk of developing RA associated with DPP-4i use among patients with T2DM compared with other second-line antidiabetic therapy.
Methods
Data Source
This study used the IMS LifeLink Plus® dataset which includes a sample of large private health insurance recipients in the USA [25]. The data is representative of the commercially insured US population and includes 11 million covered lives annually. The data includes information on medical claims, pharmacy claims, and enrollment. The enrollment file contains demographic information such as age, sex, geographic region, state, payer type, and plan type. The medical claims files contain inpatient, outpatient, and professional service claims with information on date and place of service, performed procedures (CPT), and disease diagnosis (ICD-9-CM). The pharmacy claims information includes Generic Product Identifier (GPI) of drug class, date of medication dispensed, quantity dispensed, and estimated days supply. All claims between January 2006 and June 2015 were examined. The data obtained was de-identified such that any identifiable information was removed (e.g., zip codes, race) or recoded (enrollees with age greater than 85 are truncated to the age of 85 years). Hence it is compliant with the Health Insurance Portability Act of 1996. This article is based on retrospective de-identified data and does not involve any new studies of human or animal subjects performed by any of the authors. The study was determined as exempt by the university’s investigational review board.
Nest Composition
All patients with a pharmacy claim for a second-line antidiabetic therapy [defined as a prescription for antidiabetic drug classes DPP-4i (GPI code “2755x”/“279930x”/“279925x”) or sulfonylureas (GPI code “2720x”/“279970x”/“279978x”) or thiazolidinediones (GPI codes “2760x”/“279978x”/“279980x”) or meglitinide analogues (GPI codes “2728x”) or insulin (GPI codes “2710x”)] were selected for the analysis [26, 27]. To improve specificity, all patients were required to have two or more claims for antidiabetic drugs (GPI codes “27x”). Patients who only had claims for insulin products as their antidiabetic therapy (GPI code “2749x”) were excluded to minimize the risk of including type 1 diabetics [28, 29]. Patients less than 18 years of age on the date of their first second-line therapy of antidiabetic medication in the data were excluded. The resulting cohort was defined as the nest, and the date of the first prescription for a second-line antidiabetic therapy was defined as the “index date” or cohort entry date. All patients were required to have 180 days of continuous enrollment before the index date to ensure only incident second-line therapy users were included in the study. Patients that had a diagnosis of RA (ICD-9 CM “714X”) or a prescription for RA-specific drugs prior to index date were excluded.
Study Outcomes
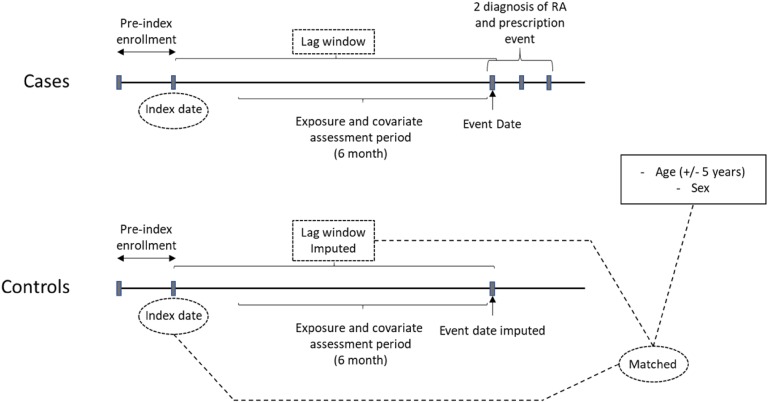
Patients with two or more diagnoses on different dates of service for RA in any setting and at least one prescription for disease-modifying agents or corticosteroids were selected as cases from the nest [30–33]. The date of first RA diagnosis or RA prescription fill date after the index date was defined as the event date. All cases were required to have 180 days of enrollment prior to event date without a diagnosis or prescription for RA, to reduce the possibility of including prevalent RA patients. Additionally, any patient with HIV or pregnancy diagnosis was also excluded [6, 34–37]. The resulting cases were matched (1:15) with potential controls on age (± 5 years), sex, and index date (± 90 days) without replacement. Incidence density sampling was used for control selection to represent the population at risk (those who have not yet developed RA) at the time when the case is developed. The event date was imputed for controls such that the time difference between the index date and event date was the same for all the matched cases and controls as shown in Fig. 1.
