Abstract
Background
Echocardiography can detect calcium deposits in heart valves and aortic root, but the relationship of echocardiographic heart calcification such as aortic valve calcification (AVC), mitral annular calcification (MAC), and aortic root calcification (ARC) with future cardiovascular disease (CVD) mortality and morbidity is not fully elucidated.
Methods
We analyzed data from 943 patients with suspected coronary heart disease (mean age, 65.7 years; 36% female). Echocardiographic total heart calcification (THC) score was determined by summing up the AVC, MAC, and ARC variables; THC-0 (N = 397), THC-1 (N = 236), THC-2 (N = 224), and THC-3 (N = 86). Subjects were followed for mean 2.9 years to assess the risk of death from CVD causes. Cardiovascular morbidity was defined as new episodes of non-fatal myocardial infarction, congestive heart failure, stroke, and surgical treatment of vascular disease.
Results
There were 43 CVD deaths and a total of 160 CVD events. Kaplan–Meier curves showed a graded CVD mortality and morbidity across increasing THC score values. With full adjustment, Cox regression hazard ratios (95% confidence intervals) for CVD mortality and morbidity, using no calcification as reference, for THC-1, THC-2, and THC-3 were 2.21 (1.31–3.74), 2.59 (1.53–4.39) and 4.14 (2.30–7.47), respectively. When THC score was added to models with CVD risk factors, C-statistics were significantly larger for CVD mortality (p = 0.048) and for CVD mortality and morbidity (p = 0.004).
Conclusions
THC score, the sum of the amounts of AVC, MAC, and ARC present as estimated by echocardiography, has an independent and incremental prognostic value in a high-risk population.
Keywords: Aortic valve calcification, Cardiovascular disease, Echocardiography, Mitral annular calcification, Morbidity/mortality
1. Introduction
Calcium deposits in heart valves, such as aortic valve calcification (AVC) and mitral annular calcification (MAC), have been considered incidental echocardiographic findings with no clinical significance unless they cause significant blood flow obstruction. However, recent data have shown that both AVC and MAC are active and highly regulated processes with histological similarities to atherosclerosis [1]. Several community-based cohort studies have suggested a strong association of AVC and MAC with traditional cardiovascular disease (CVD) risk factors 2, 3, and these valvular calcifications are strongly associated with an increased risk of CVD events and death in the general population 4, 5, 6. Similarly, as part of thoracic aortic calcification, the presence of aortic root calcification (ARC) has been independently associated with an increased risk of CVD mortality [7]. Coronary artery calcium is a marker of subclinical coronary atherosclerosis and is an established predictor of future coronary heart disease (CHD) events independent of traditional CVD risk factors [8]. However, information on an association between calcified atherosclerosis in the coronary artery, heart valves, and aortic root is currently limited 9, 10, and no large observational studies have examined the relationship of echocardiographic AVC, MAC and ARC with future CVD mortality and morbidity. The aims of this study were to evaluate the associations of AVC, MAC, and ARC with calcified coronary atherosclerosis, and to test the ability of total heart calcification (THC) score determined by echocardiography for the prediction of future CVD mortality and morbidity in a sample of individuals with suspected CHD referred for clinically indicated echocardiography and computed tomography (CT).
2. Methods
2.1. Study population
The study was performed as an observational longitudinal study of chronic complication in patients with suspected CHD attending the cardiovascular outpatient clinic at Hiroshima University Hospital. We analyzed the data from outpatients suspected to have CHD who attended our clinic and underwent coronary CT angiography during the years 2005–2011. Of these, we selected all patients with suspected CHD who had undergone a first echocardiography and CT examination no more than 1 month apart for clinical reasons (chest symptoms, N = 343; asymptomatic with ischemic findings, N = 129; and asymptomatic with multiple CVD risk factors, N = 471) at our institution. Patients with known CHD by previous invasive coronary angiography, prior coronary stenting and/or bypass surgery, advanced malignancies, and with follow-up periods shorter than 1 year were excluded. On the basis of these criteria, 943 patients (603 men and 340 women; mean age, 65.7 ± 11.2 years) suspected to have CHD were identified and included in the final analysis. The study was approved by the hospital's ethics committee, and written informed consent was obtained from all patients.
