Abstract
Understanding the cognitive processes used in creative practices is essential to design research. In this study, electroencephalography was applied to investigate the brain activations of visual designers when they responded to various types of word stimuli during design thinking. Thirty visual designers were recruited, with the top third and bottom third of the participants divided into high-creativity (HC) and low-creativity (LC) groups. The word stimuli used in this study were two short poems, adjectives with similar meanings, and adjectives with opposing meanings. The derived results are outlined as follows: (1) the brain activations of the designers increased in the frontal and right temporal regions and decreased in the right prefrontal region; (2) the negative association between the right temporal and middle frontal regions was notable; (3) the differences in activations caused by distinct word stimuli varied between HC and LC designers; (4) the spectral power in the middle frontal region of HC designers was lower than that of LC designers during the short love poem task; (5) the spectral power in the bilateral temporal regions of HC designers was higher than that of LC designers during the short autumn poem task; (6) the spectral power in the frontoparietal region of HC designers was lower than that of LC designers during the similar concept task; and (7) the spectral power in the frontoparietal and left frontotemporal regions of HC designers was higher than that of LC designers during the opposing concept task.
Keywords: Creativity, Design thinking, Electroencephalography, Text stimuli, Visual designer
Introduction
Designers are endowed with unique talents and engage in distinctive creative processes. They are typically sensitive to environmental stimuli and seek a diverse range of external references while creating a product (Liang et al. 2013). Nonetheless, few studies have examined the effects of external references other than pictures and objects. Words are one of the most essential tools people use for communication, inspiration, and persuasion. Words can influence not only the early stages of visual processing but also the later stages of reasoning and decision-making (Boutonnet and Lupyan 2015). However, research on the effects of words on design thinking is currently scant, and is rarer still regarding designers with different levels of creativity that differentiate their professional performance (Yao et al. 2017). In addition, to date, few studies have addressed the neurological basis of design—for example, by investigating inhibitory and excitatory neural oscillators (Liu et al. 2010)—or recognised or characterised design as a distinct cognitive phenomenon (Yao et al. 2017).
Designing is a dynamic process in which the hand, eye, and mind collaborate (Seitamaa-Hakkarainen et al. 2014). Understanding the cognitive processes involved in creative practices is essential to design research. Liang et al. (2017) warned that overlooking scientific evidence of cognitive reactions can lead to erroneous conclusions regarding the relationships between inspiration activities and design creativity and concerning the relationships between external stimuli and spontaneous associations. Therefore, in the current study, electroencephalography (EEG) was applied to investigate the brain activations of visual designers when they responded to different types of word stimuli during design thinking. The two major research questions were: (1) which brain regions are relatively active when visual designers engage in design thinking involving word stimuli? (2) What are the differences in brain activation between high-creativity (HC) and low-creativity (LC) designers when they engage in experimental tasks?
Prior research has suggested that both the encoding and retrieval of words are associated with increased activity in the left prefrontal and temporoparietal regions (Casasanto 2003; Davis et al. 2004; Grady et al. 1998). Maillard et al. (2010) indicated that the early stage of perception processing in words takes place in the left fusiform gyrus (in the temporooccipital lobe) and that later stages of processing corresponding to word recognition involve anterior medial temporal and ventral prefrontal structures bilaterally. A crucial transitional process between perception and recognition occurs in the left anterior rhinal cortices (in the medial temporal lobe). Previous studies suggested that there is an extended language processing network that comprises the left dorsal and ventral frontal regions, left temporal cortex, medial frontal cortex, and posterior cingulate (Perfetti and Frishkoff 2008; Schoenberg and Speckens 2015).
In addition, words can provide top-down guidance at the earliest stages of visual processing by acting as powerful categorical cues, and can also support later semantic decision processes (Boutonnet and Lupyan 2015). Wang et al. (2014) indicated that the cognitive function of information structure during language comprehension can be used to highlight the most relevant pieces of information, so that sufficient attention can be allocated. In particular, Kambe et al. (2015) suggested that beta phase resetting occurred in areas related to the subsequent stimulus, supporting the idea that the perception of multisensory stimuli is simultaneous. Neural network models have been argued to describe semantic priming effects by activating neurons that code for words that rely strongly on synaptic efficacies between pairs of neurons (Lavigne et al. 2016).
With regard to design creativity, numerous studies have suggested that the prefrontal cortex plays a critical role in creative thinking and design conceptualisation (e.g., Alexiou et al. 2009; Liu and Liang 2017). Evidence has established that the temporal cortex is also involved in creative processes (Abraham 2014; de Souza et al. 2014). Aziz-Zadeh et al. (2013) suggested that the goal-directed planning of creative solutions can be organised top-down by the left dorsolateral prefrontal cortex and by working-memory processing in the medial prefrontal cortex. De Pisapia et al. (2017) further confirmed strong connectivity between the default mode network and the executive control network during creative tasks, implying that these areas are responsible for generating novel ideas and coordinating evaluation, thereby enhancing artistic performance.
Regarding the connection between words and creativity, a prior study on the neural networks involved in artistic creativity indicated that the creativity of expert designers was quantitatively correlated with the degree of dominance of the right prefrontal cortex over the left, whereas a negative correlation with the creativity of novice designers was observed in the bilateral inferior parietal cortex (Kowatari et al. 2009). A metaanalytic study of domain-specific creativity provided evidence that verbal, musical, and visuospatial creativity activate different brain regions. In particular, verbal creativity yields activations that are mainly located in the left hemisphere, particularly in the prefrontal cortex, middle and superior temporal gyri, inferior parietal lobule, middle occipital gyrus, and insula; the right inferior frontal gyrus and the lingual gyrus were also activated (Boccia et al. 2015).
Methods
Participants and materials
In this study, 15 professional and 15 student visual designers were invited to participate in an EEG experiment. All of the expert designers had more than 10 years of design-related work experience and had led design teams specialising in graphic design. The student participants were junior or senior undergraduates majoring in design; they derived their excellent performance by following the recommendations of course instructors. Of the participants, 13 were women and 17 were men, with ages ranging from 21 to 38 years. The selection allowed for a degree of diversity in the sample, with varied levels of seniority; a broad range of design experience was thereby explored.
The participants were guided to fill out a creative personality scale (CPS; Gough 1979) questionnaire before the experiment began. Higher CPS total scores indicate higher creativity. Thus, the top and bottom thirds of the participants were subsequently divided into HC (CPS cutoff point = 6.05) and LC (CPS cutoff point = 3.96) groups for brainwave comparison analyses. Of the 10 HC designers, 4 were women and 6 were men, with ages ranging from 21 to 35 years, and 7 were right-handed and 3 were left-handed. Of the 10 LC designers, 3 were women and 7 were men, with ages ranging from 21 to 38 years, and 9 were right-handed and 1 was left-handed. All 20 participants had no history of cardiovascular disorders or of drug or alcohol abuse, and had normal or corrected-to-normal vision.
After consulting with three literature and language experts, the word stimuli used in this study were determined, namely two short poems, adjectives with similar meanings, and adjectives with opposing meanings. The eight-word short poem expresses love between couples and the 28-word short poem describes an autumn landscape from the perspective of travellers. Both are basic Classical Chinese literature texts that are learned in elementary school, so the participants were all acquainted with them. Adjectives with similar meanings that were used were ‘warm, earnest’, ‘heartfelt, sincere’, ‘friendly, loving’, and ‘tender, cordial’. Adjectives with opposite meanings were ‘warm, cold’, ‘earnest, superficial’, ‘sincere, disingenuous’, and ‘cordial, frosty’. The four sets of word stimuli were randomly presented for 60 s to the participants during the experiment.
