Abstract
Background
Prospective cohort studies have shown that seafood consumption is inversely related to fatal coronary heart disease, sudden cardiac death and stroke. We studied whether the kind of seafood consumed in addition to seafood consumption per se is associated with out-of-hospital cardiac arrests (OHCA) of cardiac origin.
Methods and results
We compared the average consumption of different kinds of seafood and other risk factors to the average incidence of age-adjusted OHCA (660,672 cases of OHCA: 55.2% of cardiac origin and 44.8% of non-cardiac origin) between 2005 and 2010 in the 47 prefectures of Japan. There were many significant correlations between the incidence of age-adjusted OHCA of cardiac origin (ad-OHCA-CO) and the consumption of many kinds of seafood, but not the total consumption of seafood. The consumption of horse mackerel (r = − 0.568, p < 0.0001) and saury (r = 0.607, p < 0.0001) showed the highest negative and positive correlations, respectively, with the age-adjusted incidence of ad-OHCA-CO.
Conclusions
In Japan, the consumption of different kinds of seafood may be an important factor in OHCA of cardiac origin. Thus, dietary habits with regard to seafood may play a role in OHCA of cardiac origin, however, the question of whether to eat fish in general or instead to eat certain kinds of fish is still unclear.
Keywords: Cardiac arrest, Ecological study, Fatty acids, Seafood consumption
1. Introduction
Dietary prevention for cardiovascular diseases (CVD) originated in animal studies in which the high intake of dietary cholesterol was shown to lead to hypercholesterolemia and promote atherosclerosis. However, dietary intervention trials in humans that have targeted the primary and secondary prevention of CVD have not always been successful 1, 2, 3, since the trials were too small or too short, or the intervention itself was too weak to produce changes in lifestyle or diet [4]. However, epidemiological surveys have shown a lower prevalence of CVD in populations around the Mediterranean, where the diet is rich in olive oil, fiber and n-3 polyunsaturated fatty acids (n-3PUFAs), and in Asia (e.g., Japan), where the diet has traditionally been low in fat and high in carbohydrates with high levels of fish consumption 5, 6, 7. Prospective cohort studies have shown that the consumption of seafood and fish oil is inversely related to fatal coronary heart disease 8, 9, sudden cardiac death (SCD) 10, 11 and stroke 10, 12, 13, and most of the reduction in SCD could be explained by the antiarrhythmic effects of n-3PUFAs in fish 14, 15. As a result, current guidelines for both the primary and secondary prevention of cardiovascular events 15, 16, 17, including Japanese guidelines 18, 19, encourage the consumption of fresh seafood or oily fish at least twice a week.
In January 2005, the Fire and Disaster Management Agency (FDMA) of Japan launched a prospective, nationwide, population-based, cohort study in subjects who had had an out-of-hospital cardiac arrest (OHCA) to evaluate the effect of the nationwide dissemination of public-access AEDs on the rate of survival among patients who had an OHCA 20, 21, 22, 23, 24, 25, and the Japanese Circulation Society (JCS) Resuscitation Science Study (JCS-ReSS) Group had a suitable database. Therefore, as a working hypothesis, we assumed that the kind of seafood consumed in each prefecture, in addition to the total consumption of seafood per se in each prefecture, would be associated with OHCA of cardiac origin. We compared the total consumption of seafood and the average consumption of different kinds of seafood to the average incidence of OHCA between 2005 and 2010 in the 47 prefectures of Japan. To the best of our knowledge, this is the first study to demonstrate the relationship between seafood consumption and OHCA.
2. Methods
2.1. Subjects
Patients who suffered from OHCA of cardiac and non-cardiac origin (n = 364,547 and 296,125, respectively, Table 1) and who were enrolled in the All-Japan Utstein Registry of the Fire and Disaster Management Agency between 2005 and 2010 were included in this analysis 20, 21, 22. The populations in the 47 prefectures in Japan were obtained from the Population Census (2005) [26] and the Annual Report on Current Population Estimates (2006–2010) [27] published by the Ministry of Internal Affairs and Communications of Japan. The study protocol for analyses was approved by the Ethics Committee of Fukuoka University (FU-#00000403), Japan.
Table 1.
Patient characteristics.
| Total (n = 660,672) |
Cardiac origin (n = 364,547) |
Non-cardiac origin (n = 296,125) |
|
|---|---|---|---|
| Age, yrs | 72 ± 18 | 75 ± 16* | 68 ± 21 |
| Male, n (%) | 387,059(58.6) | 211,623(58.1)* | 175,436(59.2) |
| ROSC, n (%) | 42,899(6.5) | 23,587(6.5) | 19,312(6.5) |
| 1-month survival, n (%) | 31,707(4.8) | 19,530(5.4)* | 12,177(4.1) |
| CPC1 or 2, n (%) | 14,337(2.2) | 10,812(3.0)* | 3525(1.2) |
| OPC1 or 2, n (%) | 14,188(2.1) | 10,714(2.9)* | 3474(1.2) |
| Initial rhythm | |||
| VF | 47,158(7.1) | 40,549(11.1)* | 6609(2.2) |
| Pulseless VT | 1627(0.2) | 1058(0.3)* | 569(0.2) |
| PEA | 140,691(21.3) | 73,357(20.1)* | 67,334(22.7) |
| Asystole | 443,209(67.1) | 235,657(64.6)* | 207,552(70.1) |
ROSC = return of spontaneous circulation.
CPC = cerebral performance category.
OPC = overall performance category.
VF = ventricular fibrillation.
VT = ventricular tachycardia.
PEA = pulseless electrical activity.
p < 0.01 vs. non-cardiac origin.
2.2. Age-adjusted incidence of OHCA
Using the Utstein Registry, we calculated the crude incidence of OHCA by determining the raw number of cases of OHCA by prefecture and then dividing these numbers by the population of the prefecture. The Japanese Model Population in 1985 was used as a standard population, and age-standardization was performed by a direct method. We determined the average yearly age-adjusted incidence of OHCA by prefecture from 2005 to 2010.
