Abstract
Background
Deficiency of 25-hydroxy vitamin D [25(OH)D] is a treatable condition that has been associated with coronary artery disease and many of its risk factors. A practical time to assess for 25(OH)D deficiency, and to initiate treatment, is at the time of an acute myocardial infarction(AMI). The prevalence of 25(OH)D deficiency and the characteristics associated with it in patients with acute myocardial infarction are unknown.
Methods
In this study 25(OH)D was assessed in 314 subjects enrolled in a Sri Jayadeva Institute of Cardiovascular Science and Research(SJICS&R). Patients enrolled from December 1, 2011 to February 28, 2012 had serum samples sent to a centralized laboratory for analysis using the ELECYS assay. Normal 25(OH)D levels are ≥ 30 ng/ml, and patients with levels < 30 and > 20 ng/ml were classified as insufficient and those with levels ≤ 20 ng/ml as deficient. Vitamin D and other baseline characteristics were analyzed with T-test and chi-squared test.
Results
Of the 314 enrolled patents, 212 (67.5%) were 25(OH)D deficient and 50(16%) were insufficient, for a total of 83.5% of patients with abnormally low 25(OH)D levels. No significant heterogeneity was observed among age or gender sub groups but 25(OH)D deficiency was more commonly seen in those with lower socioeconomic status, lower activity levels, diabetes, hypercholesterolemia(LDL), hypertriglyceridemia and in smokers.
Conclusion
Vitamin D deficiency is present in most of the patients with acute myocardial infarction and it is associated with many of its risk factors in our study.
Abbreviations: AMI, Acute myocardial infarction; RAS, Renin angiotensin system; CAD, Coronary artery disease; SJICS&R, Sri Jayadeva Institute of Cardiovascular Science and Research
Keywords: Inflammation, Diabetes, Hypercholesterolemia
1. Introduction
Vitamin D deficiency is highly prevalent worldwide [1], and is also noted to be high in India 2, 3. Low levels of 25(OH)D the principle circulating storage form of vitamin D, is present in as many as one third to one half of otherwise healthy middle aged to elderly population 1, 4, 5, 6. Limited cutaneous synthesis due to inadequate sun exposure or pigmented skin and inadequate dietary intake are the principle causes of low 25(OH)D levels.
Although the best characterised sequelae of vitamin D deficiency involve musculoskeletal system, growing evidence suggests that vitamin D axis affects vascular smooth muscle cell proliferation [6], endothelium [7] cardiomyocytes 1, 8 inflammation, vascular calcification, renin–angiotensin system (RAS) 9, 10, blood pressure and LVH 10, 11, 12 all of which affect the risk of cardiovascular diseases and myocardial infarction. However, the prevalence of vitamin D deficiency as well as the characteristics associated with it in patients presenting with acute myocardial infarction is unknown.
This is a prospective study undertaken at SJICS&R, Bangalore, India to describe the prevalence of vitamin D deficiency at the time of admission for acute myocardial infarction.
2. Methods
We evaluated the subjects enrolled in our study. We collected information of the patients admitted for AMI through chart abstraction, detailed patient interviews, and serum samples at SJICS&R from December 1, 2011 to February 28, 2012.
Inclusion criteria for the study are, age ≥ 20 years, biomarker evidence of myocardial injury (elevated troponins or CKMB), supporting evidence of AMI (eg., prolonged ischemic signs or symptoms, electrocardiographic ST-segment changes), and patients presenting within 24 h of symptoms onset. The study sample included 314 patients. Those patients with prevalent cardiovascular diseases and kidney diseases (serum creatinine > 1.6) were not included in the cohort. All the participants provided written informed consent. Detailed medical history examination and laboratory assessment of vascular risk factors were performed. patient data included demographics, age, gender, marital status, and education. Collected clinical variables included smoking, alcohol consumption, hypertension, diabetes mellitus, hypercholesterolemia, peripheral arterial disease, previous stroke, family history of coronary artery disease (CAD), chronic lung disease, chronic renal failure, chronic heart failure, previous angina, previous PTCA, and CABG. Hypertension was defined as systemic BP ≥ 140 mm of hg, diastolic BP ≥ 90 mm of hg, or use of anti hypertensive therapy [13]. Criteria for diabetes mellitus were fasting glucose ≥ 126 mg/dl or use of insulin or hypoglycaemic medications [14]. Current smoking denoted regular use of cigarettes in the preceding year. Hypercholesterolemia is defined as total cholesterol > 200 mg % and LDL cholesterol > 100 mg% [15]. Hypertriglyceridemia is defined as TGL > 150 mg.