2.2. Risk factor assessment
Overnight fasting blood samples were collected and serum levels of total cholesterol, low-density cholesterol, high-density cholesterol, and triglyceride and hemoglobin A1C level were measured. Hypertension was defined as blood pressure greater than 140/90 mm Hg or current use of hypertensive medication for this condition. Diabetes mellitus was defined as having a fasting plasma glucose level greater than 126 mg/dL or current use of hypoglycemic medication. Dyslipidemia was defined as having a fasting serum total cholesterol level greater than 200 mg/dL or current use of a lipid-lowering medication. Cigarette smoking was considered present if a subject had smoked at least one cigarette per day in the last year. A family history of early CHD was considered present if a patient's immediate family had had non-fatal myocardial infarction, coronary revascularization, or a fatal cardiovascular event before the age of 55. Glomerular filtration rate (eGFR) was estimated from the four-variable Modification of Diet in Renal Disease study equation.
2.3. Echocardiography and CT imaging
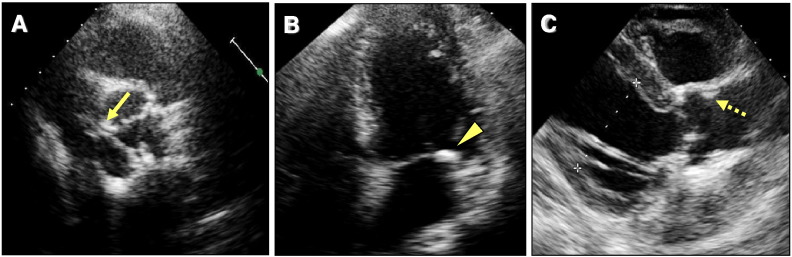
Complete echocardiographic studies of all patients were performed at our institution with a commercially available system (Vivid 7, GE Healthcare, Milwaukee, WI; or iE33, Philips Medical Systems, Andover, MA) by four experienced sonographers who were blinded to the clinical and CT information. Apical 4- and 2-chamber views were acquired for calculation of left ventricular ejection fraction and left atrial volume using the modified Simpson's formula [11]. Left ventricular mass was calculated using the area–length method and indexed to body surface area. AVC was defined as focal or diffused calcification and thickening of the aortic leaflets/annulus with or without restriction of leaflet motion using the criteria of Otto et al. [6] (Fig. 1A). MAC was defined by increased echogenicity located at the junction of the atrioventricular groove and posterior mitral leaflet on the parasternal long-axis, short-axis, or apical 4-chamber view [12] (Fig. 1B). ARC was defined as a focal area of increased echogenicity and thickening in the aortic root on the parasternal long-axis view [13] (Fig. 1C). In accord with a previous study, we calculated a THC score that was graded from 0 to a maximum of 3 according to the number of anatomic sites involved; namely, at the aortic valve, the mitral annulus, and/or the aortic root [13].
Fig. 1.
Examples of determining the THC score.
Two-dimensional echocardiographic images show aortic valve calcification (A, solid arrow), mitral annular calcification (B, arrowhead), and aortic root calcification (C, broken arrow). THC score was determined by summing these extracoronary calcifications. It was calculated to be 3.
CT examinations were performed using a 64-multidetector CT scanner (LightSpeed VCT, GE Healthcare). Prospective electrocardiogram-triggered scans were performed from the root of aorta to the apex of the heart with the following parameters: axial scan; gantry rotation times, 350 ms; X-ray exposure times, 233 ms; tube voltage, 120 kV; tube currents, 140 mA; center of imaging window, 75% of R-R. Thirty-five to 40 contiguous images of 2.5-mm thickness were obtained. Coronary artery calcium score was calculated based on the Agatston method with dedicated software (Smartscore, version 3.5, GE Healthcare) [14]. In addition, we used non-contrast CT datasets to score AVC, MAC, and ARC with the same software.
2.4. Study outcomes
Subjects were followed for a mean duration of 2.9 years (range 1.0–7.4 years). Patient information was obtained from medical records or telephone interviews with patients or their families. Ascertainment of CVD events was conducted by persons who were unaware of the baseline data and echocardiographic results. The primary outcome for the present study was the occurrence of incident CVD events [6], including death from CVD causes and CVD morbidity defined as new episodes of non-fatal myocardial infarction, unstable angina pectoris, congestive heart failure, stroke, and surgical treatment of vascular or valvular disease. Coronary revascularization was not included in CVD events.