Fig. 1.
Study design. The nest population consists of patients who had a prescription for second-line T2DM therapy. Cases were those with two or more separate diagnoses of RA and a prescription event for RA drugs. Matched controls were selected from the nest who did not have a diagnosis of RA when the case developed. Matching on age (± 5 years), sex, index date (± 90 days), and event date (lag window is equal between cases and controls). RA, rheumatoid arthritis; index date, date of first prescription for second-line T2DM therapy; event date, first RA diagnosis post index date; lag window, index date–event date
Study Exposure
The 180-day lookback period prior to the event date (for cases) or imputed event date (for controls) was used to ascertain exposure status. Patients with a prescription claim for DPP-4i (“2755x”/“279930x”/“279925x”) during this period were defined as “DPP-4i” and those with at least one prescription for any other second-line therapy agents were defined as “DPP-4i” [26].
Covariates
Information on important covariates, demographics, and enrollment from the 180-day period was collected, as was information on co-prescribed pharmacotherapy based on previous studies including hormone replacement therapy, insulin, contraceptives, ACE inhibitors, statins, and arthralgia risk-modifying drugs such as hydralazine [38, 39]. Comorbidity information for renal diseases, obesity, asthma, osteoarthritis, diabetes-related complications, and other autoimmune diseases, namely diffuse diseases of connective tissue, psoriasis and similar disorders, regional enteritis, and ulcerative colitis, was obtained from inpatient or outpatient diagnosis codes [24].
Statistical Analysis
Bivariate analyses estimated unadjusted risk and conditional logistic regression was used to analyze exposure among cases and controls while adjusting for covariates to account for the highly stratified nature of the matched data. Sensitivity analysis was used to test study assumptions first by increasing the lookback period to 1 year and second by changing the exposure definition to define exposed patients as only those with at least 60 days exposure to DPP-4i during the exposure determination period. Subgroup analyses were used to assess the effect among non-insulin users and those without other autoimmune diseases (non-AD subgroup). To account for the heterogeneity in the “other second-line therapy agent exposed” treatments, individual common second-line agents (i.e., sulfonylurea and TZD) were compared with DPP-4i. Additionally a subgroup analysis was also conducted after stratifying the data by sex. All analyses were performed using SAS® 9.3.
