Abstract
Background
There is no evidence from randomized trials for the benefit of routine non-compliant balloon (NCB) post-dilation after stent deployment. Despite being the gold standard, intravascular ultrasound is infrequently performed due to time and cost constraints and a suitable alternative technology is required for routine assessment of stent expansion. The purpose of this study was to assess the contribution of NCB post-dilation in optimizing contemporary stents by using digital stent enhancement (DSE).
Methods
We treated 120 patients with stent insertion and assessed the stents with DSE before and after NCB use. Optimal expansion was defined as the minimum stent diameter (MSD) ≥ 90% of the nominal stent diameter, an adaptation of the MUSIC and POSTIT trial criteria. Stent deployment was performed at 12 atm pressure followed by routine NCB post-dilation at ≥ 14 atm.
Results
The mean reference diameter on QCA was 2.75 mm (SD 0.63) and mean stent diameter was 3.15 mm (SD 0.46). At a mean stent deployment pressure of 11.7 atm (SD 2.4), only 21% of stents were optimally expanded. After NCB inflation at a mean of 16.9 atm (SD 2.8), MSD increased by 0.26 mm (SD 0.24), optimal stent expansion increased from 21% to 58% and mean stent symmetry ratio increased from 0.83 to 0.87 (p < 0.0001).
Conclusions
Contemporary stents are sub-optimally expanded in the majority of cases after standard deployment compared with nominal sizes. Adjunctive NCB post-dilation optimized an additional 37% of stents. DSE analysis can assist in qualitative and quantitative stent assessments and can potentially facilitate a selective NCB post-dilation strategy to achieve optimal stent expansion.
Keywords: Stent expansion, Optimal stent deployment, Digital stent enhancement, Non-compliant balloon, Percutaneous coronary intervention, Quantitative coronary angiography
1. Introduction
Optimal stent expansion and larger minimum stent area (MSA) are powerful predictors of improved long-term patency and clinical outcomes 1, 2. Coronary stents are commonly under-expanded in vivo compared with nominal stent diameters 3, 4 after standard deployment. Stent deployment strategies vary among operators from the use of moderate pressures (12–14 atm) versus high pressure deployment (16–20 atm). The use of adjunctive non-compliant balloons (NCB) also varies between operators and there are no randomized controlled trials showing the benefit from routine use. Consequently, post-dilation remains at operators' discretion in clinical practice as well as in large interventional trials. NCB post-dilation was performed in 34% of Sirolimus-eluting stents in the E-SIRIUS trial and 45% of Everolimus and Paclitaxel-eluting stents in the SPIRIT IV trial 5, 6. Some of the new stent platforms have improved in-vitro expansion and radial strength characteristics, but whether this will translate into a reduced need for NCB is not known. Angiography is not sufficiently accurate to determine which stents require post-dilation 7, 8 and routine IVUS use is not feasible. Digital stent enhancement (DSE) provides improved stent visibility for qualitative assessment and has shown good correlation with IVUS for evaluation of stent diameter and area 7, 9, 10. It may therefore be a suitable alternative technology to routinely assess contemporary stent expansion and assist with decisions regarding post-dilation. We sought to study the contribution of NCB post-dilation in optimizing expansion of current-generation stents using DSE to analyze stent expansion.
2. Materials and methods
2.1. Patient population
Patients undergoing elective or semi-urgent PCI at The Canberra Hospital, Australia were eligible for enrolment. We recruited 120 patients between May and November 2012 and all patients provided informed consent for the procedure. Patients with ST elevation myocardial infarction needing emergent PCI were excluded. There were no angiographic exclusion criteria and both native and graft PCI cases were included. The subjects were unselected but not consecutive due to limitations on human resources to recruit all PCI patients. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institutional human research committee.
2.2. Interventional procedure
PCI was undertaken via femoral or radial access. Routine lesion pre-dilation and intra-coronary nitroglycerin use were recommended followed by stent deployment at nominal pressure as per the manufacturers' compliance chart or to a maximum deployment pressure of 12 atm to reduce the risk of edge dissection. All stents were routinely post-dilated with NCB to at least 14 atm. The choice of stent and NCB type, stent size, NCB size and inflation time were at operator's discretion. DSE imaging was performed before and after NCB inflation and further NCB inflations could be performed at the operator's discretion. NCB used in this study included NC Empira, Hiryu, Pantera LEO, NC Quantum Apex, NC Sprinter, and NC Trek.
2.2.1. Digital stent enhancement analysis
We used the StentBoost DSE system (Philips Medical Systems, Nederland B.V., Best, The Netherlands). A short cine run of 2.7 s at 15 fps was acquired with the deflated NCB placed within the stent. The software superimposes 40 images of the stent centered on the balloon markers and generates a high resolution stent image which is available for review within seconds. DSE images of the stent were taken before and after NCB use in the same unforeshortened view using collimators to reduce radiation exposure. Offline DSE measurements were performed using auto-calibration from the manufacturer's software which provides tools for magnifying the enhanced stent image and tracing both stent edges. We traced the outline of the stent edges along the outer silhouette on both sides. The software then calculates the minimum, mean and maximum stent diameters and percent diameter stenosis. These data are used to calculate percent stent expansion and stent symmetry.