A 32-channel wireless BR32S EEG headset (Brain Rhythm Inc., Taiwan) was used in this experiment. The scalp markers were placed according to the international 10–20 system in line with underlying cerebral structures. This headset features spring-loaded dry electrodes and a soft cap, which render it precise and convenient. This wearable system has 16-bit quantisation and a sampling rate of 250 Hz. A single reference electrode was placed on the mastoid behind the ear and the electrode impedance was kept as low as possible (≤ 5 KΩ). The EEG data were received through Bluetooth and exported in ASCII (.txt) format for analysis.
Experimental protocol
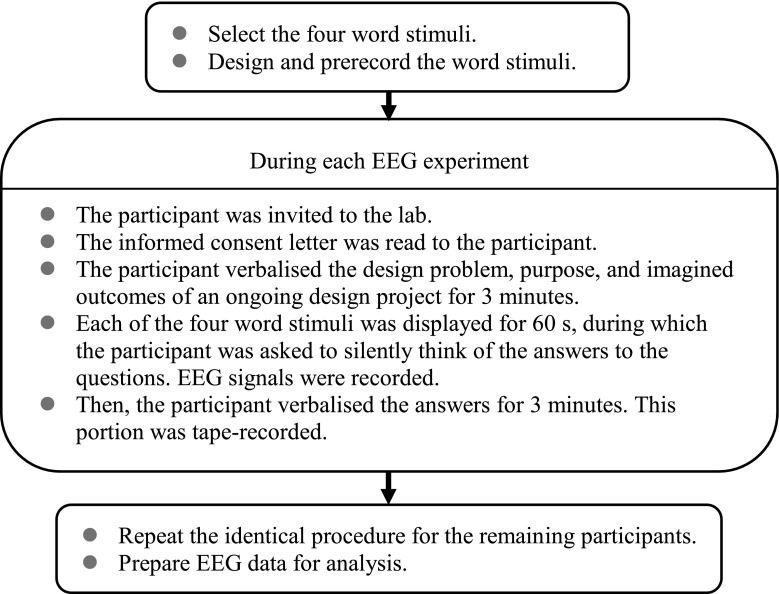
This study was approved by the Research Ethics Office of the National Taiwan University (NTU-REC No: 201706HM070). The participants were asked to sign consent forms and received a detailed explanation of the experiment. The researchers then recorded the 30-s resting-state baseline at the beginning of the experiment for potential correction. The participants watched a prerecorded presentation on a computer screen and were instructed to verbalise the design problem, purpose, and imagined outcomes of a personal design project for approximately 3 min. Each of the four word stimuli was then displayed randomly for 60 s. Then, the entire set of word stimuli was displayed on the screen. During this period, the participants were asked to silently think of answers to the questions ‘What aspect of these words can you use in the design project that was just described?’ and ‘How would you incorporate these aspects into the project?’ Their EEG signals were recorded during these sessions.
Subsequently, the participants verbalised their answers for 3 min, and were tape-recorded. The verbalised information was collected to assist the researchers in understanding the participants’ responses, rather than for a scientific comparison between brainwave activations and narrative contents. Therefore, the verbalised information was neither further used in this study nor scored as an estimate of the creativity in the design task itself. The verbalisation sessions were also treated as intertrial intervals to avoid recording overlapping brain responses. The experiment lasted approximately 40 min, including the time required for explaining the experiment, testing the EEG headset, and describing the project. The process was identical for all participants to ensure the consistency of the investigation. A conceptual diagram of the research procedure is illustrated as Fig. 1.
Fig. 1.
Conceptual diagram of the research procedure
Data analyses
A low-pass filter with a cutoff frequency of 50 Hz and a high-pass filter with a cutoff frequency of 1 Hz were applied to all signals based on FIR filters to remove line noise, oculomotor activities, and muscle movement contamination. Bad channels were deleted by kurtosis in EEGlab, using 5 standard deviations from the mean as thresholds. Accordingly, the differences between the baseline and 60-s time slots reached statistical significance. The 60-s EEG signals of each condition were split into 1.6-s epochs which were not overlapped and were trimmed to avoid edge artifacts. Some epochs were rejected manually because of electrical artifacts. EEGlab was then used to decompose the filtered EEG signals through independent component analysis (ICA) with the Infomax algorithm. ICA, which attempts to reverse the superposition by separating the EEG into mutually independent scalp maps, was applied to the concatenated epochs from all conditions. The signals of each IC were obtained from the inner product of the ICA weighting matrix and EEG signals.
In ICA, all components were grouped into several clusters according to the similarity of outcomes. The k-means clustering method on the ICs matrix was used, as well as the equivalent dipole locations. Each dipole represents the source location of an independent component as well as a specific cortex region. A best-fitting equivalent dipole was chosen for each IC. The scalp topography of each IC was used to plot the three-dimensional (3D) location of an equivalent dipole through the DIPFIT plug-in (version 2.3). DIPFIT is a unique function of the EEGlab toolbox for the localization of the IC sources of EEG data. The input of DIPFIT comprised all ICs from all participants. A threshold of residual variance from the scalp projections of these dipoles was set as 0.15 (15%); some dipole locations were rejected because of the threshold. In addition, boundary element modeling was employed.
The time-invariant correlations between clusters were obtained by averaging the component activations of each cluster, and then calculating the correlation coefficients between clusters to understand the relationship between two particular cortex regions (i.e., the correlation metric was calculated between the cluster means of component activations). Furthermore, time-domain data (the filtered EEG signals) were then transformed into frequency-domain data by using the fast Fourier transform function. A paired-sample Wilcoxon signed-rank test was applied to test the differences in the brain activity spectra of the HC and LC designers.
On the basis of previous studies (Cantero et al. 1999; Grützner et al. 2013; Tang et al. 2007), the spectra of EEG signals were separated into five frequency bands, namely delta (0.5–3.5 Hz), theta (3.5–8 Hz), alpha (range 8–13 Hz; low, 8–9 Hz; middle, 9–11 Hz; high, 11–13 Hz), beta (range 13–30 Hz; low, 13–16 Hz; middle, 16–20 Hz; high, 20–30 Hz), and gamma (range 30–100 Hz; low, 30–60 Hz; typical, 40 Hz; high > 60 Hz). Only low gamma bands ranging between 30 and 50 Hz were investigated in this study because high gamma bands rarely appear in EEG results.
Results
In this section, the scalp topography of the root cluster is illustrated first, followed by the topographies of the major component clusters and their dipole plots. The colours of scalp topographies indicate brain activity, with warm colours signifying more activation and cold colours signifying less activation. The results of the correlation analyses between components are then described. In addition, we use the Wilcoxon signed-rank test to clarify the differences in frequencies between the HC and LC designers. The significance levels of the null hypothesis at different frequencies are shown as red dots in the plots of the spectra.
Short love poem
According to the scalp map for the root cluster, when the HC and LC designers engaged in the short love poem task they had relatively low brain activation in the right prefrontal cortex and high activation in the right temporal and middle parietooccipital cortices (Fig. 2a). The scalp maps and 3D dipole plots displayed in Fig. 2b–f reveal that these brain activations could be divided into five major component clusters, namely the right temporal, right medial frontoparietal, middle occipital, left frontal, and middle frontal cortices. The 3D dipole plots indicate the equivalent dipole source locations with means and their projections onto average brain images. The correlations among these major component clusters are listed in Table 1. Accordingly, the associations were notably high for the right temporal and right medial frontoparietal, right temporal and left frontal, right medial frontoparietal and left frontal, and right medial frontoparietal and middle frontal cortices.
Fig. 2.