2.5. Statistical analysis
Continuous variables are presented as mean ± SD or median (interquartile range). Group comparisons of continuous variables were performed by 1-way analysis of variance with post-hoc analysis using the Tukey's method. Multivariate logistic analyses included valvular and aortic root calcification and were adjusted for age, gender, eGFR, body mass index, and the traditional CVD risk factors. Event rates were estimated by Kaplan–Meier curves and compared by log-rank tests stratified by the presence of AVC, MAC, and ARC or THC scores. The Cox proportional hazard model was used to calculate univariate and multivariate hazard ratios (HRs) for the occurrence of incident CVD mortality and morbidity. The independent predictive values of the presence of AVC, MAC, and ARC or THC score were assessed in the following hierarchical models: model 1, unadjusted; model 2, adjusted for age and gender; model 3, model 2 plus eGFR and the traditional CVD risk factors; and model 4, model 2 plus left ventricular ejection fraction < 50%, left atrial volume, and left ventricular mass index determined by echocardiography. In addition, we used natural log-transformed AVC, MAC, ARC, and coronary artery calcium scores for the analyses in which the quantity of calcium was used. Receiver operating characteristic analyses were conducted from logistic regression models and the probability values were compared between different models using the method described by DeLong et al. [15]. All statistical analyses were performed using SPSS software (version 16.0: SPSS Inc., Chicago, IL). A probability value of < 0.05 was considered significant.
3. Results
3.1. Baseline characteristics
There were 441 subjects (46.8%) with AVC, 145 (15.4%) with MAC, and 344 (36.5%) with ARC. Overall, 397 patients (42.1%) were free of any calcification at the valve and aortic root levels (i.e., THC-0), 236 patients (25.0%) had isolated AVC, isolated MAC, or isolated ARC (i.e., THC-1), and 224 patients (23.8%) had the combined presence of calcium deposits in any two areas (i.e., THC-2). The remaining 86 patients (9.1%) had the combined presence of calcium deposits in all three areas (i.e., THC-3). Table 1 summarizes baseline clinical characteristics, echocardiographic measurements, and CT findings stratified by THC scores. Left ventricular ejection fraction was preserved in all groups, but the THC-3 group had larger left atrial volume and higher left ventricular mass, indicating impairment of diastolic function.
Table 1.
Baseline characteristics stratified by total heart calcification (THC) score.
| THC score |
||||
|---|---|---|---|---|
| THC-0 |
THC-1 |
THC-2 |
THC-3 |
|
| (N = 397) | (N = 236) | (N = 224) | (N = 86) | |
| Age (yrs) | 59 ± 11 | 68 ± 8* | 71 ± 8*, † | 76 ± 7*, †, ‡ |
| Male, N (%) | 269 (68) | 150 (64) | 146 (65) | 38 (44)*, †, ‡ |
| Body mass index (kg/m2) | 24 ± 5 | 24 ± 3 | 24 ± 3 | 24 ± 4 |
| eGFR (mL/min/1.73 m2) | 73 ± 16 | 70 ± 16 | 67 ± 17* | 62 ± 16*, † |
| Hypertension, N (%) | 206 (52) | 177 (75)* | 178 (80)* | 74 (86)*, † |
| Diabetes mellitus, N (%) | 97 (24) | 77 (33)* | 95 (42)*, † | 41 (48)*, † |
| Dyslipidemia, N (%) | 173 (44) | 133 (56)* | 140 (63)* | 50 (58)* |
| Cigarette smoker, N (%) | 106 (27) | 73 (31) | 84 (38)* | 18 (21)‡ |
| Family history of early coronary heart disease, N (%) | 39 (10) | 34 (14) | 24 (11) | 12 (14) |
| Total cholesterol (mg/dL) | 199 ± 38 | 195 ± 37 | 195 ± 32 | 193 ± 37 |
| Triglycerides (mg/dL) | 118 (87–170) | 114 (87–171) | 127 (91–181) | 114 (82–171) |
| HDL cholesterol (mg/dL) | 62 ± 17 | 58 ± 16* | 60 ± 16 | 63 ± 22 |
| LDL cholesterol (mg/dL) | 117 ± 32 | 117 ± 32 | 114 ± 28 | 111 ± 30 |
| HbA1C (%) | 5.5 (5.2–6.0) | 5.7 (5.3–6.3)* | 5.8 (5.4–6.5)*, † | 5.7 (5.2–6.8)* |
| Left ventricular ejection fraction (%) | 64 ± 9 | 65 ± 10 | 67 ± 9* | 69 ± 11*, † |
| Left atrial volume index (mL/m2) | 31 ± 9 | 33 ± 9 | 33 ± 9 | 37 ± 12*, †, ‡ |
| Left ventricular mass index (g/m2) | 84 ± 24 | 89 ± 25 | 90 ± 22* | 103 ± 36*, †, ‡ |
| Coronary artery calcium score | 2 (0–48) | 66 (3–243)* | 272 (87–625)*, † | 344 (128–958)*, † |
p < 0.05 vs THC-0.
p < 0.05 vs THC-1.
p < 0.05 vs THC-2.