2.3. Seafood consumption
Data regarding the consumption of seafood in the 47 municipalities were obtained from the Family Income and Expenditure Survey published by the Ministry of Internal Affairs and Communications of Japan [28]. We considered 14 kinds of seafood: tuna, horse mackerel, sardine, bonito, flounder, salmon, mackerel, saury, sea bream, yellowtail, cuttlefish, octopus, shrimp, and crab. Yearly expenditures, quantities and average prices per two-or-more-person household by prefectural capital city were obtained from this Survey, which also included the quantities of different kinds of seafood and salt consumption. We calculated the daily consumption of these foods per person by dividing the yearly amounts of the different kinds of seafood by the number of household members, and determined the averages from 2005 to 2010.
2.4. Fatty acids
The yearly quantities of different kinds of seafood were determined as described in a previous section. The types of lipids that were contained in seafood were obtained from the Standard Tables of Food Composition in Japan (fifth revised and enlarged edition) published by the Ministry of Education, Culture, Sports, Science and Technology of Japan 29, 30. Although various preparation (cooking) methods and species of seafood were included, we used data for raw seafood and averages for species of seafood. For example, tuna encompasses 6 species and both red and fatty flesh, which have completely different lipid contents. In Japan, red flesh and fatty flesh account for 87.5% and 12.5%, respectively, of tuna consumption, and this was considered in the calculation. The fatty acid contents in the 47 prefectures were calculated for the 14 kinds of seafood and this was considered to be the consumption of fatty acid for fish that are mainly consumed in Japan.
2.5. Other risk factors
The sex ratio was obtained from the All-Japan Utstein Registry. Data on the consumption of salt, alcohol, and tobacco were obtained from the Family Income and Expenditure Survey [28]. We calculated the daily consumption of these foods by the approach mentioned above. The consumption of alcohol and tobacco was measured by the same method that was used to measure the consumption of seafood; i.e., in terms of money spent.
The estimated numbers of patients with hypertension and dyslipidemia were obtained from the Patient Survey published by the Ministry of Health, Labour and Welfare of Japan [31]. This survey was performed once in three years among patients of medical care institutions nationwide who were selected by random stratified sampling. The estimated numbers of patients who received medical treatment in hospitals and general clinics on the dates surveyed were used. We divided the estimated numbers of patients by the population of the prefecture for each year and expressed the results as the average of 2005 survey and 2008 survey. The rates of participation in sports were obtained from the Survey on Time Use and Leisure Activities published by the Ministry of Internal Affairs and Communications of Japan [32]. The sample was selected through a two-stage stratified sampling method, where the primary sampling unit was the enumeration district (ED) of the Population Census, and the secondary sampling unit was the household. First, the whole country was divided into its 47 prefectures, and a total of 6700 sample EDs were selected. In the selected EDs, about 80,000 households were selected from lists of households prepared by enumerators before the survey. All persons aged 10 and over in the sample households were asked to respond to the survey. Enumerators deliver the questionnaires to each household to be surveyed, collect the completed questionnaires, and interview the households as necessary. The rate of participation in sports is derived from the total participation rate in sports. Sports include all kinds of athletic activity performed for leisure, but exclude sports performed by students as part of their educational exercises and by professional athletes as their work. This survey was performed once in five years, and we used the 2006 survey. Obesity was defined as a Body Mass Index of 25 or greater. The percentage of obesity was calculated using age-adjusted data in males from 20 to 69 years old and the data were averaged between 2006 and 2010. The rate of advancement to high school was obtained from the School Basic Survey published by the Ministry of Education, Culture, Sports, Science and Technology of Japan [33]. Other data are expressed as the average of the survey between 2005 and 2010. The raw data were divided by the population of the prefecture.
2.6. Statistical analysis
The statistical analysis was performed using SAS software, version 9.3 (SAS Institute, Cary, NC, USA) at Fukuoka University. We used a t-test for continuous variables and chi-squared tests for categorical variables. The Spearman Rank Correlation Coefficient was used to evaluate associations between groups. The values are expressed as the mean ± standard deviation (SD). Statistical significance was defined as a p-value of less than 0.05.
3. Results
3.1. Patient characteristics in the All-Japan Utstein Registry
There were 670,313 cases of OHCA in the All-Japan Utstein registry between 2005 and 2010, including 9641 cases who did not receive resuscitation. Table 1 shows the patient characteristics in the All-Japan Utstein Registry between 2005 and 2010, excluding 9641 in the no-resuscitation group: 660,672 cases of OHCA: 364,547 (55.2%) of cardiac origin and 296,125 (44.8%) of non-cardiac origin. Non-cardiac origin included cerebrovascular disease, respiratory disease, malignant tumor, and exogenous disease (10.7%, 13.0%, 7.4%, and 40.8%, respectively). Patients with OHCA of cardiac origin were significantly older, and had a lower percentage of male and a higher percentage of 1-month survival, cerebral performance category (CPC) 1 or 2, and overall performance category (OPC) 1 or 2. The initial rhythms in OHCA of cardiac origin were significantly more likely to be ventricular fibrillation (VF) and pulseless ventricular tachycardia (VT), and less likely to be pulseless electrical activity (PEA) and asystole.