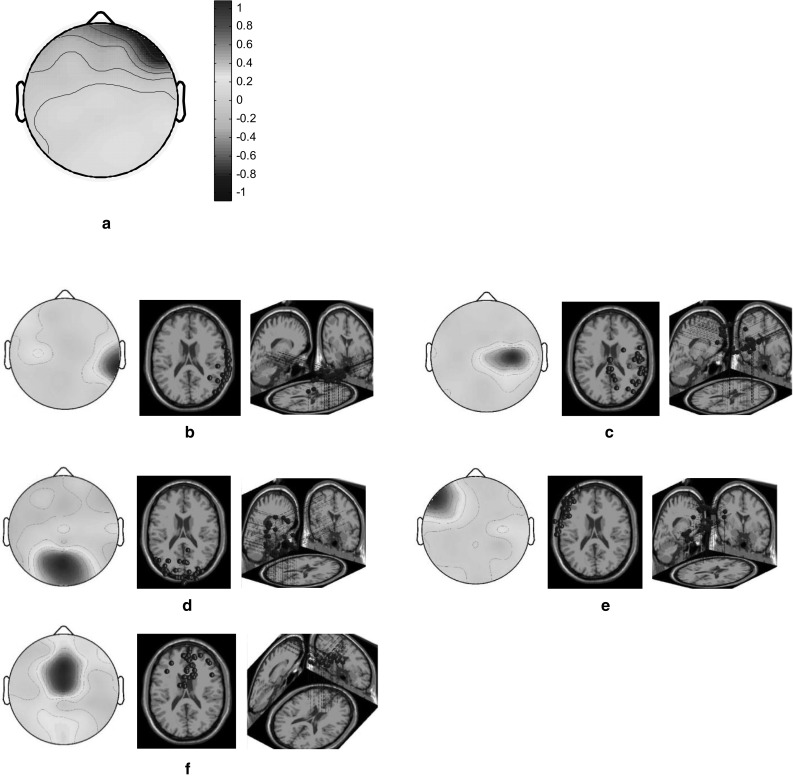
Scalp maps and 3D dipole plots for the short love poem stimulation task (a scalp map for the root cluster; b–f scalp maps for the component clusters and their 3D dipole source locations). a Root cluster, b right temporal cluster, c right medial frontoparietal cluster, d middle occipital cluster, e left frontal cluster and f middle frontal cluster
Table 1.
Correlations among the major component clusters in the short love poem stimulation task
| Component | rT | rmFP | mO | lF | mF |
|---|---|---|---|---|---|
| Right temporal cluster (rT) | 1 | −0.42*** | −0.28*** | −0.40*** | −0.22*** |
| Right medial frontoparietal cluster (rmFP) | 1 | 0.34*** | 0.51*** | 0.70*** | |
| Middle occipital cluster (mO) | 1 | 0.30*** | 0.15*** | ||
| Left frontal cluster (lF) | 1 | 0.27*** | |||
| Middle frontal cluster (mF) | 1 |
* p < 0.05; ** p < 0.01; *** p < 0.001
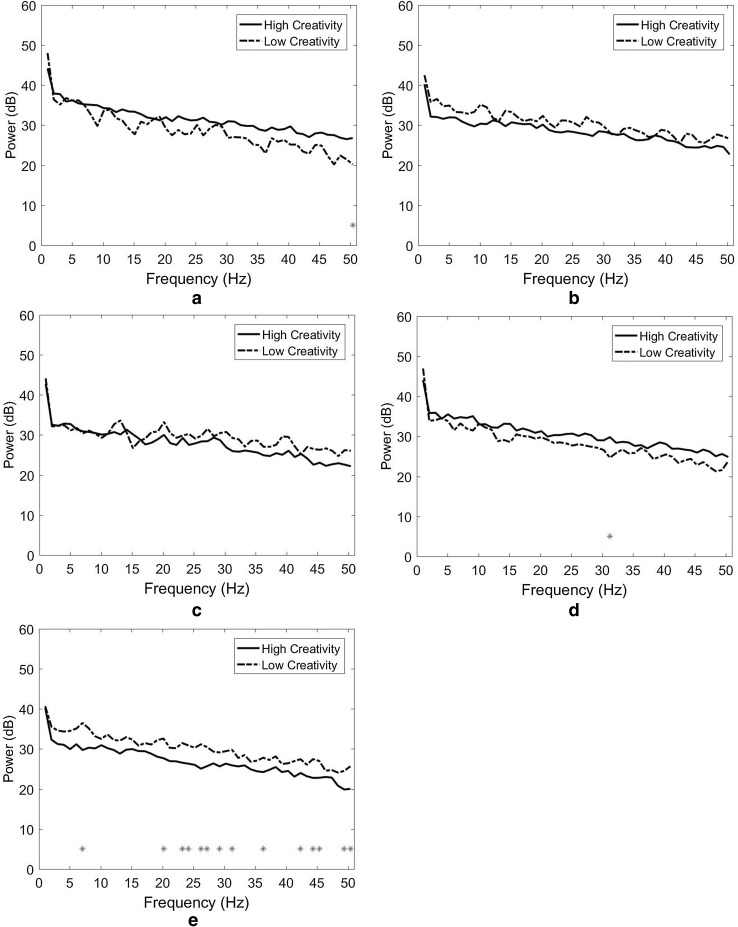
The results indicate that the differences in spectral power between HC and LC designers were limited. As depicted in Fig. 3a (the right temporal cluster), only one significant power difference was observed in the low gamma band at 50 Hz (p = 0.041). No significant power difference was observed in either the right medial frontoparietal and middle occipital clusters (Fig. 3b, c). In the left frontal cluster (Fig. 3d), only one significant power difference was observed in the low gamma band, at 31 Hz (p = 0.025). In the middle frontal cluster, significant power differences were observed in the theta, beta, and gamma bands (Fig. 3e). The largest differences appeared in the beta band at 20 Hz (p = 0.002) and 23 Hz (p = 0.009) and the low gamma band at 50 Hz (p = 0.006).
Fig. 3.
Spectral power and Wilcoxon signed-rank test for the short love poem stimulation task. a Right temporal cluster, b right medial frontoparietal cluster, c middle occipital cluster, d Left frontal cluster and e middle frontal cluster
Short autumn poem
Both the HC and LC designers exhibited high brain activation in the right temporal cortex and low activation in the left frontal cortex when they engaged in the short autumn poem task (Fig. 4a). The scalp maps and 3D dipole plots depicted in Fig. 4b–e reveal that these brain activations could be separated into four major component clusters: the left medial frontal, left occipital, right temporal, and left temporal cortices. According to Table 2, the association between right and left temporal cortices was particularly high.
Fig. 4.
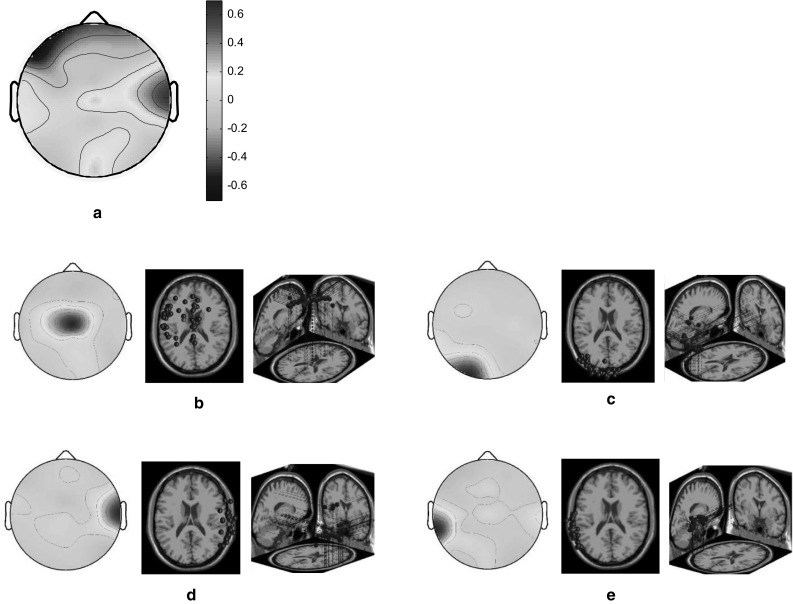
Scalp maps and 3D dipole plots for the short autumn poem stimulation results. a Root cluster, b left medial frontal cluster, c left occipital cluster, d right temporal cluster and e left temporal cluster
Table 2.