3.2. Relationship between coronary artery calcium and AVC, MAC, and ARC
As shown in Table 1, coronary artery calcium score escalated in proportion with THC scores (all p < 0.001 by ANOVA). Overall, the age- and gender-adjusted Pearson's correlation coefficient between AVC and coronary artery calcium score was 0.33 (p < 0.001). Similarly, the amount of MAC and ARC was fairly correlated with coronary artery calcium score (r = 0.18, p < 0.001; r = 0.42, p < 0.001, respectively). With adjustment for age, gender, CVD risk factors, and the presence of calcium in the other sites, AVC, MAC, and ARC detected by echocardiography were all independently correlated with coronary artery calcium score (β [95% CI] 0.17 [0.25–0.52], p < 0.001; 0.20 [0.02–0.37], p = 0.026; 0.66 [0.53–0.80], p < 0.001, respectively).
3.3. Survival analysis
The average time from initial echocardiography and CT examination to censoring due to either CVD events or end of study was 2.9 years. As of May 31, 2013, there were 43 (4.6%) deaths from CVD causes. Incident CVD events occurred in 160 (17.0%) patients. The breakdown of CVD events was as follows: CVD death in 43 patients (26.9%), non-fatal myocardial infarction in 8 (5.0%), unstable angina in 13 (8.1%), congestive heart failure in 23 (14.4%), stroke in 47 (29.4%), and surgical treatment of vascular and valvular disease in 26 (16.3%).
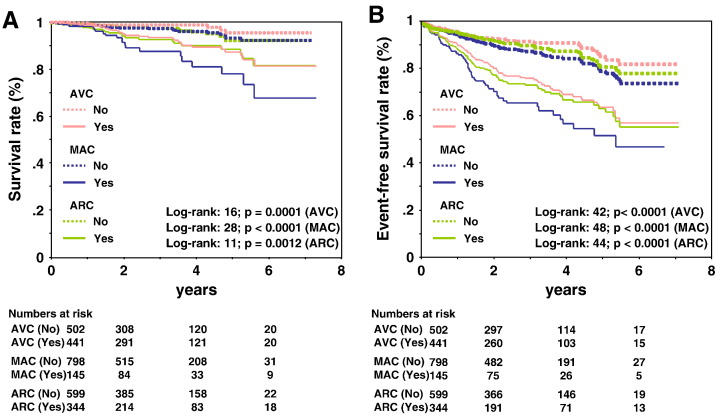
Fig. 2 shows Kaplan–Meier survival curves for incident CVD mortality and CVD mortality and morbidity as functions of valvular and aortic root calcification, respectively. For all sites of calcium deposits and compared with the absence of calcium at any site, the presence of calcium in any valve or the aortic root was associated with a significantly lower survival (p < 0.001 for all). For CVD mortality, those with MAC had the lowest probability of survival, whereas those without AVC had the highest probability. The survival curves showed the largest separation for the presence/absence of MAC. When calcium was present, the curve for MAC was significantly different from the curves for AVC and ARC (p < 0.05 for both). When calcium was absent, there were no significant differences in the survival curves among the different sites of calcium deposits. Similar results were found for CVD mortality and morbidity.
Fig. 2.
(A) Kaplan–Meier survival analysis for CVD mortality stratified by the presence of calcium deposit in different sites. Significance of site comparisons: p < 0.05 for MAC vs ARC, MAC vs AVC. (B) Kaplan–Meier survival analysis for CVD mortality and morbidity stratified by the presence of calcium deposits at different sites. Significance of site comparisons: p < 0.05 for MAC vs ARC, MAC vs AVC.
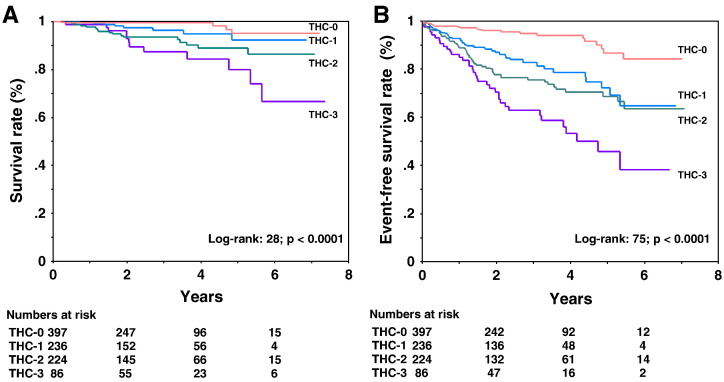
Fig. 3 shows Kaplan–Meier survival curves for incident CVD mortality and CVD mortality and morbidity as functions of the THC score. THC score was strongly associated with an increased risk of CVD death and events. The differences among these curves were statistically significant for CVD mortality (p < 0.0001 by the log-rank test). Similar results were found for CVD mortality and morbidity.
Fig. 3.
