Abstract
Heterotopic ossification (HO), acquired or hereditary, endochondral or intramembranous, is the formation of true bone outside the normal skeleton. Since perivascular Gli1+ progenitors contribute to injury induced organ fibrosis, and CD133 is expressed by a variety of populations of adult stem cells, this study utilized Cre-lox based genetic lineage tracing to test the contribution to endochondral HO of adult stem/progenitor cells that expressed either Gli1 or CD133. We found that both lineages contributed broadly to different normal tissues with distinct patterns, but that only Gli1-creERT labeled stem/progenitor cells contributed to all stages of endochondral HO in a BMP dependent, injury induced, transgenic mouse model. Hedgehog (Hh) signaling was abnormal at endochondral HO lesion sites with increased signaling surrounding the lesion but diminished signaling within it. Thus, local dysregulation of Hh signaling participates in the pathophysiology of endochondral HO. However, unlike a previous report of intramembranous HO, systemic inhibition of Hh signaling was insufficient to prevent the initiation of the endochondral HO process or to treat the existing endochondral HO, suggesting that Hh participates in, but is not essential for endochondral HO in this model. This could potentially reflect the underlying difference between intramembranous and endochondral HO. Nevertheless, identification of this novel stem/precursor cell population as a HO-contributing cell population provides a potential drugable target.
Keywords: Heterotopic ossification (HO), Adult mesenchymal stem/progenitor cells (MSC), Lineage tracing, Gli1, CD133, Hedgehog (Hh) signaling
1. Introduction
Heterotopic Ossification (HO), either acquired [1] or hereditary [2], endochondral or intramembranous, is the formation of bone in the soft tissue. Acquired HO is a serious and costly complication of traumatic tissue damage whereas hereditary HO includes rare conditions, such as fibrodysplasia ossificans progressiva (FOP) which is caused by gain-of-function mutations of a bone morphogenetic protein (BMP) type I receptor (ACVR1) [2], and Progressive osseous heteroplasia (POH) which is caused by heterozygous inactivating mutations of GNAS [3,4]. Almost all HO is endochondral except for POH, which is featured by superficial intramembranous HO that progresses to deep connective tissue. However, the fundamental mechanisms underlying HO are unknown, although numerous signaling pathways and different cellular components have been implicated [5].
Previous lineage tracing studies have shown that Glast+ [6], Tie2+ [7,8] MX1-cre and SCX-cre labeled stem/progenitor cells [9] contribute to endochondral HO. Although the contribution of many other seemingly promising candidate subpopulations has been disapproved ((5) for review), a number of additional adult stem cell populations have not been vigorously tested. For example, glioma-associated oncogene homolog 1+ (Gli1+) cells within the suture mesenchyme were recently identified as the main mesenchymal stem cell (MSC) population for craniofacial bones [10], perivascular Gli1+ progenitors were found to be the key contributor to injury-induced organ fibrosis [11], and more remarkably, activated Hh signaling was implicated in intramembranous HO in POH [3,4,12]. We therefore sought to determine whether Gli1+ progenitors also contribute to endochondral HO in BMP dependent and injury induced HO. Gli1 is a nuclear mediator of the Hedgehog (Hh) pathway which plays key roles in physiological ossification, at least partially by regulating the commitment of the stem/progenitor cells in perichondrium. However, in contrast to normal bone development, the involvement of Hh signaling in BMP dependent and injury induced HO is largely unrecognized.
A lingering question in the field has been whether circulating progenitors/stem cells, especially hematopoietic stem cells, contribute to HO. The CD133 antigen (prominin-1) is expressed by a variety of populations of different adult stem cells, including hematopoietic stem cells and endothelial progenitor cells. Further, CD133 is expressed by a small subpopulation of stem/progenitor cells which give rise to functional satellite cells after intramuscular transplantation in immunodeficient host mice [13].
In this study, we first defined the normal tissue distribution of the two candidate populations, i.e., Gli1-creERT and CD133-creERT labeled cells, in the adult, and then directly tested the contributions of these two conditionally labeled populations to endochondral HO in a well-established genetic (not generic) mouse HO model, Nse-BMP4 transgenic mice [4,10]. This line overexpresses BMP4 under the control of the neuron-specific enolase (Nse) promoter and develops injury induced HO robustly. We found that conditionally labeled Gli1-creERT stem/progenitor cells contributed significantly to HO, while CD133-creERT labeled stem/progenitor cells did not. The Gli1-creERT labeled stem/progenitor cells overlapped at least partially with MSC. Further, we found that Hh-Gli1 signaling was abnormal in HO lesion sites with increased signaling surrounding the lesion but with diminished signaling within it. However, systemic inhibition of Hh signaling was insufficient to prevent the initiation of HO process or treat the existing HO in this model. Overall, this study identified a novel subpopulation of cells that contributes to BMP dependent and injury induced HO, and provided further mechanistic insights into the pathophysiology of HO.
2. Materials and method
2.1. Animals and injury models
The Nse-BMP4 transgenic mice used in this study have been described previously [6,14]. The Gli1tm3(cre/ERT2)Alj/J (Gli1-creERT), B6N;129S-Prom1tm1(cre/ERT2)Gilb/J (CD133-creERT), and the B6.Cg-Gt(ROSA)26Sortm6(CAG-ZsGreen1)Hze/J lines (Zsgreen reporter) were from the Jackson Laboratory (Bar Harbor, ME). The Zsgreen reporter mouse has a loxP-flanked STOP cassette that prevents transcription of the downstream enhanced green fluorescent protein (ZsGreen1) in the absence of cre. However, when bred to mice that express cre, the STOP cassette is permanently deleted in cre-expressing cells resulting in constitutive expression of ZsGreen1 in those cells and all their progeny. To induce CD133-creERT or Gli1-creERT expression, 100 μl of tamoxifen (10 mg/ml) was injected intraperitoneally into adult (>1 month) mice as previously reported [6]. All animal experiments in this study were approved by the Animal Care and Use Committees at Anhui Medical University and Northwestern University.
