Abstract
Background
Fibrosis after myocardial damage can be determined by cardiac magnetic resonance (CMR) with late gadolinium enhancement (LGE). We studied whether ventricular LGE is visible in the ventricles of pediatric and adolescent TOF (tetralogy of Fallot) patients by measuring LGE and investigating whether fibrosis correlated with right ventricular volume, pulmonary regurgitation, N-terminal pro-brain natriuretic peptide (NT-proBNP) or the aminoterminal propeptide of type III procollagen (PIIINP). We also studied if the patient's age, post-operative follow-up time or surgical history would affect LGE.
Methods
A total of 40 pediatric patients who had undergone TOF repair and 43 healthy age and gender matched controls underwent a CMR study, whereby LGE was scored in the right (RV) and the left ventricle. To exclude the possible iatrogenic scarring we calculated the LGE score by excluding the right ventricular outflow tract and VSD patch region.
Results
All patients had RV LGE and in 39 of 40 it was seen also outside the surgically affected areas. The amount of LGE correlated positively with the RV end-diastolic volume (r = 0.44, P = 0.0045), pulmonary regurgitation (r = 0.40, P = 0.013), and with NT-proBNP. The presence of LGE also depended on post-operative follow-up time (r = 0.53, P = 0.006). PIIINP levels of TOF patients were significantly higher than in the control subjects but it did not correlate with LGE or with any of the studied clinical markers.
Conclusions
LGE is present globally in the right ventricular muscle in children and adolescents with TOF. The longer the follow-up time the more common was the LGE in the right ventricle.
Keywords: Tetralogy of Fallot, Pediatric cardiology, Late gadolinium enhancement, Cardiac magnetic resonance, Right ventricular volume, Pulmonary regurgitation
1. Introduction
Congenital heart defect is present in 0.5–1% of newborn infants and 3–5% of the patients are born with tetralogy of Fallot (TOF) 1, 2, 3, 4. The outcome of these patients has improved dramatically during the last decades but the long-term prognosis is still compromised by important chronic sequelae like right ventricular (RV) scarring, dysfunction, dilation and arrhythmias leading to higher morbidity and mortality than in their peers 4, 5. Surgical correction of right ventricular outflow tract (RVOT) obstruction often results in pulmonary regurgitation causing chronic volume loading, and eventually RV dilatation and deterioration of its function 6, 7, 8, 9, 10. The pathophysiology behind these hemodynamic conditions has remained elusive.
Late gadolinium enhancement (LGE) is a method where cardiovascular magnetic resonance (CMR) images are obtained after the administration of gadolinium contrast material that accumulates into a tissue with increased extra cellular space. This method is suggestive of fibrosis in both the left and right ventricles 11, 12. In previous studies the importance of RVOT fibrosis detected by MRI LGE has been highlighted in adult and adolescent series of patients 13, 14. In a patient cohort of 9–67 year old individuals with TOF, regional RV dysfunction was associated with LGE in many segments of myocardium [15]. Another recent study demonstrated a relation of fragmented QRS complex to RV LGE [16]. However, little is known about LGE in pediatric TOF patients.
The goal of this study was to establish whether LGE was present in children and adolescents with surgically repaired TOF. Our special interest was to study those myocardial segments not directly injured and scarred by the corrective surgery. We studied if RV end-diastolic volume (EDV ml/m2), ejection fraction (EF) or pulmonary regurgitation (PR) measured by CMR would correlate with LGE. We also studied whether LGE depended on surgical method of repair, post-operative follow-up time or patient age. In addition, we studied whether LGE correlated with plasma N-terminal pro-brain natriuretic peptide (NT-proBNP), a well-established marker for heart failure 17, 18, 19, found elevated with increased RV volume and pulmonary regurgitation in adult and adolescent series of patients 20, 21, 22. Finally, since LGE has previously been demonstrated in processes with increasing fibrosis 13, 14, 15, 24 we studied whether aminoterminal propeptide of the type III procollagen (PIIINP), a biochemical marker of this fibrillar collagen turnover, would correlate with right ventricular LGE, its end diastolic volume, or the extent of the pulmonary regurgitation.
2. Methods
Forty-five patients who had undergone surgical repair of TOF between 1990 and 2003 were recruited to the study. 39 patients arrived from the Helsinki University Hospital district, serving a population base of 1.7 million. The inclusion criteria were suspicion of a severe pulmonary regurgitation in echocardiography. Six additional patients arrived from other hospitals referred by their own pediatric cardiologist according to the same criteria. Three patients declined from the study and two patients were rejected because of insufficient MRI image quality. Accordingly, forty patients were admitted to the study and were examined during an annual ambulatory visit to the clinic. For control subjects, 43 healthy age and gender matched pediatric and adolescent volunteers were recruited around the Helsinki area. They were accepted to the study if they had no medical history of any cardiovascular disease and no other pre-existing condition that would affect the cardiovascular system. No subjects had any other known conditions associated with elevated levels of plasma concentrations of PIIINP such as pulmonary fibrosis, chronic liver disease or renal dysfunction. All of the patients and control subjects underwent a clinical examination by the pediatric cardiologist. The study protocol included an echocardiogram, a CMR study, and a blood sample, from each of the subjects.