2.3. Angiographic analysis
Quantitative Coronary Angiography (QCA) measurements were performed offline independently of DSE analysis using contrast-filled guide catheters for calibration. Angiography was also used to assess the degree of vessel calcification at the lesion as nil–mild or moderate–severe [11]. All DSE and angiographic measurements were performed by the same operator. A 10% random sample of DSE measurements was repeated by an independent operator to assess for inter-observer variability.
2.4. Endpoints
The primary endpoint of the study was the change in the minimum stent diameter (MSD) after NCB post-dilation measured using DSE. The secondary endpoint was optimal stent expansion, defined as MSD ≥ 90% of the nominal stent diameter, which was a non-IVUS adaptation of the MUSIC and POSTIT trial criteria 12, 13. We also assessed the change in the radial stent symmetry post-NCB which was defined as the minimum/maximum stent diameter with a symmetry ratio ≥ 0.7 regarded as optimal [12]. We further explored correlates of optimal stent expansion before and after NCB use in current generation stents.
2.5. Clinical outcomes
Procedural success was defined as successful stenting of the lesion with < 10% angiographic residual stenosis and TIMI 3 flow. Myocardial infarction was defined according to the third universal definition of MI [14]. Major adverse cardiovascular event (MACE) outcomes of death, myocardial infarction (MI), stroke and urgent target vessel revascularization (TVR) in-hospital and at 12 month follow-up were recorded. Follow-up was by clinic review, phone or letter.
2.6. Statistical analysis
Based on the POSTIT trial [13], we hypothesized an increase in MSD of 0.15 mm (SD 0.5) with NCB post-dilation in current-generation stents and it was calculated that a sample size of 102 patients would be required with 85% power to show this increase (alpha 0.05). We assumed a 10% loss to analyses and enrolled a total of 120 patients.
In addition to descriptive statistics, DSE measurements before and after NCB were compared using paired Student's t-tests. Categorical data were compared using the Chi-square statistic. Logistic regression analyses using the likelihood ratio method were performed to explore correlates of sub-optimal stent expansion after deployment. Probabilities of 0.05 or less were considered significant. Inter-observer agreement on a 10% random sub-sample was determined using intraclass correlation assuming absolute agreement.
3. Results
We enrolled 120 patients in this study with a mean age of 63.8 years (SD 10.9) and 84% were male. Indication for the procedure was acute coronary syndrome in 56%. Baseline characteristics including risk factors and procedure indication are outlined in Table 1. Lesion, device and deployment properties are outlined in Table 2. Interventions were performed on the left anterior descending/diagonal artery in 52% of cases followed by the right coronary artery in 25%. Complex lesions (B2/C type) accounted for 67.5% of cases according to the ACC/AHA lesion classification system and moderate or severe calcification was noted in 40% of lesions. Drug eluting stents (DES) were used in 63% of cases.
Table 1.
Baseline patient characteristics.
| Characteristic | N (%) |
|---|---|
| Age, mean (SD) years | 63.84 (10.9) |
| Male | 101 (84%) |
| Risk factors | |
| Hypertension | 82 (68.3%) |
| Diabetes mellitus | 30 (25.0%) |
| Dyslipidemia | 75 (62.5%) |
| Smoking | 20 (16.6%) |
| Reformed smoker | 51 (42.5%) |
| Family history of IHD | 45 (37.5%) |
| BMI ≥ 30 (kg/m2) | 59 (49.2%) |
| Procedure indication | |
| Recent STEMI | 12 (10.0%) |
| NSTEMI | 49 (40.8%) |
| Unstable angina | 6 (5.0%) |
| Stable angina | 53 (44.2%) |
IHD, ischemic heart disease; BMI, body mass index; STEMI, ST elevation myocardial infarction; NSTEMI, non-ST elevation myocardial infarction.
Table 2.
Lesion, device and deployment properties.