2.2. Genetic lineage tracing
Genetic lineage tracing was performed as described previously [6, 14]. Briefly, Zsgreen conditional reporter mice were first mated with Nse-BMP4 mice. The Zsgreen;Nse-BMP4 double transgenic mice were selected and subsequently mated with the two cre lines, respectively. Adult (>1 month old) triple transgenic mice, i.e., CD133-creERT;Zsgreen;Nse-BMP4 and CD133-creERT;Zsgreen;Nse-BMP4 were selected and the cre expression was tamoxifen-induced before the injury induced HO and further experiments. To induce Glastcre expression, tamoxifen was injected intraperitoneally into adult (>1 month) mice as previously reported [15]. For the purpose of further characterizing the cre-labeled cells, adult double transgenic mice, CD133-creERT;Zsgreen and Gli1-creERT;Zsgreen mice were also selected and were treated similarly as triple transgenic mice, i.e., the cre expression was tamoxifen-induced, and at the designated time-points, tissues were harvested and fixed in 4% PFA overnight for histological studies.
To evaluate the contributions of labeled cells to HO, injuries were performed on adult triple transgenic mice, as described previously [6, 14]. Briefly, adult (>1 month old) transgenic mice were shaved first, and then a 5-mm sharp, shallow cut was made through the skin but sparing the muscles. The injured and control hind 1egs were harvested at different time points (1, 2, 3 and 4 weeks after injury) after the injuries for further histological and immunohistological examinations. In addition, lesional tissues from triple transgenic mice that developed spontaneous HO were also used as complimentary source of lineage tracing after the standard tamoxifen-induction.
Adult triple transgenic mice without tamoxifen-induction, i.e., naïve Nse-BMP4;Gli1-creERT;Zsgreen and Nse-BMP4;CD133-creERT;Zsgreen, were also used to test the possibility of spontaneous (background) cre expression. Contralateral uninjured limbs of tamoxifen-induced Nse-BMP4;Gli1-creERT;Zsgreen were also selected to study the normal patterns of labeled cells.
2.3. Treatment with Hh inhibitor
Hh inhibitor (eggmanone, Tocris) was i.p. injected (4 mg/kg, twice a week for two weeks) into triple transgenic mice (Nse-BMP4;Glast-creERT;Zsgreen) with de novo muscle injury or preexisting HO. Vehicle (DMSO) injection into the same mice (as treatment group) serves as controls. X-ray images were taken 4 weeks after treatment, and then mice were scarified and the tissues were harvested for further histological analysis.
2.4. Histology and immunohistochemistry
Immunostaining for different markers were performed as previously described [6,14]. Briefly, sections were pre-fixed with 4% paraformaldehyde in PBS. Nonspecific binding was blocked with 10% normal serum diluted in 1% bovine serum albumin (BSA; Jackson ImmunoResearch Laboratories, West Grove, PA) and 0.25% Triton X-100 (Sigma) for 1 h in room temperature. The sections were then incubated with primary antibodies diluted with 1% BSA + 0.25% Triton X-100 at 4 °C overnight. The sections were then incubated with appropriate secondary antibodies (Alexa Fluor 488, Alexa Fluor 594, or Alexa 647 conjugated antibodies diluted with 1% BSA + 0.25% Triton X-100 in the dark at room temperature for 2 h. Counterstaining was then performed with 4,6-diamidino-2-phenylindole (1:5000).
Primary antibodies against S100A4 (mIgG1, Novus, 1F12-1 G7), STRO1 (mIgM, Santa Cruz, sc-47733), ALP (Rb, Abcam, ab65834), ColII (Rb, Abcam, ab34712), IHH (goat, R&D, AF1705,), Gli1 (Abcam, ab151796), Patched (Rat, R&D, MAB41051), PTHLH (or PTHrP) (Rabbit, proteintech, 10817-1-AP), were used in this study.
3. Results
3.1. Gli1-creERT mediated recombination in most tested adult tissues, including skeletal bones
The Gli1-creERT line has been previously used in Cre-lox based lineage tracing studies [11,16], but the general labeling activities in other tissues, especially in our target tissues [4,10], are largely unknown. We therefore first characterized the labeling patterns of this line with the Zsgreen reporter to further understand the labeling activities. We first tested whether the labeling activities are strictly tamoxifen dependent and found that all sections from adult double (i.e., CD133-creERT;Zsgreen and Gli-creERT;Zsgreen) and triple mutant (i.e., CD133-creERT;Zsgreen;Nse-BMP4 and Gli-creERT;Zsgreen;Nse-BMP4) mice displayed negligible florescence signal without tamoxifen induction (data not shown). As a positive control, based on previous report [16], we examined Gli1-creERT labeling in teeth, and found that a majority of cells in teeth are Gli1-creERT+ in our model (Suppl. Fig. 1A), thus validating the labeling system.
We then systematically examined the Gli1-creERT labeled cells in many different adult tissues, i.e., head structures (including ear, skull and brain), spinal cord, spleen, thymus, liver, jejunum, kidney, heart, lung, tail, sternum, rib, paw, and hind limbs (skin, long bone and muscles), of double transgenic (Gli1-creERT;Zsgreen) and uninjured triple transgenic (Gli1-creERT;Zsgreen;Nse-BMP4) mice. In the nasal cavity, some Gli1-creERT labeled cells were located in the submucosal mesenchymal tissue that is closely associated with nerve bundles, blood vessels, and connective tissue, and others were located in the single epithelial layer with the morphology of columnar epithelial cells (Suppl. Fig. 1B). In the outer ear, Gli1-creERT labeled cells were observed in hair follicles and in subcutaneous connective tissue where many labeled cells are associated with vasculature (data not shown). Interestingly, in the brain, the Gli1-creERT labeled cells were rarely associated with vasculature; instead, some were located in neural stem cell niches, and most labeled cells had the morphology of terminally differentiated cells, such as astrocytes (in brain parenchyma), and Purkinje cells (in cerebellum) (Suppl. Fig. 1C–F).
In spleen (Suppl. Fig. 1 G & H) and liver (Suppl. Fig. 1I & J), Gli1-creERT labeled cells were a rare population, and the morphological feature and anatomical context strongly suggested that the most of the labeled cells closely associated with vasculature. In kidney (Suppl. Fig. 1K & L) and heart (not shown), the overall frequency of Gli1-creERT labeled cells was higher, and the labeled cells could be generally divided into two categories: 1) isolated Gli1-creERT labeled cells that are closely associated with vasculature, and 2) morphologically elongated fibroblast-like cells that form clusters in interstitial space.