3.2. Incidence of OHCA of cardiac and non-cardiac origin in the 47 prefectures of Japan
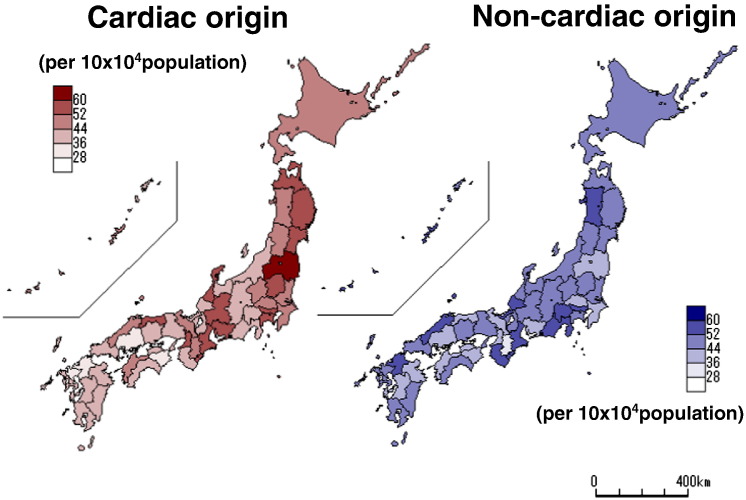
Fig. 1 shows the mean age-adjusted incidence of OHCA of cardiac and non-cardiac origin in the 47 prefectures of Japan between 2005 and 2010. Northern Japan tended to show a high incidence of cardiac arrest of both cardiac and non-cardiac origin.
Fig. 1.
Mean age-adjusted incidence of OHCA of cardiac origin (left panel) or non-cardiac origin (right panel) in the 47 prefectures of Japan between 2005 and 2010.
3.3. Time trends for the incidence of OHCA and total seafood consumption
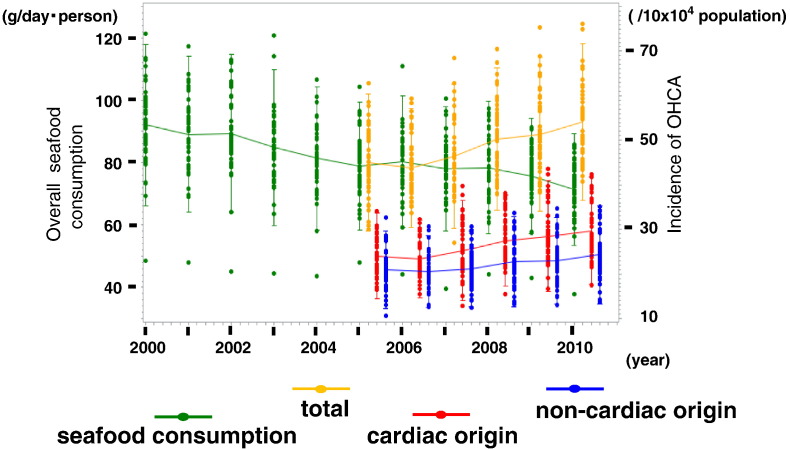
Fig. 2 shows the yearly changes in total seafood consumption and the incidence of OHCA (total, cardiac and non-cardiac origin) in the 47 prefectures of Japan from 2000 to 2010. Although the incidence of OHCA of both total and cardiac origin has been increasing since 2005, total seafood consumption has decreased yearly since 2000.
Fig. 2.
Yearly changes in overall seafood consumption and the incidence of OHCA in the 47 prefectures of Japan from 2000 to 2010.
Green line indicates the consumption of overall fish. Orange, red, and blue lines indicate the incidence of OHCA in all patients, cardiac and non-cardiac origin, respectively.
3.4. Correlations between the consumption of overall seafood, different kinds of seafood, and the incidence of OHCA of cardiac and non-cardiac origin in the 47 prefectures
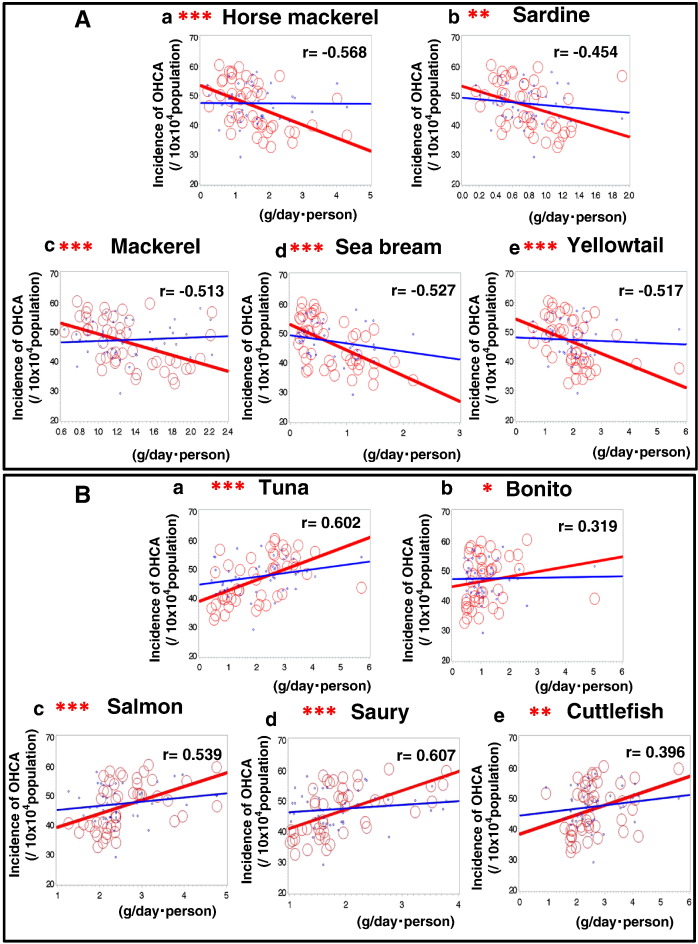
Northern and Western Japan tend to show a high consumption of seafood (data not tabulated). The overall consumption of raw fish (47 prefectures) (g/day/person) was not significantly correlated with the age-adjusted incidence of total OHCA (r = − 0.036, p = 0.81), the age-adjusted incidence of OHCA of cardiac origin (r = 0.132, p = 0.38), or the age-adjusted incidence of OHCA of non-cardiac origin (r = 0.075, p = 0.62) (data not tabulated). Fourteen kinds of seafood were included in the analysis. According to the Spearman Rank Correlation Coefficient between the consumption of each type of seafood and the age-adjusted incidence of OHCA, there were significantly different correlations (Table 2A). The consumption of horse mackerel (r = − 0.568, p < 0.0001), sardine (r = − 0.454, p = 0.001), mackerel (r = − 0.513, p = 0.0002), sea bream (r = − 0.527, p = 0.0001), and yellowtail (r = − 0.517, p = 0.0002) showed significant negative correlations with the incidence of OHCA of cardiac origin (Table 2A, Fig. 3-A). The consumption of tuna (r = 0.602, p < 0.0001), bonito (r = 0.319, p = 0.029), salmon (r = 0.539, p < 0.0001), saury (r = 0.607, p < 0.0001), and cuttlefish (r = 0.396, p = 0.006) showed significant positive correlations with the incidence of OHCA of cardiac origin (Table 2A, Fig. 3-B). The consumption of flounder (r = 0.026, p = 0.862), octopus (r = 0.390, p = 0.390), shrimp (r = − 0.258, p = 0.080), and crab (r = − 0.007, p = 0.966) showed no significant correlations with the incidence of OHCA of cardiac origin (Table 2A). The associations between the consumption of different kinds of seafood and salt consumption in the 47 prefectures of Japan are shown in Table 2B. Salt consumption was significantly and positively associated with the consumption of flounder (r = 0.388, p = 0.007), salmon (r = 0.359, p = 0.013), saury (r = 0.386, p = 0.007), and cuttlefish (r = 0.503, p = 0.0003), and negatively associated with the consumption of sea bream (r = − 0.386, p = 0.008).