(A) Kaplan–Meier survival analysis for CVD mortality stratified by THC score. (B) Kaplan–Meier survival analysis for CVD mortality and morbidity stratified by THC score.
3.4. Association of the presence of AVC, MAC, and ARC with CVD mortality and morbidity
Table 2 lists the univariate and multivariate HRs for CVD mortality and for CVD mortality and morbidity in the presence of valvular and aortic root calcification. In Cox univariate analyses, the presence of calcium in any of the sites was associated with a significantly increased HR for CVD mortality (model 1). Using THC-0 as reference, the univariate HRs were 2.75 (0.90–8.40), 5.83 (2.16–15.7) and 9.87 (3.47–28.0) for CVD mortality for THC-1, THC-2, and THC-3, respectively. Additional adjustment for eGFR and the traditional CVD risk factors attenuated the magnitudes of the associations such that only the presence of MAC or THC-3 remained statistically significantly associated with incident CVD mortality, with the largest magnitude of associations being for THC-3 (models 2 and 3). The magnitudes of the associations with CVD mortality and morbidity were stronger than those with CVD mortality. THC score and presence of calcium at any of the sites were associated with a significantly increased HR even after adjustment for CVD risk factors. The largest HR was found for the THC-3 (HR, 4.14 [2.30–7.47]). The results remained essentially unchanged after adjustment for age, gender, and other baseline echocardiographic parameters (model 4).
Table 2.
Univariate and multivariate-adjusted hazard ratios (HRs) for cardiovascular disease (CVD) mortality and morbidity.
| Cox regression analyses using hierarchical models |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 3 |
Model 4 |
|||||||||
| HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | |
| CVD mortality | ||||||||||||
| AVC presence | 4.02 | 1.93–8.39 | < 0.001 | 1.98 | 0.92–4.25 | 0.082 | 1.72 | 0.79–3.73 | 0.170 | 1.70 | 0.78–3.70 | 0.182 |
| MAC presence | 4.41 | 2.42–8.06 | < 0.001 | 2.58 | 1.37–4.85 | 0.003 | 2.51 | 1.31–4.78 | 0.005 | 2.45 | 1.29–4.63 | 0.006 |
| ARC presence | 2.65 | 1.44–4.88 | 0.002 | 1.37 | 0.72–2.60 | 0.343 | 1.17 | 0.60–2.28 | 0.655 | 1.53 | 0.79–2.95 | 0.207 |
| THC-0 | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | ||||
| THC-1 | 2.75 | 0.90–8.40 | 0.076 | 1.52 | 0.49–4.75 | 0.473 | 1.24 | 0.39–3.91 | 0.713 | 1.32 | 0.42–4.16 | 0.634 |
| THC-2 | 5.83 | 2.16–15.7 | < 0.001 | 2.47 | 0.86–7.09 | 0.093 | 2.03 | 0.70–5.90 | 0.196 | 2.71 | 0.92–7.94 | 0.069 |
| THC-3 | 9.87 | 3.47–28.0 | < 0.001 | 3.62 | 1.18–11.2 | 0.025 | 2.95 | 1.04–9.18 | 0.045 | 3.27 | 1.05–10.2 | 0.041 |
| CVD mortality and morbidity | ||||||||||||
| AVC presence | 2.94 | 2.08–4.15 | < 0.001 | 1.98 | 1.36–2.87 | < 0.001 | 1.80 | 1.24–2.63 | 0.002 | 1.74 | 1.16–2.62 | 0.007 |
| MAC presence | 2.97 | 2.13–4.13 | < 0.001 | 2.12 | 1.48–3.02 | < 0.001 | 2.06 | 1.44–2.94 | < 0.001 | 1.95 | 1.33–2.84 | 0.001 |
| ARC presence | 2.75 | 2.00–3.78 | < 0.001 | 1.93 | 1.38–2.71 | < 0.001 | 1.75 | 1.24–2.48 | 0.002 | 1.95 | 1.36–2.80 | < 0.001 |
| THC-0 | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | ||||
| THC-1 | 3.15 | 1.91–5.20 | < 0.001 | 2.41 | 1.43–4.05 | 0.001 | 2.21 | 1.31–3.74 | 0.003 | 1.97 | 1.13–3.44 | 0.016 |
| THC-2 | 4.27 | 2.65–6.87 | < 0.001 | 2.93 | 1.76–4.90 | < 0.001 | 2.59 | 1.53–4.39 | < 0.001 | 2.94 | 1.71–5.05 | < 0.001 |
| THC-3 | 7.34 | 4.36–12.3 | < 0.001 | 4.74 | 2.65–8.48 | < 0.001 | 4.14 | 2.30–7.47 | < 0.001 | 3.72 | 1.96–7.08 | < 0.001 |
Model 1: unadjusted; model 2: adjusted for age and gender; model 3: adjusted for age, gender, estimated glomerular filtration rate, body mass index, hypertension, diabetes mellitus, dyslipidemia, cigarette smoking, and family history of early coronary heart disease; model 4: adjusted for age, gender, left ventricular ejection fraction < 50%, left atrial volume index, and left ventricular mass index as detected by means of echocardiography.