In lung (Suppl. Fig. 1 M & N), the labeled cells could also be generally divided into the same two categories with slightly different anatomic contexts: 1) isolated Gli1-creERT labeled cells were similarly associated with vasculature; however, 2) the majority of the Gli1-creERT labeled cells seem to be closely associated with the mesenchymal component of secondary and tertiary bronchi. Consistently, in jejunum (Suppl. Fig. 1 O & P), most labeled cells were closely associated with the mesenchymal component and vasculature inside intestinal villi.
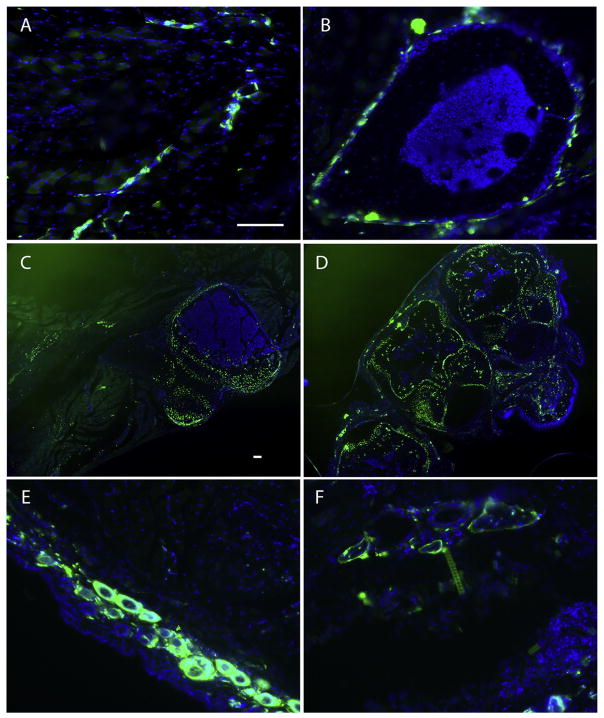
In hind limbs, our target tissues, Gli1-creERT labeled cells were observed in hair follicles, subcutaneous connective tissue and in the interstitium of skeletal muscles (Fig. 1), which is largely consistent with the finding that HO is often first found in subcutaneous connective tissue and the interstitium of skeletal muscles. In skeletal muscles, most Gli-creERT labeled cells were closely associated with vasculature (Fig. 1A). Gli1-creERT labeled cells were found in normal endogenous bone, i.e., in long bone (Fig. 1B) (most in periosteum and perichondrium), sternum (Fig. 1C) (cartilage), and footpad (Fig. 1D), but were rarely found in bone marrow (Fig. 1B). In response to injury, in double transgenic (Gli1-creERT;Zsgreen) mice, only a few Gli-creERT labeled cells were observed in the lesional tissue (compare Fig. 1E & F), indicating that Gli-creERT labeled cells are not a major contributor to the injury response without concurrent high BMP signaling.
Fig. 1.
Gli1-creERT labeled cells were found in target tissues. (A & B) cross-section images from hind limbs of Gli1-creERT; Zsgreen mice show the general pattern of labeled cells in subcutaneous connective tissue muscles and long bones. (A) Most labeled cells in subcutaneous connective tissue, or in the interstitium of muscle appear closely associated with vasculature. (B) Representative cross-section of long bone shows that most labeled cells are closely associated with periosteum. (C & D) low power images from sternum (C) and paw (D) show the general pattern of labeled cells in these structures, and support the idea that labeled cells contribute to bone, periosteum, cartilage and perichondrium, in addition to subcutaneous connective tissue. (E) Representative cross-section image from an uninjured hind limb shows the general pattern of labeled cells in skin and subcutaneous connective tissue. Note that hair follicles are Gli1-creERT labeled, in addition to some isolated labeled cells in subcutaneous connective tissue. (F) Representative cross-section image from a hind limb three days after injury, showing the general pattern of labeled cells in injury site. Note that there are not many labeled cells in the injury site. Scale bar = 80 μm, A, B, E & F share the same scale bar, and C & D share the same scale bar.
3.2. CD133-creERT mediated recombination in most tested adult tissues, but not in skeletal bones
We next examined the CD133-creERT labeled cells in adult tissues of double transgenic (CD133-creERT;Zsgreen) and uninjured triple transgenic mice (CD133-creERT;Zsgreen;Nse-BMP4) in a similar manner. No labeling was detected in the absence of tamoxifen administration. The salivary gland was used as positive control after Tamoxifen treatment (Suppl. Fig. 2A), based on previous report for CD133 labeling activities [17]. As expected, we observed strong labeling in the salivary gland. CD133-creERT labeled cells were essentially undetectable in heart (Suppl. Fig. 2B) and spleen (Suppl. Fig. 2C). In thymus (Suppl. Fig. 2D) and liver (Suppl. Fig. 2E), few CD133-creERT labeled cells were found, most frequently in association with vasculature or central vein, respectively. In lung (Suppl. Fig. 2F), many CD133-creERT labeled cells with the morphology of columnar epithelial cells were located in the epithelial compartment of secondary and tertiary bronchi, but few were found in the mesenchymal compartment.
In contrast to the pattern of Gli1-creERT labeled cells, in kidney (Suppl. Fig. 2G) and jejunum (Suppl. Fig. 2H), there were many CD133-creERT labeled cells with the morphology of columnar epithelial cells located in the epithelial compartment. However, in the brain (Suppl. Fig. 2I & J), even though the frequency of the CD133-creERT labeled cells also was high, most labeled cells associated with the microvasculature and with stem cells in the subventricular zone (SVZ).
The pattern of labeled cells in other head structures was complex. For example, in nasal cavity (Suppl. Fig. 2K), CD133-creERT labeled cells were found not only in mesenchymal but also epithelial compartments. Interestingly, in contrast to Gli1-creERT labeled cells, there were essentially no CD133-creERT labeled cells in tooth (data not shown). In ear (Suppl. Fig. 2L), there were isolated labeled cells in skin, and few muscle fibers are also labeled.
Importantly, in our target tissues, we only found some scattered single CD133-creERT labeled cells in skin and subcutaneous connective tissue, and in deep skeletal muscles (Suppl. Fig. 2M), there were very few CD133-creERT labeled cells. Further, CD133-creERT labeled cells were essentially undetectable in all tested normal bones (Suppl. Fig. 2N & O), except in bone marrow (Suppl. Fig. 2P) where a small portion of the presumably hematopoietic stem/progenitor cells was CD133-creERT labeled.