Results
Sample Characteristics
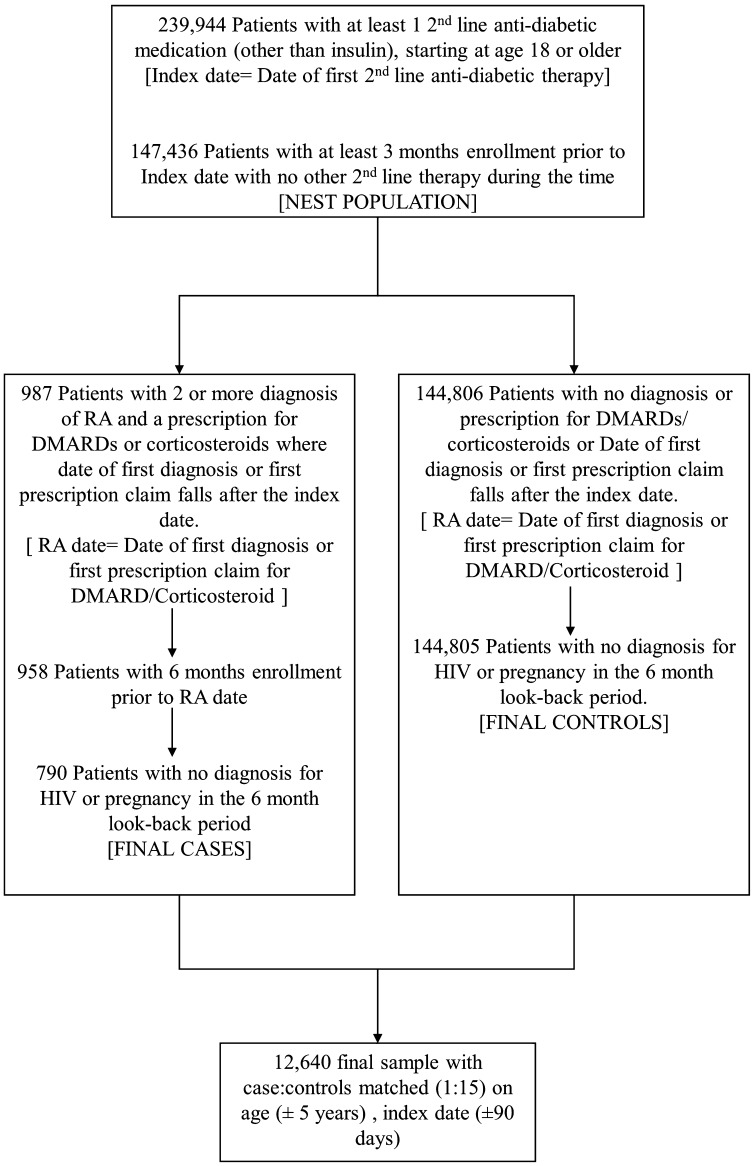
The sample consisted of 239,944 patients with claims for second-line antidiabetic medications (besides insulin) between January 2006 and June 2015. After applying inclusion and exclusion criteria, the nest consisted of 147,436 diabetic patients from which cases and controls were selected. From the nest, 790 cases were drawn that met study criteria and were matched to 11,850 controls (final sample after matching = 12,640; Fig. 2, Table 1). The final matched sample had a mean age of 60.70 years, and 63.04% were female. A total of 151 cases (19.11%) and 2177 (18.37%) matched controls were exposed to DPP-4i therapy. The cases and controls differed on the presence of comorbidities as well as concomitant therapies (Table 1). The cases were more likely to suffer from other autoimmune disorders and cardiovascular diseases and to have prescriptions for corticosteroids and anti-asthmatics.
Fig. 2.
Flowchart showing the application of inclusion, exclusion to create the nest population, selection of rheumatoid arthritis cases and matched controls. RA, rheumatoid arthritis; HIV, human immunodeficiency virus; DMARDs, disease-modifying antirheumatic drugs/RA-specific drugs
Table 1.
Sample characteristics of case and controls, unadjusted and adjusted odds ratios for the association between RA and sample characteristics
| Case (n = 790) | Control (n = 11,850) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|---|---|
| Age, mean (SD) | 60.70 (10.49) | 61.36 (6.32) | ||
| Female, n (%) | 498 (63.04) | 7470 (63.04) | ||
| Prescription drugs, n (%) | ||||
| DPP-4 inhibitor use (exposure) | 151 (19.11) | 2177 (18.37) | 1.056 (0.869–1.284) | 1.156 (0.936–1.429) |
| Insulin | 289 (36.58) | 3416 (28.83) | 1.459 (1.249–1.704) | 1.357 (1.125 –1.637) |
| Contraceptives | 9 (1.14) | 149 (1.26) | 0.892 (0.433–1.838) | 0.879 (0.423–1.826) |
| Hormone replacement therapy | 43 (5.44) | 473 (3.99) | 1.396 (1.008–1.933) | 1.367 (0.980–1.907) |
| Hydralazine | 8 (1.01) | 113 (0.95) | 1.062 (0.518–2.181) | 0.740 (0.347–1.577) |
| ACE inhibitor/ARB | 468 (59.24) | 6682 (56.39) | 1.129 (0.972–1.311) | 1.111 (0.953–1.297) |
| Statin | 373 (47.22) | 6068 (51.21) | 0.845 (0.729–0.980) | 0.807 (0.692–0.941) |
| Comorbidities, n (%) | ||||
| Other AD | 54 (6.84) | 215 (1.81) | 3.943 (2.902–5.359) | 3.340 (2.434–4.583) |