3.5. Model comparisons using receiver operating characteristic analyses
Compared with the areas under the curve when the presence of each valvular and aortic root calcification was separately added to the CVD risk factors, the area under the curve was larger for all comparisons when THC score was added to the CVD risk factors (area under the curve, 0.833 for CVD mortality, p = 0.048 vs CVD risk factors; 0.770 for CVD mortality and morbidity, p = 0.004 vs CVD risk factors) (Table 3).
Table 3.
Areas under the curve from receiver operating characteristic analyses.
| CVD mortality |
CVD mortality and morbidity |
|||||
|---|---|---|---|---|---|---|
| Model | AUC | 95% CI | p value | AUC | 95% CI | p value |
| Traditional risk factors (TRFs)a | 0.818 | 0.755–0.880 | Ref. | 0.735 | 0.693–0.778 | Ref. |
| TRFs + AVC presence | 0.824 | 0.761–0.888 | 0.14 | 0.754 | 0.714–0.794 | 0.02 |
| TRFs + MAC presence | 0.824 | 0.760–0.888 | 0.15 | 0.748 | 0.705–0.791 | 0.05 |
| TRFs + ARC presence | 0.818 | 0.755–0.880 | 0.49 | 0.751 | 0.709–0.793 | 0.04 |
| TRFs + THC score | 0.833 | 0.773–0.893 | 0.048 | 0.770 | 0.731–0.810 | 0.004 |
Includes age, gender, hypertension, diabetes mellitus, dyslipidemia, cigarette smoking, and family history of early coronary heart disease.
4. Discussion
In this “real-world” cohort study of individuals with suspected CHD, valvular and aortic root calcification detected by echocardiography were associated with calcified atherosclerosis in the coronary arteries and higher risk of CVD mortality and morbidity. We demonstrate that the prognostic value of echocardiographic THC score is independent of CVD risk factors, renal function (eGFR), and baseline echocardiographic variables. C-statistics from receiver operating characteristic analyses revealed that THC score had an incremental ability to predict CVD mortality and CVD mortality and morbidity compared to traditional risk factors.
4.1. Valvular and aortic root calcification and coronary atherosclerosis
AVC, MAC, and ARC are common abnormal findings on echocardiography in the elderly 3, 6, 13. Previous studies have suggested that these calcifications are not simply a degenerative change but an active and highly regulated process with several pathogenic features in common with atherosclerosis, including the process of lipid deposition, macrophage and T-cell infiltration, and induction of osteoblast-like phenotype [16]. Until now, several electron-beam CT studies have investigated the relationship among AVC, MAC, ARC, and CAC, mainly in the general population 9, 10. In addition, these valvular and aortic root calcification are not only related to coronary atherosclerosis, but also to atherosclerosis in other vascular beds, such as the carotid, abdominal, or iliac arteries in asymptomatic patients without known CHD [17]. The findings in those studies, however, were not sufficiently validated in a high-risk population such as patients with suspected CHD and the prognostic value of echocardiographic heart calcification remains to be investigated. The results of our study provide novel information on AVC, MAC, and ARC as reliable markers of the severity of systemic atherosclerosis, including in the coronary arteries. It should also be noted that in our study, AVC, MAC, and ARC scores were only fairly correlated with coronary artery calcium score. It is also possible to speculate that the two different phases of extracoronary calcium progression: the early phase, when calcium deposits appear de novo with progressive atherosclerosis, and the secondary phase, when established calcium deposits progress independently of CVD risk factors and grow faster with other conditions such as chronic inflammation which may predispose patients to an active calcification process.
4.2. Valvular and aortic root calcification as an indicator of future CVD events
Until now, there is limited information on echocardiographic heart calcification and future CVD events in the literature. The Cardiovascular Health Study showed that the presence of AVC is associated with an approximately 50% increase in the risk of CVD mortality and morbidity in adults age > 65 years [6]. Echocardiographic presence and severity of MAC have been directly related to increased risk of CVD events, including acute myocardial infarction, stroke, and vascular diseases 5, 12. In contrast, several studies have shown that ARC is closely associated with CVD events but that the prognostic value of ARC generally diminishes with adjustment for age, CVD risk factors, and CAC 7, 18. Also in the present study, the predictive value of ARC for CVD mortality altered nonsignificantly after full adjustment. Meanwhile, AVC, MAC, and ARC are strongly associated with CVD mortality and morbidity independent of CVD risk factors, renal function, and other echocardiographic variables.
