Abstract
Introduction
Previous analyses concluded that patients initiating treatment with sitagliptin are older and have more comorbidities than patients initiating treatment with other oral antihyperglycemic agents (OAHAs). However, these studies focused on the general population or subjects ≤ 65 years of age. We sought to compare differences in baseline characteristics of elderly patients (≥ 65 years of age) with T2DM initiating sitagliptin vs. non-DPP-4 inhibitor (non-DPP-4i) OAHA in the MarketScan® Medicare Supplemental Database.
Methods
Relevant patients were identified in the MarketScan® Medicare Supplemental Database and categorized according to the complexity of their antihyperglycemic treatment: initiating monotherapy, escalating to dual combination therapy, or escalating to triple combination therapy. Within each category, the comparison between patients initiating use of sitagliptin or non-DPP-4i OAHA was made within three age groups: 65–74, 75–84, and ≥ 85 years. Gender and comorbidity recorded within the 12 months prior to the index date (date of initiation/escalation of treatment) were assessed as baseline characteristics in each group. Between-treatment group differences in each covariate were compared using standardized differences.
Results
Patients with T2DM who initiated treatment with sitagliptin tended to be older and were more likely to have a pre-treatment history of arrhythmia, congestive heart failure, peripheral vascular disease, renal failure, and stroke than those initiating non-DPP-4i OAHAs, with the most pronounced differences observed between patients initiating monotherapy in all three age groups. As treatment complexity advanced to dual combination therapy, the differences were attenuated and mostly observed in the 75–84 and ≥ 85 age groups. In patients aged 65–74 years initiating triple therapy, no differences were observed between groups.
Conclusion
Patients ≥ 65 years with T2DM initiating sitagliptin tend to be older and have more comorbidities than those prescribed other classes of OAHA. Appropriate adjustment is required to minimize the impact of potential confounding and channeling bias in any comparative analyses including users of sitagliptin.
Funding
Merck & Co., Inc., Kenilworth, NJ, USA.
Keywords: Channeling bias, DPP-4-inhibitors, Elderly patients, Oral antihyperglycemic agents, Type 2 diabetes
Introduction
It is currently estimated that 415 million people worldwide, approximately 8.8% of all adults, are affected by type 2 diabetes mellitus (T2DM) [1]. The prevalence of T2DM is highest in older adults [2, 3]. In the US, from 2001 to 2010, the percentage of people 65–74 years of age with diabetes increased from 9.1% to 20.7% (a 127% increase) and of those 75 years or older from 8.9% to 20.1% (a 126% increase) [4]. T2DM not only disproportionately affects a large and heterogeneous older patient population, but it is also associated with a variety of complications, including macrovascular (cardiovascular) and microvascular (retinopathy, nephropathy, neuropathy) complications, as well as other conditions such as depression [5]. In addition, older adults with diabetes are more likely to have a variety of geriatric syndromes and conditions distinct from younger patients with diabetes, including polypharmacy, cognitive impairment, injurious falls, and persistent pain [2, 5].
A variety of treatment options are available for patients with T2DM, including metformin, sulfonylureas, PPAR-gamma activators, alpha-glucosidase inhibitors, insulin, dipeptidyl peptidase 4 inhibitors (DPP-4i), glucagon-like peptide 1 receptor agonists, and sodium-glucose-linked transport-2 inhibitors [6]. A physician’s choice of treatment for a given patient is often based on the patient’s medical condition (severity and duration of T2DM, presence of diabetic complications, other comorbidities, and/or propensity for hypoglycemia) as well as the efficacy and safety profiles of available treatments. Treatment choice can be influenced by physician experience, treatment guidelines, patient preference, formulary access and/or restrictions, and medication cost. Differences in baseline demographic and clinical characteristics among patients initiating different therapies can create systematic differences between treatment groups in clinical practice (i.e., channeling [7]), which may introduce bias in observational studies.