The serum samples were obtained as soon as the patient got admitted to the hospital and before initiating medication (treatment). Data collected included, complete hemogram, random blood sugar, glycosylated hemoglobin, renal function tests, lipid profile, cardiac enzymes, troponins, CKMB, calcium, phosphorous and 25 (OH)D levels. The serum 25(OH)D was determined by ECLIA from Elecsys Analyser. We classified the patients as per Table 1 16, 17, 18.
Table 1.
Vitamin D status.
| Serum 25 (OH)D (ng/ml) | Vitamin D status |
|---|---|
| ≤ 20 | Deficient |
| 21–29 | Insufficient |
| ≥ 3 0 | Sufficient |
An Elecsys vitamin D total assay is intended for quantitative determination of the total 25-hydroxy vitamin D in human serum and plasma. The electrochemiluminescence immunoassay “ECLIA” is intended for use in Elecsys and cobas e immunoassay analysers. Levels of 25 (OH)D and other baseline characteristics were analyzed using T-test and chi-squared test.
3. Results
During the enrolment period of December 1, 2011 to February 28, 2012, 314 patients admitted to SJICS&R Hospital consented to baseline blood work and had 25 (OH)D levels assessed via ECLIA method.
The mean age for the total population in deficient group was 54.09 ± 12.26 years and for insufficient and sufficient groups was 52.23 ± 12.13 years (Table 2). 82.48% of the enrolled subjects were men and 17.5% were women (Table 2).
Table 2.
Univariate association with vitamin D deficiency and insufficiency or sufficiency.
| Variable | 25 (OH)D (ng/ml) |
P value | |
|---|---|---|---|
| ≤ 20 |
> 20 |
||
| (n = 202) | (n = 112) | ||
| Age | 54.09 ± 12.26 | 52.23 ± 12.13 | 0.198 |
| Men | 157 (78%) | 102 (91%) | |
| Women | 45 (22%) | 10 (9%) | |
| Smoking | 108 (53%) | 46 (41%) | 0.035 |
| Alcohol | 3 (1%) | 4 (4%) | 0.230 |
| Diabetes mellitus | 101 (50%) | 16 (14%) | 0.002 |
| Hyper tension | 71 (35%) | 35 (31%) | 0.484 |
| Total cholesterol (high) | 146 (72%) | 53 (47%) | 0.006 |
| HDL (low) | 116 (57%) | 55 (45%) | 0.052 |
| LDL (high) | 146 (72%) | 73 (65%) | 0.190 |
| TGL (high) | 109 (54%) | 48 (43%) | 0.059 |
Data are expressed as mean ± SD or as number (percentage). Continuous variables were compared using T-tests. Categorical variables were compared using chi-square test.
No significant heterogeneity was observed among age or gender sub groups, but vitamin D deficiency was more commonly seen in patients with low social support and lower activity level and diabetes (Table 2). Vitamin D deficiency was also noted in patients with hypercholesterolemia, hypertriglyceridemia and smokers with AMI (Table 2). At baseline 212 subjects (67.5%) were found to have 25(OH)D levels ≤ 20 ng/ml, which is in the deficient range. Another 50 subjects (16%) were in the insufficient range, with vitamin D levels of 20 to 30 ng/ml. Majority of the subjects (83.5%) were in the suboptimal range of 25(OH)D (normal ≥ 30 ng/ml). (Fig 1)
Fig. 1.