| Asthma | 140 (17.72) | 1509 (12.73) | 1.483 (1.224–1.796) | 1.256 (1.029–1.532) |
| Renal | 69 (8.73) | 6.02 (5.08) | 1.818 (1.395–2.370) | 1.468 (1.107–1.947) |
| Obesity | 87 (11.01) | 1044 (8.81) | 1.286 (1.018–1.624) | 0.984 (0.772–1.253) |
| Osteoarthritis | 210 (26.58) | 1208 (10.19) | 3.324 (2.795–3.953) | 3.021 (2.529–3.610) |
| Diabetes with complications | 234 (29.62) | 2662 (22.46) | 1.465 (1.248–1.721) | 1.181 (0.993–1.404) |
| Heart diseases | 183 (23.16) | 1989 (16.78) | 1.533 (1.283–1.831) | 1.266 (1.048–1.530) |
SD standard deviation, DPP-4 dipeptidyl peptidase-4, AD autoimmune disease, OR odds ratio, ACE angiotensin converting enzyme, ARB angiotensin receptor blocker
Unadjusted Analysis
In an unadjusted analysis, the odds for exposure to DPP-4i among cases was found to be 1.056 (95% CI 0.869–1.284). However, other patient characteristics such as having hormone replacement therapy (OR = 1.396, 95% CI 1.008–1.933) and insulin use (OR = 1.459, 95% CI 1.249–1.704) were associated with the development of RA.
Adjusted Analysis
After controlling for concomitant therapies and comorbidities, DPP-4i therapy was not significantly associated with developing RA (OR = 1.156, 95% CI 0.936–1.429). However, other factors such as the use of hormone replacement therapies, asthma, the presence of other autoimmune disorders, osteoarthritis, diabetes with complications, and heart conditions were associated with RA.
Sensitivity and Subgroup Analysis
The results of the subgroup analyses, as seen in Table 2, followed a trend similar to the primary analysis. In the non-AD subgroup (OR = 1.054, 95% CI 0.854–1.300) the exposure to DPP-4i was not associated with RA. In the non-insulin subgroup (OR = 1.299, 95% CI 1.001–1.985) patients on DPP-4i were more likely to develop RA. In the subgroup analysis comparing DPP-4i exposure to individual second-line therapies, namely TZD (OR = 0.695, 95% CI 0.403–1.199) and sulfonylurea (OR = 1.074, 95% CI 0.769–1.500), the results were again non-significant. Additionally, when the data was analyzed after stratifying on sex, the results were not significant for men (OR = 1.099, 95% CI 0.781–1.546) or women (OR = 1.190, 95% 0.907–1.562). The sensitivity analysis also showed a similar trend when the definition for DPP-4i exposure was changed from having one prescription for the drug to having at least 60 days of therapy (OR = 1.170, 95% CI 0.938–1.461) or when the exposure assessment was changed from 180 days to 1 year (OR = 1.098, 95% CI 0.867–1.423).
Table 2.