The Ethics Committee of the Children's Hospital of the Helsinki University Central Hospital approved the study. A written informed consent was obtained from all the participants and/or their parents.
CMR images were obtained with a 1.5 T Philips Achieva imager (Philips Healthcare, The Netherlands) and a 5 channel cardiac coil. For right-ventricular-volume analysis, transaxial and left ventricle (LV) short axis balanced turbo field echo (B TFE) breath-hold cine images were obtained throughout the whole heart. Slice thickness was 5–8 mm depending on the patient size, gap was 20% and temporal resolution was 26–43 ms. For RV end diastolic volume measurements, both the transaxial and the LV short axis cine images were manually planimetered with the cardiac analysis tool of a Philips ViewForum workstation (Philips Healthcare, The Netherlands). Pulmonary arterial flow and regurgitation were assessed using a through plane phase contrast measurement. The phase contrast plane was positioned perpendicular to the pulmonary artery flow one 1 cm above the valve. A retrospectively gated free breathing acquisition with three averages lasting typically for one minute was obtained with a temporal resolution of 24 ms and VENC 200. The field of view was kept as small as possible for optimal spatial resolution and slice thickness = 10 mm, TR = 4.019, TE = 2.427, and ET = 4. The flow images were analyzed with the workstation provided by the MR scanner vendor. A radiologist experienced in CMR performed all CMR analysis.
For LGE analysis, transaxial and sagittal late enhancement images were obtained from both ventricles after 10 min of Gd-DOTA, Gadoterate meglumine 279.3 mg/ml (Dotarem®) 0.2 ml/kg injection. A gradient echo sequence with TR = 3.239 ms, TE = 1.628 ms and ET = 49 was used. Slice thickness was 7 mm with no gap. The inversion time (TI) was determined with the use of TI-scout sequence and varied between 260 and 360 ms.
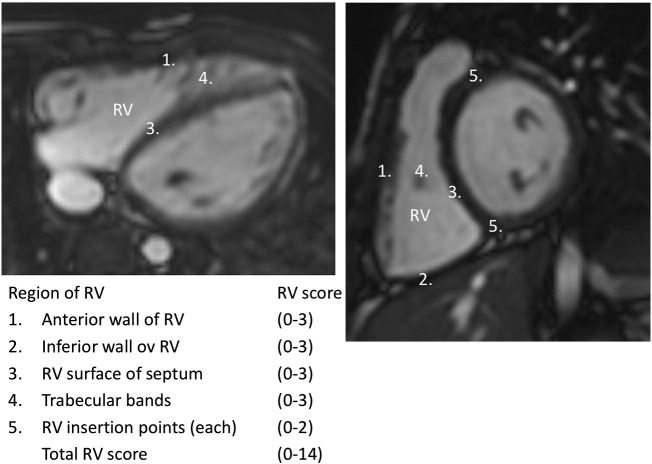
RV LGE grading protocol with seven RV segments including the target cites of repair served as a basis for our grading method [13]. For the present work we performed scoring of LGE similarly to this earlier protocol, but adapted the method by omitting 2 surgically manipulated segments (VSD patch region and the anterior wall of RVOT) to quantitate remote LGE within the RVs. Accordingly, 5 remote segments were used in the analysis: anterior wall of RV, inferior wall of RV, RV surface of septum, trabecular bands and RV insertion points. The late enhancement images were visually assessed and each segment was graded according to the linear extent of enhanced myocardium. The protocol is described in detail in Fig. 1. Meticulous care was taken to confirm RV LGE from different views. The radiologist was blinded to the clinical status and other findings of the patients. Typical late enhancement findings are shown in Fig. 2, Fig. 3. For the LV LGE scoring, a standard 17-segment model was used [23].
Fig. 1.
Five-step segmental late gadolinium enhancement (LGE) grading protocol: in the first three segments measuring right ventricular (RV) free wall and the interventricular septum the scoring was done as follows: 0 points = no enhancement, 1 point < 2 cm, 2 points = 2–3 cm and 3 points = > 3 cm in length. RV trabeculations were scored as 0 = no enhancement, 1 = enhancement of one trabeculation, 2 = enhancement of 2–4 trabeculations and 3 = enhancement of more than 4 trabeculations. RV-left ventricular insertion points were scored either 0 or 1 for absence or presence of enhancements.