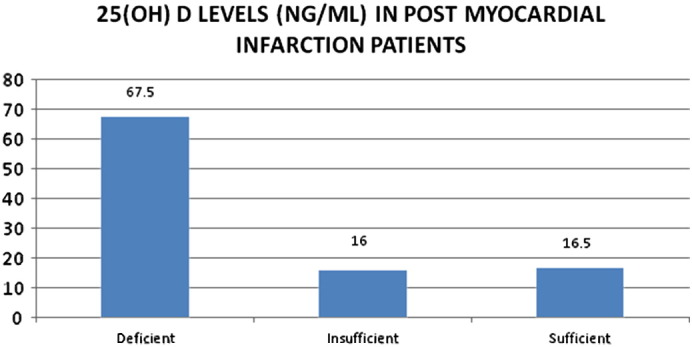
Vitamin D levels in post-AMI patients.
4. Discussion
This study evaluated vitamin D status in patients admitted for AMI at SJICS&R—a tertiary care centre. Our data suggested that there is high prevalence of vitamin D deficiency or insufficiency (83.5%) in AMI patients. This data is consistent with data associating CAD and many of its risk factors with 25(OH)D deficiency 16, 19. Studies have shown that individuals with vitamin D deficiency were at higher risk of ischemic heart disease 20, 21, 22. There is a significant association between osteoporosis and vascular calcification and vitamin D levels are inversely related to vascular calcification which in turn may affect AMI risk [23].
Our data also noted that there is more vitamin D deficiency in patients who are having diabetes mellitus, confirming previously described associations [24]. Vitamin D deficiency with dietary calcium deficiency has been associated with impaired fasting glucose and possibly type 2 diabetes mellitus which is a risk factor for cardiovascular disease 25, 26 which possibly explains the increased prevalence of vitamin D deficiency in our diabetic subgroup.
Animal studies have shown that vitamin D regulates RAS by suppressing renin gene expression, deficiency leading to increased renin production and hypertension 27, 12, though in our study hypertension was equally distributed between the two groups.
Skin pigmentation has a vital role in vitamin D production. Darker skin individuals produce less vitamin D with same ultraviolet B radiation as compared to fairer skin [28]. This explains the higher prevalence of vitamin D deficiency in our cohort as all the study subjects were Indians with dark skin.
Patients with high cholesterol high triglyceride levels and who smoke are high risk factors for cardiovascular disease and these are associated with vitamin D deficiency and myocardial infarction.
Other observations made from the study were lower socioeconomic status patients and sedentary life style were other risk factors for vitamin D deficiency. Low vitamin D levels in lower socioeconomic status patients may be because of poor dietary intake of nutritional supplements and sedentary patients may have less sun exposure for adequate 25(OH)D production.
Vitamin D can adversely affect many aspects of health, especially musculoskeletal and immunological function. Although there are no randomised trials to explain that normalising vitamin D levels will improve cardiovascular health and prognosis, it is reasonable to screen AMI patients for vitamin D deficiency and to correct deficient levels according to nationally established consensus guidelines to optimize overall health.
Limitations of the study include small number of study group done especially during the early part of the year. The study was not conducted throughout the year, so that we are unable to comment on the seasonal variation and its influence on the vitamin D levels.
Another limitation of the study is lack of an adequate control group with normal vitamin D levels. Because of large proportion of patients who were vitamin D deficient or insufficient, we did not have an adequate group for comparison.
5. Conclusions
Vitamin D deficiency is present in most of the patients with acute myocardial infarction and it is associated with many of its risk factors in our study. Prospective studies are needed to investigate benefits of screening and treatment of this very common vitamin deficiency for prevention of cardiovascular diseases.