| Characteristic | N (%) |
|---|---|
| PCI artery | |
| Circumflex | 20 (16.6%) |
| Diagonal | 5 (4.2%) |
| LAD | 62 (51.6%) |
| RCA | 30 (25.0%) |
| SVG | 3 (25.0%) |
| Lesion type | |
| A | 6 (5.0%) |
| B1 | 33 (27.5%) |
| B2 | 61 (50.8%) |
| C | 20 (16.7%) |
| Calcification | |
| Nil–mild | 72 (60.0%) |
| Moderate–severe | 48 (40.0%) |
| Lesion QCA, mean (SD) | |
| Reference diameter, mm | 2.75 (0.63) |
| Minimum diameter, mm | 0.98 (0.35) |
| Diameter stenosis, % | 63 (13.2) |
| Stent type, BMS | 45 (37.5%) |
| Integrity | 4 (3.3%) |
| Kaname | 12 (10.0%) |
| Multilink 8 | 14 (11.6%) |
| Omega | 15 (12.5%) |
| Stent type, DES | 75 (62.5%) |
| Promus Element | 21 (17.5%) |
| Resolute Integrity | 21 (17.5%) |
| Xience Prime | 33 (27.5%) |
| Stent and NCB properties | Mean (SD) |
| Stent diameter, mm | 3.15 (0.46) |
| Stent length, mm | 18.05 (6.49) |
| Stent deployment pressure, atm | 11.7 (2.4) |
| Stent deployment time, s | 27 (8.6) |
| NCB diameter, mm | 3.37 (0.52) |
| NCB deployment pressure, atm | 16.85 (2.81) |
| NCB deployment time, s | 26.5 (8.0) |
LAD, left anterior descending artery; RCA, right coronary artery; SVG, saphenous vein graft; QCA, quantitative coronary angiography; BMS, bare metal stent and DES, drug eluting stent; NCB, non-compliant balloon.
Stent measurements on DSE before and after NCB inflation are shown in Table 3. The mean reference diameter on QCA was 2.75 mm (SD 0.63) and mean stent diameter was 3.15 mm (SD 0.46). After stent deployment to a maximum pressure of 12 atm, 21% of stents achieved optimal expansion and 65% of stents achieved MSD ≥ 80% on DSE analysis. Following NCB inflation at a mean pressure of 16.9 atm (SD 2.8), the minimum stent diameter (MSD) increased by 0.26 mm (SD 0.24) [2.61 mm (SD 0.05) to 2.88 mm (SD 0.48), p < 0.0001] and mean stent diameter increased by 0.22 mm (SD 0.18). The mean percentage expansion increased from 83.1% (SD 9.8) to 91.6% (SD 8.5) (p < 0.0001). The proportion of stents with optimal expansion following NCB increased from 21% to 58% (p < 0.0001) and 89.16% stents achieved MSD ≥ 80% of the nominal stent diameter. The stent symmetry increased from a mean of 0.83 (SD 0.09) pre-NCB to a mean of 0.87 (SD 0.06) post-NCB (p < 0.0001).
Table 3.
DSE Stent measurements before and after non-compliant balloon inflation.
| Characteristic | Pre-NCB Mean (SD) |
Post-NCB Mean (SD) |
p |
|---|---|---|---|
| Minimum stent diameter, mm | 2.61 (0.50) | 2.88 (0.48) | < 0.0001 |
| Mean diameter, mm | 2.88 (0.50) | 3.10 (0.49) | < 0.0001 |
| Diameter stenosis, % | 5.98 (9.5) | 4.5 (6.8) | 0.001 |
| Mean stent expansion, % | 83.1 (9.8) | 91.6 (8.5) | < 0.0001 |
| Optimal expansion, % | 21 | 58 | < 0.0001 |
| Mean stent symmetry | 0.83 (0.09) | 0.87 (0.06) | < 0.0001 |
DSE, digital stent enhancement; NCB, non-compliant balloon.
There was strong inter-observer agreement in DSE measurements before and after NCB inflation (pre-NCB, intraclass correlation 0.97, 95% CI = 0.9–0.99; post-NCB, 0.96, 95% CI = 0.86–0.99).
Table 4 shows the incidence of sub-optimal stent expansion in various subsets. Correlates of sub-optimal stent expansion after stent deployment, on univariate analysis included LAD/diagonal lesion location (p = 0.025), B2/C lesion type (p = 0.022) and moderate–severe calcification (p = 0.017). The mean percent nominal expansion for DES was significantly lower than for bare metal stents (BMS) (81% versus 86%, p 0.018) after standard stent deployment. DSE images and DSE software analysis are shown in Fig. 1, Fig. 2, Fig. 3.
Table 4.
Correlates of sub-optimal expansion after stent deployment.
| Factor | Suboptimal expansion N (%) |
Odds ratio (95% CI) |
p | |
|---|---|---|---|---|
| Factor present | Factor absent | |||
| LAD/D lesion | 58 (87%) | 37 (70%) | 2.80 (1.12–6.96) | 0.025 |
| B2/C lesion | 69 (84%) | 32 (71%) | 2.87 (1.16–7.11) | 0.022 |
| Heavy calcification | 43 (90%) | 52 (72%) | 3.31 (1.15–9.55) | 0.017 |
| Stent type: DES | 63 (84%) | 32 (71%) | 2.13 (0.87–5.21) | 0.10 |
Suboptimal expansion refers to MSD < 90% of nominal stent diameter. LAD/D, left anterior descending or diagonal artery; B2/C, lesion type based on AHA/ACC lesion classification; heavy calcification refers to moderate–severe calcification (see the Materials and methods section).