Table 2A.
Associations between the consumption of kinds of seafood and the age-adjusted incidence of out-of-hospital cardiac arrest of cardiac origin.
| Consumption | r | p | |
|---|---|---|---|
| Tuna, g | 2.1 ± 1.2 | 0.602 | < 0.0001 |
| Horse mackerel, g | 1.5 ± 0.9 | − 0.568 | < 0.0001 |
| Sardine, g | 0.8 ± 0.4 | − 0.454 | 0.001 |
| Bonito, g | 1.2 ± 0.8 | 0.319 | 0.029 |
| Flounder, g | 1.3 ± 0.9 | 0.026 | 0.862 |
| Salmon, g | 2.6 ± 0.8 | 0.539 | < 0.0001 |
| Mackerel, g | 1.3 ± 0.4 | − 0.513 | 0.0002 |
| Saury, g | 1.9 ± 0.7 | 0.607 | < 0.0001 |
| Sea bream, g | 0.7 ± 0.5 | − 0.527 | 0.0001 |
| Yellowtail, g | 2.0 ± 0.9 | − 0.517 | 0.0002 |
| Cuttlefish, g | 2.6 ± 0.9 | 0.396 | 0.006 |
| Octopus, g | 0.7 ± 0.2 | 0.390 | 0.390 |
| Shrimp, g | 1.8 ± 0.4 | − 0.258 | 0.080 |
| Crab, g | 0.9 ± 0.7 | 0.007 | 0.966 |
Fig. 3.
A and B: Correlations between the age-adjusted incidence of OHCA and the consumption of different kinds of seafood in the 47 prefectures of Japan. Red lines and circles indicate the correlation to OHCA of cardiac origin. Blue lines and circles indicate the correlation to OHCA of non-cardiac origin.
*p < 0.05, **p < 0.01, ***p < 0.001.
Table 2B.
Associations between the consumption of kinds of seafood and salt consumption in the 47 prefectures of Japan.
| Consumption | r | p | |
|---|---|---|---|
| Tuna, g | 2.1 ± 1.2 | − 0.004 | 0.978 |
| Horse mackerel, g | 1.5 ± 0.9 | − 0.083 | 0.578 |
| Sardine, g | 0.8 ± 0.4 | − 0.14 | 0.345 |
| Bonito, g | 1.2 ± 0.8 | 0.002 | 0.991 |
| Flounder, g | 1.3 ± 0.9 | 0.388 | 0.007 |
| Salmon, g | 2.6 ± 0.8 | 0.359 | 0.013 |
| Mackerel, g | 1.3 ± 0.4 | − 0.042 | 0.781 |
| Saury, g | 1.9 ± 0.7 | 0.386 | 0.007 |
| Sea bream, g | 0.7 ± 0.5 | − 0.386 | 0.008 |
| Yellowtail, g | 2.0 ± 0.9 | − 0.116 | 0.436 |
| Cuttlefish, g | 2.6 ± 0.9 | 0.503 | 0.0003 |
| Octopus, g | 0.7 ± 0.2 | − 0.245 | 0.097 |
| Shrimp, g | 1.8 ± 0.4 | − 0.206 | 0.165 |
| Crab, g | 0.9 ± 0.7 | − 0.067 | 0.653 |
3.5. Associations between the consumption of fatty acids from 14 kinds of seafood and the incidence of OHCA of cardiac origin
Table 3 shows the associations between the consumption of fatty acids calculated from 14 kinds of seafood and the age-adjusted incidence of OHCA of cardiac origin. There were significant negative associations between the consumption of fatty acids including saturated fatty acid, palmitic acid, stearic acid, oleic acid, arachidonic acid, and eicosapentaenoic acid (EPA, trend of p = 0.052) and the age-adjusted incidence of OHCA of cardiac origin, while no associations were observed in alpha linolenic acid, docosahexaenoic acid (DHA) et al.
Table 3.