Footnotes
Available online 19 March 2014
References
- 1.Holick M.F. Prevalence of vitamin D inadequacy and implication for health. Mayo Clin Proc. 2006;81:355–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 2.Harinarayan C.V., Ramalakshmi T., Venkataprasad u. High prevalence of low dietary calcium and low vitamin D status in healthy Indians. Asia Pac J Clin Nutr. 2004;13(4):359–364. [PubMed] [Google Scholar]
- 3.Goswami R., Gupta N., Goswami D., Marwaha R.K., Tandon N., Kochupillai N. Prevalence and significance of low 25(OH)D concentration in healthy subjects in Delhi. Am J Clin Nutr. 2000;72:472–475. doi: 10.1093/ajcn/72.2.472. [DOI] [PubMed] [Google Scholar]
- 4.Malabanan A., veronikis I.E., Holick M.F. Redefining vit D insufficiency. Lancet. 1998;351:805–806. doi: 10.1016/s0140-6736(05)78933-9. [DOI] [PubMed] [Google Scholar]
- 5.Chapuy M.C., Preziosi P., Maamer M., Arnald S., Galan P., Herebergs S., Mernier P.J. Prevalence of vit D insufficiency in an adult normal population. Osteoporas Int. 1997;7:439–443. doi: 10.1007/s001980050030. [DOI] [PubMed] [Google Scholar]
- 6.Merke J., Hoffman W., Goldsmidt D., Ritz E. Demonstration of 1,25(OH)2 vit D3 receptors and actions in vascular smooth mussel cells in vitro. Calcif Tissue Int. 1987;41:112–114. doi: 10.1007/BF02555253. [DOI] [PubMed] [Google Scholar]
- 7.Merk J., Milde P., Lewicka S., Hugel U., Klaus G., Mangelsdorf D.J., Haussler M.R., Ranterberg E.W., Ritz E. Identification and regulation of 1,25(OH)2D3 receptors activity and biosynthesis of 1,25(OH)2D3; studies in cultured bovine aortic endothelial cells and human dermal capillaries. J Clin Invest. 1989;83:1903–1915. doi: 10.1172/JCI114097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Connell T.U., Berry J.E., Jarris A.K., Simpson R.U. 1,25,(OH)2D3 regulation of cardiac myocyte proliferation and hypertrophy. Am J Physiol. 1997;272:H1751–H1758. doi: 10.1152/ajpheart.1997.272.4.H1751. [DOI] [PubMed] [Google Scholar]
- 9.Sigmund C.D., Okuyama K., Ingelfinger J., Jones C.A., Mullins J.J., Kane C. Isolation and characterization of renin-expressing cell lines from transgenic mice containing a renin-promoter viral oncogene fusion construct. J Biol Chem. 1990 Nov 15;265(32):19916–19922. [PubMed] [Google Scholar]
- 10.Li Y.C., Kong J., Wei M., Chen Z.F., Liu S.Q., Cao L.P. 1,25-Dihydroxy vitamin D(3) is a negative endocrine regulator of the renin–angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu J., Garami M., Cao L., Li Q., Gardner D.G. 1,25(OH)2D3 suppresses expression and secretion of ANP from cardiac myocytes. Am J Physiol. 1995;268(pt1):E1108–E1113. doi: 10.1152/ajpendo.1995.268.6.E1108. [DOI] [PubMed] [Google Scholar]
- 12.Xiang W., Kong J., Chen S., Cao L.P., Qiao G., Zheng W. Cardiac hypertrophy in vit D receptor knockout mice: role of the systemic and cardiac renin–angiotensin systems. Am J Physiol Endocrinol Metab. 2005;288:E125–E132. doi: 10.1152/ajpendo.00224.2004. [DOI] [PubMed] [Google Scholar]
- 13.Chobamian A.V., Ballin G.l., Black Hr, GreenLa, Jl Izzo, Jone D.W., Matron Wright J.T., Roccella E.J. The seventh report of the Joint Nation Committee on prevention, detection, evaluation and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 14.Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 15.NIH publication No. 01-3670; may 2001. Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation and treatment of high blood cholesterol in adults (Adult treatment panel III) [PubMed] [Google Scholar]