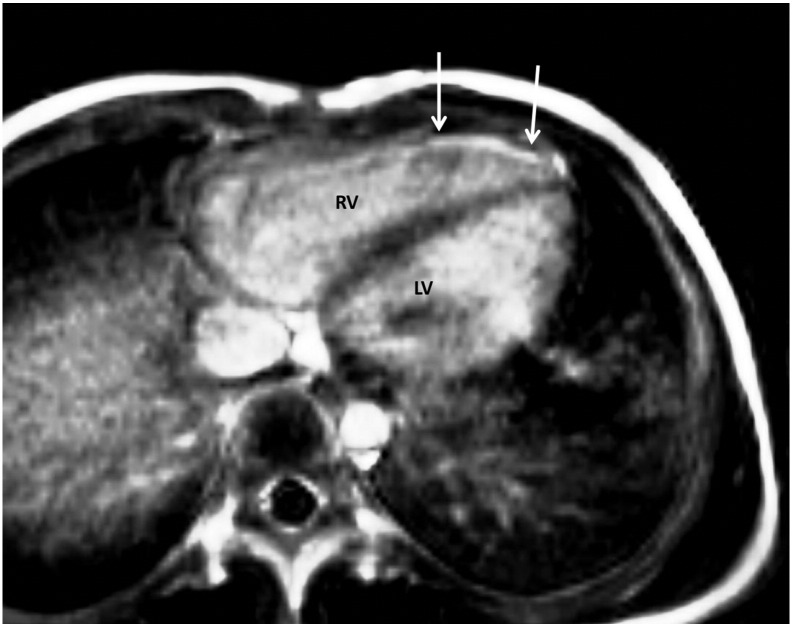
Fig. 2.
Transaxial late gadolinium enhancement (LGE) image of a 14-year-old girl with tetralogy of Fallot (TOF). The arrows indicate anterior right ventricular (RV) wall enhancements 10 min after gadolinium-injection.
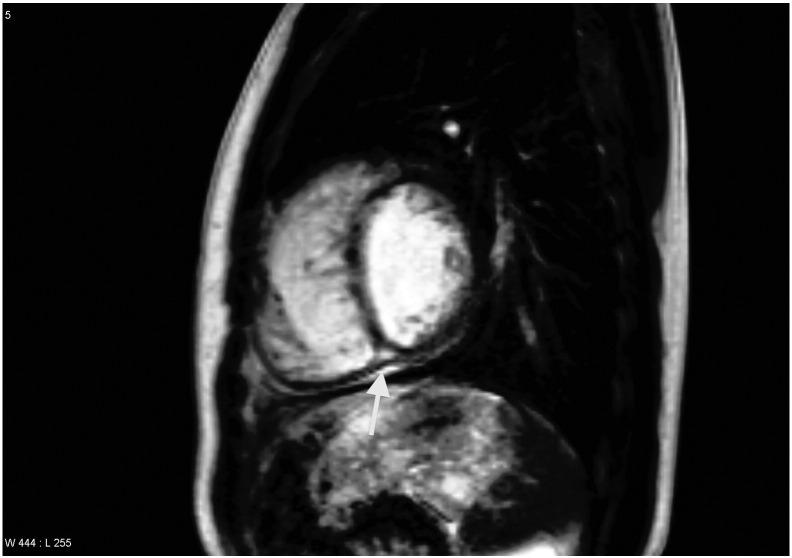
Fig. 3.
Sagittal late gadolinium enhancement (LGE) image of a 15 year old boy with tetralogy of Fallot (TOF). The arrow indicates posterior ventricular junction enhancement 10 min after gadolinium-injection.
The radiologist blinded to the primary results later repeated scoring of 23 patients' LGE images. A good correlation (R2 = 0.77) was found between the two assessments. Bland–Altman method was used to study intra-observer variability and found a difference in average score of 0.47. Difference of the individual scores was between − 1 and + 1 in 95% of the measurements.
Blood samples from a peripheral vein were drawn from all the subjects into vials with an appropriate anticoagulant agent followed by plasma separation by centrifugation. NT-proBNP was measured from the plasma by immunochemiluminometric assay at the biochemical laboratory immediately after the sample was taken. Another part of the separated plasma was stored at − 70 °C for later analysis, from which plasma levels of PIIINP were measured using a commercially available radioimmunological method (Orion Diagnostica UniQ PIIINP RIA, Orion Corporation Orion Diagnostica, Espoo, Finland). All analyses were performed in duplicate, and the average was used as a single datum. The analytic data of the control patients demonstrated that circulating PIIINP did not change between 7 and 19 years of age. Accordingly, no age-dependent correction was needed to the analytic data on PIIINP.
Statistical analysis was performed using GraphPad Prism 5.0a (GraphPad Software, Inc. San Diego, California, USA). All results are reported as a mean ± SD. Comparisons of continuous data between the groups were performed using an unpaired t test. Correlations were calculated by using linear regression. A two-tailed P-value of less than 0.05 was considered statistically significant. Intra-observer variability was tested by using Spearman's test and Bland–Altman method.
3. Results
The average age of our TOF patients was 13.1 ± 3.3 (range 7.7–18.7 years). The mean post-operative follow-up time of our patients was 11.8 ± 2.9 years from the corrective surgical procedure (range 7.2–18.4). All patients were of NYHA class I–II and all control patients were of NYHA class I. A total of 16 (40%) of the patients had undergone palliative procedure in the early childhood before the corrective surgery. Transannular patch (TAP) was used in 23 (58%) patients. All the corrective operations were done on cardiopulmonary bypass with aortic crossclamp. The myocardium was protected with cold blood cardioplegia. During the follow-up time a reoperation was done to 6 (15%) of our patients. Table 1.summarizes the baseline characteristics of TOF patients and healthy controls. There were no time-related trends in surgical techniques and all methods were used constantly during the operative period from 1990 to 2003.