3.3. Gli1-creERT labeled cells but not CD133-creERT labeled cells stem/progenitor cells contribute significantly to HO at all stages
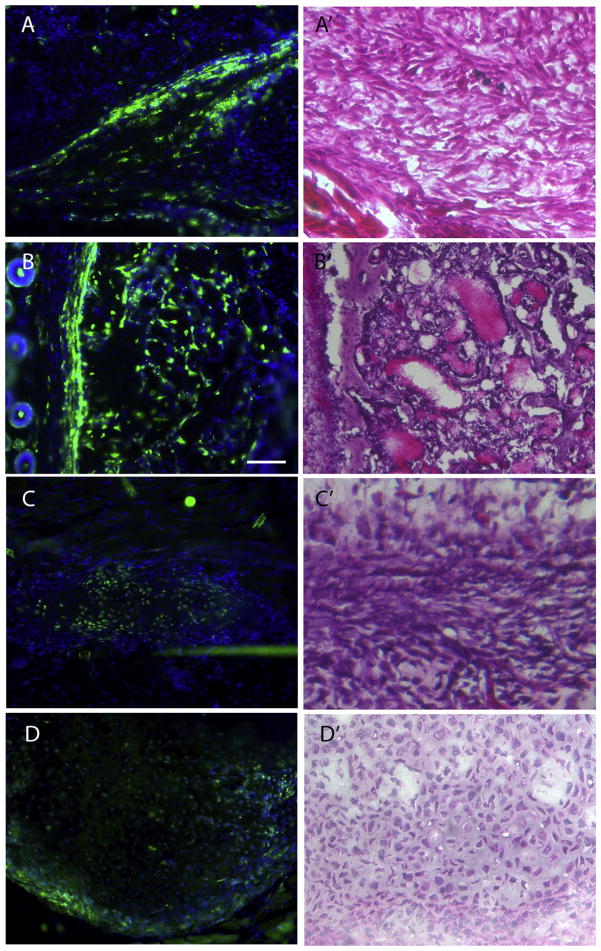
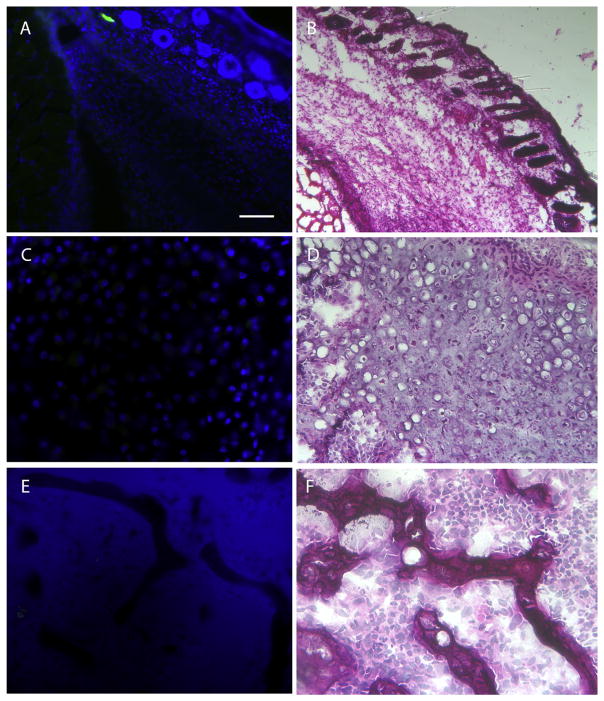
To directly test if Gli1-creERT labeled stem/progenitor cells contribute to HO, we first induced cre expression in adult triple transgenic mice (Nse-BMP4; Gli1-creERT; Zsgreen) by tamoxifen injection, and we then performed skin injuries on these mice according to previously established procedures [4,10]. The mice were then sacrificed at different time points (1, 2, 3 and 4 weeks) to trace the contribution of labeled cells at different stages of HO. Gli1-creERT labeled progenitors contributed significantly to HO at all stages (Fig. 2) (early fibroproliferative lesions, chondrocytes, and later bone formation), even though the efficiency of tamoxifen dependent labeling varied among different stages and individual animal. The overall contribution rate of labeled cells to HO was apparently ≤50% in current test conditions, which could reflect the labeling efficiency, or true rate of contribution. In contrast, in CD133-creERT;Zsgreen;Nse-BMP4 triple transgenic mice, CD133-creERT labeled stem/progenitor cells contributed negligibly to HO at all stages, both in the skin/muscle injury model and in spontaneous HO (Fig. 3). We concluded that CD133+ adult stem/progenitor cells do not contribute appreciably to HO.
Fig. 2.
Gli1-creERT labeled cells contribute to HO. Representative images from skin injury induced (A–B') and “spontaneous” (C–D') HO, showing that Gli1-creERT labeled cells contribute to all stages of HO. (A) Representative image from the lesion of the early fibroproliferative stage in the skin injury model showed that Gli1-creERT labeled cells contribute significantly to HO at this stage. (A') representative image of H&E staining of an adjacent section, (B) Representative image from the lesion of late mature HO stage in the skin injury model showed that Gli1-creERT labeled cells also contribute significantly to HO at this stage. (B') H & E staining of an adjacent section, (C) Representative image from the lesion of the early fibroproliferative stage in the spontaneous HO model showed that Gli1-creERT labeled cells contribute significantly to HO at this stage. (C') H&E staining of an adjacent section, (D) Representative image from the lesion at the chondrogenic stage in the spontaneous HO model showed that Gli1-creERT labeled cells contribute significantly to HO at this stage. (D') H & E staining of an adjacent section. Scale bar = 80 μm.
Fig. 3.
CD133-creERT labeled cells do not contribute to HO. Representative images from skin injury induced HO, showing that CD133-creERT labeled cells do not contribute to any stage of HO. (A) Representative image from the lesion of the early fibroproliferative stage in the skin injury model shows that CD133-creERT labeled cells do not contribute significantly to HO at this stage. (B) H & E staining of an adjacent section, (C) Representative image from the lesion of the chondrogenic stage shows that CD133-creERT labeled cells do not contribute significantly to HO at this stage. (D) H&E staining of an adjacent section, (E) Representative image from mature HO shows that CD133-creERT labeled cells do not contribute significantly to HO at this stage. (F') H & E staining of an adjacent section. Scale bar = 80 μm.