- 16.Lee J.H., O'Keefe J.H., Bell D., Hensrudd D.D., Holick M.F. Vitamin D deficiency: an important, common and easily treatable cardiovascular risk factor. J Am Coll Cardiol. 2008;52:1949–1956. doi: 10.1016/j.jacc.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 17.Bischoff-Ferrari H.A., Gioranncci E., Willet W.C., Dawson hughes B. Estimation of optimal serum concentrations of 25-hydroxy vitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 18.Chapuy M.C., Preziosi P., Maaner M., Arnaud S., Galan P., Hercberg S. Prevalence of vitamin D deficiency in an adult normal population. Osteoporos Int. 1997;7:439–443. doi: 10.1007/s001980050030. [DOI] [PubMed] [Google Scholar]
- 19.Carl J., Lavic M.D., John H., Lee M.D., Richard V., Milani M.D. Vitamin D and cardiovascular disease. JACC. 2011;58(15):1547–1556. doi: 10.1016/j.jacc.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Lund B., Badskjaer J., Lund B., Soerensen O.H. Vitamin D and ischemic heart disease. Horm Metab Res. 1978;10(6):553–556. doi: 10.1055/s-0028-1093390. [DOI] [PubMed] [Google Scholar]
- 21.Scragg R., Jackson R., Holdaway I.M., Lim T., Beaglehole R. Myocardial infarction is inversely associated with plasma 25-hydroxy vitamin D3 levels: a community based study. Int J Epidemiol. 1990;19(3):559–563. doi: 10.1093/ije/19.3.559. [DOI] [PubMed] [Google Scholar]
- 22.Vik B., Try K., Thelle D.S. Forde OH Tromso Heart study; Vitamin D metabolism and myocardial infarction. Br Med J. 1979;2(6183):176. doi: 10.1136/bmj.2.6183.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watson K.E., Abrolat M.L., Malone L.L., Hoeg J.M., Doherty T., Detrano R. Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation. 1997;96(6):1755–1760. doi: 10.1161/01.cir.96.6.1755. [DOI] [PubMed] [Google Scholar]
- 24.Cigolini M., Iagulli M.P., Miconi V., Galiotto M., Lombardi S., Targher G. Serum 25-hydroxy vitamin D3 concentrations and prevalence of cardiovascular diseases among type 2 diabetic patients. Diabetes Care. 2006;29:722–724. doi: 10.2337/diacare.29.03.06.dc05-2148. [DOI] [PubMed] [Google Scholar]
- 25.Pittas A.G., Harris H.S., Stark P.C., Dawson-Hughes B. The effect of calcium and vitamin D supplementation on blood glucose and markers of inflammation in non diabetic adults. Diabetes Care. 2007;30(4):980–986. doi: 10.2337/dc06-1994. [DOI] [PubMed] [Google Scholar]
- 26.Maghbooli Z., Hossein-Nezhad A., Karimi F., Shafaei A.R., Larijani B. Correlation between vitamin D3 deficiency and insulin resistance in pregnancy. Diabetes Metab Res Rev. 2008;24(1):27–32. doi: 10.1002/dmrr.737. [DOI] [PubMed] [Google Scholar]
- 27.Li Y.C., Qiao G., Uskokovic M., Xiang W., Zheng W., Kong J. Vitamin D: a negative endocrine regulator of the renin angiotensin system and blood pressure. J Steroid Biochem Mol Biol. 2004;89–90(1–5):387–392. doi: 10.1016/j.jsbmb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Matsuoka L.Y., Wortsman J., Hadda J.G., Kolm P., Hollis B.W. Racial pigmentation and the cutaneous synthesis of vitamin D. Arch Dermatol. 1991;127:536–538. [PubMed] [Google Scholar]