4.3. Clinical implication
Our study also provides an interesting finding that THC score — the sum of the amount of AVC, MAC, and ARC present as estimated by echocardiography — has an independent prognostic value associated with CVD mortality and morbidity. Importantly, based on the receiver operating characteristic analyses, this study indicates that THC score can provide additional information for discriminating CVD events beyond that provided by traditional CVD risk factors. Recent studies demonstrated that the combination of AVC and MAC is indicative of more extensive coronary atherosclerotic plaques [19] and improves the predictive power with respect to all-cause mortality and CVD mortality 20, 21. Furthermore, echocardiographic THC score is correlated with the presence of obstructive CHD and the presence of inappropriate high left ventricular mass index [22]. The result of our study suggests that the combined presence of AVC, MAC, and ARC (i.e. THC-3) is indicative of more critical disease states, such as extensive systemic atherosclerosis, concomitant diastolic dysfunction due to high left ventricular mass, and valve insufficiency, and may therefore be significantly associated with incident CVD mortality and morbidity.
Thus far, great attention has been paid to the prognostic value of coronary artery calcium score as measured by CT for both all-cause and CVD mortality 7, 8, 18. However, the evaluation of coronary artery calcium has several disadvantages, such as radiation exposure and high cost. Echocardiographic THC score overcomes these limitations and requires no special equipment, extra imaging time, or considerable effort to analyze, so this index is easy to implement for routine applications. Therefore, from a clinical perspective, heart calcification on routine echocardiography should not be overlooked and can be used for risk stratification. It can help identify patients appropriate for intensive medical treatment to inhibit an active atherosclerotic process.
4.4. Study limitations
First, all patients were suspected to have CHD and had clinical reasons for coronary CT assessment; thus, they had relatively high pretest probability. Thus, our results do not apply to patients with a lower probability of CHD. However, patients such as those from our study are the most likely to need detailed risk stratification in daily clinical practice. The second limitation of this study is its retrospective and longitudinal design, which does not allow us to draw any conclusions about causality. Therefore, the results should be interpreted with extreme caution. Larger prospective follow-up studies are necessary to confirm our findings and to determine whether therapeutic intervention in the progression of calcific aortic and valvular disease can decrease risk of developing CVD death and cardiac events in a high-risk population. Third, in this study, detailed data regarding medication use and plasma levels of inflammatory markers, such as C-reactive protein and adipokines, were not collected. The potential effect of this information on the results is not clear.
Finally, the results of this study should not be construed as support for coronary CT angiography scans for the sole purpose of CVD risk assessment. Rather, routine echocardiography examinations may be most relevant as “gatekeepers of the application of coronary CT angiography” because the echocardiographic images of heart calcification were found in our study to have strong and significant associations with extensive coronary atherosclerosis and increased risk of future CVD events.
5. Conclusions
Our study demonstrates that AVC, MAC, and ARC detected by echocardiography, singly or in combination, are independently and additively predictive of CVD mortality and morbidity in patients with suspected CHD.
Acknowledgments
This study was in part by a Grant-in Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (Tokyo, Japan, No. 23591044).