Sitagliptin, approved in 2006 in the US, was the first available DPP-4 inhibitor [8]. The efficacy and safety of sitagliptin have been evaluated with an extensive clinical trial program [9–12]. Previous analyses of sitagliptin-prescribing patterns concluded that new users of sitagliptin were more likely to be older and have more comorbidities and complications, greater use of prescription medications, and more physician visits compared to new users of other oral antihyperglycemic agents (OAHAs) [13–16]. However, some studies [13, 16] used databases that were limited to people carrying commercial insurance and significantly underestimated elderly patients who became eligible for Medicare coverage at 65 years of age. Zhang et al. [14] assessed the relationship of baseline characteristics and medication use in patients with T2DM who were prescribed sitagliptin vs. other oral antihyperglycemic agents using the GE Healthcare’s EMR database. However, this study focused on the general population and did not analyze treatment patterns in older subjects (≥ 65 years of age). As such, we sought to compare the baseline characteristics of elderly patients with T2DM initiating sitagliptin vs. non-DPP-4i OAHAs in the Medicare Supplemental Database.
Methods
Data Source and Subjects
This study used information from the Truven MarketScan® Medicare Supplemental Database (MarketScan®, Truven Health Analytics, Ann Arbor, MI, USA). The database contains the healthcare experience of retirees with Medicare supplemental insurance paid by employers. It also includes the portion of the claim paid by Medicare (represented as “Coordination of Benefits Amount,” or COB) in addition to the portions paid by the employer-sponsored supplemental plan and the patient. During 2006–2011, the Medicare Supplemental Database included an average of 2.9 million such patients annually and included information on demographics, health plan membership, medical claims, and pharmacy claims.
Patients ≥ 65 years of age with T2DM were identified if records for the patient indicated at least one inpatient or outpatient diagnosis of diabetes and at least one prescription for OAHA medication. Patients diagnosed with type 1 diabetes, ketoacidosis, malnutrition-associated diabetes, drug-induced diabetes, or gestational diabetes without a subsequent T2DM diagnosis code were excluded from the analysis. Patients on injectable diabetes medications were also excluded.
The time period evaluated for this study was 1 January 2006–31 December 2014. Patients were required to have at least 1 year of continuous enrollment in the database prior to initiation/escalation of antihyperglycemic treatment (i.e., index date) to ensure all patients had 365 days of baseline data available before the index date for each group. Patients initiating sitagliptin were compared to patients initiating any other non-DPP-4i OAHA.
Eligible patients were placed in one of the following three categories according to the complexity of their antihyperglycemic treatment: (1) initiating monotherapy (≥ 1 new outpatient prescription record on or after the T2DM diagnosis); (2) escalating to dual combination therapy (≥ 1 new prescription for a 2nd class of OAHA ≥ 90 days after initiating the 1st class, with the prescription for the 1st class overlapping the index date of 2nd class and with a minimum duration of use of the 1st class ≥ 90 days following the initiation of 2nd class); (3) escalating to triple combination therapy (≥ 1 new prescription for a 3rd class ≥ 90 days after the 2nd class, with prescription for 1st and 2nd classes overlapping the index date of 3rd class, and with the minimum duration of use of the 1st and 2nd classes ≥ 90 days following the initiation of 3rd class).
This article is based on previously conducted studies using a Health Insurance Portability and Accountability Act of 1996 (US) (HIPAA)-compliant database and does not involve any new studies of human or animal subjects performed by any of the authors.
Analysis
Within each category of OAHA use described above, comparison between sitagliptin and non-DPP-4i OAHA was made across three different age groups: 65–74, 75–84, and ≥ 85 years.
Gender and comorbidity [arrhythmia, congestive heart failure, cognitive impairment, fracture, hearing loss, hypertension, hypoglycemia, myocardial infarction, neuropathy, peripheral vascular disease, proteinuria, renal failure, retinopathy, stroke/transient ischemic event, and all eye disease (blindness and vision loss, macular edema, retinopathy, cataracts)] recorded within the 12 months prior to the index date (defined as date of initiation/escalation of antihyperglycemic treatment) were assessed as baseline characteristics in each group. Baseline comorbidities in the population were identified by the presence of any ICD-9-CM diagnosis code, procedure code, or CPT code indicating existence of the condition during the 1 year baseline period prior to index date.
Differences between sitagliptin and non-DPP-4i OAHA treatment groups were compared using standardized difference [17]. Standardized difference is the difference of two means or proportions divided by the pooled estimate of the standard deviation. Unlike the traditional p value, standardized difference is a measure of difference that is not influenced by large sample sizes and has been demonstrated to be a better measure of covariate balance. A standardized difference of at least 10% was used to indicate a meaningful difference between treatment groups [18].