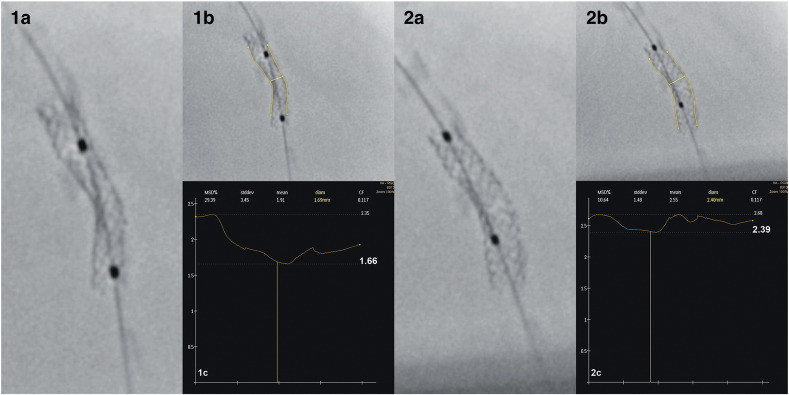
Fig. 1.
Digital stent enhancement images and software analysis before and after non-compliant balloon post-dilation. 1a—3.0 × 16 mm stent deployed at 12 atm. 1b and 1c—Software analysis tracing stent outline to measure minimum stent diameter at 1.66 mm. 2a—Stent after 3.5 mm non-compliant balloon post-dilation to 16 atm. 2b and 2c—Software analysis measuring minimum stent diameter at 2.39 mm.
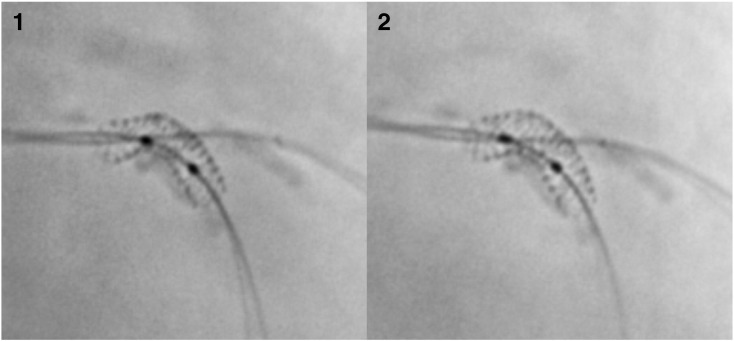
Fig. 2.
Digital stent enhancement images before and after non-compliant balloon post-dilation in the main branch of a bifurcation lesion. 1—3.0 × 16 mm stent deployed at 12 atm in the LAD artery across diagonal branch which also has a guidewire in place. The measured minimal stent diameter was 2.4 mm, mean stent diameter 2.65 mm, maximum stent diameter 2.76 mm, stent symmetry ratio 0.87, and nominal stent expansion 80%. 2—Stent after 3.0 mm non-compliant balloon post-dilation to 20 atm. The measured minimal stent diameter was 2.85 mm, mean stent diameter 3.03, maximum stent diameter 3.13 mm, stent symmetry ratio 0.91, and nominal stent expansion 95%.
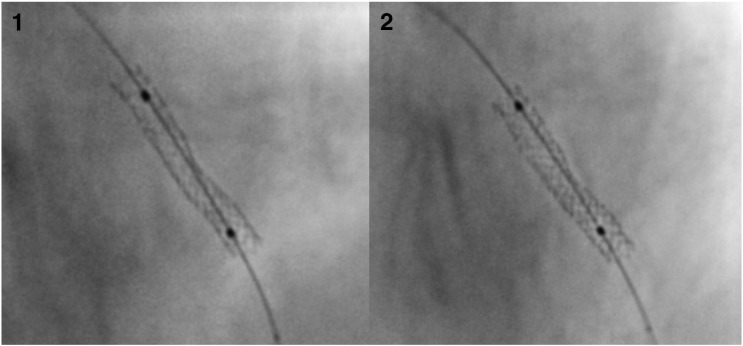
Fig. 3.
Digital stent enhancement images before and after non-compliant balloon post-dilation for assessment of stent expansion and symmetry. 1—3.0 × 20 mm stent deployed at 12 atm. The measured minimal stent diameter was 2.17 mm, mean stent diameter 2.52 mm, maximum stent diameter 2.77 mm, stent symmetry ratio 0.78, and nominal stent expansion 72.33%. 2—Stent after 3.0 mm non-compliant balloon inflation to 20 atm. The measured minimal stent diameter was 2.7 mm, mean stent diameter 3.03 mm, maximum stent diameter 3.25 mm, stent symmetry ratio 0.83, and nominal stent expansion 90%.
Procedural success rate was 100%. In-hospital MACE was 2.5% including 1 death post-operatively from heart failure and 2 peri-procedural MI. Patients were followed for a mean of 389 days apart from 4 patients who could not be contacted. One patient died from lung cancer. Repeat angiography was performed in 15 patients (13%) for clinical indications of whom 1 required repeat PCI and 1 underwent CABG.