Table 1.
Characteristics of the study population.
| Patients | Controls | P value | |
|---|---|---|---|
| N | 40 | 43 | |
| Male gender, n (%) | 25 (63) | 27 (63) | ns. |
| Mean age (years) | 13.1 ± 3.2 | 14.1 ± 3.4 | ns. |
| Age range (years) | 8.2–18.9 | 7.7–19.3 | ns. |
| Weight (kg) | 43.0 ± 14.6 | 50.9 ± 15.9 | ns. |
| Height (cm) | 153 ± 14.9 | 160 ± 16.0 | ns. |
| Body surface area (m2) | 1.3 ± 0.3 | 1.5 ± 0.3 | ns. |
| Age at TOF repair (years) | 1.4 ± 1.2 | – |
Visible RV LGE was present in all of our patients when all segments of RV where studied. Our interest was to explore whether RV LGE could be seen as remote enhancements outside the surgical scars. Therefore, we performed also a LGE score calculation by excluding enhanced areas of the right ventricular outflow tract (RVOT) and the ventricular septal defect (VSD) patch. This remote LGE was found in 39 out of 40 of our patients. Both LV and RV LGE were absent in the control subjects.
Remote LGE was frequently found in all 5 remote segments of RV. Most commonly it was found in the anterior wall (33 of 40 patients) followed by RV insertion points (27/40). In three other segments it was found as follows: the inferior wall of RV in 16, the RV surface of septum in 15 and in the trabecular bands in 9 patients out of 40.
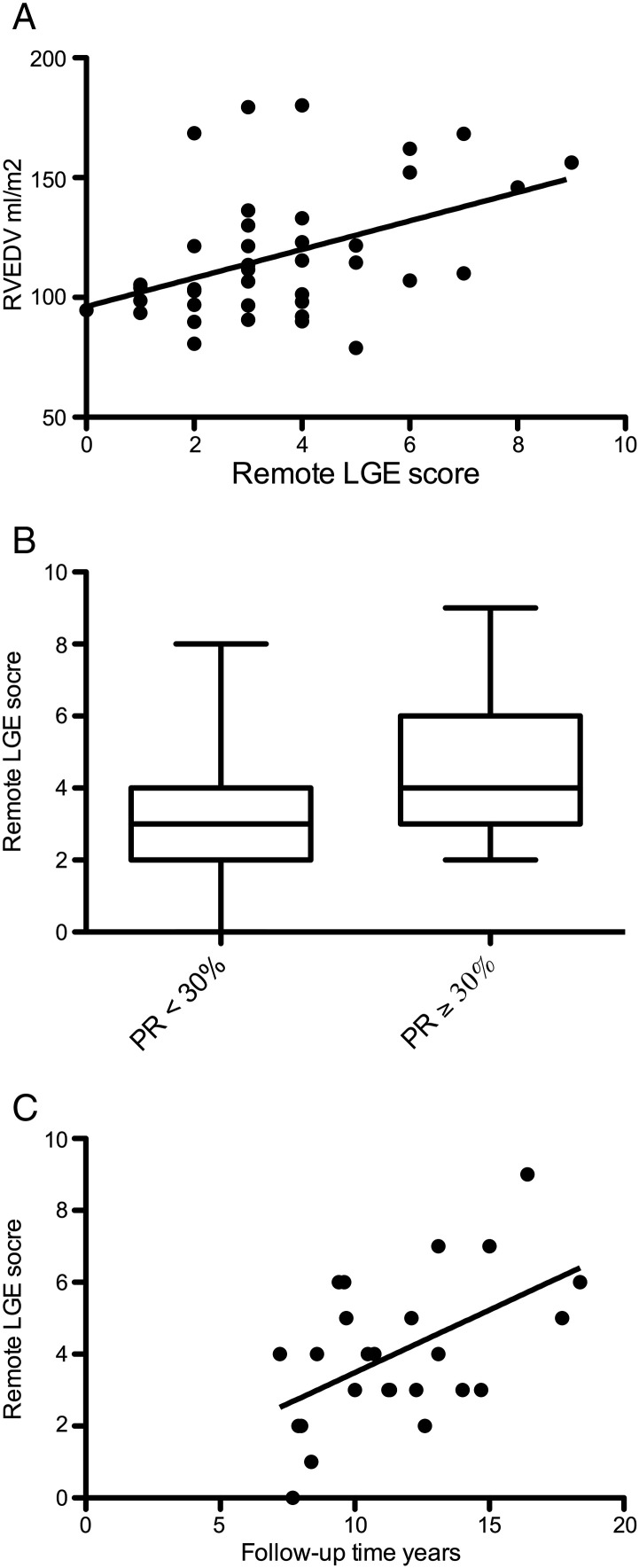
We found a significant positive correlation between remote RV LGE and RV end-diastolic volume (Fig. 4A). The remote LGE score was significantly higher in patients with severe PR when compared to those without (Fig. 4B). The amount of pulmonary regurgitation also correlated positively with RV EDV (r = 0.40, P = 0.01).