Correlations among the major component clusters in the short autumn poem stimulation task
| Component | lmF | lO | rT | lT |
|---|---|---|---|---|
| Left medial frontal cluster (lmF) | 1 | −0.13** | −0.32*** | −0.17*** |
| Left occipital cluster (lO) | 1 | −0.15*** | 0.00 | |
| Right temporal cluster (rT) | 1 | −0.54*** | ||
| Left temporal cluster (lT) | 1 |
* p < 0.05; ** p < 0.01; *** p < 0.001
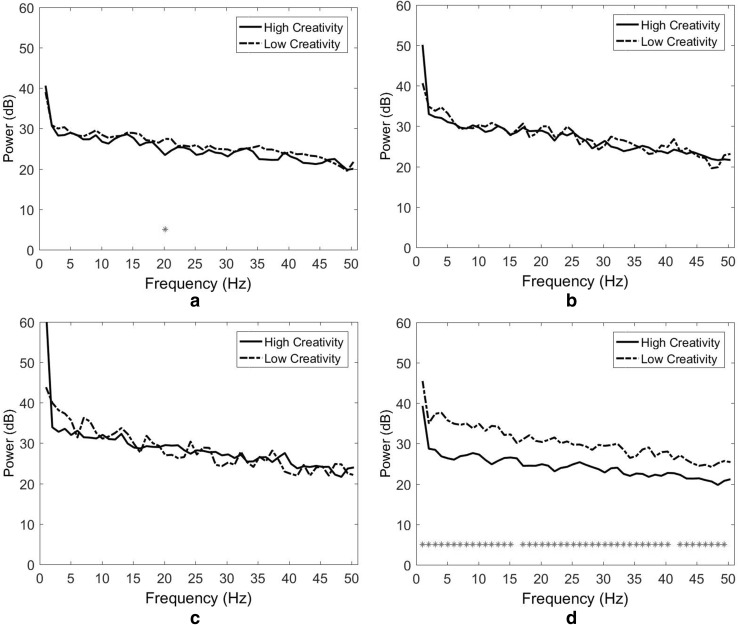
The results further revealed that the spectral power of the HC designers was generally higher than that of the LC designers. As depicted in Fig. 5a (the left medial frontal cluster), significant power differences were observed in the low gamma band. The largest differences appeared at 50 Hz (p = 0.005), 38 Hz (p = 0.010), and 46 Hz (p = 0.031). In the left occipital cluster (Fig. 5b), only one significant power difference was observed, in the delta band at 1 Hz (p = 0.032). In the right temporal cluster (Fig. 5c), significant power differences were observed at all frequencies. The largest differences appeared in the low gamma band at 49 Hz (p = 0.000), and the alpha band at 9 Hz (p = 0.000) and 10 Hz (p = 0.000). Finally, in the left temporal cluster, significant power differences were observed in the beta and gamma bands (Fig. 5d). The largest differences appeared in the low gamma band at 38 Hz (p = 0.007), 42 Hz (p = 0.009), and 45 Hz (p = 0.009).
Fig. 5.
Spectral power and Wilcoxon signed-rank tests for the short autumn poem stimulation task. a Left medial frontal cluster, b left occipital cluster, c right temporal cluster and d left temporal cluster
Adjectives with similar meanings
The HC and LC designers had relatively low brain activations in the left frontal cortex and high activations in the right temporal and middle parietal cortices when they engaged in the task involving adjectives with similar meanings (Fig. 6a). The scalp maps and 3D dipole plots displayed in Fig. 6b–e reveal that these brain activations could be divided into four major component clusters, namely the right temporal, right medial parietal, left parietooccipital, and middle frontoparietal cortices. The correlations among these major component clusters are listed in Table 3. Accordingly, the associations of the right temporal and middle frontoparietal cortices, as well as those of the left parietooccipital and middle frontoparietal cortices, were notably high.
Fig. 6.
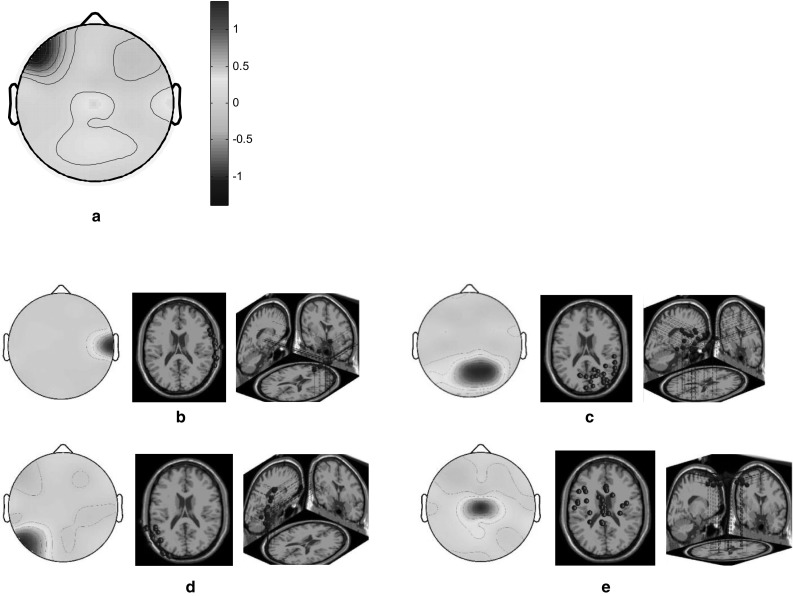
Scalp maps and 3D dipole plots for the similar concept stimulation results. a Root cluster, b right temporal cluster, c right medial parietal cluster, d left parietooccipital cluster and e middle frontoparietal cluster
Table 3.