4. Discussion
In this study we used digital stent enhancement (DSE) as a novel tool to evaluate the contribution of NCB post-dilation in optimal expansion of contemporary bare metal and drug eluting stents. We found that the minimum stent diameter (MSD) increased by a mean of 0.26 mm and the rate of optimal stent expansion from 21% to 58% following routine NCB post-dilation and this was associated with good clinical outcomes at 12 months. While the accuracy of DSE in stent analysis has previously been shown, this study documents the feasibility of using DSE technology in clinical practice to identify underexpanded stents and facilitate stent optimization with NCB post-dilation 7, 9, 10.
4.1. Strategies for optimal stent deployment
There are no published guidelines on techniques for optimal stent deployment and no evidence for a routine post-dilation strategy from randomized trials. Some operators deploy stents at 16–20 atm, others deploy stents at moderate pressures of 12–14 atm followed by routine use of high pressure NCB post-dilation, while still others deploy stents at moderate pressures and use visual assessment with angiography to decide on the need for post-dilation. High pressure stent deployment using the stent delivery system incurs the risk of “dog-boning”, edge dissection and edge restenosis [15]. It is also thought to be a possible contributor to late malapposition in DES, from damage to normal vessel wall contiguous to stent and delayed healing from the drug [16]. On the other hand, there is a better likelihood of uniform and controlled expansion using a NCB at high pressure as the dilating force is focal to the lesion. This is the rationale for stent deployment at moderately high pressures (12–14 atm) followed by post-dilation with a shorter NCB which we employed in this study [15]. Our study used DSE measurements to document the adequacy of stent expansion using this deployment strategy. One small IVUS study has shown superior DES expansion with high pressure NCB when compared with stent balloon post-dilation [17].
4.2. Prevalence and implications of stent underexpansion
Incidence of stent underexpansion after standard deployment is around 20–30% for both BMS 3, 18 and DES [19] and is a consequence of semi-compliant balloon (SCB) under-expansion against calcified or fibrotic lesions or vessel recoil [20]. In a study of second generation BMS, Costa et al. found that only 3.8% of stents achieved ≥ 90% and 24.6% of stents achieved ≥ 80% of nominal stent diameters at pressures recommended by the manufacturers' charts [4]. At moderate deployment pressures in our study, we found that 21% of stents achieved ≥ 90% and 65% of all stents achieved ≥ 80% of nominal diameters. Therefore despite improvements in stent balloon technologies in recent years, suboptimal expansion following standard deployment remains common.
Stent underexpansion has been shown to be associated with an increased incidence of in-stent restenosis, stent thrombosis, TLR and MACE at up to 12 months 1, 21, 22, 23. The recently reported AVIO randomized study of IVUS versus angiography guided stent deployment in complex lesions showed a 0.25 mm larger MSD in the IVUS group although no difference in clinical outcomes was detected up to 2 years [24]. In the larger ADAPT-DES trial, IVUS guidance was associated with a 0.2 mm larger maximum vessel diameter and a lower incidence of stent thrombosis, TVR and MACE at 12 months compared to angiographic guidance. Larger stents, larger balloons or higher inflation pressures were used in the IVUS guidance group and minimizing stent underexpansion is likely to have been a significant factor in improved outcomes in this group [25]. However, although desirable, IVUS imaging is not feasible for every case due to economic and time constraints [26]. DSE may prove to be a useful inexpensive alternative to assess stent expansion and judge the need for post-dilation.
4.3. Impact of non-compliant balloon post-dilation
In the POSTIT trial, the use of NCB at a mean of 13.8 atm increased optimal expansion in an additional 22% of stents. In our study at a mean of 16.9 atm, NCB optimized an additional 37% of all stents. It is not known however whether NCB post-dilation should be advocated selectively in sub-optimally expanded stents or routinely in all stents. The concern with routine use of NCB apart from time and economic considerations is that it can be associated with myocardial injury as indicated by a rise in serum troponin. In the recent TWENTE trial post-dilation was encouraged and was performed in 82% of stents. This may have contributed to a higher incidence of peri-procedural MI (4.1%), although the 12 month clinical target lesion revascularization (TLR) rates were lower (2.1%) compared to historic controls [27]. In our study with mandated post-dilation, only 1.6% had a peri-procedural MI. Additional data are required to investigate the association of post-dilation with troponin elevation and long term outcomes post-PCI. The routine use of digital stent enhancement to select cases for adjunctive post-dilation may potentially reduce the incidence of myocardial injury.
4.4. Predictors of stent expansion
On exploratory analyses, we found that correlates for sub-optimal stent expansion following standard deployment were LAD/diagonal lesion location, complex lesion type and moderate to severe calcification. The mean percent nominal expansion for DES was significantly lower than for BMS (81.0% versus 86%) after routine stent deployment. This may be related to physical characteristics of different stent platforms and also the lesions being treated. The small nature of our study does not allow definitive conclusions to be drawn from these findings.