Fig. 4.
A correlation between right ventricular end diastolic volume (RV EDV) and remote late gadolinium enhancement (LGE) score (r = 0.44, P = 0.0045, n = 40).
B: remote late gadolinium enhancement (LGE) score of patients with severe pulmonary regurgitation (PR) (≥ 30%) when compared to patients with PR < 30% (P = 0.008, n = 40).
C: correlation between post-operative follow-up time and remote late gadolinium enhancement (LGE) score (r = 0.53, P = 0.006, n = 25). Only patients with ≥ 20% pulmonary regurgitation were included to this analysis.
Since remote LGE was related to pulmonary regurgitation and right ventricular dilation, we studied if post-operative follow-up time would affect LGE. In patients with moderate (> 20%) or severe (≥ 30%) pulmonary regurgitation a linear regression analysis demonstrated a correlation between postoperative follow-up time and remote LGE (r = 0.53, P = 0.006, Fig. 4C). There was a suggestive negative trend between RV EF and LGE score but it did not reach statistical significance.
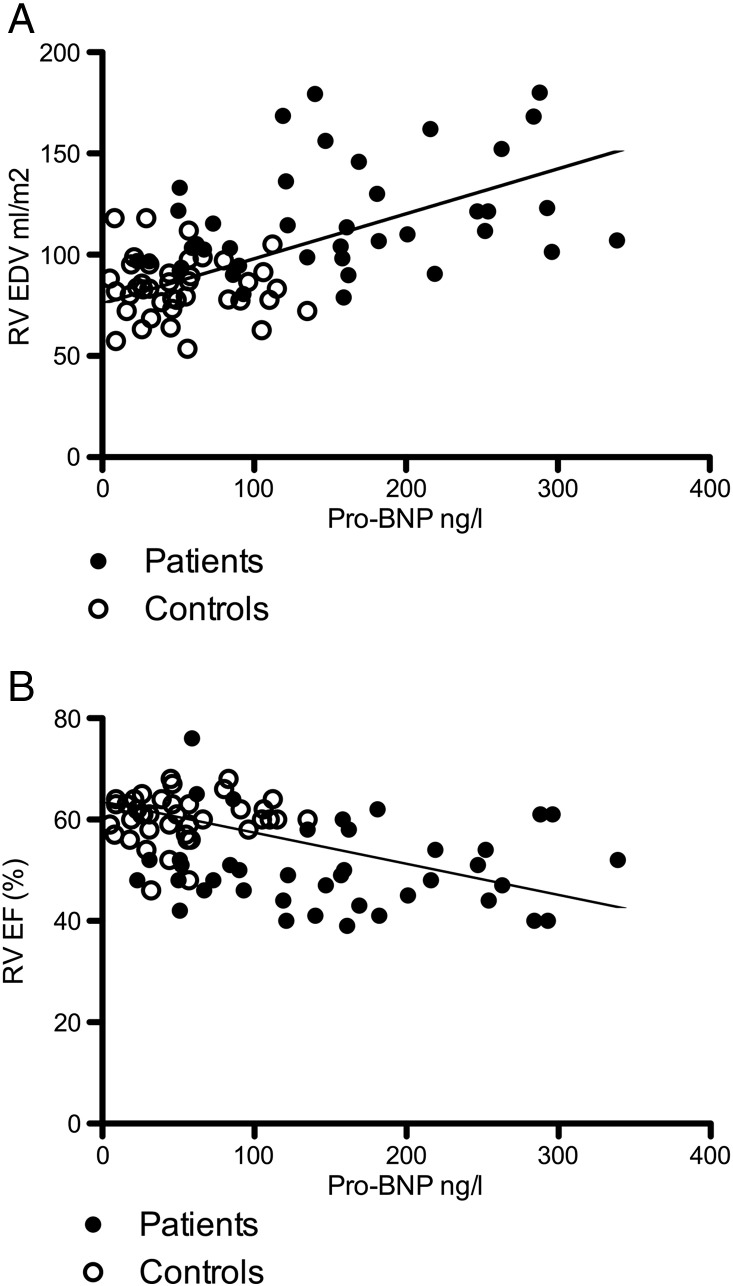
We then studied whether the levels of two circulating biochemical markers available for diagnostics and follow-up of myocardial disease, namely the NT-proBNP and PIIINP, would parallel RV fibrosis or RV size and function. The levels of NT-proBNP were clearly elevated in our patient group as shown in Table 2. A linear regression analysis demonstrated a correlation between remote LGE score and NT-proBNP levels (r = 0.38, P = 0.015). In addition, after pooling the data from the control subjects and patients, significant positive correlation existed between NT-proBNP and RV EDV and a negative correlation between NT-proBNP and RV EF (Fig. 5A and B).
Table 2.