3.4. Identity of Gli1-creERT labeled stem/progenitor cells and their osteogenic and chondrogenic differentiation
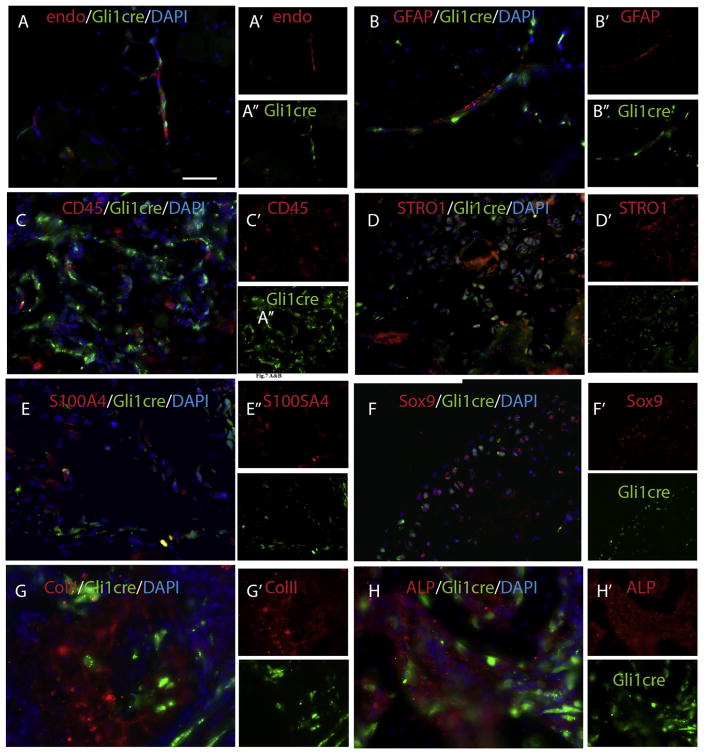
Based on morphological features and anatomic contexts, Gli1-creERT predominately labeled mesenchymal populations in our target tissues (Suppl. Fig. 1 & Fig. 1). To further clarify and confirm the identity of these labeled cells, we examined the co-localization of the cre-labeled cells with endothelial, hematopoietic, mesenchymal cell and other available markers (Fig. 4). Gli1-creERT labeled cells were closely associated, but not co-localized, with endo (an endothelial cell marker) (Fig. 4A), GFAP (a Schwann cell marker) (Fig. 4B), or CD45 (pan-hematopoietic terminally differentiated cell marker) (Fig. 4C). Notably, Gli1-creERT labeled cells extensively co-labeled with STRO1 (Fig. 4D) and S100A4 (also called FSP1) (Fig. 4E), putative markers of mesenchymal progenitors/stem cells. The co-localization of Gli1-creERT labeled cells with mesenchymal progenitor/stem cell markers was remarkably diminished at later stages of the lesion or mature HO, suggesting the loss of stemness of Gli1-creERT labeled cells in these contexts.
Fig. 4.
Gli1-creERT labeled stem/progenitor cells in early lesions express common MSC markers and contribute to HO through osteogenic and chondrogenic differentiation in later stages. (A) double staining of Gli1-creERT labeled cells with Endomucin (endo, an endothelial marker) in skeletal muscle demonstrated that many Gli1-creERT labeled cells are closely associated with endo + endothelial cells, but do not express the endothelial marker. (B) similarly, double staining of Gli1-creERT labeled cells with GFAP (a proposed Schwann cell marker) in skeletal muscle demonstrated that some Gli1-creERT labeled cells are closely associated with Schwann cells, but do not express Schwann cells marker. (C) Double staining of Gli1-creERT labeled cells with CD45 (a differentiated pan-hematopoietic cell marker) in an early lesion demonstrates that there is no significant co-localization with CD45. (D) Double staining of Gli1-creERT labeled cells with STRO1 (a proposed MSC marker) in an early lesion demonstrates that some Gli1-creERT labeled cells co-label with STRO1. (E) Double staining of Gli1-creERT labeled cells with S100A4 (a proposed MSC marker) in an early lesion demonstrates that some Gli1-creERT labeled cells co-label with S100A4. (F) Double staining of Gli1-creERT labeled cells with Sox9 (a proposed chondrocyte marker) in a mature lesion demonstrates that some Gli1-creERT labeled cells co-label with Sox9. (G) Double staining of Gli1-creERT labeled cells with col. II (a proposed chondrocyte marker) in a mature lesion demonstrates that some Gli1-creERT labeled cells co-label with Sox9. (H) Double staining of Gli1-creERT labeled cells with ALP (a proposed osteocyte marker) in a mature lesion demonstrates that some Gli1-creERT labeled cells co-label with ALP. Scale bar = 80 μm.
Further double staining was performed with lineage specific markers, Sox9 (Fig. 4F) and ColII (chondrocyte markers) (Fig. 4G), Runx2 (data not shown) and ALP (an osteoblast marker) (Fig. 4H), using sections from different time points. At the later stages, a substantial subset of cre labeled cells co-labeled with osteoblast or chondrocyte markers, indicating that Gli1-creERT labeled cells truly contribute to HO. However, there was no obvious co-localization of Gli1-creERT labeled cells with Glast antibody staining (data not shown), suggesting that Gli1-creERT labeled cells might not significantly overlap with this previously identified HO contributing population [6].