4.5. Use of DSE for assessment of stent expansion
Digital stent enhancement has shown a good correlation with IVUS for measurement of stent diameter and area 7, 9 and can be used for a rapid assessment of stent expansion in every case. With automatic software analysis, it can assist with measurement of the minimum stent diameter, stent expansion and symmetry, thereby potentially facilitating an informed decision for the need to post-dilate. The technology is safe and user friendly at no added cost. Once operators became familiar with the technique of DSE analysis, the time taken for analysis per stent was under 1 min. DSE can also assist with correct stent placement in case of overlapping stents, bifurcations and in identification of strut fracture 28, 29. Stentboost guided PCI has recently been shown by Oh et al. to be associated with lower 12 month TLR and MACE rates compared with angiography alone [30]. Given these results and the high proportion of stents that are sub-optimally expanded following standard deployment, we suggest the use of DSE to assess stent expansion and to guide post-dilation strategy.
4.6. Limitations of DSE
DSE provides only 2D assessment of stent geometry in a single projection. Acquisition must therefore be obtained in the best unforeshortened view. DSE does not provide information on vessel wall, dissection and stent malapposition. In case of long stents and tortuous vessels, more than one DSE run may be required to obtain clear images of different segments of the stent. Sternal wires and other metallic objects in the field of view could interfere with the recognition of balloon markers by the software producing distorted images.
DSE imaging can increase the cumulative radiation dose per case and operators need to be vigilant about the potential radiation risk. Our mean procedural estimated effective dose was 15.75 mSv (SD 11.63) which is comparable with the reference level of 15 mSv quoted for PCI procedures by the ACC/AHA Advisory Committee [31]. This is despite the fact that 56% of our cases included diagnostic angiography prior to intervention. Recent data from Jin et al. showed that with sufficient operator experience and optimal radiation protection, Stentboost subtract imaging did not significantly increase patient radiation dose [32].
In our study, DSE measurements were performed offline. It is envisaged that future studies would investigate whether intra-operative quantitative DSE assessment can facilitate more objective post-dilation strategies to optimize stent expansion.
4.7. Study limitations
This was a single center non-randomized study that was powered to detect a change in stent expansion with NCB post-dilation. We observed significant correlations and some trends with various lesion and stent characteristics that may affect stent expansion. These are hypothesis-generating observations that merit further investigation in future studies. We did not set out to evaluate our DSE findings with IVUS as several recent studies have shown a good correlation between the two technologies for MSD. DSE imaging was assessed in a single projection and stent under-expansion in an orthogonal plane may not have been detected.
5. Conclusions
Contemporary stents are commonly sub-optimally expanded after standard deployment. Non-compliant balloon post-dilation increased the minimum stent diameter by a mean of 0.26 mm and the rate of optimal stent expansion from 21% to 58% which was associated with a low 12 month MACE rate. LAD/diagonal lesions, complex lesions and those with moderate to severe calcification as well as drug eluting stents were associated with higher sub-optimal stent expansion rates with standard deployment. Digital stent enhancement has the potential for rapid qualitative and quantitative stent assessments to facilitate a selective rather than routine post-dilation strategy.
Footnotes
Available online 19 March 2014
References
- 1.Fitzgerald P.J., Oshima A., Hayase M., Metz J.A., Bailey S.R., Baim D.S. Final results of the can routine ultrasound influence stent expansion (CRUISE) study. Circulation. 2000;102(5):523–530. doi: 10.1161/01.cir.102.5.523. [DOI] [PubMed] [Google Scholar]
- 2.Sonoda S., Morino Y., Ako J., Terashima M., Hassan A.H., Bonneau H.N. Impact of final stent dimensions on long-term results following sirolimus-eluting stent implantation: serial intravascular ultrasound analysis from the SIRIUS trial. J Am Coll Cardiol. 2004;43(11):1959–1963. doi: 10.1016/j.jacc.2004.01.044. [DOI] [PubMed] [Google Scholar]