Correlations among the major component clusters in the similar concept stimulation task
| Component | rT | rmP | lPO | mFP |
|---|---|---|---|---|
| Right temporal cluster (rT) | 1 | −0.17*** | −0.27*** | −0.39*** |
| Right medial parietal cluster (rmP) | 1 | −0.05 | 0.06 | |
| Left parietooccipital cluster (lPO) | 1 | 0.39*** | ||
| Middle frontoparietal cluster (mFP) | 1 |
* p < 0.05; ** p < 0.01; *** p < 0.001
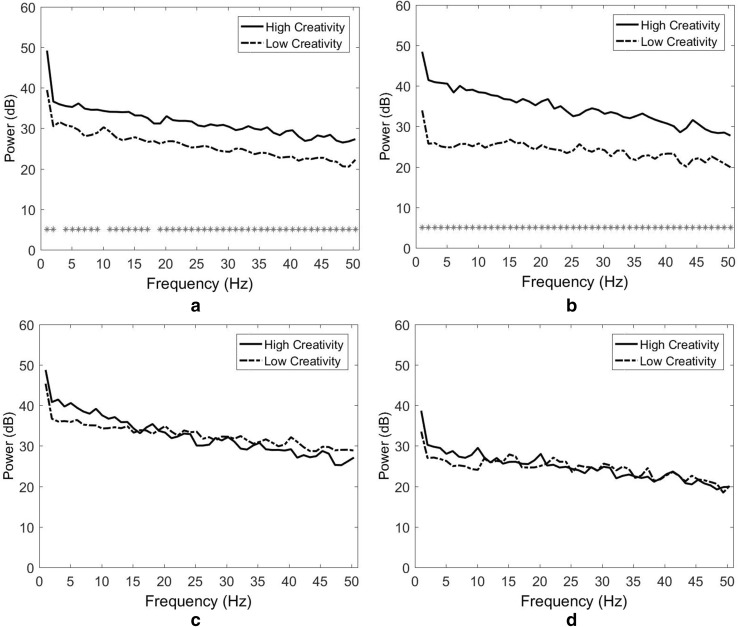
The results indicate that the differences in spectral power between HC and LC designers were limited, with exception of the middle frontoparietal cortex. As depicted in Fig. 7a (the right temporal cluster), only one significant power difference was observed in the middle beta band at 20 Hz (p = 0.035). In both the right medial parietal and left parietooccipital clusters (Fig. 7b, c) no significant power difference was observed. In the middle frontoparietal cluster (Fig. 7d) significant power differences appeared at most frequencies. The largest differences appeared in the theta band at 4 Hz (p = 0.000) and the high alpha bands at 12 Hz (p = 0.000) and 13 Hz (p = 0.000).
Fig. 7.
Spectral power and Wilcoxon signed-rank tests for the similar concept stimulation. a Right temporal cluster, b right medial parietal cluster, c left parietooccipital cluster and d middle frontoparietal cluster
Adjectives with opposing meanings
Both the HC and LC designers exhibited relatively high brain activations in the left frontal and right temporal cortices when they engaged in the task involving adjectives with opposing meanings (Fig. 8a). The scalp maps and 3D dipole plots displayed in Fig. 8b–e reveal that these brain activations can be divided into four major component clusters, namely the left frontal, middle frontoparietal, right temporal, and left temporal cortices. The correlations among these major component clusters are listed in Table 4. Accordingly, the association between the left frontal and left temporal cortices is notably high.
Fig. 8.
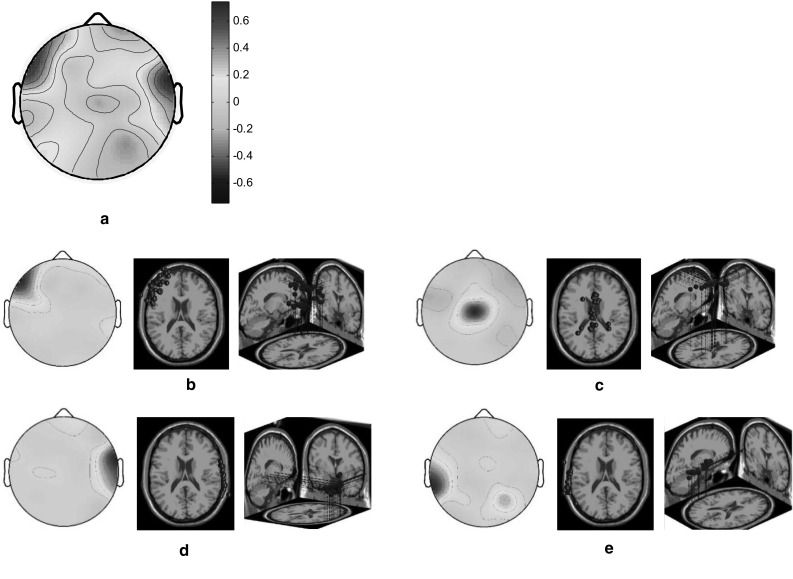
Scalp maps and 3D dipole plots for the opposing concept stimulation results. a Root cluster, b left frontal cluster, c middle frontoparietal cluster, d right temporal cluster and e left temporal cluster
Table 4.
Correlations among the major component clusters in the opposing concept stimulation task
| Component | lF | mFP | rT | lT |
|---|---|---|---|---|
| Left frontal cluster (lF) | 1 | −0.26*** | 0.22*** | 037*** |
| Middle frontoparietal cluster (mFP) | 1 | −0.22*** | −0.15*** | |
| Right temporal cluster (rT) | 1 | −0.27*** | ||
| Left temporal cluster (lT) | 1 |
* p < 0.05; ** p < 0.01; *** p < 0.001
As depicted in Fig. 9a (the left frontal cluster), significant power differences appeared at most frequencies. The largest differences appeared in the high beta band at 24 Hz (p = 0.000), the low gamma band at 46 Hz (p = 0.000), and the low beta band at 14 Hz (p = 0.001). In the middle frontoparietal cluster, significant power differences were observed at all frequencies (Fig. 9b). The largest differences appeared in the theta band at 5 Hz (p = 0.000), the middle alpha band at 11 Hz (p = 0.000), and the high beta band at 21 Hz (p = 0.000). In both the right temporal and left temporal clusters (Fig. 9b, c), no significant power difference was observed.
Fig. 9.
Spectral power and Wilcoxon signed-rank tests for the opposing concept stimulation. a Left frontal cluster, b middle frontoparietal cluster, c right temporal cluster and d left temporal cluster
Discussion
Brain activations during the short love poem task
The EEG results indicate that during the short love poem task, the right prefrontal region was deactivated and the right temporal and middle parietooccipital regions were activated. In particular, the activations of the left frontal, middle occipital, right temporal, right medial frontoparietal, and middle frontal regions were prominent. The observed deactivation of the right prefrontal cortex is in accordance with prior research (Casasanto 2003; Davis et al. 2004; Kelley et al. 1998) and indicates that nonverbal materials (pictures) activate the right prefrontal cortex. The left frontal cortex is involved in analogical reasoning (Aichelburg et al. 2016; Fine et al. 2009), revealing the corresponding reaction of designers to the selected word stimuli. The occipital region is the core of the primary visual cortex. This finding concurs with those of previous studies (Boccia et al. 2015; Maillard et al. 2010), suggesting the occipital cortex plays a key role in the perception processing and verbal creativity of visual designers.
The right temporal cortex is responsible for nonverbal memory and communication (Wisniewski et al. 2012) and is strongly activated in HC designers when they engage in visual association (Liu et al. 2017). The current study focused on design thinking caused by word stimuli, implying that visual designers tend to transform text materials into visual forms to enrich their designs. In addition, both regions of the right medial frontoparietal and middle frontal cortices are parts of the anterior cingulate cortex (ACC), which is required for detecting conflicts and resolving them by monitoring differential familiarity (Bunge et al. 2004). The short love poem used in this study was Classical Chinese poetry, which is characterised by certain traditional forms, genres, and modes and has connections with particular historical periods. The participants may have experienced mnemonic conflicts because they seldom engaged with the poem in their daily lives.
The correlations among the major components reveal the complex functional connectivity of these brain regions during the short love poem stimulation task. The negative relationship between the right temporal and left frontal cortices indicates the opposed and robustly associated reactions of nonverbal memory and verbal information processes. The negative relationship between the right temporal and right medial frontoparietal cortices demonstrates the strong, opposed response between nonverbal memory and conflict monitoring during the word stimulation task. The positive relationship between the right medial frontoparietal and left frontal cortices suggests that a closer collaboration between verbal information encoding and mnemonic conflict resolution may increase semiotic inspiration. The right medial frontoparietal and middle frontal cortices are critical parts of the ACC and the dorsal stream supports their strong positive relationship.