3.5. The roles of Hedgehog (Hh) signaling in HO
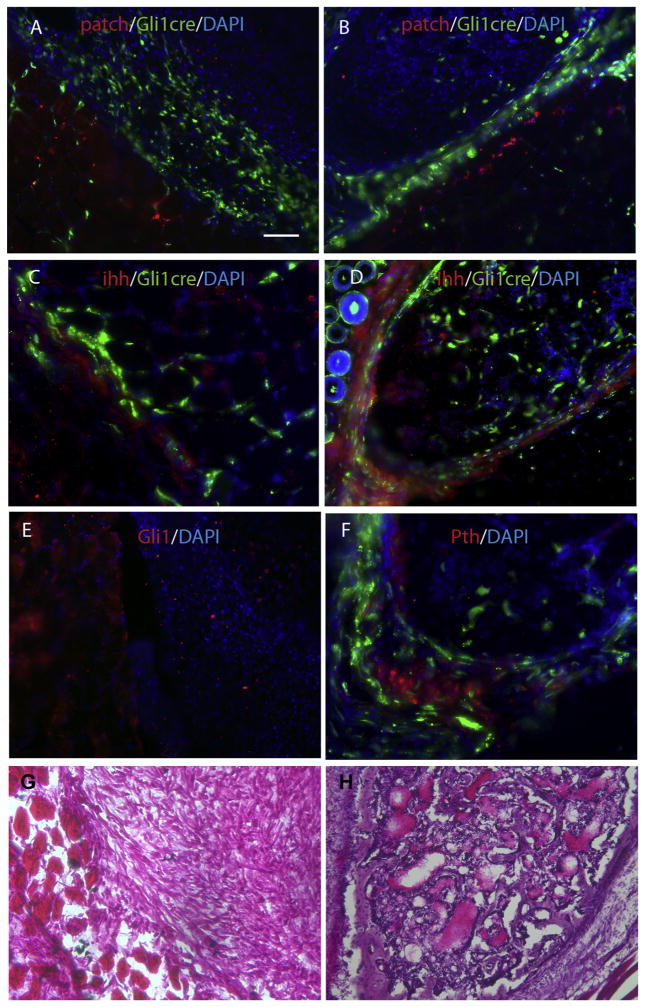
The evidence that Gli1-creERT labeled cells contributed to HO suggested a role for Hh signaling in injury induced, BMP dependent HO. We therefore examined the key components of Hh signaling, i.e., Patched (receptor) (Fig. 5A & B), IHH (ligand) (Fig. 5C & D), Gli1 (a readout of Hh signaling) (Fig. 5E) and a key regulatory hormone, PTHLH (or PTHrP) (Fig. 5F), in both early and later stages of HO. Patched expression was dramatically increased in areas adjacent to lesion sites, but was decreased within the lesion sites at all stages. Similarly, but with slight variation, IHH expression also was increased at all stages in perilesional tissues but the high IHH domain seemed to be somewhat broader with apparently shallower gradient than that of Patched, especially in the mature HO stage. In this stage, IHH was increased in the outer ring of mature HO and the area immediately adjacent to it. We also examined the expression of Gli1, a final readout of the Hh signaling, and we found a similar expression pattern i.e., Gli1 was dramatically increased in areas adjacent to lesion sites, but decreased within the lesion sites. Thus the pattern of Hh signaling marked the boundary between normal and lesional tissue.
Fig. 5.
Hh signaling is dysregulated. Representative images demonstrate dysregulation of Hh signaling at different stages of HO. (A & B) double staining of Gli1-creERT labeled cells with Patched at the early (A, fibroproliferative) and late (B, mature HO) stages of HO, demonstrating that Patched expression is markedly increased in muscle immediately adjacent to the lesion, but decreased in the lesion itself. (C & D) similarly, double staining of Gli1-creERT labeled cells with Ihh (a ligand for the receptor) in early (C, fibroproliferative) and late (D, mature HO) stages of HO, demonstrate that Ihh expression also is increased adjacent to the lesion but decreased in the lesion itself. Note that the Ihh domain is broader than that of Patched, since it labels both Gli1-creERT labeled and unlabeled cells in the early stage, and in the late stage it's expression is also increased in the outer ring of the mature HO. (E) direct Gli1 antibody staining demonstrates that Gli1expression is increased adjacent to the lesion, but decreased within the lesion. (F) double staining of Gli1-creERT labeled cells at mature stages of HO, demonstrates that PTHLH expression is significantly increased. Note that PTHLH domain almost mutually exclusive with Gli1-creERT labeled cells. (G) H & E staining from serial sections of A, C & E. (G) H & E staining from serial sections of B, D & F. Scale bar = 80 μm.
To start to understand how the local Hh signaling is regulated in our model, and to explore potential feedback regulation, we studied a key hormone, PTHLH, also called Parathyroid hormone-related protein or PTHrP. In early stages PTHLH was undetectable anywhere around lesion sites (data not shown), but in later stages the PTHLH expression was increased in a small isolated domain (Fig. 5). The high PTHLH expression domain and the Gli1-creERT labeled cells were almost always mutually exclusive, as would be expected.
3.6. Global inhibition of Hh signaling seems insufficient to block HO
To directly test whether inhibition of Hh signaling could prevent and treat HO, and understand how Hh signaling affects other than HO contributing population, we i.p. injected Hh inhibitor (eggmanone, Tocris, 4 mg/kg, twice a week for two weeks) into triple transgenic mice (Nse-BMP4;Glast-creERT;Zsgreen) with de novo skin/muscle injury or preexisting HO, and mice with vehicle (DMSO) only injection serves as controls. Intriguingly, we found that there is no significant difference in the incidence of HO or size of HO between EGG and DMSO group, i.e., EGG treatment at this dosage apparently had no obvious effect in either preventing the initiation of HO or treating the existing HO in this model (data not shown). Further histological comparison of the Glast-creERT labeled cells between EGG and DMSO groups found no obvious differences in the numbers or the morphology of the labeled cells.
Overall, our data support the idea that the Gli1-creERT labeled MSC population is a novel subpopulation that contributes to HO significantly, and that local dysregulation of Hh signaling is part of the pathophysiology of injury induced, BMP dependent HO; however, global inhibition of Hh signaling seems insufficient to prevent or treat HO.
4. Discussion
There is still substantial debate about the cellular origins of HO. Previous lineage tracing studies have established that Tie2-cre [8], Glast-creERT [6], MX1-cre [9] and SCX-cre [9] labeled populations contribute to injury induced or spontaneous endochondral HO. This study established that a separate stem/progenitor cell population (overlapping with MSCs) that expresses Gli1 (Gli1-creERT) also contributes to HO in a genetic mouse model.