- 3.de Ribamar Costa J., Jr., Mintz G.S., Carlier S.G., Costa R.A., Fujii K., Sano K. Intravascular ultrasonic assessment of stent diameters derived from manufacturer's compliance charts. Am J Cardiol. 2005;96(1):74–78. doi: 10.1016/j.amjcard.2005.02.049. [DOI] [PubMed] [Google Scholar]
- 4.de Ribamar Costa J., Jr., Mintz G.S., Carlier S.G., Fujii K., Sano K., Kimura M. Intravascular ultrasound assessment of drug-eluting stent expansion. Am Heart J. 2007;153(2):297–303. doi: 10.1016/j.ahj.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 5.Schofer J., Schluter M., Gershlick A.H., Wijns W., Garcia E., Schampaert E. Sirolimus-eluting stents for treatment of patients with long atherosclerotic lesions in small coronary arteries: double-blind, randomised controlled trial (E-SIRIUS) Lancet. 2003;362(9390):1093–1099. doi: 10.1016/S0140-6736(03)14462-5. [DOI] [PubMed] [Google Scholar]
- 6.Kereiakes D.J., Sudhir K., Hermiller J.B., Gordon P.C., Ferguson J., Yaqub M. Comparison of everolimus-eluting and paclitaxel-eluting coronary stents in patients undergoing multilesion and multivessel intervention: the SPIRIT III (a clinical evaluation of the investigational device XIENCE V everolimus eluting coronary stent system [EECSS] in the Treatment of subjects with de novo native coronary artery lesions) and SPIRIT IV (clinical evaluation of the XIENCE V everolimus eluting coronary stent system in the treatment of subjects with de novo native coronary artery lesions) randomized trials. JACC Cardiovasc Interv. 2010;3(12):1229–1239. doi: 10.1016/j.jcin.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Mishell J.M., Vakharia K.T., Ports T.A., Yeghiazarians Y., Michaels A.D. Determination of adequate coronary stent expansion using StentBoost, a novel fluoroscopic image processing technique. Catheter Cardiovasc Interv. 2007;69(1):84–93. doi: 10.1002/ccd.20901. [DOI] [PubMed] [Google Scholar]
- 8.Briguori C., Tobis J., Nishida T., Vaghetti M., Albiero R., Di Mario C. Discrepancy between angiography and intravascular ultrasound when analysing small coronary arteries. Eur Heart J. 2002;23(3):247–254. doi: 10.1053/euhj.2001.2730. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka N., Pijls N.H., Koolen J.J., Botman K.J., Michels H.R., Brueren B.R. Assessment of optimum stent deployment by stent boost imaging: comparison with intravascular ultrasound. Heart Vessels. 2013;28(1):1–6. doi: 10.1007/s00380-011-0202-9. [DOI] [PubMed] [Google Scholar]
- 10.Sanidas E.A., Maehara A., Barkama R., Mintz G.S., Singh V., Hidalgo A. Enhanced stent imaging improves the diagnosis of stent underexpansion and optimizes stent deployment. Catheter Cardiovasc Interv. 2013;81(3):438–445. doi: 10.1002/ccd.24353. [DOI] [PubMed] [Google Scholar]
- 11.Mintz G.S., Popma J.J., Pichard A.D., Kent K.M., Satler L.F., Chuang Y.C. Patterns of calcification in coronary artery disease. A statistical analysis of intravascular ultrasound and coronary angiography in 1155 lesions. Circulation. 1995;91(7):1959–1965. doi: 10.1161/01.cir.91.7.1959. [DOI] [PubMed] [Google Scholar]
- 12.de Jaegere P., Mudra H., Figulla H., Almagor Y., Doucet S., Penn I. Intravascular ultrasound-guided optimized stent deployment. Immediate and 6 months clinical and angiographic results from the multicenter ultrasound stenting in coronaries study (MUSIC Study) Eur Heart J. 1998;19(8):1214–1223. doi: 10.1053/euhj.1998.1012. [DOI] [PubMed] [Google Scholar]
- 13.Brodie B.R., Cooper C., Jones M., Fitzgerald P., Cummins F. Is adjunctive balloon postdilatation necessary after coronary stent deployment? Final results from the POSTIT trial. Catheter Cardiovasc Interv. 2003;59(2):184–192. doi: 10.1002/ccd.10474. [DOI] [PubMed] [Google Scholar]
- 14.Thygesen K., Alpert J.S., Jaffe A.S., Simoons M.L., Chaitman B.R., White H.D. Third universal definition of myocardial infarction. Circulation. 2012;126(16):2020–2035. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 15.Romagnoli E., Sangiorgi G.M., Cosgrave J., Guillet E., Colombo A. Drug-eluting stenting: the case for post-dilation. JACC Cardiovasc Interv. 2008;1(1):22–31. doi: 10.1016/j.jcin.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Ako J., Morino Y., Honda Y., Hassan A., Sonoda S., Yock P.G. Late incomplete stent apposition after sirolimus-eluting stent implantation: a serial intravascular ultrasound analysis. J Am Coll Cardiol. 2005;46(6):1002–1005. doi: 10.1016/j.jacc.2005.05.068. [DOI] [PubMed] [Google Scholar]
- 17.Muraoka Y., Sonoda S., Tsuda Y., Tanaka S., Okazaki M., Otsuji Y. Effect of intravascular ultrasound-guided adjuvant high-pressure non-compliant balloon post-dilation after drug-eluting stent implantation. Heart Vessels. 2011;26(6):565–571. doi: 10.1007/s00380-010-0094-0. [DOI] [PubMed] [Google Scholar]