Right and left ventricular size and function, plasma levels of N-terminal pro-brain natriuretic peptide (NT-proBNP) and pro-collagen III (PIIINP) of 40 pediatric patients with tetralogy of Fallot (TOF) and their age-matched controls.
| Patients | Controls | P value | |
|---|---|---|---|
| Number of subjects | 40 | 43 | |
| RV EDV ml/m2 | 115 ± 26.5 | 83.6 ± 14.8 | < 0.0001 |
| RV EF (%) | 50.1 ± 8.6 | 60.0 ± 4.8 | < 0.0001 |
| Pulmonary regurgitation (%) | 28.5 ± 17.7 | 0.5 ± 2.50 | < 0.0001 |
| LV EDV ml/m2 | 75.3 ± 15.1 | 80.9 ± 13.7 | ns. |
| LV EF (%) | 61.6 ± 8.6 | 63.0 ± 4.8 | ns. |
| Aortic regurgitation (%) | 1.3 ± 2.9 | 0 | |
| NT-proBNP levels (ng/l) | 147 ± 82 | 53 ± 36 | < 0.0001 |
| Plasma PIIINP levels (μg/l) | 12.3 ± 4.9 | 9.1 ± 3.9 | 0.0009 |
Fig. 5.
A correlation between N-terminal pro-brain natriuretic peptide (NT-proBNP) and right ventricular end diastolic volume (r = 0.54, P < 0.0001, n = 83).
B: correlation between N-terminal pro-brain natriuretic peptide (NT-proBNP) and right ventricular ejection fraction (r = − 0.41, P = 0.0002, n = 83).
Comparison between the patients and control subjects demonstrated that the plasma levels of PIIINP were significantly higher in TOF-patients. However, there was no correlation between PIIINP and CMR-findings or other clinical and biochemical data.
4. Discussion
An important finding of our study was that the RV LGE was present in children with surgically repaired TOF and these LGE changes were more abundant in patients as the right ventricle dilation, age, and follow-up time progressed.
Another new finding of this study was that RV myocardial LGE was found in remote areas of RV of these young patients. We propose that pulmonary regurgitation, significantly present in our patients, and previously demonstrated as an important determinant of the abnormal loading physiology seen in tetralogy of Fallot, might be an important factor in causing these global findings within the RV-myocardium. However, since LGE was present even outside the areas surgically manipulated during RVOT-resection and VSD patching, non-specific factors, including the extent of pre-operative cyanosis or perioperative or post-operative ischemic insults, might have enhanced the pathophysiological mechanism leading to RV LGE and dilation. In addition, myocardial LGE might also be at least partly a result of fetal programming of the extra cellular matrix framework, which has previously been described to differ in RV muscular wall of neonatal patients with hypoplastic left heart syndrome and truncus arteriosus [24].
LGE has become a marker of fibrotic, scarred or otherwise abnormal myocardium. It has been previously studied in both ischemic and non-ischemic heart disease, and CMR LGE has contributed to understanding LV dysfunction and the pathophysiology behind in these diseases 12, 25, 26, 27. Correlation between LGE and myocardial fibrosis has also been validated in earlier histological studies 28, 29. In adult patients with TOF, RV LGE has correlated with adverse clinical markers such as ventricular dysfunction and arrhythmias [13]. Accordingly, parallel to these previously published data we suggest that the adverse hemodynamic loading conditions induced by the post-operative anatomic and hemodynamic conditions are deleterious to the myocardium. In future investigations, it would be interesting to study whether, in the chronic stage of TOF, RV LGE is different in patients with restrictive RV-physiology with attenuated RV-remodeling [30].
LV LGE was not found in any of our patients. No LV venting was done in any of the patients during the TOF repair. In adult patients with TOF, LGE findings vary: in one study where patients were operated on during adult life, LV LGE was found in 53% of the patients [13]. In another study, no significant LV LGE was encountered in a cohort of 9–67 year old individuals after repair of TOF [15]. Differences in surgical era and the surgical approach, including the omittance of left ventricle venting, could explain these differences. Since our patients were children and adolescents, it is also possible that LV LGE develops later in life.
Consistent with previous observations, our present findings demonstrated an increased amount of plasma PIIINP in patients with TOF compared to their age-matched control population. However, PIIINP did not show correlation with LGE, which generally is anticipated to derive at least for some extent from increased fibrosis within the myocardium. In light of the recent literature we feel that this is not surprising since the extracardiac synthesis of fibrillar collagens is significant [31]. In addition, extracardiac turnover of collagens varies not only in noncardiac diseases but also in cardiac disease associated with abnormal loading conditions [32]. These observations may somewhat decrease attractiveness of the circulating collagen-derived peptides as diagnostic and follow-up tools in clinical cardiology [33].
Our study demonstrated a correlation between NT-proBNP and LGE. In parallel with previously published studies in somewhat older patients with TOF 20, 21, 22 we found significant correlations between NT-proBNP and RV EDV, pulmonary regurgitation, and RV function. Thus, our present findings add weight on the hypothesis that gradual dilation of RV after TOF-repair provokes fibrosis within the myocardium, which can be detected by RV LGE.
5. Conclusion
We conclude that RV LGE is present in children after repair of tetralogy of Fallot and it is found also outside the surgically injured areas. The amount of LGE correlates with the severity of PR and RV dilation. Our data on serum NT-proBNP suggests that RV LGE reflects right ventricular dilation. We propose that the study of LGE might provide valuable additional information in decision-making for the timing of the operative treatment.