By contrast, we found that CD133+ stem cells do not contribute appreciably. This is perhaps a surprising finding since CD133 is expressed by a subpopulation of stem/progenitor cells in muscle, a target tissue in HO studies. However this observation is consistent with our previous finding that myogenic stem/progenitor cells do not contribute significantly to HO [14]. More importantly, since it is well-known that HSCs are CD133+ [18], these negative lineage tracing data essentially disapprove the idea that the circulating hematopoietic stem/progenitors cells (HSC) contribute to HO. In fact, this conclusion is supported by the transplantation studies in mouse and human [19], which suggest that cells of hematopoietic origin contribute to the early inflammatory and late marrow-repopulating stages but do not contribute to the fibroproliferative, chondrogenic, or osteogenic stages of HO. This conclusion is also supported by lineage tracing study with Mx1-Cre labeled bone marrow (BM) populations in another animal model [9].
Remarkably, there are a number of similarities among the confirmed HO-contributing subpopulations: 1) though different promoter-cres induced recombinations have variable labeling efficiencies and specificities, data so far indicate that subsets of all five cre-labeled subpopulations that have been tested (Tie2-cre, Glast-creERT, MX1-cre, SCX-cre and Gli1-creERT) co-label with mesenchymal stem/progenitor cell markers, 2) all HO-contributing subpopulations are tissue resident (not circulatory), 3) the majority of the labeled subpopulations are closely associated with the vasculature, 4) all contribute to HO significantly, but none of them can replace all others in contributing to HO, 5) all subpopulations seem to be heterogeneous.
Despite these similarities there are important differences in the biologic roles of these subpopulations of cells. For example, our current study found that Gli1-creERT labeled cells contribute extensively to normal skeletal bone, whereas Glast-creERT labeled cells do not contribute to normal skeletal bone [6], while Tie2-cre and SCX-cre labeled cells are somewhere between these two extremes [5]. Tie2 is expressed by at least 3 distinct cell types: endothelial cells of endoderm origin, proangiogenic monocytes cells of hematopoietic origin, and pericyte precursors of mesenchymal origin, while Mx1-Cre marked muscle interstitial, BM, and microvascular endothelial lineages. In contrast, Glast-creERT labeled predominately mesenchymal stem/progenitor cells in target tissues in experimental HO [6], but in other experimental settings Glast labeled subpopulations might be neural stem/progenitor cells, astrocytes or Schwan cells. Furthermore, even though the Mx1 + Lin − Sca1 + PDGFRα + population that contributes to HO [9] overlaps substantially with these nonendothelial Tie2-lineage interstitial cells [7], there is no evidence to support these two populations completely overlap with each other.
Overall, since the cre lines generally label more than one cell population, it is hard to dissect the exact lineage relationship between these confirmed cre labeled populations; nevertheless, the currently available data suggest that all these populations overlap with each other to some extent. Taken together, these data, including our current findings, strongly support the hypothesis that local, tissue resident, multipotential mesenchymal progenitors/stem cells contribute to endochondral HO.
Regarding the role of Hh signaling, previous studies of normal skeletogenesis have generated conflicting data. For example, in a metatarsal organ culture, activation of BMP signaling enhanced bone collar formation cooperatively with Hh input, whereas single-cell quantitative RT-PCR analyses showed heterogeneity of perichondrial cells, i.e., Hh signaling suppressed BMP-induced chondrogenic differentiation, at least partially by inhibiting the expression of Sox5, Sox6, and Sox9 [20]. Other studies indicate that Hh-Gli1 itself can induce early osteoblast differentiation, that there is a redundant role for Gli2, and that Hh is involved in the repressor function of Gli3 in osteogenesis [21]. Further, Hh signaling enhances the osteogenic differentiation of adipose stem cells (ASCs), at the expense of adipogenesis [22].
Hh signaling reportedly also plays an important role in postnatal osteogenesis in the setting of stress fracture healing, mediating its effects directly through regulation of bone formation and angiogenesis [23]. Furthermore, Hh signaling is known to be tightly regulated by multiple factors, including PTHLH, one of the commonly accepted master regulators of skeletogenesis. In normal skeletal bone development, PTHLH regulates endochondral bone development by maintaining the endo-chondral growth plate at a constant width, and it also regulates epithelial-mesenchymal interactions in other contexts, mainly through feedback inhibition of Hh signaling [24,25]. In a pathological context, Regard et al. demonstrated that up-regulated Hh signaling could disrupt the balance between the Wnt–β-catenin and Hh signaling, which might be the key underlying mechanism of POH, suggesting that Hh inhibitors may possibly be a viable choice for treating HO in POH [3].
Intriguingly and consistent with lineage tracing data, our current study provides additional evidence that Hh signaling dysregulation participates throughout all stages of endochondral HO in our animal model, i.e., Hh signaling is elevated in the tissue bordering HO lesions but not within the lesions themselves. However, systemic administration of the Hh inhibitor, eggmanone, at tested dosage was insufficient to prevent the initiation of HO or to treat the existing HO. This negative finding seemingly conflicts with the study of intramembranous HO by Regard et al. [3], but there are numerous possible explanations for the divergent findings: 1) since Hh signaling is not ubiquitously up-regulated even in the lesions, global inhibition of Hh signaling might not be an efficient way to prevent or treat HO. Thus Hh signaling may participate in, but is not be essential for, HO; 2) alternatively, the underlying mechanisms of endochondral and intramembranous HO (such as in POH) may be fundamentally different; 3) it is possible that the type/dose/duration of inhibition used in our experiment was insufficient, and that combining Hh inhibitors with other drugs, such as the nuclear retinoic acid receptor-γ agonists, might be needed to completely block the pathogenesis of HO.
In summary, the current study utilized Cre-lox based genetic lineage tracing to test the contribution of adult stem/progenitor cells that expressed either Gli1 or CD133 to HO. We found that both lineages contributed broadly to different normal tissues with different patterns, but that only Gli1-creERT labeled stem/progenitor cells contributed to all stages of HO.
Supplementary Material
Acknowledgments
We appreciate the help from many members of the Kessler lab. LK was supported in part by National Natural Science Foundation of China (81472087) and Natural Science Foundation of Anhui Province (1508085MC45). This work was supported by NIH grant RO1 AR066539 (JAK).
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.bone.2017.06.014.