Sensitivity analysis and subgroup analysis
| Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|
| Alternative exposure definition | 1.079 (0.877–1.327) | 1.170 (0.938–1.461) |
| 1-year exposure ascertainment time window | 1.148 (0.902–1.398) | 1.098 (0.867–1.423) |
| Non-insulin subgroup | 1.173 (0.936–1.471) | 1.299 (1.001–1.985) |
| Non-AD subgroup | 1.074 (0.877–1.314) | 1.054 (0.854–1.300) |
| Sulfonyl urea comparison subgroup | 1.097 (0.884–1.362) | 1.074 (0.769–1.500) |
| Thiazolidinedione comparison subgroup | 1.083 (0.800–1.465) | 0.695 (0.403–1.199) |
| Male subgroup | 1.019 (0.745–1.394) | 1.099 (0.781–1.546) |
| Female subgroup | 1.081 (0.843–1.387) | 1.190 (0.907–1.562) |
Adjusted odds ratio are presented while controlling for covariates similar to the base-case analysis
AD autoimmune disease, OR odds ratio, Alternative exposure definition at least 60 days of DPP-4 therapy
Discussion
This study is the first to assess the association between DPP-4i and development of RA among T2DM patients initiating second-line therapies. In this study, exposure to DPP-4i was not significantly associated with the development of RA when compared to other second-line T2DM therapies. Except in the non-insulin-using subgroup, the association was not significant among subgroups tested; sensitivity analysis around the length of the lookback period yielded similar results. Among the non-insulin subgroup of patients, the likelihood of RA was 30% higher.
This study examines a nationally representative sample of commercially insured patients. To study an adverse event with low incidence in the population of interest a case–control study design nested in the T2DM population was employed. The cases and controls were nested within an appropriate sample of patients initiating second-line antidiabetic therapy. Validated definitions of cases and controls were used in the analysis. The study also employed appropriate methods to select incident cases and used incidence density sampling to select controls which approximates the baseline risk of exposure in the population. Important confounders such as diabetes severity, comorbidities, and important concomitant pharmacotherapies were included in the adjusted analysis. While data on HbA1c was not available to capture diabetes severity, proxy measures such as insulin use and the presence of diabetic complications were used.
This study approach differed from the approach used by Kim et al. [24], which evaluated some autoimmune disorders (including RA) by following patients until event development or therapy discontinuation using a cohort study design. Censoring patients at the development of the first autoimmune disorder would blind the analysis to other disorders with delayed onset. The cohort study only allowed a 1-month window after exposure termination for development of RA while the current study can observe delayed onset after exposure. To account for these differences, we performed sensitivity analysis using a lookback period of 1 year. The study population of the current study was older (mean age 61 vs. 55 years) and included people on add-on insulin therapy which were excluded in the study by Kim et al. Add-on insulin therapy is prescribed to diabetics who have inadequate glycemic control with oral agents. The inclusion of such patients makes the population more representative. A subgroup analysis using only non-insulin cases and controls was performed to account for the difference.
The study findings are not consistent with the findings by Kim et al., which reported a 33% reduced risk of RA. The results obtained by Kim et al. may be affected by the differences in the study design elements. Importantly, the results were not significant in most of the subgroup analyses conducted by Kim et al. In the current study, the results from sensitivity analyses were similar to the primary analysis, but the non-insulin subgroup analysis found an increased risk of RA development. This finding is not surprising since severe T2DM patients are prescribed insulin and may be at increased baseline risk of developing RA. At a lower baseline risk of developing RA among the non-insulin subgroup the added risk associated with DPP-4i becomes significant. However, these results are the opposite of the results obtained by Kim et al., which reported significant protective effect among non-insulin patients. The discordance between these study results may be due to the presence of other autoimmune diseases. The cohort study by Kim et al. censored patients on the development of other autoimmune diseases which could then lead to the development of RA [40]. The significant association between other autoimmune disorders prior to the development of RA in this study provides further support for the explanation. This study contributes to the limited evidence on the risk of development of RA due to DPP-4i therapy and attempts to address the concerns raised by case studies and the FDA warning [12]. Numerous case reports and case studies are evaluating the association between DPP-4i use and RA. Although the study’s primary analysis results are non-significant, they are consistent in direction with the case studies and case reports [22, 34].