Results
A total of 155,388 patients with T2DM were identified as appropriate for this analysis. Over 52% of patients (52.1%; n = 80,929) initiated sitagliptin (n = 3234) or a non-DPP-4i OAHA monotherapy (n = 77,695), 36.8% (n = 57,206) initiated an escalation to dual combination therapy (sitagliptin, n = 7652; OAHA, n = 49,554), and 11.1% (n = 17,253) initiated an escalation to triple combination therapy (sitagliptin, n = 4429; OAHA, n = 12,824). Patients with T2DM who initiated treatment with sitagliptin tended to be older than those initiating other non-DPP-4 OAHAs (Fig. 1).
Fig. 1.
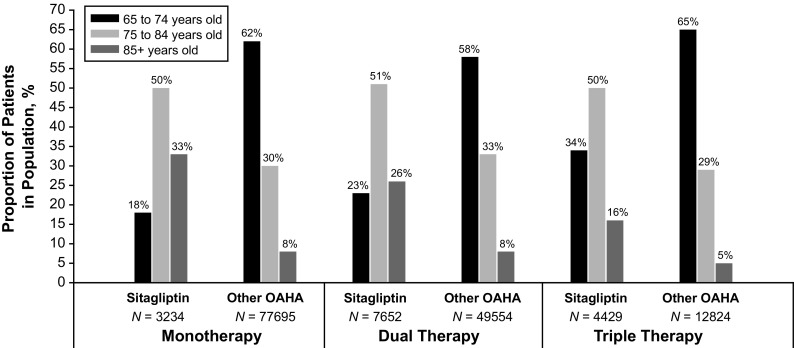
Age distribution of patients ≥ 65 years of age with type 2 diabetes initiating treatment with sitagliptin or a non-DPP-4i oral antihyperglycemic agent (OAHA)
Standardized differences of baseline characteristics of patients with T2DM up to 1 year before initiating treatment with sitagliptin or a non-DPP-4i OAHA were stratified by age and treatment complexity and are presented in Fig. 2. The greatest differences between treatment groups were observed in patients initiating monotherapy in all three age groups. Compared to patients initiating monotherapy with non-DPP-4i OAHAs, patients initiating monotherapy with sitagliptin were more likely to have a history of arrhythmia (Fig. 2a–c), congestive heart failure, peripheral vascular disease, renal failure, and stroke. As treatment complexity advanced to dual combination therapy, the differences were attenuated and mostly observed in the 75–84 and ≥ 85 age groups (Fig. 2d–f). Compared to non-DPP-4i OAHAs, patients initiating an escalation to dual combination therapy with sitagliptin were more likely to have a history of arrhythmia, congestive heart failure, renal failure, and stroke.
Fig. 2.
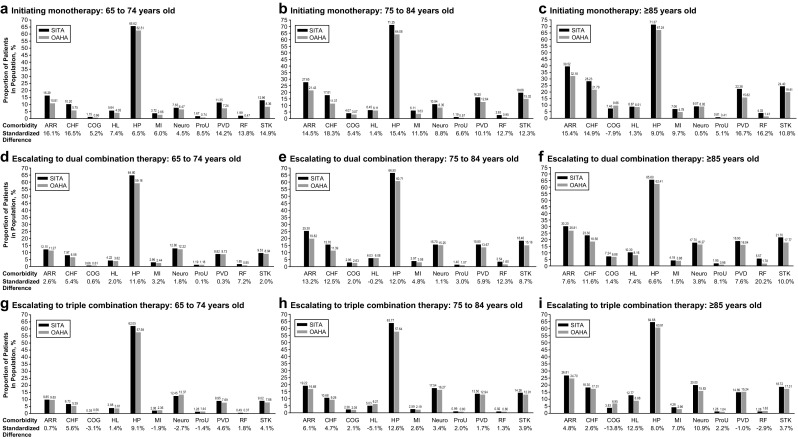
Standardized differences of baseline characteristics of patients with type 2 diabetes up to 1 year before initiating treatment with sitagliptin or a non-DPP-4i oral antihyperglycemic agent: stratified by treatment complexity and age. ARR arrhythmia, CHF congestive heart failure, COG cognition impairment, HL hearing loss, HP hypertension, MI myocardial infarction, Neuro neuropathy, ProU proteinuria, PVD peripheral vascular disease, RF renal failure, and STK stroke
In patients initiating an escalation to triple combination therapy, the differences between treatment groups were not as pronounced as those seen in patients initiating monotherapy or escalation to dual therapy (Fig. 2g–i). In patients aged 65–74 years initiating triple therapy, all between-group differences were < 10%.