6. Study limitations
Our study was cross-sectional, and a longer follow-up with repeated clinical, radiological and biochemical assessments would be needed to confirm whether the right ventricle fibrosis in children after TOF repair is progressive. Only one marker of fibrous collagen turnover was used. However, inclusion of type I collagen derived PICP and PINP was not considered due to the potential confounding factors due to the ubiquitous content of type I collagen within the parenchymal organs, skin and the skeleton.
Acknowledgments
We acknowledge the skilful work of research nurse TiinaJärvinen, X-ray technicians Inka-Mari Granlund, MarikaMyöhänen, NelliVaris, and laboratory technician TaruJokinen.
Footnotes
None of the authors declare any conflicts of interests. This study was supported by the Finnish Governmental Subsidy for health Sciences, the Finnish Cardiac Foundation, and the Academy of Finland (O.M.P.). The sponsors had no involvement in any of the following: study design, the collection, analysis or interpretation of data, the writing of the report or the decision to submit the paper for publication.
Available online 12 February 2014
References
- 1.Hoffman J.I., Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell S.C., Korones S.B., Berendes H.W. Congenital heart disease in 56,109 births. Incidence and natural history. Circulation. 1971;43(3):323–332. doi: 10.1161/01.cir.43.3.323. [DOI] [PubMed] [Google Scholar]
- 3.Moons P., Sluysmans T., De Wolf D. Congenital heart disease in 111 225 births in Belgium: birth prevalence, treatment and survival in the 21st century. Acta Paediatr. 2009;98(3):472–477. doi: 10.1111/j.1651-2227.2008.01152.x. [DOI] [PubMed] [Google Scholar]
- 4.Nieminen H.P., Jokinen E.V., Sairanen H.I. Late results of pediatric cardiac surgery in Finland: a population-based study with 96% follow-up. Circulation. 2001;104(5):570–575. doi: 10.1161/hc3101.093968. [DOI] [PubMed] [Google Scholar]
- 5.Murphy J.G., Gersh B.J., Mair D.D. Long-term outcome in patients undergoing surgical repair of tetralogy of Fallot. N Engl J Med. 1993;329(9):593–599. doi: 10.1056/NEJM199308263290901. [DOI] [PubMed] [Google Scholar]
- 6.D'Andrea A., Caso P., Sarubbi B. Right ventricular myocardial dysfunction in adult patients late after repair of tetralogy of fallot. Int J Cardiol. 2004;94(2–3):213–220. doi: 10.1016/j.ijcard.2003.04.033. [DOI] [PubMed] [Google Scholar]
- 7.Gatzoulis M.A., Balaji S., Webber S.A. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet. 2000;356(9234):975–981. doi: 10.1016/S0140-6736(00)02714-8. [DOI] [PubMed] [Google Scholar]
- 8.Geva T., Sandweiss B.M., Gauvreau K., Lock J.E., Powell A.J. Factors associated with impaired clinical status in long-term survivors of tetralogy of Fallot repair evaluated by magnetic resonance imaging. J Am Coll Cardiol. 2004;43(6):1068–1074. doi: 10.1016/j.jacc.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 9.Redington A.N., Oldershaw P.J., Shinebourne E.A., Rigby M.L. A new technique for the assessment of pulmonary regurgitation and its application to the assessment of right ventricular function before and after repair of tetralogy of Fallot. Br Heart J. 1988;60(1):57–65. doi: 10.1136/hrt.60.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Therrien J., Marx G.R., Gatzoulis M.A. Late problems in tetralogy of Fallot-recognition, management, and prevention. Cardiol Clin. 2002;20(3):395–404. doi: 10.1016/s0733-8651(02)00010-3. [DOI] [PubMed] [Google Scholar]
- 11.Babu-Narayan S.V., Goktekin O., Moon J.C. Late gadolinium enhancement cardiovascular magnetic resonance of the systemic right ventricle in adults with previous atrial redirection surgery for transposition of the great arteries. Circulation. 2005;111(16):2091–2098. doi: 10.1161/01.CIR.0000162463.61626.3B. [DOI] [PubMed] [Google Scholar]
- 12.Kim R.J., Wu E., Rafael A. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343(20):1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 13.Babu-Narayan S.V., Kilner P.J., Li W. Ventricular fibrosis suggested by cardiovascular magnetic resonance in adults with repaired tetralogy of fallot and its relationship to adverse markers of clinical outcome. Circulation. 2006;113(3):405–413. doi: 10.1161/CIRCULATIONAHA.105.548727. [DOI] [PubMed] [Google Scholar]
- 14.Oosterhof T., Mulder B.J., Vliegen H.W., de Roos A. Corrected tetralogy of Fallot: delayed enhancement in right ventricular outflow tract. Radiology. 2005;237(3):868–871. doi: 10.1148/radiol.2373041324. [DOI] [PubMed] [Google Scholar]