Associations between the consumption of fatty acids and the age-adjusted incidence of out-of-hospital cardiac arrest of cardiac origin.
| Fatty acid | r | p | |
|---|---|---|---|
| Lipid | 1.73 ± 0.27 | − 0.17 | 0.253 |
| Total fatty acid, g | 1.27 ± 0.20 | − 0.117 | 0.435 |
| Saturated fatty acids, g | 0.36 ± 0.06 | − 0.321 | 0.028 |
| Monounsaturated fatty acid, g | 0.55 ± 0.09 | 0.039 | 0.797 |
| Polyunsaturated fatty acid, g | 0.37 ± 0.06 | − 0.201 | 0.177 |
| Omega-3 fatty acid, g | 0.32 ± 0.05 | − 0.195 | 0.189 |
| Omega-6 fatty acid, g | 0.04 ± 0.01 | − 0.155 | 0.298 |
| Lauric acid, mg | 0.41 ± 0.08 | − 0.227 | 0.125 |
| Myristic acid, mg | 65.4 ± 11.3 | 0.087 | 0.561 |
| Palmitic acid, mg | 206.9 ± 36.2 | − 0.353 | 0.015 |
| Stearic acid, mg | 51.6 ± 9.7 | − 0.426 | 0.003 |
| Oleic acid, mg | 189.4 ± 32.1 | − 0.403 | 0.005 |
| Linoleic acid, mg | 21.2 ± 3.1 | − 0.086 | 0.566 |
| Alpha linolenic acid, mg | 12 ± 1.8 | − 0.059 | 0.693 |
| Gamma linolenic acid, mg | 1.3 ± 0.2 | − 0.117 | 0.434 |
| Arachidonic acid, mg | 12.4 ± 2.3 | − 0.384 | 0.008 |
| Eicosapentaenoic acid, mg | 88.3 ± 16.0 | − 0.286 | 0.052 |
| Docosahexaenoic acid, mg | 152.1 ± 23.0 | − 0.166 | 0.266 |
3.6. Correlations between the incidence of OHCA of cardiac origin and other risk factors in the 47 prefectures
Sex ratio, consumption of salt, alcohol and tobacco, age-adjusted prevalence of hypertension, or dyslipidemia, participation in sports, % obesity, and rate of advancement to high school were included in the analysis (Table 4). There were no significant correlations between the incidence of OHCA of cardiac origin by prefecture and sex ratio, consumption of salt, alcohol and tobacco, hypertension, dyslipidemia, participation in sports, or rate of advancement to high school, while the percentage of obesity tended (p = 0.064) to be associated with OHCA of cardiac origin.
Table 4.
Associations between risk factors and age-adjusted incidence of out-of-hospital cardiac arrest of cardiac origin.
| Risk factors | r | p | |
|---|---|---|---|
| Sex ratio, % | 58.0 | − 0.241 | 0.102 |
| Salt consumption, g | 2.1 ± 0.5 | − 0.123 | 0.411 |
| Alcohol consumption, yen | 38.2 ± 5.0 | 0.046 | 0.761 |
| Tobacco consumption, yen | 9.5 ± 2.0 | 0.122 | 0.414 |
| Age-adjusted prevalence of hypertensiona | 570.0 ± 77.0 | − 0.142 | 0.340 |
| Age-adjusted prevalence of dyslipidemiaa | 114.9 ± 23.3 | − 0.091 | 0.544 |
| Participation rate in sports, % | 63.5 | 0.108 | 0.470 |
| Percentage of obesity, % | 31.6 | 0.273 | 0.064 |
| Rate of advancement to high school, % | 98.0 | − 0.090 | 0.548 |
/10 × 104 person.
3.7. Trends in the consumption of different kinds of seafood
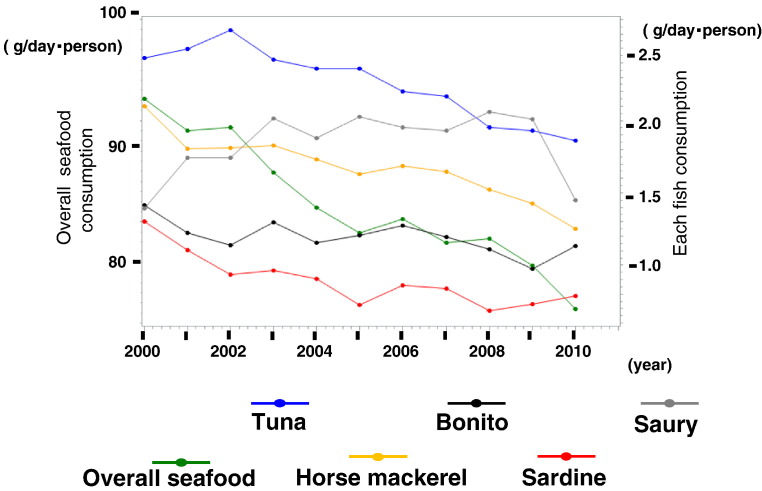
Yearly changes in the consumption of each seafood in the 47 prefectures of Japan from 2000 to 2010 are shown in Fig. 4. The consumption of many of the seafoods has decreased, while the consumption of saury has seemed to be increased.
Fig. 4.
Yearly changes in the consumption of each seafood in the 47 prefectures of Japan from 2000 to 2010. The trends in the consumption of most of the seafoods were negative. Green shows total seafood consumption, while the consumption of each type of fish is shown in blue (tuna), gray (saury), orange (horse mackerel), black (bonito), and red (sardine).
4. Discussion
The main finding in the present study was that the consumption of certain kinds of seafood, but not total seafood consumption per se, was significantly associated with the age-adjusted incidence of OHCA of cardiac origin, and the incidence of OHCA of cardiac origin increased from 2005 to 2010 and total seafood consumption decreased yearly from 2000, as shown in Fig. 2. Epidemiological data have shown unequivocally that an increased intake of fish/fish oil is associated with lower CVD morbidity and mortality 8, 9. In the JPHC study by Iso et al. [34], compared to modest fish intake, higher fish intake was associated with a substantially reduced risk of CAD and non-fatal cardiac events among middle-aged subjects. Thus, we and others have thought that total seafood consumption might be correlated with the incidence of OHCA of cardiac origin.
The incidence of OHCA of cardiac origin was positively associated with the consumption of tuna, salmon, saury, and cuttlefish, while negative correlations were found for the consumption of horse mackerel, sardines, mackerel, sea bream, and yellowtail, and these seafoods were not significantly associated with OHCA of non-cardiac origin. Thus, the kind of seafood consumed, rather than the total consumption of seafood, may be important for predicting the age-adjusted incidence of OHCA of cardiac origin. The yearly changes in the consumption of each seafood gradually decreased except saury, and the trends were similar to the yearly change in total fish consumption (Fig. 2, Fig. 4).