- 18.Johansson B., Olsson H., Wennerblom B. Angiography-guided routine coronary stent implantation results in suboptimal dilatation. Angiology. 2002;53(1):69–75. doi: 10.1177/000331970205300109. [DOI] [PubMed] [Google Scholar]
- 19.Javaid A., Chu W.W., Cheneau E., Clavijo L.C., Satler L.F., Kent K.M. Comparison of paclitaxel-eluting stent and sirolimus-eluting stent expansion at incremental delivery pressures. Cardiovasc Revasc Med. 2006;7(4):208–211. doi: 10.1016/j.carrev.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Aziz S., Morris J.L., Perry R.A., Stables R.H. Stent expansion: a combination of delivery balloon underexpansion and acute stent recoil reduces predicted stent diameter irrespective of reference vessel size. Heart. 2007;93(12):1562–1566. doi: 10.1136/hrt.2006.107052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X., Doi H., Maehara A., Mintz G.S., Costa Jde R., Jr., Sano K. A volumetric intravascular ultrasound comparison of early drug-eluting stent thrombosis versus restenosis. JACC Cardiovasc Interv. 2009;2(5):428–434. doi: 10.1016/j.jcin.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Mintz G.S., Weissman N.J. Intravascular ultrasound in the drug-eluting stent era. J Am Coll Cardiol. 2006;48(3):421–429. doi: 10.1016/j.jacc.2006.04.068. [DOI] [PubMed] [Google Scholar]
- 23.Oemrawsingh P.V., Mintz G.S., Schalij M.J., Zwinderman A.H., Jukema J.W., van der Wall E.E. Intravascular ultrasound guidance improves angiographic and clinical outcome of stent implantation for long coronary artery stenoses: final results of a randomized comparison with angiographic guidance (TULIP Study) Circulation. 2003;107(1):62–67. doi: 10.1161/01.cir.0000043240.87526.3f. [DOI] [PubMed] [Google Scholar]
- 24.Chieffo A., Latib A., Caussin C., Presbitero P., Galli S., Menozzi A. A prospective, randomized trial of intravascular-ultrasound guided compared to angiography guided stent implantation in complex coronary lesions: the AVIO trial. Am Heart J. 2013;165(1):65–72. doi: 10.1016/j.ahj.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 25.Witzenbichler B., Maehara A., Weisz G., Neumann F.J., Rinaldi M.J., Metzger D.C. Relationship between intravascular ultrasound guidance and clinical outcomes after drug-eluting stents: the ADAPT-DES study. Circulation. 2013 doi: 10.1161/CIRCULATIONAHA.113.003942. [DOI] [PubMed] [Google Scholar]
- 26.Puri R., Kapadia S.R., Nicholls S.J., Harvey J.E., Kataoka Y., Tuzcu E.M. Optimizing outcomes during left main percutaneous coronary intervention with intravascular ultrasound and fractional flow reserve: the current state of evidence. JACC Cardiovasc Interv. 2012;5(7):697–707. doi: 10.1016/j.jcin.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 27.von Birgelen C., Basalus M.W., Tandjung K., van Houwelingen K.G., Stoel M.G., Louwerenburg J.H. A randomized controlled trial in second-generation zotarolimus-eluting Resolute stents versus everolimus-eluting Xience V stents in real-world patients: the TWENTE trial. J Am Coll Cardiol. 2012;59(15):1350–1361. doi: 10.1016/j.jacc.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 28.Ramegowda R.T., Chikkaswamy S.B., Bharatha A., Radhakrishna J., Krishnanaik G.B., Nanjappa M.C. Circumferential stent fracture: novel detection and treatment with the use of StentBoost. Tex Heart Inst J. 2012;39(3):431–434. [PMC free article] [PubMed] [Google Scholar]
- 29.Agostoni P., Verheye S., Vermeersch P., Cornelis K., Van Langenhove G. “Virtual” in-vivo bench test for bifurcation stenting with “StentBoost”. Int J Cardiol. 2009;133(2):e67–e69. doi: 10.1016/j.ijcard.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 30.Oh D.J., Choi C.U., Kim S., Im S.I., Na J.O., Lim H.E. Effect of StentBoost imaging guided percutaneous coronary intervention on mid-term angiographic and clinical outcomes. Int J Cardiol. 2013;168(2):1479–1484. doi: 10.1016/j.ijcard.2012.12.051. [DOI] [PubMed] [Google Scholar]
- 31.Gerber T.C., Carr J.J., Arai A.E., Dixon R.L., Ferrari V.A., Gomes A.S. Ionizing radiation in cardiac imaging: a science advisory from the American Heart Association Committee on Cardiac Imaging of the Council on Clinical Cardiology and Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention. Circulation. 2009;119(7):1056–1065. doi: 10.1161/CIRCULATIONAHA.108.191650. [DOI] [PubMed] [Google Scholar]
- 32.Jin Z., Yang S., Jing L., Liu H. Impact of StentBoost subtract imaging on patient radiation exposure during percutaneous coronary intervention. Int J Cardiovasc Imaging. 2013;29(6):1207–1213. doi: 10.1007/s10554-013-0200-3. [DOI] [PubMed] [Google Scholar]