Discussion
Patients in observational studies are not randomized with regard to treatment assignment, which may result in an imbalance in one or more important characteristics between treatment groups. This bias, often called confounding by indication, or channeling bias [8], can affect outcomes of interest and lead to inaccurate conclusions regarding the prescribed treatment.
In this study of patients ≥ 65 years of age with T2DM from the Medicare Supplemental Database, we found that patients with T2DM initiating sitagliptin tend to be older and have more comorbidities than those prescribed other classes of OAHA. These observations of channeling in patients receiving treatment with sitagliptin are similar to those previously reported [13, 14, 16].
Furthermore, we found that the differences in baseline characteristics between treatment groups vary by age and by the complexity of antihyperglycemic treatment, the latter likely reflecting the limitation of alternative treatment choices with increasing treatment complexity. Patients initiating treatment with sitagliptin were more likely to have a pre-treatment history of arrhythmia, congestive heart failure, peripheral vascular disease, renal failure, and stroke. However, this pattern varied across subgroups defined by age and treatment complexity, with the most pronounced differences observed between patients initiating monotherapy in all three age groups. As treatment complexity advanced to dual combination therapy, the differences were attenuated and mostly observed in the 75–84 and ≥ 85 age groups. In patients aged 65–74 years initiating triple therapy, no meaningful differences were observed between groups.
Features of the US healthcare system are likely influencing the patterns observed in this analysis. Utilization of diabetes medications is influenced by access to healthcare, access to branded therapies, socioeconomic status, insurance coverage, and cost-sharing structure. The study populations were identified in MarketScan® Medicare Supplemental Database, a large, diverse population from the US. However, it contains only Medicare beneficiaries who have employer-sponsored supplemental coverage. As such, these results may not be generalizable to the overall US population or ex-US populations. In addition, the primary uses of these data are for administrative purposes, not research. Consequently, the database has missing or limited data on a number of important disease characteristics and comorbidities, such as duration of diabetes, body weight/body mass index, results of laboratory tests, and adherence to life style modifications.
Conclusion
This study further documents the presence of channeling in patients initiating treatment with sitagliptin. The analysis reported here suggests important systematic differences between treatment groups are also present in elderly patients initiating OAHA therapy. This should be recognized and addressed appropriately in comparative observational analyses.
Acknowledgements
Funding
This analysis and article processing charges were funded by Merck & Co., Inc., Kenilworth, NJ, USA.
Authorship
Tongtong Wang, Ann Marie McNeill, Yong Chen, Edward A. O’Neill, and Samuel S. Engel meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Author Contribution
Tongtong Wang, Ann Marie McNeill, Yong Chen, Edward A. O’Neill, and Samuel S. Engel are responsible for the work described in this paper. Tongtong Wang, Ann Marie McNeill, Yong Chen, and Samuel S. Engel conceived, designed, and/or planned the study. Tongtong Wang, Ann Marie McNeill, Yong Chen, Edward A. O’Neill, and Samuel S. Engel interpreted the results. Tongtong Wang drafted the manuscript. Tongtong Wang, Ann Marie McNeill, Yong Chen, Edward A. O’Neill, and Samuel S. Engel critically reviewed and/or revised the manuscript for important intellectual content. All authors provided final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The authors wish to acknowledge the contributions of the statistical programmer Michael Sendarak, PhD (Merck & Co., Inc., Kenilworth, NJ, USA), to the data analysis.
Medical Writing Assistance
Editorial assistance was provided by Jennifer Rotonda, PhD, and Michele McColgan, BA, of Merck & Co., Inc., Kenilworth, NJ, USA.
Disclosures
Tongtong Wang is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and may own stock and/or hold stock options in the company. Ann Marie McNeill is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and may own stock and/or hold stock options in the company. Edward A. O’Neill is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and may own stock and/or hold stock options in the company. Samuel S. Engel is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and may own stock and/or hold stock options in the company. Yong Chen was a former employee of Merck & Co., Inc., Kenilworth, NJ USA and is a current employee and stockholder of GSK.
Compliance with Ethics Guidelines
This article is based on previously conducted studies using a Health Insurance Portability and Accountability Act of 1996 (US) (HIPAA) compliant database and does not involve any new studies of human or animal subjects performed by any of the authors.