The results indicate that there are no differences between HC and LC designers in both the right medial frontoparietal and middle occipital regions. The power differences (HC > LC) in the right temporal cortex appear in the low gamma band. This experimental finding is explained by the notion that an increase in gamma power in the right temporal cortex is an indicator of conscious attention to nonverbal information processing and is associated with object comparison and perceptual grouping (Brunet et al. 2015; Herrmann et al. 2010). Gamma waves are also implicated during rapid eye movement, which involves visualisations (Vanderwolf 2000). This finding may imply that HC designers have an advantage over LC designers in visualising verbal information and conducting feature binding of word stimuli with design targets.
In addition, the power differences (HC > LC) in the left frontal cortex appear in the low gamma band. This result bolsters the findings of a previous study that left frontal gamma power increases when participants suppress, maintain, or amplify their emotions (Kang et al. 2014). This finding could imply that HC designers are capable of optimising the emotions caused by specific stimuli to achieve their design aims. Finally, the largest power differences (LC > HC) in the middle frontal cortex (located at the core of the ACC) predominantly appear in the beta band. The ACC is involved in conflict detection and emotion evaluation. This finding could be taken to indicate that HC designers more efficiently consume cognitive resources than do LC designers in the short love poem task. In addition, an increase of midfrontal beta oscillatory activity represents reward delivery (Mas-Herrero et al. 2015). This finding further suggests that LC designers are more strongly affected by rewarding types of verbal stimuli than HC designers.
Brain activations during the short autumn poem task
Our results indicate that the left prefrontal region is deactivated and the bilateral temporal and left occipital regions are activated during the short autumn poem task. The activations of the left medial frontal, left occipital, right temporal, and left temporal regions are particularly evident. Previous research has indicated that the left prefrontal cortex is closely associated with semantic processing, filtering out irrelevant information to ensure the efficiency of daily tasks and simultaneously blocking creative thoughts (Mayseless and Shamay-Tsoory 2015; Thompson-Schill et al. 2009). Accordingly, the deactivation of the left prefrontal cortex allowed participants to improve their creative performance.
The left medial frontal cortex is a critical part of the ACC, which is responsible for mnemonic conflict detection and resolution (Bunge et al. 2004). The short autumn poem used in this study is a renowned work describing an autumn landscape from the perspective of travellers. The scenery is described literally but the artistic conception is highly philosophical. The designer participants may have experienced conflicts when they were asked to transform the sophisticated conceptions into designs. The left occipital region is related to verbal perception and visual creativity (Boccia et al. 2015), explaining the activation of this region. The temporal lobe is involved in processing sensory input into derived meanings for the appropriate retention of visual memory, language comprehension, and emotion association (Perfetti and Frishkoff 2008; Wisniewski et al. 2012), indicating that the short autumn poem used in this study aroused not only verbal but also nonverbal memory during the short autumn poem stimulation task. Although the bilateral temporal regions can cognise histories, their highly negative association implies that the bilateral temporal cortices of designers are strongly connected but function separately.
Furthermore, the results demonstrate that the spectral power of the HC designers is higher than that of the LC designers. The largest power differences in the left medial frontal cortex appear in the low gamma band. The left medial frontal region is a crucial part of the ACC and gamma waves are associated with perceptual grouping and feature binding, suggesting that HC designers more effectively resolved the conflicts and binding features of the short autumn poem stimuli than the LC designers did. In addition, the only power difference in the left occipital cortex appears in the delta band. However, this difference was interpreted as a measurement error because delta waves seldom appear when participants are wakeful.
The largest power differences in the right temporal cortex appear in the low gamma band. As stated, an increase in gamma power in the right temporal region is an indicator of mindful attention and is associated with perceptual grouping. Therefore, this finding also implies that HC designers are more capable of visualising verbal information and conducting feature binding than LC designers. Finally, the largest power differences in the left temporal cortex primarily appear in the low gamma band. Scholars have repeatedly identified a preferential left temporal gamma activation in verbal processing tasks (Perfetti and Frishkoff 2008; Tanji et al. 2005). This finding implies that HC designers more effectively grasp verbal information than LC designers do.
Brain activations during the similar concept task
The results indicate that the left frontal region is deactivated and the right temporal and middle parietal regions are activated during the similar concept task. The activations of the right temporal, right medial parietal, left parietooccipital, and middle frontoparietal regions are particularly evident. Neuroscientific studies have suggested that the left frontal cortex is involved in brain correlates of analogical reasoning, which is critical for making inferences and adapting to novelty (Aichelburg et al. 2016; Fine et al. 2009). Because the stimuli used in this task were adjectives expressing a similar concept (‘warm’) the participants expended minimal effort on analogical reasoning, deactivating the left frontal region.
As stated, the right temporal cortex controls nonverbal memory and communication and is strongly correlated with visual association. This implies that visual designers tend to conceptualise text stimuli as visual forms to enhance their designs. The right medial parietal cortex is a crucial part of the PCC, serving to reinstate familiar contextual information from memory (Szpunar et al. 2009), appositely explaining this experimental outcome. The left parietooccipital cortex is generally involved in verbal processing, particularly in reading music (Stewart et al. 2003). Furthermore, the middle frontoparietal cortex is required for detecting and resolving conflicts. To apply the concept ‘warm’ to a specific design necessitates particular effort to narrow the gap between the stimuli and the design target.
The negative association between the right temporal and middle frontoparietal cortices indicates the opposing reaction between nonverbal memory and conflict resolution during the word stimulation task. The positive relationship between the middle frontoparietal and left parietooccipital cortices suggests a close collaboration between verbal information processing and mnemonic conflict resolution. These results indicate that most visual designers are capable of processing nonverbal communication, but that they must initiate the conflict resolution mechanism to confront word stimuli.
The results indicate that there are no differences between HC and LC designers in either the right medial parietal or left parietooccipital region. The only difference (LC > HC) in the right temporal cortex appears in the beta band. This finding could be taken to imply that HC designers expended less nonverbal mnemonic resources than LC designers in the similar concept task. In addition, the largest power differences (LC > HC) in the middle frontoparietal cortex (near the ACC) appear in the theta and alpha bands. The ACC is involved in a range of cognitive and emotive functions such as conflict monitoring and error detection, and in evaluating the emotional significance of stimuli. This finding suggests that LC designers are more activated and consume more cognitive resources than HC designers in the similar concept task. Several scholars have indicated that an increase of beta power in this region represents reward processing (Mas-Herrero et al. 2015), further suggesting that LC designers may be more influenced by rewarding types of verbal stimuli than HC designers.
Brain activations during the opposing concept task
The results indicate that the left frontal and right temporal regions were activated during the opposing concept task. The activations of the left frontal, middle frontoparietal, right temporal, and left temporal regions are particularly evident. Neuroscientists have indicated that the left frontal cortex is involved in analogical reasoning and concept formation, including the selective attention necessary for attending to multiple stimulus features and shifting between different concepts (Aichelburg et al. 2016; Fine et al. 2009), and that the right temporal cortex is responsible for nonverbal memory and communication (Wisniewski et al. 2012). Because the stimuli used in this task were adjectives with opposed concepts, the designer participants expended substantial effort in creating similarities between words with contrasting meanings. The result that both the right and left temporal cortices were activated may indicate that the adjectives with opposing meanings used in this study aroused verbal and nonverbal memories simultaneously. Moreover, the middle frontoparietal cortex is required for monitoring and resolving mnemonic conflicts, effectively explaining the requirement for this opposed concept task.
Previous neuroimaging studies have revealed that phonology-based word retrieval is mediated primarily by the left frontal cortex, whereas semantic-based word retrieval is mediated primarily by the left temporal cortex (Baldo et al. 2006; Henry and Crawford 2004). Our result further indicates a positive relationship between these two regions, suggesting that the enhanced integrative and semantic capacities supported by functional connectivity between the left frontal and left temporal cortices may lead to increasingly efficient analogical reasoning and concept formulation.