The study findings have important implications for patients who are starting on newer second-line antidiabetic therapies. The signal generated from case reports, coupled with the findings from this study cast considerable doubts on the protective effects of DPP-4i on RA suggested by Kim et al.’s study. Physicians should exercise caution when prescribing DPP-4i therapies especially for patients with greater risk of joint pain or RA or patients with low diabetes severity and who have not initiated insulin. This study specifically focused on RA, but there may be an increased risk of arthralgia and joint pain associated with DPP-4i which warrants further investigation. Physicians need to monitor early signs of joint pain and arthralgia after initiation of DPP-4i.
The findings of the study need to be interpreted with caution in the light of certain limitations. As a result of the nature of the IMS LifeLink Plus® database, information on important patient characteristics and risk factors such as race, weight, and family history of arthritis, diagnostic and lab values were not available. There may be issues with case ascertainment due to misdiagnosis of RA even though validated definitions of RA were used [41]. Also, there is a possibility of selecting prevalent RA cases due to the left-censored nature of the data. This issue is minimized by excluding cases and controls if they had prescriptions for RA-specific drugs during the lookback period. Additionally, this study did not assess the risk of arthralgia, joint pain, other rheumatic diseases, or other autoimmune disorders among DPP-4 therapy patients or assess the risk specifically among non-insulin cohort. Hence further research on impact of DPP-4i on other outcomes besides RA is required.
Conclusion
This study demonstrated that DPP-4i use was not associated with the development of RA when compared to other second-line therapies. Physicians, however, need to be cautious while prescribing DPP-4i therapy and continuously monitor patients for RA symptoms.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article. The IMS LifeLink Plus Pharmetrics data used in our study was supported by the University of Arkansas Translational Research Institute (NIH Grant # 1UL1RR029884).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Compliance with Ethics Guidelines
This article is based on retrospective de-identified data and does not involve any new studies of human or animal subjects performed by any of the authors. The retrospective data used was de-identified such that any identifiable information was removed (e.g., zip codes, race) or recoded (enrollees with age greater than 85 are truncated to the age of 85 years). The study was determined as exempt by the university’s investigational review board.
Disclosure
Q. Said was faculty at the Division of Pharmaceutical Evaluation & Policy Department of Pharmacy, UAMS while working on the manuscript. Q. Said is currently employed by Novartis Pharmaceutical Corporation. Authors N. Kathe, A. Shah, and J. T Painter have nothing to disclose.
Data Availability
The IMS LifeLink Plus data used during the current study is a commercial product offered by IMS and as per the data use agreement, the data cannot be shared publicly.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/DBFCF0602A633FFD.
References
- 1.Centers for Disease Control and Prevention. National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014. Atlanta: US Department of Health and Human Services; 2014.
- 2.Egede L, Zheng D, Simpson K. Comorbid depression is associated with increased health care use and expenditures in individuals with diabetes. Diabetes Care. 2002;25:464–70. [DOI] [PubMed]
- 3.American Diabetes Association. Economic costs of diabetes in the US in 2012. Diabetes Care. 2013;36:1033–46. [DOI] [PMC free article] [PubMed]
- 4.Kathe N, Hayes CJ, Bhandari NR, Payakachat N (2017) Assessment of reliability and validity of SF-12v2 among a diabetic population. Value Health. 10.1016/j.jval.2017.09.007. [DOI] [PubMed]
- 5.UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854–65. [PubMed]
- 6.Raebel MA, Ellis JL, Schroeder EB, Xu S, O’Connor PJ, Segal JB, et al. Intensification of antihyperglycemic therapy among patients with incident diabetes: A Surveillance Prevention and Management of Diabetes Mellitus (SUPREME-DM) study. Pharmacoepidemiol Drug Saf. 2017;23:699–710. [DOI] [PubMed]
- 7.Turner RC, Cull CA, Frighi V, Holman RR, UK Prospective Diabetes Study (UKPDS) Group. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA. 1999;281:2005–12. [DOI] [PubMed]
- 8.Doupis J, Veves A. DPP4 inhibitors: a new approach in diabetes treatment. Adv Ther. 2008;25:627–43. [DOI] [PubMed]
- 9.Goldstein BJ, Feinglos MN, Lunceford JK, Johnson J, Williams-Herman DE, Sitagliptin 036 Study Group. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care. 2007;30:1979–87. [DOI] [PubMed]
- 10.Charbonnel B, Karasik A, Liu J, Wu M, Meininger G, Sitagliptin Study 020 Group Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care. 2006;29:2638–2643. doi: 10.2337/dc06-0706. [DOI] [PubMed] [Google Scholar]
- 11.Collet JP. Limitations of clinical trials. Rev Prat. 2000;50:833–837. [PubMed] [Google Scholar]
- 12.Center for Drug Evaluation and Research. FDA Drug Safety Communication: FDA warns that DPP-4 inhibitors for type 2 diabetes may cause severe joint pain. http://www.fda.gov/Drugs/DrugSafety/ucm459579.htm. Accessed 8 Dec 2015.