- 15.Wald R.M., Haber I., Wald R., Valente A.M., Powell A.J., Geva T. Effects of regional dysfunction and late gadolinium enhancement on global right ventricular function and exercise capacity in patients with repaired tetralogy of Fallot. Circulation. 2009;119(10):1370–1377. doi: 10.1161/CIRCULATIONAHA.108.816546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park S.J., On Y.K., Kim J.S. Relation of fragmented QRS complex to right ventricular fibrosis detected by late gadolinium enhancement cardiac magnetic resonance in adults with repaired tetralogy of fallot. Am J Cardiol. 2012;109(1):110–115. doi: 10.1016/j.amjcard.2011.07.070. [DOI] [PubMed] [Google Scholar]
- 17.Maisel A.S., Krishnaswamy P., Nowak R.M. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347(3):161–167. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 18.Doust J.A., Pietrzak E., Dobson A., Glasziou P. How well does B-type natriuretic peptide predict death and cardiac events in patients with heart failure: systematic review. BMJ. 2005;330(7492):625. doi: 10.1136/bmj.330.7492.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kruger S., Graf J., Kunz D., Stickel T., Hanrath P., Janssens U. brain natriuretic peptide levels predict functional capacity in patients with chronic heart failure. J Am Coll Cardiol. 2002;40(4):718–722. doi: 10.1016/s0735-1097(02)02032-6. [DOI] [PubMed] [Google Scholar]
- 20.Festa P., Ait-Ali L., Prontera C. Amino-terminal fragment of pro-brain natriuretic hormone identifies functional impairment and right ventricular overload in operated tetralogy of Fallot patients. Pediatr Cardiol. 2007;28(5):339–345. doi: 10.1007/s00246-007-0009-8. [DOI] [PubMed] [Google Scholar]
- 21.Cheung E.W., Lam W.W., Chiu C.S., Chau A.K., Cheung S.C., Cheung Y.F. Plasma brain natriuretic peptide levels, right ventricular volume overload and exercise capacity in adolescents after surgical repair of tetralogy of Fallot. Int J Cardiol. 2007;121(2):155–162. doi: 10.1016/j.ijcard.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 22.Oosterhof T., Tulevski I.I., Vliegen H.W., Spijkerboer A.M., Mulder B.J. Effects of volume and/or pressure overload secondary to congenital heart disease (tetralogy of fallot or pulmonary stenosis) on right ventricular function using cardiovascular magnetic resonance and B-type natriuretic peptide levels. Am J Cardiol. 2006;97(7):1051–1055. doi: 10.1016/j.amjcard.2005.10.047. [DOI] [PubMed] [Google Scholar]
- 23.Cerqueira M.D., Weissman N.J., Dilsizian V. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105(4):539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 24.Davies B., d'Udekem Y., Ukoumunne O.C., Algar E.M., Newgreen D.F., Brizard C.P. Differences in extra-cellular matrix and myocyte homeostasis between the neonatal right ventricle in hypoplastic left heart syndrome and truncus arteriosus. Eur J Cardiothorac Surg. 2008;34(4):738–744. doi: 10.1016/j.ejcts.2008.06.037. [DOI] [PubMed] [Google Scholar]
- 25.Choudhury L., Mahrholdt H., Wagner A. Myocardial scarring in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002;40(12):2156–2164. doi: 10.1016/s0735-1097(02)02602-5. [DOI] [PubMed] [Google Scholar]
- 26.McCrohon J.A., Moon J.C., Prasad S.K. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108(1):54–59. doi: 10.1161/01.CIR.0000078641.19365.4C. [DOI] [PubMed] [Google Scholar]
- 27.Simonetti O.P., Kim R.J., Fieno D.S. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218(1):215–223. doi: 10.1148/radiology.218.1.r01ja50215. [DOI] [PubMed] [Google Scholar]
- 28.Maceira A.M., Joshi J., Prasad S.K. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2005;111(2):186–193. doi: 10.1161/01.CIR.0000152819.97857.9D. [DOI] [PubMed] [Google Scholar]
- 29.Moon J.C., Reed E., Sheppard M.N. The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2004;43(12):2260–2264. doi: 10.1016/j.jacc.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 30.Gatzoulis M.A., Clark A.L., Cullen S., Newman C.G., Redington A.N. Right ventricular diastolic function 15 to 35 years after repair of tetralogy of Fallot. Restrictive physiology predicts superior exercise performance. Circulation. 1995;91(6):1775–1781. doi: 10.1161/01.cir.91.6.1775. [DOI] [PubMed] [Google Scholar]
- 31.Risteli J., Risteli L. Analysing connective tissue metabolites in human serum. Biochemical, physiological and methodological aspects. J Hepatol. 1995;22(2 Suppl.):77–81. [PubMed] [Google Scholar]
- 32.Lopez B., Gonzalez A., Diez J. Circulating biomarkers of collagen metabolism in cardiac diseases. Circulation. 2010;121(14):1645–1654. doi: 10.1161/CIRCULATIONAHA.109.912774. [DOI] [PubMed] [Google Scholar]
- 33.Kupari M., Laine M., Turto H., Lommi J., Werkkala K. Circulating collagen metabolites, myocardial fibrosis and heart failure in aortic valve stenosis. J Heart Valve Dis. 2013;22(2):166–176. [PubMed] [Google Scholar]