Horse mackerel and saury showed the highest negative and positive correlations with the age-adjusted incidence of OHCA of cardiac origin (Table 2A, Figs. 3A and B). Interestingly, although whole salt consumption was not significantly associated with the age-adjusted incidence of OHCA of cardiac origin, salt consumption was significantly and positively associated with the consumption of flounder (r = 0.388, p = 0.007), salmon (r = 0.359, p = 0.013), saury (r = 0.386, p = 0.007), and cuttlefish (r = 0.503, p = 0.0003), and significantly and negatively associated with the consumption of sea bream (r = − 0.383, p = 0.008) (Table 2B). These data suggest that the method used to cook seafood might affect the association with the incidence of OHCA of cardiac origin. In fact, Belin et al. [35] showed that, in a diverse population of postmenopausal women who were at increased risk for heart failure (HF), a higher intake of baked/broiled fish was associated with a lower risk for incident HF, whereas a higher intake of fried fish was linked to a higher risk for incident HF, which suggests that dietary modification with an increase in the consumption of baked/broiled fish, and/or a decrease in the consumption of fried fish, could play a role in these associations. Although saury showed a positive association and is rich in fatty acids, it is not mostly eaten raw but with salt in Japan. The effects of saury, salmon, and cuttlefish may be reduced by the cooking methods used.
The influence of mercury in seafood should also be considered. It has been reported that exposure to high levels of mercury might reduce the beneficial effects of n-3PUFA on sudden cardiac death [36]. The Japanese Ministry of Health, Labour and Welfare has advised pregnant women to avoid consuming seafood that contains a large amount of mercury. Tuna and bonito are predators that occupy positions high in the food chain, and may contain large amounts of mercury through bioaccumulation. Mercury concentrations in tuna are positively correlated with body size 37, 38. Differences between regions, which could be associated with different lifestyles and different harvests of seafood, may have also affected the results. Northern Japan tends to show a high incidence of OHCA of cardiac origin, and this could explain the positive association between the consumption of seafood, which was higher in northern Japan than in southern Japan, and OHCA of cardiac origin. Thus, we cannot exclude confounding factors.
In this study, we calculated the consumption of fatty acids in the 47 prefectures of Japan in terms of the fish that are mainly consumed. There were several significant negative associations between the consumption of fatty acids including saturated fatty acids, palmitic acid, stearic acid or arachidonic acid, and the age-adjusted incidence of OHCA of cardiac origin. Over the past decade, an inverse relationship has been observed between fish intake and fatal CVD or sudden cardiac death, which suggests that n-3 polyunsaturated fatty acids (e.g., [EPA] + [DHA]) may have an antiarrhythmic effect 10, 11, 14, in addition to any platelet aggregatory 39, 40, anti-atherosclerotic [41], anti-inflammatory [42], or vasodilatory effects [42]. In the JELIS study [43] in Japan, daily treatment with EPA together with statin was associated with a relative reduction in major coronary events. The DART [44], GISSI-Prevenzione (P) 10, 11 and GISSI-HF [45] studies have also shown that supplementation with n-3 polyunsaturated fatty acids has beneficial effects on cardiovascular outcomes in different patient populations. Our results cannot be compared to those of studies involving supplementation with fatty acids. In particular, the amount of fatty acids in our analysis was significantly lower than the dose used in supplementation, since fatty acid contents were simply calculated from the consumption of each kind of seafood, not from total intake of diet. In addition, the amount of fatty acids contained in seafood can change dramatically with preparation, and in our study we did not know how the different seafoods were cooked. Many nutrients such as proteins, calcium, vitamins A, B and D, iron and taurine should be considered together.
5. Limitations
Since this is an ecological study and the baseline characteristics in these surveys are different, we may need to consider an ecological fallacy, and thus the results may not be completely accurate, although the data from 660,672 individuals should be sufficient to discuss our hypothesis. The data on the average consumption of seafood, salt, alcohol, and tobacco in the 47 prefectures of Japan do not include single-person households and only included information for the prefectural capital. However, this information should reflect the characteristics of the prefectures. Furthermore, the results were estimated from data on consumer spending rather than from a dietary questionnaire. Therefore, salt consumption should be considered in the intake of other foods, such as when eating out. Other confounding factors, such as the climate or lifestyle in the 47 prefectures, should also be considered. Unfortunately, we could not stratify other risks. Although the amount of fatty acids varies according to the cooking style, in our data it is not clear how the seafood was cooked, and our study did not consider relations with other foods.
6. Conclusion
In Japan, the consumption of different kinds of seafood may be an important factor in OHCA of cardiac origin. Thus, dietary habits with regard to seafood may play a role in the incidence of OHCA of cardiac origin, however, the question of whether to eat fish in general or instead to eat certain kinds of fish is still unclear.
Disclosures
Disclosures (KS): KS has an Endowed Department of “Advanced Therapeutics for Cardiovascular Disease” supported by Boston Scientific Japan Co. LTD, Japan Medtronic Co. LTD, Japan Lifeline Co. LTD, Nihon Koden. Co. LTD, and St. Jude Medical Japan Co. LTD (these 5 companies relate to OHCA: devices and AED, et al), and the Department of Community and Emergency Medicine (supported by Izumi General Medical Center) which relates Emergency Medicine Supports by Fukuoka Prefecture. KS and MS are Directors of Nonprofit Organization Clinical and Applied Science, Fukuoka, Japan.
Footnotes
This work was supported by a grant-in-aid from the AIG Collaborative Research Institute of Cardiovascular Medicine (2011–2013), Fukuoka University 814-0180, Japan, the Baccalaureate Degree Program “Learning that Life is Precious” of Fukuoka University (2011–2013), and the JCS-ReSS group of the Japanese Circulation Society.