- 13.Ahrén B. DPP-4 inhibitors. Best Pract Res Clin Endocrinol Metab. 2007;21:517–33. [DOI] [PubMed]
- 14.Cuchacovich M, Gatica H, Pizzo S V, Gonzalez-Gronow M. Characterization of human serum dipeptidyl peptidase IV (CD26) and analysis of its autoantibodies in patients with rheumatoid arthritis and other autoimmune diseases. Clin Exp Rheumatol. 2001;19:673–80. [PubMed]
- 15.Cordero OJ, Salgado FJ, Mera-Varela A, Nogueira M. Serum interleukin-12, interleukin-15, soluble CD26, and adenosine deaminase in patients with rheumatoid arthritis. Rheumatol Int. 2001;21:69–74. [DOI] [PubMed]
- 16.Yazbeck R, Howarth GS, Abbott CA. Dipeptidyl peptidase inhibitors, an emerging drug class for inflammatory disease? Trends Pharmacol Sci. 2009;30:600–7. [DOI] [PubMed]
- 17.Blackburn SCF, Ellis R, George CF, Kirwan JR. The impact and treatment of arthritis in general practice. Pharmacoepidemiol Drug Saf. 1994;3:123–38.
- 18.Amin M, Suksomboon N. Pharmacotherapy of type 2 diabetes mellitus: an update on drug–drug interactions. Drug Saf. 2014;37:903–19. [DOI] [PubMed]
- 19.Chou H-C, Chen W-W, Hsiao F-Y. Acute pancreatitis in patients with type 2 diabetes mellitus treated with dipeptidyl peptidase-4 inhibitors: a population-based nested case-control study. Drug Saf. 2014;37:521–8. [DOI] [PubMed]
- 20.Salam A, Henry R, Sheeran T. Acute onset polyarthritis in older people: is it RS3PE syndrome? Cases J. 2008;1:132. [DOI] [PMC free article] [PubMed]
- 21.Yokota K, Igaki N. Sitagliptin (DPP-4 inhibitor)-induced rheumatoid arthritis in type 2 diabetes mellitus: a case report. Intern Med. 2012;51:2041–4. [DOI] [PubMed]
- 22.Yamauchi K, Sato Y, Yamashita K, et al. RS3PE in association with dipeptidyl peptidase-4 inhibitor: report of two cases. Diabetes Care. 2012;35:e7. [DOI] [PMC free article] [PubMed]
- 23.Mascolo A, Rafaniello C, Sportiello L, et al. Dipeptidyl peptidase (DPP)-4 inhibitor-induced arthritis/arthralgia: a review of clinical cases. Drug Saf. 2016;39:401–7. [DOI] [PubMed]
- 24.Kim SC, Schneeweiss S, Glynn RJ, Doherty M, Goldfine AB, Solomon DH. Dipeptidyl peptidase-4 inhibitors in type 2 diabetes may reduce the risk of autoimmune diseases: a population-based cohort study. Ann Rheum Dis. 2015;74:1968–75. [DOI] [PMC free article] [PubMed]
- 25.MS Real-World Data Adjudicated Claims: USA [IMS PharMetrics Plus]. Bridge to Data, Burlington. http://www.bridgetodata.org/node/824. Accessed 1 Jan 2016.