Accordingly, there are no differences between HC and LC designers with respect to both the right and left temporal regions. The largest power differences (HC > LC) in the left frontal cortex appear in the beta and gamma bands. Our study concurs with prior findings that left frontal gamma power (also beta power) is increased when participants suppress or amplify their emotions (Kang et al. 2014). Again, this finding implies that HC designers can transform emotions to enhance their design more effectively than LC designers can. In addition, the largest power differences (HC > LC) in the middle frontoparietal cortex (near the ACC) appeared in the theta, alpha, and beta bands. ACC encodes prediction error following feedback, thereby updating predictions and improving performance (Bunge et al. 2004). This finding suggests that HC designers are more capable of detecting and resolving conflicts in opposing concept stimuli than LC designers.
Research limitations
Although EEG is a promising neuroscientific tool for investigating design cognition, its use and the experimental design have certain limitations. First, although design thinking is a complex process, neuroscience studies have typically investigated simple and repeatable cognitive processes. There are numerous similarities in the activity patterns and proposed functions of these different brain regions, which complicates the determination of whether there are systematic differences between the roles of different word stimuli. Second, the present study focused only on visual designers and was limited by the number of participating designers. Thus, whether the findings would also hold true for a general population of designers that includes a full range of ages and levels of expertise is unknown. Finally, the experimental stimuli used in this study were limited to the four types of text representations, for which only Chinese words were used. Additional types of texts and other languages could be employed in future studies.
Conclusions and reflections
Despite the limitations of this study, we believe that the results provide insights into the complexities of design thinking during word stimulation and that six conclusions can be drawn. The following results serve to answer the first research question: (1) the brain activations of the visual designers notably increased in the frontal and right temporal regions and decreased markedly in the right prefrontal region during the word stimulation tasks; (2) the negative association between the right temporal and middle frontal regions is notable, irrespective of which types of word stimuli are provided; (3) the differences in activations caused by distinct word stimuli varied between the HC and LC designers.
Regarding the second research question, the four findings of this study are outlined as follows: (1) the spectral power in the middle frontal region of the HC designers was lower than that of the LC designers during the short love poem task; (2) the spectral power in the bilateral temporal regions of the HC designers was higher than that of the LC designers during the short autumn poem task; (3) the spectral power in the frontoparietal region of the HC designers was lower than that of the LC designers during the similar concept task; and (4) the spectral power in the frontoparietal and left frontotemporal regions of the HC designers was higher than that of the LC designers during the opposing concept task.
In conjunction, the research findings imply that most visual designers tend to transform text information into visual forms through the conflict resolution mechanism, thereby enriching their designs. HC designers appear to grasp verbal information more effectively, resolving mnemonic conflicts, consuming cognitive resources, conducting feature binding, and optimising their emotions more efficiently than LC designers. LC designers may be more influenced by rewarding types of verbal stimuli than HC designers. Further research is necessary to deepen our understanding of the roles the identified regions play in creative thinking during word stimulation and the relationships between these regions, and thereby clarify how they interact through their functional connectivity. Understanding these relationships can facilitate talent development in creative designers. Few studies have been conducted on the effect of words on creative thinking. The findings of this pioneering study offer new structural targets for future research on this topic.
Contributor Information
Yu-Cheng Liu, Email: brad.ycliu@gmail.com.
Chi-Cheng Chang, Email: samchang@ntnu.edu.tw.
Yu-Hsuan Sylvia Yang, Email: Sylvia.Yang@quantatw.com.
Chaoyun Liang, Email: cliang@ntu.edu.tw.
References
- Abraham A (2014) Creative thinking as orchestrated by semantic processing vs. cognitive control brain networks. Front Hum Neurosci 8, Article 95 [DOI] [PMC free article] [PubMed]
- Aichelburg C, Urbanski M, de Schotten MT, Humbert F, Levy R, Volle E. Morphometry of left frontal and temporal poles predicts analogical reasoning abilities. Cereb Cortex. 2016;26:915–932. doi: 10.1093/cercor/bhu254. [DOI] [PubMed] [Google Scholar]
- Alexiou K, Zamenopoulos T, Johnson JH, Gilbert SJ. Exploring the neurological basis of design cognition using brain imaging: some preliminary results. Des Stud. 2009;30(6):623–647. doi: 10.1016/j.destud.2009.05.002. [DOI] [Google Scholar]
- Aziz-Zadeh L, Liew S-L, Dandekar F. Exploring the neural correlates of visual creativity. Soc Cogn Affect Neurosci. 2013;8(4):475–480. doi: 10.1093/scan/nss021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo JV, Schwartz S, Wilkins D, Dronkers NF. Role of frontal versus temporal cortex in verbal fluency as revealed by voxel-based lesion symptom mapping. J Int Neuropsychol Soc. 2006;12:896–900. doi: 10.1017/S1355617706061078. [DOI] [PubMed] [Google Scholar]
- Boccia M, Piccardi L, Palermo L, Nori R, Palmiero M (2015) Where do bright ideas occur in our brain? Meta-analytic evidence from neuroimaging studies of domain-specific creativity. Front Psychol 6, Article 1195 [DOI] [PMC free article] [PubMed]
- Boutonnet B, Lupyan G. Words jump-start vision: a label advantage in object recognition. J Neurosci. 2015;35(25):9329–9335. doi: 10.1523/JNEUROSCI.5111-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet N, Bosman CA, Roberts M, Oostenveld R, Womelsdorf T, De Weerd P, Fries P. Visual cortical gamma-band activity during free viewing of natural images. Cereb Cortex. 2015;25(4):918–926. doi: 10.1093/cercor/bht280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Burrows B, Wagner AD. Prefrontal and hippocampal contributions to visual associative recognition: Interactions between cognitive control and episodic retrieval. Brain Cogn. 2004;56(2):141–152. doi: 10.1016/j.bandc.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Cantero JL, Atienza M, Gómez CM, Salas RM. Spectral structure and brain mapping of human alpha activities in different arousal states. Neuropsychobiology. 1999;39(2):110–116. doi: 10.1159/000026569. [DOI] [PubMed] [Google Scholar]
- Casasanto D. Hemispheric specialization in prefrontal cortex: Effects of verbalizability, imageability and meaning. J Neurolinguist. 2003;16:361–382. doi: 10.1016/S0911-6044(03)00020-4. [DOI] [Google Scholar]
- Davis MH, Meunier F, Marslen-Wilson WD. Neural responses to morphological, syntactic, and semantic properties of single words: an fMRI study. Brain Lang. 2004;89:439–449. doi: 10.1016/S0093-934X(03)00471-1. [DOI] [PubMed] [Google Scholar]
- De Pisapia N, Bacci F, Parrott D, Melcher D (2017) Brain networks for visual creativity: a functional connectivity study of planning a visual artwork. Sci Rep 6, Article 39185 [DOI] [PMC free article] [PubMed]