References
- 1.Kraft CT, Agarwal S, Ranganathan K, Wong VW, Loder S, Li J, et al. Trauma-induced heterotopic bone formation and the role of the immune system: a review. J Trauma Acute Care Surg. 2016;80(1):156–165. doi: 10.1097/TA.0000000000000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH, et al. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006;38(5):525–527. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 3.Regard JB, Malhotra D, Gvozdenovic-Jeremic J, Josey M, Chen M, Weinstein LS, et al. Activation of Hedgehog signaling by loss of GNAS causes heterotopic ossification. Nat Med. 2013;19(11):1505–1512. doi: 10.1038/nm.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pignolo RJ, Ramaswamy G, Fong JT, Shore EM, Kaplan FS. Progressive osseous heteroplasia: diagnosis, treatment, and prognosis. Appl Clin Genet. 2015;8:37–48. doi: 10.2147/TACG.S51064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kan L, Kessler JA. Evaluation of the cellular origins of heterotopic ossification. Orthopedics. 2014;37(5):329–340. doi: 10.3928/01477447-20140430-07. [DOI] [PubMed] [Google Scholar]
- 6.Kan L, Peng CY, McGuire TL, Kessler JA. Glast-expressing progenitor cells contribute to heterotopic ossification. Bone. 2013;53(1):194–203. doi: 10.1016/j.bone.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wosczyna MN, Biswas AA, Cogswell CA, Goldhamer DJ. Multipotent progenitors resident in the skeletal muscle interstitium exhibit robust BMP-dependent osteogenic activity and mediate heterotopic ossification. J Bone Miner Res Off J Am Soc Bone Miner Res. 2012;27(5):1004–1017. doi: 10.1002/jbmr.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lounev VY, Ramachandran R, Wosczyna MN, Yamamoto M, Maidment AD, Shore EM, et al. Identification of progenitor cells that contribute to heterotopic skeletogenesis. J Bone Joint Surg Am. 2009;91(3):652–663. doi: 10.2106/JBJS.H.01177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dey D, Bagarova J, Hatsell SJ, Armstrong KA, Huang L, Ermann J, et al. Two tissue-resident progenitor lineages drive distinct phenotypes of heterotopic ossification. Sci Transl Med. 2016;8:366366ra163. doi: 10.1126/scitranslmed.aaf1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao H, Feng J, Ho TV, Grimes W, Urata M, Chai Y. The suture provides a niche for mesenchymal stem cells of craniofacial bones. Nat Cell Biol. 2015;17(4):386–396. doi: 10.1038/ncb3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, et al. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell. 2015;16(1):51–66. doi: 10.1016/j.stem.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shore EM, Ahn J, de Beur Jan S, Li M, Xu M, Gardner RJ, et al. Paternally inherited inactivating mutations of the GNAS1 gene in progressive osseous heteroplasia. N Engl J Med. 2002;346(2):99–106. doi: 10.1056/NEJMoa011262. [DOI] [PubMed] [Google Scholar]
- 13.Meng J, Chun S, Asfahani R, Lochmuller H, Muntoni F, Morgan J. Human skeletal muscle-derived CD133(+) cells form functional satellite cells after intramuscular transplantation in immunodeficient host mice. Mol Ther. 2014;22(5):1008–1017. doi: 10.1038/mt.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kan L, Liu Y, McGuire TL, Berger DM, Awatramani RB, Dymecki SM, et al. Dys-regulation of local stem/progenitor cells as a common cellular mechanism for heterotopic ossification. Stem Cells. 2009;27(1):150–156. doi: 10.1634/stemcells.2008-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisen J. A pericyte origin of spinal cord scar tissue. Science. 2011;333(6039):238–242. doi: 10.1126/science.1203165. [DOI] [PubMed] [Google Scholar]
- 16.Zhao H, Feng J, Seidel K, Shi S, Klein O, Sharpe P, et al. Secretion of shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem Cell. 2014;14(2):160–173. doi: 10.1016/j.stem.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karbanova J, Laco J, Marzesco AM, Janich P, Vobornikova M, Mokry J, et al. Human prominin-1 (CD133) is detected in both neoplastic and non-neoplastic salivary gland diseases and released into saliva in a ubiquitinated form. PLoS One. 2014;9(6):e98927. doi: 10.1371/journal.pone.0098927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alakel N, Jing D, Muller K, Bornhauser M, Ehninger G, Ordemann R. Direct contact with mesenchymal stromal cells affects migratory behavior and gene expression profile of CD133+ hematopoietic stem cells during ex vivo expansion. Exp Hematol. 2009;37(4):504–513. doi: 10.1016/j.exphem.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan FS, Glaser DL, Shore EM, Pignolo RJ, Xu M, Zhang Y, et al. Hematopoietic stem-cell contribution to ectopic skeletogenesis. J Bone Joint Surg Am. 2007;89(2):347–357. doi: 10.2106/JBJS.F.00472. [DOI] [PubMed] [Google Scholar]
- 20.Hojo H, Ohba S, Taniguchi K, Shirai M, Yano F, Saito T, et al. Hedgehog-Gli activators direct osteochondrogenic function of bone morphogenetic protein toward osteogenesis in the perichondrium. J Biol Chem. 2013;288(14):9924–9932. doi: 10.1074/jbc.M112.409342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hojo H, Ohba S, Yano F, Saito T, Ikeda T, Nakajima K, et al. Gli1 protein participates in Hedgehog-mediated specification of osteoblast lineage during endochondral ossification. J Biol Chem. 2012;287(21):17860–17869. doi: 10.1074/jbc.M112.347716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.James AW, Leucht P, Levi B, Carre AL, Xu Y, Helms JA, et al. Sonic Hedgehog influences the balance of osteogenesis and adipogenesis in mouse adipose-derived stromal cells. Tissue Eng Part A. 2010;16(8):2605–2616. doi: 10.1089/ten.tea.2010.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kazmers NH, McKenzie JA, Shen TS, Long F, Silva MJ. Hedgehog signaling mediates woven bone formation and vascularization during stress fracture healing. Bone. 2015;81:524–532. doi: 10.1016/j.bone.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strewler GJ. The parathyroid hormone-related protein. Endocrinol Metab Clin N Am. 2000;29(3):629–645. doi: 10.1016/s0889-8529(05)70154-7. [DOI] [PubMed] [Google Scholar]
- 25.Strewler GJ. The physiology of parathyroid hormone-related protein. N Engl J Med. 2000;342(3):177–185. doi: 10.1056/NEJM200001203420306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.