Available online 14 November 2013
References
- 1.Frantz I.D., Jr., Dawson E.A., Ashman P.L., Gatewood L.C., Bartsch G.E., Kuba K. Test of effect of lipid lowering by diet on cardiovascular risk. The Minnesota Coronary Survey. Arteriosclerosis. 1989;9:129–135. doi: 10.1161/01.atv.9.1.129. [DOI] [PubMed] [Google Scholar]
- 2.Miettinen M., Turpeinen O., Karvonen M.J., Elosuo R., Paavilainen E. Effect of cholesterol-lowering diet on mortality from coronary heart-disease and other causes. A twelve-year clinical trial in men and women. Lancet. 1972;2:835–838. doi: 10.1016/s0140-6736(72)92208-8. [DOI] [PubMed] [Google Scholar]
- 3.Low-fat diet in myocardial infarction: A controlled trial. Lancet. 1965;2:501–504. [PubMed] [Google Scholar]
- 4.Noda K., Zhang B., Iwata A., Nishikawa H., Ogawa M., Nomiyama T. Lifestyle changes through the use of delivered meals and dietary counseling in a single-blind study. The STYLIST study. Circ J. 2012;76:1335–1344. doi: 10.1253/circj.cj-12-0164. [DOI] [PubMed] [Google Scholar]
- 5.Kromhout D., Keys A., Aravanis C., Buzina R., Fidanza F., Giampaoli S. Food consumption patterns in the 1960s in seven countries. Am J Clin Nutr. 1989;49:889–894. doi: 10.1093/ajcn/49.5.889. [DOI] [PubMed] [Google Scholar]
- 6.Japanese Circulation Society Guidelines for the Primary Prevention of Ischemic Heart Disease Revised Version. 2006. http://www.j-circ.or.jp/guideline/pdf/JCS2006_kitabatake_h.pdf [accessed January 6th, 2013]
- 7.Ueshima H., Sekikawa A., Miura K., Turin T.C., Takashima N., Kita Y. Cardiovascular disease and risk factors in Asia: a selected review. Circulation. 2008;118:2702–2709. doi: 10.1161/CIRCULATIONAHA.108.790048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daviglus M.L., Stamler J., Orencia A.J., Dyer A.R., Liu K., Greenland P. Fish consumption and the 30-year risk of fatal myocardial infarction. N Engl J Med. 1997;336:1046–1053. doi: 10.1056/NEJM199704103361502. [DOI] [PubMed] [Google Scholar]
- 9.Hu F.B., Bronner L., Willett W.C., Stampfer M.J., Rexrode K.M., Albert C.M. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA. 2002;287:1815–1821. doi: 10.1001/jama.287.14.1815. [DOI] [PubMed] [Google Scholar]
- 10.GISSI-Prevenzione I. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 11.Marchioli R., Barzi F., Bomba E., Chieffo C., Di Gregorio D., Di Mascio R. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105:1897–1903. doi: 10.1161/01.cir.0000014682.14181.f2. [DOI] [PubMed] [Google Scholar]
- 12.Iso H., Sato S., Umemura U., Kudo M., Koike K., Kitamura A. Linoleic acid, other fatty acids, and the risk of stroke. Stroke. 2002;33:2086–2093. doi: 10.1161/01.str.0000023890.25066.50. [DOI] [PubMed] [Google Scholar]
- 13.Simon J.A., Fong J., Bernert J.T., Jr., Browner W.S. Serum fatty acids and the risk of stroke. Stroke. 1995;26:778–782. doi: 10.1161/01.str.26.5.778. [DOI] [PubMed] [Google Scholar]
- 14.Reiffel J.A., McDonald A. Antiarrhythmic effects of omega-3 fatty acids. Am J Cardiol. 2006;98:50i–60i. doi: 10.1016/j.amjcard.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 15.Catapano A.L., Reiner Z., De Backer G., Graham I., Taskinen M.R., Wiklund O. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Atherosclerosis. 2011;217(Suppl. 1):S1–S44. doi: 10.1016/j.atherosclerosis.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Smith S.C., Jr., Benjamin E.J., Bonow R.O., Braun L.T., Creager M.A., Franklin B.A. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association. J Am Coll Cardiol. 2011;58:2432–2446. doi: 10.1016/j.jacc.2011.10.824. [DOI] [PubMed] [Google Scholar]
- 17.Mosca L., Benjamin E.J., Berra K., Bezanson J.L., Dolor R.J., Lloyd-Jones D.M. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: a guideline from the American Heart Association. Circulation. 2011;123:1243–1262. doi: 10.1161/CIR.0b013e31820faaf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Japan Atherosclerosis Society. Japan Atherosclerosis Society (JAS) 2012. Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases. [PubMed] [Google Scholar]
- 19.Japanese Circulation Society Guidelines for Secondary Prevention of Myocardial Infarction. 2011. http://www.j-circ.or.jp/guideline/pdf/JCS2011_ogawah_h.pdf [accessed January 5th, 2013] [DOI] [PubMed]
- 20.Kitamura T., Iwami T., Kawamura T., Nagao K., Tanaka H., Hiraide A. Nationwide public-access defibrillation in Japan. N Engl J Med. 2010;362:994–1004. doi: 10.1056/NEJMoa0906644. [DOI] [PubMed] [Google Scholar]
- 21.Kitamura T., Iwami T., Kawamura T., Nitta M., Nagao K., Nonogi H. Nationwide improvements in survival from out-of-hospital cardiac arrest in Japan. Circulation. 2012;126:2834–2843. doi: 10.1161/CIRCULATIONAHA.112.109496. [DOI] [PubMed] [Google Scholar]
- 22.Iwami T., Kitamura T., Kawamura T., Mitamura H., Nagao K., Takayama M. Chest compression-only cardiopulmonary resuscitation for out-of-hospital cardiac arrest with public-access defibrillation: a nationwide cohort study. Circulation. 2012;126:2844–2851. doi: 10.1161/CIRCULATIONAHA.112.109504. [DOI] [PubMed] [Google Scholar]