Footnotes
Available online 26 November 2013
References
- 1.Otto C.M., Kuusisto J., Reichenbach D.D., Gown A.M., O'Brien K.D. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90:844–853. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]
- 2.Kanjanauthai S., Nasir K., Katz R., Rivera J.J., Takasu J., Blumenthal R.S. Relationships of mitral annular calcification to cardiovascular risk factors: the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2011;213:558–562. doi: 10.1016/j.atherosclerosis.2010.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewart B.F., Siscovick D., Lind B.K., Gardin J.M., Gottdiener J.S., Smith V.E. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 4.Owens D.S., Budoff M.J., Katz R., Takasu J., Shavelle D.M., Carr J.J. Aortic valve calcium independently predicts coronary and cardiovascular events in a primary prevention population. JACC Cardiovasc Imaging. 2012;5:619–625. doi: 10.1016/j.jcmg.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox C.S., Vasan R.S., Parise H., Levy D., O'Donnell C.J., D'Agostino R.B. Mitral annular calcification predicts cardiovascular morbidity and mortality: the Framingham Heart Study. Circulation. 2003;107:1492–1496. doi: 10.1161/01.cir.0000058168.26163.bc. [DOI] [PubMed] [Google Scholar]
- 6.Otto C.M., Lind B.K., Kitzman D.W., Gersh B.J., Siscovick D.S. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341:142–147. doi: 10.1056/NEJM199907153410302. [DOI] [PubMed] [Google Scholar]
- 7.Allison M.A., Hsi S., Wassel C.L., Morgan C., Ix J.H., Wright C.M. Calcified atherosclerosis in different vascular beds and the risk of mortality. Arterioscler Thromb Vasc Biol. 2012;32:140–146. doi: 10.1161/ATVBAHA.111.235234. [DOI] [PubMed] [Google Scholar]
- 8.Detrano R., Guerci A.D., Carr J.J., Bild D.E., Burke G., Folsom A.R. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 9.Hamirani Y.S., Nasir K., Blumenthal R.S., Takasu J., Shavelle D., Kronmal R. Relation of mitral annular calcium and coronary calcium (from the Multi-Ethnic Study of Atherosclerosis [MESA]) Am J Cardiol. 2011;107:1291–1294. doi: 10.1016/j.amjcard.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Nasir K., Katz R., Al-Mallah M., Takasu J., Shavelle D.M., Carr J.J. Relationship of aortic valve calcification with coronary artery calcium severity: the Multi-Ethnic Study of Atherosclerosis (MESA) J Cardiovasc Comput Tomogr. 2010;4:41–46. doi: 10.1016/j.jcct.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Lang R.M., Bierig M., Devereux R.B., Flachskampf F.A., Foster E., Pellikka P.A. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Adler Y., Herz I., Vaturi M., Fusman R., Shohat-Zabarski R., Fink N. Mitral annular calcium detected by transthoracic echocardiography is a marker for high prevalence and severity of coronary artery disease in patients undergoing coronary angiography. Am J Cardiol. 1998;82:1183–1186. doi: 10.1016/s0002-9149(98)00596-7. [DOI] [PubMed] [Google Scholar]
- 13.Jeon D.S., Atar S., Brasch A.V., Luo H., Mirocha J., Naqvi T.Z. Association of mitral annulus calcification, aortic valve sclerosis and aortic root calcification with abnormal myocardial perfusion single photon emission tomography in subjects age < or = 65 years old. J Am Coll Cardiol. 2001;38:1988–1993. doi: 10.1016/s0735-1097(01)01678-3. [DOI] [PubMed] [Google Scholar]
- 14.Agatston A.S., Janowitz W.R., Hildner F.J., Zusmer N.R., Viamonte M., Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 15.DeLong E.R., DeLong D.M., Clarke-Pearson D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 16.Rajamannan N.M., Subramaniam M., Rickard D., Stock S.R., Donovan J., Springett M. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. 2003;107:2181–2184. doi: 10.1161/01.CIR.0000070591.21548.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allison M.A., Cheung P., Criqui M.H., Langer R.D., Wright C.M. Mitral and aortic annular calcification are highly associated with systemic calcified atherosclerosis. Circulation. 2006;113:861–866. doi: 10.1161/CIRCULATIONAHA.105.552844. [DOI] [PubMed] [Google Scholar]
- 18.Budoff M.J., Nasir K., Katz R., Takasu J., Carr J.J., Wong N.D. Thoracic aortic calcification and coronary heart disease events: the multi-ethnic study of atherosclerosis (MESA) Atherosclerosis. 2011;215:196–202. doi: 10.1016/j.atherosclerosis.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Utsunomiya H., Yamamoto H., Kunita E., Kitagawa T., Ohashi N., Oka T. Combined presence of aortic valve calcification and mitral annular calcification as a marker of the extent and vulnerable characteristics of coronary artery plaque assessed by 64-multidetector computed tomography. Atherosclerosis. 2010;213:166–172. doi: 10.1016/j.atherosclerosis.2010.08.070. [DOI] [PubMed] [Google Scholar]
- 20.Rossi A., Targher G., Zoppini G., Cicoira M., Bonapace S., Negri C. Aortic and mitral annular calcifications are predictive of all-cause and cardiovascular mortality in patients with type 2 diabetes. Diabetes Care. 2012;35:1781–1786. doi: 10.2337/dc12-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volzke H., Haring R., Lorbeer R., Wallaschofski H., Reffelmann T., Empen K. Heart valve sclerosis predicts all-cause and cardiovascular mortality. Atherosclerosis. 2010;209:606–610. doi: 10.1016/j.atherosclerosis.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 22.Corciu A.I., Siciliano V., Poggianti E., Petersen C., Venneri L., Picano E. Cardiac calcification by transthoracic echocardiography in patients with known or suspected coronary artery disease. Int J Cardiol. 2010;142:288–295. doi: 10.1016/j.ijcard.2009.01.021. [DOI] [PubMed] [Google Scholar]