Data Availability
Data used in this analysis were extracted from the Truven MarketScan® Medicare Supplemental Database (owned by Truven Health Analytics, Ann Arbor, MI, USA) on February 15, 2016.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/9C1DF0604FA8308A.
References
- 1.International Diabetes Federation . IDF diabetes. 7. Brussels: International Diabetes Federation; 2015. [PubMed] [Google Scholar]
- 2.Kirkman MS, Briscoe VJ, Clark N, Florez H, Haas LB, Halter JB, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650–2664. doi: 10.2337/dc12-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Diabetes Control and Prevention . National diabetes fact sheet: general information and national estimates on diabetes in the United States. Atlanta: US Department of Health and Human Services Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 4.Statistical analysis by the Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Division of Diabetes Translation (2011). http://diabetes.niddk.nih.gov/dm/pubs/statistics/.
- 5.Cigolle CT, Lee PG, Langa KM, Lee YY, Tian Z, Blaum CS. Geriatric conditions develop in middle-aged adults with diabetes. J Gen Intern Med. 2011;26:272–279. doi: 10.1007/s11606-010-1510-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 7.Petri H, Urquhart J. Channeling bias in the interpretation of drug effects. Stat Med. 1991;10:577–581. doi: 10.1002/sim.4780100409. [DOI] [PubMed] [Google Scholar]
- 8.Thornberry NA, Weber AE. Discovery of JANUVIA (Sitagliptin), a selective dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. Curr Top Med Chem. 2007;7:557–568. doi: 10.2174/156802607780091028. [DOI] [PubMed] [Google Scholar]
- 9.Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–242. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 10.Engel SS, Golm GT, Shapiro D, Davies MJ, Kaufman KD, Goldstein BJ. Cardiovascular safety of sitagliptin in patients with type 2 diabetes mellitus: a pooled analysis. Cardiovasc Diabetol. 2013;12:3. doi: 10.1186/1475-2840-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engel SS, Williams-Herman DE, Golm GT, Clay RJ, Machotka SV, Kaufman KD, et al. Sitagliptin: review of preclinical and clinical data regarding incidence of pancreatitis. Int J Clin Pract. 2010;64:984–990. doi: 10.1111/j.1742-1241.2010.02382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engel SS, Round E, Golm GT, Kaufman KD, Goldstein BJ. Safety and tolerability of sitagliptin in type 2 diabetes: pooled analysis of 25 clinical studies. Diabetes Ther. 2013;4:119–145. doi: 10.1007/s13300-013-0024-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai B, Katz L, Alexander CM, Williams-Herman D, Girman CJ. Characteristics of patients prescribed sitagliptin and other oral antihyperglycaemic agents in a large US claims database. Int J Clin Pract. 2010;64:1601–1608. doi: 10.1111/j.1742-1241.2010.02516.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Q, Rajagopalan S, Mavros P, Engel SS, Davies MJ, Yin D, et al. Baseline characteristic differences between patients prescribed sitagliptin vs. other oral antihyperglycemic agents: analysis of a US electronic medical record database. Curr Med Res Opin. 2010;26:1697–1703. doi: 10.1185/03007995.2010.489029. [DOI] [PubMed] [Google Scholar]
- 15.Brodovicz KG, Chen Y, Liu Z, Ritchey ME, Liao J, Engel SS. Characterization of sitagliptin use in patients with Type 2 diabetes and chronic kidney disease by cross-sectional analysis of a medical insurance claims database. Diabetes Ther. 2015;6:627–634. doi: 10.1007/s13300-015-0133-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brodovicz KG, Kou TD, Alexander CM, O’Neill EA, Senderak M, Engel SS, et al. Recent trends in the characteristics of patients prescribed sitagliptin and other oral antihyperglycaemic agents in a large US claims database. Int J Clin Pract. 2013;67:449–454. doi: 10.1111/ijcp.12090. [DOI] [PubMed] [Google Scholar]
- 17.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ali MS, Groenwold RH, Pestman WR, Belitser SV, Roes KC, Hoes AW, et al. Propensity score balance measures in pharmacoepidemiology: a simulation study. Pharmacoepidemiol Drug Saf. 2014;23:802–811. doi: 10.1002/pds.3574. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used in this analysis were extracted from the Truven MarketScan® Medicare Supplemental Database (owned by Truven Health Analytics, Ann Arbor, MI, USA) on February 15, 2016.