- 26.Zhang Y, McCoy RG, Mason JE, et al. Second-line agents for glycemic control for type 2 diabetes: are newer agents better? Diabetes Care. 2014;37:1338–45. [DOI] [PubMed]
- 27.Noel RA, Braun DK, Patterson RE, et al. Increased risk of acute pancreatitis and biliary disease observed in patients with type 2 diabetes: a retrospective cohort study. Diabetes Care. 200932:834–8. [DOI] [PMC free article] [PubMed]
- 28.Pickup J, Keen H. Continuous subcutaneous insulin infusion at 25 years: evidence base for the expanding use of insulin pump therapy in type 1 diabetes. Diabetes Care. 2002;25:593–8. [DOI] [PubMed]
- 29.DeWitt D, Hirsch I. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: scientific review. JAMA. 2003;289:2254–64. [DOI] [PubMed]
- 30.Schmajuk G, Trivedi A, Solomon D, Yelin E, et al. Receipt of disease-modifying antirheumatic drugs among patients with rheumatoid arthritis in Medicare managed care plans. JAMA. 2011;305:480–6. [DOI] [PMC free article] [PubMed]
- 31.Kim S, Servi A, Polinski J, et al. Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis Res Ther. 2011;13:R32. [DOI] [PMC free article] [PubMed]
- 32.Singh J, Holmgren A, Noorbaloochi S. Accuracy of Veterans Administration databases for a diagnosis of rheumatoid arthritis. Arthritis Rheum. 2004;51:952–7. [DOI] [PubMed]
- 33.Bernard M-A, Bénichou J, Blin P, et al. Use of health insurance claim patterns to identify patients using nonsteroidal anti-inflammatory drugs for rheumatoid arthritis. Pharmacoepidemiol Drug Saf. 2012;21:573–83. [DOI] [PubMed]
- 34.Crickx E, Marroun I, Veyrie C, et al. DPP4 inhibitor-induced polyarthritis: a report of three cases. Rheumatol Int. 2014;34:291–2. [DOI] [PubMed]
- 35.Masi AT, Feigenbaum SL, Chatterton RT. Hormonal and pregnancy relationships to rheumatoid arthritis: convergent effects with immunologic and microvascular systems. Semin Arthritis Rheum. 1995;25:1–27. [DOI] [PubMed]
- 36.Berman A, Reboredo G, Spindler A. Rheumatic manifestations in populations at risk for HIV infection: the added effect of HIV. Rheumatol. 1991;18:1564–7. [PubMed]
- 37.McIntosh B, Cameron C, Singh S, et al. Second-line therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a systematic review and mixed treatment. Open Med. 2011;5:e35–48. [PMC free article] [PubMed]
- 38.Forsblad d’Elia H, Carlsten H. Hormone replacement therapy in rheumatoid arthritis. Curr Rheumatol Rev. 2006;2:251–60.
- 39.Aho K, Heliövaara M. Risk factors for rheumatoid arthritis. Ann Med. 2004;36:242–251. doi: 10.1080/07853890410026025. [DOI] [PubMed] [Google Scholar]
- 40.Jawaheer D, Seldin MF, Amos CI, et al. A genomewide screen in multiplex rheumatoid arthritis families suggests genetic overlap with other autoimmune diseases. Am J Hum Genet. 2001;68:927–36. [DOI] [PMC free article] [PubMed]
- 41.Losina E, Barrett J, Baron JA, Katz JN. Accuracy of Medicare claims data for rheumatologic diagnoses in total hip replacement recipients. J Clin Epidemiol. 2003;56:515–519. doi: 10.1016/S0895-4356(03)00056-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The IMS LifeLink Plus data used during the current study is a commercial product offered by IMS and as per the data use agreement, the data cannot be shared publicly.