- 23.Sasaki M., Iwami T., Kitamura T., Nomoto S., Nishiyama C., Sakai T. Incidence and outcome of out-of-hospital cardiac arrest with public-access defibrillation. A descriptive epidemiological study in a large urban community. Circ J. 2011;75:2821–2826. doi: 10.1253/circj.cj-11-0316. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi Y., Iwami T., Kitamura T., Nishiuchi T., Kajino K., Sakai T. Impact of early intravenous epinephrine administration on outcomes following out-of-hospital cardiac arrest. Circ J. 2012;76:1639–1645. doi: 10.1253/circj.cj-11-1433. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi N., Hata N., Shimura T., Yokoyama S., Shirakabe A., Shinada T. Characteristics of patients with cardiac arrest caused by coronary vasospasm. Circ J. 2013;77:673–678. doi: 10.1253/circj.cj-12-0846. [DOI] [PubMed] [Google Scholar]
- 26.Ministry of Internal Affairs and Communications 2005. http://www.stat.go.jp/data/kokusei/2005/index.htm [accessed January 5th, 2013]
- 27.Ministry of Internal Affairs and Communications 2006–2010. http://www.stat.go.jp/data/jinsui/ [accessed January 5th, 2013]
- 28.Ministry of Internal Affairs and Communications 2005–2010. http://www.stat.go.jp/data/kakei/index.htm [accessed January 5th, 2013]
- 29.Japan Diabetes Society . 2002. Food Substitution Table for Diabetes Mellitus Diet Therapy 6th Version of the Japan Diabetes Society. [in Japanese] [Google Scholar]
- 30.Ministry of Education C, Sports, Science of Japan 2005. http://www.mext.go.jp/b_menu/shingi/gijyutu/gijyutu3/toushin/05031802.htm [accessed January 5th, 2013]
- 31.Ministry of Health Labour and Welfare of Japan 2010. http://www.mhlw.go.jp/toukei/list/10-20.html [accessed January 5th, 2013]
- 32.Ministry of Internal Affairs and Communications 2010. http://www.stat.go.jp/data/shakai/2006/index.htm [accessed January 5th, 2013]
- 33.Ministry of Education C, Sports, Science of Japan 2010. http://www.mext.go.jp/b_menu/toukei/chousa01/kihon/1267995.htm [accessed January 5th, 2013]
- 34.Iso H., Kobayashi M., Ishihara J., Sasaki S., Okada K., Kita Y. Intake of fish and n3 fatty acids and risk of coronary heart disease among Japanese: the Japan Public Health Center-Based (JPHC) Study Cohort I. Circulation. 2006;113:195–202. doi: 10.1161/CIRCULATIONAHA.105.581355. [DOI] [PubMed] [Google Scholar]
- 35.Belin R.J., Greenland P., Martin L., Oberman A., Tinker L., Robinson J. Fish intake and the risk of incident heart failure: the Women's Health Initiative. Circ Heart Fail. 2011;4:404–413. doi: 10.1161/CIRCHEARTFAILURE.110.960450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Virtanen J.K., Laukkanen J.A., Mursu J., Voutilainen S., Tuomainen T.P. Serum long-chain n-3 polyunsaturated fatty acids, mercury, and risk of sudden cardiac death in men: a prospective population-based study. PLoS One. 2012;7:e41046. doi: 10.1371/journal.pone.0041046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Storelli M.M., Marcotrigiano G.O. Mercury speciation and relationship between mercury and selenium in liver of Galeus melastomus from the Mediterranean sea. Bull Environ Contam Toxicol. 2002;69:516–522. doi: 10.1007/s00128-002-0092-8. [DOI] [PubMed] [Google Scholar]
- 38.Nakata H., Shimada H., Yoshimoto M., Narumi R., Akimoto K., Yamashita T. Concentrations and distribution of mercury and other heavy metals in surface sediments of the Yatsushiro Sea including Minamata Bay. Japan. Bull Environ Contam Toxicol. 2008;80:78–84. doi: 10.1007/s00128-007-9320-6. [DOI] [PubMed] [Google Scholar]
- 39.Hirai A., Terano T., Hamazaki T., Sajiki J., Kondo S., Ozawa A. The effects of the oral administration of fish oil concentrate on the release and the metabolism of [14C]arachidonic acid and [14C]eicosapentaenoic acid by human platelets. Thromb Res. 1982;28:285–298. doi: 10.1016/0049-3848(82)90112-8. [DOI] [PubMed] [Google Scholar]
- 40.Tamura Y., Hirai A., Terano T., Takenaga M., Saitoh H., Tahara K. Clinical and epidemiological studies of eicosapentaenoic acid (EPA) in Japan. Prog Lipid Res. 1986;25:461–466. doi: 10.1016/0163-7827(86)90092-5. [DOI] [PubMed] [Google Scholar]
- 41.Thies F., Garry J.M., Yaqoob P., Rerkasem K., Williams J., Shearman C.P. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial. Lancet. 2003;361:477–485. doi: 10.1016/S0140-6736(03)12468-3. [DOI] [PubMed] [Google Scholar]
- 42.Cawood A.L., Ding R., Napper F.L., Young R.H., Williams J.A., Ward M.J. Eicosapentaenoic acid (EPA) from highly concentrated n-3 fatty acid ethyl esters is incorporated into advanced atherosclerotic plaques and higher plaque EPA is associated with decreased plaque inflammation and increased stability. Atherosclerosis. 2010;212:252–259. doi: 10.1016/j.atherosclerosis.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 43.Yokoyama M., Origasa H., Matsuzaki M., Matsuzawa Y., Saito Y., Ishikawa Y. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 44.Burr M.L., Fehily A.M., Gilbert J.F., Rogers S., Holliday R.M., Sweetnam P.M. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART) Lancet. 1989;2:757–761. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- 45.Tavazzi L., Maggioni A.P., Marchioli R., Barlera S., Franzosi M.G., Latini R. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]