- De Souza LC, Guimarães HC, Teixeira AL, Caramelli P, Levy R, Dubois B, Volle E (2014) Frontal lobe neurology and the creative mind. Front Psychol 5, Article 761 [DOI] [PMC free article] [PubMed]
- Fine EM, Delis DC, Dean D, Beckman V, Miller BL, Rosen HJ, Kramer JH. Left frontal lobe contributions to concept formation: a quantitative MRI study of D-KEFS sorting test performance. J Clin Exp Neuropsychol. 2009;31(5):624–631. doi: 10.1080/13803390802419017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough HG. A creative personality scale for the adjective checklist. J Pers Soc Psychol. 1979;37:1398–1405. doi: 10.1037/0022-3514.37.8.1398. [DOI] [Google Scholar]
- Grady CL, McIntosh AR, Rajah MN, Craik IM. Neural correlates of the episodic encoding of pictures and words. Proc Nat Acad Sci PNAS. 1998;95:2703–2708. doi: 10.1073/pnas.95.5.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grützner C, Wibral M, Sun M, Rivolta D, Singer W, Maurer K, Uhlhaas PJ (2013) Deficits in high- (>60 Hz) gamma-band oscillations during visual processing in schizophrenia. Front Hum Neurosci 7, Article 88 [DOI] [PMC free article] [PubMed]
- Henry JD, Crawford JR. A meta-analytic review of verbal fluency performance following focal cortical lesions. Neuropsychology. 2004;18:284–295. doi: 10.1037/0894-4105.18.2.284. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Fründ I, Lenz D. Human gamma-band activity: A review on cognitive and behavioural correlates and network models. Neurosci Biobehav Rev. 2010;34(7):981–992. doi: 10.1016/j.neubiorev.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Kambe J, Kakimoto Y, Araki O. Phase reset affects auditory-visual simultaneity judgment. Cogn Neurodyn. 2015;9(5):487–493. doi: 10.1007/s11571-015-9342-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JH, Jeong JW, Kim HT, Kim SH, Kim SP. Representation of cognitive reappraisal goals in frontal gamma oscillations. PLoS ONE. 2014;9(11):e113375. doi: 10.1371/journal.pone.0113375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WM, Miezin FM, McDermott KB, et al. Hemispheric specialization in human dorsl frontal cortex and medial temporal lobe for verbal and nonverbal encoding. Neuron. 1998;20:927–936. doi: 10.1016/S0896-6273(00)80474-2. [DOI] [PubMed] [Google Scholar]
- Kowatari Y, Lee SH, Yamamura H, Nagamori Y, Levy P, Yamane S, Yamamoto M. Neural networks involved in artistic creativity. Hum Brain Mapp. 2009;30(5):1678–1690. doi: 10.1002/hbm.20633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne F, Longrée D, Mayaffre D, Mellet S. Semantic integration by pattern priming: experiment and cortical network model. Cogn Neurodyn. 2016;10(6):513–533. doi: 10.1007/s11571-016-9410-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C, Hsu Y, Chang C-C, Lin L-J. In search of an index of imagination for virtual experience designers. Int J Technol Des Educ. 2013;23(4):1037–1046. doi: 10.1007/s10798-012-9224-6. [DOI] [Google Scholar]
- Liang C, Lin C-T, Yao S-N, Chang W-S, Liu Y-C, Chen S-A. Visual attention and association: an electroencephalography study in expert designers. Des Stud. 2017;48:76–95. doi: 10.1016/j.destud.2016.11.002. [DOI] [Google Scholar]
- Liu Y-C, Liang C. Investigating how the brain activations of visual attention differ among designers with different levels of creativity. In: Delagarza D, editor. New developments in visual attention research. Hauppauge: Nova Science Publishers; 2017. pp. 167–194. [Google Scholar]
- Liu Y, Wang R, Zhang Z, Jiao X. Analysis on stability of neural network in the presence of inhibitory neurons. Cogn Neurodyn. 2010;4(1):61–68. doi: 10.1007/s11571-009-9100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y-C, Yang Y-H, Liang C (2017) How do creativity levels and stimulus types matter? A preliminary investigation of designer visual association. J Neurol Neurosci 8(2), article 185, 1–13
- Maillard L, Barbeau EJ, Baumann C, Koessler L, Bénar C, Chauvel P, Liégeois-Chauvel C. From perception to recognition memory: Time course and lateralization of neural substrates of word and abstract picture processing from perception to recognition memory. J Cogn Neurosci. 2010;23(4):782–800. doi: 10.1162/jocn.2010.21434. [DOI] [PubMed] [Google Scholar]
- Mas-Herrero E, Ripollés P, HajiHosseini A, Rodríguez-Fornells A, Marco-Pallarés J. Beta oscillations and reward processing: coupling oscillatory activity and hemodynamic responses. Neuroimage. 2015;119:13–19. doi: 10.1016/j.neuroimage.2015.05.095. [DOI] [PubMed] [Google Scholar]
- Mayseless N, Shamay-Tsoory SG. Enhancing verbal creativity: modulating creativity by altering the balance between right and left inferior frontal gyrus with tDGS. Neuroscience. 2015;291:167–176. doi: 10.1016/j.neuroscience.2015.01.061. [DOI] [PubMed] [Google Scholar]
- Perfetti CA, Frishkoff GA. The neural bases of text and discourse processing. In: Stemmer B, Whitaker HA, editors. Handbook of the neuroscience of language. New York: Elsevier; 2008. pp. 165–174. [Google Scholar]
- Schoenberg PLA, Speckens AEM. Multi-dimensional modulations of α and γ cortical dynamics following mindfulness-based cognitive therapy in Major Depressive Disorder. Cogn Neurodyn. 2015;9(1):13–29. doi: 10.1007/s11571-014-9308-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitamaa-Hakkarainen P, Huotilainen M, Mäkelä M, Groth C, Hakkarainen K (2014) The promise of cognitive neuroscience in design studies. In: Lim Y, Niedderer K, Redström J, Stolterman E, Valtonen A (eds) Proceedings of DRS 2014: design’s big debates: design research society biennial international conference, 16–19 June, Umeå, Sweden, pp 834–846
- Stewart L, Henson R, Kampe K, Walsh V, Turner R, Frith U. Brain changes after learning to read and play music. Neuroimage. 2003;20:71–83. doi: 10.1016/S1053-8119(03)00248-9. [DOI] [PubMed] [Google Scholar]
- Szpunar KK, Chan JC, McDermott KB. Contextual processing in episodic future thought. Cereb Cortex. 2009;19(7):1539–1548. doi: 10.1093/cercor/bhn191. [DOI] [PubMed] [Google Scholar]
- Tang Y, Chorlian DB, Rangaswamy M, Porjesz B, Bauer L, Kuperman S, O’Connor S, Rohrbaugh J, Schuckit M, Stimus A, Begleiter H. Genetic influences on bipolar EEG power spectra. Int J Psychophysiol. 2007;65(1):2–9. doi: 10.1016/j.ijpsycho.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Tanji K, Suzuki K, Delorme A, Shamoto H, Nakasato N. High-frequency gamma-band activity in the basal temporal cortex during picture-naming and lexical-decision tasks. J Neurosci. 2005;25(13):3287–3293. doi: 10.1523/JNEUROSCI.4948-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, Ramscar M, Chrysikou GE. Cognition without control: when a little frontal lobe goes a long way. Curr Dir Psychol Sci. 2009;18(5):259–263. doi: 10.1111/j.1467-8721.2009.01648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwolf CH. Are neocortical gamma waves related to consciousness? Brain Res. 2000;855(2):217–224. doi: 10.1016/S0006-8993(99)02351-3. [DOI] [PubMed] [Google Scholar]
- Wang L, Li X, Yang Y. A review on the cognitive function of information structure during language comprehension. Cogn Neurodyn. 2014;8(5):353–361. doi: 10.1007/s11571-014-9305-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski I, Wendling AS, Manning L, Steinhoff BJ. Visuo-spatial memory tests in right temporal lobe epilepsy foci: clinical validity. Epilepsy Behav. 2012;23(3):254–260. doi: 10.1016/j.yebeh.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Yao S-N, Lin C-T, King J-T, Liu Y-C, Liang C. Learning in the visual association of novice and expert designers. Cogn Syst Res. 2017;43:76–88. doi: 10.1016/j.cogsys.2017.01.005. [DOI] [Google Scholar]